Iron Chelate Improves Rooting in Indole-3-Butyric Acid-Treated Rosemary (Rosmarinus officinalis) Stem Cuttings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Greenhouse and Propagation Conditions
2.2. Plant Material and Growth Conditions
2.3. Plant Morphological Traits
2.4. Pigment Analysis
2.5. Statistical Analysis
3. Results
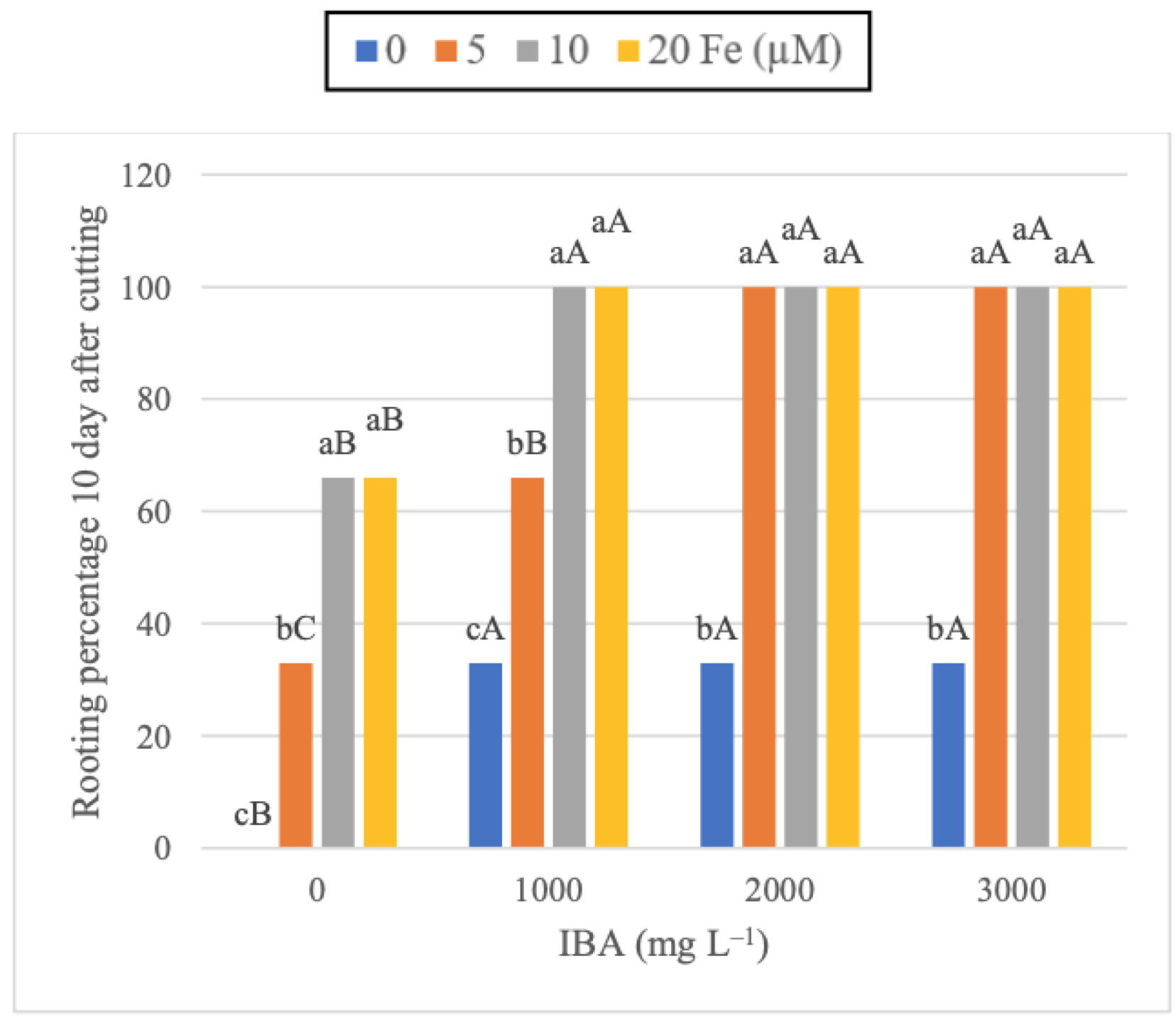
3.1. Application of Fe Enhance the Rooting Performance in IBA-Treated Cuttings
3.2. Application of Fe Increase Biomass in IBA-Treated Root Cuttings
3.3. Application of Fe Increase Root Length and Number in IBA-Treated Root Cuttings
3.4. Application of Fe-Chelate Causes Positive Changes in Shoot Parameters by IBA
3.5. Fe-Chelate Increase Leaf Photosynthetic Pigment Concentration of Cuttings
3.6. Cutting Survival Percentage Increased by Applying IBA and Fe-Chelate Simultaneously
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hartmann, H.T.; Kester, D.E. Plant Propagation: Principles and Practices, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1975. [Google Scholar]
- Bassuk, N.L.; Hunter, L.D.; Howard, B.H. The apparent involvement of polyphenol oxidase and phloridzin in the production of apple rooting cofactors. J. Hortic. Sci. 1981, 56, 313–322. [Google Scholar] [CrossRef]
- Ling, W.X.; Zhong, Z. Seasonal variation in rooting of the cuttings from tetraploid locust in relation to nutrients and endogenous plant hormones of the shoot. Turk. J. Agric. For. 2010, 36, 257–266. [Google Scholar]
- Hilo, A.; Shahinnia, F.; Druege, U.; Franken, P.; Melzer, M.; Rutten, T.; von Wirén, N.; Hajirezaei, M.-R. A specific role of iron in promoting meristematic cell division during adventitious root formation. J. Exp. Bot. 2017, 68, 4233–4247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.C.; du Toit, E.S.; Reinhardt, C.F.; Rimando, A.M.; van der Kooy, F.; Meyer, J.J.M. The phenolic, 3,4-dihydroxybenzoic acid, is an endogenous regulator of rooting in Protea cynaroides. Plant Growth Regul. 2007, 52, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Epstein, E.; Lavee, S. Conversion of indole-3-butyric acid to indole-3-acetic acid by cuttings of grapevine (Vitis vinifera) and olive (Olea europaea). Plant Cell Physiol. 1984, 25, 697–703. [Google Scholar]
- Al-Zebari, S.M.K.; Al-Brifkany, A.-A.A.M. Effect of cutting type and IBA on rooting and growth of citron (Citrus medica L). J. Exp. Agric. Int. 2015, 5, 134–138. [Google Scholar] [CrossRef]
- Nasri, F.; Fadakar, A.; Saba, M.K.; Yousefi, B. Study of indole butyric acid (IBA) effects on cutting rooting improving some of wild genotypes of damask roses (Rosa damascena Mill.). J. Agric. Sci. 2015, 60, 263–275. [Google Scholar] [CrossRef]
- Izadi, Z.; Zarei, H. Evaluation of propagation of chinese hibiscus (Hibiscus rosa-sinensis) through stenting method in response to different IBA concentrations and rootstocks. Am. J. Plant Sci. 2014, 5, 1836–1841. [Google Scholar] [CrossRef] [Green Version]
- Peixe, A.; Raposo, A.; Lourenço, R.; Cardoso, H.; Macedo, E. Coconut water and BAP successfully replaced zeatin in olive (Olea europaea L.) micropropagation. Sci. Hortic. 2007, 113, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hussain, K.; Qadri, R.; Akram, M.T.; Nisar, N.; Iqbal, A.; Yang, Y.; Khan, M.M.; Haq, I.U.; Khan, R.I.; Iqbal, M.A. Clonal propagation of olive (Olea europaea) through semi-hardwood cuttings using IBA under shaded polyethylene tunnels (SPTS). Fresenius Environ. Bull. 2020, 29, 8131–8137. [Google Scholar]
- Shao, F.; Wang, S.; Huang, W.; Liu, Z. Effects of IBA on the rooting of branch cuttings of Chinese jujube (Zizyphus jujuba Mill.) and changes to nutrients and endogenous hormones. J. For. Res. 2017, 29, 1557–1567. [Google Scholar] [CrossRef]
- Amini, A.; Tabari Kouchaksaraei, M.; Hosseini, S.M.; Yousefzadeh, H. Influence of Hormones Of IAA, IBA, And NAA On Improvement of rooting and early growth gf Tilia Rubra Subsp. Caucasica Form Angulata (Rupr.) V. Engler. Ecopersia 2019, 3, 169–174. [Google Scholar]
- Schwambach, J.; Fadanelli, C.; Fett-Neto, A.G. Mineral nutrition and adventitious rooting in microcuttings of Eucalyptus globulus. Tree Physiol. 2005, 25, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Brondani, G.E.; Baccarin, F.J.B.; de Wit Ondas, H.W. Low temperature, IBA concentrations and optimal time for adventitious rooting of Eucalyptus benthamii mini-cutting. J. For. Res. 2012, 23, 583–592. [Google Scholar] [CrossRef]
- Azad, M.S.; Alam, M.J.; Mollick, A.S.; Khan, M.N.I. Rooting of cuttings of the Indian almond (Sterculia foetida) enhanced by the application of the indole-3-butyric acid (IBA) under leafy and non-leafy conditions. Rhizosphere 2017, 5, 8–15. [Google Scholar] [CrossRef]
- Vielba, J.M.; Vidal, N.; José, M.C.S.; Rico, S.; Sánchez, C. Recent advances in adventitious root formation in chestnut. Plants 2020, 9, 1543. [Google Scholar] [CrossRef]
- Bannoud, F.; Bellini, C. Adventitious rooting in Populus species: Update and perspectives. Front Plant. Sci. 2021, 12, 918. [Google Scholar] [CrossRef]
- Xiao, Z.; Jin, Z.; Zhang, B.; Li, F.; Yu, F.; Zhang, H.; Lu, X.; Zhang, J. Effects of IBA on rooting ability of Cinnamomum bodinieri citral type micro-shoots from transcriptomics analysis. Plant Biotechnol. Rep. 2020, 14, 467–477. [Google Scholar] [CrossRef]
- Otiende, M.A.; Nyabundi, J.O.; Ngamau, K.; Opala, P. Effects of cutting position of rose rootstock cultivars on rooting and its relationship with mineral nutrient content and endogenous carbohydrates. Sci. Hortic. 2017, 225, 204–212. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Boston, MA, USA, 2012. [Google Scholar]
- Landsberg, E.-C. Hormonal regulation of iron-stress response in sunflower roots: A morphological and cytological investigation. Protoplasma 1996, 194, 69–80. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wang, B.; Song, W.F.; Zheng, S.J.; Shen, R.F. Putrescine alleviates iron deficiency via NO-dependent reutilization of root cell-wall Fe in Arabidopsis. Plant Physiol. 2016, 170, 558–567. [Google Scholar] [CrossRef]
- Johnson, C.R.; Hamilton, D.F. Effects of media and controlled-release fertilizers on rooting and leaf nutrient composition of Juniperus conferta and Ligustrum japonicum cuttings. J. Am. Soc. Hortic. Sci. 1977, 102, 320–322. [Google Scholar]
- Ward, J.D.; Whitcomb, C.E. Nutrition of Japanese holly during propagation and production. J. Am. Soc. Hortic. Sci. 1979, 104, 523–526. [Google Scholar]
- Tsipouridis, C.; Thomidis, T.; Zakinthinos, Z. Iron deficiency and adventitious rooting in peach hardwood cuttings (cv. Early Crest). Austr. J. Exp. Agric. 2006, 46, 1629–1632. [Google Scholar] [CrossRef]
- De Pasquale, C.; La Bella, S.; Cammalleri, I.; Gennaro, M.C.; Licata, M.; Leto, C.; Tuttolomondo, T. Agronomical and postharvest evaluation of the essential oils of Sicilian rosemary (Rosmarinus officinalis L.) biotypes. Acta Hortic. 2019, 1255, 139–144. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.M.; Cicero, N.; Gervasi, T.; Virga, G.; Leone, R.; Licata, M.; et al. Study of quantitative and qualitative variations in essential oils of Sicilian Rosmarinus officinalis L. Nat. Prod. Res. 2015, 29, 1928–1934. [Google Scholar] [CrossRef]
- La Bella, S.; Virga, G.; Iacuzzi, N.; Licata, M.; Sabatino, L.; Consentino, B.B.; Leto, C.; Tuttolomondo, T. Effects of irrigation, peat-alternative substrate and plant habitus on the morphological and production characteristics of Sicilian rosemary (Rosmarinus officinalis L.) biotypes grown in pot. Agriculture 2021, 11, 13. [Google Scholar] [CrossRef]
- Gil, C.S.; Kwon, S.J.; Jeong, H.Y.; Lee, C.; Lee, O.J.; Eom, S.H. Blue light upregulates auxin signaling and stimulates root formation in irregular rooting of rosemary cuttings. Agronomy 2021, 11, 1725. [Google Scholar] [CrossRef]
- Kiuru, P.; Muriuki, S.; Wepukhulu, S.; Muriuki, S. Influence of growth media and regulators on vegetative propagation of rosemary (Rosmarinus officinalis L.). East Afr. Agric. For. J. 2015, 81, 105–111. [Google Scholar] [CrossRef]
- Koleva Gudeva, L.; Trajkova, F.; Mihajlov, L.; Troiciki, J. Influence of different auxins on rooting of rosemary, sage and elderberry. Ann. Res. Rev. Biol. 2017, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Poornima, K.; Chandregowda, M.; Pushpa, T.; Srikantaprasad, D. Studies on effect of growth regulators on rooting of two rosemary types and estimation of biochemical changes associated with rooting. Crop Res. 2012, 43, 245–248. [Google Scholar]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T., Jr.; Geneve, R.L. Plant Propagation: Principles and Practice, 7th ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 2002. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Björkman, T. Effect of Trichoderma colonization on auxin-mediated regulation of root elongation. Plant Growth Regul. 2004, 43, 89–92. [Google Scholar] [CrossRef]
- Sun, W.-Q.; Bassuk, N.L. Effects of banding and IBA on rooting and budbreak in cuttings of apple rootstock ‘MM. 106′ and Franklinia. J. Environ. Hortic. 1991, 9, 40–43. [Google Scholar] [CrossRef]
- Trejgell, A.; Libront, I.; Tretyn, A. The effect of Fe-EDDHA on shoot multiplication and in vitro rooting of Carlina onopordifolia Besser. Acta Physiol. Plant. 2012, 34, 2051–2055. [Google Scholar] [CrossRef]
- Svenson, S.E.; Davies, F.T. Change in tissue mineral elemental concentration during root initiation and development of poinsettia cuttings. HortScience 1995, 30, 617–619. [Google Scholar] [CrossRef]
- Rowe, D.B.; Blazich, F.A.; Weir, R.J. Mineral nutrient and carbohydrate status of loblolly pine during mist propagation as influenced by stock plant nitrogen fertility. HortScience 1999, 34, 1279–1285. [Google Scholar] [CrossRef]
- Abadía, J.; Vázquez, S.; Rellán-Álvarez, R.; El-Jendoubi, H.; Abadía, A.; Álvarez-Fernández, A.; López-Millán, A.F. Towards a knowledge-based correction of iron chlorosis. Plant Physiol. Biochem. 2011, 49, 471–482. [Google Scholar] [CrossRef]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trend. Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Morales, F.; Abadía, A.; Belkhodja, R.; Abadía, J. Iron-deficiency-induced changes on the photosynthetic pigment composition of field-grown pear (Pyrus communis L.) leaves. Plant Cell Environ. 1994, 17, 1153–1160. [Google Scholar] [CrossRef]
- Abadía, J.; Tagliavini, M.; Grasa, R.; Belkhodja, R.; Abadía, A.; Sanz, M.; Faria, E.A.; Tsipouridis, C.; Marangoni, B. Using the flower Fe concentration for estimating chlorosis status in fruit tree orchards: A summary report. J. Plant Nutr. 2000, 23, 2024–2033. [Google Scholar] [CrossRef]
- Terry, N.; Abadía, J. Biochemistry and physiology of iron. J. Plant Nutr. 1986, 9, 609–646. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Ahn, Y.O.; Ahn, M.-J.; Lee, H.-S.; Kwak, S.-S. Down-regulation of β-carotene hydroxylase increases β-carotene and total carotenoids enhancing salt stress tolerance in transgenic cultured cells of sweetpotato. Phytochemistry 2012, 74, 69–78. [Google Scholar] [CrossRef]
- Vigani, G.; Zocchi, G.; Bashir, K.; Philippar, K.; Briat, J.-F. Signals from chloroplasts and mitochondria for iron homeostasis regulation. Trends Plant Sci. 2013, 18, 305–311. [Google Scholar] [CrossRef]
- Izadi, Z.; Rezaei Nejad, A.; Abadía, J. Physio-morphological and biochemical responses of pot marigold (Calendula officinalis L.) to split iron nutrition. Acta Physiol. Plant. 2020, 42, 6. [Google Scholar] [CrossRef]
- Izadi, Z.; Rezaei Nejad, A.; Abadía, J. Foliar applications of thidiazuron and putrescine increase leaf iron and chlorophyll concentrations in iron-deficient pot marigold (Calendula officinalis L.). Acta Physiol. Plant. 2021, 43, 122. [Google Scholar] [CrossRef]
- El-Jendoubi, H.; Melgar, J.C.; Álvarez-Fernández, A.; Sanz, M.; Abadía, A.; Abadía, J. Setting good practices to assess the efficiency of iron fertilizers. Plant Physiol. Biochem. 2011, 49, 483–488. [Google Scholar] [CrossRef]
- Izadi, Z.; Zarei, H.; Alizadeh, M. Effect of time, cultivar and rootstock on success of rose propagation through stenting technique. Am. J. Plant Sci. 2014, 5, 1644–1650. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Skalák, J.; Vercruyssen, L.; Claeys, H.; Hradilová, J.; Černý, M.; Novák, O.; Plačková, L.; Saiz-Fernández, I.; Skaláková, P.; Coppens, F. Multifaceted activity of cytokinin in leaf development shapes its size and structure in Arabidopsis. Plant J. 2019, 97, 805–824. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izadi, Z.; Rezaei Nejad, A.; Abadía, J. Iron Chelate Improves Rooting in Indole-3-Butyric Acid-Treated Rosemary (Rosmarinus officinalis) Stem Cuttings. Agriculture 2022, 12, 210. https://doi.org/10.3390/agriculture12020210
Izadi Z, Rezaei Nejad A, Abadía J. Iron Chelate Improves Rooting in Indole-3-Butyric Acid-Treated Rosemary (Rosmarinus officinalis) Stem Cuttings. Agriculture. 2022; 12(2):210. https://doi.org/10.3390/agriculture12020210
Chicago/Turabian StyleIzadi, Zeinab, Abdolhossein Rezaei Nejad, and Javier Abadía. 2022. "Iron Chelate Improves Rooting in Indole-3-Butyric Acid-Treated Rosemary (Rosmarinus officinalis) Stem Cuttings" Agriculture 12, no. 2: 210. https://doi.org/10.3390/agriculture12020210
APA StyleIzadi, Z., Rezaei Nejad, A., & Abadía, J. (2022). Iron Chelate Improves Rooting in Indole-3-Butyric Acid-Treated Rosemary (Rosmarinus officinalis) Stem Cuttings. Agriculture, 12(2), 210. https://doi.org/10.3390/agriculture12020210





