Engineered Fungus Thermothelomyces thermophilus Producing Plant Storage Proteins
Abstract
:1. Introduction
2. Materials and Methods
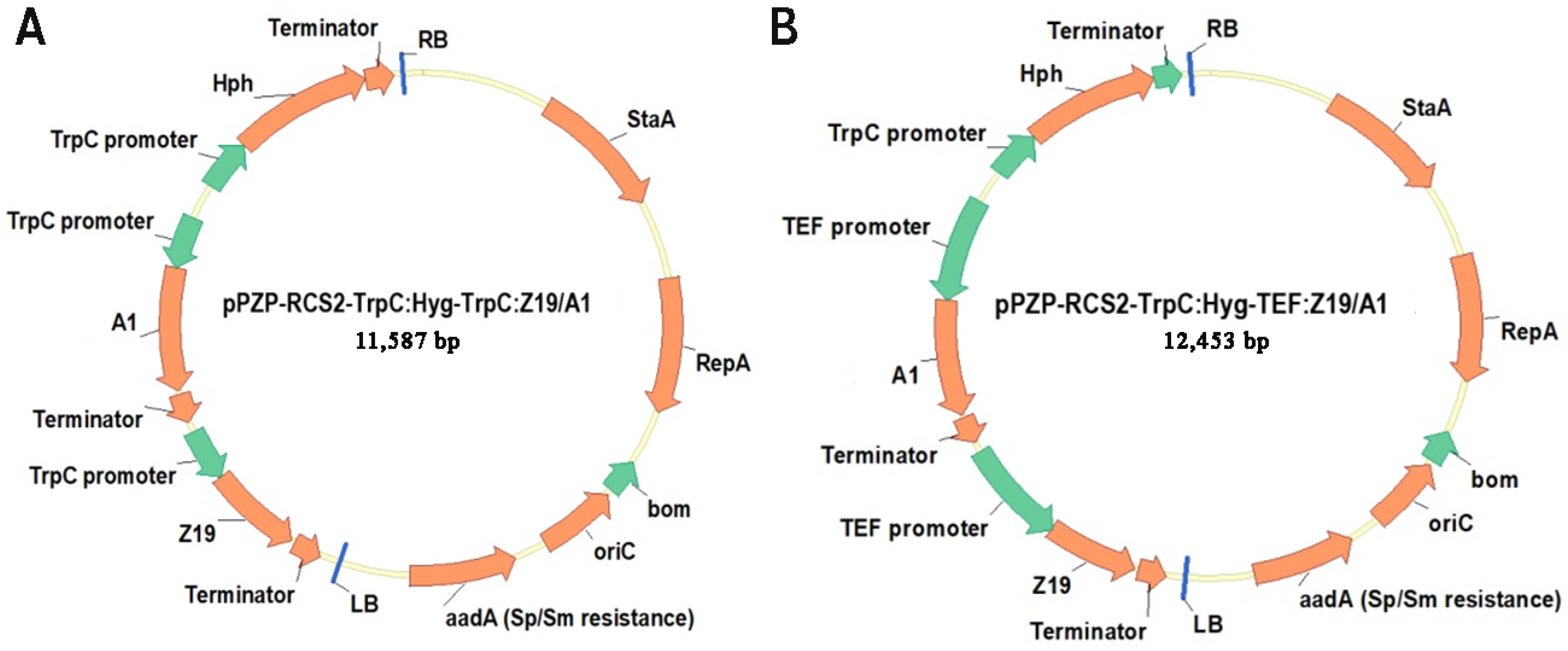
2.1. Gene Cloning and Vector Construction
2.2. Transformation of Th. thermophilus Mediated by Agrobacterium tumefaciens
2.3. Real-Time PCR Analysis
2.4. Protein Isolation and Analysis
2.5. Western Blotting
2.6. Determination of Amino Acid Composition
2.7. Microscopy
2.8. Statistical Analysis
3. Results and Discussions
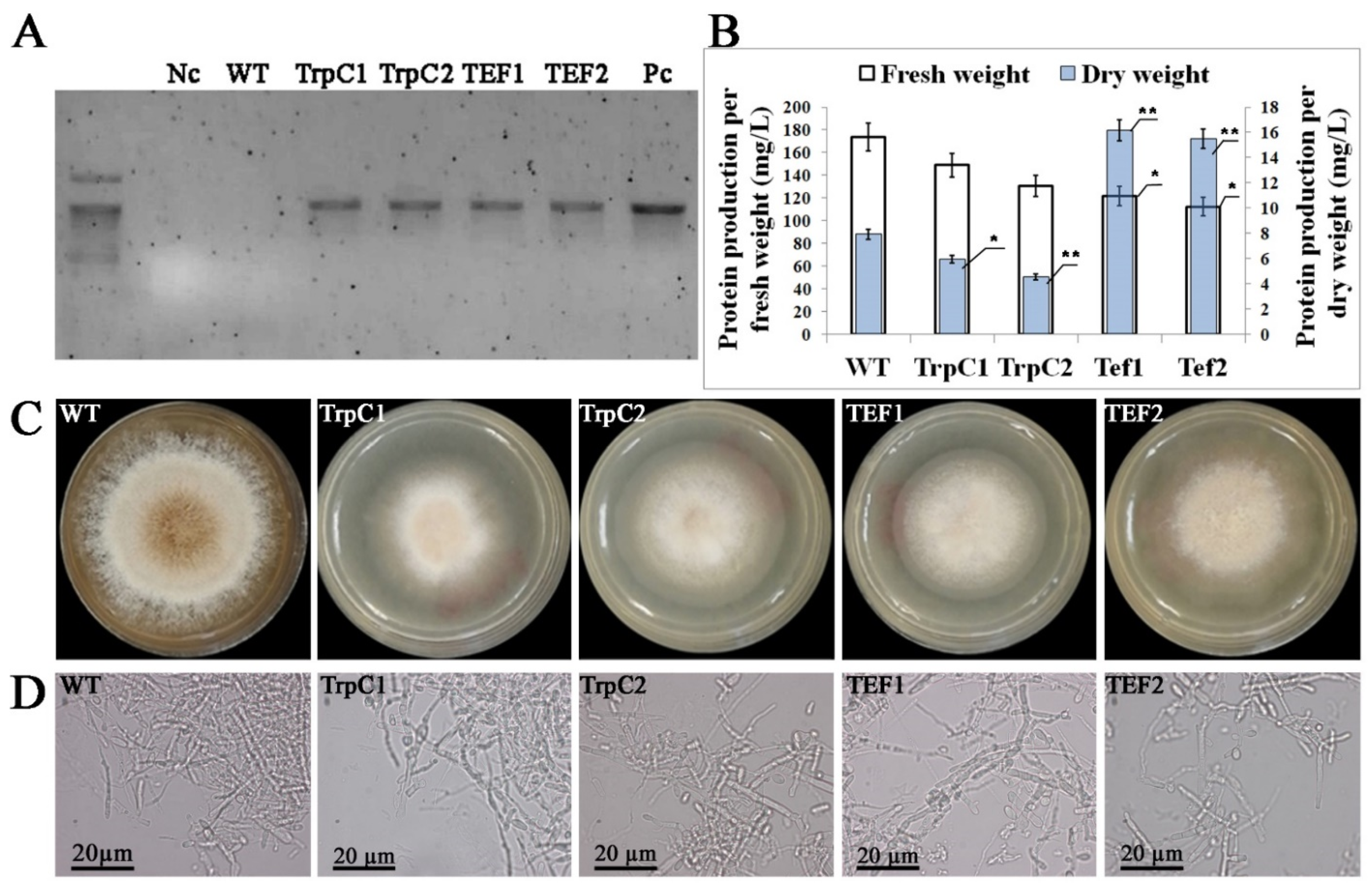
3.1. Characterization of Transgenic Th. thermophilus rF-859
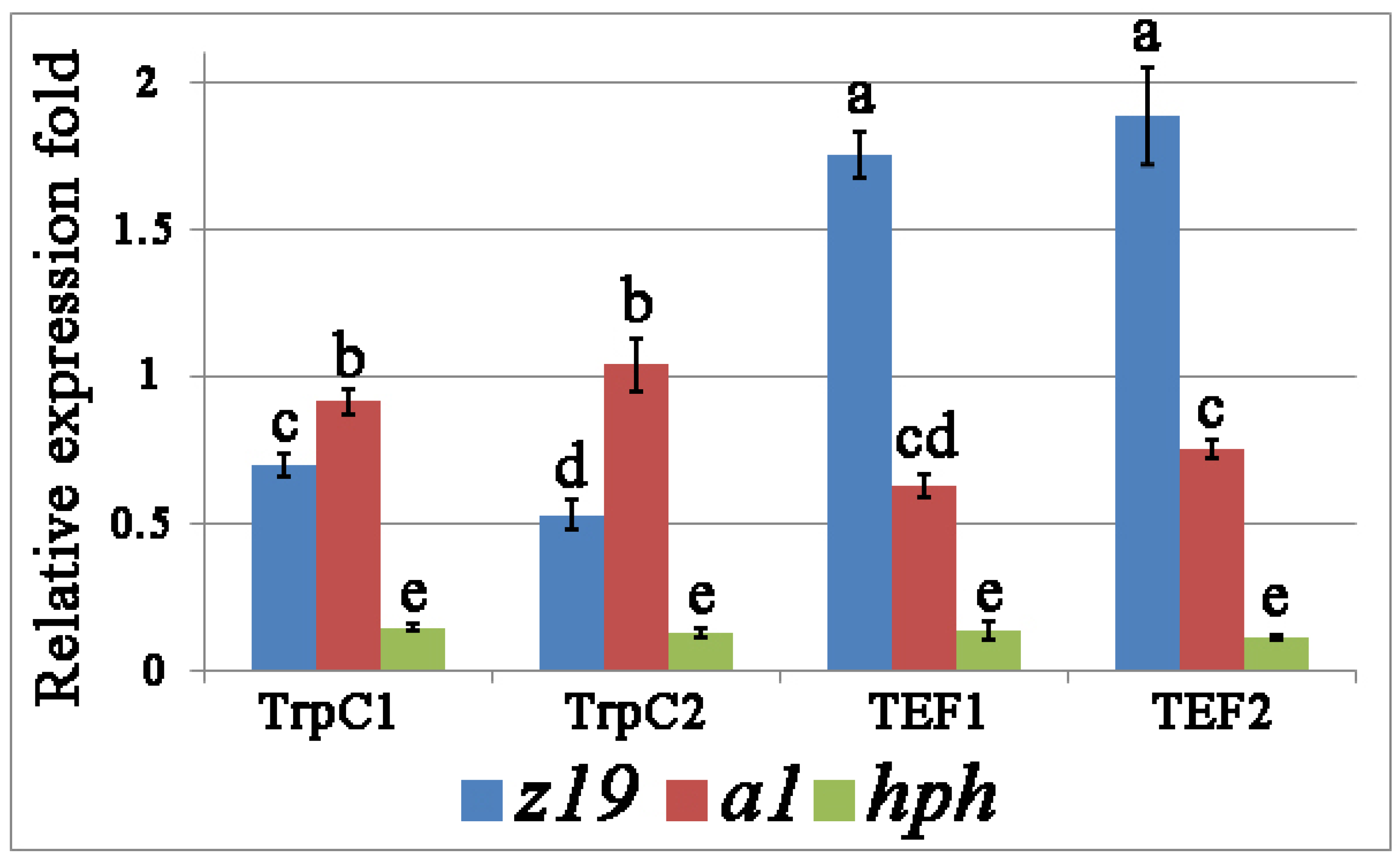
3.2. Heterologous Expression of z19 and a1 in Transgenic Strains Th. thermophilus rF-859
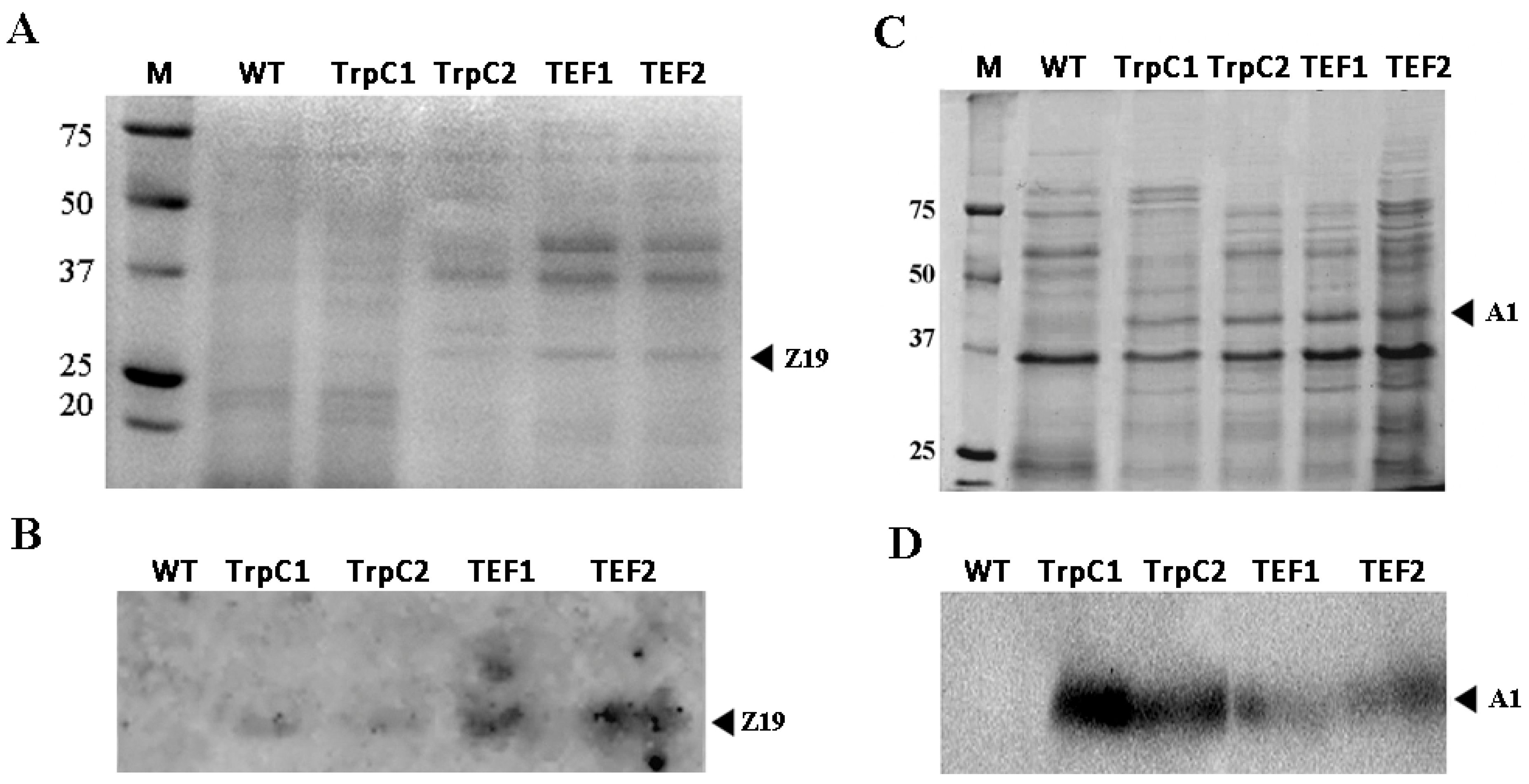
3.3. Accumulation of Plant Storage Proteins in Th. thermophilus rF-859 Strains
3.4. Amino Acid Profile of Th. thermophilus rF-859
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parisi, G.; Tulli, F.; Fortina, R.; Marino, R.; Bani, P.; Dalle Zotte, A.; De Angelis, A.; Piccolo, G.; Pinotti, L.; Schiavone, A.; et al. Protein Hunger of the Feed Sector: The Alternatives Offered by the Plant World. Ital. J. Anim. Sci. 2020, 19, 1204–1225. [Google Scholar] [CrossRef]
- Zhang, S.; Qiao, M.; Trottier, N.L. Feeding a Reduced Protein Diet with a near Ideal Amino Acid Profile Improves Amino Acid Efficiency and Nitrogen Utilization for Milk Production in Sows. J. Anim. Sci. 2019, 97, 3882–3897. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S. Protein Byproducts. Transformation from Environmental Burden into Value-Added Products; Dhillon, G.S., Ed.; Academic Press: Camridge, MA, USA, 2016; ISBN 978-0-12-802391-4. [Google Scholar]
- Li, M.H.; Robinson, E.H.; Bosworth, B.G.; Oberle, D.F.; Lucas, P.M. Use of Corn Gluten Feed and Cottonseed Meal to Replace Soybean Meal and Corn in Diets for Pond-Raised Channel Catfish. N. Am. J. Aquac. 2011, 73, 153–158. [Google Scholar] [CrossRef]
- Schweiggert-Weisz, U.; Eisner, P.; Bader-Mittermaier, S.; Osen, R. Food Proteins from Plants and Fungi. Curr. Opin. Food Sci. 2020, 32, 156–162. [Google Scholar] [CrossRef]
- Loy, D.D.; Lundy, E.L. Chapter 23—Nutritional Properties and Feeding Value of Corn and Its Coproducts. In Corn, 3rd ed.; AACC International Press: Oxford, UK, 2019; pp. 633–659. ISBN 978-0-12-811971-6. [Google Scholar]
- Gibbon, B.C.; Larkins, B.A. Molecular Genetic Approaches to Developing Quality Protein Maize. Trends Genet. 2005, 21, 227–233. [Google Scholar] [CrossRef]
- Sánchez-López, F.; Robles-Olvera, V.J.; Hidalgo-Morales, M.; Tsopmo, A. Characterization of Amaranthus hypochondriacus Seed Protein Fractions, and Their Antioxidant Activity after Hydrolysis with Lactic Acid Bacteria. J. Cereal Sci. 2020, 95, 103075. [Google Scholar] [CrossRef]
- Manyelo, T.G.; Sebola, N.A.; van Rensburg, E.J.; Mabelebele, M. The Probable Use of Genus Amaranthus as Feed Material for Monogastric Animals. Animals 2020, 10, 1504. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Chakraborty, N.; Datta, A. Increased Nutritive Value of Transgenic Potato by Expressing a Nonallergenic Seed Albumin Gene from Amaranthus hypochondriacus. Proc. Natl. Acad. Sci. USA 2000, 97, 3724–3729. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Agrawal, L.; Mishra, D.; Buragohain, A.K.; Unnikrishnan, M.; Mohan, C.; Chakraborty, S.; Chakraborty, N. Ectopic Expression of Amaranth Seed Storage Albumin Modulates Photoassimilate Transport and Nutrient Acquisition in Sweetpotato. Sci. Rep. 2016, 6, 25384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Milgen, J.; Dourmad, J.-Y. Concept and Application of Ideal Protein for Pigs. J. Anim. Sci. Biotechnol. 2015, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Sari, Y.W. Biomass and Its Potential for Protein and Amino Acids: Valorizing Agricultural By-Products; Wageningen University: Wageningen, The Netherlands, 2015; p. 152. [Google Scholar]
- Sarnklong, C.; Cone, J.; Pellikaan, W.F.; Hendriks, W. Utilization of Rice Straw and Different Treatments to Improve Its Feed Value for Ruminants: A Review. Anim. Biosci. 2010, 23, 680–692. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein—State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef] [PubMed]
- Ntana, F.; Mortensen, U.H.; Sarazin, C.; Figge, R. Aspergillus: A Powerful Protein Production Platform. Catalysts 2020, 10, 1064. [Google Scholar] [CrossRef]
- Karimi, S.; Mahboobi Soofiani, N.; Lundh, T.; Mahboubi, A.; Kiessling, A.; Taherzadeh, M.J. Evaluation of Filamentous Fungal Biomass Cultivated on Vinasse as an Alternative Nutrient Source of Fish Feed: Protein, Lipid, and Mineral Composition. Fermentation 2019, 5, 99. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.E. Protein Hyperproduction in Fungi by Design. Appl. Microbiol. Biotechnol. 2018, 102, 8621–8628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnau, J.; Yaver, D.; Hjort, C.M. Strategies and Challenges for the Development of Industrial Enzymes Using Fungal Cell Factories. In Grand Challenges in Fungal Biotechnology; Nevalainen, H., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 179–210. ISBN 978-3-030-29541-7. [Google Scholar]
- Wang, Q.; Zhong, C.; Xiao, H. Genetic Engineering of Filamentous Fungi for Efficient Protein Expression and Secretion. Front. Bioeng. Biotechnol. 2020, 8, 293. [Google Scholar] [CrossRef]
- Brancoli, P.; Gmoser, R.; Taherzadeh, M.J.; Bolton, K. The Use of Life Cycle Assessment in the Support of the Development of Fungal Food Products from Surplus Bread. Fermentation 2021, 7, 173. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Lin, L.; Liu, Q.; Sun, W.; Huang, B.; Tian, C. Development of Genetic Tools for Myceliophthora thermophila. BMC Biotechnol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Gao, R.; Li, J.; Lin, L.; Zhao, J.; Sun, W.; Tian, C. Development of a Genome-Editing CRISPR/Cas9 System in Thermophilic Fungal Myceliophthora Species and Its Application to Hyper-Cellulase Production Strain Engineering. Biotechnol. Biofuels 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, Y.; Li, J.; Sun, T.; Tian, C. Metabolic Engineering of the Cellulolytic Thermophilic Fungus Myceliophthora thermophila to Produce Ethanol from Cellobiose. Biotechnol. Biofuels 2020, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Kern, A.; Shanahan, D.; Buesen, R.; Geiger, D. Safety Evaluation of a β-Mannanase Enzyme Preparation Produced with Thermothelomyces thermophilus Expressing a Protein-Engineered β-Mannanase Gene. PLoS ONE 2020, 15, e0243647. [Google Scholar] [CrossRef] [PubMed]
- Visser, H.; Joosten, V.; Punt, P.J.; Gusakov, A.V.; Olson, P.T.; Joosten, R.; Bartels, J.; Visser, J.; Sinitsyn, A.P.; Emalfarb, M.A.; et al. Development of a Mature Fungal Technology and Production Platform for Industrial Enzymes Based on a Myceliophthora thermophila Isolate, Previously Known as Chrysosporium lucknowense C1. Ind. Biotechnol. 2011, 7, 214–223. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Veremeichik, G.N.; Makhazen, D.S.; Silantieva, S.A.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; Bulgakov, V.P. Increase of Anthraquinone Content in Rubia cordifolia Cells Transformed by Native and Constitutively Active Forms of the AtCPK1 Gene. Plant Cell Rep. 2016, 35, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Balabanova, L.A.; Shkryl, Y.N.; Slepchenko, L.V.; Yugay, Y.A.; Gorpenchenko, T.Y.; Kirichuk, N.N.; Khudyakova, Y.V.; Bakunina, I.Y.; Podvolotskaya, A.B.; Bulgakov, V.P.; et al. Development of Host Strains and Vector System for an Efficient Genetic Transformation of Filamentous Fungi. Plasmid 2019, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tzfira, T.; Tian, G.-W.; Lacroix, B.; Vyas, S.; Li, J.; Leitner-Dagan, Y.; Krichevsky, A.; Taylor, T.; Vainstein, A.; Citovsky, V. PSAT Vectors: A Modular Series of Plasmids for Autofluorescent Protein Tagging and Expression of Multiple Genes in Plants. Plant Mol. Biol. 2005, 57, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Veremeichik, G.N.; Shkryl, Y.N. The rolB Gene Activates the Expression of Genes Encoding MicroRNA Processing Machinery. Biotechnol. Lett. 2015, 37, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Hood, E.E.; Gelvin, S.B.; Melchers, L.S.; Hoekema, A. New Agrobacterium Helper Plasmids for Gene Transfer to Plants. Transgenic Res. 1993, 2, 208–218. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fic, E.; Kedracka-Krok, S.; Jankowska, U.; Pirog, A.; Dziedzicka-Wasylewska, M. Comparison of Protein Precipitation Methods for Various Rat Brain Structures Prior to Proteomic Analysis. Electrophoresis 2010, 31, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.-M.; Hu, D.W.-N.; Larkins, B.A.; Jung, R. Genomics Analysis of Genes Expressed in Maize Endosperm Identifies Novel Seed Proteins and Clarifies Patterns of Zein Gene Expression. Plant Cell 2001, 13, 2297–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisarikova, B.; Kracmar, S.; Herzig, I. Amino Acid Contents and Biological Value of Protein in Various Amaranth Species. Czech J. Anim. Sci. 2005, 50, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Rantasalo, A.; Landowski, C.P.; Kuivanen, J.; Korppoo, A.; Reuter, L.; Koivistoinen, O.; Valkonen, M.; Penttilä, M.; Jäntti, J.; Mojzita, D. A Universal Gene Expression System for Fungi. Nucleic Acids Res. 2018, 46, e111. [Google Scholar] [CrossRef] [PubMed]
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wöstena, H.A.B. Fabrication Factors Influencing Mechanical, Moisture- and Water-Related Properties of Mycelium-Based Composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; De Laet, L. Mechanical, Physical and Chemical Characterisation of Mycelium-Based Composites with Different Types of Lignocellulosic Substrates. PLoS ONE 2019, 14, e0213954. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.; Charles, T.C. The Variation of Promoter Strength in Different Gene Contexts. bioRxiv 2020. [Google Scholar] [CrossRef]
- Duzenli, O.F.; Okay, S.; Duzenli, O.F.; Okay, S. Promoter Engineering for the Recombinant Protein Production in Prokaryotic Systems. AIMS Bioeng. 2020, 7, 62–81. [Google Scholar] [CrossRef]
- He, X.; Fütterer, J.; Hohn, T. Contribution of Downstream Promoter Elements to Transcriptional Regulation of the Rice Tungro Bacilliform Virus Promoter. Nucleic Acids Res. 2002, 30, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Wenz, P.; Schwank, S.; Hoja, U.; Schüller, H.J. A Downstream Regulatory Element Located within the Coding Sequence Mediates Autoregulated Expression of the Yeast Fatty Acid Synthase Gene FAS2 by the FAS1 Gene Product. Nucleic Acids Res. 2001, 29, 4625–4632. [Google Scholar] [CrossRef]
- Lee, N.; Iyer, S.S.; Mu, J.; Weissman, J.D.; Ohali, A.; Howcroft, T.K.; Lewis, B.A.; Singer, D.S. Three Novel Downstream Promoter Elements Regulate MHC Class I Promoter Activity in Mammalian Cells. PLoS ONE 2010, 5, e15278. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.D.; Brown, A.J.; Zhu, J.; Rosignoli, G.; Gibson, S.J.; Hatton, D.; James, D.C. Control of Multigene Expression Stoichiometry in Mammalian Cells Using Synthetic Promoters. ACS Synth. Biol. 2021, 10, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Tokuoka, M.; Tanaka, M.; Ono, K.; Takagi, S.; Shintani, T.; Gomi, K. Codon Optimization Increases Steady-State MRNA Levels in Aspergillus oryzae Heterologous Gene Expression. Appl. Environ. Microbiol. 2008, 74, 6538–6546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, C.E.; Herman, E.M.; Takasaki, K.; Larkins, B.A. The Maize Gamma-Zein Sequesters Alpha-Zein and Stabilizes Its Accumulation in Protein Bodies of Transgenic Tobacco Endosperm. Plant Cell 1996, 8, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Bharadwaj, G.; Bhat, M.K. Thermophilic Fungi: Their Physiology and Enzymes. Microbiol. Mol. Biol. Rev. 2000, 64, 461–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rendsvig, J.K.H.; Workman, C.T.; Hoof, J.B. Bidirectional Histone-Gene Promoters in Aspergillus: Characterization and Application for Multi-Gene Expression. Fungal Biol. Biotechnol. 2019, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Aladdin, A.; Sahly, N.; Faty, R.; Youssef, M.M.; Salem, T.Z. The Baculovirus Promoter OpIE2 Sequence Has Inhibitory Effect on the Activity of the Cytomegalovirus (CMV) Promoter in HeLa and HEK-293 T Cells. Gene 2021, 781, 145541. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębowska, K.; Gabriel, I. Inhibitors of Amino Acids Biosynthesis as Antifungal Agents. Amino Acids 2015, 47, 227–249. [Google Scholar] [CrossRef] [Green Version]
- Bischof, R.H.; Horejs, J.; Metz, B.; Gamauf, C.; Kubicek, C.P.; Seiboth, B. L-Methionine Repressible Promoters for Tuneable Gene Expression in Trichoderma reesei. Microb. Cell Fact. 2015, 14, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nai, Y.-S.; Huang, Y.-C.; Yen, M.-R.; Chen, P.-Y. Diversity of Fungal DNA Methyltransferases and Their Association with DNA Methylation Patterns. Front. Microbiol. 2021, 11, 3614. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, L.G.; Sakomura, N.K.; Suzuki, R.M.; Dorigam, J.C.P.; Viana, G.S.; Van Milgen, J.; Denadai, J.C. Methionine to Cystine Ratio in the Total Sulfur Amino Acid Requirements and Sulfur Amino Acid Metabolism Using Labelled Amino Acid Approach for Broilers. BMC Vet. Res. 2018, 14, 364. [Google Scholar] [CrossRef]
- Atri, N.S. Amino Acid Profile of a Basidiomycetous Edible Mushroom—Lentinus sajor-caju. Int. J. Pharm. Pharm. Sci. 2017, 9, 252–257. [Google Scholar] [CrossRef]
- Tan, J.; Sastry, A.V.; Fremming, K.S.; Bjørn, S.P.; Hoffmeyer, A.; Seo, S.; Voldborg, B.G.; Palsson, B.O. Independent Component Analysis of E. Coli’s Transcriptome Reveals the Cellular Processes That Respond to Heterologous Gene Expression. Metab. Eng. 2020, 61, 360–368. [Google Scholar] [CrossRef] [PubMed]
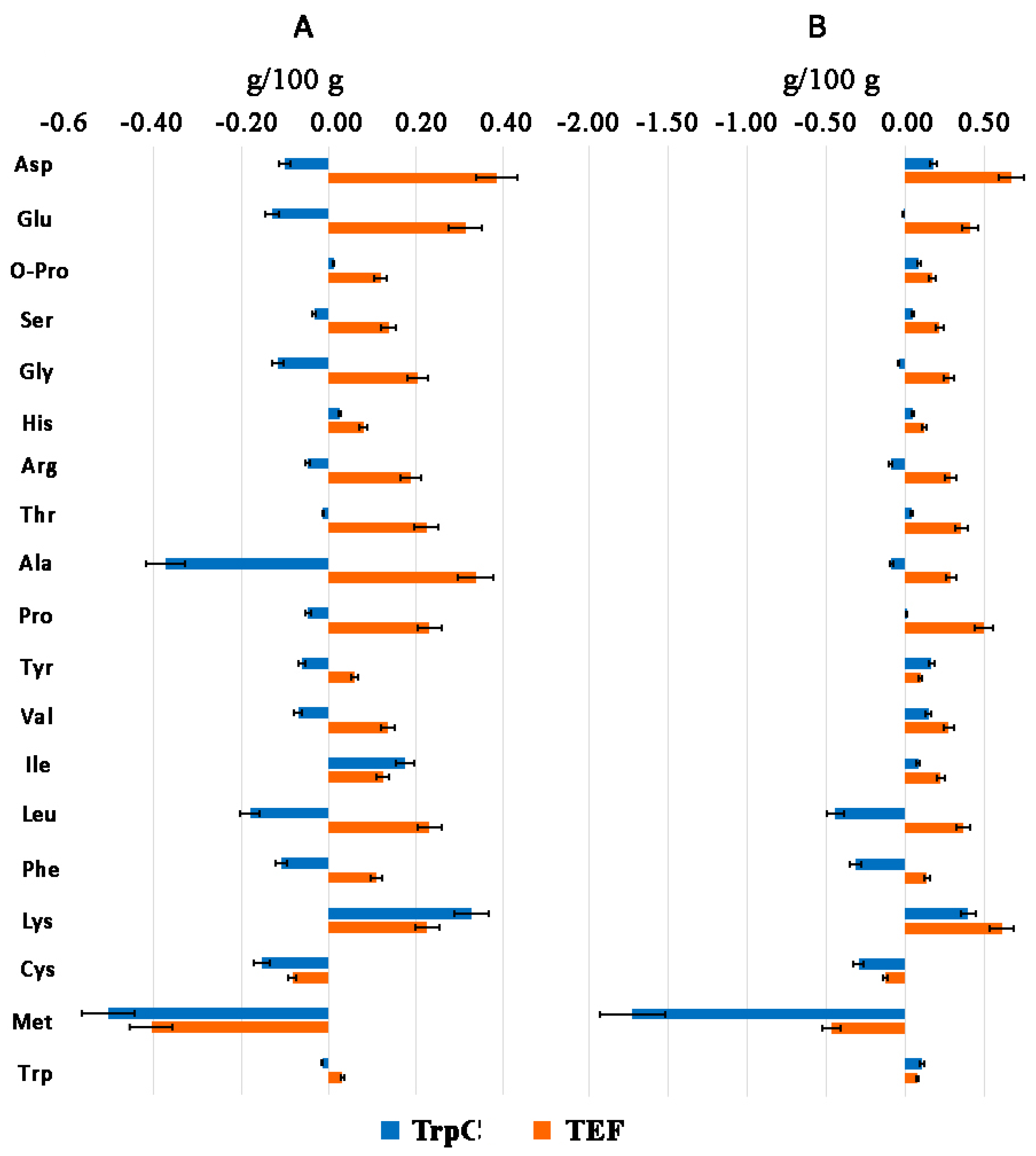
| Amino Acids | g/kg DW | % of Total AA Content | |||||
|---|---|---|---|---|---|---|---|
| Wild Type * | TrpC * | TEF * | AO/FM/SBM ** | Wild Type * | TrpC * | TEF * | |
| Essential Amino Acids | |||||||
| Histidine | 6.7 ± 0.8 | 7.2 ± 0.9 | 7.9 ± 0.9 | 7.51/15.9/11.5 | 2.63 | 3.02 | 2.64 |
| Isoleucine | 9.2 ± 1.1 | 10.0 ± 1.2 | 11.4 ± 1.4 | 13.8/25.0/19.9 | 3.60 | 4.21 | 3.82 |
| Leucine | 14.8 ± 1.8 | 10.4 ± 1.2 | 18.5 ± 2.2 | 24.6/44.5/31.9 | 5.82 | 4.37 | 6.16 |
| Lysine | 12.3 ± 1.5 | 16.4 ± 2.1 | 18.5 ± 2.2 | 21.4/46.4/26.6 | 4.86 | 6.88 | 6.16 |
| Methionine | 64.0 ± 7.7 | 46.7 ± 5.6 | 59.3 ± 7.1 | 5.66/16.3/6.2 | 25.17 | 19.66 | 19.80 |
| Phenylalanine | 9.2 ± 1.1 | 6.1 ± 0.7 | 10.6 ± 1.3 | 14.2/24.3/21.7 | 3.60 | 2.55 | 3.52 |
| Threonine | 10.9 ± 1.4 | 11.4 ± 1.5 | 14.5 ± 1.7 | 16.9/25.6/17.7 | 4.30 | 4.78 | 4.84 |
| Tryptophan | 3.2 ± 0.5 | 4.3 ± 0.7 | 4.0 ± 0.7 | - | 1.27 | 1.82 | 1.34 |
| Valine | 14.4 ± 1.7 | 15.9 ± 1.9 | 17.1 ± 2.1 | 17.2/30.0/20.8 | 5.66 | 6.68 | 5.72 |
| Non-Essential Amino Acids | |||||||
| Arginine | 9.9 ± 1.4 | 9.0 ± 1.3 | 12.8 ± 1.8 | 19.9/38.2/32.0 | 3.88 | 3.77 | 4.26 |
| Alanin | 27.9 ± 3.3 | 27.0 ± 3.2 | 30.8 ± 3.7 | 23.7/39.7/19.0 | 10.96 | 11.36 | 10.28 |
| Aspartic acid | 14.8 ± 1.9 | 16.6 ± 2.2 | 21.5 ± 2.8 | - | 5.82 | 6.99 | 7.19 |
| Cystein | 8.8 ± 1.1 | 5.8 ± 0.8 | 7.6 ± 1.0 | 2.75/5.1/0.5 | 3.45 | 2.46 | 2.53 |
| Glutamic acid | 9.5 ± 1.1 | 9.4 ± 1.1 | 13.6 ± 1.6 | 43.3/77.3/77.0 | 3.75 | 3.96 | 4.55 |
| Glycin | 7.7 ± 0.9 | 7.4 ± 0.9 | 10.6 ± 1.3 | 17.2/48.0/18.1 | 3.05 | 3.10 | 3.52 |
| Hydroxyprolin | 5.3 ± 1.0 | 6.2 ± 1.1 | 7.0 ± 1.3 | - | 2.08 | 2.60 | 2.35 |
| Proline | 13.1 ± 1.6 | 13.2 ± 1.6 | 18.0 ± 2.2 | 18.5/28.3/21.6 | 5.13 | 5.53 | 6.02 |
| Serine | 7.0 ± 0.8 | 7.5 ± 0.9 | 9.2 ± 1.1 | 17.2/24.5/21.8 | 2.77 | 3.18 | 3.08 |
| Tyrosine | 5.6 ± 0.7 | 7.3 ± 0.9 | 6.6 ± 0.8 | 12.8/19.0/14.6 | 2.21 | 3.07 | 2.20 |
| Total amino acids | 254.2 | 237.8 | 299.5 | 168.7/299.6/221.6 | 100 | 100 | 100 |
| Nutritional Quality * | Results | ||
|---|---|---|---|
| Wild Type | TrpC | TEF | |
| Total Amino Acid (TAA), % | 25.42 | 23.78 | 29.95 |
| Total Essential Amino Acid/Total Non-Essential Amino Acid | 1.32 | 1.17 | 1.17 |
| Total Sulphur Amino Acid (TSAA = Meth + Cys), % | 7.28 | 5.25 | 6.69 |
| Total Aromatic Essential Amino Acid (TArEAA = Phe + Tyr), % | 1.48 | 1.37 | 1.72 |
| Total Essential Amino Acids, % | 56.91 | 53.97 | 54.00 |
| Total Non-Essential Amino Acid (TNEAA)/Total Amino Acid (TAA) | 43.10 | 46.02 | 45.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balabanova, L.; Seitkalieva, A.; Yugay, Y.; Rusapetova, T.; Slepchenko, L.; Podvolotskaya, A.; Yatsunskaya, M.; Vasyutkina, E.; Son, O.; Tekutyeva, L.; et al. Engineered Fungus Thermothelomyces thermophilus Producing Plant Storage Proteins. J. Fungi 2022, 8, 119. https://doi.org/10.3390/jof8020119
Balabanova L, Seitkalieva A, Yugay Y, Rusapetova T, Slepchenko L, Podvolotskaya A, Yatsunskaya M, Vasyutkina E, Son O, Tekutyeva L, et al. Engineered Fungus Thermothelomyces thermophilus Producing Plant Storage Proteins. Journal of Fungi. 2022; 8(2):119. https://doi.org/10.3390/jof8020119
Chicago/Turabian StyleBalabanova, Larissa, Aleksandra Seitkalieva, Yulia Yugay, Tatiana Rusapetova, Lubov Slepchenko, Anna Podvolotskaya, Margarita Yatsunskaya, Elena Vasyutkina, Oksana Son, Liudmila Tekutyeva, and et al. 2022. "Engineered Fungus Thermothelomyces thermophilus Producing Plant Storage Proteins" Journal of Fungi 8, no. 2: 119. https://doi.org/10.3390/jof8020119
APA StyleBalabanova, L., Seitkalieva, A., Yugay, Y., Rusapetova, T., Slepchenko, L., Podvolotskaya, A., Yatsunskaya, M., Vasyutkina, E., Son, O., Tekutyeva, L., & Shkryl, Y. (2022). Engineered Fungus Thermothelomyces thermophilus Producing Plant Storage Proteins. Journal of Fungi, 8(2), 119. https://doi.org/10.3390/jof8020119






