Blue Light Alters the Composition of the Jejunal Microbiota and Promotes the Development of the Small Intestine by Reducing Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Sampling
2.3. Chick Normal Small Intestinal Epithelial Cells Culture and Treatment
2.4. Antioxidant Parameters Measurements
2.5. Enzyme-Linked Immunosorbent Assay
2.6. Intestinal Permeability
2.7. Histological Staining and Immunohistochemical Staining
2.8. Real-Time Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.9. Western Blot Analysis
2.10. Microbial Sequencing and Analysis
2.11. SCFAs Extraction and Analysis
2.12. Statistical Analysis
3. Results
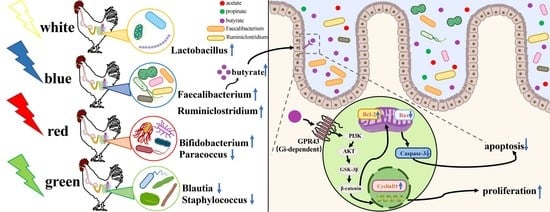
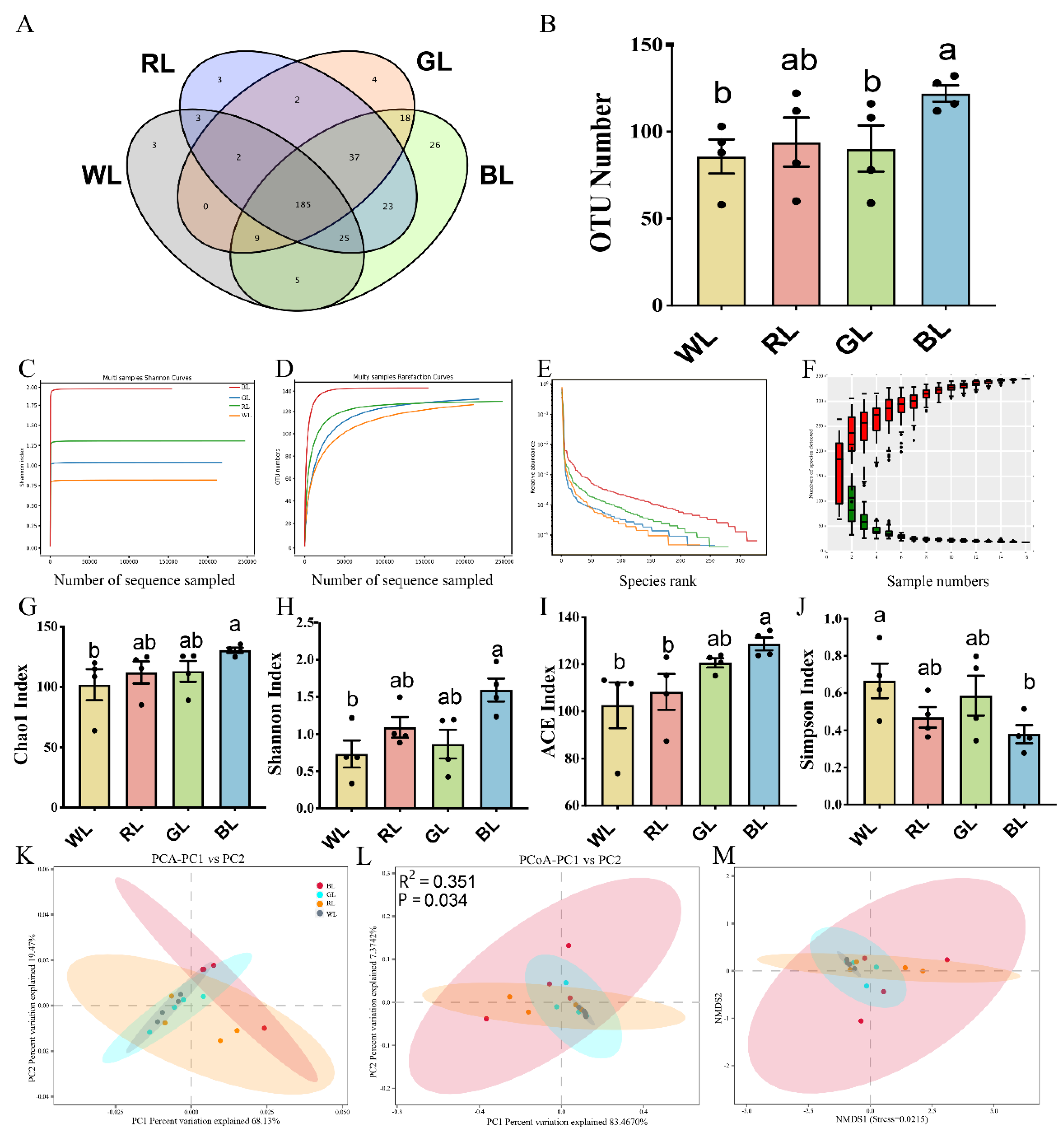
3.1. Effect of Various Monochromatic Lights on Altered Gut Microbiota Composition
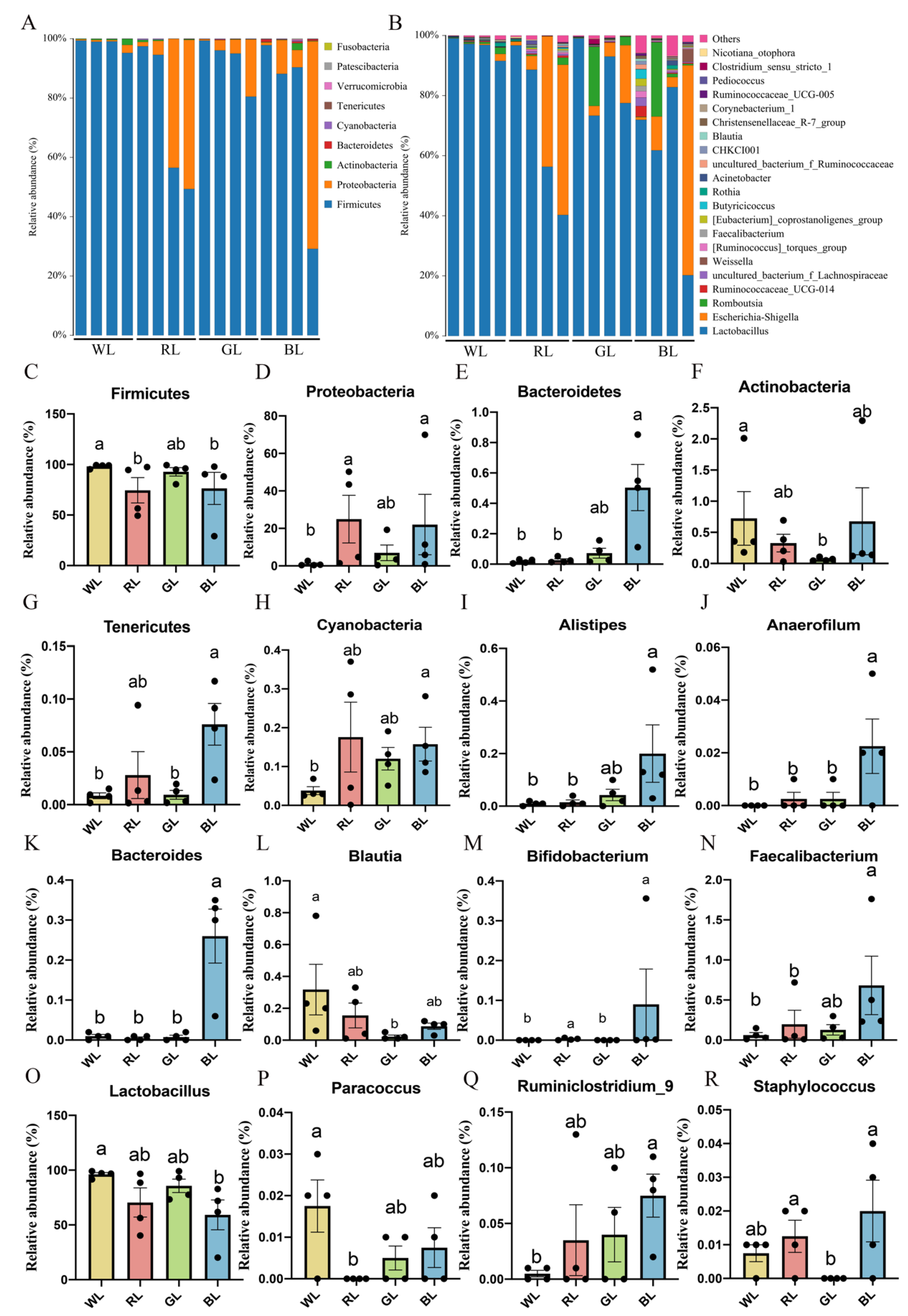
3.2. Abundance and Significant Difference between Four Groups at the Phylum Level
3.3. Abundance and Significant Differences between the Four Groups at the Genus Level
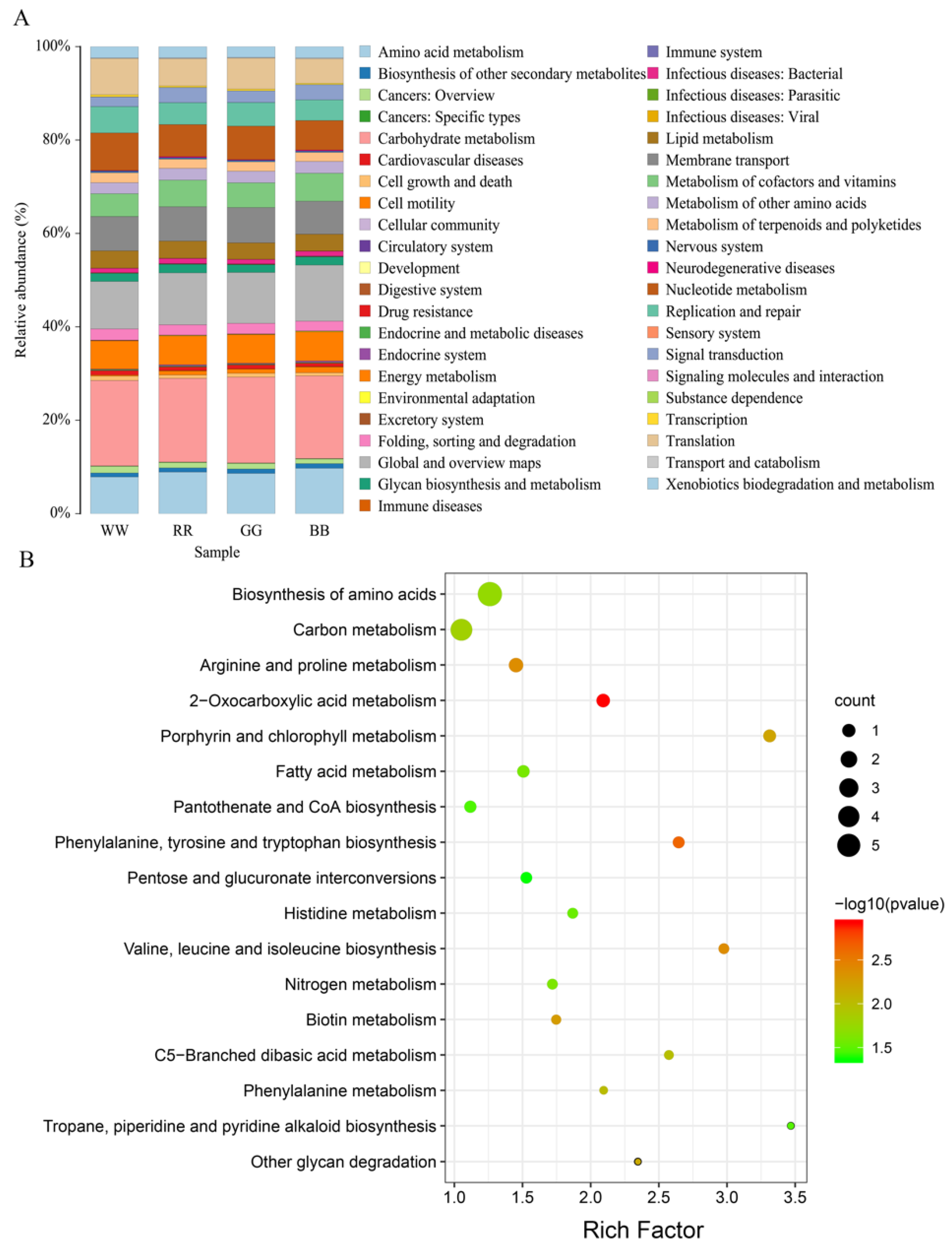
3.4. Differences in Predicted Functional Properties between the Four Groups
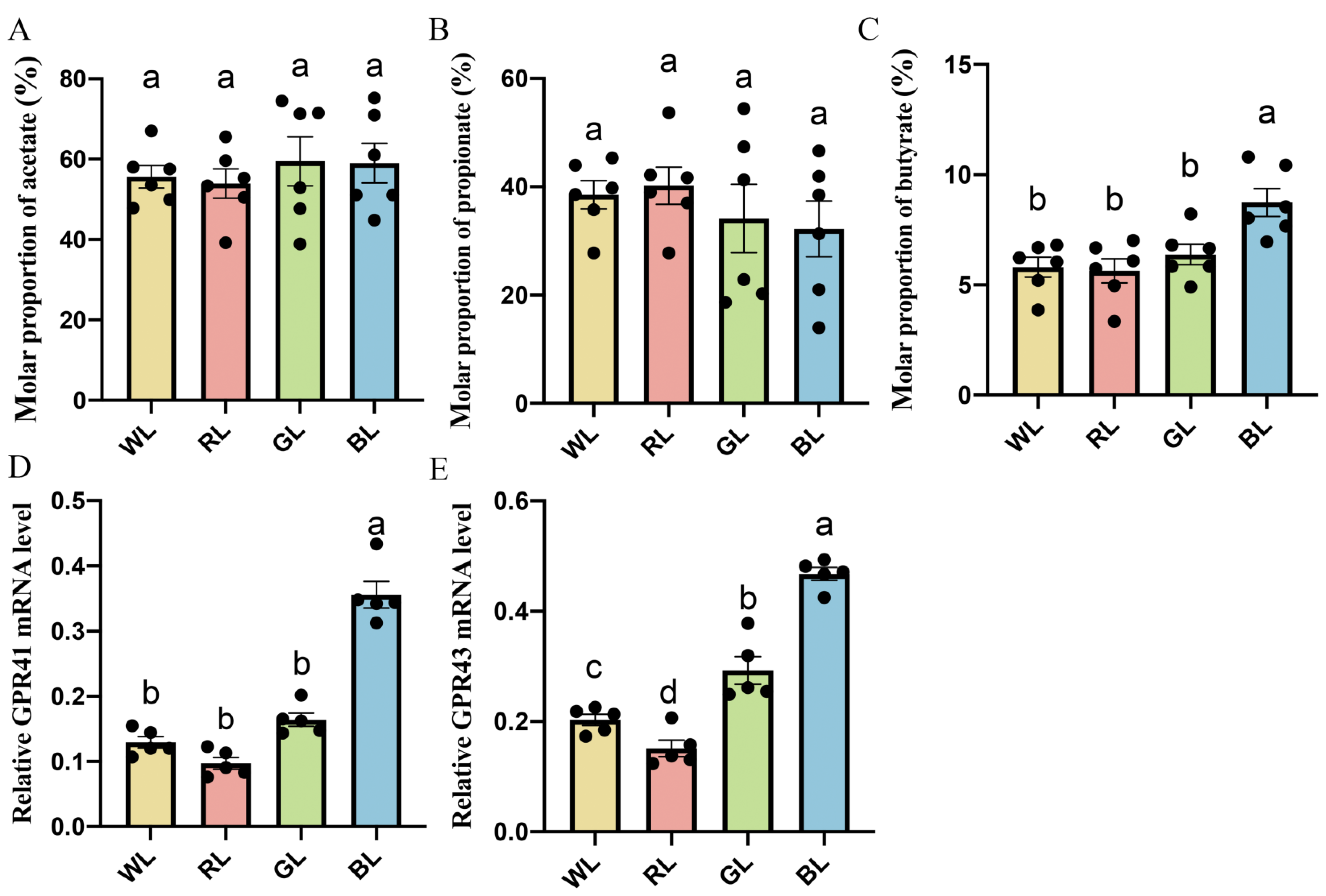
3.5. Effect of Various Monochromatic Lights on Jejunal Butyrate Concentrations and Membrane Receptor GPR41 and GPR43 mRNA Expression in the Jejunum
3.6. Effects of Different Monochromatic Lights on Jejunal Cyclin d1, Caspase-3, Bcl-2 and Bax Protein Expression
3.7. Effects of Various Monochromatic Lights on the Expression of p-AKT, p-GSK-3β and β-Catenin Protein in the Jejunum
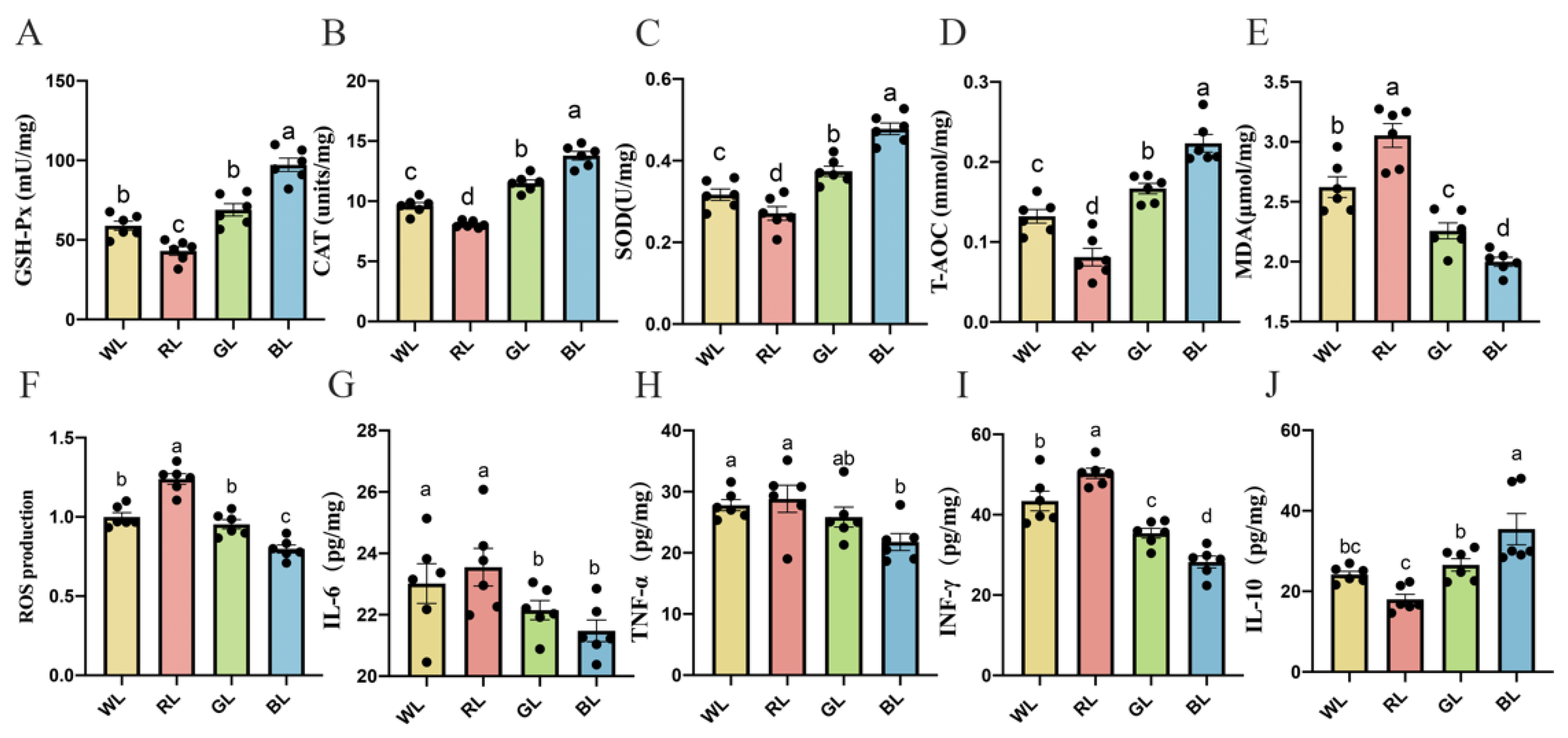
3.8. Effects of Different Monochromatic Lights on the Antioxidant Capacity of the Jejunum
3.9. Effects of Different Monochromatic Lights on Jejunal Cytokine Levels
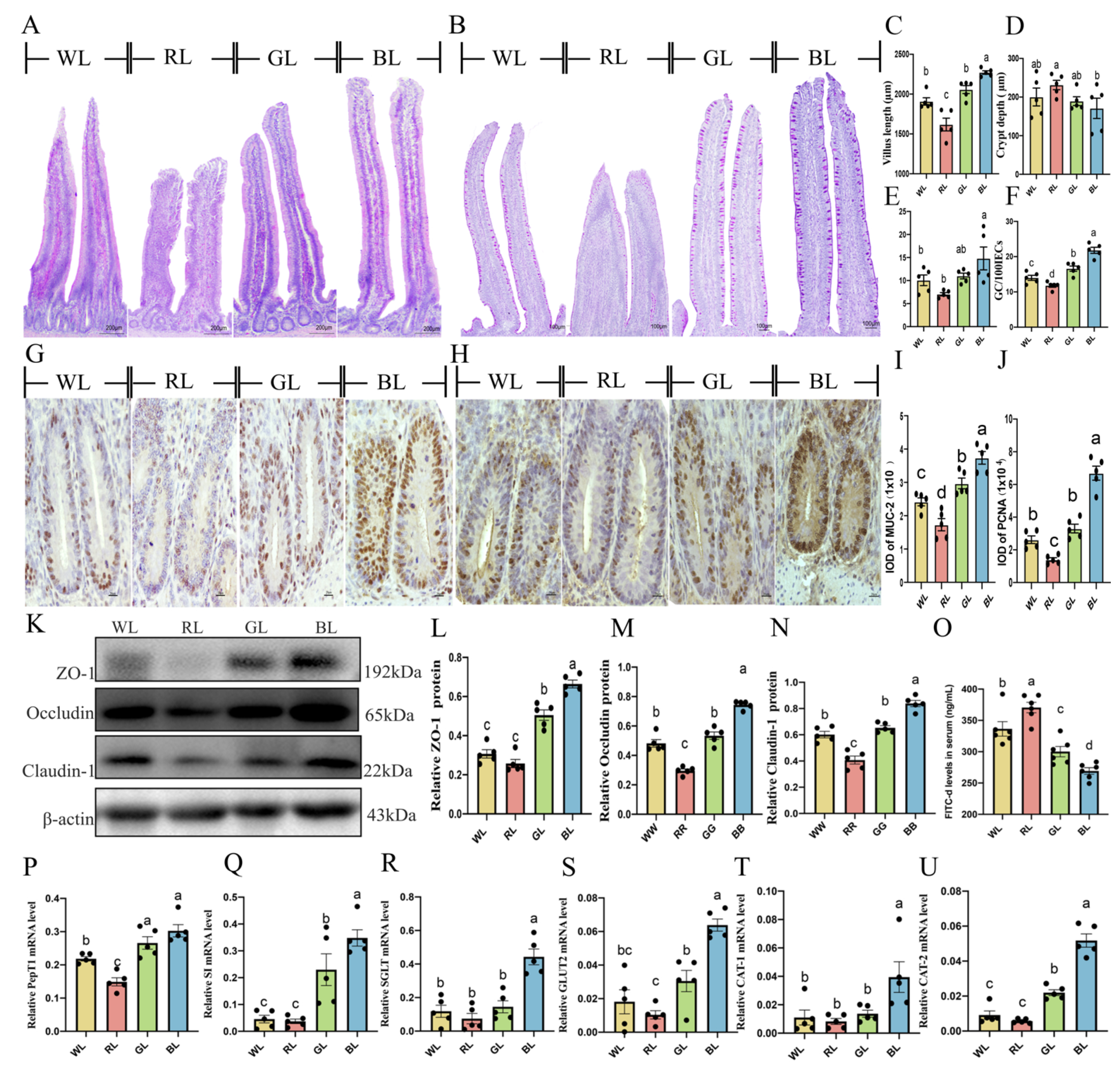
3.10. Effects of Different monochromatic Lights on Jejunal Development and Jejunal Mucosa Function in Chicks
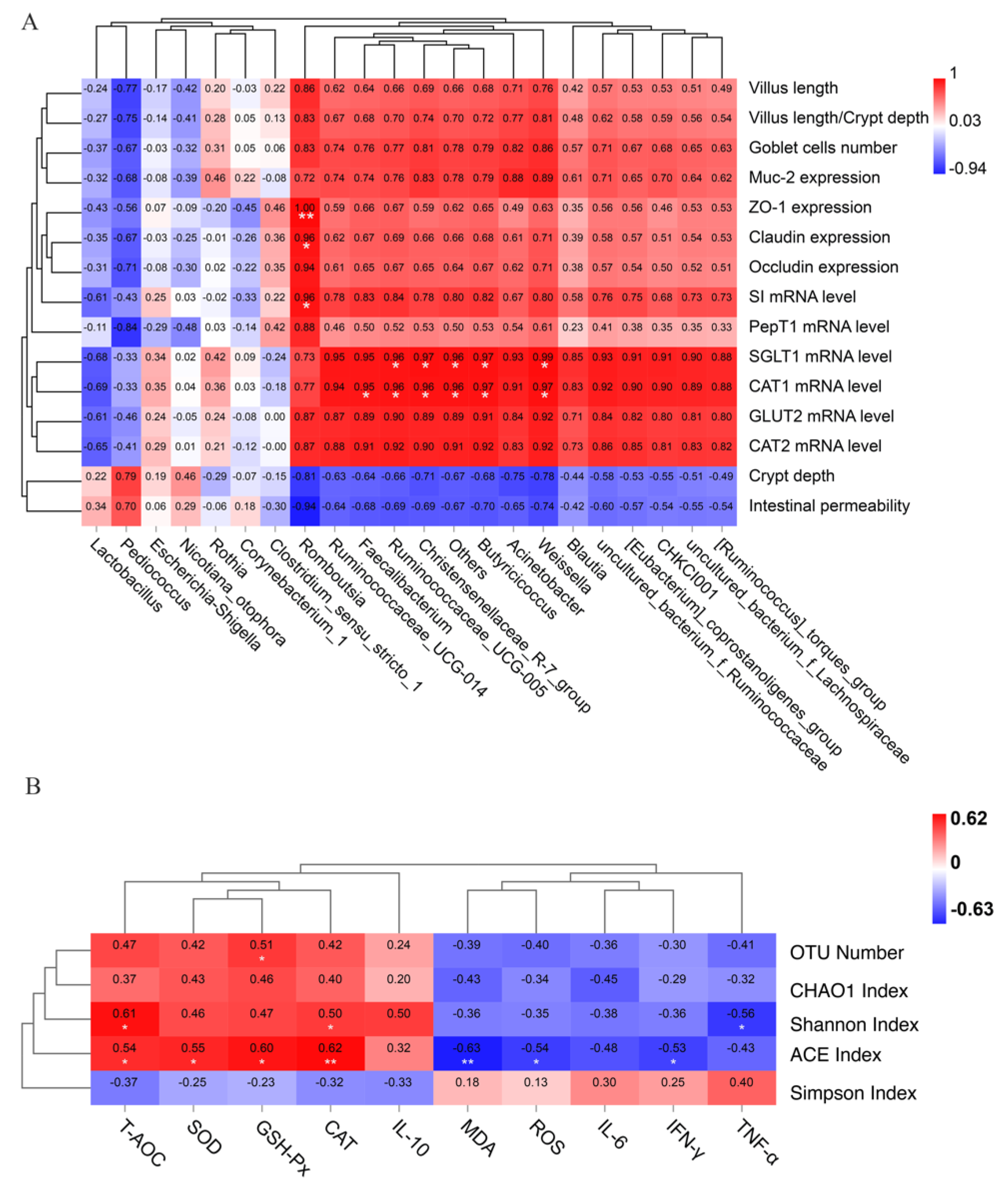
3.11. Correlation Analysis between Gut Health, Oxidative Stress Level and Jejunal Microbiota
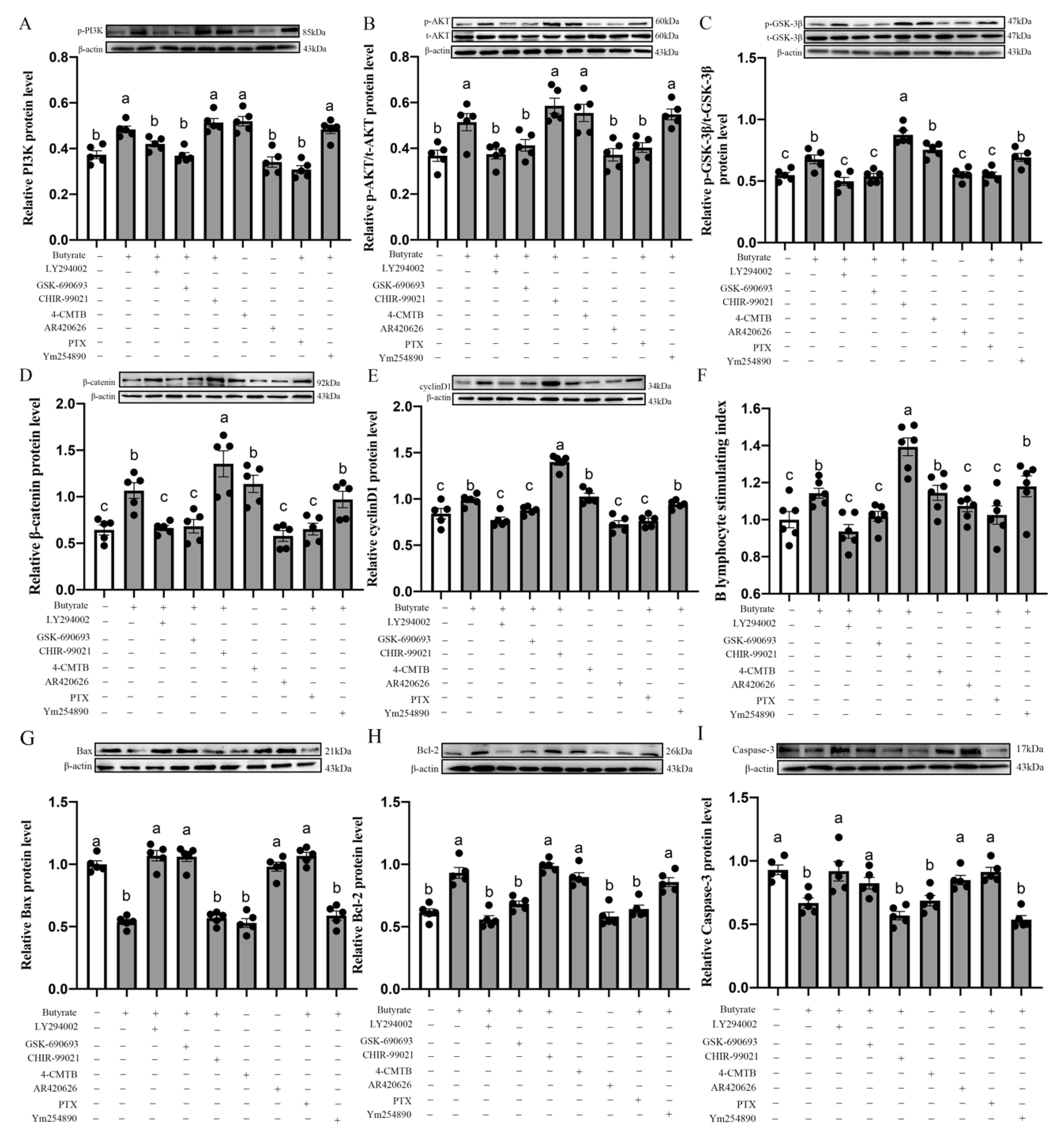
3.12. Butyrate Modulates BL-Induced CIECs Proliferation in Chicks via the GPR43/Gi/PI3K/AKT/GSK-3β/β-Catenin Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, D.; Wang, Z.X.; Dong, Y.L.; Cao, J.; Wang, J.F.; Chen, J.L.; Chen, Y.X. Effects of monochromatic light on immune response of broilers. Poult. Sci. 2008, 87, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Effect of pinealectomy on the circadian clock of the chick retina under different monochromatic lights. Chronobiol. Int. 2019, 36, 548–563. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Wang, T.; Pan, J. Wavelength-specific artificial light disrupts molecular clock in avian species: A power-calibrated statistical approach. Environ. Pollut. 2020, 265, 114206. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Wang, Z.X.; Chen, Y.X. Effects of monochromatic light on developmental changes in satellite cell population of pectoral muscle in broilers during early posthatch period. Anat. Rec. (Hoboken) 2010, 293, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Physiological crosstalk between the AC/PKA and PLC/PKC pathways modulates melatonin-mediated, monochromatic-light-induced proliferation of T-lymphocytes in chickens. Cell Tissue Res. 2017, 369, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, J.; Wang, Z.; Dong, Y.; Chen, Y. Melatonin plays a critical role in inducing B lymphocyte proliferation of the bursa of Fabricius in broilers via monochromatic lights. J. Photochem. Photobiol. B 2015, 142, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Q.; Liu, S.; Sun, R.; Zhou, Y.; Li, Y. Age-related variations in intestinal microflora of free-range and caged hens. Front. Microbiol. 2017, 8, 1310. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, C.J.; Chia, N.; Jeraldo, P.; Sipos, M.; Goldenfeld, N.D.; White, B.A. The microbiome of the chicken gastrointestinal tract. Anim. Health Res. Rev. 2012, 13, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, gut health and chicken productivity: What is the connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef] [Green Version]
- Waite, D.W.; Taylor, M.W. Characterizing the avian gut microbiota: Membership, driving influences, and potential function. Front. Microbiol. 2014, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, D.; Bai, L.; Qu, Q.; Zhou, S.; Yang, M.; Guo, S.; Li, Q.; Liu, C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019, 98, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.B.; Vasconcelos, E.J.R.; Diniz, P.; Calloway, K.N.; Richardson, E.; Meinersmann, R.J.; Cox, N.A.; Berrang, M.E. The cecal microbiome of commercial broiler chickens varies significantly by season. Poult. Sci. 2018, 97, 3635–3644. [Google Scholar] [CrossRef]
- Guardia, S.; Konsak, B.; Combes, S.; Levenez, F.; Cauquil, L.; Guillot, J.F.; Moreau-Vauzelle, C.; Lessire, M.; Juin, H.; Gabriel, I. Effects of stocking density on the growth performance and digestive microbiota of broiler chickens. Poult. Sci. 2011, 90, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Reese, A.T.; Cho, E.H.; Klitzman, B.; Nichols, S.P.; Wisniewski, N.A.; Villa, M.M.; Durand, H.K.; Jiang, S.; Midani, F.S.; Nimmagadda, S.N.; et al. Antibiotic-induced changes in the microbiota disrupt redox dynamics in the gut. Elife 2018, 7, e35987. [Google Scholar] [CrossRef]
- Hieke, A.C.; Hubert, S.M.; Athrey, G. Circadian disruption and divergent microbiota acquisition under extended photoperiod regimens in chicken. PeerJ 2019, 7, e6592. [Google Scholar] [CrossRef] [Green Version]
- Frankel, W.L.; Zhang, W.; Singh, A.; Klurfeld, D.M.; Don, S.; Sakata, T.; Modlin, I.; Rombeau, J.L. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology 1994, 106, 375–380. [Google Scholar] [CrossRef]
- Hass, R.; Busche, R.; Luciano, L.; Reale, E.; Engelhardt, W.V. Lack of butyrate is associated with induction of Bax and subsequent apoptosis in the proximal colon of guinea pig. Gastroenterology 1997, 112, 875–881. [Google Scholar] [CrossRef]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential amino acids and protein synthesis: Insights into maximizing the muscle and whole-body response to feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef] [PubMed]
- Corrochano, L.M. Light in the fungal world: From photoreception to gene transcription and beyond. Annu. Rev. Genet. 2019, 53, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Tisch, D.; Schmoll, M. Light regulation of metabolic pathways in fungi. Appl. Microbiol. Biotechnol. 2010, 85, 1259–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hlker, F.; Wurzbacher, C.; Weienborn, C.; Monaghan, M.T.; Holzhauer, S.; Premke, K. Microbial diversity and community respiration in freshwater sediments influenced by artificial light at night. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.Y.; Antonopoulos, D.A.; Kalra, A.; Tonelli, A.; Khalife, W.T.; Schmidt, T.M.; Young, V.B. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J. Infect. Dis. 2008, 197, 435–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffeis, C.; Martina, A.; Corradi, M.; Quarella, S.; Nori, N.; Torriani, S.; Plebani, M.; Contreas, G.; Felis, G.E. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 700–709. [Google Scholar] [CrossRef]
- Brown, J.R.M.; Flemer, B.; Joyce, S.A.; Zulquernain, A.; Sheehan, D.; Shanahan, F.; O’Toole, P.W. Changes in microbiota composition, bile and fatty acid metabolism, in successful faecal microbiota transplantation for Clostridioides difficile infection. BMC Gastroenterol. 2018, 18, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Zhang, Y.; Xiao, K.; Jiang, F.; Wang, H.; Tang, D.; Liu, D.; Liu, B.; Liu, Y.; He, X.; et al. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 2018, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Y.; Li, J.; Xing, T.; Jiang, Y.; Zhang, L.; Gao, F. Dietary resistant starch modifies the composition and function of caecal microbiota of broilers. J. Sci. Food Agric. 2020, 100, 1274–1284. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Baumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Xue, F.; Xin, H.; Zhao, Y.; Wang, Y.; Xiong, B. Effects of monochromatic lights on the growth performance, carcass characteristics, eyeball development, oxidation resistance, and cecal bacteria of Pekin ducks. Anim. Biosci. 2021, 34, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Battermann, A.; Disse-Kromker, C.; Dreiseikelmann, B. A functional plasmid-borne rrn operon in soil isolates belonging to the genus Paracoccus. Microbiology 2003, 149, 3587–3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Tsai, A.; Sze, M.A.; Vucic, E.A.; Shaipanich, T.; Harris, M.; Guillemi, S.; Yang, J.; Sinha, S.; Nislow, C.; et al. Decreased microbiome diversity in the HIV small airway epithelium. Respir. Res. 2018, 19, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bry, L.; Falk, P.G.; Midtvedt, T.; Gordon, J.I. A model of host-microbial interactions in an open mammalian ecosystem. Science 1996, 273, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.L. Gut reaction environmental effects on the human microbiota. Environ. Health Perspect. 2009, 117, A198–A205. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Gao, H.; Wu, G.; Qin, W.; Song, P.; Wang, L.; Chen, J.; Cai, Z.; Zhang, T. Comparison of gut microbiota diversity between wild and captive bharals (Pseudois nayaur). BMC Vet. Res. 2019, 15, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Lilburn, M.; Yu, Z. Intestinal microbiota of broiler chickens as affected by litter management regimens. Front. Microbiol. 2016, 7, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zellner, G.; Stackebrandt, E.; Nagel, D.; Messner, P.; Weiss, N.; Winter, J. Anaerofilum pentosovorans gen. nov., sp. nov., and Anaerofilum agile sp. nov., two new, strictly anaerobic, mesophilic, acidogenic bacteria from anaerobic bioreactors. Int. J. Syst. Bacteriol. 1996, 46, 871–875. [Google Scholar] [CrossRef] [Green Version]
- Sheng, T.; Zhao, L.; Gao, L.F.; Liu, W.Z.; Cui, M.H.; Guo, Z.C.; Ma, X.D.; Ho, S.H.; Wang, A.J. Lignocellulosic saccharification by a newly isolated bacterium, Ruminiclostridium thermocellum M3 and cellular cellulase activities for high ratio of glucose to cellobiose. Biotechnol. Biofuels. 2016, 9, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsanius, B.W.; Vaas, L.; Gharaie, S.; Karlsson, M.E.; Rosberg, A.K.; Wohanka, W.; Khalil, S.; Windstam, S. Dining in blue light impairs the appetite of some leaf epiphytes. Front. Microbiol. 2021, 12, 725021. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Idris, U.; Harmon, B.; Hofacre, C.; Maurer, J.J.; Lee, M.D. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 2003, 69, 6816–6824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Liu, W.; Wang, Z.; Xie, D.; Jia, L.; Chen, Y. Green and blue monochromatic lights promote growth and development of broilers via stimulating testosterone secretion and myofiber growth. J. Appl. Poult. Res. 2008, 17, 211–218. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-carbon metabolism in health and disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Abdo, S.E.; El-Kassas, S.; El-Nahas, A.F.; Mahmoud, S. Modulatory effect of monochromatic blue light on heat stress response in commercial broilers. Oxid. Med. Cell. Longev. 2017, 2017, 1351945. [Google Scholar] [CrossRef] [Green Version]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Li, C.L.; Wang, J.; Zhang, H.J.; Wu, S.G.; Hui, Q.R.; Yang, C.B.; Fang, R.J.; Qi, G.H. Intestinal Morphologic and Microbiota Responses to Dietary Bacillus spp. in a Broiler Chicken Model. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Lenoir, M.; Martín, R.; Torres-Maravilla, E.; Chadi, S.; González-Dávila, P.; Sokol, H.; Langella, P.; Chain, F.; Bermúdez-Humarán, L.G. Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3. Gut Microbes 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Liu, B.; Kleinsteuber, S.; Centler, F.; Harms, H.; Sträuber, H. Competition between butyrate fermenters and chain-elongating bacteria limits the efficiency of medium-chain carboxylate production. Front. Microbiol. 2020, 11, 336. [Google Scholar] [CrossRef]
- Melaku, M.; Zhong, R.; Han, H.; Wan, F.; Yi, B.; Zhang, H. Butyric and citric acids and their salts in poultry nutrition: Effects on gut health and intestinal microbiota. Int. J. Mol. Sci. 2021, 22, 10392. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhao, K.N.; Vitetta, L. Effects of intestinal microbial-elaborated butyrate on oncogenic signaling pathways. Nutrients 2019, 11, 1026. [Google Scholar] [CrossRef] [Green Version]
- Birchenough, G.M.; Nyström, E.E.; Johansson, M.E.; Hansson, G.C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 2016, 352, 1535–1542. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef] [Green Version]
- Mroz, M.S.; Lajczak, N.K.; Goggins, B.J.; Keely, S.; Keely, S.J. The bile acids, deoxycholic acid and ursodeoxycholic acid, regulate colonic epithelial wound healing. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G378–G387. [Google Scholar] [CrossRef]
- Metidji, A.; Omenetti, S.; Crotta, S.; Li, Y.; Nye, E.; Ross, E.; Li, V.; Maradana, M.R.; Schiering, C.; Stockinger, B. The environmental sensor ahr protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity 2018, 49, 353–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Ingredients | Content |
|---|---|
| Dry matter | 86.05 |
| Crude protein | 19.09 |
| Crude ash | 8.04 |
| Sodium chloride | 0.67 |
| Calcium | 0.85 |
| Phosphorus | 0.42 |
| Methionine + Cystine | 0.71 |
| Gene | Product Size | Primer Sequences (5′–3′) | Accession No. |
|---|---|---|---|
| PepT1 | 180 | F: TTTCCTTTACATCCCTCTCCCG R: ATCACAGCATCTACAACTGGGACC | AY029615 |
| SI | 132 | F: TGGATTGTCATCACCCGTTC R: CCAAAGAGACTGAACTCCATCATACC | XM_422811 |
| SGLT1 | 124 | F: TGGCGGGCTTCTACCGCAGCGAG R: CCCGGTAGGTCACCAGTCCCCAG | XM_415247 |
| GLUT2 | 107 | F: GCCTTGAGGAAACATCTGCT R: GGACTGGATGGACGTTATGG | Z22932 |
| CAT-1 | 127 | F: TCTGCTCATCTGCTTTGTGG R: GGCTCCATCCCAACCTACAT | XM_417116 |
| CAT-2 | 106 | F: GCTAACTTGGAGCCCTGGAG R: CCACTTTCTGCTGGTTCTGC | XM_420685 |
| GPR43 | 149 | F: AACGCCAACCTCAACAAGTC R: TGGGAGAAGTCATCGTAGCA | NM_001318430.1 |
| GPR41 | 103 | F: GAAGGTGGTTTGGGAGTGAA R: CAGAGGATTTGAGGCTGGAG | XM_040693461.1 |
| β-actin | 169 | F: TCCACCGCAAATGCTTCTAAAC R: CTGCTGACACCTTCACCATTCC | NM_205518 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Blue Light Alters the Composition of the Jejunal Microbiota and Promotes the Development of the Small Intestine by Reducing Oxidative Stress. Antioxidants 2022, 11, 274. https://doi.org/10.3390/antiox11020274
Zhang Y, Wang Z, Dong Y, Cao J, Chen Y. Blue Light Alters the Composition of the Jejunal Microbiota and Promotes the Development of the Small Intestine by Reducing Oxidative Stress. Antioxidants. 2022; 11(2):274. https://doi.org/10.3390/antiox11020274
Chicago/Turabian StyleZhang, Yijia, Zixu Wang, Yulan Dong, Jing Cao, and Yaoxing Chen. 2022. "Blue Light Alters the Composition of the Jejunal Microbiota and Promotes the Development of the Small Intestine by Reducing Oxidative Stress" Antioxidants 11, no. 2: 274. https://doi.org/10.3390/antiox11020274
APA StyleZhang, Y., Wang, Z., Dong, Y., Cao, J., & Chen, Y. (2022). Blue Light Alters the Composition of the Jejunal Microbiota and Promotes the Development of the Small Intestine by Reducing Oxidative Stress. Antioxidants, 11(2), 274. https://doi.org/10.3390/antiox11020274