Mechanisms Linking Obesity with Non-Alcoholic Fatty Liver Disease (NAFLD) and Cardiovascular Diseases (CVDs)—The Role of Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
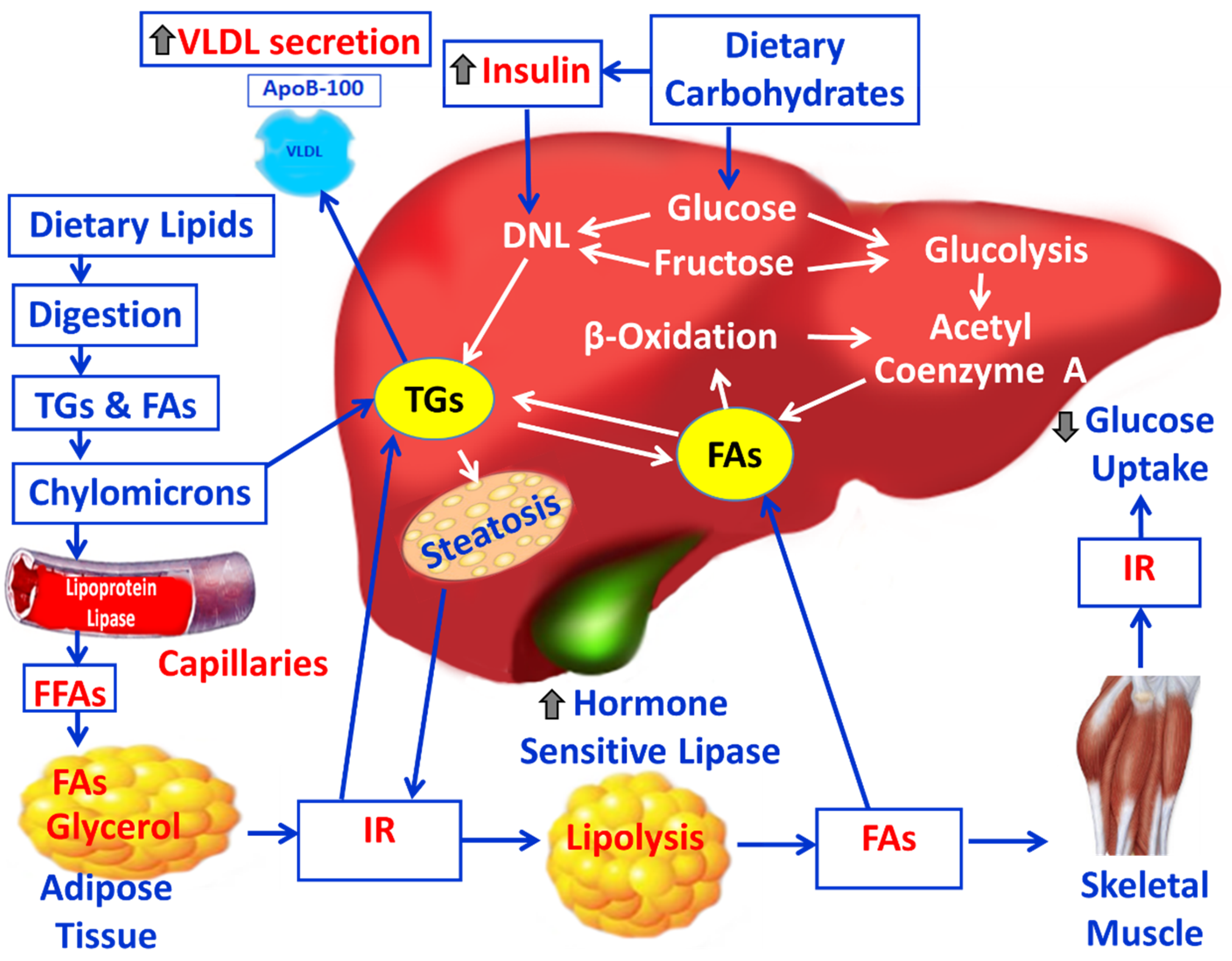
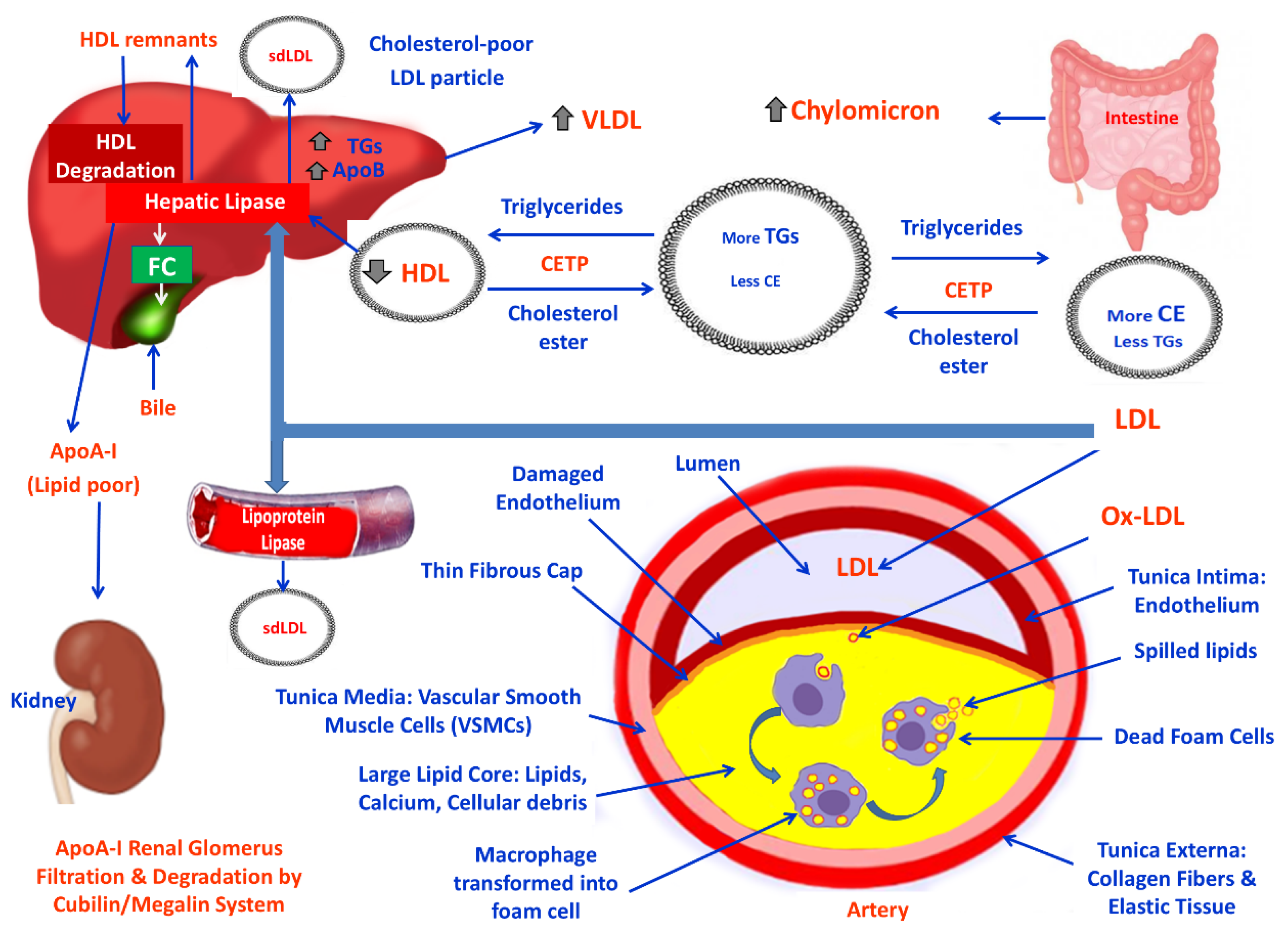
3. Lipid Derangement and NAFLD
3.1. Pathophysiology and Mechanisms of NAFLD and the Impact of OS
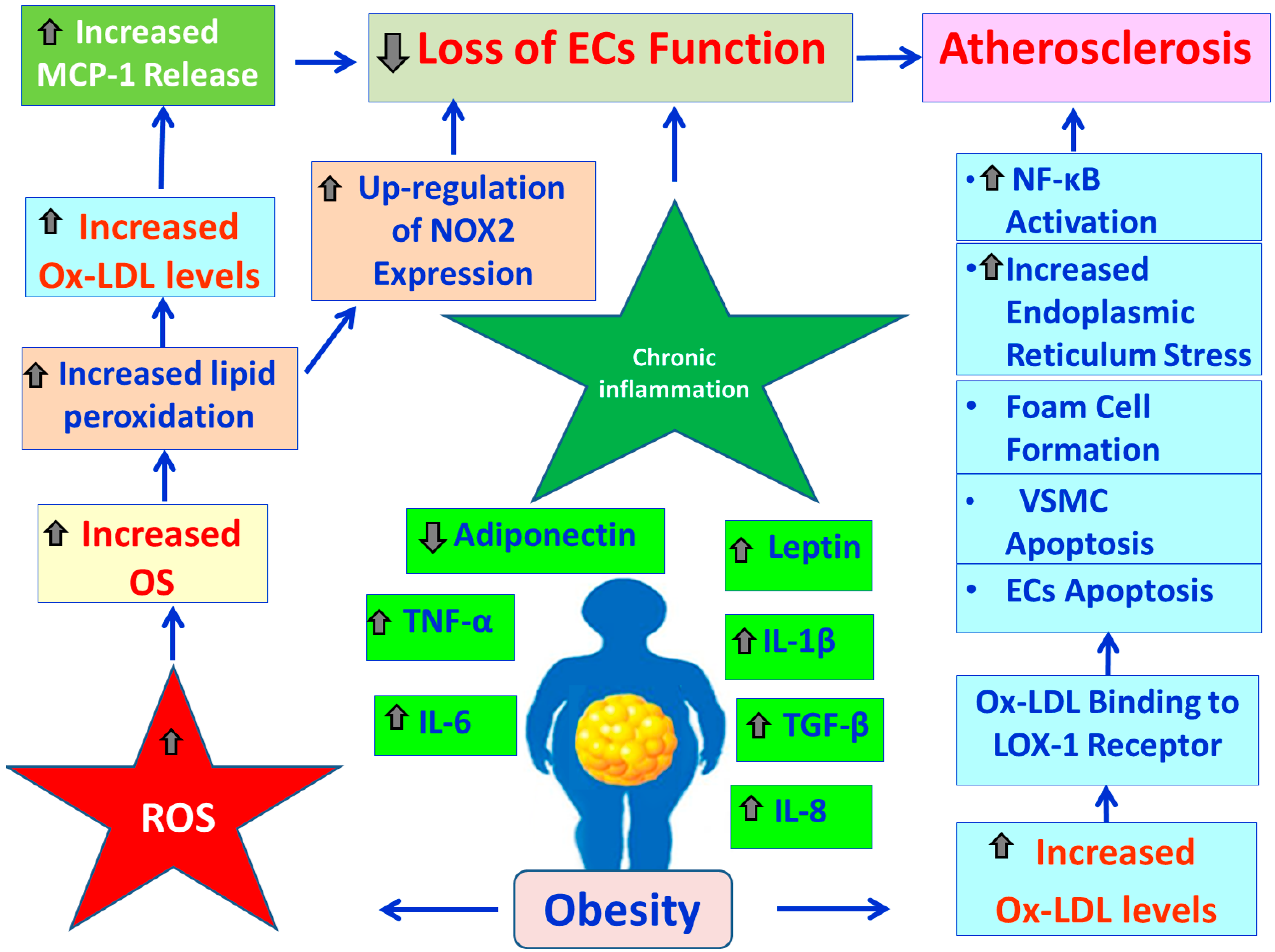
3.2. Obesity, Lipid Derangement, OS and CVDs
3.3. Obesity, OS and Endothelial Blood Cells Function
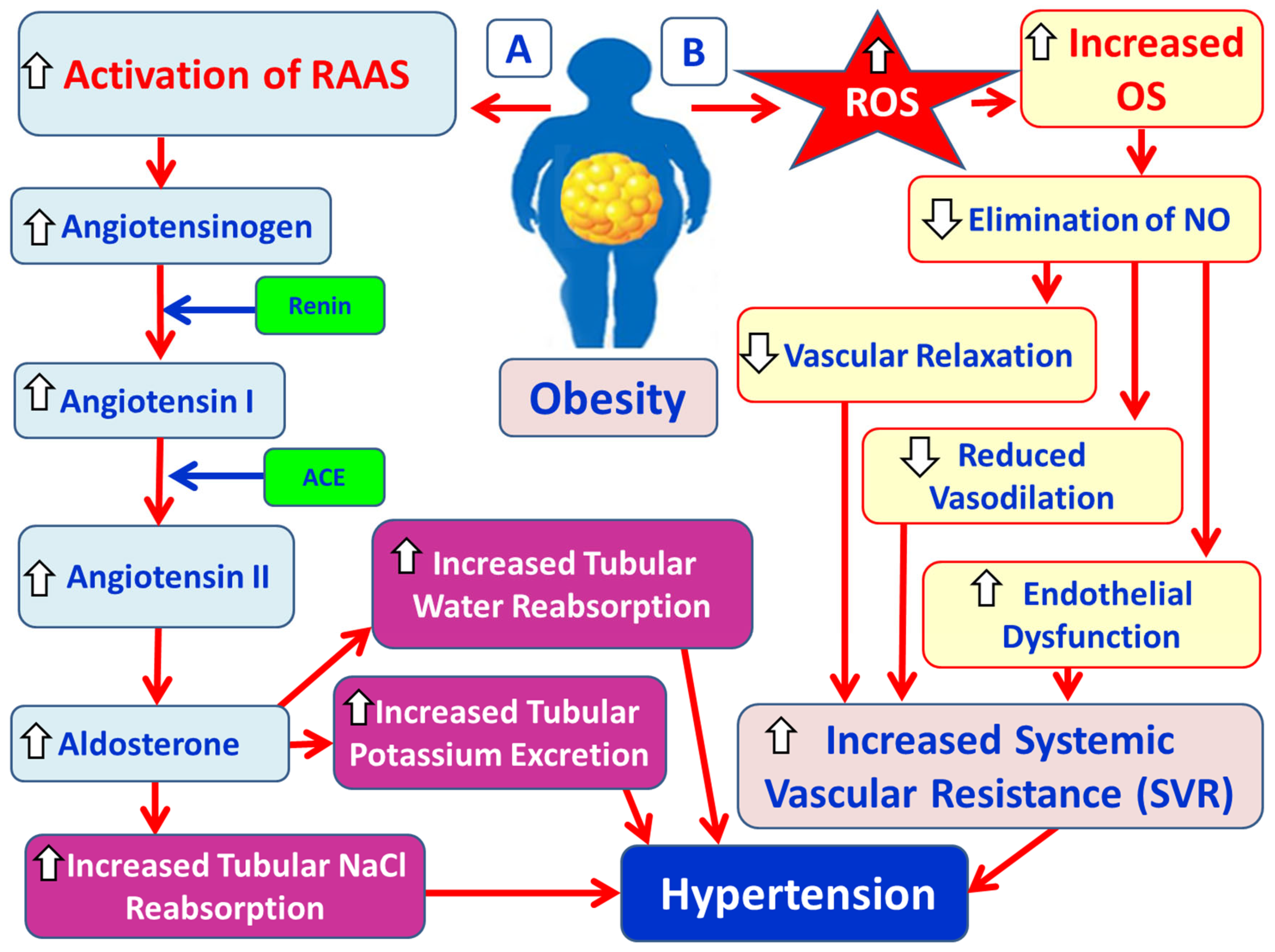
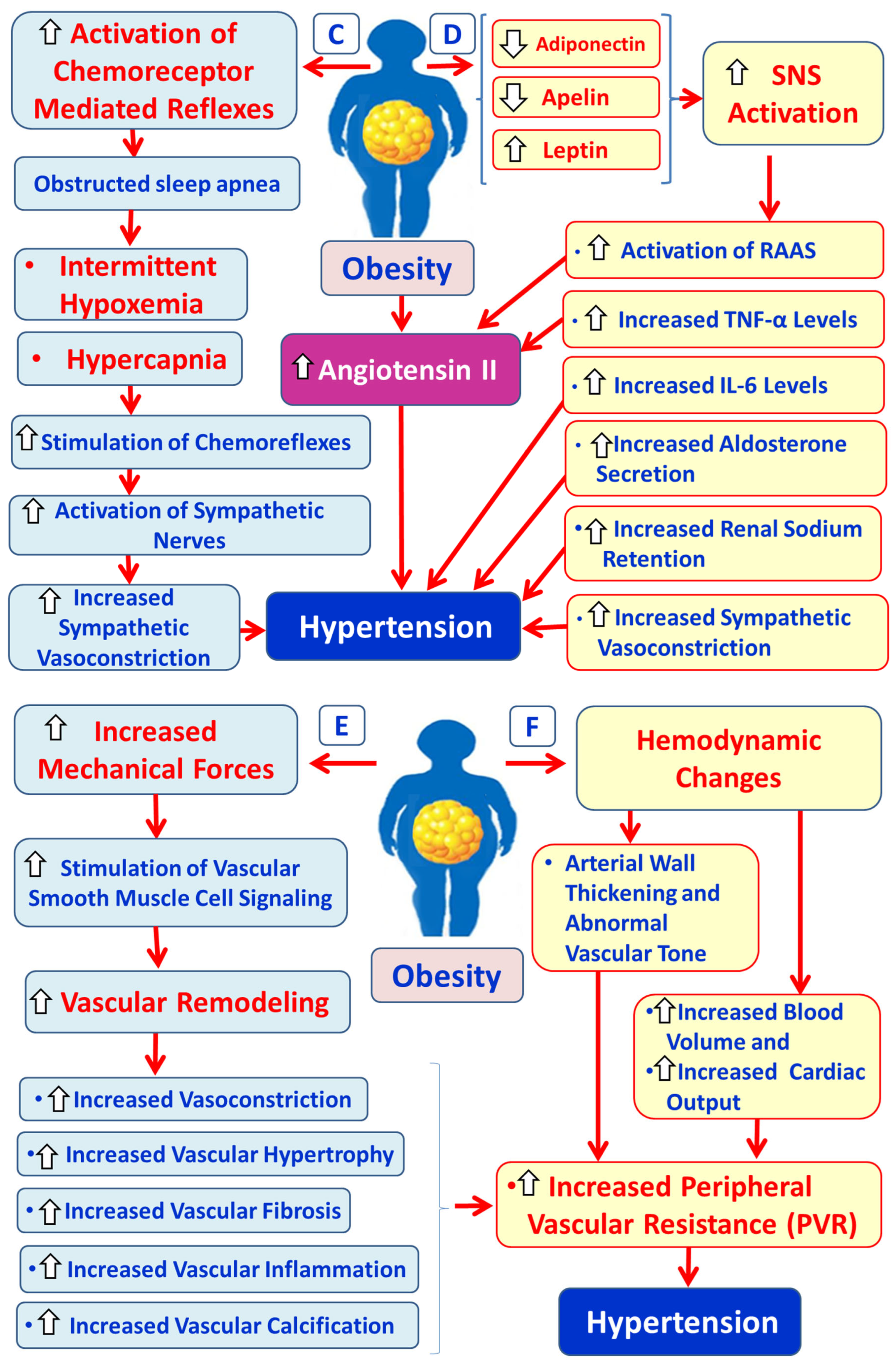
3.4. Obesity, OS and Hypertension
3.4.1. Physiology and Pathophysiology of RAAS
3.4.2. Hypertension Due to Over-Activation of SNS in Obesity
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 32–42. [Google Scholar] [CrossRef]
- Sanyal, A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 377–386. [Google Scholar] [CrossRef]
- Miyake, T.; Kumagi, T.; Hirooka, M.; Furukawa, S.; Kawasaki, K.; Koizumi, M.; Todo, Y.; Yamamoto, S.; Nunoi, H.; Tokumoto, Y.; et al. Significance of exercise in nonalcoholic fatty liver disease in men: A community-based large crosss-sectional study. J. Gastroenterol. 2015, 50, 230–237. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the study of liver diaseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]
- Burnt, E.M.; Tiniakos, D.G. Histopatholygy of nonalcoholic fatty liver disease. World J. Gastroenterol. 2010, 16, 5286–5296. [Google Scholar] [CrossRef]
- Cariou, B.; Byrne, C.D.; Loomba, R.; Sanyal, A. Nonalcoholic fatty liver disease as a metabolic disease in humans: A literature review. Diabetes Obes. Metab. 2021, 23, 1069–1083. [Google Scholar] [CrossRef]
- Sobczak, A.I.S.; Blindauer, C.A.; Stewart, A.J. Changes in plasma free fatty acids associated with type-2 diabetes. Nutrients 2019, 11, 2022. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride metabolism in the liver. Comp. Physiol. 2017, 8, 1–8. [Google Scholar]
- Fisher, E.A.; Feig, J.E.; Hewing, B.; Hazen, S.L.; Smith, J.D. High-density lipoprotein function, dysfunction, and reserve cholesterol trasport. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2813–2820. [Google Scholar] [CrossRef]
- Sapanaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The subtle balance between lipolysis and lipogenesis: A critical point in metabolic homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [PubMed]
- Price, E.R. The physiology of lipid storage and use in reptiles. Biol. Rev. Cam. Philos. Soc. 2017, 92, 1406–1426. [Google Scholar] [CrossRef]
- Nielsen, T.S.; Jessen, N.; Jørgensen, J.O.L.; Møller, N.; Lund, S. Dissecting adipose tissue lipolysis: Molecular regulation and implications for metabolic disease. J. Mol. Endocrinol. 2014, 52, R199–R222. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 17–197. [Google Scholar] [CrossRef]
- Peverill, W.; Powell, L.W.; Skoien, R. Evolving concepts in the pathgenesis of NASH: Beyond steatosis and inflammation. Int. J. Mol. Sci. 2014, 15, 8591–8638. [Google Scholar] [CrossRef]
- Ma, Y.; Lee, G.; Heo, S.-Y.; Roh, Y.-S. Oxidative stress is a key modulator in the development of nonalcoholic fatty liver disease. Antioxidants 2022, 11, 91. [Google Scholar] [CrossRef]
- Mavilia, M.G.; Wu, G.Y. Liver and serum adiponectin levels in non-alcoholic fatty liver disease. J. Dig. Dis. 2021, 22, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tang, W.J.; Yin, J.J.; Zhou, B. Signal transductions and nonalcoholic fatty liver: A mini-review. Int. J. Clin. Exp. Med. 2014, 7, 1624–1631. [Google Scholar] [PubMed]
- Doulberis, M.; Kotronis, G.; Gialamprinou, D.; Kountouras, J.; Katsinelos, P. Non-alcoholic fatty liver disease: An update with special focus on the role of gut microbiota. Metabolism 2017, 71, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M.; Day, C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N. Engl. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef]
- Sun, Z.; Lazar, M.A. Dissociating fatty liver and diabetes. Trends Endocrinol. Metab. 2013, 24, 4–12. [Google Scholar] [CrossRef]
- Pérez-Carreras, M.; Del Hoyo, P.; Martín, M.A.; Rudio, J.C.; Martín, A.; Castellano, G.; Colina, F.; Arenas, J.; Solis-Herruzo, J.A. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 2003, 38, 999–1007. [Google Scholar] [CrossRef]
- Peng, K.-Y.; Watt, M.J.; Pensen, S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef]
- Leadsham, J.E.; Sanders, G.; Giannaki, S.; Bastow, E.L.; Hutton, R.; Naeimi, W.R.; Breitenbach, M.; Gourlay, C.W. Loss of cytochrome c oxidase promotes RAS-dependent ROS production from the ER resident NADPH oxidase, Yno1p, in yeast. Cell Metab. 2013, 18, 279–286. [Google Scholar] [CrossRef]
- Hensley, K.; Robinson, K.A.; Gabbita, S.P.; Salsman, S.; Flyod, R.A. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biphys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Klimova, N.; Fearnow, A.; Kristiann, T. Role of NAD+—Modulated free fadical generation in mechanisms o facute brain injury. Brain Sci. 2020, 10, 449. [Google Scholar] [CrossRef]
- Begriche, K.; Massart, J.; Robin, M.A.; Bonnet, F.; Fromenty, B. Mitochondrial adaptation and dysfunctions in nonalcoholic fatty liver disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef]
- Ramos-Tovar, E.; Muriel, P. Molecular mechanisms that link oxidative stress, inflammation, and fibrosis in the liver. Antioxidants 2020, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Manti, S.; Maseglia, L.; D’Angelo, G.; Filippelli, M.; Cuppari, C.; Gitto, E.; Romano, C.; Arrigo, T.; Salpietro, C. Portal hypertension as immune mediate disease. Hepat. Mon. 2014, 14, e18625. [Google Scholar] [CrossRef] [PubMed]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic treatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef] [PubMed]
- Basaronoglu, M.; Kayacetin, S.; Yilmaz, N.; Kayacetin, E.; Tarcin, O.; Sonsuz, A. Understanding mechanisms o the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2010, 16, 2223–2226. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Youssef, T.M.; Abdullah, E.E.; Ahmed, A.E. Correlation between adiponectin level and the degree of fibrosis in patients with non-alcoholic fatty liver disease. Egypt. Liver J. 2021, 2021, 78. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, X. The role of gut-liver axis in gut microbiome dysbiosis associated NAFL and NAFLD-HCC. Biomedicines 2022, 10, 524. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Bolkent, S.; Yanardag, R.; Bolkent, S.; Doger, M.M. Beneficial effects of combined treatment with niacin and chromium on the liver of hyperlipemic rats. Biol. Trace Elem. Res. 2004, 101, 219–229. [Google Scholar] [CrossRef]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American College of Endocrinologyy Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr. Pract. 2017, 23 (Suppl. S2), 1–87. [Google Scholar] [CrossRef]
- Matey-Hernandez, M.; Williams, F.M.K.; Potter, T.; Valdes, A.M.; Spector, T.D.; Menni, C. Genetic and microbiome influence on lipid metabolism and dyslipidemia. Physiol. Genom. 2018, 50, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bijland, S.; Mancini, S.J.; Salt, I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013, 124, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, S.-Y.; Coi, C.S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-K.; Jun, W.; Lee, J. Mechanism of ER Stress and Inflammation for Hepatic Insulin Resistance in Obesity. Ann. Nutr. Metab. 2015, 67, 218–227. [Google Scholar] [CrossRef]
- Cariello, A.; Toboga, C.; Tonutti, L.; Quagliaro, L.; Piconi, L.; Bais, D.; da Ros, R.; Motz, E. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyper-glycemia on endothelia dysfunction and oxidative stress generation: Effects of short- and long-term simvastatin treatment. Circulation 2002, 106, 1211–1218. [Google Scholar] [CrossRef]
- Tall, A.R.; Thomas, D.G.; Gonzalez-Cabodevilla, A.G.; Goldberg, I.J. Adrressing dyslipidemic risk beyond LDL-cholesterol. J. Clin. Investig. 2022, 132, e148559. [Google Scholar] [CrossRef]
- Nelson, A.J.; Sniderman, A.D.; Ditmarsch, M.; Dicklin, M.R.; Nicholls, S.J.; Davidson, M.H.; Kastelein, J.J.P. Cholesteryl ester transfer protein inhibition reduces major adverse cardiovascular events by lowering apolipoprotein B levels. Int. J. Mol. Sci. 2022, 23, 9417. [Google Scholar] [CrossRef]
- Sandhofer, A.; Kaser, S.; Ritch, A.; Laimer, M.; Engl, J.; Paulweber, B.; Pathch, J.R.; Ebenbichler, C.F. Cholesteryl ester transfer protein in metabolic syndrome. Obesity 2006, 14, 812–818. [Google Scholar] [CrossRef]
- Carmena, R.; Duriez, P.; Fruchart, J.-C. Atherogenic lipoprotein particles in atherosclerosis. Circulation 2004, 109 (Suppl. S1), III2-7. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid. Med. Cell Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef] [PubMed]
- Nazar, A.; Anush, J.; Mathew, R.; Prabhakar, P.K. Clinical Utility of small, dense LDL as an atherogenic risk marker. Biointerface Res. Appl. Chem. 2023, 13, 199. [Google Scholar]
- Vekic, J.; Zeljkovic, A.; Cicero, A.R.G.; Janez, A.; Stoian, A.P.; Sonmez, A.; Rizzo, M. Atherosclerosis development and progression:the role of atherogenic small, dense LDL. Medicina 2022, 58, 299. [Google Scholar] [CrossRef]
- Jin, X.; Yang, S.; Lu, J.; Wu, M. Small, dense low-density lipoprotein-cholesterol and atherosclerosis: Relationship and therapeutic strategies. Front. Cardiovasc. Med. 2021, 8, 804214. [Google Scholar] [CrossRef]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Invest. 2000, 106, 453–458. [Google Scholar] [CrossRef]
- Hirano, T. Pathophysiology of diabetic dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef]
- Khatana, C.; Sain, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid. Med. Cell Long. 2020, 2020, 5245308. [Google Scholar] [CrossRef]
- Daghem, M.; Bing, R.; Fayad, Z.A.; Dweck, M.R. Noninvasive imaging to assess atherosclerotic plaque composition and disease acrivity. Coronary and carotid applications. J. Am. Coll. Cardiol. Img. 2020, 13, 1055–1068. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Raghavamenon, A.; Garelnabi, M.O.; Santanam, N. Oxidized low—Density lipoprotein. Methods Mol. Biol. 2010, 610, 403–417. [Google Scholar]
- Poznyak, A.V.; Nikoforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and its impact on cardiovascular health: Focus on atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Y.; Zhao, X.; Ru, J.; Kang, N.; Tian, T.; Tang, L.; An, Y.; Li, P. oxLDL-mediated cellular senescence is associated with increased NADPH oxidase p47phox recruitment to caveolae. Biosci. Rep. 2018, 38, BSR20180283. [Google Scholar] [CrossRef]
- Manea, A.; Simionescu, M. Nox enzymes and oxidative stress in atherosclerosis. Front. Biosci. 2012, 4, 651–670. [Google Scholar] [CrossRef]
- Gaens, K.H.; Sthouwer, C.D.; Schalkwijk, C.G. Advanced glycation endproducts and its receprot for advanced glycation endproducts in obesity. Curr. Opin. Lipidol. 2013, 24, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-Garcia, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martin, C. Pathophysiology of atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahabkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of oxidized LDL-mediated endothelial dysfunction and its consequences for the development of atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef] [PubMed]
- Beck-Joseph, J.; Tabrizian, M.; Lehoux, S. Molecular interactions between vascular smooth muscle cells and macrophages in atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 737934, Correction in Front. Cardiovasc. Med. 2021, 11, 1462284. [Google Scholar] [CrossRef]
- Pirillo, A.; Notara, G.D.; Catapano, A.L. LOX-1, OxLDL and atherosclerosis. Mediat. Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef]
- Li, W.; Jin, K.; Luo, J.; Xu, W.; Wu, Y.; Zhou, J.; Wang, Y.; Xu, R.; Jiao, L.; Wang, T.; et al. NF-κB and its crosstalk with endoplasmic reticulum stress in atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 988266. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Kyler, K.; Xu, J. Endothelial–vascular smooth muscle cells interactions in atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 151. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Li, C.-J.; Hou, M.-F.; Chu, P.-Y. New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef]
- Stengel, D.; Antonucci, M.; Groua, W.; Dachet, C.; Lesnik, P.; Hourton, D.; Ninio, E.; Chapman, M.J.; Griglio, S. Inhibition of LPL expression in human monocyte-derived macrophages is dependent on LDL oxidation state: A key role for lysophosphatidylcholine. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1172–1180. [Google Scholar] [CrossRef]
- Merkel, M.; Heeren, J.; Dudeck, W.; Rinninger, F.; Radner, H.; Breslow, J.L.; Goldberg, I.J.; Zachner, R.; Greten, H. Inactive lipoprotein lipase (LPL) alone increases selective cholesterol ester uptake in vivo, whereas in the presence of active LPL it also increases triglyceride hydroysis and whole particle lipoprotein uptake. J. Biol. Chem. 2002, 277, 7405–7411. [Google Scholar] [CrossRef]
- Zani, I.A.; Stephen, S.L.; Mughal, N.A.; Russell, D.; Homer-Vanniasinkam, S.; Wheatcroft, S.B.; Ponnambalam, S. Scavenger report structure and function in health and disease. Cells 2015, 4, 178–201. [Google Scholar] [CrossRef]
- Toledo-Ibelles, P.; Mas-Oliva, J. Antioxidants in the fight against atherosclerosis: Is this a dead end? Curr. Atheroscler. Rep. 2018, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Kume, N.; Miyamoto, S.; Minami, M.; Morimoto, M.; Hayashida, K.; Hashimoto, N.; Kita, T. Oxidized LDL modulates Bax/Bcl-2 through the lectinlike Ox-LDL receptor-1 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 955–960. [Google Scholar] [CrossRef]
- Vykoukal, D.; Davies, M.G. Vascular biology of metabolic syndrome. J. Vasc. Surg. 2011, 54, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S. Hypertension in the metabolic syndrome. Metab. Syndr. Relat. Disord. 2006, 4, 287–298. [Google Scholar] [CrossRef]
- Bray, G.A.; Bellanger, T. Epidemiology, trends and morbidities of obesity and the metabolic syndrome. Endocrine 2006, 29, 109–117. [Google Scholar] [CrossRef]
- daSilva-deAbreu, A.; Alhafez, B.A.; Lavie, C.J.; Milani, R.V.; Ventura, H.O. Interactions of hypertension, obesity, left ventricular hypertrophy, and heart failure. Curr. Opin. Cardiol. 2021, 36, 453–460. [Google Scholar] [CrossRef]
- Intengan, H.D.; Schiffrin, E.L. Vascular remodeling in hypertension. Roles of apoptosis, inflammation and fibrosis. Hypertension 2001, 38, 581–587. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free redicals: Properties, sources, targets and their implication in various diseases. Indian. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Leong, X.-F.; Mustafa, M.R.; Jaarin, K. Nigella sativa and its protective role in oxidative stress and hypertension. Evid. Based Complement. Altern. Med. 2013, 2013, 120732, Erratum in Evid. Based Complement. Altern. Med. 2013, 2013, 253479. [Google Scholar] [CrossRef]
- Jefimow, M.; Przybylska-Piech, A.S.; Wojciechowski, M.S. Predictive and reactive changes in antioxidant defence system in a heterothermic rodent. J. Comp. Physiol. B 2020, 190, 479–492. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and detrimental effects of reactive oxygen species of lifespan: A comprehensive review of comparative and experimental studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef]
- Higashi, Y.; Maruhashi, T.; Noma, K.; Kihara, Y. Oxidative stress and endothelial dysfunction: Clinical evidence and therapeutic implications. Treds Cardiovasc. Med. 2014, 24, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Elmasry, S.A.; Al-Azzawi, M.A.; Ghoneim, A.H.; Nasr, M.Y.; AboZaid, M.M.N. Role of oxidant-antioxidant imbalance in the pathogenesis of chronic obstructive pulmonary disease. Egypt. J. Chest Dis. Tuberc. 2015, 64, 813–820. [Google Scholar] [CrossRef]
- Reid, I.A. Interanctions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am. J. Physiol. 1992, 262, E763–E778. [Google Scholar]
- Ranjbar, T.; Oza, P.P.; Kashfi, K. The renin-angiotensin-aldosterone system, nitric oxide, and hydrogen sulfide at the crossroads of hypertension and COVID-19: Radical disparities and outcomes. Int. J. Mol. Sci. 2022, 23, 13895. [Google Scholar] [CrossRef]
- Miller, A.J.; Arnold, A.C. The renin-angiotensin system in cardiovascular autonomic control: Recent development and clinical implications. Clin. Auton. Res. 2019, 29, 231–234. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Khan, Z.; Giani, J.F.; Cao, D.Y.; Bernstein, E.A.; Shen, X.Z. Angiotensin-converting enzyme in innate and adaptive immunity. Nat. Rev. Nephrol. 2018, 14, 325–336. [Google Scholar] [CrossRef]
- Harrison-Bernard, L.M. The renal renin-angiotensin system. Adv. Physiol. Educ. 2009, 33, 270–274. [Google Scholar] [CrossRef]
- Rossi, G.P.; Lenzini, L.; Caroccia, B.; Rossitto, G.; Seccia, T.M. Angiotensin peptides in the regulation of adrenal cortical function. Explor. Med. 2021, 2, 294–304. [Google Scholar] [CrossRef]
- Weiner, I.D. Endocrine and hypetensive disorders of potassium regulation: Primary aldosterone. Semin. Nephrol. 2013, 33, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Meneton, P.; Loffing, J.; Warnock, D.G. Sodium and potassium hadling by the aldosterone-sensitive distal nephron: The pivotal role of the distal and connectin tubule. Am. J. Physiol.-Ren. Physiol. 2004, 287, F593–F601. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K. Renin-angiotensin system and sympathetic neurotransmitter release in the central nervous system of hypertension. Int. J. Hypertens. 2012, 2012, 474870. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, Z.; Roncari, C.F.; Guo, F.; Johnson, A.K. Aldosterone acting through the central nervous system sensitized angiotensin II-induced hypertension. Hypertension 2012, 60, 1023–1030. [Google Scholar] [CrossRef]
- Lohmeier, T.E.; Iliescu, R. The sympathetic nervous system in obesity hypertension. Curr. Hypertens. Rep. 2013, 15, 409–416. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Thethi, T.; Kamiyama, M.; Kobori, H. The link between renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr. Hypertens. Rep. 2012, 14, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Varra, F.-N.; Varras, M.; Varra, V.-K.; Theodosis-Nobelos, P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation-mediating treatment options (Review). Mol. Med. Rep. 2024, 29, 95. [Google Scholar] [CrossRef] [PubMed]
- Schütten, M.T.J.; Houben, A.J.H.M.; de Leeuw, P.W.; Stehouwer, C.D.A. The link between adipose tissue renin-angiotensine-aldosterone system signaling and obesity-associated hypertension. Physiology 2017, 32, 197–209. [Google Scholar] [CrossRef]
- Muniyappa, R.; Yavuz, S. Metabolic actions of angiotensin II and insulin: A microvascular endothelial balancing act. Mol. Cell Endocrinol. 2013, 378, 59–69. [Google Scholar] [CrossRef]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef]
- Yavuzer, H.; Yavuzer, S.; Cengiz, M.; Erman, H.; Doventas, A.; Balci, H.; Erdincler, D.S.; Uzun, H. Biomarkers of lipid peroxidation related to hypertension in aging. Hypertens. Res. 2016, 39, 342–348. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Hao, X.-H.; Tan, F.-F.; Pei, X.; Shang, L.-M.; Jiang, X.-L.; Yang, F. The elements of human cyclin D1 promoter and regulation involved. Clin. Epigenetics 2011, 2, 63–76. [Google Scholar] [CrossRef]
- Wang, M.-L.; Yu, X.-J.; Li, X.-G.; Pang, D.-Z.; Su, Q.; Saahene, R.O.; Li, H.-B.; Mao, X.-Y.; Liu, K.-L.; Fu, L.-Y.; et al. Blockade of TLR4 within the paraventricular nucleus attenuates blood pressure by regulating ROS and inflammatory cytokines in prehypertensive rats. Am. J. Hypertens. 2018, 32, 1013–1023. [Google Scholar] [CrossRef]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative stress: A unifying paradigm in hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef]
- Canale, M.P.; di Villahermosa, S.M.; Martino, G.; Rovella, V.; Noce, A.; de Lorenzo, A.; Daniele, N.D. Obesity-related metabolic syndrome: Mechanisms of sympathetic overactivity. Int. J. Endocrinol. 2013, 2013, 865965. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, V.G.; Aroor, A.R.; Sowers, J.R. The pathophysiology of hypertension in patients with obesity. Nat. Rev. Endocrinol. 2014, 10, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Liakos, C.I.; Sanidas, E.A.; Perrea, D.N.; Grassos, C.A.; Chantziara, V.; Viniou, N.-A.; Barbetseas, J.D.; Papadopoulos, D.P. Apelin and visfatin plasma levels in healthy individuals with high normal blood pressure. Am. J. Hypertens. 2016, 29, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Minson, C.T. Obesity and adipokines: Effects on sympathetic overactivity. J. Physiol. 2012, 590, 1787–1801. [Google Scholar] [CrossRef]
- Rahmouni, K. Leptin-induced sympathetic nerve activation: Signaling mechanisms and cardiovascular consequences in obesity. Curr. Hypertens. Rev. 2010, 6, 104–109. [Google Scholar] [CrossRef]
- Okamoto, L.E.; Raj, S.R.; Gamboa, A.; Shibao, C.A.; Arnold, A.C.; Garland, E.M.; Black, B.K.; Farley, G.; Giedrich, A.; Biaggioni, I. Sympathetic activation is associated with increased IL-6, but not CRP in the absence of obesity: Lessons from postural tachycardia syndrome and obesity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H2098–H2107. [Google Scholar] [CrossRef]
- Huang, B.; Cheng, Y.; Usa, K.; Liu, Y.; Baker, M.A.; Mattson, D.L.; He, Y.; Wang, N.; Liang, M. Renal tumor necrosis factor α contributes to hypertension in Dahl salt-sensitive rats. Sci. Rep. 2016, 6, 21960. [Google Scholar] [CrossRef]
- Harte, A.; McTernal, P.; Chetty, R.; Coppack, S.; Kartz, J.; Smith, S.; Kumar, S. Insulin-mediated upregulation of the renin angiotensin system in human subcutaneous adipocytes is reduced by rosiglitazone. Circulation 2005, 111, 1954–1961. [Google Scholar] [CrossRef]
- Briones, A.M.; Nguyen Dinh Cat, A.; Callera, G.E.; Yogi, A.; Burger, D.; He, Y.; Corrêa, J.W.; Gagnon, A.M.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: Implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012, 59, 1069–1078. [Google Scholar] [CrossRef]
- Wang, Y.; Seto, S.-W.; Golledge, J. Angiotensin II, sympathetic nerve activity and chronic heart failure. Heart Fail. Rev. 2014, 19, 187–198. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, L.; Wu, B.; Wang, G.; Zhan, X.; Wang, Q.; Bayorh, M.A.; Song, Q. C-reactive protein causes adult-onset obesity through chronic inflammatory mechanism. Front. Cell Dev. Biol. 2020, 8, 18. [Google Scholar]
- Tank, J.; Diedrich, A.; Schoeder, C.; Stoffels, M.; Franke, G.; Sharma, A.M.; Luft, F.C.; Jordan, J. Limited effect of systemic β-blockade on sympathetic outflow. Hypertension 2001, 38, 1377–1381. [Google Scholar] [CrossRef]
- Nakagaki, T.; Hirooka, Y.; Matsukawa, R.; Nishihara, M.; Nakano, M.; Ito, K.; Hoka, S.; Sunagawa, K. Activation of mineralocorticoid receptors in the rostral ventrolateral medulla is involved in hypertensive mechanisms in stroke-prone spontaneously hypertensive rats. Hypertens. Res. 2012, 35, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Mansukhani, M.P.; Wangs Somers, V.K. Chemoreflex physiology and implications for sleep apnea-insights from studies in humans. Exp. Physiol. 2015, 100, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Narkiewicz, K.; Somers, V.K. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand. 2003, 177, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological molecular mechanisms of obesity: A link between MAFLD and NASH with cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef]
- Grander, C.; Grabherr, F.; Tilg, H.; Notes, A. Non-alcoholic fatty liver disease: Pathophysiological concepts and treatment options. Cardiovasc. Res. 2023, 119, 1787–1798. [Google Scholar] [CrossRef]
- Boulos, M.; Mousa, R.S.; Jeries, N.; Simaan, E.; Alam, K.; Bulus, B.; Assy, N. Hidden in the fat: Unpacking the metabolic tango between metabolic dysfunction associated steatotic liver disease and metabolic syndrome. Int. J. Mol. Sci. 2025, 26, 3448. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, J.; Cai, J.; Zhang, X.-J.; Zhang, P.; She, Z.-G.; Li, H. The Transition of Cardiovascular Disease Risks from NAFLD to MAFLD. Rev. Cardiovasc. Med. 2023, 24, 157. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef]
- Gancheva, S.; Roden, M.; Castera, L. Diabetes as a risk factor for MASH progression. Res. Clin. Pr. 2024, 217, 111846. [Google Scholar] [CrossRef] [PubMed]
- Finney, A.C.; Das, S.; Kumar, D.; McKinney, M.P.; Cai, B.; Yurdagul, A., Jr.; Rom, O. The interplay between nonalcoholic fatty liver disease and atherosclerotic cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1116861. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Y.; Wang, D.; Huang, Z.; Xiao, X.; Zheng, Q.; Li, S.; Long, D.; Feng, L. Mitochondrial dysfunction in metabolic dysfunction fatty liver disease (MAFLD). Int. J. Mol. Sci. 2023, 24, 17514. [Google Scholar] [CrossRef] [PubMed]
- Tauil, R.B.; Golono, P.T.; de Lima, E.P.; de Alvares Goulart, R.; Guiguer, E.L.; Bechara, M.D.; Nicolau, C.C.T.; Junior, J.L.Y.; Fiorini, A.M.R.; Méndez-Sánchez, N.; et al. Metabolic-Associated Fatty Liver Disease: The influence of oxidative stress, inflammation, mitochondrial dysfunctions, and the role of polyphenols. Pharmaceuticals 2024, 17, 1354. [Google Scholar] [CrossRef]
- Xu, H.-L.; Wan, S.-R.; An, Y.; Wu, Q.; Xing, Y.-H.; Deng, C.-H.; Zhang, P.-P.; Long, Y.; Xu, B.-T.; Jiang, Z.-Z. Targeting cell death in NAFLD: Mechanisms and targeted therapies. Cell Death Discov. 2024, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Oladipupo, S.O.; Ezenabor, E.H.; Ojo, A.B.; Ogunlakin, A.D.; Ojo, O.A. Interplay of the pathophysiological mechanisms of non-alcoholic fatty liver disease, diabetes mellitus, and inflammation: A growing threat to public health. Obes. Med. 2025, 55, 100613. [Google Scholar] [CrossRef]
- Zheng, H.; Sechi, L.A.; Navarese, E.R.; Casu, G.; Vidili, G. Metabolic dysfunction-associated steatotic liver disease and cardiovascular risk: A comprehensive review. Cardiovasc. Diabetol. 2024, 23, 346. [Google Scholar] [CrossRef]
- Bansal, S.; Vachher, M.; Arora, T.; Kumar, B.; Burman, A. Visceral fat: A key mediator of NAFLD development and progression. Hum. Nutr. Metab. 2023, 33, 200210. [Google Scholar] [CrossRef]
- He, Y.; Chen, Y.; Qian, S.; van Der Merwe, S.; Dhar, D.; Brenner, D.A.; Tacke, F. Immunopathogenic mechanisms and immunoregulatory therapies in MASLD. Cell. Mol. Immunol. 2025, 1–19. [Google Scholar] [CrossRef]
- Pepin, M.E.; Gupra, R.M. The Role of Endothelial Cells in Atherosclerosis-Insights from genetic association studies. Am. J. Pathol. 2024, 194, 499–509. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.; Yu, J.; Tu, Y.; Zhao, Y.; Zhang, Y.; Hu, Y.; Yang, H.; Yan, H.; Zheng, C. Prognostic impact of systemic inflammation indicators on all-cause and CVD mortality in adults with MASLD. BMC Gastroenterol. 2025, 25, 448. [Google Scholar] [CrossRef]
- Song, C.; Long, X.; He, J.; Huang, Y. Recent evaluation about inflammatory mechanisms in nonalcoholic fatty liver disease. Front. Pharmacol. 2023, 14, 1081334. [Google Scholar] [CrossRef] [PubMed]
- Baral, A. Endoplasmic reticulum stress signaling in the regulation of hepatic pathological responses. Stresses 2024, 4, 481–504. [Google Scholar] [CrossRef]
- Kwaifa, K.; Mainasara, A.S.; Jidda, M.L.; Amin, A.M.; Abdullahi, G.; Ladan, F.; Danyaro, M. Non-alcoholic fatty liver disease: Pathogenesis and the significance of high-density lipoprotein as a molecular modifier. In Non-Alcoholic Fatty Liver Disease-New Insight and Glance into Disease Pathogenesis; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Pădureanu, V.; Forţofoiu, M.C.; Pîrşcoveanu, M.; Pădureanu, R.; Rădulescu, D.; Donoiu, I.; Pîrşcoveanu, D.F.V. Cardiovascular manifestations of patients with non-alcoholic fatty liver disease. Metabolites 2025, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Iturbe-Rey, S.; Maccali, C.; Arrese, M.; Aspichueta, P.; Oliveira, C.P.; Castro, R.E.; Lapitz, A.; Izquierdo-Sanchez, L.; Bujanda, L.F.; Perugorría, M.J.; et al. Lipotoxicity-driven metabolic dysfunction-associated steatotic liver disease (MASLD). Atherosclerosis 2025, 400, 119053. [Google Scholar] [CrossRef]
- Guo, X.; Yin, X.; Liu, Z.; Wang, J. Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 15489. [Google Scholar] [CrossRef]
- Mann, V.; Sundaresan, A.; Shishodia, S. Overnutrition and Lipotoxicity: Impaired Efferocytosis and Chronic Inflammation as Precursors to Multifaceted Disease Pathogenesis. Biology 2024, 13, 241. [Google Scholar] [CrossRef]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef]
- Tu, Z.; Yang, J.; Fan, C. The role of different nutrients in the prevention and treatment of cardiovascular diseases. Front. Immunol. 2024, 15, 1393378. [Google Scholar] [CrossRef]
- Rogóż, W.; Karp, A.; Kulig, K.; Szkudlarek, A.; Owczarzy, A.; Maciążek-Jurczyk, M. The differences between the antioxidant activity of vitamin products. Med. Res. J. 2021, 6, 281–287. [Google Scholar] [CrossRef]
- Bender, D.A. The antioxidant paradox: Damage and defence. Biochem 2006, 28, 9–12. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef]
- Młynarska, E.; Hajdys, J.; Czarnik, W.; Fularski, P.; Leszto, K.; Majchrowicz, G.; Lisińska, W.; Rysz, J.; Franczyk, B. The role of antioxidants in the therapy of cardiovascular diseases—A literature review. Nutrients 2024, 16, 2587. [Google Scholar] [CrossRef] [PubMed]
- Baharuddin, B. Antioxidant Protection Mechanisms in the Cardiovascular System. Asian J. Med. Health 2024, 22, 140–146. [Google Scholar] [CrossRef]
- Riccioni, G.; Bucciarelli, T.; Mancini, B.; Corradi, F.; Di Ilio, C.; Mattei, P.A.; D’Orazio, N. Antioxidant vitamin supplementation in cardiovascular diseases. Ann. Clin. Lab. Sci. 2007, 37, 89–95. [Google Scholar] [PubMed]
- Huang, X.; Chen, H.; Wen, S.; Dong, M.; Zhou, L.; Yuan, X. Therapeutic Approaches for Nonalcoholic Fatty Liver Disease: Established Targets and Drugs. Diabetes Metab. Syndr. Obes. 2023, 16, 1809–1819. [Google Scholar] [CrossRef]
- Baradeiya, A.M.; Taghlabi, K.M.; Saleh, A.N.; Manikonda, S.; Salim, S.S. Can Nutritional Supplements Benefit Patients with Nonalcoholic Steatohepatitis and Nonalcoholic Fatty Liver Disease? Cureus 2023, 15, e40849. [Google Scholar] [CrossRef]
- Mohammadian, K.; Fakhar, F.; Keramat, S.; Stanek, A. The role of antioxidants in the treatment of metabolic dysfunction-associated fatty liver disease: A systematic review. Antioxidants 2024, 13, 797. [Google Scholar] [CrossRef]
- Sun, J.; Jin, X.; Li, Y. Current strategies for nonalcoholic fatty liver disease treatment (Review). Int. J. Mol. Med. 2024, 54, 88. [Google Scholar] [CrossRef]
- Svobodava, G.; Hornv, M.; Velecka, E.; Boušovα, I. Metabolic dysfunction-associated steatotic liver disease-induced changes in the antioxidant system: A review. Arch. Toxicol. 2025, 99, 1–22. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varra, F.-N.; Varras, M.; Varra, V.-K.; Theodosis-Nobelos, P. Mechanisms Linking Obesity with Non-Alcoholic Fatty Liver Disease (NAFLD) and Cardiovascular Diseases (CVDs)—The Role of Oxidative Stress. Curr. Issues Mol. Biol. 2025, 47, 766. https://doi.org/10.3390/cimb47090766
Varra F-N, Varras M, Varra V-K, Theodosis-Nobelos P. Mechanisms Linking Obesity with Non-Alcoholic Fatty Liver Disease (NAFLD) and Cardiovascular Diseases (CVDs)—The Role of Oxidative Stress. Current Issues in Molecular Biology. 2025; 47(9):766. https://doi.org/10.3390/cimb47090766
Chicago/Turabian StyleVarra, Fani-Niki, Michail Varras, Viktoria-Konstantina Varra, and Panagiotis Theodosis-Nobelos. 2025. "Mechanisms Linking Obesity with Non-Alcoholic Fatty Liver Disease (NAFLD) and Cardiovascular Diseases (CVDs)—The Role of Oxidative Stress" Current Issues in Molecular Biology 47, no. 9: 766. https://doi.org/10.3390/cimb47090766
APA StyleVarra, F.-N., Varras, M., Varra, V.-K., & Theodosis-Nobelos, P. (2025). Mechanisms Linking Obesity with Non-Alcoholic Fatty Liver Disease (NAFLD) and Cardiovascular Diseases (CVDs)—The Role of Oxidative Stress. Current Issues in Molecular Biology, 47(9), 766. https://doi.org/10.3390/cimb47090766





