Reactive Sulfur Species and Protein Persulfidation: An Emerging Redox Axis in Human Health and Disease
Abstract
1. Introduction
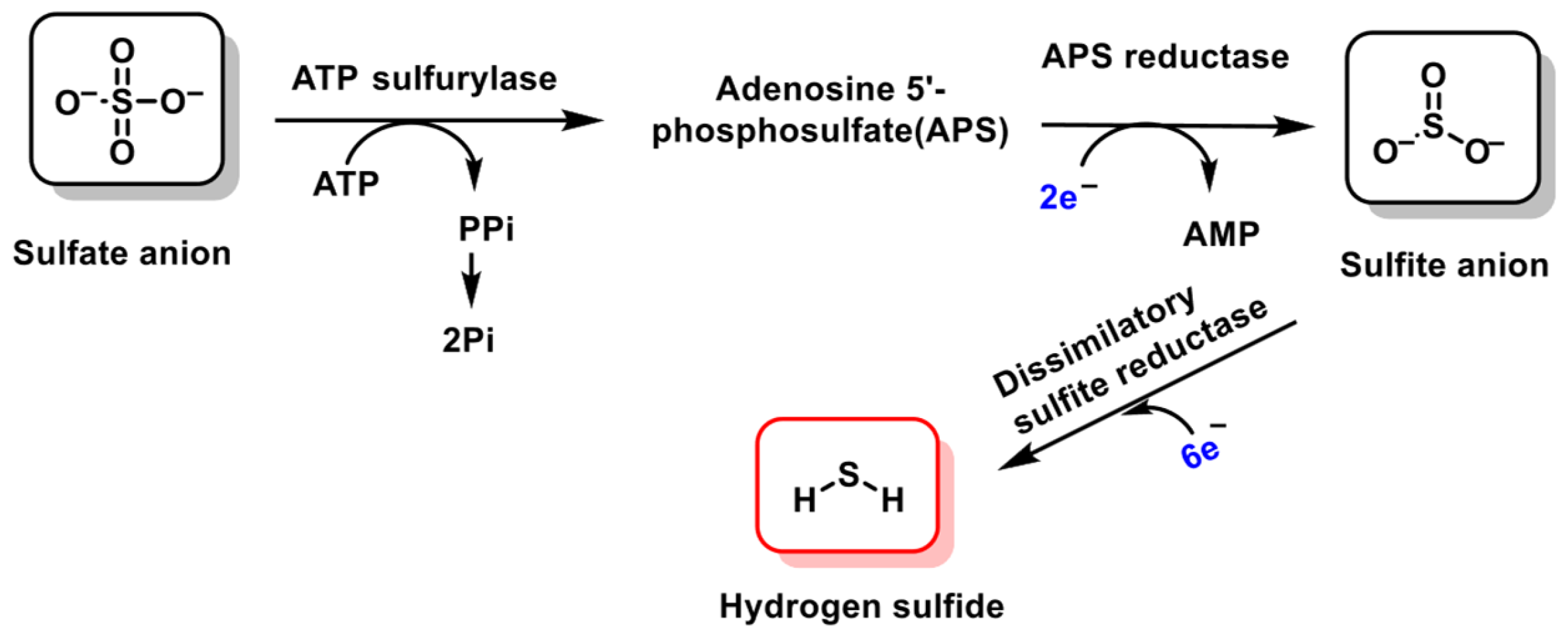
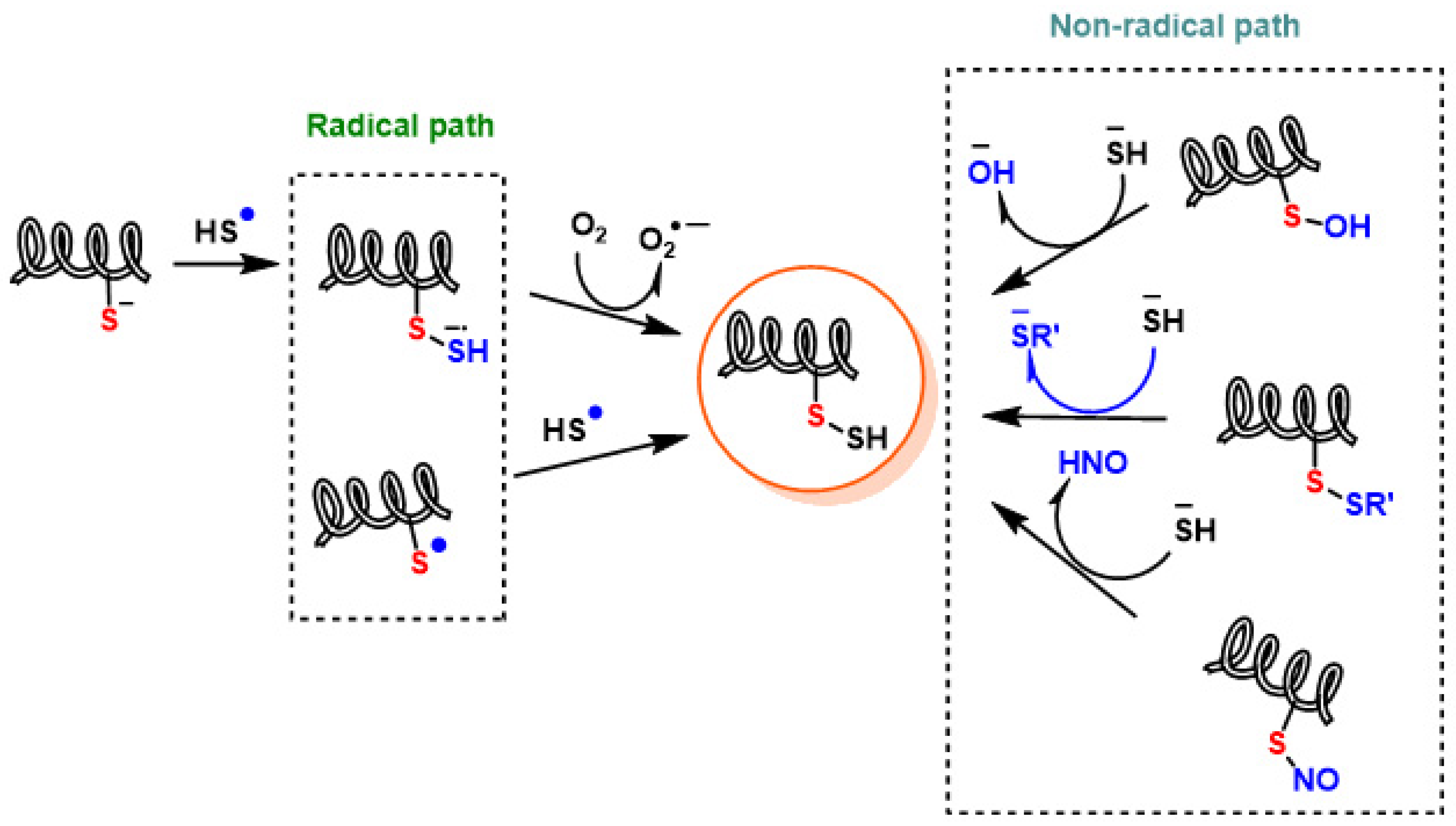
2. Chemistry of RSS. Endogenous Generation (CBS/CSE, MQO), Polysulfide Formation and Electrophilic/Nucleophilic Characteristics
Quantitative Kinetics: Persulfide Formation Versus Oxidation
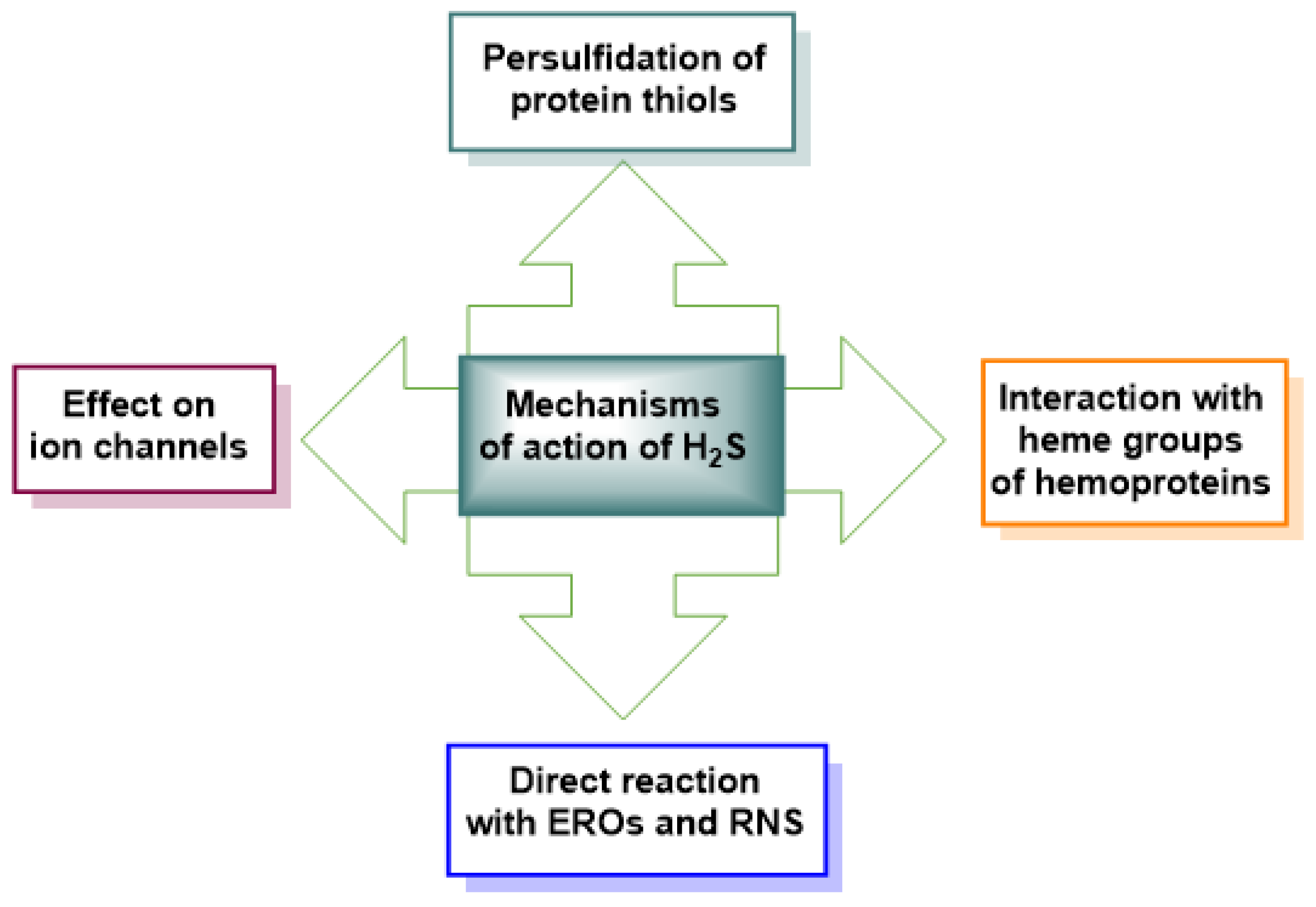
3. Protein Persulfidation. Mechanisms, Detection Techniques, Comparison with Nitrosylation and Glutathionylation
3.1. Reproducibility and Standardization in RSS Analytics
- ○
- Minimal sample-handling SOPs that stabilize RSS speciation: immediate cold quench, metal chelation and standardized alkylation/blocking prior to lysis to prevent artifactual interconversion (free H2S vs. low-MW persulfides/polysulfides vs. protein-SSH); report timing, temperature and pH at derivatization.
- ○
- Community “reference protocol” for the improved tag-switch (reagent identity/concentration, reaction times, click handles, and positive/negative controls), with site-level quantification via isotopically labeled iodoacetamide–alkyne probes to enable inter-lab comparability of occupancy changes.
- ○
- Benchmarking panels for SSP4 and related probes: define dynamic range, response factors for persulfides vs. polysulfides and mandatory orthogonal validation (e.g., parallel tag-switch or MS) to mitigate false positives; include reference datasets and spike-in materials (e.g., GSSH).
- ○
- Reporting standards for speciation and units: always distinguish free H2S, acid-labile sulfide, bound/persulfidic pools and protein-SSH; specify tissue compartment, subcellular fraction and donor release kinetics when applicable.
- ○
- Calibration/QA for real-time sensors: two-point calibration before/after runs, interference testing against SO2/NO2 and other acid gases, and recommended redundant sensing (dual cartridges) in exposure studies, until interferences and passivation are formally resolved
4. Crosstalk RSS-ROS/RNS. Synergies and Antagonisms in Mitochondria, NADPH-Oxidase and •NO-Synthases
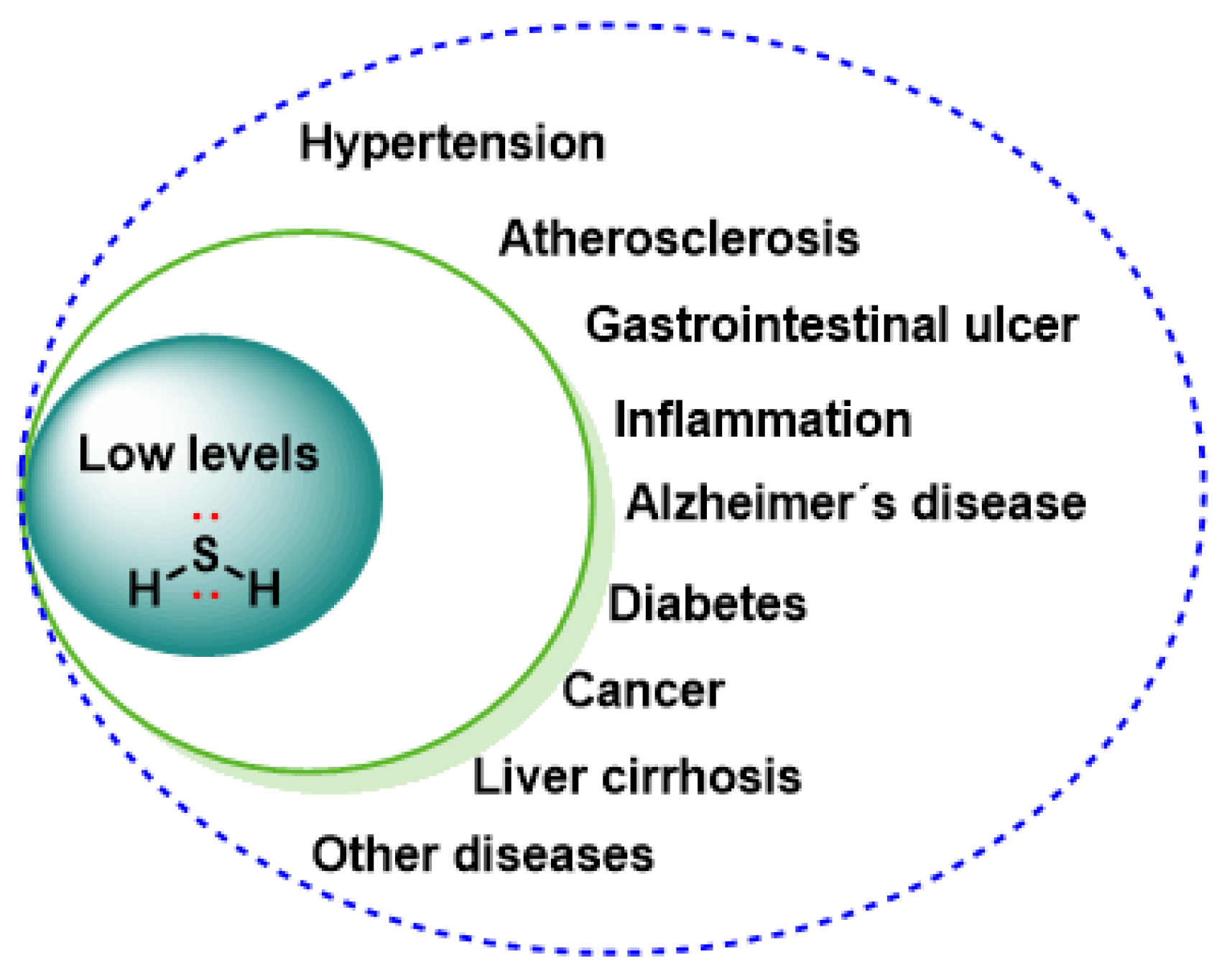
5. Physiological Role of RSS
6. RSS in Cardiovascular Disease, Aging, Sarcopenia, Neurodegeneration, Cancer and Long COVID
- Cardiovascular disease. Evidence: robust preclinical cardioprotection (ischemia–reperfusion, remodeling) and early interventional clinical signals with oral slow-release donors (e.g., SG1002) showing biomarker shifts (↑H2S/NO surrogates, ↓BNP) and acceptable safety. Uncertainties/controversies: paucity of hard outcomes, optimal dose and kinetics (salt vs. slow donors vs. mitochondria-targeted), responder phenotypes, and interaction with standard HF therapies. The bioavailability of H2S is reduced proportionally to the severity of heart failure: a study of 124 patients with varying degrees of congestion showed total sulfur concentrations of ≈5.3 [2.2–8.0] μM versus 8.5 [6.0–14.0] μM in healthy controls, and the decline was accompanied by reduced endogenous capacity to produce H2S and depressed CSE activity [91]. Preclinical models confirm the causal relationship: mice with cardiac overexpression of CSE or treated with inorganic donors (NaHS 3 mg kg−1 day−1) have smaller infarct size, better diastolic relaxation and less remodeling after ischemia–reperfusion, while genetic CSE deficiency aggravates functional impairment [92,93]. Clinical translation has begun with the oral prodrug SG1002: in a phase I/II trial in patients with NYHA II–III, step doses of 200–800 mg bid steadily increased plasma H2S, elevated nitrite (indicator of •NO), and attenuated the increase in BNP without causing symptomatic hypotension or electrocardiographic abnormalities. endorsing its safety and potential hemodynamic benefit [18]. Taken together, these data support that strengthening the CSE/H2S axis—either through gene therapy or drug donors—offers a promising strategy for limiting ischemic injury, improving diastolic function and slowing the progression of heart failure.
- Aging and sarcopenia. Evidence: preclinical data indicate that restoring H2S supports autophagy, mitochondrial biogenesis, and myofiber integrity; limited ex vivo human myotube findings. Uncertainties: translatability to older adults, long-term safety and net effects on proteostasis under comorbidities.During aging, the expression of CSE—the main enzyme that generates H2S in muscle—decreases steadily, and its loss aggravates age-related atrophy: in old mice, CSE deficiency triggers loss of lean mass and slows regeneration after cardiotoxin, while NaHS supplementation restores myogenic genes and accelerates fiber repair [94]. In parallel, reduced sulfur synthesis raises muscle ROS and contributes to the blockade of basal autophagy; recent studies have shown that replenishing the “H2S axis” with slow donors such as GYY4137—or, genetically, overexpressing CSE/CBS—reactivates the AMPK pathway → PGC-1α/ULK1, stimulates autophagy and mitochondrial biogenesis, reduces proteolysis and preserves fiber diameter, effects that translate into a significant prolongation of the half-life of senescent mouse models [95,96]. In humans, the relevance is parallel: myotubes derived from vaso-lateral biopsies exposed to sulfide or polysulfide donors show lower expression of the ubiquitin MuRF1/atrogin-1 ligases and greater induction of mitochondrial genes dependent on PGC-1α, indicating that the reactive sulfur signal retains the ability to slow down proteolysis and improve energy quality also in human tissue in vitro [97]. These findings place RSS as a promising target to counteract primary sarcopenia, either by boosting endogenous production (cysteine-rich nutraceuticals, CSE/CBS activators) or by administering controlled release clinical donors of H2S.
- Neurodegeneration. Evidence: predominantly preclinical (Parkinson’s/Alzheimer’s models: anti-inflammatory, anti-aggregative, BDNF/TrkB support). Uncertainties: brain delivery and chronic dosing of donors, cell-type specificity (neurons vs. microglia), and disease-stage heterogeneity.In the brain, physiological levels of H2S < 1 μM insulate the neuron from the double excitotoxic-oxidative stress: the gas neutralizes the excess Ca2+ that follows the overactivation of NMDA receptors and, in parallel, prevents α-synuclein from undergoing nitration and aggregating. In the MPTP model of Parkinson’s, the slow donor GYY4137 (50 mg kg−1 i.p.) preserves tyrosine hydroxylase activity, reduces α-synuclein nitration, and maintains the density of dopaminergic neurons in the substantia nigra; Under the same conditions, motor coordination improves in the Rotarod test [98]. Complementary studies with 6-OHDA show that chronic administration of NaHS (30–100 μmol kg−1) or polysulfides increases the survival of nigro-striatal neurons through the opening of K_ATP channels and the suppression of microgliosis [99].
- Cancer. Evidence level: preclinical and dominant signals: CBS/CSE/3-MST overexpression, tumor “sulfur addiction”, and xenograft sensitivity to enzyme inhibition (e.g., AOAA) or high-load donors (e.g., GYY4137). Key uncertainties/controversies: bell-shaped (biphasic) dose–response; tumor-type heterogeneity; effects on the immune microenvironment; optimal dosing kinetics and delivery; predictive biomarkers for patient selection.
- Long COVID. Evidence level: human observational + interventional pilot; dominant signals: sustained thiol (R-SH) depletion correlating with dyspnea/fatigue, and improved FEV1, mMRC, IL-6/CRP after double-blind sulfurous inhalations (~30 ppm). Key uncertainties/controversies: durability of benefit; generalizability beyond respiratory endpoints; replication in larger, multi-center cohorts; head-to-head vs. classical H2S donors; standardized analytics for target engagement.
7. Exogenous Modulation. H2S Pharmacological Donors, Smart Releasers, Dietary Compounds and Sulfur-Producing Microbiota
- Classic and slow-release drug donors. The inorganic salts sodium-hydrosulfide (NaHS) and sodium-sulfide (Na2S) continue to be the most widely used tools in vitro because they dissociate almost instantaneously and reach millimolar peaks of H2S; however, this brief and difficult to dose “burst” moves away from physiology and limits its clinical translation [114]. To overcome this obstacle, oral prodrugs and extended-release donors have been developed. SG1002 polysulfide—formulated in 200–800 mg capsules twice daily—was safe in both healthy volunteers and patients with heart failure (NYHA II-III); the 21-day treatment stably raised plasma levels of H2S and nitrite, and attenuated the rise in BNP and systolic blood pressure, results that supported its move to larger phase II studies [18]. Preclinically, thiobenzide GYY4137 releases less than 20 μM of H2S over several hours-days; this profile better mimics endogenous synthesis and has been linked to post-ischemic cardioprotection, antineoplastic action and anti-inflammatory effects with a much lower toxicity than that of fast salts [115,116]. With this evidence, slow-release donors are emerging as the most promising platform to restore the H2S pool in chronic pathologies without exceeding the cytotoxic threshold.
- “Smart” and organelle-directed donors. The second wave of compounds incorporates chemical “triggers” that deliver H2S only in specific micro-environments, avoiding the toxic spikes of fast salts. A first example is the aromatic gem-dithiol, which remain inert until they react with cellular thiols (cysteine, GSH); In doing so, they release the gas in a stoichiometric and controllable way both in physiological buffer and inside the cell [117].
- Sulfur dietary compounds. Sulfur-rich foods illustrate how nutrition can modulate the RSS pool without resorting to drugs. In garlic, lipophilic polysulfides—notably diallyl trisulfide (DATS)—are stable donors that release H2S in situ: in a mouse model of myocardial ischemia–reperfusion, DATS elevated plasma sulfide, reduced infarct size, and preserved ventricular function [125]; in addition, in rats with metabolic syndrome it improved lipid profile and attenuated cardiac dysfunction, effects linked to the sustained increase in H2S and the fall of pro-oxidant species [126].
- Sulfur-producing microbiota. In the colon, sulfate-reducing bacteria (SRB), led by Desulfovibrio spp., metabolize sulfates and sulfur amino acids to release H2S. When production is maintained in the nanomolar range, the gas supports the integrity of the intestinal barrier—for example, the CBS-H2S persulfidal pathway to the HuR protein reduces COX-2 expression and protects the epithelium against lipopolysaccharide [84]. However, the overpopulation of SRB typical of dysbiosis in ulcerative colitis or metabolic syndrome raises sulfur to millimolar concentrations that damage colonocytes, increase permeability, and fuel neuroinflammation at a distance [135,136].
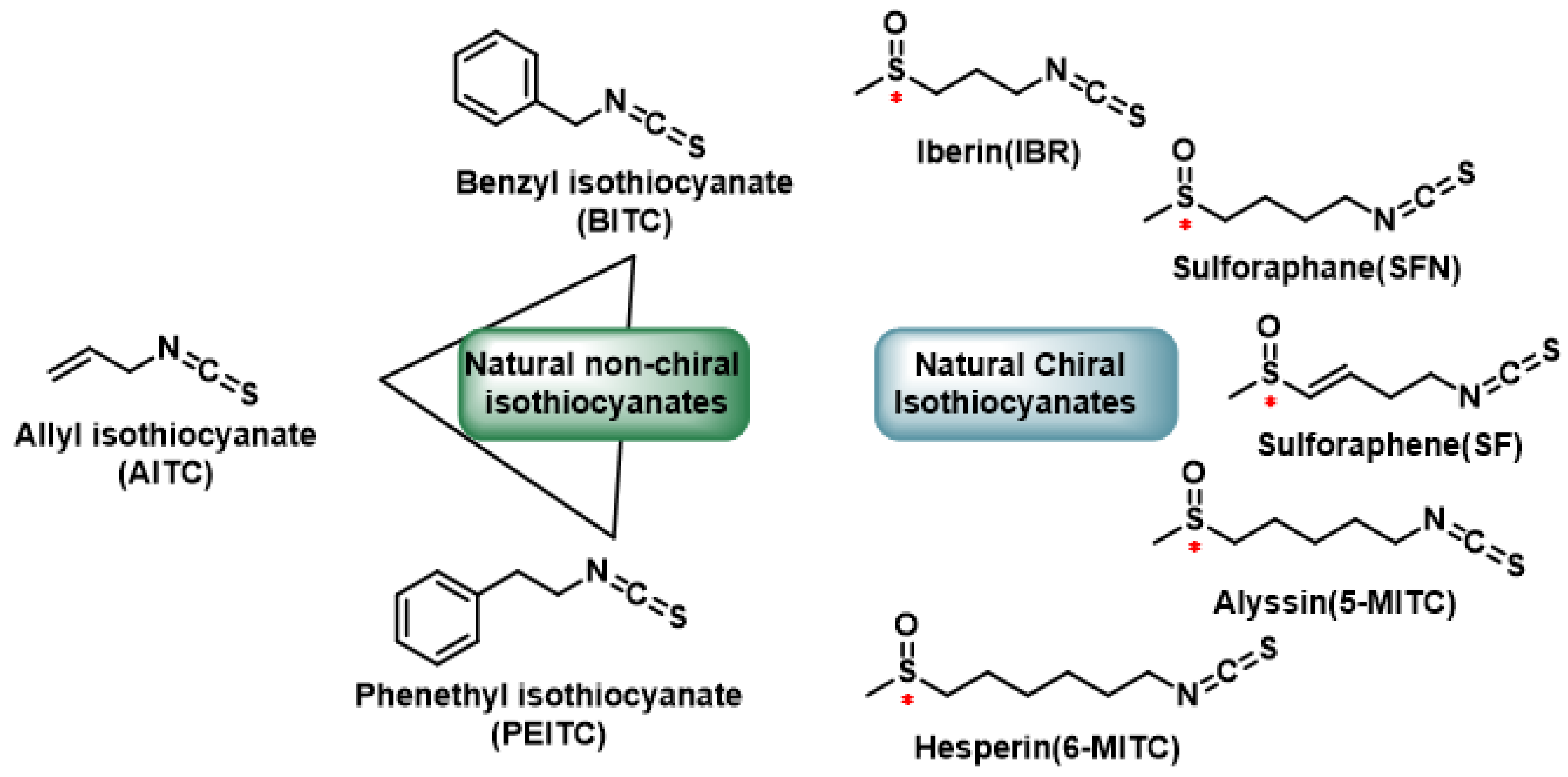
- Operational synthesis. The current therapeutic repertoire allows several layers of intervention to be superimposed on the H2S axis:(1) Endogenous production can be enhanced with a diet rich in alliums (garlic, leek) and cruciferous vegetables: the lipophilic polysulfides of garlic and the isothiocyanates of sulforaphane release H2S in situ and raise the RSS “pool” in peripheral tissues without generating toxic peaks, as has been proven both in cell assays and in studies on myocardial perfusion and tumor viability [128,139].(2) To stabilize circulating levels, slow-release oral donors—for example, thiobenzamide GYY4137—maintain sub-micromolar concentrations of H2S for hours and have already demonstrated cardioprotection and fibrosis reduction in hypertensive models, with a toxicity profile lower than that of inorganic salts [140].(3) When organelle-cellular specificity is required, “smart” platforms such as AP39 are used, which directs sulfide to the mitochondrial matrix and improves endothelial bioenergetics or neuronal survival in various injury models [141]. (4) Finally, the colonic microbiota can be modulated with fiber, polyphenols, or strains designed to oxidize excess sulfur, so that bacterial production is maintained in the protective nanomolar window without escalating to cytotoxic levels associated with colitis and neuroinflammation [142].
8. Selective Persulfidation and S-Alkylation of Reactive Cysteines of Proteins and Transcription Factors
8.1. Selective Persulfidation and S-Alkylation of Reactive Cysteines in Keap1
8.2. Selective Persulfidation and S-Alkylation of Reactive Cysteines in NF-kB
- Persulfidation as the default rheostat. Persulfidation has emerged as the fast “volume-control” that tunes every tier of the canonical NF-κB pathway. Stopped-flow competition experiments on a well-characterised sulfenic-acid model (Mt AhpE–SOH) give a pH-independent second-order rate constant of 1.4 × 103 M−1 s−1 for attack by HS−, after correction for protonation equilibria. This value sits squarely inside the 1–3 × 104 M−1 s−1 window quoted for persulfide formation on IKKβ Cys179 [146].
- Protection against irreversible damage. Persulfidation safeguards the same hotspots from progression to sulfinic/sulfonic acid during oxidative bursts. Cells exposed to 100 µM H2O2 for 5 min accumulate 20–25% sulfinylated Cys38, but co-treatment with 1 µM polysulfides diverts > 80% of the modification to persulfide, which the thioredoxin system then clears within 10 min, restoring full DNA-binding capacity and curbing apoptotic signaling [53,166,167].
- S-Alkylation as an emergency brake. S-Alkylation functions as a rapid, irreversible brake on NF-κB signaling. Electrophilic inhibitors—such as the sesquiterpene lactone parthenolide, the vinyl sulfone BAY 11-7082, sulforaphane and dimethyl fumarate (DMF), (Figure 13), and the isothiocyanate sulforaphane—attack the critical Cys38/Cys179/Lys171 motif of RelA(p65) and IKKβ with second-order rate constants of approximately 102 M−1 s−1—some 102–103-fold slower than protective persulfidation reactions [168,169]. These Michael additions generate stable C–S adducts that cannot be reversed by cellular glutathione. As a result, they produce (i): cys38 alkylation on RelA blocks its DNA-binding capability, and (ii) cys179 alkylation on IKKβ abolishes kinase activity and forms a reactive sulfonium, which can cross-link to Lys171, “locking” the activation loop in an open, inactive conformation [170,171].
8.3. Selective Persulfidation and S-Alkylation of Reactive Cysteines in STAT3
9. Therapeutic Perspectives. Clinical Trials, Combined Strategy with Classic Antioxidants, Limitations and Analytical Challenges
9.1. Clinical Trials
9.2. Combined Strategy
9.3. Limitations and Analytical Challenges
9.4. Safety, Contraindications and Drug–Drug Interactions
10. Combination and Sequential Therapies Based on Reactive Sulfur Species/Persulfidation
- Intravenous “flash” donors such as IK-1001 (Na2S) deliver a sharp H2S/RSS peak lasting only a few hours—useful in peri-ischemic settings but unsuited to longer phases (Clinical trial NCT00858936).
10.1. Condensed Clinical Roadmap
10.2. Clinical Sequence
- If H2S < 0.3 µM → increase SG1002 by 200 mg steps (max 1 g BID).
- If H2S 5–10 µM and lactate steady → maintain.
- If H2S > 10 µM or lactate rises → halve or hold one dose and re-check in 48 h [195].
- Sustained free H2S above ~10 µM blocks cytochrome-c oxidase and flips the gas from cytoprotective to cytotoxic.
- STS can transiently chelate calcium; check ionised Ca2+ and QTc in renally impaired patients.
- Combining any sulfide donor with high-dose nitrates or PDE-5 inhibitors may amplify hypotension—space administrations by several hours.
- Sprinter—a brief i.v. sulfide or thiosulfate surge buys time during the first 24 h of ischemia–reperfusion or fulminant inflammation.
- Middle-distance runner—slow-release SG1002 (±NAC) keeps micromolar persulfidation humming through the sub-acute week.
- Marathoner—diet and sulfur-rich spa interventions retrain endogenous and microbiome sulfur metabolism for the long haul.
11. Conclusions
- Slow-release oral donors (SG1002, GYY4137) sustain safe H2S elevations.
- Organelle-targeted vectors (AP39, gem-dithiol pro-drugs) fine-tune delivery within mitochondria.
- Multi-gas hybrids synergize sulfur with NO or CO.
- Dietary interventions (garlic polysulfides, cruciferous isothiocyanates) and microbiota engineering reinforce endogenous RSS tone.
Author Contributions
Funding
Conflicts of Interest
References
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Radomski, M.W.; Palmer, R.M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem. Pharmacol. 1988, 37, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.; Pluth, M.D. Reactive sulfur species (RSS): Persulfides, polysulfides, potential, and problems. Curr. Opin. Chem. Biol. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Vignane, T.; Filipovic, M.R. Emerging Chemical Biology of Protein Persulfidation. Antioxid. Redox Signal. 2023, 39, 19–39, Correction in Antioxid. Redox Signal. 2023, 39, 620. https://doi.org/10.1089/ars.2023.0352.correx. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Sen, U.; Sathnur, P.B.; Kundu, S.; Givvimani, S.; Coley, D.M.; Mishra, P.K.; Qipshidze, N.; Tyagi, N.; Metreveli, N.; Tyagi, S.C. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. Am. J. Physiol. Cell Physiol. 2012, 303, C41–C51. [Google Scholar] [CrossRef]
- Wade Wolfe, M.M.; Pluth, M.D. Understanding Reactive Sulfur Species through P/S Synergy. Inorg. Chem. 2023, 62, 14339–14343. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Kevil, C.G. Reactive Sulfur Species: A New Redox Player in Cardiovascular Pathophysiology. Arterioscler. Thromb Vasc. Biol. 2020, 40, 874–884. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide: Its production and functions. Exp. Physiol. 2011, 96, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.I.; Nasim, M.J.; Ali, W.; Jacob, C. The Reactive Sulfur Species Concept: 15 Years On. Antioxidants 2017, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef] [PubMed]
- Tiranti, V.; Viscomi, C.; Hildebrandt, T.; Di Meo, I.; Mineri, R.; Tiveron, C.; Levitt, M.D.; Prelle, A.; Fagiolari, G.; Rimoldi, M.; et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 2009, 15, 200–205. [Google Scholar] [CrossRef]
- Wedmann, R.; Onderka, C.; Wei, S.; Szijártó, I.A.; Miljkovic, J.L.; Mitrovic, A.; Lange, M.; Savitsky, S.; Yadav, P.K.; Torregrossa, R.; et al. Improved tag-switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem. Sci. 2016, 7, 3414–3426. [Google Scholar] [CrossRef]
- Rusetskaya, N.Y.; Loginova, N.Y.; Pokrovskaya, E.; Chesovskikh, Y.S.; Titova, L. Redox regulation of the NLRP3-mediated inflammation and pyroptosis. Biomeditsinskaya Khimiya 2023, 69, 333–352. [Google Scholar] [CrossRef]
- Polhemus, D.J.; Li, Z.; Pattillo, C.B.; Gojon, G., Sr.; Gojon, G., Jr.; Giordano, T.; Krum, H. A Novel Hydrogen Sulfide Prodrug, SG1002, Promotes Hydrogen Sulfide and Nitric Oxide Bioavailability in Heart Failure Patients. Cardiovasc. Ther. 2015, 33, 216–226. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Sulfide-Pathological and Physiological Functions in Mammalian Cells. Cells 2023, 12, 2684. [Google Scholar] [CrossRef]
- Zuhra, K.; Augsburger, F.; Majtan, T.; Szabo, C. Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition. Biomolecules 2020, 10, 697. [Google Scholar] [CrossRef]
- Zhu, H.; Blake, S.; Chan, K.T.; Pearson, R.B.; Kang, J. Cystathionine β-Synthase in Physiology and Cancer. BioMed Res. Int. 2018, 2018, 3205125. [Google Scholar] [CrossRef]
- Xia, H.; Li, Z.; Sharp, T.E., 3rd; Polhemus, D.J.; Carnal, J.; Moles, K.H.; Tao, Y.X.; Elrod, J.; Pfeilschifter, J.; Beck, K.F.; et al. Endothelial Cell Cystathionine γ-Lyase Expression Level Modulates Exercise Capacity, Vascular Function, and Myocardial Ischemia Reperfusion Injury. J. Am. Heart Assoc. 2020, 9, e017544. [Google Scholar] [CrossRef]
- Mistry, R.K.; Murray, T.V.A.; Prysyazhna, O.; Martin, D.; Burgoyne, J.R.; Santos, C.; Eaton, P.; Shah, A.M.; Brewer, A.C. Transcriptional Regulation of Cystathionine-γ-Lyase in Endothelial Cells by NADPH Oxidase 4-Dependent Signaling. J. Biol. Chem. 2016, 291, 1774–1788. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.P.; Dobariya, P.; Bellamkonda, H.; More, S.S. Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease. Antioxidants 2023, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.D.; Sanchez-Aranguren, L.; Marwah, M.; Wang, K.; Spickett, C.M.; Griffiths, H.R.; Dias, I.H.K. Exploring mitochondrial hydrogen sulfide signalling for therapeutic interventions in vascular diseases. Adv. Redox Res. 2022, 4, 100030. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef]
- Landry, A.P.; Moon, S.; Kim, H.; Yadav, P.K.; Guha, A.; Cho, U.-S.; Banerjee, R. A Catalytic Trisulfide in Human Sulfide Quinone Oxidoreductase Catalyzes Coenzyme A Persulfide Synthesis and Inhibits Butyrate Oxidation. Cell Chem. Biol. 2019, 26, 1515–1525.e1514. [Google Scholar] [CrossRef]
- Motl, N.; Skiba, M.A.; Kabil, O.; Smith, J.L.; Banerjee, R. Structural and biochemical analyses indicate that a bacterial persulfide dioxygenase-rhodanese fusion protein functions in sulfur assimilation. J. Biol. Chem. 2017, 292, 14026–14038. [Google Scholar] [CrossRef]
- Luna-Sánchez, M.; Hidalgo-Gutiérrez, A.; Hildebrandt, T.M.; Chaves-Serrano, J.; Barriocanal-Casado, E.; Santos-Fandila, Á.; Romero, M.; Sayed, R.K.; Duarte, J.; Prokisch, H.; et al. CoQ deficiency causes disruption of mitochondrial sulfide oxidation, a new pathomechanism associated with this syndrome. EMBO Mol. Med. 2017, 9, 78–95. [Google Scholar] [CrossRef]
- Carbonero, F.; Benefiel, A.C.; Alizadeh-Ghamsari, A.H.; Gaskins, H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012, 3, 448. [Google Scholar] [CrossRef] [PubMed]
- Loubinoux, J.; Bronowicki, J.P.; Pereira, I.A.; Mougenel, J.L.; Faou, A.E. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol. Ecol. 2002, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-D.; Jin, S.; Cheng, R.-X.; Cai, W.-J.; Xue, W.-L.; Zhang, Q.-Q.; Yang, L.-J.; Zhu, Q.; Li, M.-Y.; Lin, G.; et al. Hydrogen sulfide functions as a micro-modulator bound at the copper active site of Cu/Zn-SOD to regulate the catalytic activity of the enzyme. Cell Rep. 2023, 42, 112750. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Tanaka, T.; Kato, Y.; Nishiyama, K.; Nishida, M. Cardiac robustness regulated by reactive sulfur species. J. Clin. Biochem. Nutr. 2022, 70, 1–6. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Benchoam, D.; Semelak, J.A.; Möller, M.N.; Zeida, A.; Trujillo, M.; Alvarez, B.; Estrin, D.A. Possible molecular basis of the biochemical effects of cysteine-derived persulfides. Front. Mol. Biosci. 2022, 9, 975988. [Google Scholar] [CrossRef]
- Bolton, S.G.; Pluth, M.D. Efficient inhibition of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by sulfuration with solubilized elemental sulfur. Free Radic. Biol. Med. 2022, 185, 46–51. [Google Scholar] [CrossRef]
- Xie, L.; Gu, Y.; Wen, M.; Zhao, S.; Wang, W.; Ma, Y.; Meng, G.; Han, Y.; Wang, Y.; Liu, G.; et al. Hydrogen Sulfide Induces Keap1 S-sulfhydration and Suppresses Diabetes-Accelerated Atherosclerosis via Nrf2 Activation. Diabetes 2016, 65, 3171–3184. [Google Scholar] [CrossRef]
- Murray, J.S. The Formation of σ-Hole Bonds: A Physical Interpretation. Molecules 2024, 29, 600. [Google Scholar] [CrossRef]
- Dóka, É.; Pader, I.; Bíró, A.; Johansson, K.; Cheng, Q.; Ballagó, K.; Prigge, J.R.; Pastor-Flores, D.; Dick, T.P.; Schmidt, E.E.; et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016, 2, e1500968. [Google Scholar] [CrossRef]
- Zheng, C.; Guo, S.; Tennant, W.G.; Pradhan, P.K.; Black, K.A.; Dos Santos, P.C. The Thioredoxin System Reduces Protein Persulfide Intermediates Formed during the Synthesis of Thio-Cofactors in Bacillus subtilis. Biochemistry 2019, 58, 1892–1904. [Google Scholar] [CrossRef]
- Benchoam, D.; Cuevasanta, E.; Möller, M.N.; Alvarez, B. Hydrogen Sulfide and Persulfides Oxidation by Biologically Relevant Oxidizing Species. Antioxidants 2019, 8, 48. [Google Scholar] [CrossRef]
- Zhang, D.; Du, J.; Tang, C.; Huang, Y.; Jin, H. H2S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017, 8, 608. [Google Scholar] [CrossRef]
- Benchoam, D.; Cuevasanta, E.; Möller, M.N.; Alvarez, B. Persulfides, at the crossroads between hydrogen sulfide and thiols. Essays Biochem. 2020, 64, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R. Persulfidation (S-sulfhydration) and H2S. Handb. Exp. Pharmacol. 2015, 230, 29–59. [Google Scholar] [CrossRef] [PubMed]
- Kouroussis, E.; Adhikari, B.; Zivanovic, J.; Filipovic, M.R. Measurement of Protein Persulfidation: Improved Tag-Switch Method. Methods Mol. Biol. 2019, 2007, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Shieh, M.; Ni, X.; Xu, S.; Lindahl, S.P.; Yang, M.; Matsunaga, T.; Flaumenhaft, R.; Akaike, T.; Xian, M. Shining a light on SSP4: A comprehensive analysis and biological applications for the detection of sulfane sulfurs. Redox Biol. 2022, 56, 102433. [Google Scholar] [CrossRef]
- Abo, M.; Li, C.; Weerapana, E. Isotopically-Labeled Iodoacetamide-Alkyne Probes for Quantitative Cysteine-Reactivity Profiling. Mol. Pharm. 2018, 15, 743–749. [Google Scholar] [CrossRef]
- Fu, L.; Liu, K.; He, J.; Tian, C.; Yu, X.; Yang, J. Direct Proteomic Mapping of Cysteine Persulfidation. Antioxid. Redox Signal. 2020, 33, 1061–1076. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, X.; Li, Z.; Yan, H.; Tian, X.; Luo, C.; Ma, K.; Li, L.; Zhang, L. Hydrogen sulfide mitigates ox-LDL-induced NLRP3/caspase-1/GSDMD dependent macrophage pyroptosis by S-sulfhydrating caspase-1. Mol. Med. Rep. 2024, 30, 135. [Google Scholar] [CrossRef]
- Cao, X.; Xiong, S.; Zhou, Y.; Wu, Z.; Ding, L.; Zhu, Y.; Wood, M.E.; Whiteman, M.; Moore, P.K.; Bian, J.S. Renal Protective Effect of Hydrogen Sulfide in Cisplatin-Induced Nephrotoxicity. Antioxid. Redox Signal. 2018, 29, 455–470. [Google Scholar] [CrossRef]
- Altaany, Z.; Ju, Y.; Yang, G.; Wang, R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci. Signal. 2014, 7, ra87. [Google Scholar] [CrossRef] [PubMed]
- Marcolongo, J.P.; Venâncio, M.F.; Rocha, W.R.; Doctorovich, F.; Olabe, J.A. NO/H2S “Crosstalk” Reactions. The Role of Thionitrites (SNO–) and Perthionitrites (SSNO–). Inorg. Chem. 2019, 58, 14981–14997. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Fang, H.; Gao, R.; Liao, W. Protein Persulfidation in Plants: Function and Mechanism. Antioxidants 2021, 10, 1631. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Fernandez, B.O.; Santos, J.L.; Mergia, E.; Grman, M.; Nagy, P.; Kelm, M.; Butler, A.; Feelisch, M. Nitrosopersulfide (SSNO–) accounts for sustained NO bioactivity of S-nitrosothiols following reaction with sulfide. Redox Biol. 2014, 2, 234–244. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Z.W.; Singh, S.; Townsend, D.M.; Tew, K.D. An evolving understanding of the S-glutathionylation cycle in pathways of redox regulation. Free Radic. Biol. Med. 2018, 120, 204–216. [Google Scholar] [CrossRef]
- Tossounian, M.A.; Zhang, B.; Gout, I. The Writers, Readers, and Erasers in Redox Regulation of GAPDH. Antioxidants 2020, 9, 1288. [Google Scholar] [CrossRef]
- Gao, X.H.; Li, L.; Parisien, M.; Wu, J.; Bederman, I.; Gao, Z.; Krokowski, D.; Chirieleison, S.M.; Abbott, D.; Wang, B.; et al. Discovery of a Redox Thiol Switch: Implications for Cellular Energy Metabolism. Mol. Cell Proteom. 2020, 19, 852–870. [Google Scholar] [CrossRef]
- Yang, C.T.; Devarie-Baez, N.O.; Hamsath, A.; Fu, X.D.; Xian, M. S-Persulfidation: Chemistry, Chemical Biology, and Significance in Health and Disease. Antioxid. Redox Signal. 2020, 33, 1092–1114. [Google Scholar] [CrossRef]
- Carballal, S.; Trujillo, M.; Cuevasanta, E.; Bartesaghi, S.; Möller, M.N.; Folkes, L.K.; García-Bereguiaín, M.A.; Gutiérrez-Merino, C.; Wardman, P.; Denicola, A.; et al. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic. Biol. Med. 2011, 50, 196–205. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Zeida, A.; Carballal, S.; Wedmann, R.; Morzan, U.N.; Trujillo, M.; Radi, R.; Estrin, D.A.; Filipovic, M.R.; Alvarez, B. Insights into the mechanism of the reaction between hydrogen sulfide and peroxynitrite. Free Radic. Biol. Med. 2015, 80, 93–100. [Google Scholar] [CrossRef]
- Nagy, P.; Winterbourn, C.C. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem. Res. Toxicol. 2010, 23, 1541–1543. [Google Scholar] [CrossRef]
- Munteanu, C.; Galaction, A.I.; Onose, G.; Turnea, M.; Rotariu, M. The Janus Face of Oxidative Stress and Hydrogen Sulfide: Insights into Neurodegenerative Disease Pathogenesis. Antioxidants 2025, 14, 360. [Google Scholar] [CrossRef]
- Huang, D.; Jing, G.; Zhu, S. Regulation of Mitochondrial Respiration by Hydrogen Sulfide. Antioxidants 2023, 12, 1644. [Google Scholar] [CrossRef]
- Stubbert, D.; Prysyazhna, O.; Rudyk, O.; Scotcher, J.; Burgoyne, J.R.; Eaton, P. Protein kinase G Iα oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension 2014, 64, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Armstrong, J.S.; Chu, S.H.; Jia-Ling, S.; Wong, B.S.; Cheung, N.S.; Halliwell, B.; Moore, P.K. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ’scavenger’? J. Neurochem. 2004, 90, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Streeter, E.; Ng, H.H.; Hart, J.L. Hydrogen sulfide as a vasculoprotective factor. Med. Gas Res. 2013, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, H.; Guo, N. Protective effect of hydrogen sulfide on the kidney (Review). Mol. Med. Rep. 2021, 24, 696. [Google Scholar] [CrossRef]
- Montanaro, R.; Vellecco, V.; Torregrossa, R.; Casillo, G.M.; Manzo, O.L.; Mitidieri, E.; Bucci, M.; Castaldo, S.; Sorrentino, R.; Whiteman, M.; et al. Hydrogen sulfide donor AP123 restores endothelial nitric oxide-dependent vascular function in hyperglycemia via a CREB-dependent pathway. Redox Biol. 2023, 62, 102657. [Google Scholar] [CrossRef]
- Altaany, Z.; Yang, G.; Wang, R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J. Cell Mol. Med. 2013, 17, 879–888. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kuhnle, G.G.C.; Dyson, A.; Fernandez, B.O.; Grman, M.; DuMond, J.F.; Barrow, M.P.; McLeod, G.; Nakagawa, H.; Ondrias, K.; et al. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. USA 2015, 112, E4651–E4660. [Google Scholar] [CrossRef]
- Kumar, R.; Landry, A.P.; Guha, A.; Vitvitsky, V.; Lee, H.J.; Seike, K.; Reddy, P.; Lyssiotis, C.A.; Banerjee, R. A redox cycle with complex II prioritizes sulfide quinone oxidoreductase-dependent H2S oxidation. J. Biol. Chem. 2022, 298, 101435. [Google Scholar] [CrossRef]
- Kanemaru, E.; Shimoda, K.; Marutani, E.; Morita, M.; Miranda, M.; Miyazaki, Y.; Sinow, C.; Sharma, R.; Dong, F.; Bloch, D.B.; et al. Exclusion of sulfide:quinone oxidoreductase from mitochondria causes Leigh-like disease in mice by impairing sulfide metabolism. J. Clin. Investig. 2024, 134, 170994. [Google Scholar] [CrossRef]
- Baruah, P.; Padhi, D.; Moorthy, H.; Ramesh, M.; Govindaraju, T. Navigating the dichotomy of reactive oxygen, nitrogen, and sulfur species: Detection strategies and therapeutic interventions. RSC Chem. Biol. 2025, 6, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Turnea, M.A.; Rotariu, M. Hydrogen Sulfide: An Emerging Regulator of Oxidative Stress and Cellular Homeostasis—A Comprehensive One-Year Review. Antioxidants 2023, 12, 1737. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Gadalla, M.M.; Snyder, S.H. Signaling by gasotransmitters. Sci. Signal. 2009, 2, re2. [Google Scholar] [CrossRef]
- Shimizu, Y.; Polavarapu, R.; Eskla, K.L.; Nicholson, C.K.; Koczor, C.A.; Wang, R.; Lewis, W.; Shiva, S.; Lefer, D.J.; Calvert, J.W. Hydrogen sulfide regulates cardiac mitochondrial biogenesis via the activation of AMPK. J. Mol. Cell Cardiol. 2018, 116, 29–40. [Google Scholar] [CrossRef]
- Munteanu, C.; Popescu, C.; Vlădulescu-Trandafir, A.I.; Onose, G. Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review. Antioxidants 2024, 13, 1158. [Google Scholar] [CrossRef]
- Castelblanco, M.; Lugrin, J.; Ehirchiou, D.; Nasi, S.; Ishii, I.; So, A.; Martinon, F.; Busso, N. Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J. Biol. Chem. 2018, 293, 2546–2557. [Google Scholar] [CrossRef]
- Bai, X.; Batallé, G.; Martínez-Martel, I.; Pol, O. Hydrogen Sulfide Interacting with Cannabinoid 2 Receptors during Sciatic Nerve Injury-Induced Neuropathic Pain. Antioxidants 2023, 12, 1179. [Google Scholar] [CrossRef]
- Predmore, B.L.; Julian, D.; Cardounel, A.J. Hydrogen sulfide increases nitric oxide production from endothelial cells by an akt-dependent mechanism. Front. Physiol. 2011, 2, 104. [Google Scholar] [CrossRef] [PubMed]
- Macabrey, D.; Longchamp, A.; Déglise, S.; Allagnat, F. Clinical Use of Hydrogen Sulfide to Protect Against Intimal Hyperplasia. Front. Cardiovasc. Med. 2022, 9, 876639. [Google Scholar] [CrossRef] [PubMed]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Módis, K.; Panopoulos, P.; Asimakopoulou, A.; Gerö, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, Z.; Zhu, J.; Yue, T.; Wang, X.; Pan, Y.; Bu, D.; Liu, Y.; Wang, P.; Chen, S. CBS-H2S axis preserves the intestinal barrier function by inhibiting COX-2 through sulfhydrating human antigen R in colitis. J. Adv. Res. 2023, 44, 201–212. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, P.; Yang, Y.; Ge, H.; Chen, W.; Qu, J.; Shi, J.; Cui, G.; Liu, X.; Feng, H.; et al. Endogenous hydrogen sulphide attenuates NLRP3 inflammasome-mediated neuroinflammation by suppressing the P2X7 receptor after intracerebral haemorrhage in rats. J. Neuroinflammation 2017, 14, 163. [Google Scholar] [CrossRef]
- Hu, M.; Zou, W.; Wang, C.Y.; Chen, X.; Tan, H.Y.; Zeng, H.Y.; Zhang, P.; Gu, H.F.; Tang, X.Q. Hydrogen Sulfide Protects against Chronic Unpredictable Mild Stress-Induced Oxidative Stress in Hippocampus by Upregulation of BDNF-TrkB Pathway. Oxid. Med. Cell Longev. 2016, 2016, 2153745. [Google Scholar] [CrossRef]
- Gu, B.; Li, T.; Zhao, H.; Yue, R.; Luo, Q.; Yu, S.; Li, T.; Zhao, Y.; Liu, D.; Wang, Z.; et al. Age-dependent effects of H2S on post-traumatic stress disorder in adolescent and adult mice. Front. Psychiatry 2025, 16, 1546737. [Google Scholar] [CrossRef]
- Bao, P.; Gong, Y.; Wang, Y.; Xu, M.; Qian, Z.; Ni, X.; Lu, J. Hydrogen Sulfide Prevents LPS-Induced Depression-like Behavior through the Suppression of NLRP3 Inflammasome and Pyroptosis and the Improvement of Mitochondrial Function in the Hippocampus of Mice. Biology 2023, 12, 1092. [Google Scholar] [CrossRef]
- Zhao, S.; Li, X.; Lu, P.; Li, X.; Sun, M.; Wang, H. The Role of the Signaling Pathways Involved in the Effects of Hydrogen Sulfide on Endoplasmic Reticulum Stress. Front. Cell Dev. Biol. 2021, 9, 646723. [Google Scholar] [CrossRef]
- Hacioglu, G.; Cirrik, S.; Tezcan Yavuz, B.; Tomruk, C.; Keskin, A.; Uzunoglu, E.; Takir, S. The BDNF-TrkB signaling pathway is partially involved in the neuroprotective effects of hydrogen sulfide in Parkinson’s disease. Eur. J. Pharmacol. 2023, 944, 175595. [Google Scholar] [CrossRef]
- Kovačić, D.; Glavnik, N.; Marinšek, M.; Zagožen, P.; Rovan, K.; Goslar, T.; Marš, T.; Podbregar, M. Total plasma sulfide in congestive heart failure. J. Card. Fail 2012, 18, 541–548. [Google Scholar] [CrossRef]
- Kondo, K.; Bhushan, S.; King, A.L.; Prabhu, S.D.; Hamid, T.; Koenig, S.; Murohara, T.; Predmore, B.L.; Gojon, G., Sr.; Gojon, G., Jr.; et al. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 2013, 127, 1116–1127. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Guo, W.; Zhu, Y.Z. Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure: A mechanism through cardiac mitochondrial protection. Biosci. Rep. 2011, 31, 87–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Masters, L.; Wang, Y.; Wu, L.; Pei, Y.; Guo, B.; Parissenti, A.; Lees, S.J.; Wang, R.; Yang, G. Cystathionine gamma-lyase/H2S signaling facilitates myogenesis under aging and injury condition. FASEJ J. 2021, 35, e21511. [Google Scholar] [CrossRef]
- Xu, M.; Liu, X.; Bao, P.; Wang, Y.J.; Lu, J.; Liu, Y.J. H2S Protects Against Immobilization-Induced Muscle Atrophy via Reducing Oxidative Stress and Inflammation. Front. Physiol. 2022, 13, 844539. [Google Scholar] [CrossRef]
- Yang, J.H.; Gao, J.; E, Y.Q.; Jiao, L.J.; Wu, R.; Yan, Q.Y.; Wei, Z.Y.; Yan, G.L.; Liang, J.L.; Li, H.Z. Hydrogen sulfide inhibits skeletal muscle ageing by up-regulating autophagy through promoting deubiquitination of adenosine 5’-monophosphate (AMP)-activated protein kinase α1 via ubiquitin specific peptidase 5. J. Cachexia Sarcopenia Muscle 2024, 15, 2118–2133. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, M.; Wang, R.; Wang, X.; Jiao, H.; Zhao, J.; Zhou, Y.; Li, H.; Lin, H. Hydrogen Sulfide Regulates Glucose Uptake in Skeletal Muscles via S-Sulfhydration of AMPK in Muscle Fiber Type-Dependent Way. J. Nutr. 2023, 153, 2878–2892. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.; Schwarz, D. Vegetable Grafting: The Implications of a Growing Agronomic Imperative for Vegetable Fruit Quality and Nutritive Value. Front. Plant. Sci. 2017, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Minaei, A.; Sarookhani, M.R.; Haghdoost-Yazdi, H.; Rajaei, F. Hydrogen sulfide attenuates induction and prevents progress of the 6-hydroxydopamine-induced Parkinsonism in rat through activation of ATP-sensitive potassium channels and suppression of ER stress. Toxicol. Appl. Pharmacol. 2021, 423, 115558. [Google Scholar] [CrossRef]
- Munteanu, C.; Onose, G.; Rotariu, M.; Poștaru, M.; Turnea, M.; Galaction, A.I. Role of Microbiota-Derived Hydrogen Sulfide (H2S) in Modulating the Gut–Brain Axis: Implications for Alzheimer’s and Parkinson’s Disease Pathogenesis. Biomedicines 2024, 12, 2670. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Bian, J.S. Hydrogen sulfide protects amyloid-β induced cell toxicity in microglia. J. Alzheimers Dis. 2010, 22, 1189–1200. [Google Scholar] [CrossRef]
- Szabo, C.; Coletta, C.; Chao, C.; Módis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474–12479. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, Y.; Zhou, J.; Tan, M.; Xue, Y.; Tao, Y. Hydrogen Sulfide Expression May Affect Ovarian Cancer Progression and Drug Resistance. Int. J. Womens Health 2025, 17, 1533–1545. [Google Scholar] [CrossRef]
- Khattak, S.; Rauf, M.A.; Khan, N.H.; Zhang, Q.Q.; Chen, H.J.; Muhammad, P.; Ansari, M.A.; Alomary, M.N.; Jahangir, M.; Zhang, C.Y.; et al. Hydrogen Sulfide Biology and Its Role in Cancer. Molecules 2022, 27, 3389. [Google Scholar] [CrossRef] [PubMed]
- Untereiner, A.A.; Pavlidou, A.; Druzhyna, N.; Papapetropoulos, A.; Hellmich, M.R.; Szabo, C. Drug resistance induces the upregulation of H2S-producing enzymes in HCT116 colon cancer cells. Biochem. Pharmacol. 2018, 149, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Zhang, K.; Zhou, Z.T.; Jiang, Z.L.; Liu, Y.; Zhang, Y.X.; Liu, Z.H.; Ji, X.Y.; Wu, D.D. The Role of Hydrogen Sulfide in the Development and Progression of Lung Cancer. Molecules 2022, 27, 9005. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ding, L.; Xie, Z.Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y. Hydrogen sulfide: A rising star for cancer treatment. Med. Gas Res. 2025, 15, 114–116. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Y.-F.; Zhang, Y.-X.; Wang, Y.; Jin, Y.-Q.; Yuan, H.; Liang, X.-Y.; Ji, X.-Y.; Jiang, Q.-Y.; Wu, D.-D. The potential role of hydrogen sulfide in cancer cell apoptosis. Cell Death Discov. 2024, 10, 114. [Google Scholar] [CrossRef]
- Hellmich, M.R.; Szabo, C. Hydrogen Sulfide and Cancer. Handb. Exp. Pharmacol. 2015, 230, 233–241. [Google Scholar] [CrossRef]
- Vlaming-van Eijk, L.E.; Bulthuis, M.L.C.; van der Gun, B.T.F.; Wold, K.I.; Veloo, A.C.M.; Vincenti González, M.F.; de Borst, M.H.; den Dunnen, W.F.A.; Hillebrands, J.L.; van Goor, H.; et al. Systemic oxidative stress associates with the development of post-COVID-19 syndrome in non-hospitalized individuals. Redox Biol. 2024, 76, 103310. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Pal, V.K.; Suhas, K.S.; Menon, G.J.; Singh, I.R.; Malhotra, N.; Naren, C.S.; Ganesh, K.; Rajmani, R.S.; Narain Seshasayee, A.S.; et al. Hydrogen sulfide (H2S) coordinates redox balance, carbon metabolism, and mitochondrial bioenergetics to suppress SARS-CoV-2 infection. PLoS Pathog. 2025, 21, e1013164. [Google Scholar] [CrossRef] [PubMed]
- Crucianelli, S.; Mariano, A.; Valeriani, F.; Cocomello, N.; Gianfranceschi, G.; Baseggio Conrado, A.; Moretti, F.; Scotto d’Abusco, A.; Mennuni, G.; Fraioli, A.; et al. Effects of sulphur thermal water inhalations in long-COVID syndrome: Spa-centred, double-blinded, randomised case-control pilot study. Clin. Med. 2024, 24, 100251. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.; Wray, S. A new slow releasing, H2S generating compound, GYY4137 relaxes spontaneous and oxytocin-stimulated contractions of human and rat pregnant myometrium. PLoS ONE 2012, 7, e46278. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, Z.; Luo, S.; Guo, W.; Zhu, Y.Z. The Cardioprotective Effects of Hydrogen Sulfide in Heart Diseases: From Molecular Mechanisms to Therapeutic Potential. Oxid. Med. Cell Longev. 2015, 2015, 925167. [Google Scholar] [CrossRef]
- Meng, G.; Wang, J.; Xiao, Y.; Bai, W.; Xie, L.; Shan, L.; Moore, P.K.; Ji, Y. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J. Biomed. Res. 2015, 29, 203–213. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, J.; Park, C.M.; Bagdon, P.E.; Peng, B.; Xian, M. Thiol-activated gem-dithiols: A new class of controllable hydrogen sulfide donors. Org. Lett. 2014, 16, 4536–4539. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Liu, N.; Liu, B. AP39, a novel mitochondria-targeted hydrogen sulfide donor ameliorates doxorubicin-induced cardiotoxicity by regulating the AMPK/UCP2 pathway. PLoS ONE 2024, 19, e0300261. [Google Scholar] [CrossRef]
- Kodela, R.; Chattopadhyay, M.; Velázquez-Martínez, C.A.; Kashfi, K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem. Pharmacol. 2015, 98, 564–572. [Google Scholar] [CrossRef]
- Kodela, R.; Chattopadhyay, M.; Kashfi, K. NOSH-Aspirin: A Novel Nitric Oxide-Hydrogen Sulfide-Releasing Hybrid: A New Class of Anti-inflammatory Pharmaceuticals. ACS Med. Chem. Lett. 2012, 3, 257–262. [Google Scholar] [CrossRef]
- Lee, M.; McGeer, E.; Kodela, R.; Kashfi, K.; McGeer, P.L. NOSH-aspirin (NBS-1120), a novel nitric oxide and hydrogen sulfide releasing hybrid, attenuates neuroinflammation induced by microglial and astrocytic activation: A new candidate for treatment of neurodegenerative disorders. Glia 2013, 61, 1724–1734. [Google Scholar] [CrossRef]
- Klán, P.; Slanina, T.; Štacko, P. Light-Activatable H 2 S Donors. In Hydrogen Sulfide; Wiley: Hoboken, NJ, USA, 2022; pp. 347–372. [Google Scholar]
- Chaudhuri, A.; Venkatesh, Y.; Jena, B.C.; Behara, K.K.; Mandal, M.; Singh, N.D.P. Real-time monitoring of a photoactivated hydrogen persulfide donor for biological entities. Org. Biomol. Chem. 2019, 17, 8800–8805. [Google Scholar] [CrossRef] [PubMed]
- Štacko, P.; Muchová, L.; Vítek, L.; Klán, P. Visible to NIR Light Photoactivation of Hydrogen Sulfide for Biological Targeting. Org. Lett. 2018, 20, 4907–4911. [Google Scholar] [CrossRef] [PubMed]
- Predmore, B.L.; Kondo, K.; Bhushan, S.; Zlatopolsky, M.A.; King, A.L.; Aragon, J.P.; Grinsfelder, D.B.; Condit, M.E.; Lefer, D.J. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2410–H2418. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, J.N.; Jakovljevic, V.L.; Zivkovic, V.I.; Srejovic, I.M.; Bradic, J.V.; Milosavljevic, I.M.; Mitrovic, S.L.; Jovicic, N.U.; Bolevich, S.B.; Svistunov, A.A.; et al. Garlic Derived Diallyl Trisulfide in Experimental Metabolic Syndrome: Metabolic Effects and Cardioprotective Role. Int. J. Mol. Sci. 2020, 21, 9100. [Google Scholar] [CrossRef]
- Liu, M.; Wu, L.; Montaut, S.; Yang, G. Hydrogen Sulfide Signaling Axis as a Target for Prostate Cancer Therapeutics. Prostate Cancer 2016, 2016, 8108549. [Google Scholar] [CrossRef]
- Pei, Y.; Wu, B.; Cao, Q.; Wu, L.; Yang, G. Hydrogen sulfide mediates the anti-survival effect of sulforaphane on human prostate cancer cells. Toxicol. Appl. Pharmacol. 2011, 257, 420–428. [Google Scholar] [CrossRef]
- Testai, L.; Montanaro, R.; Flori, L.; Pagnotta, E.; Vellecco, V.; Gorica, E.; Ugolini, L.; Righetti, L.; Brancaleone, V.; Bucci, M.; et al. Persulfidation of mitoKv7.4 channels contributes to the cardioprotective effects of the H2S-donor Erucin against ischemia/reperfusion injury. Biochem. Pharmacol. 2023, 215, 115728. [Google Scholar] [CrossRef]
- Piragine, E.; Citi, V.; Lawson, K.; Calderone, V.; Martelli, A. Potential Effects of Natural H2S-Donors in Hypertension Management. Biomolecules 2022, 12, 581. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Breschi, M.C.; Lawson, K.; McKay, N.G.; Miceli, F.; Taglialatela, M.; Calderone, V. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol. Res. 2013, 70, 27–34. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Munguira, E.B.; Juan, C.A.; Pérez-Lebeña, E. The Multifaceted Health Benefits of Broccoli—A Review of Glucosinolates, Phenolics and Antimicrobial Peptides. Molecules 2025, 30, 2262. [Google Scholar] [CrossRef] [PubMed]
- Bouranis, J.A.; Beaver, L.M.; Ho, E. Metabolic Fate of Dietary Glucosinolates and Their Metabolites: A Role for the Microbiome. Front. Nutr. 2021, 8, 748433. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D.A. Influence of Cooking Methods on Glucosinolates and Isothiocyanates Content in Novel Cruciferous Foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Carroll-Portillo, A.; Lin, H.C. Desulfovibrio in the Gut: The Enemy within? Microorganisms 2023, 11, 1772. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 2021, 27, 55–69. [Google Scholar] [CrossRef]
- Teigen, L.; Biruete, A.; Khoruts, A. Impact of diet on hydrogen sulfide production: Implications for gut health. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 55–58. [Google Scholar] [CrossRef]
- Hayes, J.A.; Lunger, A.W.; Sharma, A.S.; Fernez, M.T.; Carrier, R.L.; Koppes, A.N.; Koppes, R.; Woolston, B.M. Engineered bacteria titrate hydrogen sulfide and induce concentration-dependent effects on the host in a gut microphysiological system. Cell Rep. 2023, 42, 113481. [Google Scholar] [CrossRef]
- Melino, S.; Leo, S.; Toska Papajani, V. Natural Hydrogen Sulfide Donors from Allium sp. as a Nutraceutical Approach in Type 2 Diabetes Prevention and Therapy. Nutrients 2019, 11, 1581. [Google Scholar] [CrossRef]
- Meng, G.; Zhu, J.; Xiao, Y.; Huang, Z.; Zhang, Y.; Tang, X.; Xie, L.; Chen, Y.; Shao, Y.; Ferro, A.; et al. Hydrogen Sulfide Donor GYY4137 Protects against Myocardial Fibrosis. Oxid. Med. Cell Longev. 2015, 2015, 691070. [Google Scholar] [CrossRef]
- Fox, B.C.; Slade, L.; Torregrossa, R.; Pacitti, D.; Szabo, C.; Etheridge, T.; Whiteman, M. The mitochondria-targeted hydrogen sulfide donor AP39 improves health and mitochondrial function in a C. elegans primary mitochondrial disease model. J. Inherit. Metab. Dis. 2021, 44, 367–375. [Google Scholar] [CrossRef]
- Riglar, D.T.; Giessen, T.W.; Baym, M.; Kerns, S.J.; Niederhuber, M.J.; Bronson, R.T.; Kotula, J.W.; Gerber, G.K.; Way, J.C.; Silver, P.A. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat. Biotechnol. 2017, 35, 653–658. [Google Scholar] [CrossRef]
- Park, C.-M.; Weerasinghe, L.; Day, J.J.; Fukuto, J.M.; Xian, M. Persulfides: Current knowledge and challenges in chemistry and chemical biology. Mol. BioSystems 2015, 11, 1775–1785. [Google Scholar] [CrossRef]
- Huang, F.; Han, X.; Xiao, X.; Zhou, J. Covalent Warheads Targeting Cysteine Residue: The Promising Approach in Drug Development. Molecules 2022, 27, 7728. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Lobo, F.; Pérez de la Lastra, J.M.; Munguira, E.B.; Juan, C.A.; Pérez-Lebeña, E. Cysteine Alkylation in Enzymes and Transcription Factors: A Therapeutic Strategy for Cancer. Cancers 2025, 17, 1876. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Reyes, A.M.; Zeida, A.; Mastrogiovanni, M.; De Armas, M.I.; Radi, R.; Alvarez, B.; Trujillo, M. Kinetics of formation and reactivity of the persulfide in the one-cysteine peroxiredoxin from Mycobacterium tuberculosis. J. Biol. Chem. 2019, 294, 13593–13605. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Rouhier, N.; Couturier, J. Dual Roles of Reducing Systems in Protein Persulfidation and Depersulfidation. Antioxidants 2025, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Suzuki, T.; Hiramoto, K.; Asami, S.; Naganuma, E.; Suda, H.; Iso, T.; Yamamoto, H.; Morita, M.; Baird, L.; et al. Characterizations of Three Major Cysteine Sensors of Keap1 in Stress Response. Mol. Cell Biol. 2016, 36, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Lange, M.; Bonanata, J.; Coitiño, E.L.; Ferrer-Sueta, G.; Filipovic, M.R.; Alvarez, B. Reaction of Hydrogen Sulfide with Disulfide and Sulfenic Acid to Form the Strongly Nucleophilic Persulfide. J. Biol. Chem. 2015, 290, 26866–26880. [Google Scholar] [CrossRef]
- Khoo, N.K.H.; Li, L.; Salvatore, S.R.; Schopfer, F.J.; Freeman, B.A. Electrophilic fatty acid nitroalkenes regulate Nrf2 and NF-κB signaling: A medicinal chemistry investigation of structure-function relationships. Sci. Rep. 2018, 8, 2295. [Google Scholar] [CrossRef]
- Jamaluddin, M.; Haas de Mello, A.; Tapryal, N.; Hazra, T.K.; Garofalo, R.P.; Casola, A. NRF2 Regulates Cystathionine Gamma-Lyase Expression and Activity in Primary Airway Epithelial Cells Infected with Respiratory Syncytial Virus. Antioxidants 2022, 11, 1582. [Google Scholar] [CrossRef]
- Sauerland, M.; Mertes, R.; Morozzi, C.; Eggler, A.L.; Gamon, L.F.; Davies, M.J. Kinetic assessment of Michael addition reactions of alpha, beta-unsaturated carbonyl compounds to amino acid and protein thiols. Free Radic. Biol. Med. 2021, 169, 1–11. [Google Scholar] [CrossRef]
- Brennan, M.S.; Matos, M.F.; Li, B.; Hronowski, X.; Gao, B.; Juhasz, P.; Rhodes, K.J.; Scannevin, R.H. Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS ONE 2015, 10, e0120254. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, M.; Ammal Kaidery, N.; Yang, L.; Calingasan, N.; Smirnova, N.; Gaisin, A.; Gaisina, I.N.; Gazaryan, I.; Hushpulian, D.M.; Kaddour-Djebbar, I.; et al. Distinct Nrf2 Signaling Mechanisms of Fumaric Acid Esters and Their Role in Neuroprotection against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Experimental Parkinson’s-Like Disease. J. Neurosci. 2016, 36, 6332–6351. [Google Scholar] [CrossRef] [PubMed]
- Dayalan Naidu, S.; Dinkova-Kostova, A.T. KEAP1, a cysteine-based sensor and a drug target for the prevention and treatment of chronic disease. Open Biol. 2020, 10, 200105. [Google Scholar] [CrossRef] [PubMed]
- Higdon, A.N.; Landar, A.; Barnes, S.; Darley-Usmar, V.M. The electrophile responsive proteome: Integrating proteomics and lipidomics with cellular function. Antioxid. Redox Signal. 2012, 17, 1580–1589. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Byun, M.S.; Choi, J.; Jue, D.M. Cysteine-179 of IkappaB kinase beta plays a critical role in enzyme activation by promoting phosphorylation of activation loop serines. Exp. Mol. Med. 2006, 38, 546–552. [Google Scholar] [CrossRef]
- García-Piñeres, A.J.; Castro, V.; Mora, G.; Schmidt, T.J.; Strunck, E.; Pahl, H.L.; Merfort, I. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J. Biol. Chem. 2001, 276, 39713–39720. [Google Scholar] [CrossRef]
- Matthews, J.R.; Kaszubska, W.; Turcatti, G.; Wells, T.N.; Hay, R.T. Role of cysteine62 in DNA recognition by the P50 subunit of NF-kappa B. Nucleic Acids Res. 1993, 21, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Huang, Y.; Yan, H.; Zhang, Q.; Zhao, M.; Zhu, M.; Liu, J.; Chen, S.X.; Bu, D.; Tang, C.; et al. Hydrogen sulfide suppresses oxidized low-density lipoprotein (ox-LDL)-stimulated monocyte chemoattractant protein 1 generation from macrophages via the nuclear factor κB (NF-κB) pathway. J. Biol. Chem. 2014, 289, 9741–9753. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Lin, X.L.; Xiao, L.L. Hydrogen sulfide attenuates TMAO-induced macrophage inflammation through increased SIRT1 sulfhydration. Mol. Med. Rep. 2023, 28, 129. [Google Scholar] [CrossRef] [PubMed]
- García-Calderón, M.; Vignane, T.; Filipovic, M.R.; Ruiz, M.T.; Romero, L.C.; Márquez, A.J.; Gotor, C.; Aroca, A. Persulfidation protects from oxidative stress under nonphotorespiratory conditions in Arabidopsis. New Phytol. 2023, 238, 1431–1445. [Google Scholar] [CrossRef]
- Braunstein, I.; Engelman, R.; Yitzhaki, O.; Ziv, T.; Galardon, E.; Benhar, M. Opposing effects of polysulfides and thioredoxin on apoptosis through caspase persulfidation. J. Biol. Chem. 2020, 295, 3590–3600. [Google Scholar] [CrossRef]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- Sauerland, M.B.; Davies, M.J. Electrophile versus oxidant modification of cysteine residues: Kinetics as a key driver of protein modification. Arch. Biochem. Biophys. 2022, 727, 109344. [Google Scholar] [CrossRef]
- Ha, K.H.; Byun, M.S.; Choi, J.; Jeong, J.; Lee, K.J.; Jue, D.M. N-tosyl-L-phenylalanine chloromethyl ketone inhibits NF-kappaB activation by blocking specific cysteine residues of IkappaB kinase beta and p65/RelA. Biochemistry 2009, 48, 7271–7278. [Google Scholar] [CrossRef]
- Kwok, B.H.; Koh, B.; Ndubuisi, M.I.; Elofsson, M.; Crews, C.M. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem. Biol. 2001, 8, 759–766. [Google Scholar] [CrossRef]
- Jung, T.; Höhn, A.; Grune, T. The proteasome and the degradation of oxidized proteins: Part II—Protein oxidation and proteasomal degradation. Redox Biol. 2014, 2, 99–104. [Google Scholar] [CrossRef]
- Raynes, R.; Pomatto, L.C.; Davies, K.J. Degradation of oxidized proteins by the proteasome: Distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol. Asp. Med. 2016, 50, 41–55. [Google Scholar] [CrossRef]
- Rachakonda, G.; Xiong, Y.; Sekhar, K.R.; Stamer, S.L.; Liebler, D.C.; Freeman, M.L. Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3. Chem. Res. Toxicol. 2008, 21, 705–710. [Google Scholar] [CrossRef]
- Weerapana, E.; Wang, C.; Simon, G.M.; Richter, F.; Khare, S.; Dillon, M.B.; Bachovchin, D.A.; Mowen, K.; Baker, D.; Cravatt, B.F. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 2010, 468, 790–795. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Novichkova, M.D. S-Glutathionylation and S-Nitrosylation as Modulators of Redox-Dependent Processes in Cancer Cell. Biochemistry 2023, 88, 924–943. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kole, S.; Precht, P.; Pazin, M.J.; Bernier, M. S-glutathionylation impairs signal transducer and activator of transcription 3 activation and signaling. Endocrinology 2009, 150, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, D.; Bursac, B.; Sbodio, J.I.; Nalluru, S.; Vignane, T.; Snowman, A.M.; Albacarys, L.M.; Sedlak, T.W.; Torregrossa, R.; Whiteman, M.; et al. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA 2021, 118, e2017225118. [Google Scholar] [CrossRef]
- Butturini, E.; Carcereri de Prati, A.; Mariotto, S. Redox Regulation of STAT1 and STAT3 Signaling. Int. J. Mol. Sci. 2020, 21, 7034. [Google Scholar] [CrossRef]
- Qu, J.; Ren, X.; Xue, F.; He, Y.; Zhang, R.; Zheng, Y.; Huang, H.; Wang, W.; Zhang, J. Specific Knockdown of α-Synuclein by Peptide-Directed Proteasome Degradation Rescued Its Associated Neurotoxicity. Cell Chem. Biol. 2020, 27, 763. [Google Scholar] [CrossRef]
- Pönisch, W.; Yanakieva, I.; Salbreux, G.; Paluch, E.K. Cell shape noise strength regulates the rate of shape change during EMT-associated cell spreading. bioRxiv 2024. [Google Scholar] [CrossRef]
- Hill, C.N.; Hernández-Cáceres, M.P.; Asencio, C.; Torres, B.; Solis, B.; Owen, G.I. Deciphering the Role of the Coagulation Cascade and Autophagy in Cancer-Related Thrombosis and Metastasis. Front. Oncol. 2020, 10, 605314. [Google Scholar] [CrossRef]
- Sabnis, R.W.; Sabnis, A.R. Novel Heterocyclic Compounds for Treating Huntington’s Disease. ACS Med. Chem. Lett. 2024, 15, 324–325. [Google Scholar] [CrossRef]
- Peter, E.A.; Shen, X.; Shah, S.H.; Pardue, S.; Glawe, J.D.; Zhang, W.W.; Reddy, P.; Akkus, N.I.; Varma, J.; Kevil, C.G. Plasma free H2S levels are elevated in patients with cardiovascular disease. J. Am. Heart Assoc. 2013, 2, e000387. [Google Scholar] [CrossRef]
- Merz, T.; Denoix, N.; Wepler, M.; Gäßler, H.; Messerer, D.A.C.; Hartmann, C.; Datzmann, T.; Radermacher, P.; McCook, O. H2S in acute lung injury: A therapeutic dead end(?). Intensive Care Med. Exp. 2020, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Marinko, M.; Novaković, A. Hydrogen sulfide-releasing therapeutics-how far have we come in clinical studies? Arh. Farm. 2023, 73, 173–189. [Google Scholar] [CrossRef]
- de Koning, M.L.Y.; van Dorp, P.; Assa, S.; Pundziute-Do Prado, G.; Voskuil, M.; Anthonio, R.L.; Veen, D.; Leiner, T.; Sibeijn-Kuiper, A.J.; van Goor, H.; et al. Sodium Thiosulfate in Acute Myocardial Infarction: A Randomized Clinical Trial. JACC Basic Transl. Sci. 2023, 8, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Zhou, W.; Guo, Y.; Hu, G.; Chu, J.; Xie, F.; Li, Y.; Wang, W. H2S attenuates oxidative stress via Nrf2/NF-κB signaling to regulate restenosis after percutaneous transluminal angioplasty. Exp. Biol. Med. 2021, 246, 226–239. [Google Scholar] [CrossRef]
- Ali Qaba, M.A.M.; Saleem, M.K.; Ali Qaba, N.K.; Alani, M.A.; Ahmed, M.M.; Sabry, S.M. Assessment of Inhaled Hydrogen Sulfide in Suppressing Deterioration in Patients With COVID-19. Shock 2021, 56, 868–869. [Google Scholar] [CrossRef]
- Dattilo, M. The role of host defences in Covid 19 and treatments thereof. Mol. Med. 2020, 26, 90. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Nicholls, P.; Marshall, D.C.; Cooper, C.E.; Wilson, M.T. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem. Soc. Trans. 2013, 41, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.R.; Schoenfisch, M.H. Direct electrochemical sensing of hydrogen sulfide without sulfur poisoning. Anal. Chem. 2018, 90, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide 2013, 35, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Ransy, C.; Módis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef]
- Shieh, M.; Xu, S.; Lederberg, O.L.; Xian, M. Detection of sulfane sulfur species in biological systems. Redox Biol. 2022, 57, 102502. [Google Scholar] [CrossRef]
- Pickens, C.J.; Johnson, S.N.; Pressnall, M.M.; Leon, M.A.; Berkland, C.J. Practical Considerations, Challenges, and Limitations of Bioconjugation via Azide-Alkyne Cycloaddition. Bioconjug. Chem. 2018, 29, 686–701. [Google Scholar] [CrossRef]
- Xie, F.; Wei, W. [64Cu]Cu-ATSM: An emerging theranostic agent for cancer and neuroinflammation. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3964–3972. [Google Scholar] [CrossRef]
- Gojon, G.; Morales, G.A. SG1002 and Catenated Divalent Organic Sulfur Compounds as Promising Hydrogen Sulfide Prodrugs. Antioxid. Redox Signal. 2020, 33, 1010–1045. [Google Scholar] [CrossRef]
- Predmore, B.L.; Lefer, D.J. Hydrogen sulfide-mediated myocardial pre- and post-conditioning. Expert. Rev. Clin. Pharmacol. 2011, 4, 83–96. [Google Scholar] [CrossRef]
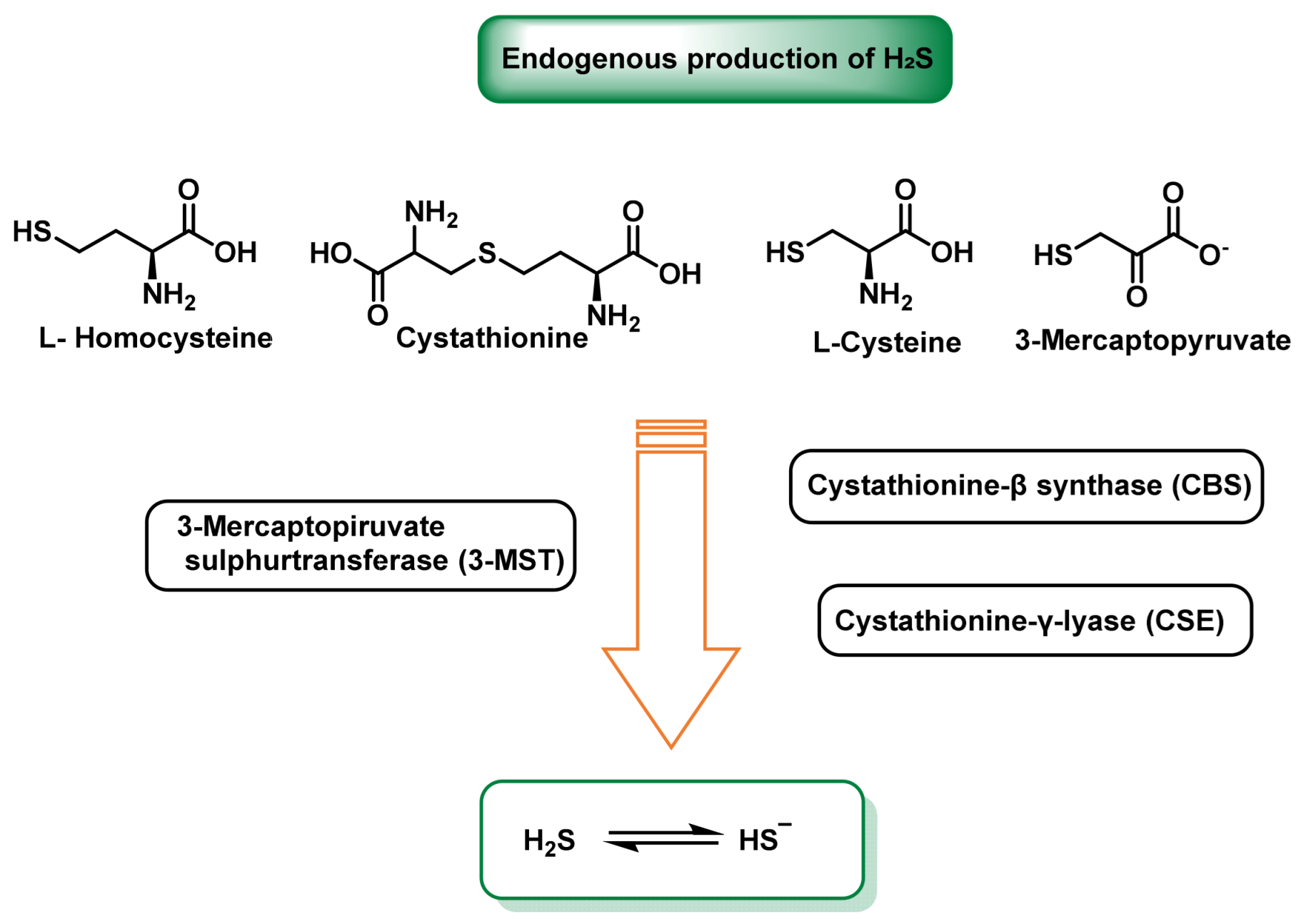
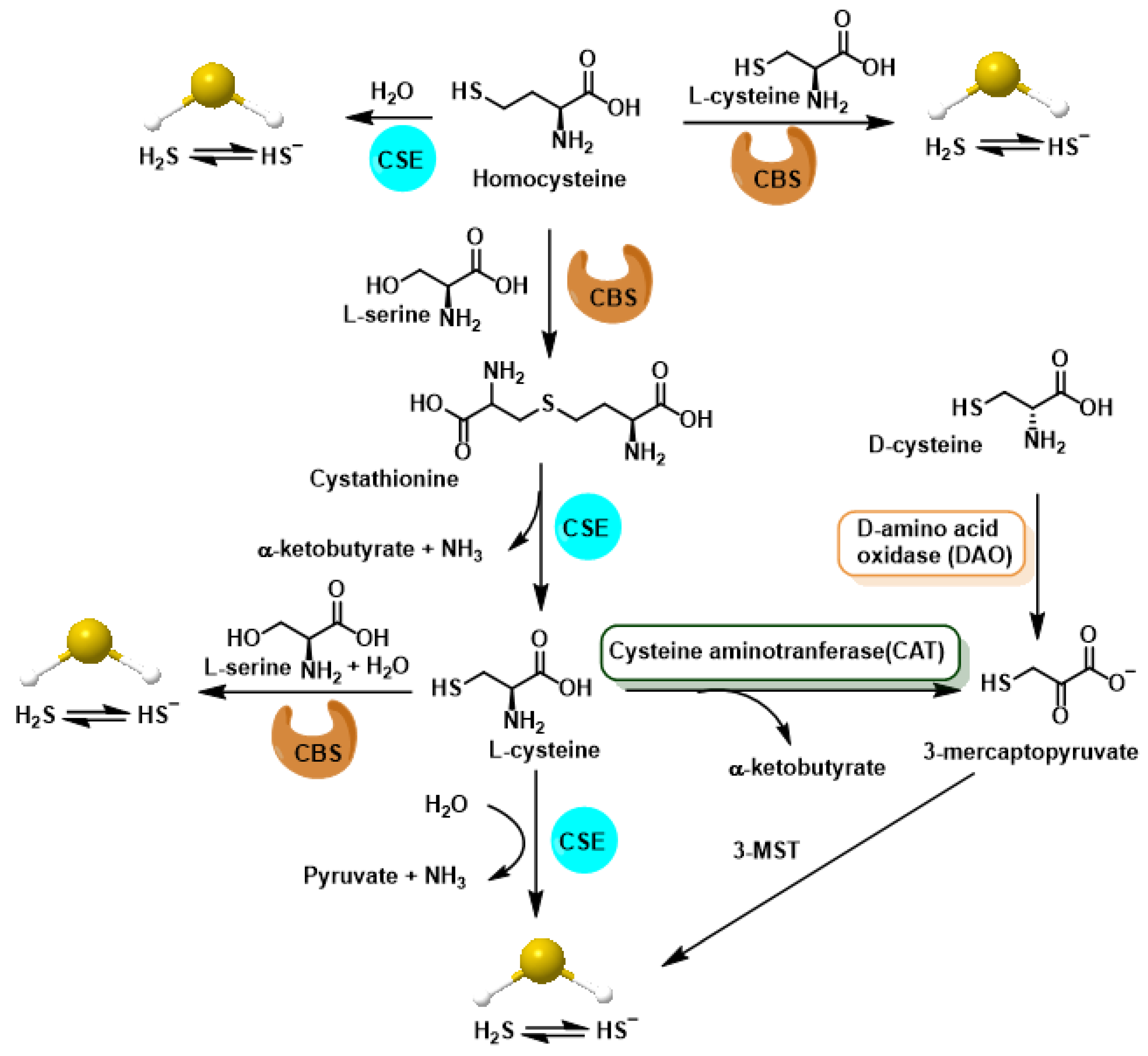
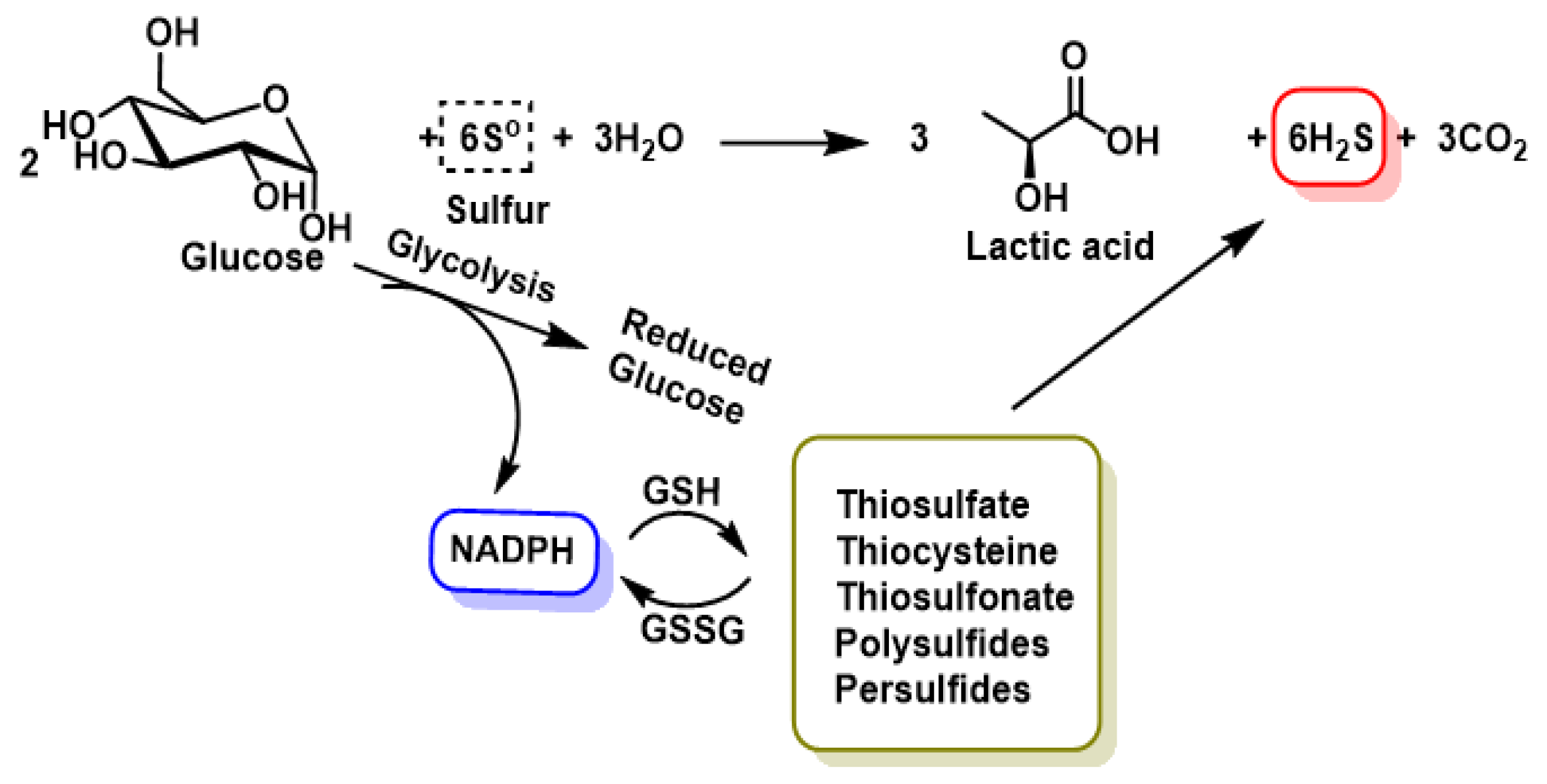




| Property | ROS | RNS | RHS | RSS |
|---|---|---|---|---|
| Central atom | O | N | Cl/Br/I | S |
| Canonical examples | O2•−, H2O2, •OH | •NO, ONOO−, NO2• | HOCl, HOBr, Cl2•− | H2S, RSSH, S42− |
| Half-life | ns–ms (•OH) a ms–s (H2O2) | µs–ms (ONOO−) | μs (HOCl) | MS–min (polysulfides) |
| Biosynthetic pathways | Mitochondria, NADPH oxidase | US + ROS | MPO + halide | CBS/CSE, MPST |
| Prevailing changes | Oxidation (-OH, =O) | Nitro-/nitrous- | Chlor(in)ation, bromination | Persulfidation (-SSH) |
| Physiological functions | Signaling, immunity | Vasorrelaxation, defense | Microbicide inflammation | Bioenergy, mitochondrial protection |
| Process/Reaction (Representative) | k2 (M−1 s−1) | pH (BUFFER) | T (°C) | Notes |
|---|---|---|---|---|
| HS− + protein–SOH → protein–SSH (Mt AhpE–SOH model) | 1.4 × 103 | ~7.4 (pH-independent after correction) | n.r. | Nucleophilic attack of HS− on sulfenic acid |
| Hydrophilic LMW-persulfide + protein-Cys → protein–SSH | 104–105 | ~7–7.5 | n.r. | Stopped-flow; faster than Michael additions |
| Michael electrophile + reactive Cys (comparative) | ~102 | ~7.4 | n.r. | Irreversible C–S adduct formation; comparator scale |
| H2S + H2O2 → HSOH → (polysulfides/S0/SO42−) | 0.73 | 7.4 | 37 | Product distribution depends on [H2O2]:[H2S] |
| HS− + ONOOH → HSOH + NO2− (±HSSH) | 6.7 × 103 | 7.4 | 37 | Nucleophilic substitution pathway |
| HS− + HOCl → HSCl → HSOH (rapid hydrolysis) | 0.8–20 × 108 | 7.4 | n.r. | Among the fastest oxidations of HS− |
| HS− + taurine-chloramine → oxidized products | 3 × 102 | 7.4 | 37 | Slower than HOCl; higher selectivity |
| Oxidizing | k2 M−1 s−1 | pH | Temperature | Reference |
|---|---|---|---|---|
| Hydrogen peroxide | 0.73 | 7.4 | 37 °C | [59] |
| Peroxynitrous acid | 6.7 × 103 | 7.4 | 37 °C | [60] |
| Hypochlorous acid | (0.8–20) × 108 | 7.4 | [61] | |
| Taurine chloramine | 3 × 102 | 7.4 | 37 °C | [41] |
| NCT ID | Agent/ Modality | Indication and Design | Phase | Current Status | Results/Reference | Reason for Termination/Note | Practical Implication |
|---|---|---|---|---|---|---|---|
| NCT00879645 | IK-1001 (sodium sulfide, IV) | PK/renal function stratification, single 3-h infusion | I | Terminated | No posted results [185] | Program hit a core analytical barrier: inability to reliably determine sulfide levels in vivo | Future IV “flash” donors require validated, rapid speciation assays before dose-escalation. |
| NCT00858936 | IK-1001 (IV) | Peri-operative CABG, single pre-reperfusion dose | I/II | Terminated | No posted results [185] | Reasons not reported publicly; part of broader assay/measurement challenges | De-risk peri-ischemic studies by pre-specifying orthogonal exposure readouts (e.g., thiosulfate, bound sulfane sulfur). |
| NCT01007461 | IK-1001 (IV) | STEMI, post-infarction infusion | IIa | Withdrawn | [185] | Company decision (non-safety-related) | Confirms strategic, not toxicity-driven, halt; emphasizes the need for assay-ready platforms. |
| NCT01989208 | SG1002 (oral slow-release H2S donor) | HF patients; dose-escalation, 200–800 mg BID | I (HF) | Completed | Published [18] | — | Supports feasibility of sustained donors; motivates phase II designs with standardized endpoints. |
| NCT02278276 | SG1002 (oral) | Heart failure; randomized, placebo-controlled | I/II | Status: unknown/no results posted (record not recently updated) | [186] | No public readout | Consolidate HF endpoints and ensure assay-anchored target engagement in future trials |
| NCT01407172 | Biomarker study (plasma free H2S) | Peripheral arterial disease; observational | — | Completed | Published [184] | — | Demonstrates clinical biomarker feasibility but highlights pre-analytical sensitivity; standardization needed |
| NCT01232257 | N-acetylcysteine (NAC) (endogenous H2S modulator) | 4 cohorts (healthy, CKD 3–4, HD, PD); single-group | — | Completed (2011) | No posted results | — | Keep as background evidence of feasibility; pursue modern speciation and kinetic sampling in any replication. |
| NCT02899364 | Sodium thiosulfate (STS) (indirect H2S donor) | STEMI, proof-of-principle | II | Completed | Published [187] | — | STS remains attractive for safety; refine timing, dose, and analytics to capture cardioprotection signal. |
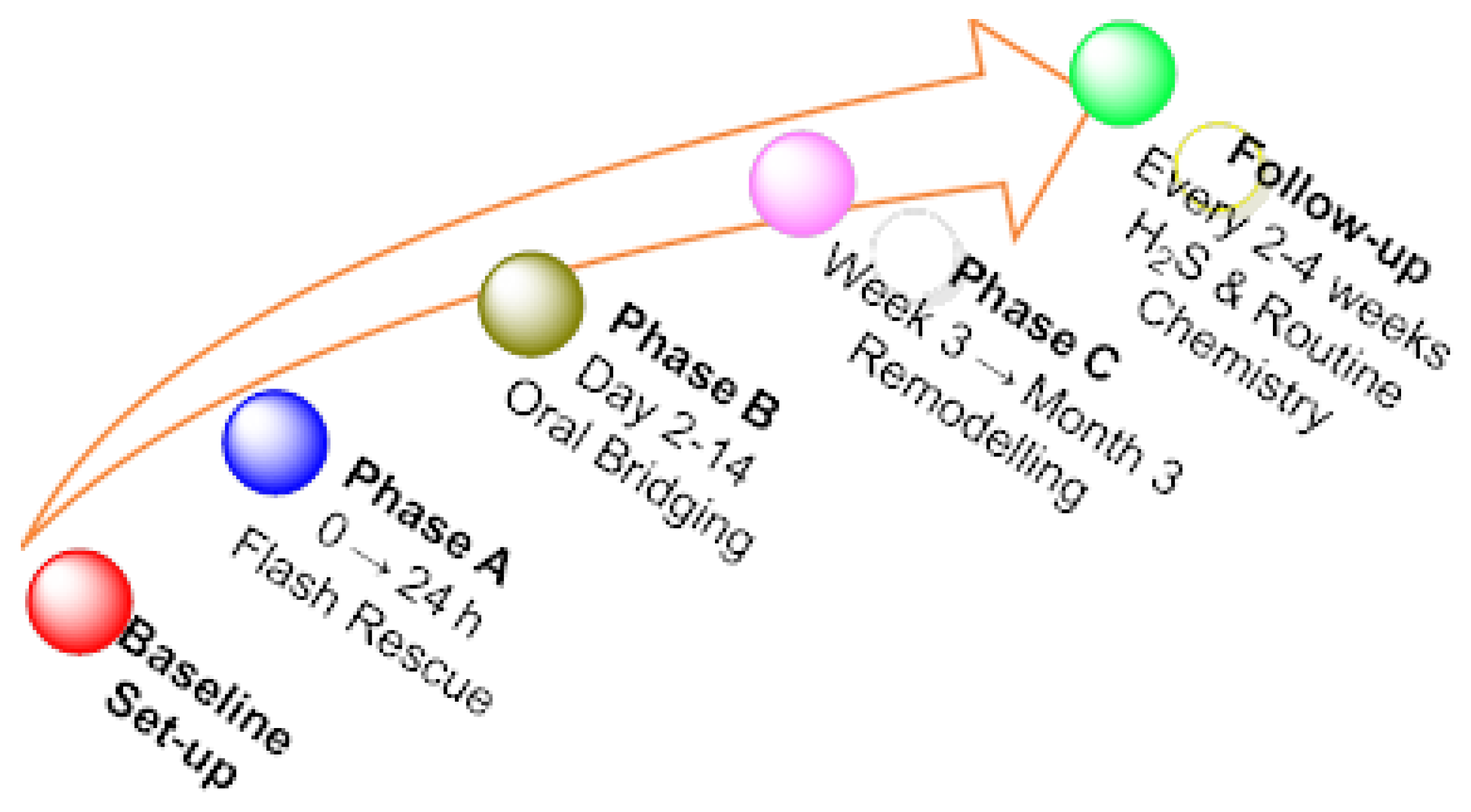
| Layer | Time-Window | Main Objective | Prototype Interventions | Key Clinical Evidence |
|---|---|---|---|---|
| Rescue (“flash”) | 0–24 h around infarction, CABG, early ARDS | Rapid mitochondrial rescue/IPC mimicry | IV IK-1001 1.0–1.5 mg kg−1 h−1 × 3 h; IV sodium thiosulfate 6 g bolus → 4 g q12h | IK-1001 tested in CABG (n = 6) with no serious AEs, programme halted for analytical—not safety—issues; STS dose-escalation in ventilated COVID-ARDS is under way (NCT05277285) |
| Sustained catalytic | Day 2 → 14 | Maintain systemic persulfidation | Oral SG1002 400–800 mg BID (phase I/II); GYY4137 slow-donor (pre-clinical) | SG1002 raised plasma H2S and nitrite and lowered BNP in heart-failure patients without safety signals (NCT02278276) |
| Chronic remodelling | ≥3 weeks (rehab/long-COVID/CVD risk) | Re-educate endogenous and microbiome sulfur routes | Protein-calorie restriction (PCR) diet 4 d pre-surgery; H2S thermal-water aerosol 12 d; Sulfurous spa immersion 14 d; High-allium and crucifer diet ± pro-/pre-biotics | PCR trial in vascular surgery (n = 226) ongoing (NCT05457881); aerosol H2S raised circulating levels without safety issues (NCT06095349); spa immersion pilot recruiting (NCT06725797) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés, C.M.C.; Lobo, F.; Lastra, J.M.P.d.l.; Munguira, E.B.; Juan, C.A.; Pérez Lebeña, E. Reactive Sulfur Species and Protein Persulfidation: An Emerging Redox Axis in Human Health and Disease. Curr. Issues Mol. Biol. 2025, 47, 765. https://doi.org/10.3390/cimb47090765
Andrés CMC, Lobo F, Lastra JMPdl, Munguira EB, Juan CA, Pérez Lebeña E. Reactive Sulfur Species and Protein Persulfidation: An Emerging Redox Axis in Human Health and Disease. Current Issues in Molecular Biology. 2025; 47(9):765. https://doi.org/10.3390/cimb47090765
Chicago/Turabian StyleAndrés, Celia María Curieses, Fernando Lobo, José Manuel Pérez de la Lastra, Elena Bustamante Munguira, Celia Andrés Juan, and Eduardo Pérez Lebeña. 2025. "Reactive Sulfur Species and Protein Persulfidation: An Emerging Redox Axis in Human Health and Disease" Current Issues in Molecular Biology 47, no. 9: 765. https://doi.org/10.3390/cimb47090765
APA StyleAndrés, C. M. C., Lobo, F., Lastra, J. M. P. d. l., Munguira, E. B., Juan, C. A., & Pérez Lebeña, E. (2025). Reactive Sulfur Species and Protein Persulfidation: An Emerging Redox Axis in Human Health and Disease. Current Issues in Molecular Biology, 47(9), 765. https://doi.org/10.3390/cimb47090765






