In Silico Study of Natural Polyphenols as Potential Metabolic Modulators in Mitigating Lipotoxicity in Non-Alcoholic Fatty Liver Disease via Thyroid Hormone Receptor Alpha Activation
Abstract
1. Introduction
2. Materials and Methods
3. Results
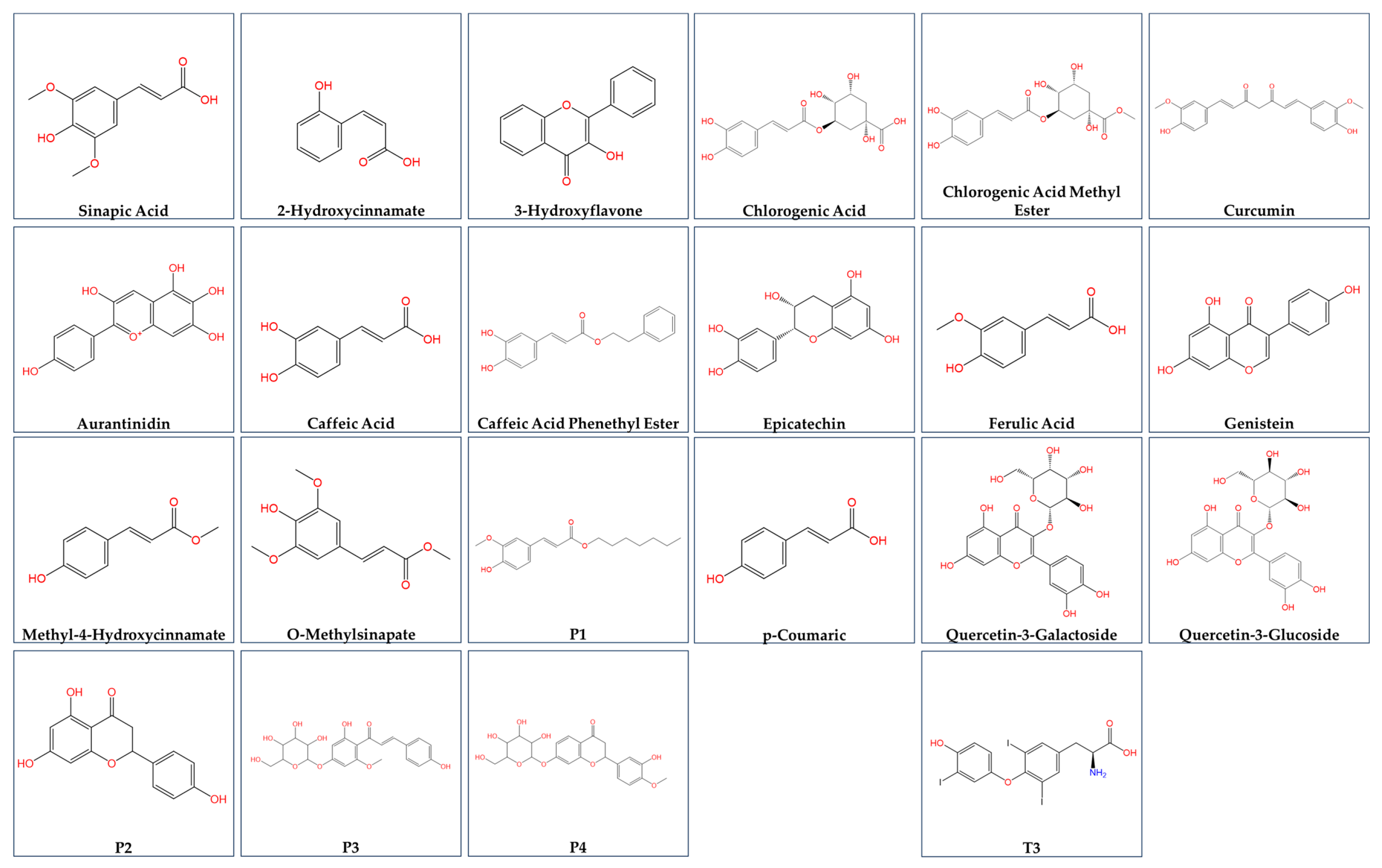
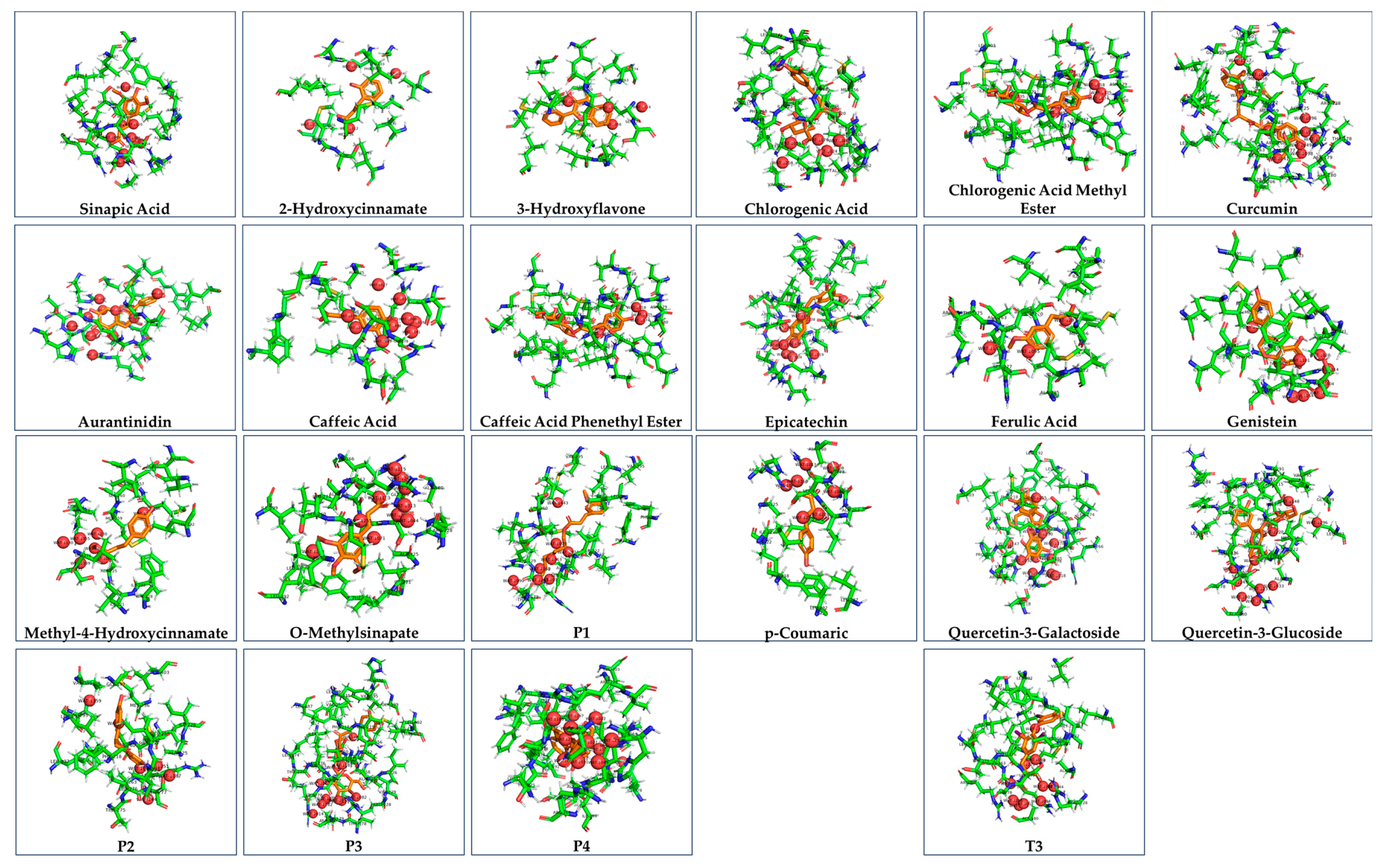

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yin, X.; Liu, Z.; Wang, J. Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 15489. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Cable, E.E.; Finn, P.D.; Stebbins, J.W.; Hou, J.; Ito, B.R.; van Poelje, P.D.; Linemeyer, D.L.; Erion, M.D. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology 2009, 49, 407–417. [Google Scholar] [CrossRef]
- Schoenmakers, N. Genetic Causes of Congenital Hypothyroidism. Encycl. Endocr. Dis. 2019, 42, 379–389. [Google Scholar]
- Fernández-Real, J.M.; Corella, D.; Goumidi, L.; Mercader, J.M.; Valdés, S.; Rojo Martínez, G.; Ortega, F.; Martinez-Larrad, M.T.; Gómez-Zumaquero, J.M.; Salas-Salvadó, J.; et al. Thyroid hormone receptor alpha gene variants increase the risk of developing obesity and show gene-diet interactions. Int. J. Obes. (2005) 2013, 37, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Cevallos, P.; Murúa-Beltrán Gall, S.; Uribe, M.; Chávez-Tapia, N.C. Understanding the Relationship between Nonalcoholic Fatty Liver Disease and Thyroid Disease. Int. J. Mol. Sci. 2023, 24, 14605. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xie, H.; Shan, H.; Zheng, Z.; Li, G.; Li, M.; Hong, L. Development of Thyroid Hormones and Synthetic Thyromimetics in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 1102. [Google Scholar] [CrossRef]
- Damiano, F.; Rochira, A.; Gnoni, A.; Siculella, L. Action of Thyroid Hormones, T3 and T2, on Hepatic Fatty Acids: Differences in Metabolic Effects and Molecular Mechanisms. Int. J. Mol. Sci. 2017, 18, 744. [Google Scholar] [CrossRef]
- Paisdzior, S.; Knierim, E.; Kleinau, G.; Biebermann, H.; Krude, H.; Straussberg, R.; Schuelke, M. A New Mechanism in THRA Resistance: The First Disease-Associated Variant Leading to an Increased Inhibitory Function of THRA2. Int. J. Mol. Sci. 2021, 22, 5338. [Google Scholar] [CrossRef]
- Park, S.W.; Li, G.; Lin, Y.P.; Barrero, M.J.; Ge, K.; Roeder, R.G.; Wei, L.N. Thyroid hormone-induced juxtaposition of regulatory elements/factors and chromatin remodeling of Crabp1 dependent on MED1/TRAP220. Mol. Cell 2005, 19, 643–653. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 2024, 52, D368–D375. [Google Scholar] [CrossRef] [PubMed]
- Seok, C.; Baek, M.; Steinegger, M.; Park, H.; Lee, G.R.; Won, J. Accurate protein structure prediction: What comes next? BioDesign 2021, 9, 47–50. [Google Scholar] [CrossRef]
- Heo, L.; Park, S.; Seok, C. GalaxyWater-wKGB: Prediction of Water Positions on Protein Structure Using wKGB Statistical Potential. J. Chem. Inf. Model. 2021, 61, 2283–2293. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- O'Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef]
- Lee, G.R.; Seok, C. Galaxy7TM: Flexible GPCR-ligand docking by structure refinement. Nucleic Acids Res. 2016, 44, W502–W506. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Ghoorah, A.W.; Devignes, M.-D.; Smaïl-Tabbone, M.; Ritchie, D.W. Protein docking using case-based reasoning. Proteins: Struct. Funct. Bioinform. 2013, 81, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Bender, B.J.; Gahbauer, S.; Luttens, A.; Lyu, J.; Webb, C.M.; Stein, R.M.; Fink, E.A.; Balius, T.E.; Carlsson, J.; Irwin, J.J.; et al. A practical guide to large-scale docking. Nat. Protoc. 2021, 16, 4799–4832. [Google Scholar] [CrossRef]
- Ko, J.; Park, H.; Seok, C. GalaxyTBM: Template-based modeling by building a reliable core and refining unreliable local regions. BMC Bioinform. 2012, 13, 198. [Google Scholar] [CrossRef]
- Bugnon, M.; Röhrig, U.F.; Goullieux, M.; Perez Marta, A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-García, B.; Pons, C.; Fernández-Recio, J. pyDockWEB: A web server for rigid-body protein-protein docking using electrostatics and desolvation scoring. Bioinformatics 2013, 29, 1698–1699. [Google Scholar] [CrossRef]
- Korlepara, D.B.; Vasavi, C.S.; Jeurkar, S.; Pal, P.K.; Roy, S.; Mehta, S.; Sharma, S.; Kumar, V.; Muvva, C.; Sridharan, B.; et al. PLAS-5k: Dataset of Protein-Ligand Affinities from Molecular Dynamics for Machine Learning Applications. Sci. Data 2022, 9, 548. [Google Scholar] [CrossRef]
- Allam, A.E.; Assaf, H.K.; Hassan, H.A.; Shimizu, K.; Elshaier, Y.A.M.M. An in silico perception for newly isolated flavonoids from peach fruit as privileged avenue for a countermeasure outbreak of COVID-19. RSC Adv. 2020, 10, 29983–29998. [Google Scholar] [CrossRef]
- Byrnes, K.; Blessinger, S.; Bailey, N.T.; Scaife, R.; Liu, G.; Khambu, B. Therapeutic regulation of autophagy in hepatic metabolism. Acta Pharm. Sin. B 2022, 12, 33–49. [Google Scholar] [CrossRef]
- Hu, L.; Gu, Y.; Liang, J.; Ning, M.; Yang, J.; Zhang, Y.; Qu, H.; Yang, Y.; Leng, Y.; Zhou, B. Discovery of Highly Potent and Selective Thyroid Hormone Receptor β Agonists for the Treatment of Nonalcoholic Steatohepatitis. J. Med. Chem. 2023, 66, 3284–3300. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Lee, H.Y.; Jurczak, M.J.; Alves, T.C.; Guebre-Egziabher, F.; Guigni, B.A.; Zhang, D.; Samuel, V.T.; Silva, J.E.; Shulman, G.I. Thyroid hormone receptor-α gene knockout mice are protected from diet-induced hepatic insulin resistance. Endocrinology 2012, 153, 583–591. [Google Scholar] [CrossRef]
- Singh, B.K.; Sinha, R.A.; Yen, P.M. Novel Transcriptional Mechanisms for Regulating Metabolism by Thyroid Hormone. Int. J. Mol. Sci. 2018, 19, 3284. [Google Scholar] [CrossRef]
- Zucchi, R. Thyroid Hormone Analogues: An Update. Thyroid Off. J. Am. Thyroid Assoc. 2020, 30, 1099–1105. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, Q.; Fu, J. Dysfunction of autophagy in high-fat diet-induced non-alcoholic fatty liver disease. Autophagy 2024, 20, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.X.; Ito, M.; Fondell, J.D.; Fu, Z.Y.; Roeder, R.G. The TRAP220 component of a thyroid hormone receptor—Associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc. Natl. Acad. Sci. USA 1998, 95, 7939–7944. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, T.; Li, J.; Wang, S.; Qiu, F.; Yu, H.; Zhang, Y.; Wang, T. Effects of Natural Products on Fructose-Induced Nonalcoholic Fatty Liver Disease (NAFLD). Nutrients 2017, 9, 96. [Google Scholar] [CrossRef]
- Zhu, L.R.; Li, S.S.; Zheng, W.Q.; Ni, W.J.; Cai, M.; Liu, H.P. Targeted modulation of gut microbiota by traditional Chinese medicine and natural products for liver disease therapy. Front. Immunol. 2023, 14, 1086078. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Rustichelli, A.; Dongiovanni, P. Nutrition and Genetics in NAFLD: The Perfect Binomium. Int. J. Mol. Sci. 2020, 21, 2986. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, Y.; Wu, L.; Peng, J. Natural products in non-alcoholic fatty liver disease (NAFLD): Novel lead discovery for drug development. Pharmacol. Res. 2023, 196, 106925. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Zhang, L.; Li, Z.P.; Ji, G. Natural Products on Nonalcoholic Fatty Liver Disease. Curr. Drug Targets 2015, 16, 1347–1355. [Google Scholar] [CrossRef]
- Zemel, M.B. Natural Products: New Hope for Nonalcoholic Steatohepatitis? J. Med. Food 2019, 22, 1187–1188. [Google Scholar] [CrossRef]
- Cao, P.; Wang, Y.; Zhang, C.; Sullivan, M.A.; Chen, W.; Jing, X.; Yu, H.; Li, F.; Wang, Q.; Zhou, Z.; et al. Quercetin ameliorates nonalcoholic fatty liver disease (NAFLD) via the promotion of AMPK-mediated hepatic mitophagy. J. Nutr. Biochem. 2023, 120, 109414. [Google Scholar] [CrossRef]
- Li, L.; Qin, Y.; Xin, X.; Wang, S.; Liu, Z.; Feng, X. The great potential of flavonoids as candidate drugs for NAFLD. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 164, 114991. [Google Scholar] [CrossRef]
- Wang, K.; Tan, W.; Liu, X.; Deng, L.; Huang, L.; Wang, X.; Gao, X. New insight and potential therapy for NAFLD: CYP2E1 and flavonoids. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 137, 111326. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Changes in phenolics and antioxidant property of peach fruit during ripening and responses to 1-methylcyclopropene. Postharvest Biol. Technol. 2015, 108, 111–118. [Google Scholar] [CrossRef]
- Williamson, K.; Pao, S.; Dormedy, E.; Phillips, T.; Nikolich, G.; Li, L. Microbial evaluation of automated sorting systems in stone fruit packinghouses during peach packing. Int. J. Food Microbiol. 2018, 285, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.; Sánchez, C.; Romero, J.; Alfaro, P.; Batlle, R.; Nerín, C. Development and Application of an Active Package to Increase the Shelf-Life of “Calanda Peach”; National Research Council—IMCB University of Naples—DSA and DIMP: Naples, Italy, 2009. [Google Scholar]
- Abidi, W.; Akrimi, R. Phenotypic diversity of nutritional quality attributes and chilling injury symptoms in four early peach [Prunus persica (L.) Batsch] cultivars grown in west central Tunisia. J. Food Sci. Technol. 2022, 59, 3938–3950. [Google Scholar] [CrossRef]
- Drincovich, M.F. Identifying sources of metabolomic diversity and reconfiguration in peach fruit: Taking notes for quality fruit improvement. FEBS Open Bio 2021, 11, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, R.M.; Carle, R. Carotenoid deposition in plant and animal foods and its impact on bioavailability. Crit. Rev. Food Sci. Nutr. 2017, 57, 1807–1830. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.; Gonçalves, A.C.; Silva, B.; Silva, L.R. Peach (Prunus persica): Phytochemicals and Health Benefits. Food Rev. Int. 2022, 38, 1703–1734. [Google Scholar] [CrossRef]
- Yuan, Z.; Lu, X.; Lei, F.; Sun, H.; Jiang, J.; Xing, D.; Du, L. Novel Effect of p-Coumaric Acid on Hepatic Lipolysis: Inhibition of Hepatic Lipid-Droplets. Molecules 2023, 28, 4641. [Google Scholar] [CrossRef]
- Prša, P.; Karademir, B.; Biçim, G.; Mahmoud, H.; Dahan, I.; Yalçın, A.S.; Mahajna, J.; Milisav, I. The potential use of natural products to negate hepatic, renal and neuronal toxicity induced by cancer therapeutics. Biochem. Pharmacol. 2020, 173, 113551. [Google Scholar] [CrossRef]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxidative Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Gao, J.; Gu, X.; Zhang, M.; Zu, X.; Shen, F.; Hou, X.; Hao, E.; Bai, G. Ferulic acid targets ACSL1 to ameliorate lipid metabolic disorders in db/db mice. J. Funct. Foods 2022, 91, 105009. [Google Scholar] [CrossRef]
- Mu, H.N.; Zhou, Q.; Yang, R.Y.; Tang, W.Q.; Li, H.X.; Wang, S.M.; Li, J.; Chen, W.X.; Dong, J. Caffeic acid prevents non-alcoholic fatty liver disease induced by a high-fat diet through gut microbiota modulation in mice. Food Res. Int. 2021, 143, 110240. [Google Scholar] [CrossRef]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Malik, A.; Malik, M. Effects of curcumin in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Can. Liver J. 2024, 7, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jamal, A.; Jamil, D.A.; Al-Aubaidy, H.A. A systematic review exploring the mechanisms by which citrus bioflavonoid supplementation benefits blood glucose levels and metabolic complications in type 2 diabetes mellitus. Diabetes Metab. Syndr. 2023, 17, 102884. [Google Scholar] [CrossRef]
- Mi, J.; Liu, D.; Qin, C.; Yan, X.; Yang, L.; Xu, X.; Nie, G. (−)-Epigallocatechin-3-O-gallate or (−)-epicatechin enhances lipid catabolism and antioxidant defense in common carp (Cyprinus carpio L.) fed a high-fat diet: Mechanistic insights from the AMPK/Sirt1/PGC-1α signaling pathway. Aquaculture 2024, 587, 740876. [Google Scholar] [CrossRef]
- Lee, C.W.; Seo, J.Y.; Lee, J.; Choi, J.W.; Cho, S.; Bae, J.Y.; Sohng, J.K.; Kim, S.O.; Kim, J.; Park, Y.I. 3-O-Glucosylation of quercetin enhances inhibitory effects on the adipocyte differentiation and lipogenesis. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 95, 589–598. [Google Scholar] [CrossRef]
- Čižmárová, B.; Tomečková, V.; Hubková, B.; Birková, A. Anti-obesity properties and mechanism of action of genistein. Food Funct. Food Sci. Obes. 2023, 1, 26. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Souza, P.C.; Puhl, A.C.; Martínez, L.; Aparício, R.; Nascimento, A.S.; Figueira, A.C.; Nguyen, P.; Webb, P.; Skaf, M.S.; Polikarpov, I. Identification of a new hormone-binding site on the surface of thyroid hormone receptor. Mol. Endocrinol. 2014, 28, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Pramfalk, C.; Pedrelli, M.; Parini, P. Role of thyroid receptor β in lipid metabolism. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2011, 1812, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.B.; Bortolini, M.; Tadayyon, M.; Bopst, M. Minireview: Challenges and Opportunities in Development of PPAR Agonists. Mol. Endocrinol. 2014, 28, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.A.; Asghar, R.; Makwana, S.; Yahya, M.; Kumar, N.; Khawar, M.H.; Ahmed, A.; Islam, T.; Kumari, K.; Shadmani, S.; et al. Thyroid and Its Ripple Effect: Impact on Cardiac Structure, Function, and Outcomes. Cureus 2024, 16, e51574. [Google Scholar] [CrossRef] [PubMed]

| Ligand | ΔG of Binding to THRA (kcal/mol) | MMGBSA ΔG Binding Energy (kcal/mol) of GalaxyWEB Model | ΔG of THRA-Liganded Binding to TRAP220 (kcal/mol) |
|---|---|---|---|
| p-Coumaric | −7.948 | −7.381 | −812.60 |
| P4 * | −17.269 | −17.953 | −694.70 |
| 2-Hydroxycinnamate | −5.804 | −6.201 | −669.50 |
| P2 * | −12.110 | −11.995 | −665.11 |
| P1 * | −10.579 | −11.003 | −654.50 |
| Caffeic Acid | −8.499 | −8.421 | −651.59 |
| Curcumin | −15.491 | −16.113 | −649.71 |
| Methyl-4-Hydroxycinnamate | −7.914 | −8.004 | −641.87 |
| Chlorogenic Acid | −13.616 | −13.412 | −627.93 |
| Chlorogenic Acid Methyl Ester | −15.155 | −14.950 | −617.52 |
| Quercetin-3-Galactoside | −17.321 | −17.117 | −599.31 |
| Epicatechin | −11.952 | −12.021 | −596.21 |
| Genistein | −9.671 | −10.423 | −595.28 |
| O-Methylsinapate | −9.628 | −9.527 | −588.62 |
| T3 | −13.388 | −13.216 | −582.45 |
| Quercetin-3-Glucoside | −15.370 | −15.444 | −576.29 |
| 3-Hydroxyflavone | −9.231 | −9.785 | −570.24 |
| Ferulic Acid | −8.980 | −9.075 | −563.35 |
| Caffeic Acid Phenethyl Ester | −12.300 | −11.889 | −562.89 |
| Sinapic Acid | −9.274 | −10.004 | −559.81 |
| Aurantinidin | −14.221 | −12.925 | −559.73 |
| P3 * | −18.465 | −17.563 | −525.58 |
| Ligand | ΜW (g/mol) | TPSA (Å2) | Consensus LogP | Bioavailability Score |
|---|---|---|---|---|
| p-Coumaric | 164.16 | 57.53 | 1.26 | 0.85 |
| P4 * | 448.42 | 155.14 | 0.28 | 0.55 |
| 2-Hydroxycinnamate | 163.15 | 60.36 | 1.49 | 0.85 |
| P2 * | 272.25 | 86.99 | 1.84 | 0.55 |
| P1 * | 292.37 | 55.76 | 3.83 | 0.55 |
| Caffeic Acid | 180.16 | 77.76 | 0.93 | 0.56 |
| Curcumin | 368.38 | 93.06 | 3.03 | 0.55 |
| Methyl-4-Hydroxycinnamate | 178.18 | 46.53 | 1.81 | 0.55 |
| Chlorogenic Acid | 354.31 | 164.75 | −0.39 | 0.11 |
| Chlorogenic Acid Methyl Ester | 368.34 | 153.75 | 0.00 | 0.55 |
| Quercetin-3-Galactoside | 464.38 | 210.51 | −0.38 | 0.17 |
| Epicatechin | 290.27 | 110.38 | 0.85 | 0.55 |
| Genistein | 270.24 | 90.90 | 2.04 | 0.55 |
| O-Methylsinapate | 238.24 | 64.99 | 1.75 | 0.55 |
| T3 | 650.97 | 92.78 | 2.93 | 0.55 |
| Quercetin-3-Glucoside | 463.37 | 213.34 | −0.23 | 0.11 |
| 3-Hydroxyflavone | 238.24 | 50.44 | 2.84 | 0.55 |
| Ferulic Acid | 194.18 | 66.76 | 1.36 | 0.85 |
| Caffeic Acid Phenethyl Ester | 284.31 | 66.76 | 3.09 | 0.55 |
| Sinapic Acid | 224.21 | 75.99 | 1.31 | 0.56 |
| Aurantinidin | 287.24 | 114.29 | 0.53 | 0.55 |
| P3 * | 448.42 | 166.14 | 0.42 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinou, E.K.; Panagiotopoulos, A.A.; Dimitriou, M. In Silico Study of Natural Polyphenols as Potential Metabolic Modulators in Mitigating Lipotoxicity in Non-Alcoholic Fatty Liver Disease via Thyroid Hormone Receptor Alpha Activation. Curr. Issues Mol. Biol. 2025, 47, 777. https://doi.org/10.3390/cimb47090777
Konstantinou EK, Panagiotopoulos AA, Dimitriou M. In Silico Study of Natural Polyphenols as Potential Metabolic Modulators in Mitigating Lipotoxicity in Non-Alcoholic Fatty Liver Disease via Thyroid Hormone Receptor Alpha Activation. Current Issues in Molecular Biology. 2025; 47(9):777. https://doi.org/10.3390/cimb47090777
Chicago/Turabian StyleKonstantinou, Evangelia K., Athanasios A. Panagiotopoulos, and Maria Dimitriou. 2025. "In Silico Study of Natural Polyphenols as Potential Metabolic Modulators in Mitigating Lipotoxicity in Non-Alcoholic Fatty Liver Disease via Thyroid Hormone Receptor Alpha Activation" Current Issues in Molecular Biology 47, no. 9: 777. https://doi.org/10.3390/cimb47090777
APA StyleKonstantinou, E. K., Panagiotopoulos, A. A., & Dimitriou, M. (2025). In Silico Study of Natural Polyphenols as Potential Metabolic Modulators in Mitigating Lipotoxicity in Non-Alcoholic Fatty Liver Disease via Thyroid Hormone Receptor Alpha Activation. Current Issues in Molecular Biology, 47(9), 777. https://doi.org/10.3390/cimb47090777







