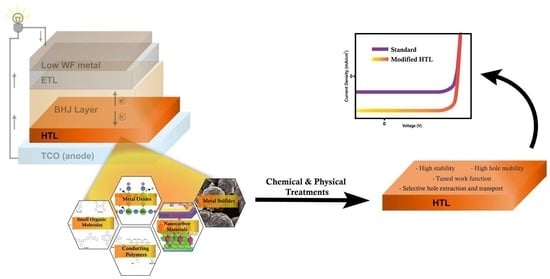
Recent Advances in Hole-Transporting Layers for Organic Solar Cells
Abstract
:1. Introduction
2. Structure and Characterization of Organic Solar Cells
3. Hole-Transporting Layers
4. Hole-Transporting Materials as HTLs in OSCs
4.1. Metal Oxides
4.1.1. Molybdenum Oxide
4.1.2. Tungsten Oxide
4.1.3. Vanadium Oxide
4.1.4. Nickel Oxide
4.1.5. Other Oxides
4.2. Metal Sulfides
4.2.1. Molybdenum Disulfide
4.2.2. Tungsten Disulfide
4.2.3. Nickel Sulfide
4.2.4. Other Sulfides
4.3. Nanocarbons
4.3.1. Graphene Oxide
4.3.2. Other Nanocarbons
4.4. Conducting Polymers and Their Composites
4.4.1. PEDOT
4.4.2. Other Conjugated Polymers
4.5. Small Organic Molecules
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Y6 | (2,2′-((2Z,2Z)-((12,13-bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2″,3″:4′,50]thieno[2′,3′:4,5]pyrrolo[3,2-g]thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile) |
| O-IDTBR | (5Z,5′Z)-5,5′-{[7,7′-(4,4,9,9-tetraoctyl-4,9-dihydro-s-indaceno[1,2-b:5,6-b’]dithiophene-2,7-diyl)bis(benzo[c][1,2,5]thiadiazole-7,4-diyl)]bis(methanylylidene)}bis(3-ethyl-2-thioxothiazolidin-4-one) |
| PC71BM | (6,6)-phenyl-C71-butyric acid methyl ester |
| HPCzI | 1,3,4,5,6,7-hexaphenyl-2-{3′-(9-ethylcarbazolyl)}-isoindole |
| ICBA | 1′,1″,4′,4″-Tetrahydro-di[1,4]methanonaphthaleno[1,2:2′,3′,56,60:2″,3″][5,6]fullerene-C60 |
| TEMPO | 2,2,6,6-tetramethylpiperidine-1-oxoammonium |
| IDIC | 2,2′-[(4,4,9,9-tetrahexyl-4,9-dihydro-s-indaceno[1,2-b:5,6-b′]dithiophene-2,7-diyl)bis[methylidyne(3-oxo-1H-indene-2,1(3H)-diylidene)]]bis-propanedinitrile |
| DIPO-Ph4 | 2,2′,6,6′-tetraphenyl-dipyranylidene |
| F4-TCNQ | 2,3,5,6-tetrafluoro-7,7,8,8-tetracyanoquinodimethane |
| EGME | 2-methoxyethanol |
| PTCDA | 3,4,9,10-perylenetetracarboxylic dianhydride |
| IT-4F | 3,9-bis(2-methylene-((3-(1,1-dicyanomethylene)-6,7-difluoro)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b’]dithiophene |
| ITIC | 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno-[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene |
| ITIC-Th | 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone))-5,5,11,11-tetrakis(5-hexylthienyl)-dithieno-[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene |
| CBP | 4,4′-N,N′-dicarbazole-biphenyl |
| a-MWNTs | Amino-functionalized multi-walled carbon nanotubes |
| AHM | Ammonium heptamolybdate |
| AIL | Anode interfacial layer |
| AFM | Atomic force microscope |
| BPQDs | Black phosphorous quantum dots |
| bf-MWCNTs | Boronic acid functionalized multi-walled carbon nanotubes |
| BHJ | Bulk heterojunction |
| CNT | Carbon nanotubes |
| CTAB | Cetyltrimethylammonium bromide |
| CoOx | Cobalt oxide |
| CPEs | Conjugated polyelectrolytes |
| CIGS | Copper indium gallium diselenide |
| CuOx | Copper oxide |
| CuSx | Copper sulfide |
| MeOPhN-DBC | Dibenzo[g,p]chrysene derivative, 3,6,11,14-tetramethoxyphenylamine- dibenzo[g,p]chrysene |
| DMSO | Dimethyl sulfoxide |
| DMF | Dimethylformamide |
| DC | Direct current |
| DA | Dopamine |
| ETL | Electron transport layer |
| EQE | External quantum efficiency |
| FF | Fill factor |
| GSL | Grafted sulfonated-acetone-formaldehyde lignin |
| GO | Graphene oxide |
| GQDs | Graphene quantum dots |
| g-C3N4 | Graphitic carbon nitrile |
| HOMO | High occupied molecular orbital |
| HTL | Hole transport layer |
| HyMoO3-x | Hydrogenated molybdenum oxide |
| ITO | Indium tin oxide |
| Pin | Input power |
| IPA | Isopropanol |
| LIL | Laser interference lithography |
| LSPR | Localized surface plasmon resonance |
| LUMO | Lowest unoccupied molecular orbital |
| Pmax | Maximum power output |
| MO | Metal oxides |
| MW | Microwave |
| NPB | N,N′-bis(1-naphthalenyl)N,N′-bis-phenyl-(1,1′-biphenyl)-4,4′-diamine |
| BF-DPB | N,N’-((diphenyl-N,N’-bis)9,9,-dimethyl-fluoren-2-yl)-benzidine |
| NDs | Nanodots |
| NPs | Nanoparticles |
| NRs | Nanorods |
| NWs | Nanowires |
| NFD | Nickel formate dihydrate |
| NiOx | Nickel oxide |
| NiSx | Nickel sulfide |
| nGQDs | Nitrogen doped graphene quantum dots |
| Voc | Open circuit voltage |
| OLEDs | Organic light-emiting diodes |
| OSCs | Organic solar cells |
| Pout | Output power |
| PCBM | Phenyl-C61-butyric acid methyl ester |
| PMA | Phosphomolybdic acid |
| PL | Photoluminescence |
| PV | Photovoltaic |
| PC | Phthalocyanine |
| PTB7 | Poly [[4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl][3-fluoro-2-[(2-ethylhexyl)carbonyl]thieno[3,4-b]thiophenediyl]] |
| PCPDT | Poly 3,4-dithia-7H-cyclopenta[a]pentalene |
| FBT-TH4 | Poly 5,6-difluorobenzothiadiazole |
| PDMT | Poly(3,4-dimethoxythiophene) |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) |
| P3HT | Poly(3-hexylthiophene) |
| P3HT50-b-PSS23 | Poly(3-hexylthiophene)-b-poly(p-styrenesulfonate) |
| PEDOT-S | Poly(4-(2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl-methoxy)-1-butanesulfonic acid) |
| PTPCz | Poly(carbazolyl triphenylethylene) derivative |
| PDMS | Poly(dimethylsiloxane) |
| PMMA | Poly(methylmethacrylate) |
| PSS | Poly(styrenesulfonate) |
| PM6 | Poly[(2,6-(4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene))-co-(1,3-di(5-thiophene-2-yl)-5,7-bis(2-ethylhexyl)-benzo[1,2-c:4,5-c′]dithiophene-4,8-dione))] |
| PBDB-T | Poly[(2,6-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene)-co-(1,3-di(5-thiophene-2-yl)-5,7-bis(2-ethylhexyl)benzo[1,2-c:4,5-c′]dithiophene-4,8-dione)] |
| PffBT4T-2OD | Poly[(5,6-difluoro-2,1,3-benzothiadiazol-4,7-diyl)-alt-(3,3‴-di(2-octyldodecyl)-2,2′;5′,2″;5″,2‴-quaterthiophen-5,5‴-diyl)] |
| PFN | Poly[(9,9bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)-alt-2,7-(9,9dioctylfluorene)] |
| PSFP-DTBTP | Poly[(9,9-bis(4-sulfonatobutyl sodium) fluorene-alt-phenylene)-ran-(4,7-di-2-thienyl-2,1,3-benzothiadiazole-alt-phenylene)] |
| TFB | Poly[(9,9-dioctylfluorenyl-2,7-diyl)-alt-(4,4′-(N-(4-butylphenyl)))] |
| PNDIT-F3N | Poly[[2,7-bis(2-ethylhexyl)-1,2,3,6,7,8-hexahydro-1,3,6,8-tetraoxobenzo[lmn] [3,8]phenanthroline-4,9-diyl]-2,5-thiophenediyl[9,9-bis[3′((N,N-dimethyl)-N-ethylammonium)]propyl]-9H-fluorene-2,7-diyl]-2,5-thiophenediyl] |
| PhNa-1T | Poly[1,4-bis(4-sulfonatobutoxy)benzene-thiophene] |
| PCP-Na | Poly[2,6-(4,4-bis-(propane-1-sulfonate sodium)-4H-cyclopenta[2,1-b;3,4-b′ ]dithiophene)-alt-(4,4′-biphenyl)] |
| P3HTN | Poly[3-(6′-N,N,N-trimethyl ammonium)-hexylthiophene] bromide |
| PTB7-Th | Poly[4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b;4,5-b′]dithiophene-2,6-diyl-alt-(4-(2-ethylhexyl)-3-fluorothieno[3,4-b]thiophene-)-2-carboxylate-2-6-diyl)] |
| PBDT-TS1 | Poly[4,8-bis(5-(octylthio)thiophen-2-yl)benzo[1,2-b;4,5-b′]dithiophene-2,6-diyl–alt–(4-(2- ethylhexyl)-3-fluorothieno[3,4-b]thiophene-)-2-carboxylate-2-6-diyl] |
| PFtT-D | Poly[9,9-bis(4′-sulfonatobutyl)fluorene-alt-thieno[3,2-b]thiophene] |
| PFSe | Poly[9,9-bis(4′-sulfonatobutyl)fluorene-alt-selenophene] |
| PFT-D | Poly[9,9-bis(4′-sulfonatobutyl)fluorene-alt-thiophene] |
| PBDT-DTNT | Poly[benzodithiophene-bis(decyltetradecyl-thien)naphthothiadiazole] |
| PCDTBT | Poly[N-9′-heptadecanyl-2,7-carbazole-alt-5,5(4′,7′-di-2-thienyl-2′,1′,3′-benzothiadiazole)] |
| PANI | Polyaniline |
| PEG | Polyethylene glycol |
| PSC | Polymer solar cell |
| PMC | Polynuclear metal-oxo clusters |
| p-TPCF | Polytriphenylcarbazole fluoranthene |
| PCE | Power conversion efficiency |
| QDs | Quantum dots |
| rrP3HT | Regioregular poly(3-hexylthiophene-2,5-diyl) |
| RMS | Root meand square |
| SEM | Scanning electron microscopy |
| Rs | Series resistance |
| Jsc | Short circuit current |
| Rsh | Shunt resistance |
| SWCNTs | Single-walled carbon nanotubes |
| SILAR | Successive ionic layer adsorption and reaction |
| F4TCNQ | Tetrafluorotetracyanoquino-dimethane |
| TTA | Tetrathiafulvalene |
| TTF-py | Tetrathiafulvalene pyridine derivative |
| P3HT-Si | Triethoxysilane terminated poly(3-hexylthiophene) |
| WOx | Tungsten oxide |
| WSx | Tungsten sulfide |
| UVO | Ultraviolet ozone |
| uSWNT | Unzipped single-walled carbon nanotubes |
| VOx | Vanadium oxide |
| VoPC | Vanadylphthalocyanine |
| WF | Work function |
References
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Luqman, M.; Ahmad, S.R.; Khan, S.; Ahmad, U.; Raza, A.; Akmal, F. Estimation of Solar Energy Potential from Rooftop of Punjab Government Servants Cooperative Housing Society Lahore Using GIS. Smart Grid Renew. Energy 2015, 6, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar energy: Potential and future prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Hosenuzzaman, M.; Rahim, N.A.; Selvaraj, J.; Hasanuzzaman, M.; Malek, A.B.M.A.; Nahar, A. Global prospects, progress, policies, and environmental impact of solar photovoltaic power generation. Renew. Sustain. Energy Rev. 2015, 41, 284–297. [Google Scholar] [CrossRef]
- Kannan, N.; Vakeesan, D. Solar energy for future world:—A review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Goswami, D.Y.; Vijayaraghavan, S.; Lu, S.; Tamm, G. New and emerging developments in solar energy. Sol. Energy 2004, 76, 33–43. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Lin, Y.; Shao, Y.; Dai, J.; Li, T.; Liu, Y.; Dai, X.; Xiao, X.; Deng, Y.; Gruverman, A.; Zeng, X.C.; et al. Metallic surface doping of metal halide perovskites. Nat. Commun. 2021, 12, 7. [Google Scholar] [CrossRef]
- Ur Rehman, S.; Noman, M.; Khan, A.D.; Saboor, A.; Ahmad, M.S.; Khan, H.U. Synthesis of polyvinyl acetate /graphene nanocomposite and its application as an electrolyte in dye sensitized solar cells. Optik 2020, 202, 163591. [Google Scholar] [CrossRef]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Yuan, J.; Deng, W.; Luo, M.; Xie, Y.; Liang, Q.; Zou, Y.; He, Z.; Wu, H.; Cao, Y. High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder. Nat. Photonics 2020, 14, 300–305. [Google Scholar] [CrossRef]
- Chuang, C.-H.M.; Brown, P.R.; Bulović, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernède, J.C. Organic photovoltaic cells: History, principle and techniques. J. Chil. Chem. Soc. 2008, 53. [Google Scholar] [CrossRef] [Green Version]
- Kearns, D.; Calvin, M. Photovoltaic Effect and Photoconductivity in Laminated Organic Systems. J. Chem. Phys. 1958, 29, 950–951. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Semiconducting polymers (as donors) and buckminsterfullerene (as acceptor): Photoinduced electron transfer and heterojunction devices. Synth. Met. 1993, 59, 333–352. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Wei, J.; Zheng, Q. Interfacial Materials for Organic Solar Cells: Recent Advances and Perspectives. Adv. Sci. 2016, 3, 1500362. [Google Scholar] [CrossRef] [Green Version]
- Green, M.A. Recent developments in photovoltaics. Sol. Energy 2004, 76, 3–8. [Google Scholar] [CrossRef]
- Darling, S.B.; You, F. The case for organic photovoltaics. RSC Adv. 2013, 3, 17633. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 57). Prog. Photovolt. Res. Appl. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Salim, M.B.; Nekovei, R.; Jeyakumar, R. Organic tandem solar cells with 18.6% efficiency. Sol. Energy 2020, 198, 160–166. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef]
- Hou, J.; Inganas, O.; Friend, R.H.; Gao, F. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018, 17, 119–128. [Google Scholar] [CrossRef]
- Jacoby, M. The future of low-cost solar cells. Chem. Eng. News 2016, 94, 30–35. [Google Scholar]
- Riede, M.; Spoltore, D.; Leo, K. Organic Solar Cells—The Path to Commercial Success. Adv. Energy Mater. 2021, 11, 2002653. [Google Scholar] [CrossRef]
- Krebs, F.C.; Espinosa, N.; Hösel, M.; Søndergaard, R.R.; Jørgensen, M. 25th anniversary article: Rise to power—OPV-based solar parks. Adv. Mater. 2014, 26, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.Y.; Marder, S.R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003. [Google Scholar] [CrossRef]
- Duan, C.; Huang, F.; Cao, Y. Solution processed thick film organic solar cells. Polym. Chem. 2015, 6, 8081–8098. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Efficiency of bulk-heterojunction organic solar cells. Prog. Polym. Sci. 2013, 38, 1929–1940. [Google Scholar] [CrossRef] [Green Version]
- Yeh, N.; Yeh, P. Organic solar cells: Their developments and potentials. Renew. Sustain. Energy Rev. 2013, 21, 421–431. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, T.-W.; Chin, B.-D.; Wang, D.H.; Park, O.O. Roles of Interlayers in Efficient Organic Photovoltaic Devices. Macromol. Rapid Commun. 2010, 31, 2095–2108. [Google Scholar] [CrossRef]
- Lai, T.-H.; Tsang, S.-W.; Manders, J.R.; Chen, S.; So, F. Properties of interlayer for organic photovoltaics. Mater. Today 2013, 16, 424–432. [Google Scholar] [CrossRef]
- Steim, R.; Kogler, F.R.; Brabec, C.J. Interface materials for organic solar cells. J. Mater. Chem. 2010, 20, 2499. [Google Scholar] [CrossRef]
- Po, R.; Carbonera, C.; Bernardi, A.; Camaioni, N. The role of buffer layers in polymer solar cells. Energy Environ. Sci. 2011, 4, 285–310. [Google Scholar] [CrossRef]
- Roman, L.S.; Mammo, W.; Pettersson, L.A.A.; Andersson, M.R.; Inganäs, O. High Quantum Efficiency Polythiophene. Adv. Mater. 1998, 10, 774–777. [Google Scholar] [CrossRef]
- Tian, L.; Xue, Q.; Hu, Z.; Huang, F. Recent advances of interface engineering for non-fullerene organic solar cells. Org. Electron. 2021, 93, 106141. [Google Scholar] [CrossRef]
- Palilis, L.C.; Vasilopoulou, M.; Verykios, A.; Soultati, A.; Polydorou, E.; Argitis, P.; Davazoglou, D.; Mohd Yusoff, B.A.R.; Nazeeruddin, M.K. Inorganic and Hybrid Interfacial Materials for Organic and Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 2000910. [Google Scholar] [CrossRef]
- Gusain, A.; Faria, R.M.; Miranda, P.B. Polymer Solar Cells—Interfacial Processes Related to Performance Issues. Front. Chem. 2019, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Amollo, T.A.; Mola, G.T.; Nyamori, V.O. Organic solar cells: Materials and prospects of graphene for active and interfacial layers. Crit. Rev. Solid State Mater. Sci. 2020, 45, 261–288. [Google Scholar] [CrossRef]
- Wu, C.; Wang, K.; Batmunkh, M.; Bati, A.S.R.; Yang, D.; Jiang, Y.; Hou, Y.; Shapter, J.G.; Priya, S. Multifunctional nanostructured materials for next generation photovoltaics. Nano Energy 2020, 70, 104480. [Google Scholar] [CrossRef]
- Huang, Z.; Ouyang, D.; Shih, C.; Yang, B.; Choy, W.C.H. Solution-Processed Ternary Oxides as Carrier Transport/Injection Layers in Optoelectronics. Adv. Energy Mater. 2020, 10, 1900903. [Google Scholar] [CrossRef]
- Li, Y. Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Ye, L.; Zhang, H.; Li, S.; Zhang, S.; Hou, J. Molecular Design of Benzodithiophene-Based Organic Photovoltaic Materials. Chem. Rev. 2016, 116, 7397–7457. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, K.; Coates, N.E.; Moses, D.; Nguyen, T.-Q.; Dante, M.; Heeger, A.J. Efficient Tandem Polymer Solar Cells Fabricated by All-Solution Processing. Science 2007, 317, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Meillaud, F.; Shah, A.; Droz, C.; Vallat-Sauvain, E.; Miazza, C. Efficiency limits for single-junction and tandem solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 2952–2959. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-J.; Adkison, B.L.; Xu, L.; Kramer, A.A.; Hsu, J.W.P. Comparison of conventional and inverted organic photovoltaic devices with controlled illumination area and extraction layers. Sol. Energy Mater. Sol. Cells 2016, 144, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Nunzi, J.-M. Organic photovoltaic materials and devices. Comptes Rendus Phys. 2002, 3, 523–542. [Google Scholar] [CrossRef]
- Shrotriya, V.; Li, G.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. Accurate Measurement and Characterization of Organic Solar Cells. Adv. Funct. Mater. 2006, 16, 2016–2023. [Google Scholar] [CrossRef]
- Qi, B.; Wang, J. Fill factor in organic solar cells. Phys. Chem. Chem. Phys. 2013, 15, 8972. [Google Scholar] [CrossRef] [PubMed]
- Parker, I.D. Carrier tunneling and device characteristics in polymer light-emitting diodes. J. Appl. Phys. 1994, 75, 1656–1666. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Uddin, A. Open circuit voltage of organic solar cells: An in-depth review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Ratcliff, E.L.; Zacher, B.; Armstrong, N.R. Selective Interlayers and Contacts in Organic Photovoltaic Cells. J. Phys. Chem. Lett. 2011, 2, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Mihailetchi, V.D.; Blom, P.W.M.; Hummelen, J.C.; Rispens, M.T. Cathode dependence of the open-circuit voltage of polymer:fullerene bulk heterojunction solar cells. J. Appl. Phys. 2003, 94, 6849–6854. [Google Scholar] [CrossRef] [Green Version]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Street, R.A.; Song, K.W.; Cowan, S. Influence of series resistance on the photocurrent analysis of organic solar cells. Org. Electron. 2011, 12, 244–248. [Google Scholar] [CrossRef]
- Servaites, J.D.; Yeganeh, S.; Marks, T.J.; Ratner, M.A. Efficiency Enhancement in Organic Photovoltaic Cells: Consequences of Optimizing Series Resistance. Adv. Funct. Mater. 2010, 20, 97–104. [Google Scholar] [CrossRef]
- Antohe, S.; Iftimie, S.; Hrostea, L.; Antohe, V.A.; Girtan, M. A critical review of photovoltaic cells based on organic monomeric and polymeric thin film heterojunctions. Thin Solid Films 2017, 642, 219–231. [Google Scholar] [CrossRef]
- Antohe, S.; Ion, L.; Tomozeiu, N.; Stoica, T.; Barna, E. Electrical and photovoltaic properties of photosensitised ITO/a-Si:H p–i–n/TPyP/Au cells. Sol. Energy Mater. Sol. Cells 2000, 62, 207–216. [Google Scholar] [CrossRef]
- Antohe, S.; Ruxandra, V.; Tugulea, L.; Gheorghe, V.; Ionascu, D. Three-Layered Photovoltaic Cell with an Enlarged Photoactive Region of Codeposited Dyes. J. Phys. III 1996, 6, 1133–1144. [Google Scholar] [CrossRef]
- Proctor, C.M.; Nguyen, T.-Q. Effect of leakage current and shunt resistance on the light intensity dependence of organic solar cells. Appl. Phys. Lett. 2015, 106, 083301. [Google Scholar] [CrossRef]
- Wurfel, U.; Cuevas, A.; Wurfel, P. Charge Carrier Separation in Solar Cells. IEEE J. Photovolt. 2015, 5, 461–469. [Google Scholar] [CrossRef]
- Yip, H.-L.; Jen, A.K.Y. Recent advances in solution-processed interfacial materials for efficient and stable polymer solar cells. Energy Environ. Sci. 2012, 5, 5994. [Google Scholar] [CrossRef]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-Level Alignment at Organic/Metal and Organic/Organic Interfaces. Adv. Mater. 2009, 21, 1450–1472. [Google Scholar] [CrossRef]
- Tokito, S.; Noda, K.; Taga, Y. Metal oxides as a hole-injecting layer for an organic electroluminescent device. J. Phys. D Appl. Phys. 1996, 29, 2750–2753. [Google Scholar] [CrossRef]
- Meyer, J.; Hamwi, S.; Kröger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition Metal Oxides for Organic Electronics: Energetics, Device Physics and Applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef]
- Chen, L.-M.; Xu, Z.; Hong, Z.; Yang, Y. Interface investigation and engineering—Achieving high performance polymer photovoltaic devices. J. Mater. Chem. 2010, 20, 2575. [Google Scholar] [CrossRef]
- Curiel, D.; Más-Montoya, M. Hole Transporting Layers in Printable Solar Cells. In Printable Solar Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 93–161. [Google Scholar]
- Shrotriya, V.; Li, G.; Yao, Y.; Chu, C.-W.; Yang, Y. Transition metal oxides as the buffer layer for polymer photovoltaic cells. Appl. Phys. Lett. 2006, 88, 073508. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Shin, W.S.; Seo, M.; Gupta, D.; Moon, S.-J.; Yoo, S. Improving performance of organic solar cells using amorphous tungsten oxides as an interfacial buffer layer on transparent anodes. Org. Electron. 2009, 10, 791–797. [Google Scholar] [CrossRef]
- Irwin, M.D.; Buchholz, D.B.; Hains, A.W.; Chang, R.P.H.; Marks, T.J. p-Type semiconducting nickel oxide as an efficiency-enhancing anode interfacial layer in polymer bulk-heterojunction solar cells. Proc. Natl. Acad. Sci. USA 2008, 105, 2783–2787. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Ren, H.; Yi, C.; Liu, C.; Wang, H.; Huang, L.; Zhang, H.; Karim, A.; Gong, X. Solution-Processed Fe3O4 Magnetic Nanoparticle Thin Film Aligned by an External Magnetostatic Field as a Hole Extraction Layer for Polymer Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 10325–10330. [Google Scholar] [CrossRef]
- Gu, X.; Cui, W.; Li, H.; Wu, Z.; Zeng, Z.; Lee, S.-T.; Zhang, H.; Sun, B. A Solution-Processed Hole Extraction Layer Made from Ultrathin MoS2 Nanosheets for Efficient Organic Solar Cells. Adv. Energy Mater. 2013, 3, 1262–1268. [Google Scholar] [CrossRef]
- Xu, H.; Xu, H.; Yuan, F.; Zhou, D.; Liao, X.; Chen, L.; Chen, Y.; Chen, Y. Hole transport layers for organic solar cells: Recent progress and prospects. J. Mater. Chem. A 2020, 8, 11478–11492. [Google Scholar] [CrossRef]
- Kawano, K.; Pacios, R.; Poplavskyy, D.; Nelson, J.; Bradley, D.D.C.; Durrant, J.R. Degradation of organic solar cells due to air exposure. Sol. Energy Mater. Sol. Cells 2006, 90, 3520–3530. [Google Scholar] [CrossRef]
- Wang, T.; Scarratt, N.W.; Yi, H.; Dunbar, A.D.F.; Pearson, A.J.; Watters, D.C.; Glen, T.S.; Brook, A.C.; Kingsley, J.; Buckley, A.R.; et al. Fabricating High Performance, Donor-Acceptor Copolymer Solar Cells by Spray-Coating in Air. Adv. Energy Mater. 2013, 3, 505–512. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, I.; Niewczas, M.; Petric, A. Electrodeposition of hybrid organic–inorganic films containing iron oxide. Mater. Lett. 2003, 57, 1045–1050. [Google Scholar] [CrossRef]
- Krebs, F.C. Polymer solar cell modules prepared using roll-to-roll methods: Knife-over-edge coating, slot-die coating and screen printing. Sol. Energy Mater. Sol. Cells 2009, 93, 465–475. [Google Scholar] [CrossRef]
- Jung, J.W.; Jo, W.H. Annealing-Free High Efficiency and Large Area Polymer Solar Cells Fabricated by a Roller Painting Process. Adv. Funct. Mater. 2010, 20, 2355–2363. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Cui, Y.; Liu, D.; Xu, B.; Hou, J. Over 100-nm-Thick MoO x Films with Superior Hole Collection and Transport Properties for Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1800698. [Google Scholar] [CrossRef]
- Lou, X.W.; Zeng, H.C. Complex α-MoO3 Nanostructures with External Bonding Capacity for Self-Assembly. J. Am. Chem. Soc. 2003, 125, 2697–2704. [Google Scholar] [CrossRef]
- Pan, W.; Tian, R.; Jin, H.; Guo, Y.; Zhang, L.; Wu, X.; Zhang, L.; Han, Z.; Liu, G.; Li, J.; et al. Structure, Optical, and Catalytic Properties of Novel Hexagonal Metastable h-MoO3 Nano-and Microrods Synthesized with Modified Liquid-Phase Processes. Chem. Mater. 2010, 22, 6202–6208. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Sun, X.W.; Jiang, C.Y.; Lo, G.Q.; Zhao, D.W.; Kwong, D.L. An inverted organic solar cell employing a sol-gel derived ZnO electron selective layer and thermal evaporated MoO3 hole selective layer. Appl. Phys. Lett. 2008, 93, 221107. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Kim, J.; Park, G.; Bae, H.W.; An, M.; Kim, J.Y. Enhanced Operating Temperature Stability of Organic Solar Cells with Metal Oxide Hole Extraction Layer. Polymers 2020, 12, 992. [Google Scholar] [CrossRef]
- Liao, X.; Jeong, A.R.; Wilks, R.G.; Wiesner, S.; Rusu, M.; Félix, R.; Xiao, T.; Hartmann, C.; Bär, M. Tunability of MoO3 Thin-Film Properties Due to Annealing in Situ Monitored by Hard X-ray Photoemission. ACS Omega 2019, 4, 10985–10990. [Google Scholar] [CrossRef] [Green Version]
- Boudaoud, L.; Benramdane, N.; Desfeux, R.; Khelifa, B.; Mathieu, C. Structural and optical properties of MoO3 and V2O5 thin films prepared by Spray Pyrolysis. Catal. Today 2006, 113, 230–234. [Google Scholar] [CrossRef]
- Inzani, K.; Nematollahi, M.; Selbach, S.M.; Grande, T.; Waalekalv, M.L.; Brakstad, T.; Reenaas, T.W.; Kildemo, M.; Vullum-Bruer, F. Tailoring properties of nanostructured MoO3−x thin films by aqueous solution deposition. Appl. Surf. Sci. 2018, 459, 822–829. [Google Scholar] [CrossRef] [Green Version]
- Bortoti, A.A.; de Gavanski, A.F.; Velazquez, Y.R.; Galli, A.; de Castro, E.G. Facile and low cost oxidative conversion of MoS2 in α-MoO3: Synthesis, characterization and application. J. Solid State Chem. 2017, 252, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.; Cheng, J.; Yang, X.; Yu, J.; Li, L. Enhanced charge carrier transport in spray-cast organic solar cells using solution processed MoO3 micro arrays. RSC Adv. 2017, 7, 3059–3065. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Park, E.-K.; Kim, J.-H.; Cho, H.J.; Lee, D.-H.; Kim, Y.-S. Improving charge transport of P3HT:PCBM organic solar cell using MoO3 nanoparticles as an interfacial buffer layer. Electron. Mater. Lett. 2016, 12, 383–387. [Google Scholar] [CrossRef]
- Choy, W.C.H.; Ren, X. Plasmon-Electrical Effects on Organic Solar Cells by Incorporation of Metal Nanostructures. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 1–9. [Google Scholar] [CrossRef]
- Bobeico, E.; Mercaldo, L.V.; Morvillo, P.; Usatii, I.; Della Noce, M.; Lancellotti, L.; Sasso, C.; Ricciardi, R.; Delli Veneri, P. Evaporated MoOx as General Back-Side Hole Collector for Solar Cells. Coatings 2020, 10, 763. [Google Scholar] [CrossRef]
- Jagadamma, L.K.; Hu, H.; Kim, T.; Ndjawa, G.O.N.; Mansour, A.E.; El Labban, A.; Faria, J.C.D.; Munir, R.; Anjum, D.H.; McLachlan, M.A.; et al. Solution-processable MoOx nanocrystals enable highly efficient reflective and semitransparent polymer solar cells. Nano Energy 2016, 28, 277–287. [Google Scholar] [CrossRef]
- Wang, J.; Jia, X.; Zhou, J.; Pan, L.; Huang, S.; Chen, X. Improved Performance of Polymer Solar Cells by Thermal Evaporation of AgAl Alloy Nanostructures into the Hole-Transport Layer. ACS Appl. Mater. Interfaces 2016, 8, 26098–26104. [Google Scholar] [CrossRef]
- Cong, S.; Hadipour, A.; Sugahara, T.; Wei, T.; Jiu, J.; Ranjbar, S.; Hirose, Y.; Karakawa, M.; Nagao, S.; Aernouts, T.; et al. Modifying the valence state of molybdenum in the efficient oxide buffer layer of organic solar cells via a mild hydrogen peroxide treatment. J. Mater. Chem. C 2017, 5, 889–895. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Kim, U.; Park, H. Solution-Processed Molybdenum Oxide with Hydroxyl Radical-Induced Oxygen Vacancy as an Efficient and Stable Interfacial Layer for Organic Solar Cells. Sol. RRL 2020, 4, 1900420. [Google Scholar] [CrossRef]
- Kobori, T.; Kamata, N.; Fukuda, T. Effect of annealing-induced oxidation of molybdenum oxide on organic photovoltaic device performance. Org. Electron. 2016, 37, 126–133. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Huang, X.; Wu, Z.; Chen, M. A simple synthesis method to prepare a molybdenum oxide hole-transporting layer for efficient polymer solar cells. RSC Adv. 2017, 7, 7890–7900. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Wu, Y.; Shen, Y.; Cai, X.; Li, L. Enhancing the performance and stability of organic solar cells using solution processed MoO3 as hole transport layer. RSC Adv. 2017, 7, 37952–37958. [Google Scholar] [CrossRef] [Green Version]
- Cai, P.; Ren, P.; Huang, X.; Zhang, X.; Zhan, T.; Xiong, J.; Xue, X.; Wang, Z.; Zhang, J.; Chen, J. An Ultraviolet-Deposited MoO3 Film as Anode Interlayer for High-Performance Polymer Solar Cells. Adv. Mater. Interfaces 2020, 7, 1901912. [Google Scholar] [CrossRef]
- Tran, H.N.; Park, S.; Wibowo, F.T.A.; Krishna, N.V.; Kang, J.H.; Seo, J.H.; Nguyen-Phu, H.; Jang, S.; Cho, S. 17% Non-Fullerene Organic Solar Cells with Annealing-Free Aqueous MoOx. Adv. Sci. 2020, 7, 2002395. [Google Scholar] [CrossRef]
- Irfan; Ding, H.; Gao, Y.; Small, C.; Kim, D.Y.; Subbiah, J.; So, F. Energy level evolution of air and oxygen exposed molybdenum trioxide films. Appl. Phys. Lett. 2010, 96, 243307. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Soultati, A.; Argitis, P.; Stergiopoulos, T.; Davazoglou, D. Fast Recovery of the High Work Function of Tungsten and Molybdenum Oxides via Microwave Exposure for Efficient Organic Photovoltaics. J. Phys. Chem. Lett. 2014, 5, 1871–1879. [Google Scholar] [CrossRef]
- Soultati, A.; Kostis, I.; Argitis, P.; Dimotikali, D.; Kennou, S.; Gardelis, S.; Speliotis, T.; Kontos, A.G.; Davazoglou, D.; Vasilopoulou, M. Dehydration of molybdenum oxide hole extraction layers via microwave annealing for the improvement of efficiency and lifetime in organic solar cells. J. Mater. Chem. C 2016, 4, 7683–7694. [Google Scholar] [CrossRef]
- Chang, F.-K.; Huang, Y.-C.; Jeng, J.-S.; Chen, J.-S. Band offset of vanadium-doped molybdenum oxide hole transport layer in organic photovoltaics. Solid-State. Electron. 2016, 122, 18–22. [Google Scholar] [CrossRef]
- Marchal, W.; Verboven, I.; Kesters, J.; Moeremans, B.; De Dobbelaere, C.; Bonneux, G.; Elen, K.; Conings, B.; Maes, W.; Boyen, H.G.; et al. Steering the properties of MoOx hole transporting layers in OPVs and OLEDs: Interface morphology vs. electronic structure. Materials 2017, 10, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Zhao, C.; Guo, Q.; Zhang, J.; Hu, S.; Liu, J.; Hayat, T.; Alsaedi, A.; Tan, Z. Enhancing the electron blocking ability of n-type MoO3 by doping with p-type NiO for efficient nonfullerene polymer solar cells. Org. Electron. 2019, 68, 168–175. [Google Scholar] [CrossRef]
- Li, X.; Xie, F.; Zhang, S.; Hou, J.; Choy, W.C.H. MoOx and V2Ox as hole and electron transport layers through functionalized intercalation in normal and inverted organic optoelectronic devices. Light Sci. Appl. 2015, 4, e273. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xie, F.; Zhang, S.; Hou, J.; Choy, W.C.H. Over 1.1 eV Workfunction Tuning of Cesium Intercalated Metal Oxides for Functioning as Both Electron and Hole Transport Layers in Organic Optoelectronic Devices. Adv. Funct. Mater. 2014, 24, 7348–7356. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, H.; Shin, E.-Y.; Bae, I.-G.; Park, B.; Noh, Y.-Y.; Hwang, I. Enhanced hole extraction by interaction between CuI and MoO3 in the hole transport layer of organic photovoltaic devices. Org. Electron. 2016, 32, 200–207. [Google Scholar] [CrossRef]
- Li, Z.; Guo, W.; Liu, C.; Zhang, X.; Li, S.; Guo, J.; Zhang, L. Impedance investigation of the highly efficient polymer solar cells with composite CuBr2 /MoO3 hole transport layer. Phys. Chem. Chem. Phys. 2017, 19, 20839–20846. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, H.; Huang, X.; Wu, Z.; Xu, H. Improved performance for polymer solar cells using CTAB-modified MoO3 as an anode buffer layer. Sol. Energy Mater. Sol. Cells 2017, 171, 72–84. [Google Scholar] [CrossRef]
- Kang, Q.; Zu, Y.; Liao, Q.; Zheng, Z.; Yao, H.; Zhang, S.; He, C.; Xu, B.; Hou, J. An inorganic molecule-induced electron transfer complex for highly efficient organic solar cells. J. Mater. Chem. A 2020, 8, 5580–5586. [Google Scholar] [CrossRef]
- Kwon, K.C.; Lee, T.H.; Choi, S.; Choi, K.S.; Gim, S.O.; Bae, S.-R.; Lee, J.-L.; Jang, H.W.; Kim, S.Y. Synthesis of atomically thin alloyed molybdenum-tungsten disulfides thin films as hole transport layers in organic light-emitting diodes. Appl. Surf. Sci. 2021, 541, 148529. [Google Scholar] [CrossRef]
- Cheng, P.-P.; Zhou, L.; Li, J.-A.; Li, Y.-Q.; Lee, S.-T.; Tang, J.-X. Light trapping enhancement of inverted polymer solar cells with a nanostructured scattering rear electrode. Org. Electron. 2013, 14, 2158–2163. [Google Scholar] [CrossRef]
- Zhang, W.; Lan, W.; Lee, M.H.; Singh, J.; Zhu, F. A versatile solution-processed MoO3/Au nanoparticles/MoO3 hole contact for high performing PEDOT:PSS-free organic solar cells. Org. Electron. 2018, 52, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, M.; Hofmann, H.; Hagelüken, C.; Hool, A. Critical raw materials: A perspective from the materials science community. Sustain. Mater. Technol. 2018, 17, e00074. [Google Scholar] [CrossRef]
- Peñafiel-Vicuña, S.; Rendón-Enríquez, I.; Vega-Poot, A.; López-Téllez, G.; Palma-Cando, A.; Frontana-Uribe, B.A. Recovering Indium Tin Oxide Electrodes for the Fabrication of Hematite Photoelectrodes. J. Electrochem. Soc. 2020, 167, 126512. [Google Scholar] [CrossRef]
- Chen, S.; Dai, Y.; Zhao, D.; Zhang, H. ITO-free flexible organic photovoltaics with multilayer MoO3 /LiF/MoO3 /Ag/MoO3 as the transparent electrode. Semicond. Sci. Technol. 2016, 31, 055013. [Google Scholar] [CrossRef]
- Lee, H.-M.; Kim, S.-S.; Kim, H.-K. Artificially MoO3 graded ITO anodes for acidic buffer layer free organic photovoltaics. Appl. Surf. Sci. 2016, 364, 340–348. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, Q.; Cheng, S.; Zhou, H.; Lai, Y.; Yu, J. Comparing molybdenum oxide thin films prepared by magnetron sputtering and thermal evaporation applied in organic solar cells. J. Mater. Sci. Mater. Electron. 2016, 27, 3245–3249. [Google Scholar] [CrossRef]
- Chaturvedi, N.; Swami, S.K.; Dutta, V. Electric field assisted spray deposited MoO3 thin films as a hole transport layer for organic solar cells. Sol. Energy 2016, 137, 379–384. [Google Scholar] [CrossRef]
- Dong, W.J.; Ham, J.; Jung, G.H.; Son, J.H.; Lee, J.-L. Ultrafast laser-assisted synthesis of hydrogenated molybdenum oxides for flexible organic solar cells. J. Mater. Chem. A 2016, 4, 4755–4762. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.; Hamwi, S.; Bülow, T.; Johannes, H.-H.; Riedl, T.; Kowalsky, W. Highly efficient simplified organic light emitting diodes. Appl. Phys. Lett. 2007, 91, 113506. [Google Scholar] [CrossRef]
- Tao, C.; Ruan, S.; Xie, G.; Kong, X.; Shen, L.; Meng, F.; Liu, C.; Zhang, X.; Dong, W.; Chen, W. Role of tungsten oxide in inverted polymer solar cells. Appl. Phys. Lett. 2009, 94, 043311. [Google Scholar] [CrossRef] [Green Version]
- Miyake, K.; Kaneko, H.; Sano, M.; Suedomi, N. Physical and electrochromic properties of the amorphous and crystalline tungsten oxide thick films prepared under reducing atmosphere. J. Appl. Phys. 1984, 55, 2747–2753. [Google Scholar] [CrossRef]
- Vida, G.; Josepovits, V.K.; Győr, M.; Deák, P. Characterization of Tungsten Surfaces by Simultaneous Work Function and Secondary Electron Emission Measurements. Microsc. Microanal. 2003, 9, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Stubhan, T.; Li, N.; Luechinger, N.A.; Halim, S.C.; Matt, G.J.; Brabec, C.J. High Fill Factor Polymer Solar Cells Incorporating a Low Temperature Solution Processed WO 3 Hole Extraction Layer. Adv. Energy Mater. 2012, 2, 1433–1438. [Google Scholar] [CrossRef]
- Brabec, C.J. Organic photovoltaics: Technology and market. Sol. Energy Mater. Sol. Cells 2004, 83, 273–292. [Google Scholar] [CrossRef]
- Lee, Y.H.; Abdu, H.A.E.; Kim, D.H.; Kim, T.W. Enhancement of the power conversion efficiency of organic photovoltaic cells due to Au@SiO2 core shell nanoparticles embedded into a WO3 hole transport layer. Org. Electron. 2019, 68, 182–186. [Google Scholar] [CrossRef]
- Li, X.; Choy, W.C.H.; Ren, X.; Xin, J.; Lin, P.; Leung, D.C.W. Polarization-independent efficiency enhancement of organic solar cells by using 3-dimensional plasmonic electrode. Appl. Phys. Lett. 2013, 102, 153304. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Choy, W.C.H.; Huo, L.; Xie, F.; Sha, W.E.I.; Ding, B.; Guo, X.; Li, Y.; Hou, J.; You, J.; et al. Dual Plasmonic Nanostructures for High Performance Inverted Organic Solar Cells. Adv. Mater. 2012, 24, 3046–3052. [Google Scholar] [CrossRef]
- Li, X.H.; Sha, W.E.I.; Choy, W.C.H.; Fung, D.D.S.; Xie, F.X. Efficient Inverted Polymer Solar Cells with Directly Patterned Active Layer and Silver Back Grating. J. Phys. Chem. C 2012, 116, 7200–7206. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, D.H.; Kim, T.W. Enhanced power conversion efficiency of organic photovoltaic devices due to the surface plasmonic resonance effect generated utilizing Au-WO3 nanocomposites. Org. Electron. 2017, 45, 256–262. [Google Scholar] [CrossRef]
- Shen, W.; Tang, J.; Wang, D.; Yang, R.; Chen, W.; Bao, X.; Wang, Y.; Jiao, J.; Wang, Y.; Huang, Z.; et al. Enhanced efficiency of polymer solar cells by structure-differentiated silver nano-dopants in solution-processed tungsten oxide layer. Mater. Sci. Eng. B 2016, 206, 61–68. [Google Scholar] [CrossRef]
- Wang, C.C.D.; Choy, W.C.H.; Duan, C.; Fung, D.D.S.; Sha, W.E.I.; Xie, F.-X.; Huang, F.; Cao, Y. Optical and electrical effects of gold nanoparticles in the active layer of polymer solar cells. J. Mater. Chem. 2012, 22, 1206–1211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Choy, W.C.H.; Xie, F.; Sha, W.E.I.; Li, X.; Ding, B.; Zhang, K.; Huang, F.; Cao, Y. Plasmonic Electrically Functionalized TiO2 for High-Performance Organic Solar Cells. Adv. Funct. Mater. 2013, 23, 4255–4261. [Google Scholar] [CrossRef]
- Li, X.; Choy, W.C.H.; Xie, F.; Zhang, S.; Hou, J. Room-temperature solution-processed molybdenum oxide as a hole transport layer with Ag nanoparticles for highly efficient inverted organic solar cells. J. Mater. Chem. A 2013, 1, 6614. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Choy, W.C.H.; Sha, W.E.I.; Zhang, D.; Zhang, S.; Li, X.; Leung, C.; Hou, J. Enhanced charge extraction in organic solar cells through electron accumulation effects induced by metal nanoparticles. Energy Environ. Sci. 2013, 6, 3372. [Google Scholar] [CrossRef]
- Ren, X.; Cheng, J.; Zhang, S.; Li, X.; Rao, T.; Huo, L.; Hou, J.; Choy, W.C.H. High Efficiency Organic Solar Cells Achieved by the Simultaneous Plasmon-Optical and Plasmon-Electrical Effects from Plasmonic Asymmetric Modes of Gold Nanostars. Small 2016, 12, 5200–5207. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.E.I.; Zhu, H.L.; Chen, L.; Chew, W.C.; Choy, W.C.H. A General Design Rule to Manipulate Photocarrier Transport Path in Solar Cells and Its Realization by the Plasmonic-Electrical Effect. Sci. Rep. 2015, 5, 8525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sha, W.E.I.; Li, X.; Choy, W.C.H. Breaking the Space Charge Limit in Organic Solar Cells by a Novel Plasmonic-Electrical Concept. Sci. Rep. 2015, 4, 6236. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Choy, W.C.H.; Lu, H.; Sha, W.E.I.; Ho, A.H.P. Efficiency Enhancement of Organic Solar Cells by Using Shape-Dependent Broadband Plasmonic Absorption in Metallic Nanoparticles. Adv. Funct. Mater. 2013, 23, 2728–2735. [Google Scholar] [CrossRef]
- Remya, R.; Gayathri, P.T.G.; Deb, B. Studies on solution-processed tungsten oxide nanostructures for efficient hole transport in the inverted polymer solar cells. Mater. Chem. Phys. 2020, 255, 123584. [Google Scholar] [CrossRef]
- Liao, H.-H.; Chen, L.-M.; Xu, Z.; Li, G.; Yang, Y. Highly efficient inverted polymer solar cell by low temperature annealing of Cs2CO3 interlayer. Appl. Phys. Lett. 2008, 92, 173303. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-S.; Chou, C.-Y.; Liu, M.-Y.; Tsai, K.-H.; Lin, W.-H.; Lin, C.-F. Solution-processed vanadium oxide as an anode interlayer for inverted polymer solar cells hybridized with ZnO nanorods. Org. Electron. 2009, 10, 1060–1065. [Google Scholar] [CrossRef]
- Meyer, J.; Zilberberg, K.; Riedl, T.; Kahn, A. Electronic structure of Vanadium pentoxide: An efficient hole injector for organic electronic materials. J. Appl. Phys. 2011, 110, 033710. [Google Scholar] [CrossRef]
- Xu, M.-F.; Xu, T.; Wang, C.-N.; Zhai, Z.-C.; Jin, Y.-L.; Yang, X.-H. Low-temperature, aqueous solution-processed V2O5 as the hole-transport layer for high performance organic solar cells. J. Mater. Sci. Mater. Electron. 2018, 29, 14783–14787. [Google Scholar] [CrossRef]
- Jiang, Y.; Xiao, S.; Xu, B.; Zhan, C.; Mai, L.; Lu, X.; You, W. Enhancement of Photovoltaic Performance by Utilizing Readily Accessible Hole Transporting Layer of Vanadium(V) Oxide Hydrate in a Polymer-Fullerene Blend Solar Cell. ACS Appl. Mater. Interfaces 2016, 8, 11658–11666. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Han, D.; Sun, B.; Zhou, D.; Wang, C.; Liu, P.; Feng, L. Facile Approach to Preparing a Vanadium Oxide Hydrate Layer as a Hole-Transport Layer for High-Performance Polymer Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 18087–18094. [Google Scholar] [CrossRef]
- Xie, F.; Choy, W.C.H.; Wang, C.; Li, X.; Zhang, S.; Hou, J. Low-Temperature Solution-Processed Hydrogen Molybdenum and Vanadium Bronzes for an Efficient Hole-Transport Layer in Organic Electronics. Adv. Mater. 2013, 25, 2051–2055. [Google Scholar] [CrossRef]
- Vishnumurthy, K.A.; Kesavan, A.V.; Swathi, S.K.; Ramamurthy, P.C. Low band gap thienothiophene-diketopyrrolopyrole copolymers with V2O5 as hole transport layer for photovoltaic application. Opt. Mater. 2020, 109, 110303. [Google Scholar] [CrossRef]
- Ravi, R.; Deb, B. Studies on One-Step-Synthesized Hydrated Vanadium Pentoxide for Efficient Hole Transport in Organic Photovoltaics. Energy Technol. 2020, 8, 1901323. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Y.; Huang, X.; Jiang, L.; Li, Q.; Hu, X.; Huang, F.; Gong, X.; Cao, Y. Solution-processed VOx prepared using a novel synthetic method as the hole extraction layer for polymer solar cells. J. Mater. Chem. C 2016, 4, 1953–1958. [Google Scholar] [CrossRef]
- Shafeeq, K.M.; Athira, V.P.; Kishor, C.H.R.; Aneesh, P.M. Structural and optical properties of V2O5 nanostructures grown by thermal decomposition technique. Appl. Phys. A 2020, 126, 586. [Google Scholar] [CrossRef]
- Alsulami, A.; Griffin, J.; Alqurashi, R.; Yi, H.; Iraqi, A.; Lidzey, D.; Buckley, A. Thermally Stable Solution Processed Vanadium Oxide as a Hole Extraction Layer in Organic Solar Cells. Materials 2016, 9, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, M.; Yun, J.-Y.; Kim, D.-H. Highly stable inverted organic photovoltaic cells with a V2O5 hole transport layer. Korean J. Chem. Eng. 2017, 34, 1504–1508. [Google Scholar] [CrossRef]
- Teng, N.-W.; Li, C.-H.; Lo, W.-C.; Tsai, Y.-S.; Liao, C.-Y.; You, Y.-W.; Ho, H.-L.; Li, W.-L.; Lee, C.-C.; Lin, W.-C.; et al. Highly Efficient Nonfullerene Organic Photovoltaic Devices with 10% Power Conversion Efficiency Enabled by a Fine-Tuned and Solution-Processed Hole-Transporting Layer. Sol. RRL 2020, 4, 2000223. [Google Scholar] [CrossRef]
- Xia, C.; Hong, W.T.; Kim, Y.E.; Choe, W.-S.; Kim, D.-H.; Kim, J.K. Metal-Organic Decomposition-Mediated Nanoparticulate Vanadium Oxide Hole Transporting Buffer Layer for Polymer Bulk-Heterojunction Solar Cells. Polymers 2020, 12, 1791. [Google Scholar] [CrossRef] [PubMed]
- Beliatis, M.J.; Helgesen, M.; García-Valverde, R.; Corazza, M.; Roth, B.; Carlé, J.E.; Jørgensen, M.; Krebs, F.C.; Gevorgyan, S.A. Slot-Die-Coated V2O5 as Hole Transport Layer for Flexible Organic Solar Cells and Optoelectronic Devices. Adv. Eng. Mater. 2016, 18, 1494–1503. [Google Scholar] [CrossRef]
- Arbab, E.A.A.; Mola, G.T. V2O5 thin film deposition for application in organic solar cells. Appl. Phys. A Mater. Sci. Process. 2016, 122, 405. [Google Scholar] [CrossRef]
- Kavuri, H.A.; Fukuda, T.; Takahira, K.; Takahashi, A.; Kihara, S.; McGillivray, D.J.; Willmott, G. Electrospray-deposited vanadium oxide anode interlayers for high-efficiency organic solar cells. Org. Electron. 2018, 57, 239–246. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Cho, S.-P.; Na, S.-I.; Kim, S.-S. ITO-free polymer solar cells with vanadium oxide hole transport layer. J. Ind. Eng. Chem. 2017, 45, 1–4. [Google Scholar] [CrossRef]
- Ko, E.-H.; Kim, H.-K. Highly transparent vanadium oxide-graded indium zinc oxide electrodes for flexible organic solar cells. Thin Solid Films 2016, 601, 2–6. [Google Scholar] [CrossRef]
- Ai, L.; Fang, G.; Yuan, L.; Liu, N.; Wang, M.; Li, C.; Zhang, Q.; Li, J.; Zhao, X. Influence of substrate temperature on electrical and optical properties of p-type semitransparent conductive nickel oxide thin films deposited by radio frequency sputtering. Appl. Surf. Sci. 2008, 254, 2401–2405. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Lin, F.; He, H.; Mao, J.; Wong, K.S.; Jen, A.K.Y.; Choy, W.C.H. Pinhole-Free and Surface-Nanostructured NiOx Film by Room-Temperature Solution Process for High-Performance Flexible Perovskite Solar Cells with Good Stability and Reproducibility. ACS Nano 2016, 10, 1503–1511. [Google Scholar] [CrossRef]
- Manders, J.R.; Tsang, S.-W.; Hartel, M.J.; Lai, T.-H.; Chen, S.; Amb, C.M.; Reynolds, J.R.; So, F. Solution-Processed Nickel Oxide Hole Transport Layers in High Efficiency Polymer Photovoltaic Cells. Adv. Funct. Mater. 2013, 23, 2993–3001. [Google Scholar] [CrossRef]
- Parthiban, S.; Kim, S.; Tamilavan, V.; Lee, J.; Shin, I.; Yuvaraj, D.; Jung, Y.K.; Hyun, M.H.; Jeong, J.H.; Park, S.H. Enhanced efficiency and stability of polymer solar cells using solution-processed nickel oxide as hole transport material. Curr. Appl. Phys. 2017, 17, 1232–1237. [Google Scholar] [CrossRef]
- Chavhan, S.D.; Hansson, R.; Ericsson, L.K.E.; Beyer, P.; Hofmann, A.; Brütting, W.; Opitz, A.; Moons, E. Low temperature processed NiOx hole transport layers for efficient polymer solar cells. Org. Electron. 2017, 44, 59–66. [Google Scholar] [CrossRef]
- Jiang, F.; Choy, W.C.H.; Li, X.; Zhang, D.; Cheng, J. Post-treatment-Free Solution-Processed Non-stoichiometric NiOx Nanoparticles for Efficient Hole-Transport Layers of Organic Optoelectronic Devices. Adv. Mater. 2015, 27, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ouyang, D.; Ma, R.; Wu, W.; Roy, V.A.L.; Choy, W.C.H. A General Method: Designing a Hypocrystalline Hydroxide Intermediate to Achieve Ultrasmall and Well-Dispersed Ternary Metal Oxide for Efficient Photovoltaic Devices. Adv. Funct. Mater. 2019, 29, 1904684. [Google Scholar] [CrossRef]
- Kim, J.K. PEG-assisted Sol-gel Synthesis of Compact Nickel Oxide Hole-Selective Layer with Modified Interfacial Properties for Organic Solar Cells. Polymers 2019, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Rho, Y.; Kang, K.-T.; Lee, D. Highly crystalline Ni/NiO hybrid electrodes processed by inkjet printing and laser-induced reductive sintering under ambient conditions. Nanoscale 2016, 8, 8976–8985. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, S.K.; Garg, A. Inkjet printing of NiO films and integration as hole transporting layers in polymer solar cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Wang, Y.; Shen, S.; Tang, Y.; Yu, A.; Kang, B.; Silva, S.R.P.; Lu, G. Enhancing the performance of polymer solar cells using solution-processed copper doped nickel oxide nanoparticles as hole transport layer. J. Colloid Interface Sci. 2019, 535, 308–317. [Google Scholar] [CrossRef]
- Ouyang, D.; Zheng, J.; Huang, Z.; Zhu, L.; Choy, W.C.H. An efficacious multifunction codoping strategy on a room-temperature solution-processed hole transport layer for realizing high-performance perovskite solar cells. J. Mater. Chem. A 2021, 9, 371–379. [Google Scholar] [CrossRef]
- Al-Jawhari, H.A.; Caraveo-Frescas, J.A.; Hedhili, M.N.; Alshareef, H.N. P-Type Cu2O/SnO Bilayer Thin Film Transistors Processed at Low Temperatures. ACS Appl. Mater. Interfaces 2013, 5, 9615–9619. [Google Scholar] [CrossRef] [PubMed]
- Murali, D.S.; Kumar, S.; Choudhary, R.J.; Wadikar, A.D.; Jain, M.K.; Subrahmanyam, A. Synthesis of Cu2O from CuO thin films: Optical and electrical properties. AIP Adv. 2015, 5, 047143. [Google Scholar] [CrossRef]
- Fortunato, E.; Figueiredo, V.; Barquinha, P.; Elamurugu, E.; Barros, R.; Gonçalves, G.; Park, S.-H.K.; Hwang, C.-S.; Martins, R. Thin-film transistors based on p-type Cu2O thin films produced at room temperature. Appl. Phys. Lett. 2010, 96, 192102. [Google Scholar] [CrossRef] [Green Version]
- Zuo, C.; Ding, L. Solution-Processed Cu2O and CuO as Hole Transport Materials for Efficient Perovskite Solar Cells. Small 2015, 11, 5528–5532. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, W.; Fu, W.; Zhang, Z.; Yang, W.; Wang, S.; Li, H.; Xu, M.; Chen, H. An aqueous solution-processed CuO X film as an anode buffer layer for efficient and stable organic solar cells. J. Mater. Chem. A 2016, 4, 5130–5136. [Google Scholar] [CrossRef]
- Yu, R.-S.; Tasi, C.-P. Structure, composition and properties of p-type CuCrO2 thin films. Ceram. Int. 2014, 40, 8211–8217. [Google Scholar] [CrossRef]
- Han, M.; Jiang, K.; Zhang, J.; Yu, W.; Li, Y.; Hu, Z.; Chu, J. Structural, electronic band transition and optoelectronic properties of delafossite CuGa1−xCrxO2 (0 ≤ x ≤ 1) solid solution films grown by the sol–gel method. J. Mater. Chem. 2012, 22, 18463. [Google Scholar] [CrossRef]
- Xiong, D.; Xu, Z.; Zeng, X.; Zhang, W.; Chen, W.; Xu, X.; Wang, M.; Cheng, Y.-B. Hydrothermal synthesis of ultrasmall CuCrO2 nanocrystal alternatives to NiO nanoparticles in efficient p-type dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 24760. [Google Scholar] [CrossRef]
- Marquardt, M.A.; Ashmore, N.A.; Cann, D.P. Crystal chemistry and electrical properties of the delafossite structure. Thin Solid Films 2006, 496, 146–156. [Google Scholar] [CrossRef]
- Yang, B.; Ouyang, D.; Huang, Z.; Ren, X.; Zhang, H.; Choy, W.C.H. Multifunctional Synthesis Approach of In:CuCrO2 Nanoparticles for Hole Transport Layer in High-Performance Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1902600. [Google Scholar] [CrossRef]
- Wang, J.; Lee, Y.-J.; Hsu, J.W.P. Sub-10 nm copper chromium oxide nanocrystals as a solution processed p-type hole transport layer for organic photovoltaics. J. Mater. Chem. C 2016, 4, 3607–3613. [Google Scholar] [CrossRef]
- Wang, J.; Daunis, T.B.; Cheng, L.; Zhang, B.; Kim, J.; Hsu, J.W.P. Combustion Synthesis of p-Type Transparent Conducting CuCrO2+x and Cu:CrOx Thin Films at 180 °C. ACS Appl. Mater. Interfaces 2018, 10, 3732–3738. [Google Scholar] [CrossRef] [PubMed]
- Wahl, T.; Zellmer, S.; Hanisch, J.; Garnweitner, G.; Ahlswede, E. Thin indium tin oxide nanoparticle films as hole transport layer in inverted organic solar cells. Thin Solid Films 2016, 616, 419–424. [Google Scholar] [CrossRef]
- Bhargav, R.; Gairola, S.P.; Patra, A.; Naqvi, S.; Dhawan, S.K. Improved performance of organic solar cells with solution processed hole transport layer. Opt. Mater. 2018, 80, 138–142. [Google Scholar] [CrossRef]
- Kan, M.; Wang, J.Y.; Li, X.W.; Zhang, S.H.; Li, Y.W.; Kawazoe, Y.; Sun, Q.; Jena, P. Structures and Phase Transition of a MoS2 Monolayer. J. Phys. Chem. C 2014, 118, 1515–1522. [Google Scholar] [CrossRef]
- Barrera, D.; Jawaid, A.; Daunis, T.B.; Cheng, L.; Wang, Q.; Lee, Y.-J.; Kim, M.J.; Kim, J.; Vaia, R.A.; Hsu, J.W.P. Inverted OPVs with MoS2 hole transport layer deposited by spray coating. Mater. Today Energy 2017, 5, 107–111. [Google Scholar] [CrossRef]
- Martinez-Rojas, F.; Hssein, M.; El Jouad, Z.; Armijo, F.; Cattin, L.; Louarn, G.; Stephant, N.; del Valle, M.A.; Addou, M.; Soto, J.P.; et al. Mo(SxOy) thin films deposited by electrochemistry for application in organic photovoltaic cells. Mater. Chem. Phys. 2017, 201, 331–338. [Google Scholar] [CrossRef]
- Xing, W.; Chen, Y.; Wang, X.; Lv, L.; Ouyang, X.; Ge, Z.; Huang, H. MoS2 Quantum Dots with a Tunable Work Function for High-Performance Organic Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 26916–26923. [Google Scholar] [CrossRef]
- Wei, J.; Yin, Z.; Chen, S.-C.; Cai, D.; Zheng, Q. Solution-processed MoSx thin-films as hole-transport layers for efficient polymer solar cells. RSC Adv. 2016, 6, 39137–39143. [Google Scholar] [CrossRef]
- Adilbekova, B.; Lin, Y.; Yengel, E.; Faber, H.; Harrison, G.; Firdaus, Y.; El-Labban, A.; Anjum, D.H.; Tung, V.; Anthopoulos, T.D. Liquid phase exfoliation of MoS2 and WS2 in aqueous ammonia and their application in highly efficient organic solar cells. J. Mater. Chem. C 2020, 8, 5259–5264. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Adilbekova, B.; Firdaus, Y.; Yengel, E.; Faber, H.; Sajjad, M.; Zheng, X.; Yarali, E.; Seitkhan, A.; Bakr, O.M.; et al. 17% Efficient Organic Solar Cells Based on Liquid Exfoliated WS2 as a Replacement for PEDOT:PSS. Adv. Mater. 2019, 31, 1902965. [Google Scholar] [CrossRef] [PubMed]
- Ram, K.S.; Singh, J. Over 20% Efficient and Stable Non-Fullerene-Based Ternary Bulk-Heterojunction Organic Solar Cell with WS2 Hole-Transport Layer and Graded Refractive Index Antireflection Coating. Adv. Theory Simul. 2020, 3, 2000047. [Google Scholar] [CrossRef]
- Hilal, M.; Han, J.I. Preparation of hierarchical flower-like nickel sulfide as hole transporting material for organic solar cells via a one-step solvothermal method. Sol. Energy 2019, 188, 403–413. [Google Scholar] [CrossRef]
- Bhargav, R.; Patra, A.; Dhawan, S.K.; Gairola, S.P. Solution processed hole transport layer towards efficient and cost effective organic solar cells. Sol. Energy 2018, 165, 131–135. [Google Scholar] [CrossRef]
- Jose, E.; Mohan, M.; Namboothiry, M.A.G.; Kumar, M.C.S. Room temperature deposition of high figure of merit p-type transparent conducting Cu–Zn–S thin films and their application in organic solar cells as an efficient hole transport layer. J. Alloys Compd. 2020, 829, 154507. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, L.; Wei, X.; Zhao, M.; Miao, Y.; Zhang, X.; Zhang, H.; Wang, H.; Hao, Y.; Xu, B.; et al. Energy level engineering of PEDOT:PSS by antimonene quantum sheet doping for highly efficient OLEDs. J. Mater. Chem. C 2020, 8, 1796–1802. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Wang, Z.; Zhao, M.; Zhou, Y.; Zhao, B.; Miao, Y.; Liu, P.; Hao, Y.; Wang, H.; et al. Novel 2D material from AMQS-based defect engineering for efficient and stable organic solar cells. 2D Mater. 2019, 6, 045017. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Y.; Ouyang, D.; Liu, H.; Huang, Z.; Kim, J.; Choy, W.C.H. Triple Interface Passivation Strategy-Enabled Efficient and Stable Inverted Perovskite Solar Cells. Small Methods 2020, 4, 2000478. [Google Scholar] [CrossRef]
- Ma, R.; Ren, Z.; Li, C.; Wang, Y.; Huang, Z.; Zhao, Y.; Yang, T.; Liang, Y.; Sun, X.W.; Choy, W.C.H. Establishing Multifunctional Interface Layer of Perovskite Ligand Modified Lead Sulfide Quantum Dots for Improving the Performance and Stability of Perovskite Solar Cells. Small 2020, 16, 2002628. [Google Scholar] [CrossRef]
- Ma, R.; Zheng, J.; Tian, Y.; Li, C.; Lyu, B.; Lu, L.; Su, Z.; Chen, L.; Gao, X.; Tang, J.; et al. Self-Polymerization of Monomer and Induced Interactions with Perovskite for Highly Performed and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2105290. [Google Scholar] [CrossRef]
- Wijeyasinghe, N.; Eisner, F.; Tsetseris, L.; Lin, Y.-H.; Seitkhan, A.; Li, J.; Yan, F.; Solomeshch, O.; Tessler, N.; Patsalas, P.; et al. p-Doping of Copper(I) Thiocyanate (CuSCN) Hole-Transport Layers for High-Performance Transistors and Organic Solar Cells. Adv. Funct. Mater. 2018, 28, 1802055. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, J.; Guo, J.; Wang, Z.; Yan, L.; Hao, Y.; Wang, H.; Xu, B.; Yin, S. Hybrid Hole Extraction Layer Enabled High Efficiency in Polymer Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 55342–55348. [Google Scholar] [CrossRef]
- Worakajit, P.; Hamada, F.; Sahu, D.; Kidkhunthod, P.; Sudyoadsuk, T.; Promarak, V.; Harding, D.J.; Packwood, D.M.; Saeki, A.; Pattanasattayavong, P. Elucidating the Coordination of Diethyl Sulfide Molecules in Copper(I) Thiocyanate (CuSCN) Thin Films and Improving Hole Transport by Antisolvent Treatment. Adv. Funct. Mater. 2020, 30, 2002355. [Google Scholar] [CrossRef]
- Suresh Kumar, M.; Mohanta, K.; Batabyal, S.K. Solution processed Cu2CdSnS4 as a low-cost inorganic hole transport material for polymer solar cells. Sol. Energy Mater. Sol. Cells 2017, 161, 157–161. [Google Scholar] [CrossRef]
- Xu, Z. Fundamental Properties of Graphene. In Graphene; Elsevier: Amsterdam, The Netherlands, 2018; pp. 73–102. [Google Scholar]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through p K a Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Garfunkel, E.; Chhowalla, M. Evolution of Electrical, Chemical, and Structural Properties of Transparent and Conducting Chemically Derived Graphene Thin Films. Adv. Funct. Mater. 2009, 19, 2577–2583. [Google Scholar] [CrossRef]
- Sun, L. Structure and synthesis of graphene oxide. Chin. J. Chem. Eng. 2019, 27, 2251–2260. [Google Scholar] [CrossRef] [Green Version]
- Rafique, S.; Abdullah, S.M.; Alhummiany, H.; Abdel-wahab, M.S.; Iqbal, J.; Sulaiman, K. Bulk Heterojunction Organic Solar Cells with Graphene Oxide Hole Transport Layer: Effect of Varied Concentration on Photovoltaic Performance. J. Phys. Chem. C 2017, 121, 140–146. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Huang, X.; Yu, H.; Wu, Z.; Li, Y. Improving the performance of polymer solar cells by efficient optimizing the hole transport layer-graphene oxide. J. Solid-State Electrochem. 2018, 22, 317–329. [Google Scholar] [CrossRef]
- Lee, S.; Yeo, J.-S.; Yun, J.-M.; Kim, D.-Y. Water dispersion of reduced graphene oxide stabilized via fullerenol semiconductor for organic solar cells. Opt. Mater. Express 2017, 7, 2487. [Google Scholar] [CrossRef]
- Kwon, S.-N.; Jung, C.-H.; Na, S.-I. Electron-beam-induced reduced graphene oxide as an alternative hole-transporting interfacial layer for high-performance and reliable polymer solar cells. Org. Electron. 2016, 34, 67–74. [Google Scholar] [CrossRef]
- Fakharan, Z.; Naji, L.; Madanipour, K. Nd:YAG pulsed laser production of reduced-graphene oxide as hole transporting layer in polymer solar cells and the influences of solvent type. Org. Electron. 2020, 76, 105459. [Google Scholar] [CrossRef]
- Dericiler, K.; Alishah, H.M.; Bozar, S.; Güneş, S.; Kaya, F. A novel method for graphene synthesis via electrochemical process and its utilization in organic photovoltaic devices. Appl. Phys. A 2020, 126, 904. [Google Scholar] [CrossRef]
- Xia, Y.; Pan, Y.; Zhang, H.; Qiu, J.; Zheng, Y.; Chen, Y.; Huang, W. Graphene Oxide by UV-Ozone Treatment as an Efficient Hole Extraction Layer for Highly Efficient and Stable Polymer Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 26252–26256. [Google Scholar] [CrossRef]
- Rafique, S.; Abdullah, S.M.; Iqbal, J.; Jilani, A.; Vattamkandathil, S.; Iwamoto, M. Moderately reduced graphene oxide via UV-ozone treatment as hole transport layer for high efficiency organic solar cells. Org. Electron. 2018, 59, 140–148. [Google Scholar] [CrossRef]
- Zhao, F.-G.; Hu, C.-M.; Kong, Y.-T.; Pan, B.; Yao, X.; Chu, J.; Xu, Z.-W.; Zuo, B.; Li, W.-S. Sulfanilic Acid Pending on a Graphene Scaffold: Novel, Efficient Synthesis and Much Enhanced Polymer Solar Cell Efficiency and Stability Using It as a Hole Extraction Layer. ACS Appl. Mater. Interfaces 2018, 10, 24679–24688. [Google Scholar] [CrossRef]
- Ali, A.; Khan, Z.S.; Jamil, M.; Khan, Y.; Ahmad, N.; Ahmed, S. Simultaneous reduction and sulfonation of graphene oxide for efficient hole selectivity in polymer solar cells. Curr. Appl. Phys. 2018, 18, 599–610. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Zhang, M.; Ju, H.; Zhu, J.; Qiao, Q.; Wang, M.; Yang, S. Noncovalent phosphorylation of graphene oxide with improved hole transport in high-efficiency polymer solar cells. Nanoscale 2018, 10, 14840–14846. [Google Scholar] [CrossRef]
- Cheng, X.; Long, J.; Wu, R.; Huang, L.; Tan, L.; Chen, L.; Chen, Y. Fluorinated Reduced Graphene Oxide as an Efficient Hole-Transport Layer for Efficient and Stable Polymer Solar Cells. ACS Omega 2017, 2, 2010–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicasio-Collazo, J.; Maldonado, J.-L.; Salinas-Cruz, J.; Barreiro-Argüelles, D.; Caballero-Quintana, I.; Vázquez-Espinosa, C.; Romero-Borja, D. Functionalized and reduced graphene oxide as hole transport layer and for use in ternary organic solar cell. Opt. Mater. 2019, 98, 109434. [Google Scholar] [CrossRef]
- Park, J.-J.; Heo, Y.-J.; Yun, J.-M.; Kim, Y.; Yoon, S.C.; Lee, S.-H.; Kim, D.-Y. Orthogonal Printable Reduced Graphene Oxide 2D Materials as Hole Transport Layers for High-Performance Inverted Polymer Solar Cells: Sheet Size Effect on Photovoltaic Properties. ACS Appl. Mater. Interfaces 2020, 12, 42811–42820. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, H.; Yang, Q.; Xiong, C.; Li, W.; Yan, Y.; Gurney, R.S.; Wang, T. Solution-processed Graphene-MoS2 heterostructure for efficient hole extraction in organic solar cells. Carbon 2019, 142, 156–163. [Google Scholar] [CrossRef]
- Shoyiga, H.O.; Martincigh, B.S.; Nyamori, V.O. Hydrothermal synthesis of reduced graphene oxide-anatase titania nanocomposites for dual application in organic solar cells. Int. J. Energy Res. 2020, 45, 7293–7314. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, S.; Ko, M.S.; Lee, N.; Hwang, I.; Lee, M.J. Improved performance of organic photovoltaic devices by doping F4TCNQ onto solution-processed graphene as a hole transport layer. Org. Electron. 2016, 30, 302–311. [Google Scholar] [CrossRef]
- Lee, C.K.; Seo, J.G.; Kim, H.J.; Hong, S.J.; Song, G.; Ahn, C.; Lee, D.J.; Song, S.H. Versatile and Tunable Electrical Properties of Doped Nonoxidized Graphene Using Alkali Metal Chlorides. ACS Appl. Mater. Interfaces 2019, 11, 42520–42527. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, D.; Wang, C.; Liu, P.; Hao, Y.; Han, D.; Feng, L.; Zhou, Y. Copper(II) chloride doped graphene oxides as efficient hole transport layer for high-performance polymer solar cells. Org. Electron. 2017, 44, 176–182. [Google Scholar] [CrossRef]
- Capasso, A.; Salamandra, L.; Faggio, G.; Dikonimos, T.; Buonocore, F.; Morandi, V.; Ortolani, L.; Lisi, N. Chemical Vapor Deposited Graphene-Based Derivative As High-Performance Hole Transport Material for Organic Photovoltaics. ACS Appl. Mater. Interfaces 2016, 8, 23844–23853. [Google Scholar] [CrossRef]
- Sarkar, A.S.; Rao, A.D.; Jagdish, A.K.; Gupta, A.; Nandi, C.K.; Ramamurthy, P.C.; Pal, S.K. Facile embedding of gold nanostructures in the hole transporting layer for efficient polymer solar cells. Org. Electron. 2018, 54, 148–153. [Google Scholar] [CrossRef]
- Iakobson, O.D.; Gribkova, O.L.; Tameev, A.R.; Nekrasov, A.A.; Saranin, D.S.; Di Carlo, A. Graphene nanosheet/polyaniline composite for transparent hole transporting layer. J. Ind. Eng. Chem. 2018, 65, 309–317. [Google Scholar] [CrossRef]
- Aatif, M.; Patel, J.; Sharma, A.; Chauhan, M.; Kumar, G.; Pal, P.; Chand, S.; Tripathi, B.; Pandey, M.K.; Tiwari, J.P. Graphene oxide-molybdenum oxide composite with improved hole transport in bulk heterojunction solar cells. AIP Adv. 2019, 9, 075215. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Zhang, H.; Zhao, Y.; Mao, J.; Li, C.; Zhang, S.; Wong, K.S.; Hou, J.; Choy, W.C.H. Self-Assembled Quasi-3D Nanocomposite: A Novel p-Type Hole Transport Layer for High Performance Inverted Organic Solar Cells. Adv. Funct. Mater. 2018, 28, 1706403. [Google Scholar] [CrossRef]
- Dang, Y.; Wang, Y.; Shen, S.; Huang, S.; Qu, X.; Pang, Y.; Silva, S.R.P.; Kang, B.; Lu, G. Solution processed hybrid Graphene-MoO3 hole transport layers for improved performance of organic solar cells. Org. Electron. 2019, 67, 95–100. [Google Scholar] [CrossRef]
- Christopholi, L.P.; da Cunha, M.R.P.; Spada, E.R.; Gavim, A.E.X.; Hadano, F.S.; da Silva, W.J.; Rodrigues, P.C.; Macedo, A.G.; Faria, R.M.; de Deus, J.F. Reduced graphene oxide and perylene derivative nanohybrid as multifunctional interlayer for organic solar cells. Synth. Met. 2020, 269, 116552. [Google Scholar] [CrossRef]
- Lu, J.; Yeo, P.S.E.; Gan, C.K.; Wu, P.; Loh, K.P. Transforming C60 molecules into graphene quantum dots. Nat. Nanotechnol. 2011, 6, 247–252. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Ho, N.T.; Van Tam, T.; Tien, H.N.; Jang, S.-J.; Nguyen, T.K.; Choi, W.M.; Cho, S.; Kim, Y.S. Solution-Processed Transparent Intermediate Layer for Organic Tandem Solar Cell Using Nitrogen-Doped Graphene Quantum Dots. J. Nanosci. Nanotechnol. 2017, 17, 5686–5692. [Google Scholar] [CrossRef]
- Hoang, T.T.; Pham, H.P.; Tran, Q.T. A Facile Microwave-Assisted Hydrothermal Synthesis of Graphene Quantum Dots for Organic Solar Cell Efficiency Improvement. J. Nanomater. 2020, 2020, 3207909. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.; Liu, X. Amino functionalized carbon nanotubes as hole transport layer for high performance polymer solar cells. Inorg. Chem. Commun. 2019, 103, 142–148. [Google Scholar] [CrossRef]
- Rajanna, P.M.; Meddeb, H.; Sergeev, O.; Tsapenko, A.P.; Bereznev, S.; Vehse, M.; Volobujeva, O.; Danilson, M.; Lund, P.D.; Nasibulin, A.G. Rational design of highly efficient flexible and transparent p-type composite electrode based on single-walled carbon nanotubes. Nano Energy 2020, 67, 104183. [Google Scholar] [CrossRef]
- Zhang, W.; Bu, F.; Shen, W.; Qi, X.; Yang, N.; Chen, M.; Yang, D.; Wang, Y.; Zhang, M.; Jiang, H.; et al. Strongly enhanced efficiency of polymer solar cells through unzipped SWNT hybridization in the hole transport layer. RSC Adv. 2020, 10, 24847–24854. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical-Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef] [PubMed]
- Emah, J.B.; George, N.J.; Akpan, U.B. Interfacial Surface Modification via Nanoimprinting to Increase Open-Circuit Voltage of Organic Solar Cells. J. Electron. Mater. 2017, 46, 4989–4998. [Google Scholar] [CrossRef]
- Fernández Castro, M.; Mazzolini, E.; Sondergaard, R.R.; Espindola-Rodriguez, M.; Andreasen, J.W. Flexible ITO-Free Roll-Processed Large-Area Nonfullerene Organic Solar Cells Based on P3HT:O-IDTBR. Phys. Rev. Appl. 2020, 14, 034067. [Google Scholar] [CrossRef]
- Vohra, V.; Shimizu, S.; Takeoka, Y. Water-Processed Organic Solar Cells with Open-Circuit Voltages Exceeding 1.3V. Coatings 2020, 10, 421. [Google Scholar] [CrossRef]
- Hong, N.; Xiao, J.; Li, Y.; Li, Y.; Wu, Y.; Yu, W.; Qiu, X.; Chen, R.; Yip, H.-L.; Huang, W.; et al. Unexpected fluorescent emission of graft sulfonated-acetone-formaldehyde lignin and its application as a dopant of PEDOT for high performance photovoltaic and light-emitting devices. J. Mater. Chem. C 2016, 4, 5297–5306. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.M.; Younes, E.M.; Namkoong, G.; El-Maghraby, E.M.; Elsayed, A.H.; Abo Elazm, A.H. Solvents effects on the hole transport layer in organic solar cells performance. Sol. Energy 2016, 137, 337–343. [Google Scholar] [CrossRef]
- Kurukavak, Ç.K.; Polat, S. Influence of the volume of EGME-DMSO mixed co-solvent doping on the characteristics of PEDOT:PSS and their application in polymer solar cells. Polym. Polym. Compos. 2020, 29, 1222–1228. [Google Scholar] [CrossRef]
- Yi, M.; Hong, S.; Kim, J.-R.; Kang, H.; Lee, J.; Yu, K.; Kee, S.; Lee, W.; Lee, K. Modification of a PEDOT:PSS hole transport layer for printed polymer solar cells. Sol. Energy Mater. Sol. Cells 2016, 153, 117–123. [Google Scholar] [CrossRef]
- Howells, C.T.; Marbou, K.; Kim, H.; Lee, K.J.; Heinrich, B.; Kim, S.J.; Nakao, A.; Aoyama, T.; Furukawa, S.; Kim, J.-H.; et al. Enhanced organic solar cells efficiency through electronic and electro-optic effects resulting from charge transfers in polymer hole transport blends. J. Mater. Chem. A 2016, 4, 4252–4263. [Google Scholar] [CrossRef] [Green Version]
- Bhargav, R.; Bhardwaj, D.; Shahjad, A.P.; Chand, S. Poly(Styrene Sulfonate) Free Poly(3,4-Ethylenedioxythiophene) as a Robust and Solution-Processable Hole Transport Layer for Organic Solar Cells. ChemistrySelect 2016, 1, 1347–1352. [Google Scholar] [CrossRef]
- Cai, W.; Musumeci, C.; Ajjan, F.N.; Bao, Q.; Ma, Z.; Tang, Z.; Inganäs, O. Self-doped conjugated polyelectrolyte with tuneable work function for effective hole transport in polymer solar cells. J. Mater. Chem. A 2016, 4, 15670–15675. [Google Scholar] [CrossRef]
- Gribkova, O.L.; Iakobson, O.D.; Nekrasov, A.A.; Cabanova, V.A.; Tverskoy, V.A.; Tameev, A.R.; Vannikov, A.V. Ultraviolet-Visible-Near Infrared and Raman spectroelectrochemistry of poly(3,4-ethylenedioxythiophene) complexes with sulfonated polyelectrolytes. The role of inter- and intra-molecular interactions in polyelectrolyte. Electrochim. Acta 2016, 222, 409–420. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, D.J.; Kim, B.; Yun, C.; Kang, M.H. Tailoring PEDOT:PSS polymer electrode for solution-processed inverted organic solar cells. Solid-State Electron. 2020, 169, 107808. [Google Scholar] [CrossRef]
- Liu, L.; Li, F.; Zhao, C.; Bi, F.; Jiu, T.; Zhao, M.; Li, J.; Zhang, G.; Wang, L.; Fang, J.; et al. Performance Enhancement of Conventional Polymer Solar Cells with TTF-py-Modified PEDOT:PSS Film as the Hole Transport Layer. ACS Appl. Energy Mater. 2019, 2, 6577–6583. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Z.; Zhang, D.; Zhang, J.; Zhou, J.; Liu, J.; Xie, S.; Zhao, Y.; Zhang, Y.; Wei, Z.; et al. Regulating Bulk-Heterojunction Molecular Orientations through Surface Free Energy Control of Hole-Transporting Layers for High-Performance Organic Solar Cells. Adv. Mater. 2019, 31, 1806921. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Z.; Hu, Z.; Liang, Y.; Huang, F.; Cao, Y. Oxoammonium enabled secondary doping of hole transporting material PEDOT:PSS for high-performance organic solar cells. Sci. China Chem. 2020, 63, 802–809. [Google Scholar] [CrossRef]
- Khan, M.A.; Farva, U. Elucidation of hierarchical metallophthalocyanine buffer layers in bulk heterojunction solar cells. RSC Adv. 2017, 7, 11304–11311. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yu, H.; Hou, C.; Zhang, J. Solution-Processable PEDOT:PSS:α-In2Se3 with Enhanced Conductivity as a Hole Transport Layer for High-Performance Polymer Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 26543–26554. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, X.; Ma, R.; Zhu, W.; Li, Y.; Chen, Z.; Zhou, J.; Li, W.; Liu, T.; He, Z.; et al. Dopamine Semiquinone Radical Doped PEDOT:PSS: Enhanced Conductivity, Work Function and Performance in Organic Solar Cells. Adv. Energy Mater. 2020, 10, 2000743. [Google Scholar] [CrossRef]
- Roh, S.H.; Kim, J.K. Hexagonal Array Patterned PMMA Buffer Layer for Efficient Hole Transport and Tailored Interfacial Properties of FTO-Based Organic Solar Cells. Macromol. Res. 2018, 26, 1173–1178. [Google Scholar] [CrossRef]
- Kwak, C.K.; Pérez, G.E.; Freestone, B.G.; Al-Isaee, S.A.; Iraqi, A.; Lidzey, D.G.; Dunbar, A.D.F. Improved efficiency in organic solar cells via conjugated polyelectrolyte additive in the hole transporting layer. J. Mater. Chem. C 2016, 4, 10722–10730. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Scherf, U. Electrochemically Generated Thin Films of Microporous Polymer Networks: Synthesis, Properties, and Applications. Macromol. Chem. Phys. 2016, 217, 827–841. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Scherf, U. Electrogenerated Thin Films of Microporous Polymer Networks with Remarkably Increased Electrochemical Response to Nitroaromatic Analytes. ACS Appl. Mater. Interfaces 2015, 7, 11127–11133. [Google Scholar] [CrossRef] [PubMed]
- Räupke, A.; Palma-Cando, A.; Shkura, E.; Teckhausen, P.; Polywka, A.; Görrn, P.; Scherf, U.; Riedl, T. Highly sensitive gas-phase explosive detection by luminescent microporous polymer networks. Sci. Rep. 2016, 6, 29118. [Google Scholar] [CrossRef] [Green Version]
- Palma-Cando, A.; Woitassek, D.; Brunklaus, G.; Scherf, U. Luminescent tetraphenylethene-cored, carbazole- and thiophene-based microporous polymer films for the chemosensing of nitroaromatic analytes. Mater. Chem. Front. 2017, 1, 1118–1124. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Preis, E.; Scherf, U. Silicon-or Carbon-Cored Multifunctional Carbazolyl Monomers for the Electrochemical Generation of Microporous Polymer Films. Macromolecules 2016, 49, 8041–8047. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Rendón-Enríquez, I.; Tausch, M.; Scherf, U. Thin Functional Polymer Films by Electropolymerization. Nanomaterials 2019, 9, 1125. [Google Scholar] [CrossRef] [Green Version]
- Palma-Cando, A.; Brunklaus, G.; Scherf, U. Thiophene-Based Microporous Polymer Networks via Chemical or Electrochemical Oxidative Coupling. Macromolecules 2015, 48, 6816–6824. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Huang, N.; Chen, Y.; Qin, L.; Xu, H.; Zhang, S.; Li, F.; Ma, Y.; Jiang, D. π-Conjugated Microporous Polymer Films: Designed Synthesis, Conducting Properties, and Photoenergy Conversions. Angew. Chem. 2015, 127, 13798–13802. [Google Scholar] [CrossRef]
- Liu, C.; Luo, H.; Shi, G.; Nian, L.; Chi, Z.; Ma, Y. Electrochemical route to fabricate porous organic polymers film and its application for polymer solar cells. Dyes Pigments 2017, 142, 132–138. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Zhang, X.; Zhang, D.; Xia, H.; Zhou, J.; Ahmad, N.; Leng, X.; Yang, S.; Zhang, Y.; et al. Built-in voltage enhanced by in situ electrochemical polymerized undoped conjugated hole-transporting modifiers in organic solar cells. J. Mater. Chem. C 2020, 8, 2676–2681. [Google Scholar] [CrossRef]
- Lee, E.J.; Han, J.P.; Jung, S.E.; Choi, M.H.; Moon, D.K. Improvement in Half-Life of Organic Solar Cells by Using a Blended Hole Extraction Layer Consisting of PEDOT:PSS and Conjugated Polymer Electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 31791–31798. [Google Scholar] [CrossRef] [PubMed]
- Pérez, G.E.; Erothu, H.; Topham, P.D.; Bastianini, F.; Alanazi, T.I.; Bernardo, G.; Parnell, A.J.; King, S.M.; Dunbar, A.D.F. Improved Performance and Stability of Organic Solar Cells by the Incorporation of a Block Copolymer Interfacial Layer. Adv. Mater. Interfaces 2020, 7, 2000918. [Google Scholar] [CrossRef]
- Hamed, M.S.G.; Oseni, S.O.; Kumar, A.; Sharma, G.; Mola, G.T. Nickel sulphide nano-composite assisted hole transport in thin film polymer solar cells. Sol. Energy 2020, 195, 310–317. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Q.; Wan, H.; Zhang, Y.; Zheng, S.; Zhang, Y. A facile synthesis of segmented silver nanowires and enhancement of the performance of polymer solar cells. Phys. Chem. Chem. Phys. 2018, 20, 18837–18843. [Google Scholar] [CrossRef]
- Hao, J.; Xu, Y.; Chen, S.; Zhang, Y.; Mai, J.; Lau, T.-K.; Zhang, R.; Mei, Y.; Wang, L.; Lu, X.; et al. Broadband plasmon-enhanced polymer solar cells with power conversion efficiency of 9.26% using mixed Au nanoparticles. Opt. Commun. 2016, 362, 50–58. [Google Scholar] [CrossRef]
- Oh, Y.; Lim, J.W.; Kim, J.G.; Wang, H.; Kang, B.-H.; Park, Y.W.; Kim, H.; Jang, Y.J.; Kim, J.; Kim, D.H.; et al. Plasmonic Periodic Nanodot Arrays via Laser Interference Lithography for Organic Photovoltaic Cells with >10% Efficiency. ACS Nano 2016, 10, 10143–10151. [Google Scholar] [CrossRef]
- Singh, A.; Dey, A.; Das, D.; Iyer, P.K. Combined influence of plasmonic metal nanoparticles and dual cathode buffer layers for highly efficient rrP3HT:PCBM-based bulk heterojunction solar cells. J. Mater. Chem. C 2017, 5, 6578–6587. [Google Scholar] [CrossRef]
- Sah, P.-T.; Chang, W.-C.; Chen, J.-H.; Wang, H.-H.; Chan, L.-H. Bimetallic Ag‒Au‒Ag nanorods used to enhance efficiency of polymer solar cells. Electrochim. Acta 2018, 259, 293–302. [Google Scholar] [CrossRef]
- Tang, M.; Sun, B.; Zhou, D.; Gu, Z.; Chen, K.; Guo, J.; Feng, L.; Zhou, Y. Broad-band plasmonic Cu-Au bimetallic nanoparticles for organic bulk heterojunction solar cells. Org. Electron. 2016, 38, 213–221. [Google Scholar] [CrossRef]
- Adedeji, M.A.; Hamed, M.S.G.; Mola, G.T. Light trapping using copper decorated nano-composite in the hole transport layer of organic solar cell. Sol. Energy 2020, 203, 83–90. [Google Scholar] [CrossRef]
- Najeeb, M.A.; Abdullah, S.M.; Aziz, F.; Ahmad, Z.; Shakoor, R.A.; Mohamed, A.M.A.; Khalil, U.; Swelm, W.; Al-Ghamdi, A.A.; Sulaiman, K. A comparative Study on the performance of hybrid solar cells containing ZnSTe QDs in hole transporting layer and photoactive layer. J. Nanopart. Res. 2016, 18, 384. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Q.; Wang, J.; Tang, J. Black phosphorous quantum dots as an effective interlayer modifier in polymer solar cells. Sol. Energy 2020, 206, 670–676. [Google Scholar] [CrossRef]
- Ismail, Y.A.M.; Kishi, N.; Soga, T. Improvement of organic solar cells using aluminium microstructures prepared in PEDOT:PSS buffer layer by using ultrasonic ablation technique. Thin Solid Films 2016, 616, 73–79. [Google Scholar] [CrossRef]
- Kesavan, A.V.; Jagdish, A.K.; Ramamurthy, P.C. Organic Inorganic Hybrid Hole Transport Layer for Light Management in Inverted Organic Photovoltaic. IEEE J. Photovolt. 2017, 7, 787–791. [Google Scholar] [CrossRef]
- Liu, Z.; Park, J.; Li, B.; Chan, H.P.; Yi, D.K.; Lee, E.-C. Performance improvement of organic bulk-heterojunction solar cells using complementary plasmonic gold nanorods. Org. Electron. 2020, 84, 105802. [Google Scholar] [CrossRef]
- Phetsang, S.; Nootchanat, S.; Lertvachirapaiboon, C.; Ishikawa, R.; Shinbo, K.; Kato, K.; Mungkornasawakul, P.; Ounnunkad, K.; Baba, A. Enhancement of organic solar cell performance by incorporating gold quantum dots (AuQDs) on a plasmonic grating. Nanoscale Adv. 2020, 2, 2950–2957. [Google Scholar] [CrossRef]
- Haase, M.; Schäfer, H. Upconverting Nanoparticles. Angew. Chemie Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Mei, Y.; Ma, X.; Fan, Y.; Bai, Z.; Cheng, F.; Hu, Y.; Fan, Q.; Chen, S.; Huang, W. Application of β-NaYF4:Er3+ (2%), Yb3+ (18%) Up-Conversion Nanoparticles in Polymer Solar Cells and its Working Mechanism. J. Nanosci. Nanotechnol. 2016, 16, 7380–7387. [Google Scholar] [CrossRef]
- Mola, G.T.; Arbab, E.A.A. Bimetallic nanocomposite as hole transport co-buffer layer in organic solar cell. Appl. Phys. A 2017, 123, 772. [Google Scholar] [CrossRef]
- Michalska, M.; Iwan, A.; Andrzejczuk, M.; Roguska, A.; Sikora, A.; Boharewicz, B.; Tazbir, I.; Hreniak, A.; Popłoński, S.; Korona, K.P. Analysis of the surface decoration of TiO2 grains using silver nanoparticles obtained by ultrasonochemical synthesis towards organic photovoltaics. New J. Chem. 2018, 42, 7340–7354. [Google Scholar] [CrossRef]
- Truong, N.T.N.; Kim, C.D.; Minnam Reddy, V.R.; Thai, V.H.; Jeon, H.J.; Park, C. Shape control of plasmonic gold nanoparticles and its application to vacuum-free bulk hetero-junction solar cells. J. Mater. Sci. Mater. Electron. 2020, 31, 22957–22965. [Google Scholar] [CrossRef]
- Mohammad, T.; Dwivedi, C.; Kumar, V.; Dutta, V. Role of Continuous Spray Pyrolyzed synthesized MoO3 nanorods in PEDOT:PSS matrix by electric field assisted spray deposition for organic photovoltaics. Org. Electron. 2020, 77, 105525. [Google Scholar] [CrossRef]
- Pan, J.; Li, P.; Cai, L.; Hu, Y.; Zhang, Y. All-solution processed double-decked PEDOT:PSS/V2O5 nanowires as buffer layer of high performance polymer photovoltaic cells. Sol. Energy Mater. Sol. Cells 2016, 144, 616–622. [Google Scholar] [CrossRef]
- Li, J.; Qin, J.; Liu, X.; Ren, M.; Tong, J.; Zheng, N.; Chen, W.; Xia, Y. Enhanced organic photovoltaic performance through promoting crystallinity of photoactive layer and conductivity of hole-transporting layer by V2O5 doped PEDOT:PSS hole-transporting layers. Sol. Energy 2020, 211, 1102–1109. [Google Scholar] [CrossRef]
- Du, X.; Lytken, O.; Killian, M.S.; Cao, J.; Stubhan, T.; Turbiez, M.; Schmuki, P.; Steinrück, H.-P.; Ding, L.; Fink, R.H.; et al. Overcoming Interfacial Losses in Solution-Processed Organic Multi-Junction Solar Cells. Adv. Energy Mater. 2017, 7, 1601959. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Cai, L.; Li, N.; Li, Z.; Zhu, F. Efficient and stable operation of nonfullerene organic solar cells: Retaining a high built-in potential. J. Mater. Chem. A 2020, 8, 21255–21264. [Google Scholar] [CrossRef]
- Mbuyise, X.G.; Arbab, E.A.A.; Kaviyarasu, K.; Pellicane, G.; Maaza, M.; Mola, G.T. Zinc oxide doped single wall carbon nanotubes in hole transport buffer layer. J. Alloys Compd. 2017, 706, 344–350. [Google Scholar] [CrossRef]
- Zheng, Z.; Hu, Q.; Zhang, S.; Zhang, D.; Wang, J.; Xie, S.; Wang, R.; Qin, Y.; Li, W.; Hong, L.; et al. A Highly Efficient Non-Fullerene Organic Solar Cell with a Fill Factor over 0.80 Enabled by a Fine-Tuned Hole-Transporting Layer. Adv. Mater. 2018, 30, 1801801. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Jeon, S.J.; Lee, H.S.; Park, H.; Kim, K.S.; Lee, H.; Moon, D.K. Evaporation-Free Nonfullerene Flexible Organic Solar Cell Modules Manufactured by An All-Solution Process. Adv. Energy Mater. 2019, 9, 1902065. [Google Scholar] [CrossRef]
- Sun, L.; Zeng, W.; Xie, C.; Hu, L.; Dong, X.; Qin, F.; Wang, W.; Liu, T.; Jiang, X.; Jiang, Y.; et al. Flexible All-Solution-Processed Organic Solar Cells with High-Performance Nonfullerene Active Layers. Adv. Mater. 2020, 32, 1907840. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Wang, Y.; Luo, Q.; Han, K.; Xie, M.; Zhang, L.; Wu, N.; Lin, J.; Xiao, S.; Li, Y.-Q.; et al. Fully Coated Semitransparent Organic Solar Cells with a Doctor-Blade-Coated Composite Anode Buffer Layer of Phosphomolybdic Acid and PEDOT:PSS and a Spray-Coated Silver Nanowire Top Electrode. ACS Appl. Mater. Interfaces 2018, 10, 943–954. [Google Scholar] [CrossRef]
- Liu, Z.; Niu, S.; Wang, N. Oleylamine-functionalized graphene oxide as an electron block layer towards high-performance and photostable fullerene-free polymer solar cells. Nanoscale 2017, 9, 16293–16304. [Google Scholar] [CrossRef]
- Maity, S.; Thomas, T. Hole-Collecting Treated Graphene Layer and PTB7:PC 71 BM-Based Bulk-Heterojunction OPV With Improved Carrier Collection and Photovoltaic Efficiency. IEEE Trans. Electron Devices 2018, 65, 4548–4554. [Google Scholar] [CrossRef]
- Hilal, M.; Han, J.I. Enhancing the photovoltaic characteristics of organic solar cells by introducing highly conductive graphene as a conductive platform for a PEDOT:PSS anode interfacial layer. J. Mater. Sci. Mater. Electron. 2019, 30, 6187–6200. [Google Scholar] [CrossRef]
- Amollo, T.A.; Mola, G.T.; Nyamori, V.O. High-performance organic solar cells utilizing graphene oxide in the active and hole transport layers. Sol. Energy 2018, 171, 83–91. [Google Scholar] [CrossRef]
- Goumri, M.; Lucas, B.; Ratier, B.; Baitoul, M. Inverted Polymer Solar Cells with a Reduced Graphene Oxide/Poly (3,4-Ethylene Dioxythiophene):Poly(4-Styrene Sulfonate) (PEDOT:PSS) Hole Transport Layer. J. Electron. Mater. 2019, 48, 1097–1105. [Google Scholar] [CrossRef]
- Ozcan, S.; Erer, M.C.; Vempati, S.; Uyar, T.; Toppare, L.; Çırpan, A. Graphene oxide-doped PEDOT:PSS as hole transport layer in inverted bulk heterojunction solar cell. J. Mater. Sci. Mater. Electron. 2020, 31, 3576–3584. [Google Scholar] [CrossRef]
- Amollo, T.A.; Mola, G.T.; Nyamori, V.O. Polymer solar cells with reduced graphene oxide–germanium quantum dots nanocomposite in the hole transport layer. J. Mater. Sci. Mater. Electron. 2018, 29, 7820–7831. [Google Scholar] [CrossRef]
- Raj, P.G.; Rani, V.S.; Kanwat, A.; Jang, J. Enhanced organic photovoltaic properties via structural modifications in PEDOT:PSS due to graphene oxide doping. Mater. Res. Bull. 2016, 74, 346–352. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Pionteck, J. Recent Advances in Preparation, Structure, Properties and Applications of Graphite Oxide. J. Nanosci. Nanotechnol. 2015, 15, 1984–2000. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Iwan, A.; Caballero-Briones, F.; Filapek, M.; Boharewicz, B.; Tazbir, I.; Hreniak, A.; Guerrero-Contreras, J. Electrochemical and photocurrent characterization of polymer solar cells with improved performance after GO addition to the PEDOT:PSS hole transporting layer. Sol. Energy 2017, 146, 230–242. [Google Scholar] [CrossRef]
- Rafique, S.; Abdullah, S.M.; Shahid, M.M.; Ansari, M.O.; Sulaiman, K. Significantly improved photovoltaic performance in polymer bulk heterojunction solar cells with graphene oxide /PEDOT:PSS double decked hole transport layer. Sci. Rep. 2017, 7, 39555. [Google Scholar] [CrossRef] [Green Version]
- Hilal, M.; Han, J.I. Significant improvement in the photovoltaic stability of bulk heterojunction organic solar cells by the molecular level interaction of graphene oxide with a PEDOT: PSS composite hole transport layer. Sol. Energy 2018, 167, 24–34. [Google Scholar] [CrossRef]
- Moon, B.J.; Jang, D.; Yi, Y.; Lee, H.; Kim, S.J.; Oh, Y.; Lee, S.H.; Park, M.; Lee, S.; Bae, S. Multi-functional nitrogen self-doped graphene quantum dots for boosting the photovoltaic performance of BHJ solar cells. Nano Energy 2017, 34, 36–46. [Google Scholar] [CrossRef]
- Rafique, S.; Roslan, N.A.; Abdullah, S.M.; Li, L.; Supangat, A.; Jilani, A.; Iwamoto, M. UV-ozone treated graphene oxide/ PEDOT:PSS bilayer as a novel hole transport layer in highly efficient and stable organic solar cells. Org. Electron. 2019, 66, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.; Chen, Y.; Wu, X.; Xu, X.; Ye, P.; Zhu, T.; Guo, Q.; Yang, L.; Li, W.; Huang, H. PEDOT:PSS-Assisted Exfoliation and Functionalization of 2D Nanosheets for High-Performance Organic Solar Cells. Adv. Funct. Mater. 2017, 27, 1701622. [Google Scholar] [CrossRef]
- Koo, D.; Jung, S.; Oh, N.K.; Choi, Y.; Seo, J.; Lee, J.; Kim, U.; Park, H. Improved charge transport via WSe2-mediated hole transporting layer toward efficient organic solar cells. Semicond. Sci. Technol. 2018, 33, 125020. [Google Scholar] [CrossRef]
- Ramasamy, M.S.; Ryu, K.Y.; Lim, J.W.; Bibi, A.; Kwon, H.; Lee, J.-E.; Kim, D.H.; Kim, K. Solution-Processed PEDOT:PSS/MoS2 Nanocomposites as Efficient Hole-Transporting Layers for Organic Solar Cells. Nanomaterials 2019, 9, 1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, Y.; Shen, S.; Wang, Y.; Qu, X.; Huang, S.; Dong, Q.; Silva, S.R.P.; Kang, B. Hole Extraction Enhancement for Efficient Polymer Solar Cells with Boronic Acid Functionalized Carbon Nanotubes doped Hole Transport Layers. ACS Sustain. Chem. Eng. 2018, 6, 5122–5131. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, S.; Fu, P.; Yu, W.; Liu, Y.; Liu, X.; Feng, Z.; Guo, X.; Li, C. Boosting Performance of Non-Fullerene Organic Solar Cells by 2D g-C3N4 Doped PEDOT:PSS. Adv. Funct. Mater. 2020, 30, 1910205. [Google Scholar] [CrossRef]
- Hou, C.; Yu, H. Modifying the nanostructures of PEDOT:PSS/Ti3C2TX composite hole transport layers for highly efficient polymer solar cells. J. Mater. Chem. C 2020, 8, 4169–4180. [Google Scholar] [CrossRef]
- Chiou, G.-C.; Lin, M.-W.; Lai, Y.-L.; Chang, C.-K.; Jiang, J.-M.; Su, Y.-W.; Wei, K.-H.; Hsu, Y.-J. Fluorene Conjugated Polymer/Nickel Oxide Nanocomposite Hole Transport Layer Enhances the Efficiency of Organic Photovoltaic Devices. ACS Appl. Mater. Interfaces 2017, 9, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lei, H.; Xiong, L.; Li, B.; Fang, G. Organic solar cells based on a Cu2O/FBT-TH4 anode buffer layer with enhanced power conversion efficiency and ambient stability. J. Mater. Chem. C 2017, 5, 8033–8040. [Google Scholar] [CrossRef]
- Jo, W.J.; Nelson, J.T.; Chang, S.; Bulović, V.; Gradečak, S.; Strano, M.S.; Gleason, K.K. Oxidative Chemical Vapor Deposition of Neutral Hole Transporting Polymer for Enhanced Solar Cell Efficiency and Lifetime. Adv. Mater. 2016, 28, 6399–6404. [Google Scholar] [CrossRef]
- Awada, H.; Mattana, G.; Tournebize, A.; Rodriguez, L.; Flahaut, D.; Vellutini, L.; Lartigau-Dagron, C.; Billon, L.; Bousquet, A.; Chambon, S. Surface engineering of ITO electrode with a functional polymer for PEDOT:PSS-free organic solar cells. Org. Electron. 2018, 57, 186–193. [Google Scholar] [CrossRef]
- Kadem, B.Y.; Kadhim, R.G.; Banimuslem, H. Efficient P3HT:SWCNTs hybrids as hole transport layer in P3HT:PCBM organic solar cells. J. Mater. Sci. Mater. Electron. 2018, 29, 9418–9426. [Google Scholar] [CrossRef]
- Babaei, Z.; Rezaei, B.; Pisheh, M.K.; Afshar-Taromi, F. In situ synthesis of gold/silver nanoparticles and polyaniline as buffer layer in polymer solar cells. Mater. Chem. Phys. 2020, 248, 122879. [Google Scholar] [CrossRef]
- Deng, L.; Li, D.; Agbolaghi, S. Networked Conductive Polythiophene/Polyaniline Bottlebrushes with Modified Carbon Nanotubes As Hole Transport Layer in Organic Photovoltaics. J. Electron. Mater. 2020, 49, 937–948. [Google Scholar] [CrossRef] [Green Version]
- Sorkhishams, N.; Massoumi, B.; Saraei, M.; Agbolaghi, S. Electrode buffer layers via networks of polythiophene/polyaniline bottlebrushes and carbon nanotubes in organic solar cells. J. Mater. Sci. Mater. Electron. 2019, 30, 21117–21125. [Google Scholar] [CrossRef]
- Biswas, S.; You, Y.-J.; Kim, J.; Ha, S.R.; Choi, H.; Kwon, S.-H.; Kim, K.-K.; Shim, J.W.; Kim, H. Decent efficiency improvement of organic photovoltaic cell with low acidic hole transport material by controlling doping concentration. Appl. Surf. Sci. 2020, 512, 145700. [Google Scholar] [CrossRef]
- Biswas, S.; You, Y.-J.; Lee, Y.; Shim, J.W.; Kim, H. Efficiency improvement of indoor organic solar cell by optimization of the doping level of the hole extraction layer. Dyes Pigments 2020, 183, 108719. [Google Scholar] [CrossRef]
- Biswas, S.; You, Y.-J.; Shim, J.W.; Kim, H. Utilization of poly (4-styrenesulfonic acid) doped polyaniline as a hole transport layer of organic solar cell for indoor applications. Thin Solid Films 2020, 700, 137921. [Google Scholar] [CrossRef]
- Abdulrazzaq, O.A.; Bourdo, S.E.; Saini, V.; Biris, A.S. Acid-free polyaniline:graphene-oxide hole transport layer in organic solar cells. J. Mater. Sci. Mater. Electron. 2020, 31, 21640–21650. [Google Scholar] [CrossRef]
- Dong, J.; Guo, J.; Wang, X.; Dong, P.; Wang, Z.; Zhou, Y.; Miao, Y.; Zhao, B.; Hao, Y.; Wang, H.; et al. A Low-Temperature Solution-Processed CuSCN/Polymer Hole Transporting Layer Enables High Efficiency for Organic Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 46373–46380. [Google Scholar] [CrossRef]
- Houston, J.E.; Kraft, M.; Scherf, U.; Evans, R.C. Sequential detection of multiple phase transitions in model biological membranes using a red-emitting conjugated polyelectrolyte. Phys. Chem. Chem. Phys. 2016, 18, 12423–12427. [Google Scholar] [CrossRef] [Green Version]
- Meazzini, I.; Blayo, C.; Arlt, J.; Marques, A.-T.; Scherf, U.; Burrows, H.D.; Evans, R.C. Ureasil organic–inorganic hybrids as photoactive waveguides for conjugated polyelectrolyte luminescent solar concentrators. Mater. Chem. Front. 2017, 1, 2271–2282. [Google Scholar] [CrossRef] [Green Version]
- Willis-Fox, N.; Gutacker, A.; Browne, M.P.; Khan, A.R.; Lyons, M.E.G.; Scherf, U.; Evans, R.C. Selective recognition of biologically important anions using a diblock polyfluorene–polythiophene conjugated polyelectrolyte. Polym. Chem. 2017, 8, 7151–7159. [Google Scholar] [CrossRef] [Green Version]
- Balcı Leinen, M.; Berger, F.J.; Klein, P.; Mühlinghaus, M.; Zorn, N.F.; Settele, S.; Allard, S.; Scherf, U.; Zaumseil, J. Doping-Dependent Energy Transfer from Conjugated Polyelectrolytes to (6,5) Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2019, 123, 22680–22689. [Google Scholar] [CrossRef]
- Pipertzis, A.; Papamokos, G.; Sachnik, O.; Allard, S.; Scherf, U.; Floudas, G. Ionic Conductivity in Polyfluorene-Based Diblock Copolymers Comprising Nanodomains of a Polymerized Ionic Liquid and a Solid Polymer Electrolyte Doped with LiTFSI. Macromolecules 2021, 54, 4257–4268. [Google Scholar] [CrossRef]
- Martelo, L.; Fonseca, S.; Marques, A.; Burrows, H.; Valente, A.; Justino, L.; Scherf, U.; Pradhan, S.; Song, Q. Effects of Charge Density on Photophysics and Aggregation Behavior of Anionic Fluorene-Arylene Conjugated Polyelectrolytes. Polymers 2018, 10, 258. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.W.; Jung, J.W.; Bae, S.; Ko, M.J.; Kim, H.; Jo, W.H.; Jen, A.K.-Y.; Son, H.J. Development of Self-Doped Conjugated Polyelectrolytes with Controlled Work Functions and Application to Hole Transport Layer Materials for High-Performance Organic Solar Cells. Adv. Mater. Interfaces 2016, 3, 1500703. [Google Scholar] [CrossRef]
- Jo, J.W.; Yun, J.H.; Bae, S.; Ko, M.J.; Son, H.J. Development of a conjugated donor-acceptor polyelectrolyte with high work function and conductivity for organic solar cells. Org. Electron. 2017, 50, 1–6. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, B.; Yang, B.; Yao, H.; Li, S.; Hou, J. A Novel pH Neutral Self-Doped Polymer for Anode Interfacial Layer in Efficient Polymer Solar Cells. Macromolecules 2016, 49, 8126–8133. [Google Scholar] [CrossRef]
- Choi, M.-H.; Lee, E.J.; Han, J.P.; Moon, D.K. Solution-processed pH-neutral conjugated polyelectrolytes with one-atom variation (O, S, Se) as a novel hole-collecting layer in organic photovoltaics. Sol. Energy Mater. Sol. Cells 2016, 155, 243–252. [Google Scholar] [CrossRef]
- Xu, H.; Fu, X.; Cheng, X.; Huang, L.; Zhou, D.; Chen, L.; Chen, Y. Highly and homogeneously conductive conjugated polyelectrolyte hole transport layers for efficient organic solar cells. J. Mater. Chem. A 2017, 5, 14689–14696. [Google Scholar] [CrossRef]
- Moon, S.; Khadtare, S.; Wong, M.; Han, S.-H.; Bazan, G.C.; Choi, H. Hole transport layer based on conjugated polyelectrolytes for polymer solar cells. J. Colloid Interface Sci. 2018, 518, 21–26. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, J.; Xu, H.; Liao, X.; Chen, Y.; Li, Y.; Chen, L. Self-doped polymer with fluorinated phenylene as hole transport layer for efficient polymer solar cells. Org. Electron. 2018, 61, 207–214. [Google Scholar] [CrossRef]
- Lee, E.J.; Choi, M.H.; Han, Y.W.; Moon, D.K. Effect on Electrode Work Function by Changing Molecular Geometry of Conjugated Polymer Electrolytes and Application for Hole-Transporting Layer of Organic Optoelectronic Devices. ACS Appl. Mater. Interfaces 2017, 9, 44060–44069. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zou, H.; Zhou, D.; Zeng, G.; Chen, L.; Liao, X.; Chen, Y. Printable Hole Transport Layer for 1.0 cm2Organic Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 52028–52037. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-P.; Li, S.-Y.; Li, Y.; Peng, Q. Recent progress in organic hole-transporting materials with 4-anisylamino-based end caps for efficient perovskite solar cells. Rare Met. 2021, 40, 1669–1690. [Google Scholar] [CrossRef]
- Rombach, F.M.; Haque, S.A.; Macdonald, T.J. Lessons learned from spiro-OMeTAD and PTAA in perovskite solar cells. Energy Environ. Sci. 2021, 14, 5161–5190. [Google Scholar] [CrossRef]
- Li, H.; Fu, K.; Boix, P.P.; Wong, L.H.; Hagfeldt, A.; Grätzel, M.; Mhaisalkar, S.G.; Grimsdale, A.C. Hole-Transporting Small Molecules Based on Thiophene Cores for High Efficiency Perovskite Solar Cells. ChemSusChem 2014, 7, 3420–3425. [Google Scholar] [CrossRef]
- Bormann, L.; Selzer, F.; Weiß, N.; Kneppe, D.; Leo, K.; Müller-Meskamp, L. Doped hole transport layers processed from solution: Planarization and bridging the voids in noncontinuous silver nanowire electrodes. Org. Electron. 2016, 28, 163–171. [Google Scholar] [CrossRef]
- Cheng, J.; Ren, X.; Zhu, H.L.; Mao, J.; Liang, C.; Zhuang, J.; Roy, V.A.L.; Choy, W.C.H. Pre- and post-treatments free nanocomposite based hole transport layer for high performance organic solar cells with considerably enhanced reproducibility. Nano Energy 2017, 34, 76–85. [Google Scholar] [CrossRef]
- Courté, M.; Alaaeddine, M.; Barth, V.; Tortech, L.; Fichou, D. Structural and electronic properties of 2,2′,6,6′-tetraphenyl-dipyranylidene and its use as a hole-collecting interfacial layer in organic solar cells. Dye. Pigment. 2017, 141, 487–492. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Tan, T.W.; Shi, Y.; Zhao, X.Y.; Mi, B.X.; Gao, Z.Q. An organic semiconductor as an anode-buffer for the improvement of small molecular photovoltaic cells. RSC Adv. 2017, 7, 38204–38209. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zheng, Y.; Zheng, D.; Yu, J. Effect of organic small-molecule hole injection materials on the performance of inverted organic solar cells. J. Photonics Energy 2016, 6, 035502. [Google Scholar] [CrossRef]
- Li, P.; Wu, B.; Yang, Y.C.; Huang, H.S.; De Yang, X.; Zhou, G.D.; Song, Q.L. Improved charge transport ability of polymer solar cells by using NPB/MoO3 as anode buffer layer. Sol. Energy 2018, 170, 212–216. [Google Scholar] [CrossRef]
- Shi, Z.; Deng, H.; Zhao, W.; Cao, H.; Yang, Q.; Zhang, J.; Ban, D.; Qin, D. An alternative hole extraction layer for inverted organic solar cells. Appl. Phys. A 2018, 124, 676. [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Chang, K.; Lv, W.; Mi, B.; Song, J.; Zhao, X.; Gao, Z. A new dibenzo[g.p]chrysene derivative as an efficient anode buffer for inverted polymer solar cells. Org. Electron. 2019, 74, 269–275. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, S.; Zhao, C.; Jiu, T.; Bi, F.; Jian, H.; Zhao, M.; Zhang, G.; Wang, L.; Li, F.; et al. TTA as a potential hole transport layer for application in conventional polymer solar cells. J. Energy Chem. 2020, 42, 210–216. [Google Scholar] [CrossRef] [Green Version]
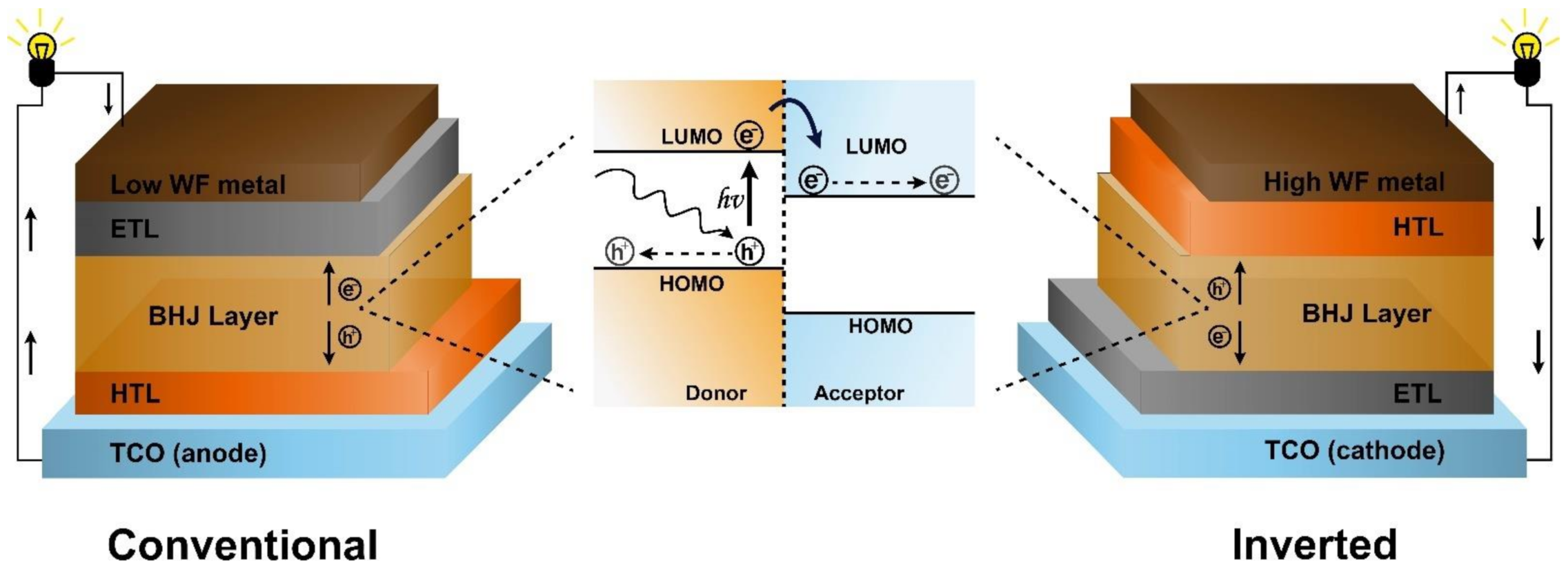
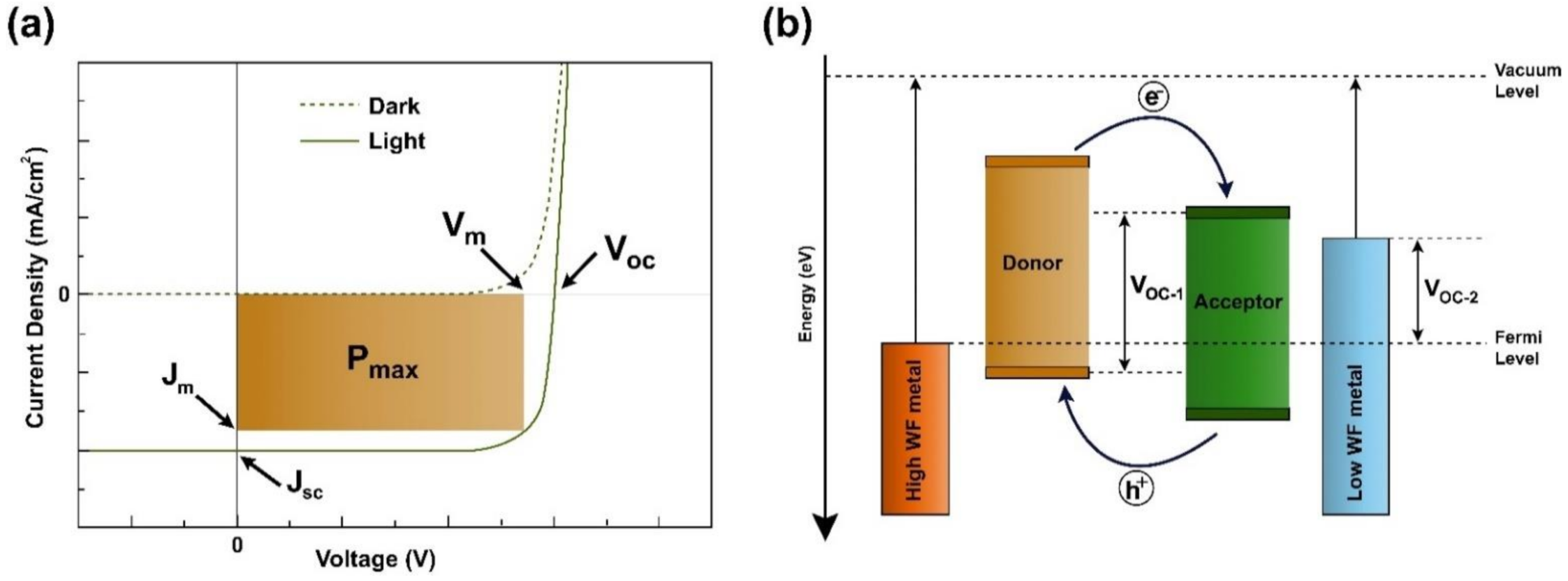
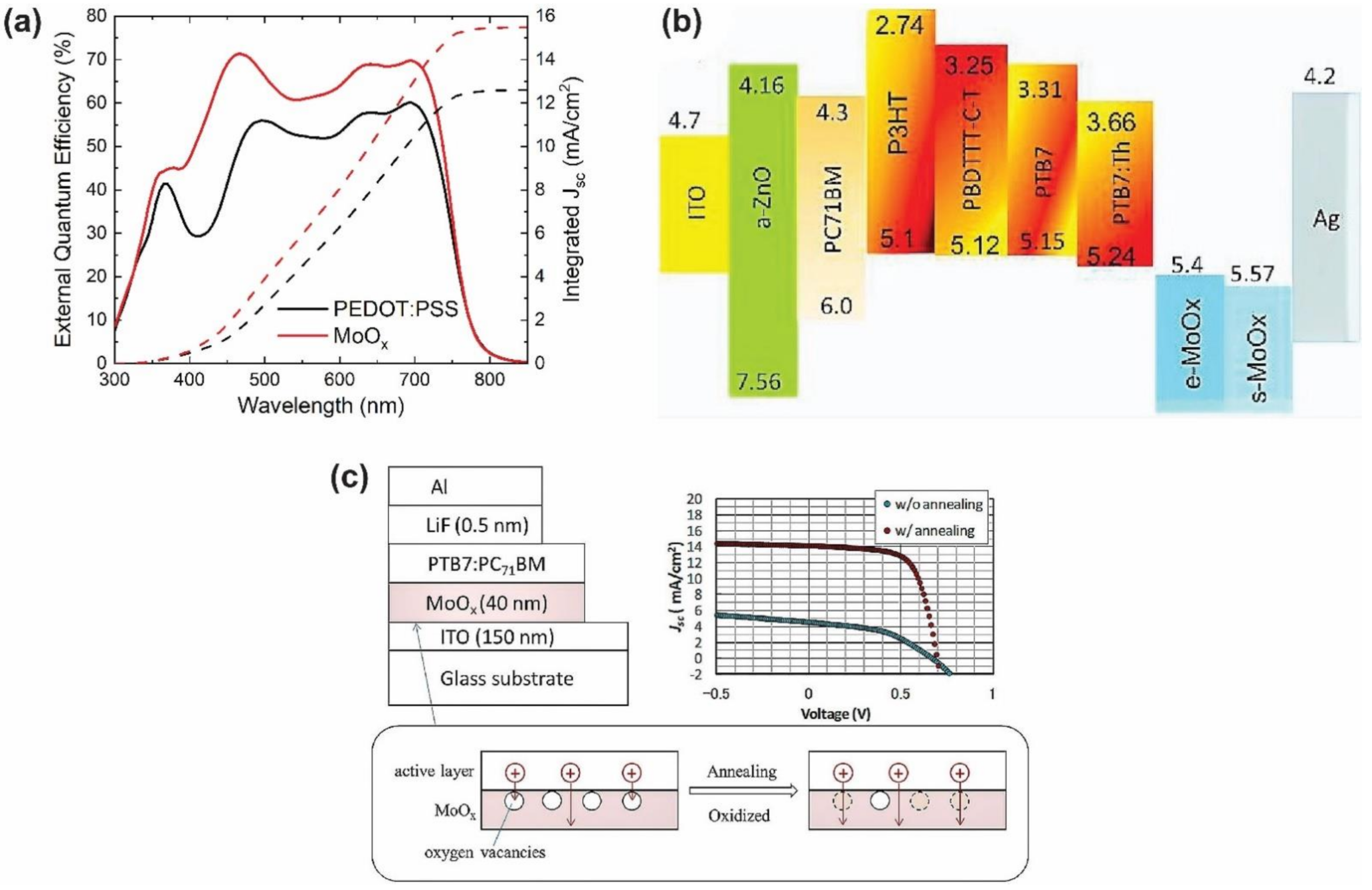
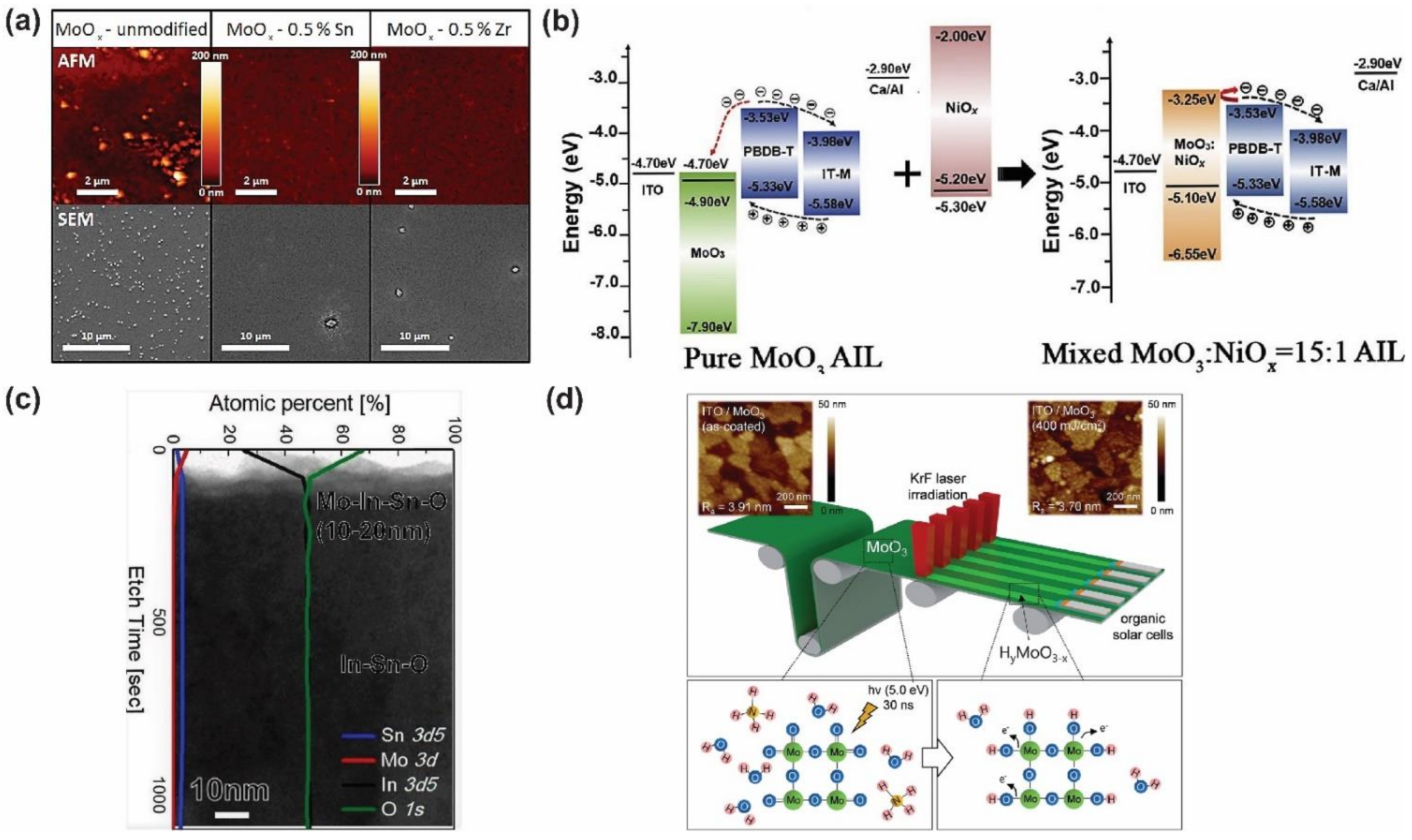
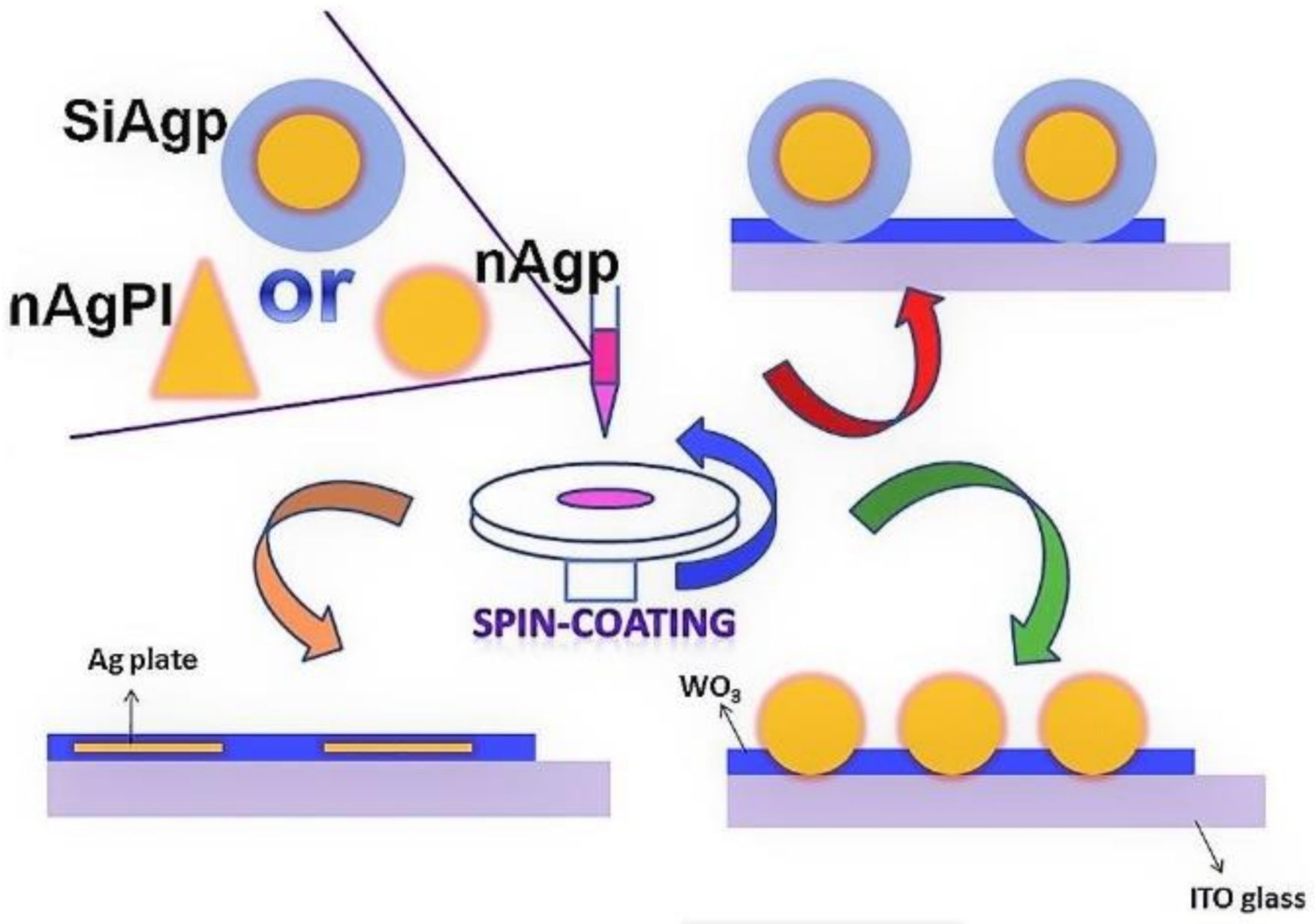
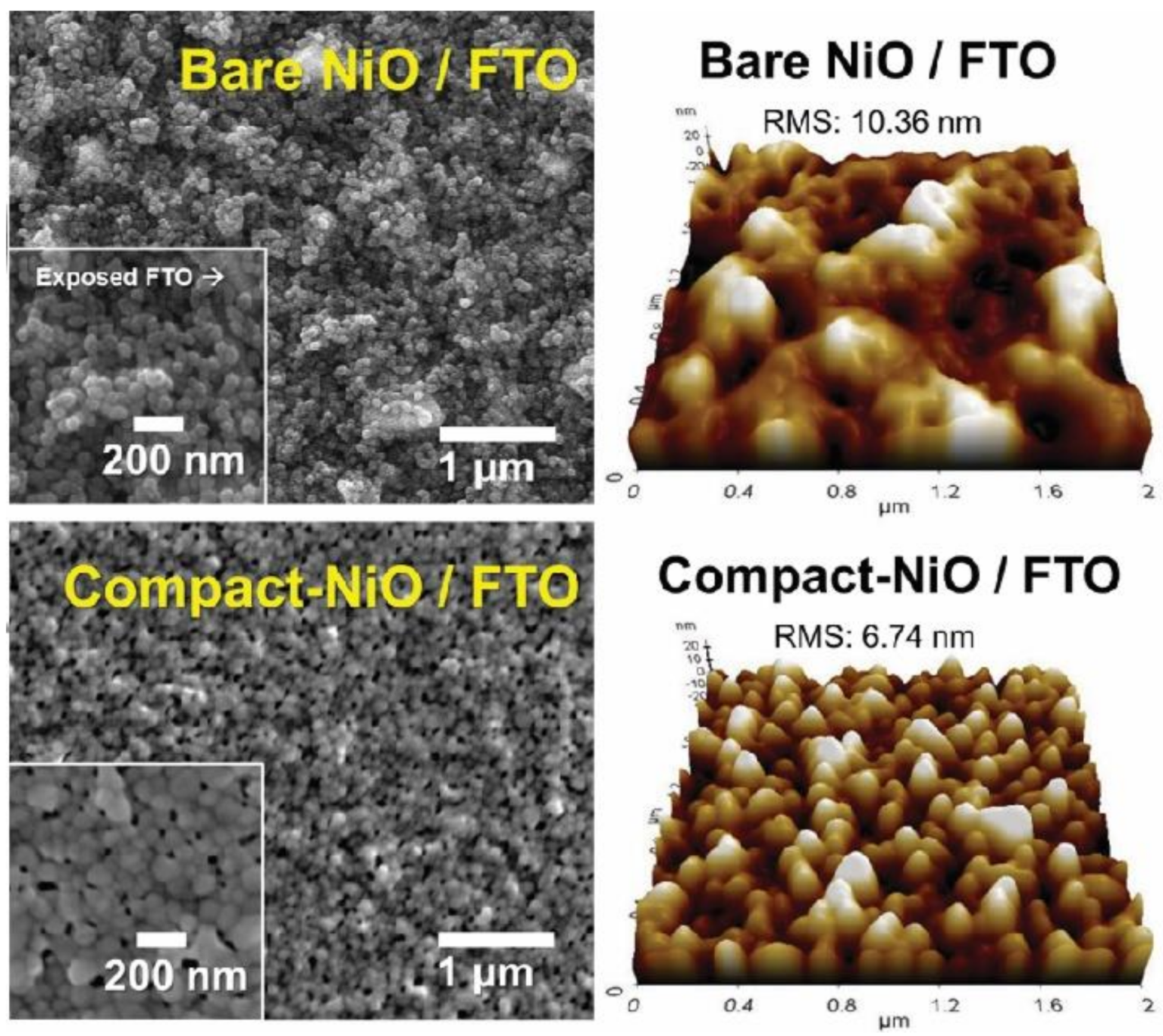
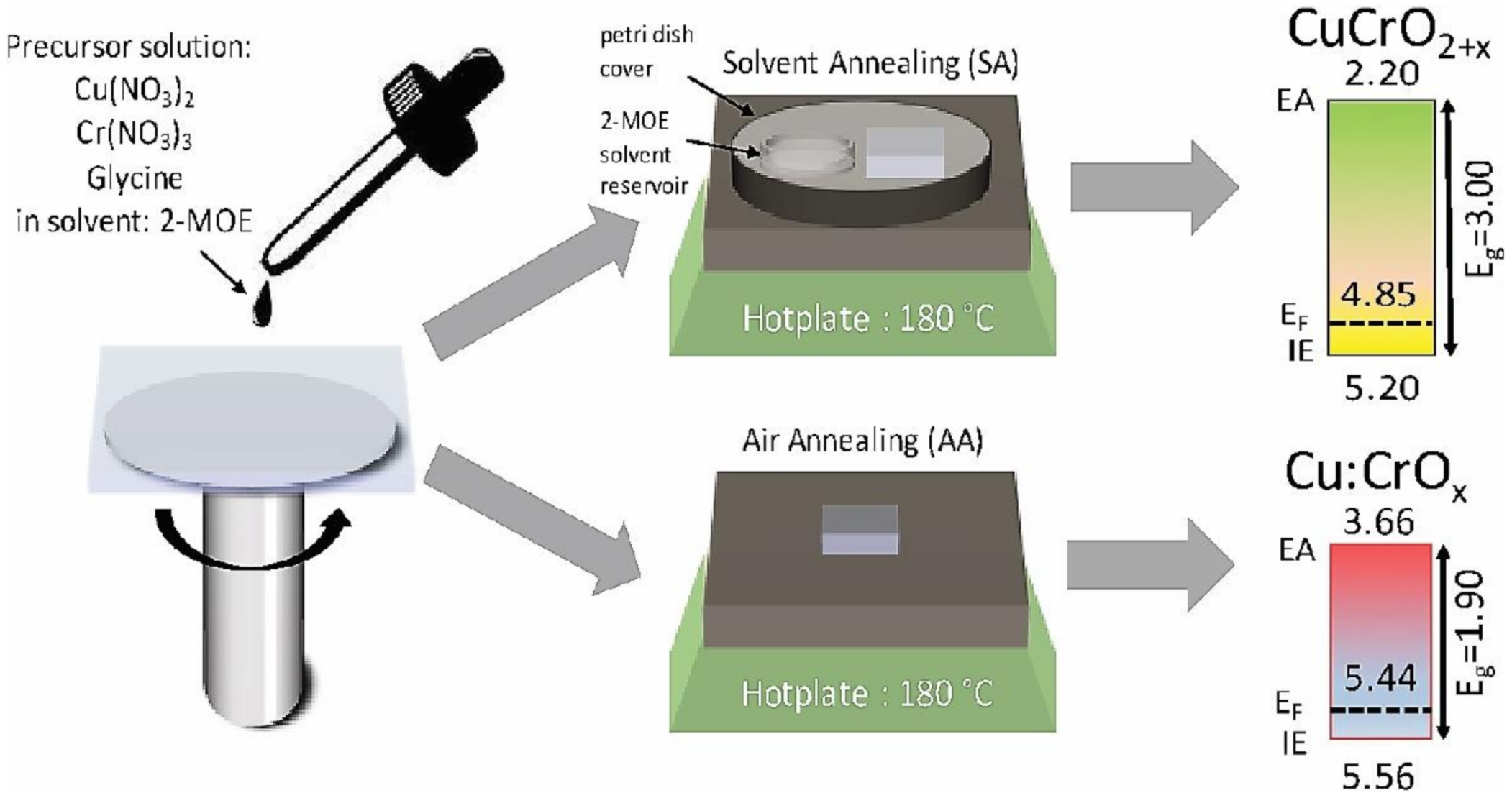
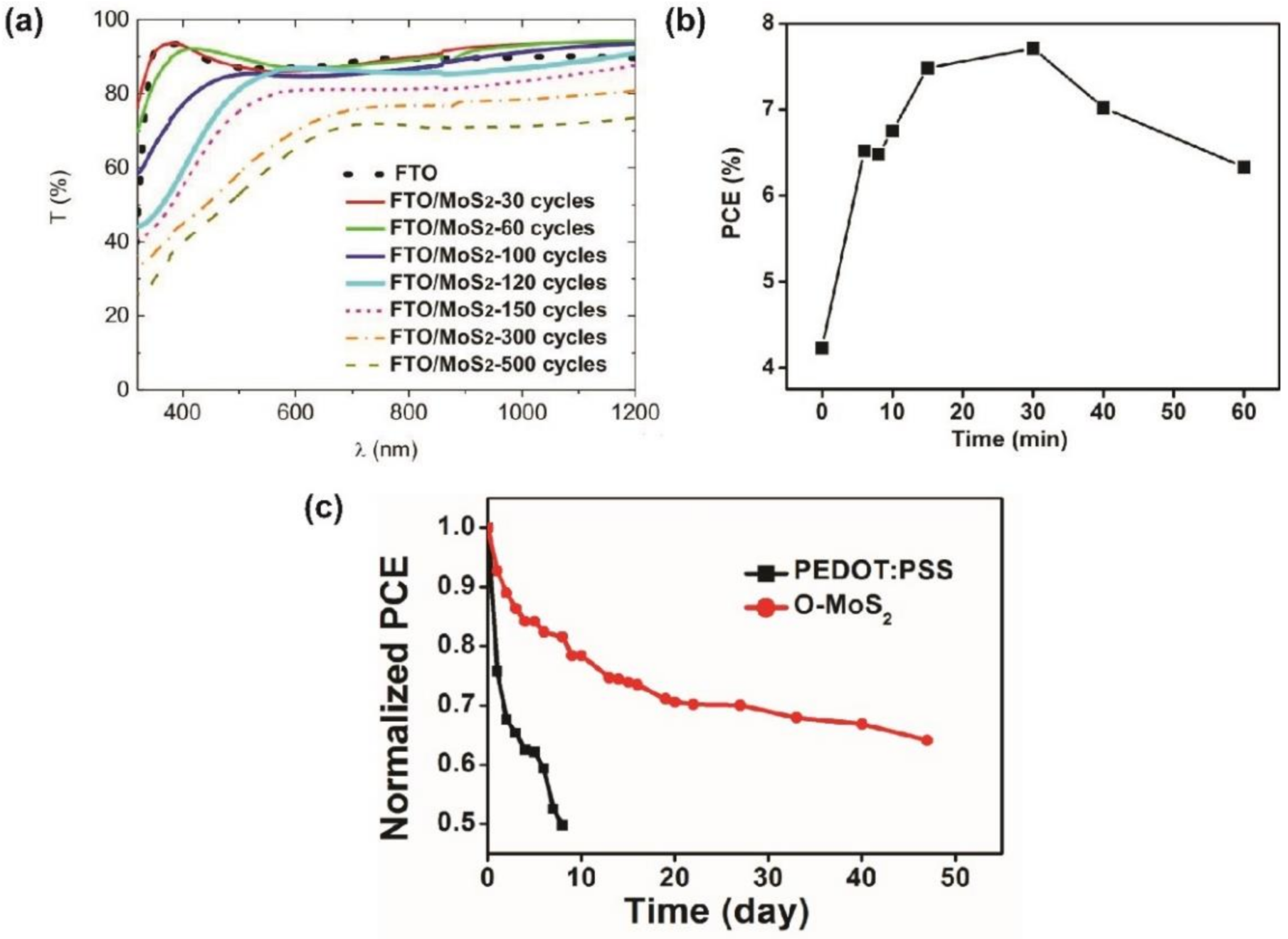
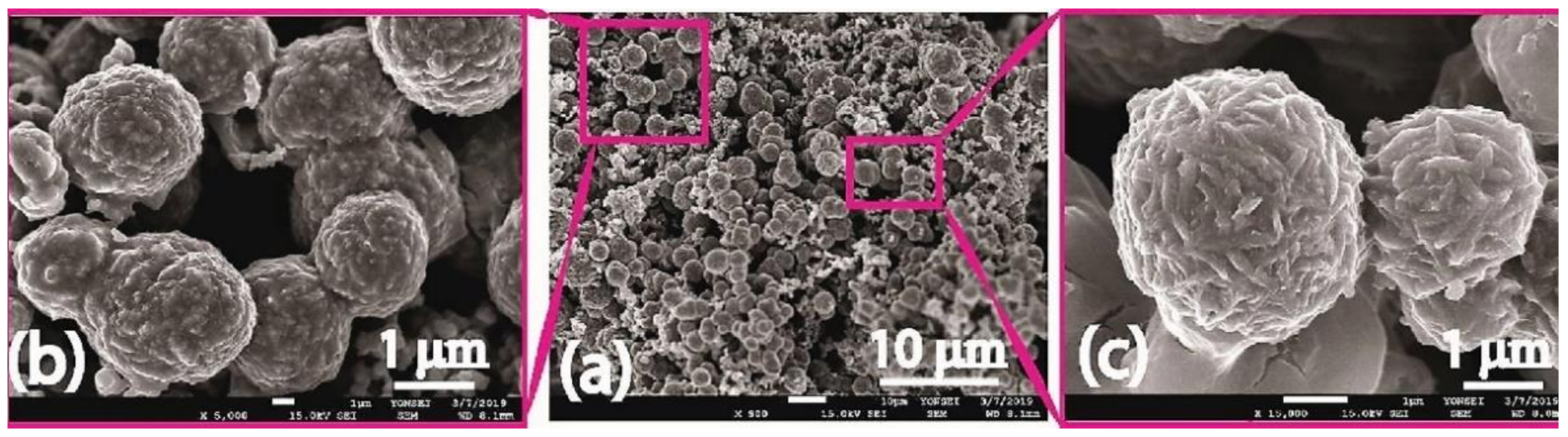
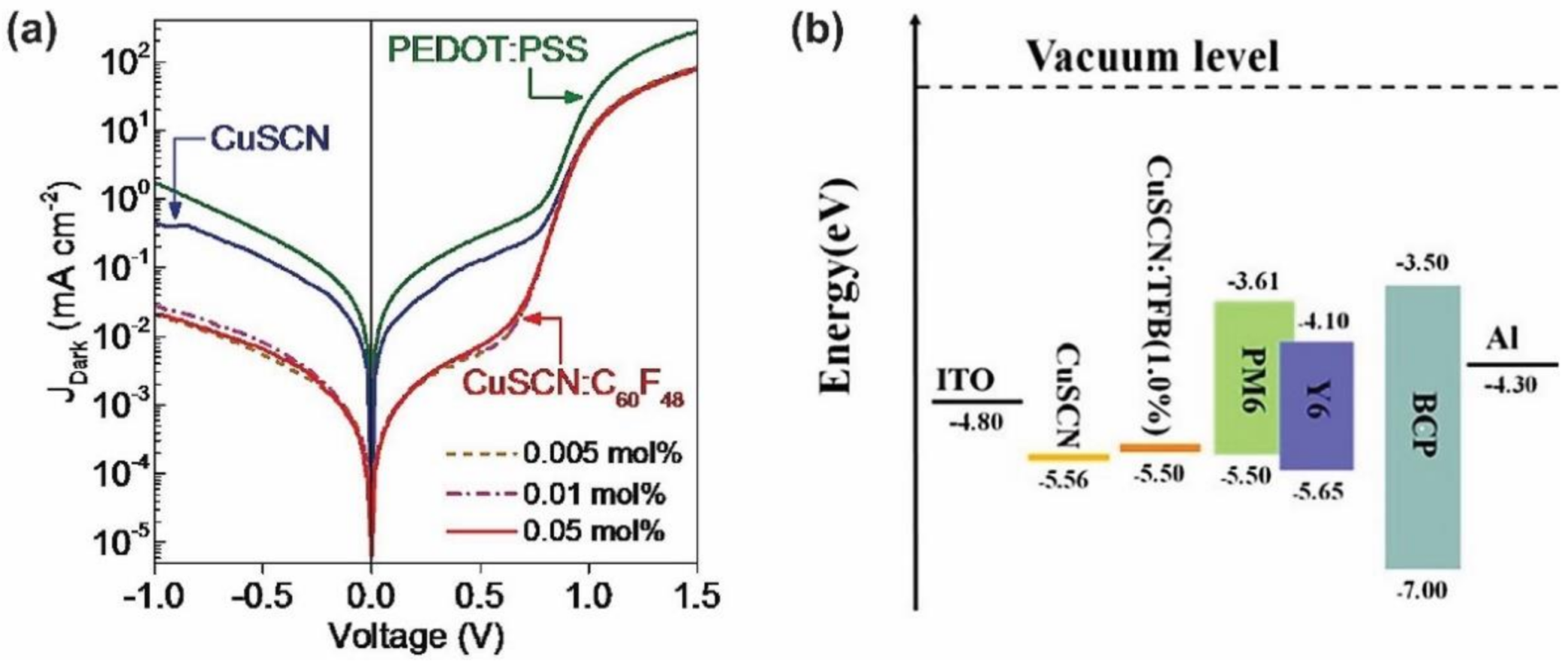

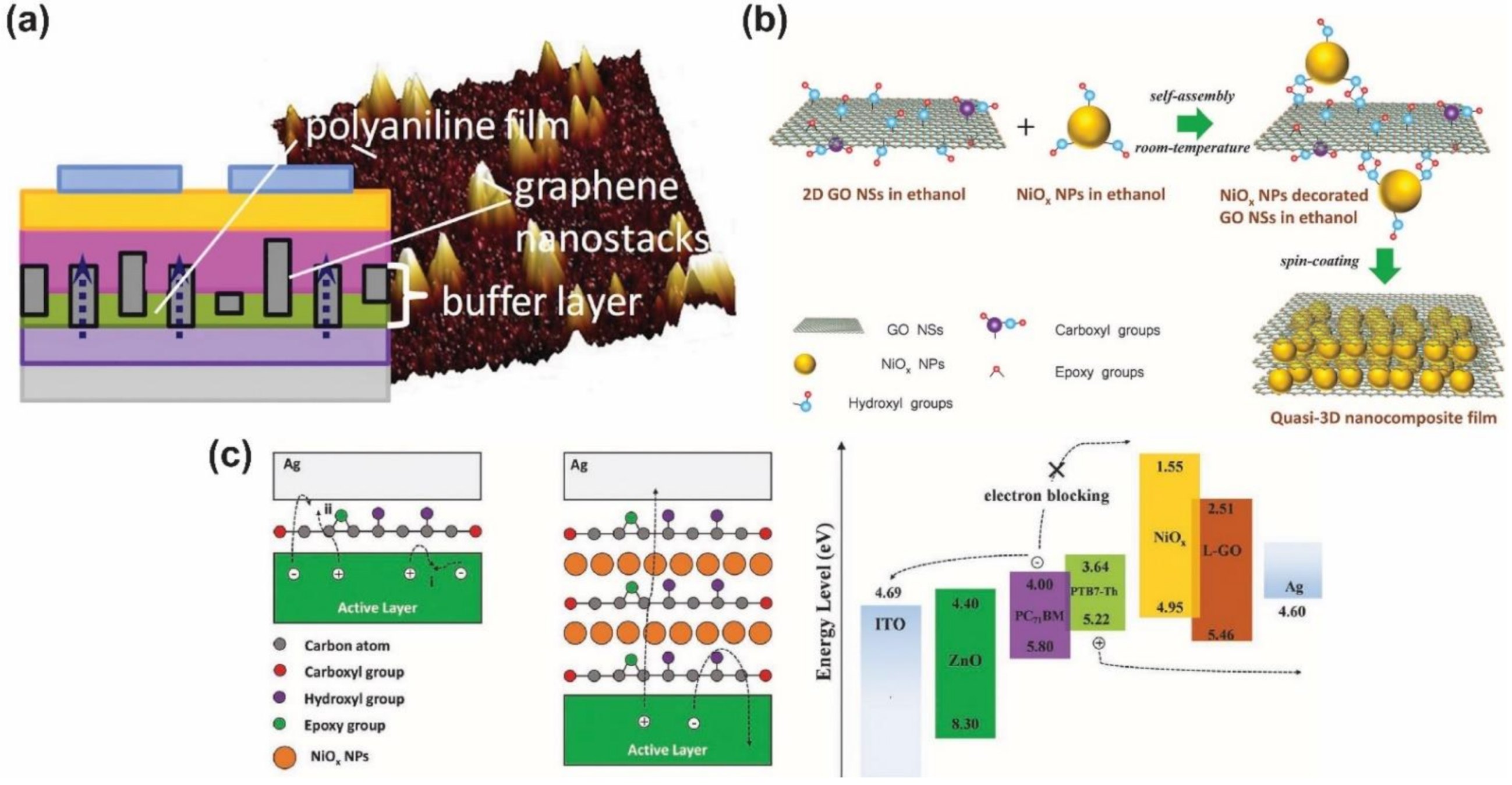
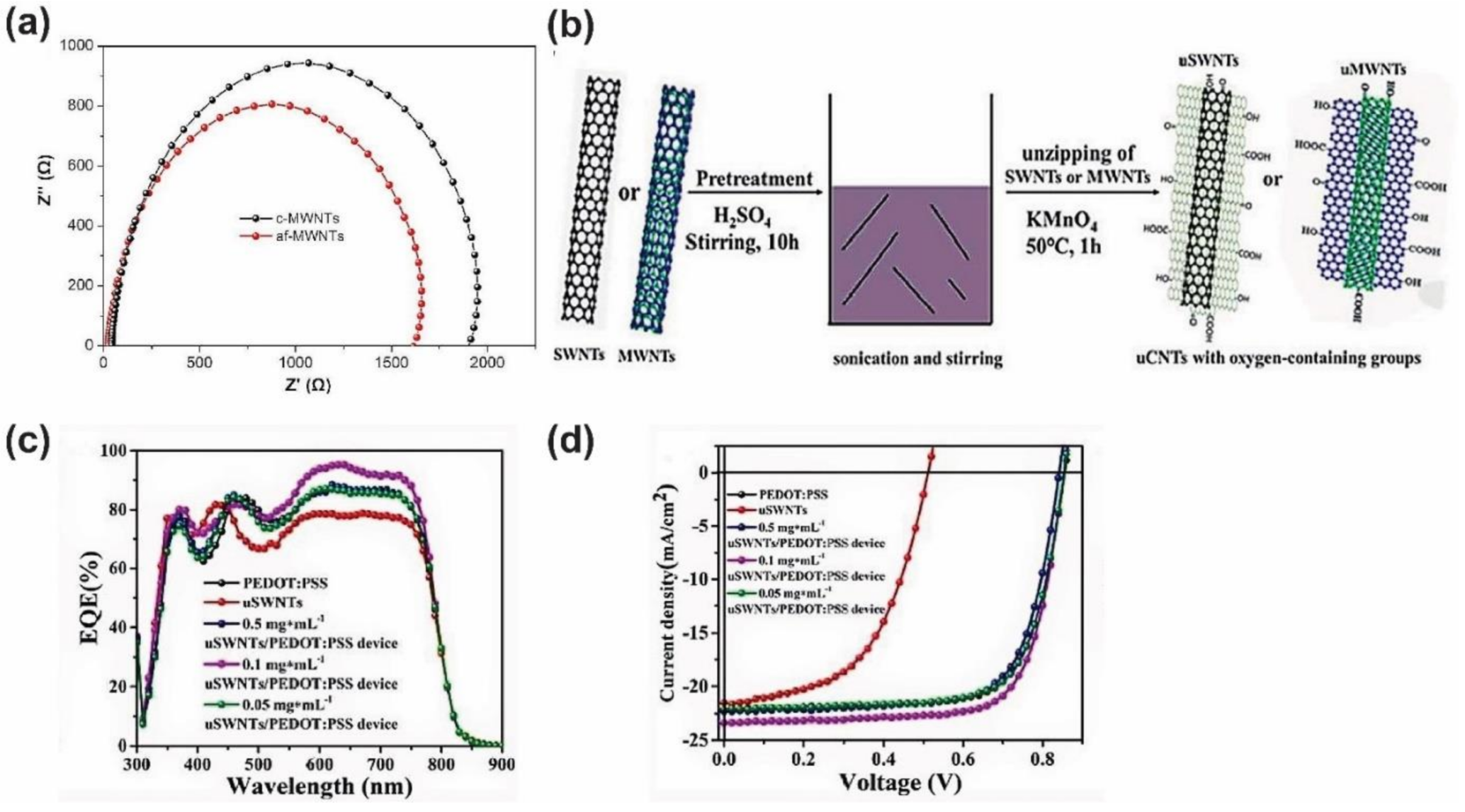
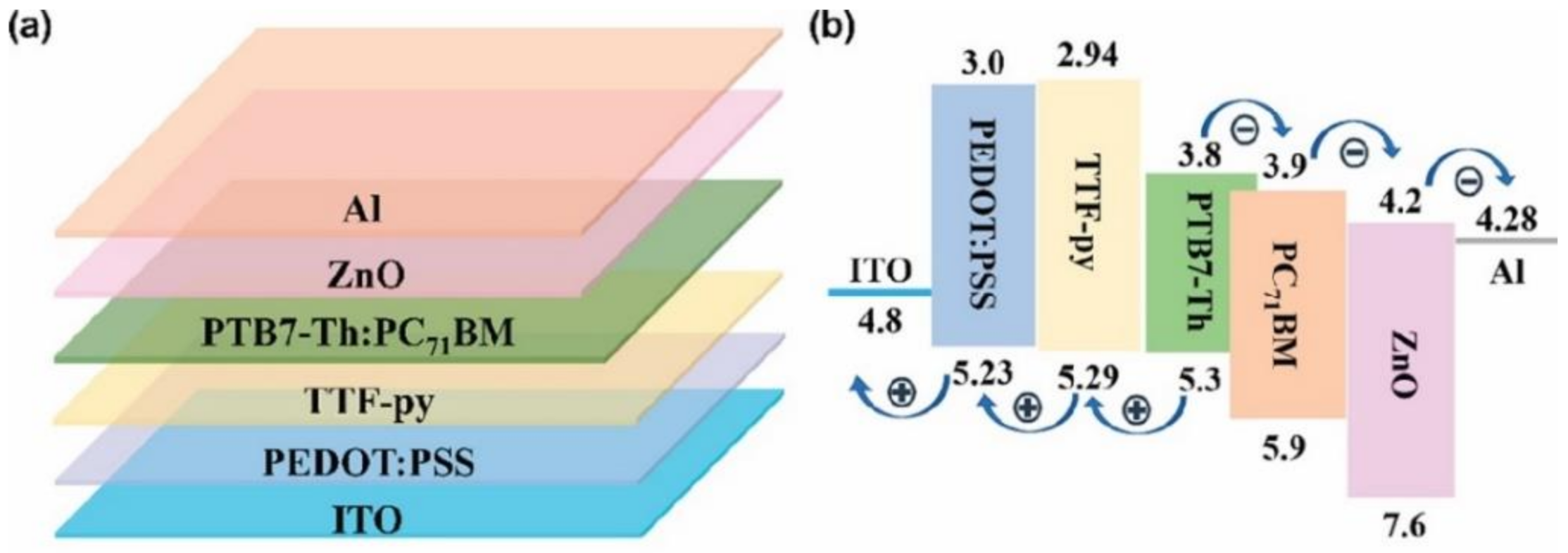
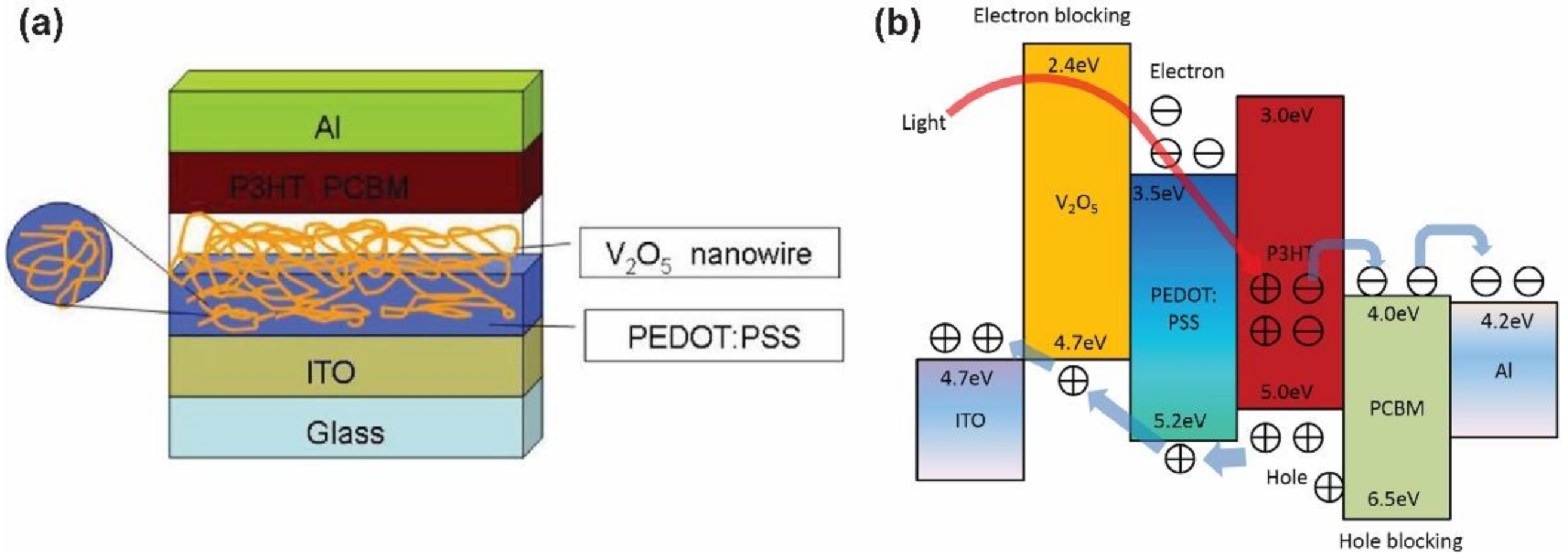


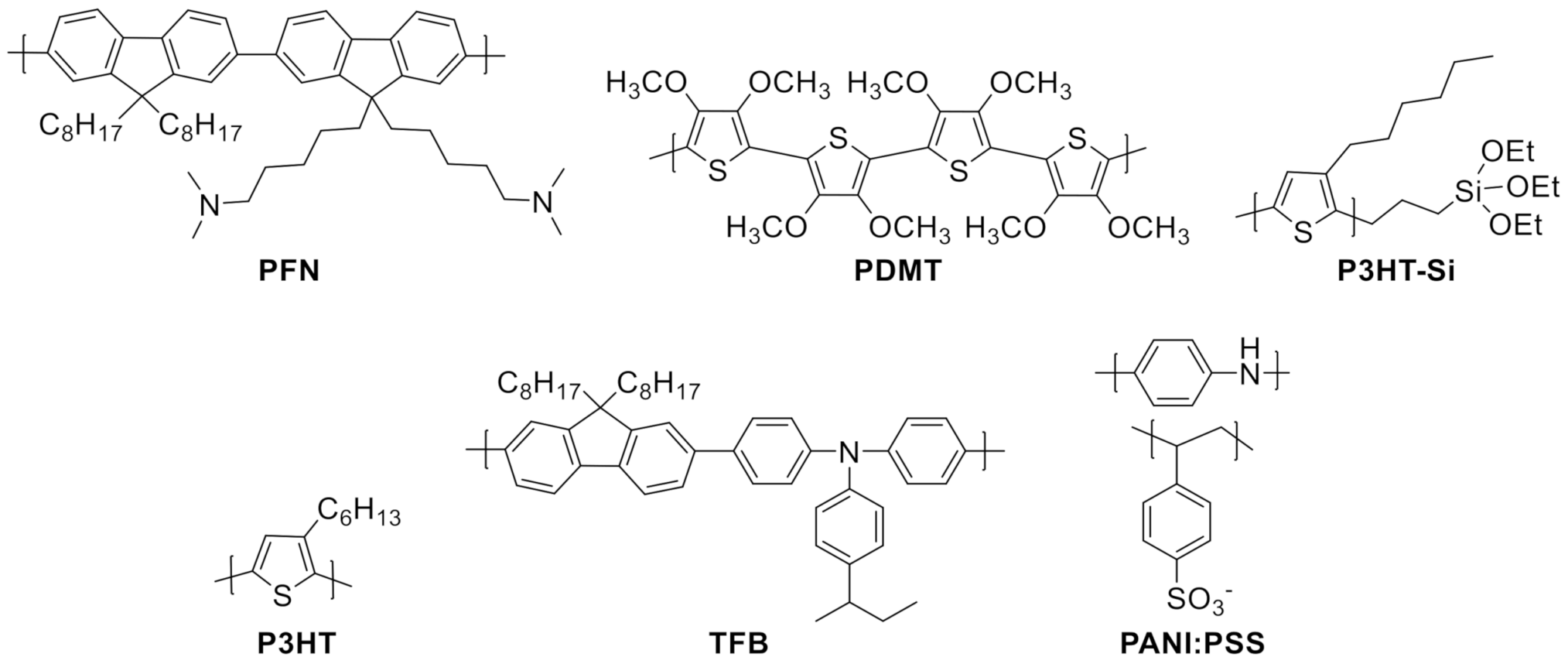
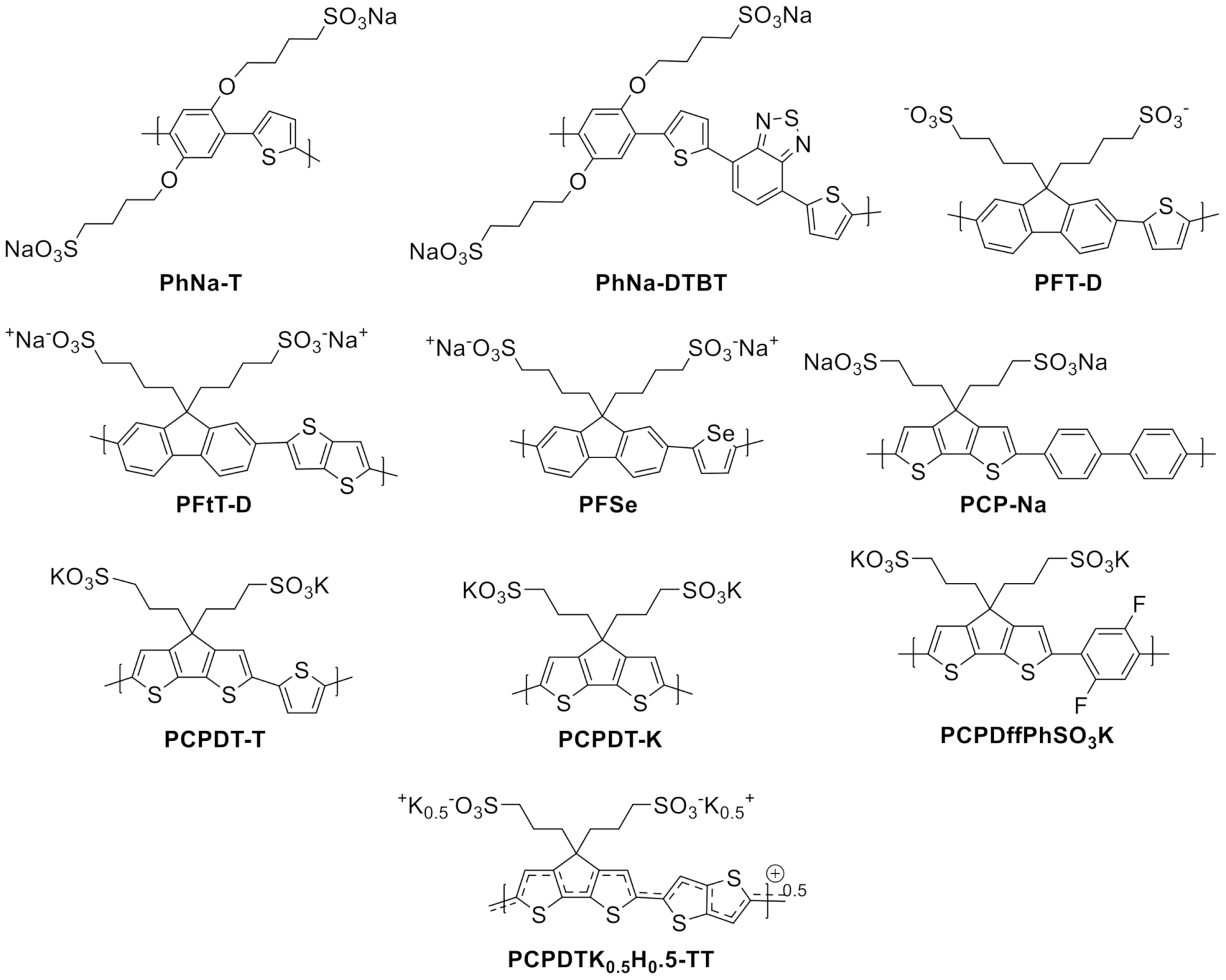
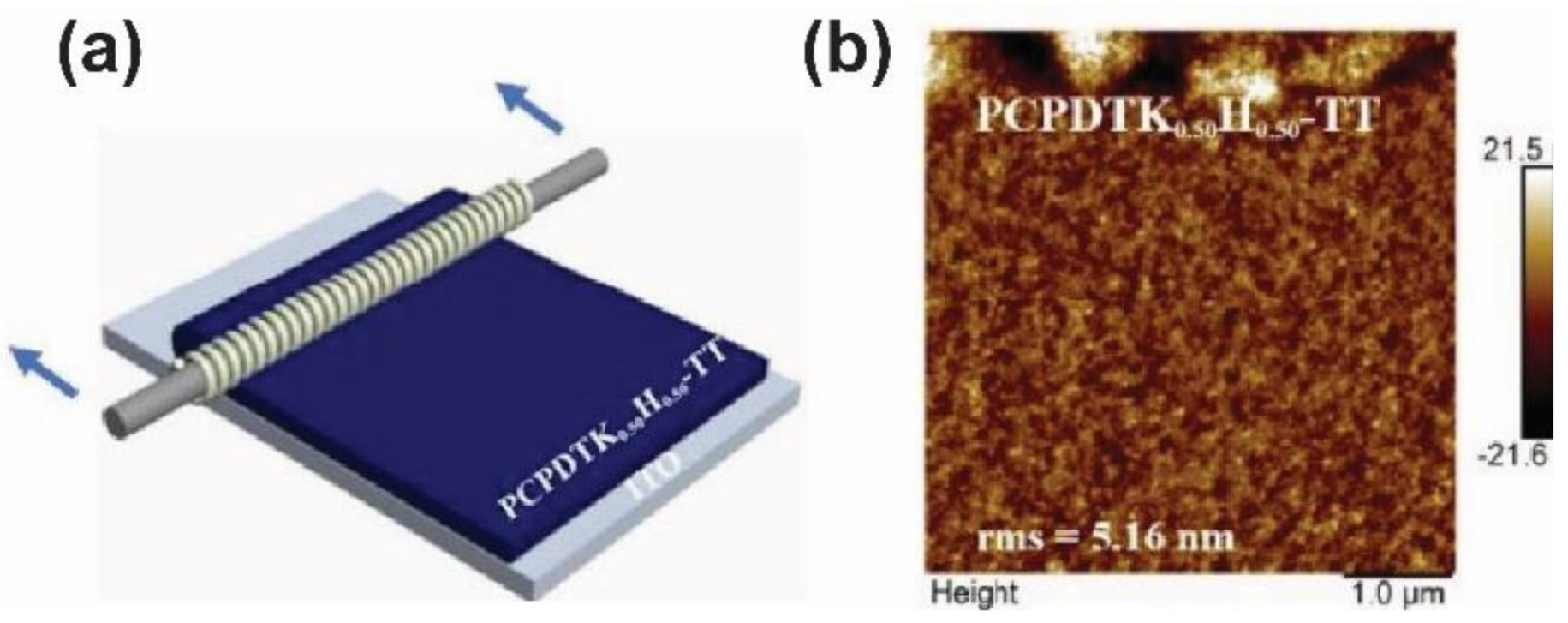
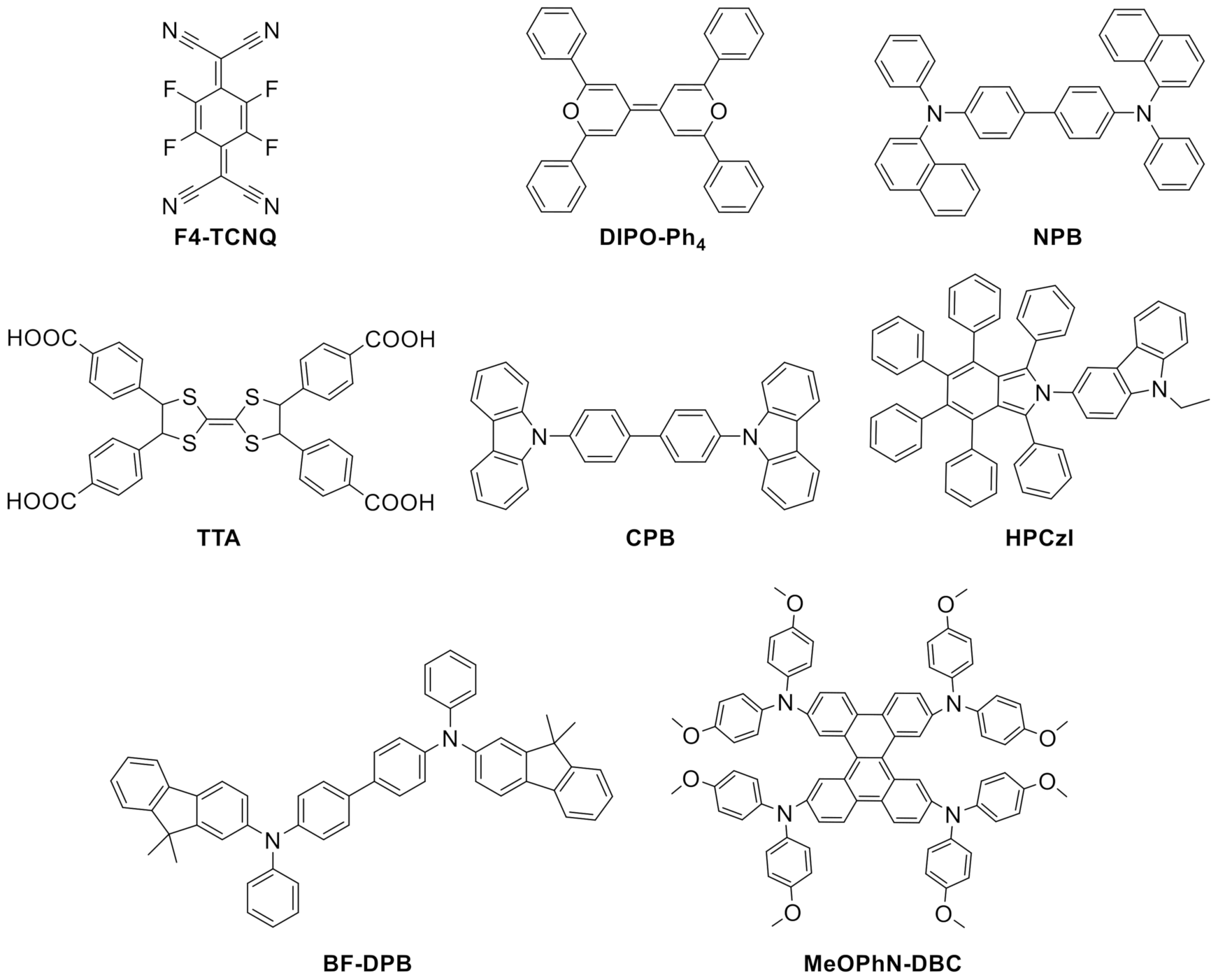

| Anode Configuration and WF (eV) | Deposition Technique | Active Layer | VOC (V) | JSC (mA cm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Metallic oxides | |||||||
| ITO/s-MoO3 (4.92) | spin coating | PBDB-T-2F:Y6 | 0.84 | 27.53 | 73.10 | 17.00 | [107] |
| ITO/PCM4 (5.4) | blade coating | PBDB-T-2F:Y6 | 0.83 | 16.06 | 68.28 | 14.30 | [119] |
| HVO/Ag (6.7) | spin coating | PBDTT-FTTE:PC71BM | 0.82 | 22.51 | 60.19 | 11.14 | [159] |
| ITO/MoO3 (5.3) | spin coating | PBDB-T:PC71BM | 0.88 | 17.48 | 71.00 | 10.86 | [102] |
| MoO3:NiOx (5.1) | spin coating | PBDB-T:IT-M | 0.94 | 17.26 | 66.63 | 10.81 | [113] |
| e-MoOx/Ag (5.4) | spin coating | PTB7-Th:PC71BM | 0.79 | 18.70 | 69.20 | 10.42 | [99] |
| VOx:PEDOT:PSS/Ag (5.28) | spin coating | TPD-3F:IT-4F | 0.87 | 16.80 | 69.10 | 10.10 | [164] |
| MoO3/AgAl/MoO3/AgAl | thermal evaporation | PTB7-Th:PC71BM | 0.78 | 19.60 | 61.90 | 9.79 | [100] |
| CuBr-MoO3/Ag (5.03) | thermal evaporation | PTB7:PC71BM | 0.75 | 19.65 | 65.20 | 9.56 | [117] |
| MoOx NPs/Ag (5.40) | spin coating | PTB7- Th:PC 71 BM | 0.79 | 18.05 | 65.20 | 9.50 | [99] |
| ITO/MoO3 (5.29) | spin coating | PBDB-T:ITIC | 0.91 | 15.19 | 66.59 | 9.17 | [106] |
| ITO/NiOx NPs(5.25) | spin coating | PTB7-Th:PC71BM | 0.79 | 18.32 | 63.10 | 9.16 | [176] |
| ITO/s-MoO3 (4.92) | spin coating | PTB7-Th:PC71BM | 0.79 | 16.69 | 67.10 | 8.90 | [107] |
| ITO/CuOx (5.06) | spin coating | PTB7:PC71BM | 0.74 | 16.44 | 71.00 | 8.68 | [187] |
| MoO3/Ag (5.52) | thermal evaporation | PTB7-Th:PC70BM | 0.81 | 15.90 | 67.80 | 8.67 | [98] |
| ITO/p-MoO3 (5.26) | spin coating | PTB7:PC71BM | 0.73 | 17.02 | 68.10 | 8.46 | [104] |
| ITO/CTAB-MoO3 (5.18) | spin coating | PTB7:PC71BM | 0.72 | 16.88 | 68.10 | 8.34 | [118] |
| ITO/VOx·nH2O (5.0) | spin coating | PTB7-th:PC71BM | 0.78 | 15.76 | 64.62 | 8.11 | [156] |
| ITO/V2O5 | spin coating | PTB7:PC70BM | 0.71 | 17.35 | 65.00 | 8.05 | [154] |
| ITO/NiOx NPs(5.25) | spin coating | PTB7:PC71BM | 0.74 | 16.10 | 66.42 | 7.96 | [176] |
| ITO/NP-V2O5 (4.7) | spin coating | PTB7:PC70BM | 0.72 | 15.81 | 69.01 | 7.89 | [165] |
| WOx nanosheets/Ag | spin coating | PTB7: PC71BM | 0.81 | 16.42 | 58.19 | 7.76 | [150] |
| ITO/s-VOx (5.3) | spin coating | PTB7:PC71BM | 0.73 | 15.79 | 66.82 | 7.70 | [160] |
| ITO/MoO3/AuNPs/MoO3 (5.6) | spin coating | PTB7:PC70BM | 0.73 | 14.40 | 73.00 | 7.68 | [122] |
| ITO/ESD-VOx (5.6) | spray casting | PTB7:PC71BM | 0.74 | 15.30 | 67.00 | 7.61 | [168] |
| ITO/V2Ox (5.42) | spin coating | PBDTDTTT-S-T:PC71BM | 0.68 | 16.29 | 67.21 | 7.44 | [114] |
| ITO/MoOx (5.30) | spin coating | PBDTDTTT-S-T:PC71BM | 0.69 | 16.14 | 66.02 | 7.35 | [114] |
| ITO/sMoOx | spin coating | PV10:PC70BM | 0.73 | 13.57 | 72.55 | 7.19 | [101] |
| FTO/Cu:NiOx | spin coating | PCDTBT:PC71BM | 0.89 | 12.40 | 63.85 | 7.05 | [181] |
| FTO/c-NiO (5.0) | spin coating | PTB7:PC71BM | 0.72 | 14.28 | 66.98 | 6.91 | [178] |
| ITO/MoOx | thermal evaporation | PTB7:PC71BM | 0.67 | 14.00 | 67.00 | 6.57 | [103] |
| ITO/HyMoO3-x (5.6) | spin coating | PTB7:PC70BM | 0.77 | 13.90 | 61.20 | 6.55 | [129] |
| ITO/NiOx NPs(5.25) | spin coating | PCDTBT:PC71BM | 0.90 | 11.36 | 62.35 | 6.42 | [176] |
| ITO/NiOx (5.6) | spin coating | TQ1:PC70BM | 0.87 | 10.30 | 71.30 | 6.39 | [175] |
| ITO/s-V2Ox (5.25) | spin coating | PFDT2BT-8:PC70BM | 0.87 | 10.20 | 67.10 | 6.30 | [162] |
| V2Ox/Ag (5.42) | spin coating | PBDTDTTT-S-T:PC71BM | 0.63 | 15.81 | 61.02 | 6.08 | [114] |
| MoOx/Ag (5.30) | spin coating | PBDTDTTT-S-T:PC71BM | 0.61 | 15.68 | 62.78 | 6.00 | [114] |
| ITO/V2O5·H2O (5.04) | spin coating | PBDSe-DT2PyT:PC71BM | 0.72 | 13.96 | 59.00 | 5.87 | [155] |
| ITO/CTAB-MoO3 (5.18) | spin coating | P3HT:ICBA | 0.82 | 10.40 | 67.40 | 5.80 | [118] |
| ITO/MoOx | thermal evaporation | PTB7-Th:PC71BM | 0.74 | 14.50 | 44.80 | 5.52 | [103] |
| FTO/s-MoO3 (5.3) | spin coating | P3HT:ICBA | 0.82 | 11.50 | 58.00 | 5.40 | [105] |
| FTO/MoOx (5.6) | spin coating | P3HT:PC71BM | 0.65 | 12.72 | 61.00 | 5.00 | [110] |
| Metallic sulfides | |||||||
| ITO/WS2 (5.3) | spin coating | PBDB-T-2F:Y6:SF(BR)4 | 0.89 | 29.31 | 80.00 | 20.87 | [204] |
| ITO/WS2 (5.5) | spin coating | PBDB-T-2F: Y6: PC71BM | 0.84 | 26.00 | 78.00 | 17.00 | [203] |
| ITO/WS2 (5.1) | spin coating | PBDB-T-2F:Y6:PC71BM | 0.83 | 26.00 | 72.00 | 15.60 | [202] |
| ITO/CuSCN:TFB (1.0%) (5.50) | spin coating | PM6:Y6 | 0.85 | 24.35 | 73.84 | 15.28 | [214] |
| ITO/MoS2 (5.04) | spin coating | PBDB-T-2F:Y6:PC71BM | 0.81 | 25.30 | 71.00 | 14.90 | [202] |
| ITO/CuSCN:AMQS | spin coating | PBDBT-2F:IT-4F | 0.80 | 18.70 | 67.80 | 10.14 | [209] |
| ITO/CuSCN:AMQS | spin coating | PTB7-Th:PC71BM | 0.79 | 17.10 | 65.20 | 8.80 | [209] |
| ITO/O-MoS2 QDs (5.2) | spin coating | PTB7-Th: PC71BM | 0.79 | 16.90 | 65.00 | 8.66 | [200] |
| ITO/MoSx (5.10) | spin coating | PTB7-Th: PC71BM | 0.77 | 18.16 | 53.56 | 7.50 | [201] |
| ITO/CuSCN:AMQSs | spin coating | PTB7-Th:ITIC | 0.82 | 15.07 | 59.06 | 7.15 | [209] |
| ITO/CuSCN:C60F48 (5.40) | spin coating | PCDTBT:PC70BM | 0.92 | 11.50 | 61.00 | 6.60 | [213] |
| Nanocarbon materials | |||||||
| ITO/uSWNTs/PEDOT:PSS | spin coating | PBDB-T-2F:IT-4F | 0.85 | 23.39 | 73.17 | 14.60 | [254] |
| ITO/FrGO (4.9) | spray casting | PM6:Y6 PSC. | 0.77 | 24.64 | 69.60 | 13.26 | [235] |
| L-GO:NiO/Ag | spin coating | PBDB-T:IT-M | 0.91 | 17.81 | 71.00 | 12.13 | [245] |
| G-MoS2/Ag (4.42) | spin coating | PTB7-Th:PC71BM | 0.80 | 17.10 | 67.70 | 9.50 | [236] |
| ITO/G-MoS2/PEDOT:PSS (5.0) | spin coating | PTB7-Th:PC71BM | 0.77 | 17.20 | 72.00 | 9.40 | [236] |
| ITO/FrGO (4.9) | spray casting | PTB7-Th:EH-IDTBR | 1.00 | 14.86 | 61.80 | 9.22 | [236] |
| ITO/FrGO (5.1) | spin coating | PTB7-Th:PC71BM | 0.79 | 16.89 | 64.80 | 8.60 | [233] |
| ITO/P-GO (4.70) | spin coating | PTB7:PC71BM | 0.71 | 16.12 | 68.40 | 7.90 | [232] |
| ITO/GO:CuCl2 (5.1) | spin coating | PTB7-Th:PC71BM | 0.79 | 15.52 | 63.00 | 7.74 | [240] |
| ITO/G-MoO3 (5.32) | spin coating | PCDTBT:PC71BM | 0.86 | 12.83 | 63.67 | 7.07 | [246] |
| af-MWNTs (5.22) | spin coating | PCDTBT:PC71BM | 0.87 | 12.65 | 63.50 | 6.97 | [252] |
| ITO/GO:NPs (4.9) | spin coating | PTB7:PC71BM | 0.75 | 11.55 | 67.91 | 5.88 | [242] |
| ITO/F5-rGO (5.1) | spin coating | PTB7:PC71BM | 0.68 | 14.78 | 57.30 | 5.82 | [234] |
| ITO/GBD (4.9) | spin coating | PBDTTT-C-T:PC70BM | 0.71 | 13.38 | 52.54 | 5.01 | [241] |
| ITO/GO:MoO3 (5.3) | spin coating | PCDTBT:PC71BM | 0.66 | 16.16 | 47.11 | 5.10 | [244] |
| Anode Configuration and WF (eV) | Deposition Technique | Active Layer | VOC (V) | JSC (mA cm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|
| PEDOT:PSS | |||||||
| ITO/PEDOT:PSS-DA (5.14) | spin coating | PM6:Y6 | 0.84 | 25.52 | 77.1 | 16.55 | [273] |
| ITO/g-C3N4:PEDOT:PSS (4.89) | spin coating | PM6:Y6 | 0.84 | 26.71 | 73.0 | 16.38 | [336] |
| ITO/PEDOT:PSS:TEMPO+Br- (4.95) | spin coating | PM6:Y6 | 0.82 | 27.18 | 72.6 | 16.10 | [270] |
| ITO/PEDOT:PSS:a-In2Se3 (5.06) | spin coating | PM6:Y6 | 0.84 | 25.47 | 74.5 | 15.89 | [272] |
| ITO/WOx:PEDOT:PSS (4.7) | spin coating | PM6:IT-4F | 0.87 | 20.73 | 80.8 | 14.57 | [313] |
| ITO/PEDOT:PSS/Ti3C2Tx (5.0) | selectively etching/spin coating | PM6:Y6 | 0.83 | 25.63 | 68.4 | 14.55 | [337] |
| ITO/PEDOT:PSS-MoO3 (5.22) | spin coating | PBDB-T-2F:IT-4F | 0.86 | 21.71 | 70.6 | 13.19 | [311] |
| ITO/PEDOT:PSS/BPQD (4.92) | spin coating | PM6:IT-4F | 0.85 | 21.14 | 71.3 | 12.81 | [297] |
| AgNWs/PEDOT:PSS/HxMoO3 (5.44) | transfer printing | PM6:IDIC:Y6 | 0.83 | 21.00 | 68.0 | 11.90 | [315] |
| ITO/PEDOT:PSS:a-In2Se3 (5.06) | spin coating | PBDB-T:ITIC | 0.91 | 17.31 | 71.1 | 11.22 | [272] |
| ITO/PEDOT:PSS/Ti3C2Tx (5.0) | selectively etching/spin coating | PBDB-T:ITIC | 0.91 | 17.08 | 70.9 | 11.02 | [337] |
| ITO/NiFD:PEDOT:PSS (5.01) | spin coating | PM6:PC71BM | 0.98 | 13.82 | 79.4 | 10.76 | [269] |
| WO3/PEDOT:PSS/Ag (5.27) | spin coating | SMD2:ITIC-Th | 0.90 | 17.30 | 66.0 | 10.30 | [314] |
| ITO/Ag ND/PEDOT:PSS | LIL/spin coating | PTB7:PC70BM | 0.73 | 23.26 | 61.0 | 10.11 | [291] |
| ITO/PEDOT:PSS-AuNRs | spin coating | PTB7-Th:PC71BM-Au NRs | 0.80 | 17.90 | 68.8 | 9.89 | [300] |
| ITO/V2O5: PEDOT:PSS | spin coating | PTB7-Th:PC71BM | 0.80 | 16.83 | 70.1 | 9.44 | [309] |
| ITO/PEDOT:PSS/TTF-py (5.29) | spin coating | PTB7-Th:PC71BM | 0.79 | 17.19 | 70.6 | 9.37 | [268] |
| ITO/PEDOT:PSS + Au NPs (5.4) | spin coating | PTB7:PC71BM | 0.74 | 18.30 | 68.0 | 9.26 | [290] |
| ITO/PEDOT:PSS/BPQD (4.92) | spin coating | PTB7-Th:PC71BM | 0.80 | 16.40 | 69.4 | 9.11 | [297] |
| ITO/PEDOT:PSS/p-TPCF (5.28) | electrochemical cyclic voltammetry | PTB7-Th:PC71BM | 0.80 | 16.98 | 66.2 | 8.99 | [285] |
| ITO/GOs/PEDOT:PSS (4.55) | spin coating | PBDB-T:ITIC | 0.90 | 15.10 | 65.7 | 8.93 | [317] |
| PMA:PEDOT:PSS/Al (5.02) | spin coating | PTB7-Th:PC71BM | 0.79 | 17.10 | 68.0 | 8.88 | [316] |
| PMA:PEDOT:PSS/Al (5.02) | spin coating | PffBT4T-2OD:PC71BM | 0.77 | 18.44 | 64.0 | 8.75 | [316] |
| ITO/PEDOT:PSS/PTPCz (5.23) | spin coating/electrodeposition | PTB7:PC71BM | 0.74 | 16.23 | 71.1 | 8.54 | [284] |
| ITO/PEDOT:PSS-WSe2 | spin coating | PTB7:PC71BM | 0.78 | 16.60 | 65.5 | 8.50 | [333] |
| ITO/PEDOT:PSS:Cu-Au NPs | spin coating | PTB7-Th:PC71BM | 0.79 | 17.78 | 60.1 | 8.48 | [294] |
| ITO/PEDOT:GSL (5.05) | spin coating | PTB7-Th:PC71BM | 0.77 | 15.82 | 68.7 | 8.47 | [259] |
| ITO/GO/PEDOT:PSS (4.9) | chemical vapor deposition/drop casting | PTB7:PC71BM | 0.75 | 16.10 | 69.5 | 8.40 | [318] |
| ITO/PEDOT:PSS:FOS (4.90) | spin coating | PTB7:PC70BM | 0.70 | 16.94 | 69.3 | 8.26 | [263] |
| ITO/PEDOT:PSS+PFT-D (5.0) | spin coating | PTB7-Th:PC71BM | 0.77 | 14.90 | 71.3 | 8.20 | [286] |
| FTO/PMMA/PEDOT:PSS | nanoimprinting/spin coating | PTB7:PC70BM | 0.73 | 16.30 | 68.2 | 8.12 | [274] |
| ITO/PSS:PEDOT:PSS (4.80) | spin coating | PDCBT:PC71BM | 0.83 | 12.44 | 77.2 | 7.97 | [269] |
| ITO/PEDOT:PSS:GO (5.1) | spin coating | PTB7:PCBM | 0.75 | 14.90 | 67.5 | 7.68 | [324] |
| PEDOT:PSS+MoO3 NPs/Ag (5.0) | spin coating/blade coated | PTB7-Th:PC60BM | 0.78 | 14.99 | 63.0 | 7.39 | [310] |
| ITO/PEDOT:PSS:Ag-Au-Au NRs | spin coating | PTB7:PC71BM | 0.73 | 16.87 | 60.0 | 7.36 | [293] |
| ITO/PEDOT:PSS/WS2 | spin coating | PTB7-Th:PC71BM | 0.79 | 15.67 | 58.6 | 7.24 | [332] |
| ITO/PEDOT:PSS:Cu-Au NPs | spin coating | PTB7-Th:PC61BM | 0.80 | 15.50 | 57.9 | 7.13 | [294] |
| ITO/PEDOT:PSS:bf-MWCNTs (5.39) | spin coating | PCDTBT:PC71BM | 0.88 | 12.51 | 63.1 | 6.95 | [335] |
| ITO/PEDOT-S (5.2) | spin coating | P3TI:PC71BM | 0.73 | 12.80 | 72.0 | 6.70 | [265] |
| ITO/PEDOT:PSS-NiS | spin coating | P3HT:PC61BM | 0.58 | 18.65 | 55.9 | 6.03 | [288] |
| ITO/PEDOT:PSS + Au NPs (5.0) | spin coating | rrP3HT:PC71BM | 0.58 | 16.10 | 61.0 | 5.65 | [292] |
| ITO/PEDOT:PSS + Au NPs (5.0) | spin coating | rrP3HT:PC71BM | 0.58 | 14.70 | 61.0 | 5.29 | [292] |
| ITO/PEDOT:PSS:PSFP-DTBTP (5.14) | spin coating | PCDTBT:PC71BM | 0.88 | 9.46 | 66.3 | 5.26 | [275] |
| ITO/GO/PEDOT:PSS (4.9) | spin coating | PCDTBT:PC71BM | 0.85 | 10.82 | 57.0 | 5.24 | [331] |
| ITO/PEDOT:PSS:GO (5.52) | spin casting | PTB7:PC71BM | 0.65 | 15.17 | 53.0 | 5.22 | [327] |
| ITO/PEDOT:PSS-MoO3 (5.3) | spray deposition | PTB7:PC71BM | 0.69 | 15.20 | 48.3 | 5.11 | [307] |
| PMA:PEDOT:PSS/Ag NWs (5.02) | doctor-blade coating | PTB7-Th:PC71BM | 0.78 | 11.28 | 57.0 | 5.01 | [316] |
| Other conjugated polymers | |||||||
| ITO/PCPDTKH-TT (5.24) | wire-bar coating | PM6:Y6:PC71BM | 0.85 | 25.10 | 75.9 | 16.30 | [365] |
| ITO/CuSCN/TFB (5.32) | spin coating | PM6:Y6 | 0.85 | 24.45 | 72.7 | 15.10 | [350] |
| ITO/PhNa-1T (5.21) | spin coating | PTB7-Th:PC71BM | 0.79 | 16.98 | 71.1 | 9.89 | [357] |
| ITO/PCP-Na (5.22) | spin coating | PBDT-TS1:PC71BM | 0.80 | 17.46 | 70.6 | 9.89 | [359] |
| ITO/Cu2O/FBT-TH4 (5.08) | sputtered method | PffBT4T-2OD:PC71BM | 0.77 | 17.50 | 70.7 | 9.56 | [339] |
| ITO/PCPDffPhSO3K (5.18) | spin coating | PTB7-Th:PC71BM | 0.79 | 18.08 | 67.0 | 9.50 | [363] |
| ITO/PCPDT-T (4.87) | spin casting | PTB7-Th:PC71BM | 0.77 | 18.92 | 63.5 | 9.30 | [361] |
| ITO/PhNa-DTBT (5.3) | spin coating | PTB7-Th:PC71BM | 0.79 | 16.92 | 69.5 | 9.29 | [358] |
| ITO/CuSCN/TFB (5.32) | spin coating | PTB7-Th:PC71BM | 0.79 | 16.42 | 66.3 | 8.56 | [350] |
| ITO/PhNa-1T (5.21) | spin coating | PTB7:PC71BM | 0.75 | 16.17 | 68.6 | 8.38 | [357] |
| ITO/PFtT-D (5.19) | spin coated | PTB7-Th:PC71BM | 0.76 | 16.00 | 68.4 | 8.30 | [364] |
| ITO/PFSe (5.1) | spin casting | PTB7:PC71BM | 0.68 | 14.40 | 69.0 | 7.20 | [360] |
| ITO/NiOx:PFN (5.34) | spin casting | PBDTTBO-C8:PC71BM | 0.71 | 13.75 | 63.7 | 6.20 | [338] |
| ITO/CNT-g-PDDT:P3ThEt-g-PANI | spin coating | PBDT-DTNT:PC61BM | 0.71 | 12.84 | 62.0 | 5.65 | [344] |
| ITO/CNt:P3ThEt-g-PANI | spin coating | P3HT:PC71BM | 0.68 | 12.85 | 60.7 | 5.30 | [345] |
| Small organic molecules | |||||||
| ITO/TTA (5.26) | spin coating | PTB7-Th:PC71BM | 0.80 | 16.56 | 69.04 | 9.09 | [377] |
| ITO/NiOx:F4-TCNQ (5.30) | spin coating | PTB7-Th:PC71BM | 0.78 | 16.80 | 65.20 | 8.59 | [370] |
| ITO/DIPO-Ph4/PEDOT:PSS (4.7) | vacuum deposition/spin coating | P3HT:PC61BM/ | 0.60 | 11.50 | 47.00 | 4.60 | [371] |
| NPB/MoO3/Ag (5.4) | thermal evaporation | P3HT:PC61BM | 0.60 | 10.04 | 63.00 | 3.94 | [374] |
| MeOPhN-DBC/MoO3/Al (5.0) | vacuum deposition | P3HT:PC61BM | 0.63 | 12.44 | 47.00 | 3.68 | [376] |
| ITO/NiOx:F4-TCNQ (5.30) | spin coating | P3HT:PC61BM | 0.59 | 9.89 | 61.60 | 3.59 | [370] |
| NPB/Ag (5.4) | vacuum deposition | P3HT:PC71BM | 0.57 | 9.49 | 48.90 | 2.63 | [373] |
| ITO/BF-DPB:NDP9 (5.23) | spin coating | ZnPC:C60 | 0.51 | 7.50 | 55.00 | 2.10 | [369] |
| Ag NWs/BF-DPB:NDP9 (5.23) | spin coating | ZnPC:C60 | 0.49 | 7.60 | 55.00 | 2.10 | [369] |
| ITO/MoO3:HPCzI (5.3/5.1) | thermal evaporation | CuPC:C60 | 0.49 | 6.63 | 53.00 | 1.71 | [372] |
| ITO/HPCzI (5.1) | thermal evaporation | CuPC:C60 | 0.49 | 6.22 | 53.00 | 1.62 | [372] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anrango-Camacho, C.; Pavón-Ipiales, K.; Frontana-Uribe, B.A.; Palma-Cando, A. Recent Advances in Hole-Transporting Layers for Organic Solar Cells. Nanomaterials 2022, 12, 443. https://doi.org/10.3390/nano12030443
Anrango-Camacho C, Pavón-Ipiales K, Frontana-Uribe BA, Palma-Cando A. Recent Advances in Hole-Transporting Layers for Organic Solar Cells. Nanomaterials. 2022; 12(3):443. https://doi.org/10.3390/nano12030443
Chicago/Turabian StyleAnrango-Camacho, Cinthya, Karla Pavón-Ipiales, Bernardo A. Frontana-Uribe, and Alex Palma-Cando. 2022. "Recent Advances in Hole-Transporting Layers for Organic Solar Cells" Nanomaterials 12, no. 3: 443. https://doi.org/10.3390/nano12030443