A Novel Yeast Genus and Two Novel Species Isolated from Pineapple Leaves in Thailand: Savitreella phatthalungensis gen. nov., sp. nov. and Goffeauzyma siamensis sp. nov.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Yeast Isolation
2.2. Yeast Identification
2.2.1. Morphological Study
2.2.2. Biochemical and Physiological Studies
2.2.3. Molecular Study
3. Results
3.1. Yeast Isolation
3.2. Molecular Analyses and Phenotypic Characterization
3.2.1. Strain DMKU-PAL186 and DMKU-PAL178
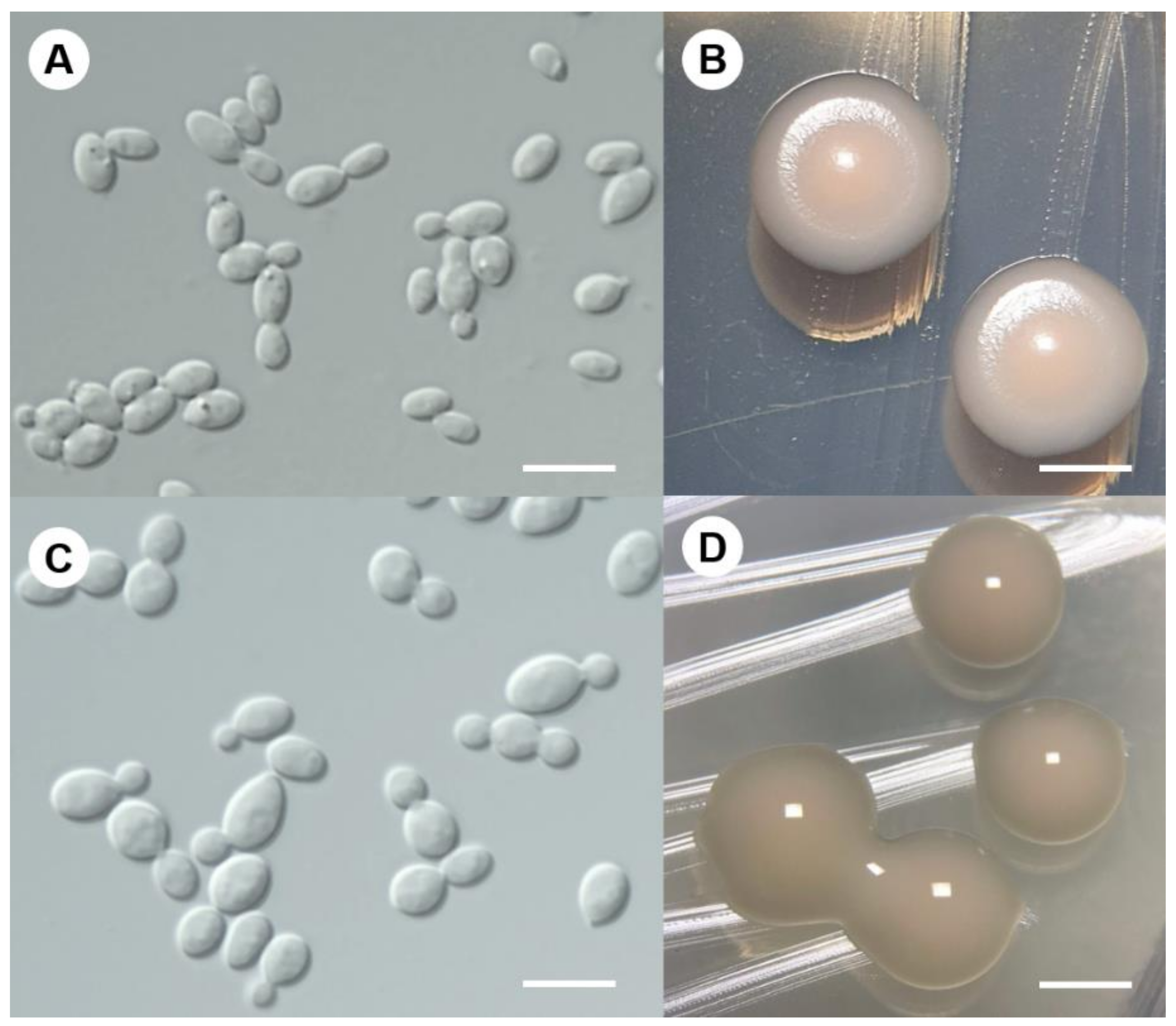
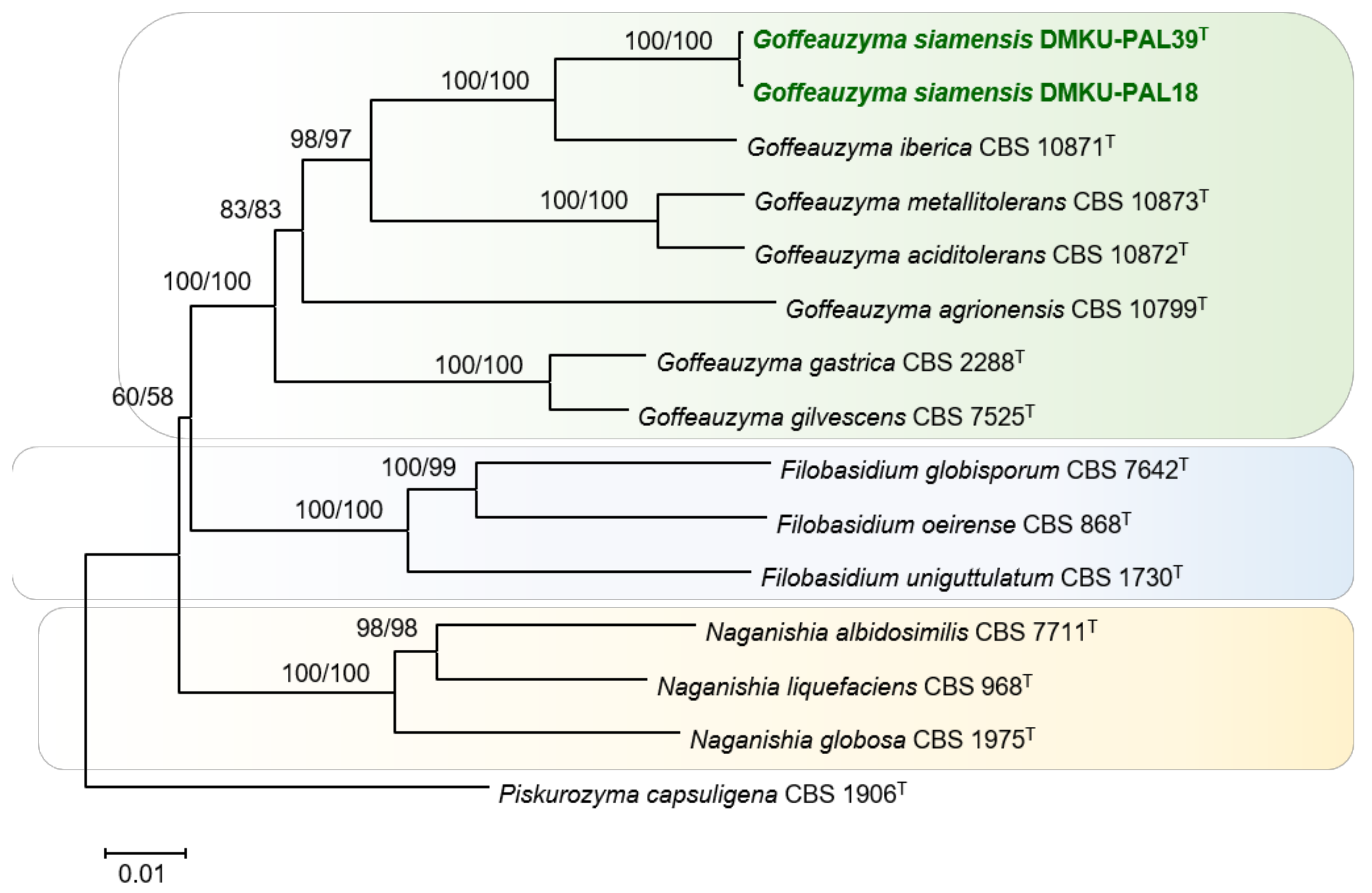
3.2.2. DMKU-PAL39 and DMKU-PAL18
3.3. Taxonomic Description of Genus and New Species
3.3.1. Savitreella P. Nutaratat, W. Boontham & P. Khunnamwong, gen. nov.
3.3.2. Savitreella phatthalungensis P. Nutaratat, W. Boontham & P. Khunnamwong, sp. nov.
3.3.3. Goffeauzyma siamensis P. Nutaratat, W. Boontham & P. Khunnamwong, sp. nov.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Medium-Term Outlook: Prospects for Global Production and Trade in Bananas and Tropical Fruits 2019 to 2028; Rome, Italy; Available online: https://www.fao.org/publications/card/en/c/CA7568EN/ (accessed on 1 December 2021).
- Prakongpan, T.; Nitithamyong, A.; Luangpituaksa, P. Extraction and application of dietary fiber and cellulose from pineapple cores. J. Food Sci. 2006, 67, 1308–1313. [Google Scholar] [CrossRef]
- Onken, J.E.; Greer, P.K.; Calingaert, B.; Hale, L.P. Bromelain treatmentdecreases secretion of pro-inflammatory cytokines and chemokines by colonbiopsies in vitro. Clin. Immunol. 2008, 126, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohsin, A.; Jabeen, A.; Majid, D.; Allai, F.M.; Dar, A.H.; Gulzar, B.; Makroo, H.A. Pineapple. In Antioxidants in Fruits: Properties and Health Benefits; Nayik, G.A., Gull, A., Eds.; Springer: Singapore, 2020; pp. 379–396. [Google Scholar]
- Chen, C.C.; Paull, R.E. Sugar metabolism and pineapple fruit translucency. J. Am. Soc. Hortic. Sci. 2000, 125, 558–562. [Google Scholar] [CrossRef] [Green Version]
- Di Cagno, R.; Cardinali, G.; Minervini, G.; Antonielli, L.; Rizzello, C.G.; Ricciuti, P.; Gobbetti, M. Taxonomic structure of the yeasts and lactic acid bacteria microbiota of pineapple (Ananas comosus L. Merr.) and use of autochthonous starters for minimally processing. Food Microbiol. 2010, 27, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.C.; Piccoli, R.H.; Duarte, W.F. Probiotic potential of yeasts isolated from pineapple and their use in the elaboration of potentially functional fermented beverages. Food Res. Int. 2018, 107, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Escriba, R.C.R.; Rodriguez, R.; López, D.; Lorente, G.Y.; Pino, Y.; Aragón, C.E.; Garza, Y.; Podestá, F.E.; González, G.Y.L. High light intensity increases the CAM expression in “MD-2” micro-propagated pineapple plants at the end of the acclimatization stage. Am. J. Plant Sci. 2015, 6, 3109–3118. [Google Scholar] [CrossRef] [Green Version]
- Kurtzman, C.P. Protomyces Unger (1833). In The Yeasts: A Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 725–731. [Google Scholar]
- Reddy, M.S.; Kramer, C. A taxonomic revision of the Protomycetales. Mycotaxon 1975, 3, 1–50. [Google Scholar]
- Wang, K.; Sipilä, T.P.; Overmyer, K. The isolation and characterization of resident yeasts from the phylloplane of Arabidopsis thaliana. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Wang, K.; Sipilä, T.; Overmyer, K. A novel Arabidopsis phyllosphere resident Protomyces species and a re-examination of genus Protomyces based on genome sequence data. IMA Fungus 2021, 12, 8. [Google Scholar] [CrossRef]
- Fonseca, Á.; Rodrigues, M.G. Taphrina Fries (1832). In The Yeasts: A Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 823–858. [Google Scholar]
- Mix, A.J. A monograph of the genus Taphrina. Univ. Kans. Sci. Bull. 1949, 33, 3–167. [Google Scholar] [CrossRef]
- Gjaerum, H.B. The genus Taphrina Fr. in Norway. Nytt Mag. Bot. 1964, 11, 5–26. [Google Scholar]
- Rodrigues, M.G.; Fonseca, A. Molecular systematics of the dimorphic ascomycete genus Taphrina. Int. J. Syst. Evol. Microbiol. 2003, 53, 607–616. [Google Scholar] [CrossRef]
- Selbmann, L.; Turchetti, B.; Andrey, Y.; Cecchini, C.; Zucconi, L.; Isola, D.; Buzzini, P.; Onofri, S. Description of Taphrina antarctica f.a. sp. nov., a new anamorphic ascomycetous yeast species associated with antarctic endolithic microbial communities and transfer of four Lalaria species in the genus Taphrina. Extremophiles 2014, 18, 707–721. [Google Scholar] [CrossRef]
- Liu, X.-Z.; Wang, Q.-M.; Theelen, B.; Groenewald, M.; Bai, F.-Y.; Boekhout, T. Phylogeny of tremellomycetous yeasts and related dimorphic and filamentous basidiomycetes reconstructed from multiple gene sequence analyses. Stud. Mycol. 2015, 81, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Z.; Wang, G.M.; Göker, M.; Groenewald, M.; Kachalkin, A.V.; Lumbsch, H.T.; Millanes, A.M.; Wedin, M.; Yurkov, A.M.; Bai, F.Y.; et al. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud. Mycol. 2015, 81, 85–147. [Google Scholar] [CrossRef] [Green Version]
- Gadanho, M.; Sampaio, J.P. Cryptococcus ibericus sp. nov., Cryptococcus aciditolerans sp. nov. and Cryptococcus metallitolerans sp. nov., a new ecoclade of anamorphic basidiomycetous yeast species from an extreme environment associated with acid rock drainage in São Domingos pyrite mine, Portugal. Int. J. Syst. Evol. Microbiol. 2009, 59, 2375–2379. [Google Scholar]
- Russo, G.; Libkind, D.; Ulloa, R.J.; de García, V.; Sampaio, J.P.; van Broock, M.R. Cryptococcus agrionensis sp. nov., a basidiomycetous yeast of the acidic rock drainage ecoclade, isolated from an acidic aquatic environment of volcanic origin. Int. J. Syst. Evol. Microbiol. 2010, 60, 996–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, Á.; Boekhout, T.; Fell, J.W. Cryptococcus Vuillemin (1901). In The Yeasts: A Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1161–1737. [Google Scholar]
- McNeill, J.; Barrie, F.; Buck, W.; Demoulin, V.; Greuter, W.; Al, E. International Code of Nomenclature for Algae, Fungi, and Plants (Melbourne Code); Regnum Vegetabile 154; A.R.G. Gantner Verlag KG: Ruggell, Liechtenstein, 2012; ISBN 978-3-87429-425-6. [Google Scholar]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.; Robert, V. Methods for isolation, phenotypic characterization and maintenance of yeasts. In The Yeasts: A Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 87–110. [Google Scholar]
- Limtong, S.; Yongmanitchai, W.; Tun, M.M.; Kawasaki, H.; Seki, T. Kazachstania siamensis sp. nov., an ascomycetous yeast species from forest soil in Thailand. Int. J. Syst. Evol. Microbiol. 2007, 57, 419–422. [Google Scholar] [CrossRef] [Green Version]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Rosa, C.A.; Lachance, M.A.; Teixeira, L.C.; Pimenta, R.S.; Morais, P.B. Metschnikowia cerradonensis sp. nov., a yeast species isolated from ephemeral flowers and their nitidulid beetles in Brazil. Int. J. Syst. Evol. Microbiol. 2007, 57, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Kurtzman, C.P.; Robnett, C.J. Relationships among genera of the Saccharomycotina (Ascomycota) from multigene phylogenetic analysis of type species. FEMS Yeast Res. 2013, 13, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diezmann, S.; Cox, C.J.; SchÖnian, G.; Vilgalys, R.J.; Mitchell, T.G. Phylogeny and evolution of medical species of Candida and related taxa: A multigenic analysis. J. Clin. Microbiol. 2004, 42, 5624–5635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.W.; Boekhout, T.; Fonseca, A.; Scorzetti, G.; StatzellTallman, A. Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int. J. Syst. Evol. Microbiol. 2000, 50, 1351–1371. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; Szöke, S.; Cardinali, G.; Eberhardt, U.; Stielow, B.; De Vries, M.; Verkleij, G.J.M.; Crous, P.W.; Robert, V.; et al. DNA barcoding analysis of more than 9000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Stud. Mycol. 2016, 85, 91–105. [Google Scholar] [CrossRef]
- Butinar, L.; Strmole, T.; Gunde-Cimerman, N. Relative incidence of ascomycetous yeasts in arctic coastal environments. Microb. Ecol. 2011, 61, 832–843. [Google Scholar] [CrossRef]
- Valverde, R.A.; Templeton, G.E. Leaf gall of Torilis japonica caused by Protomyces macrosporus in Arkansas. Plant Dis. 1984, 68, 716–717. [Google Scholar] [CrossRef]


| Species | Strain | GenBank Accession Number * | ||
|---|---|---|---|---|
| SSU | ITS | D1/D2 | ||
| Novakomyces olei | NCAIM Y.02187T | - | MW023954 | MG250349 |
| Protomyces arabidopsidicola | C29T | - | LT602858 | MK934482 |
| Protomyces inouyei | NRRL YB-4354T | AY548295 | MK937056 | NG_042406 |
| Protomyces inundatus | NRRL Y-6349T | - | MK937057 | U76528 |
| Protomyces gravidus | NRRL Y-17093T | - | MK937055 | U84342 |
| Protomyces lactucaedebilis | NRRL YB-4353T | - | MK937058 | U84343 |
| Protomyces macrosporus | NRRL Y-12879T | D85143 | MK937059 | U94939 |
| Protomyces pachydermus | NRRL YB-4355T | D85142 | MK937060 | NG_064602 |
| Saccharomyces cerevisiae | CBS 1171T | NG_063315 | AB018043 | JQ689017 |
| Savitreella phatthalungensis | DMKU-PAL186T | LC647808 | MW876306 | MW879743 |
| Savitreella phatthalungensis | DMKU-PAL178 | LC647809 | MW876305 | MW879742 |
| Saitoella complicata | NRRL Y-17804T | NG_013154 | JN161162 | JN161156 |
| Saitoella coloradoensis | CBS 12360T | - | KY105294 | KY109521 |
| Schizosaccharomyces cryophilus | CBS 11777T | - | NR_121468 | GU470882 |
| Schizosaccharomyces osmophilus | CBS 15793T | - | MK589403 | MK253005 |
| Schizosaccharomyces japonicus | CBS 354T | - | NR_121199 | U94943 |
| Schizosaccharomyces pombe | CBS 356T | - | MK749863 | U40085 |
| Taphrina alni | CBS 683.93T | - | AF492077 | AF492024 |
| Taphrina antarctica | CCFEE 5198 | - | NR_132870 | NG_059477 |
| Taphrina americana | CBS 331.55 | - | NR_155874 | AF492025 |
| Taphrina deformans | CBS 356.35T | - | MH855698 | MH867217 |
| Taphrina dearnessii | CBS 334.55T | - | MH857499 | AF492037 |
| Taphrina betulina | CBS 119536T | - | AF492080 | AF492027 |
| Taphrina bullata | TB004 | - | KC491200 | JN997390 |
| Taphrina carnea | CBS 332.55T | - | MH857498 | MH869038 |
| Taphrina carpini | PYCC 5558T | - | NR_119488 | NG_042399 |
| Taphrina caerulescens | CBS 351.35T | - | NR_155875 | NG_057691 |
| Taphrina confusa | CBS 375.39T | - | NR_155876 | NG_067349 |
| Taphrina communis | CBS 352.35T | NG_065466 | NR_160070 | MH867214 |
| Taphrina epiphylla | CBS 111109T | - | AF492096 | AF492039 |
| Taphrina flavorubra | CBS 377.39T | NG_062593 | MH856045 | MH867541 |
| Taphrina insititiae | CBS 12782T | - | KC491202 | JN997391 |
| Taphrina letifera | CBS 335.55T | NG_062594 | NR_155877 | MH869040 |
| Taphrina mirabilis | CBS 357.35T | NG_062595 | NR_155878 | MH867218 |
| Taphrina nana | CBS 336.55T | - | MH857501 | MH869041 |
| Taphrina padi | CBS 693.93T | - | NR_155879 | AF492048 |
| Taphrina populina | CBS 337.55T | NG_062683 | MH857502 | NG_057694 |
| Taphrina populi-salicis | CBS 419.54T | - | NR_155881 | NG_057695 |
| Taphrina polystichi | CBS 379.39T | - | AF492105 | AF492049 |
| Taphrina pruni-subcordatae | CBS 381.39T | NG_062596 | MH856046 | MH867542 |
| Taphrina pruni | CBS 119537T | - | AF492111 | MH867219 |
| Taphrina robinsoniana | CBS 382.39T | NG_062597 | NR_172369 | AF492059 |
| Taphrina sadebeckii | CBS 102170T | - | NR_155882 | NG_057697 |
| Taphrina sacchari | CBS 119738T | - | AF492117 | AF492061 |
| Taphrina tormentillae | CBS 339.55T | - | NR_155883 | NG_057698 |
| Taphrina tosquinetii | CBS 276.28T | - | NR_155884 | NG_057699 |
| Taphrina ulmi | CBS 420.54T | NG_062598 | NR_155885 | NG_057700 |
| Taphrina vestergrenii | CBS 679.93T | AJ496253 | NR_155886 | NG_057701 |
| Taphrina virginica | CBS 340.55T | NG_062599 | AF492125 | MH869044 |
| Taphrina veronaerambellii | PYCC 5734T | - | NR_111148 | NG_059911 |
| Taphrina wiesneri | CBS 275.28T | - | NR_160065 | MH866479 |
| Species | Strain | GenBank Accession Number * | ||||
|---|---|---|---|---|---|---|
| SSU | ITS | D1/D2 | RPB2 | TEF1 | ||
| Goffeauzyma aciditolerans | CBS 10872T | KF036609 | NR_137808 | KY107763 | KF036746 | KF037017 |
| Goffeauzyma agrionensis | CBS 10799T | KF036611 | NR_137809 | EU627786 | KF036749 | KF037020 |
| Goffeauzyma gastrica | CBS 2288T | AB032633 | NR_111048 | KY107764 | KF036785 | KF037057 |
| Goffeauzyma gilvescens | CBS 7525T | AB032634 | NR_073228 | NG_058297 | KF036786 | KF037058 |
| Goffeauzyma iberica | CBS 10871T | KF036636 | NR_137812 | NG_058298 | KF036791 | KF037063 |
| Goffeauzyma metallitolerans | CBS 10873T | KF036639 | NR_137813 | KY107771 | KF036798 | KF037070 |
| Goffeauzyma siamensis | DMKU-PAL39T | OK576181 | MW669577 | LC604627 | LC656481 | LC656484 |
| Goffeauzyma siamensis | DMKU-PAL18 | OK576179 | MZ621116 | MZ621115 | LC656482 | LC656483 |
| Filobasidium globisporum | CBS 7642T | AB075546 | KY103422 | KY107713 | KF036890 | KF037153 |
| Filobasidium oeirense | CBS 868T | KF036644 | KF036644 | KY103438 | KY107723 | KF037076 |
| Filobasidium uniguttulatum | CBS 1730T | AB032664 | AF444302 | AF075468 | KF036891 | KF037154 |
| Naganishia albidosimilis | CBS 7711T | KF036612 | AF145325 | AF137601 | KF036750 | KF037021 |
| Naganishia liquefaciens | CBS 968T | KF036638 | AF444345 | NG_057655 | KF036794 | KF037066 |
| Naganishia globosa | CBS 1975T | KF036651 | AF444372 | AF181540 | KF036814 | KF037085 |
| Piskurozyma capsuligena | CBS 1906T | AB075544 | AF444381 | AF363642 | KF036887 | KF037152 |
| Gene/ Species | Number of Nucleotide Substitution/Nucleotide Similarity (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. inouyei NRRL YB-4354T | P. pachydermus NRRL YB-4355T | P. lactucaedebilis NRRL YB-4353T | P. macrosporus NRRL Y-12879T | P. gravidus NRRL Y-17093T | P. inundatus NRRL Y-6349T | P. arabidopsidicola C29T | T. virginica CBS 340.55T | T. wiesneri CBS 275.28T | T. letifera CBS 335.55T | T. communis CBS 352.35T | T. deformans CBS 356.35T | T. carnea CBS 332.55T | |
| D1/D2 | 56/90.49 | 54/90.50 | 55/90.33 | 53/90.63 | 66/88.88 | 52/90.81 | 55/90.33 | 65/88.98 | 65/88.98 | 63/89.26 | 64/89.13 | 66/88.42 | 65/88.98 |
| ITS | 91/80.51 | 57/87.01 | 97/79.27 | 92/80.04 | 106/77.82 | 87/80.87 | 92/79.60 | 110/77.55 | 105/78.61 | 108/78.18 | 114/77.24 | 105/78.70 | 89/81.45 |
| Characteristics | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assimilation of carbon compound | |||||||||||||||
| D-Galactose | - | - | v | - | + | - | - | - | w | w | - | w | - | - | - |
| L-Sorbose | - | - | - | - | s | - | v | - | - | - | v | - | v | v | - |
| N-Acetyl glucosamine | - | - | - | - | s | - | - | - | ND | ND | ND | ND | ND | ND | ND |
| D-Ribose | - | - | - | - | s | - | - | - | - | + | - | - | v | - | v |
| L-Arabinose | - | - | s | - | s | - | - | w | - | + | - | + | - | - | + |
| D-Arabinose | - | s | s | s | s | - | v | w | - | + | - | - | v | - | v |
| Cellobiose | s | + | s | - | s | + | v | + | + | + | + | + | v | + | + |
| Salicin | w | + | w | - | - | - | - | + | - | + | + | + | v | + | + |
| D-Gluconate | + | - | - | - | - | - | - | - | - | - | v | - | - | - | w |
| Assimilation of nitrogen compound | |||||||||||||||
| Potassium nitrate | - | s | v | + | s | + | v | ND | w/s | + | + | + | + | + | + |
| Other characteristic | |||||||||||||||
| Growth 25 °C | + | + | ND | - | + | ND | - | + | - | + | + | - | + | + | v |
| Growth 30 °C | + | ND | ND | - | ND | ND | - | - | - | - | - | - | - | - | - |
| Starch formation | - | w | w | w/- | w/- | w | + | ND | + | + | + | + | + | v | + |
| Characteristics | G. siamensis | G. iberica |
|---|---|---|
| Assimilation of carbon compound | ||
| Glycerol | + | - |
| Ribitol | + | - |
| Galactitol | w | - |
| D-Gluconate | s | - |
| DL-Lactate | + | - |
| Citrate | - | + |
| Other characteristics | ||
| 0.01% Cycloheximide | - | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nutaratat, P.; Boontham, W.; Khunnamwong, P. A Novel Yeast Genus and Two Novel Species Isolated from Pineapple Leaves in Thailand: Savitreella phatthalungensis gen. nov., sp. nov. and Goffeauzyma siamensis sp. nov. J. Fungi 2022, 8, 118. https://doi.org/10.3390/jof8020118
Nutaratat P, Boontham W, Khunnamwong P. A Novel Yeast Genus and Two Novel Species Isolated from Pineapple Leaves in Thailand: Savitreella phatthalungensis gen. nov., sp. nov. and Goffeauzyma siamensis sp. nov. Journal of Fungi. 2022; 8(2):118. https://doi.org/10.3390/jof8020118
Chicago/Turabian StyleNutaratat, Pumin, Wanatchaporn Boontham, and Pannida Khunnamwong. 2022. "A Novel Yeast Genus and Two Novel Species Isolated from Pineapple Leaves in Thailand: Savitreella phatthalungensis gen. nov., sp. nov. and Goffeauzyma siamensis sp. nov." Journal of Fungi 8, no. 2: 118. https://doi.org/10.3390/jof8020118






