Resina Draconis Promotes Diabetic Wound Healing by Regulating the AGE-RAGE Pathway to Modulate Macrophage Polarization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of RD Hydrogel
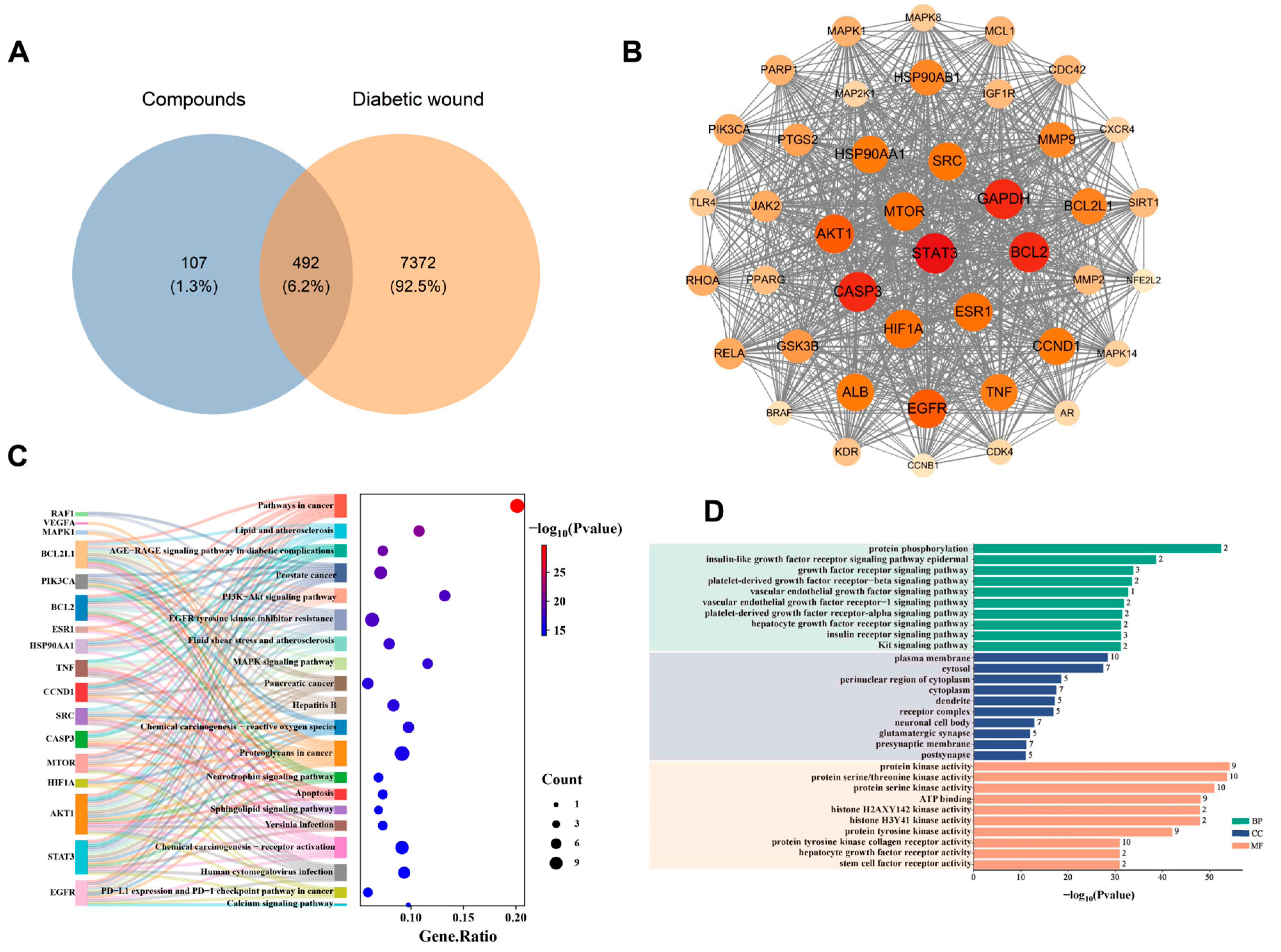
2.3. Research on Network Pharmacology
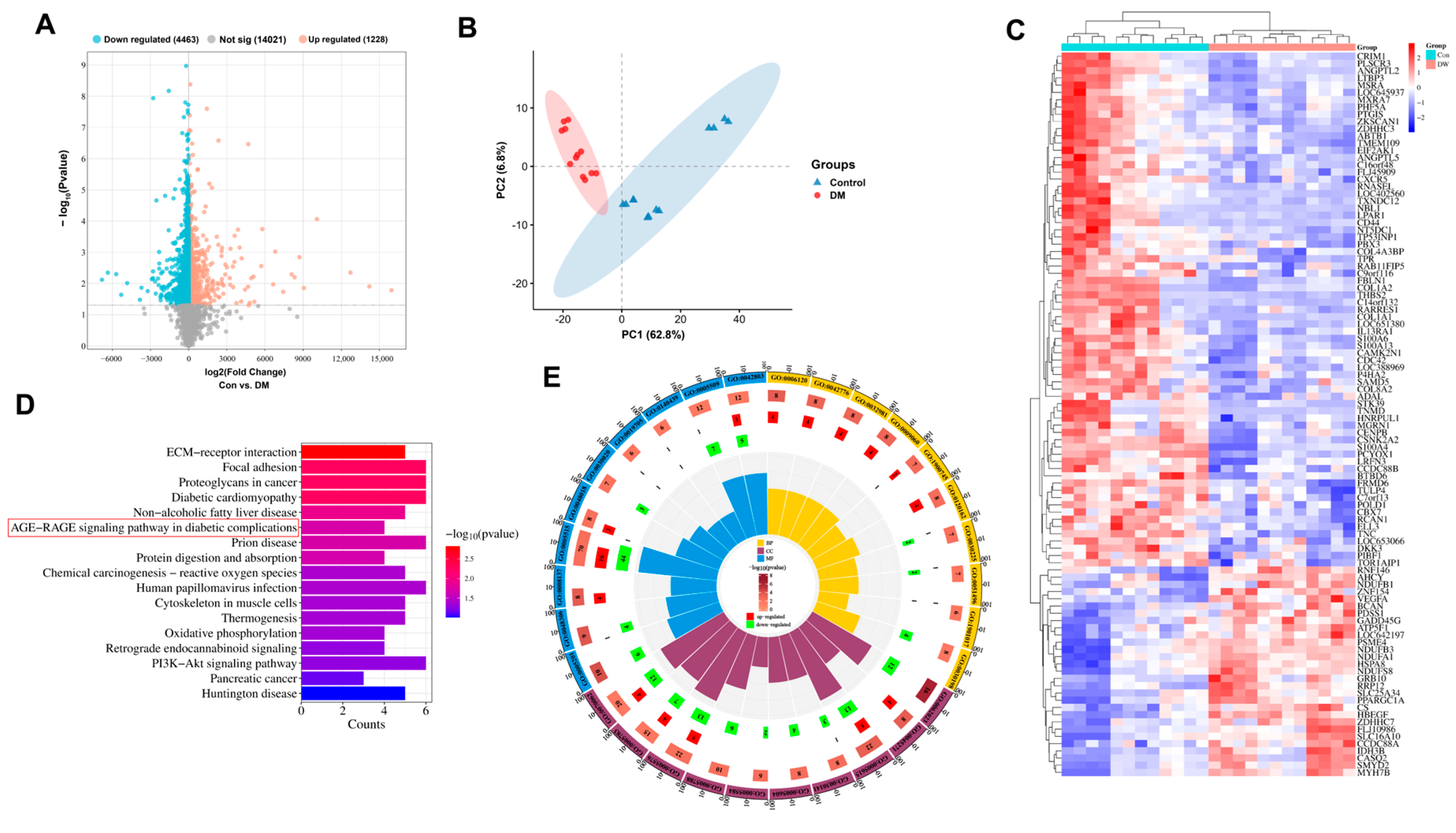
2.4. Bioinformatics Analysis of Clinical Data from the GEO Database
2.5. Molecular Docking
2.6. Cell Culture and Treatment
2.7. Western Blot (WB) Analysis
2.8. RNA Extraction and Quantitative RT-PCR Analysis
2.9. Measurement of ROS
2.10. Construction of the Diabetic Wound Mouse Model and Group Administration
2.11. Hematoxylin-Eosin Staining (H&E)
2.12. Masson’s Trichrome Staining
2.13. Immunohistochemistry
2.14. Immunofluorescence
2.15. Statistical Analysis
3. Results
3.1. Analysis of Network Pharmacology
3.2. Clinical Sample Analysis
3.3. The Main Components of RD Can Tightly Bind to the Extracellular Domain of RAGE
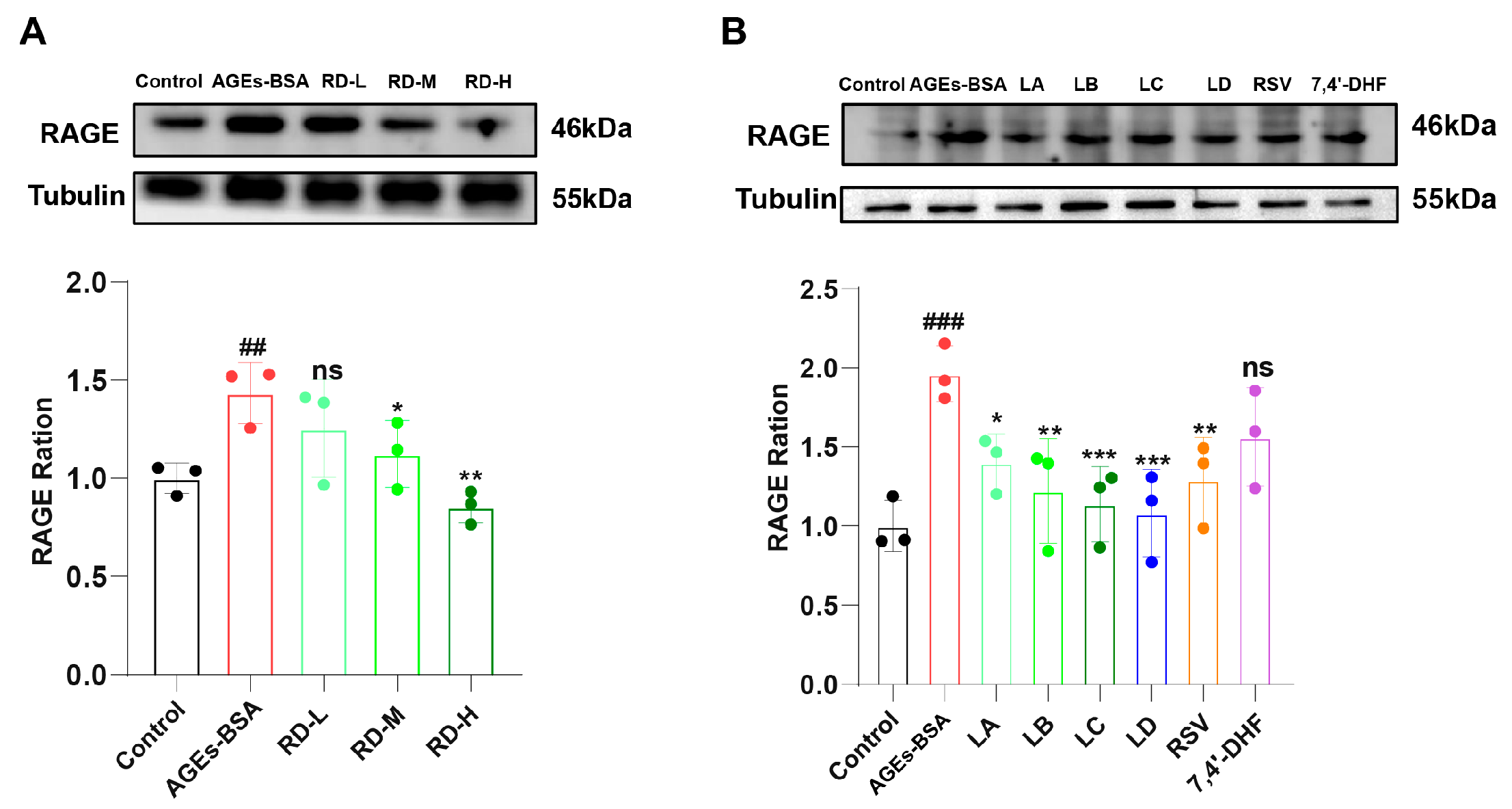
3.4. RD and Its Active Components Attenuate AGEs-Induced RAGE Upregulation
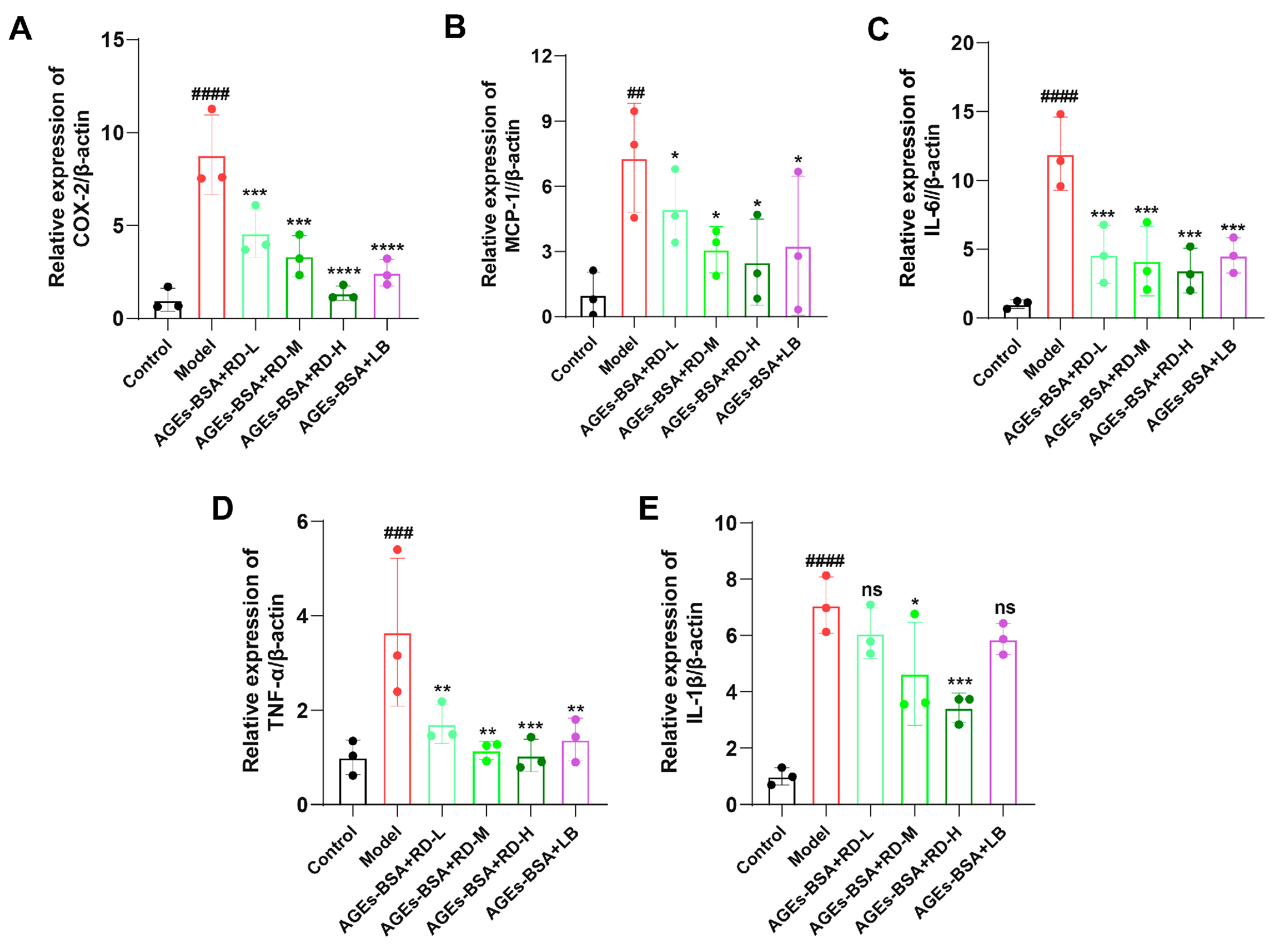
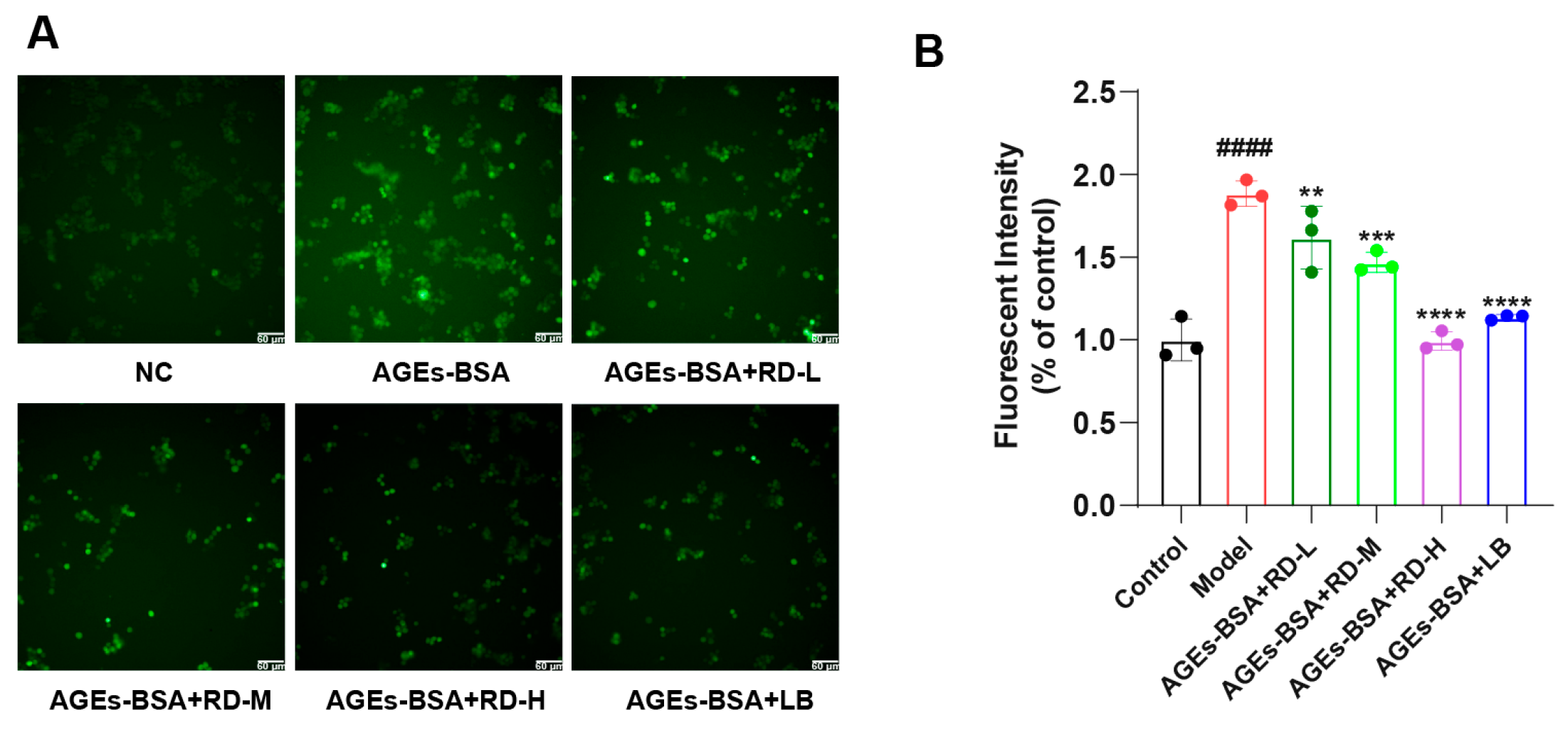
3.5. RD and LB Reduce Inflammatory Responses and ROS Generation
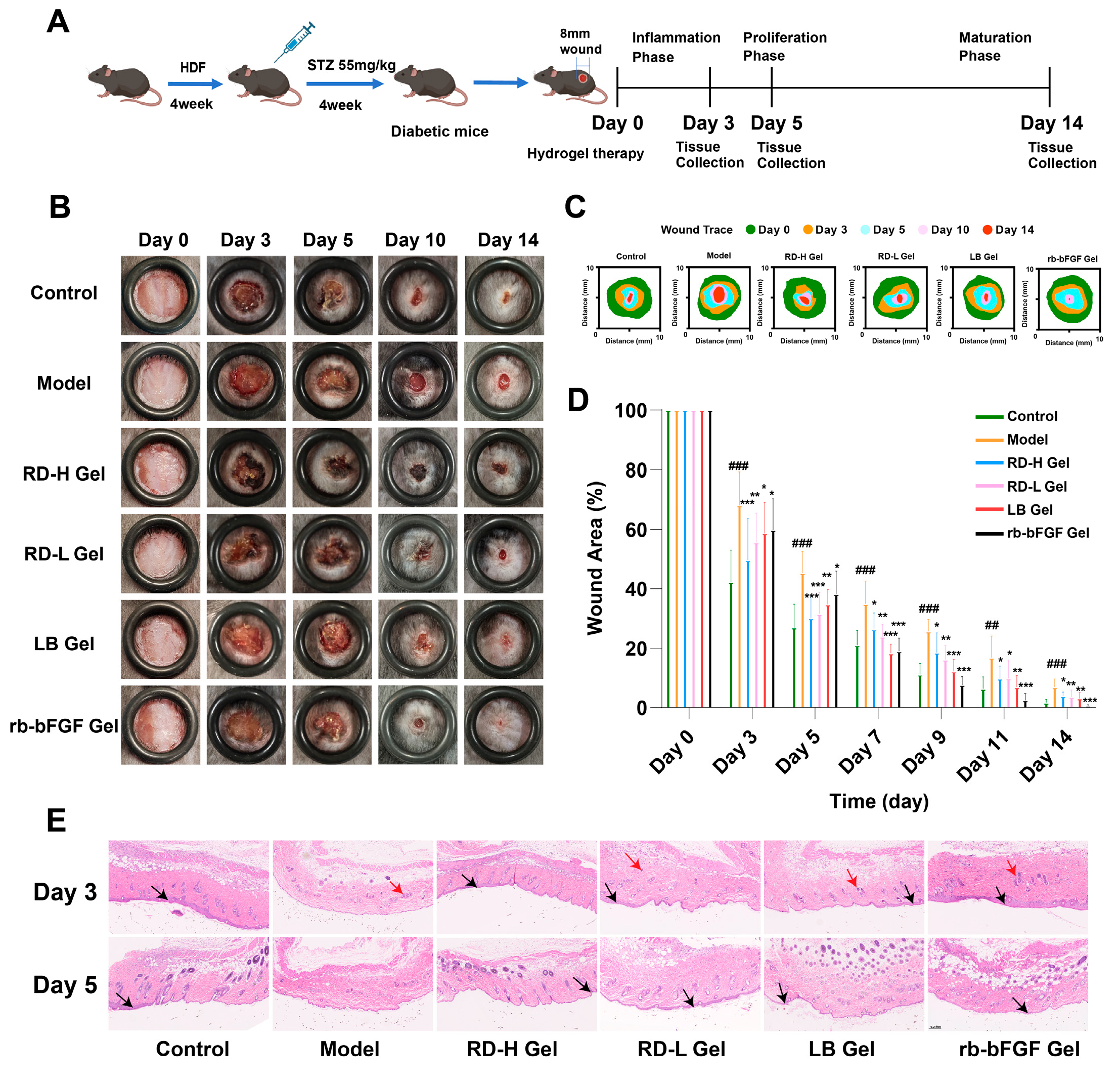
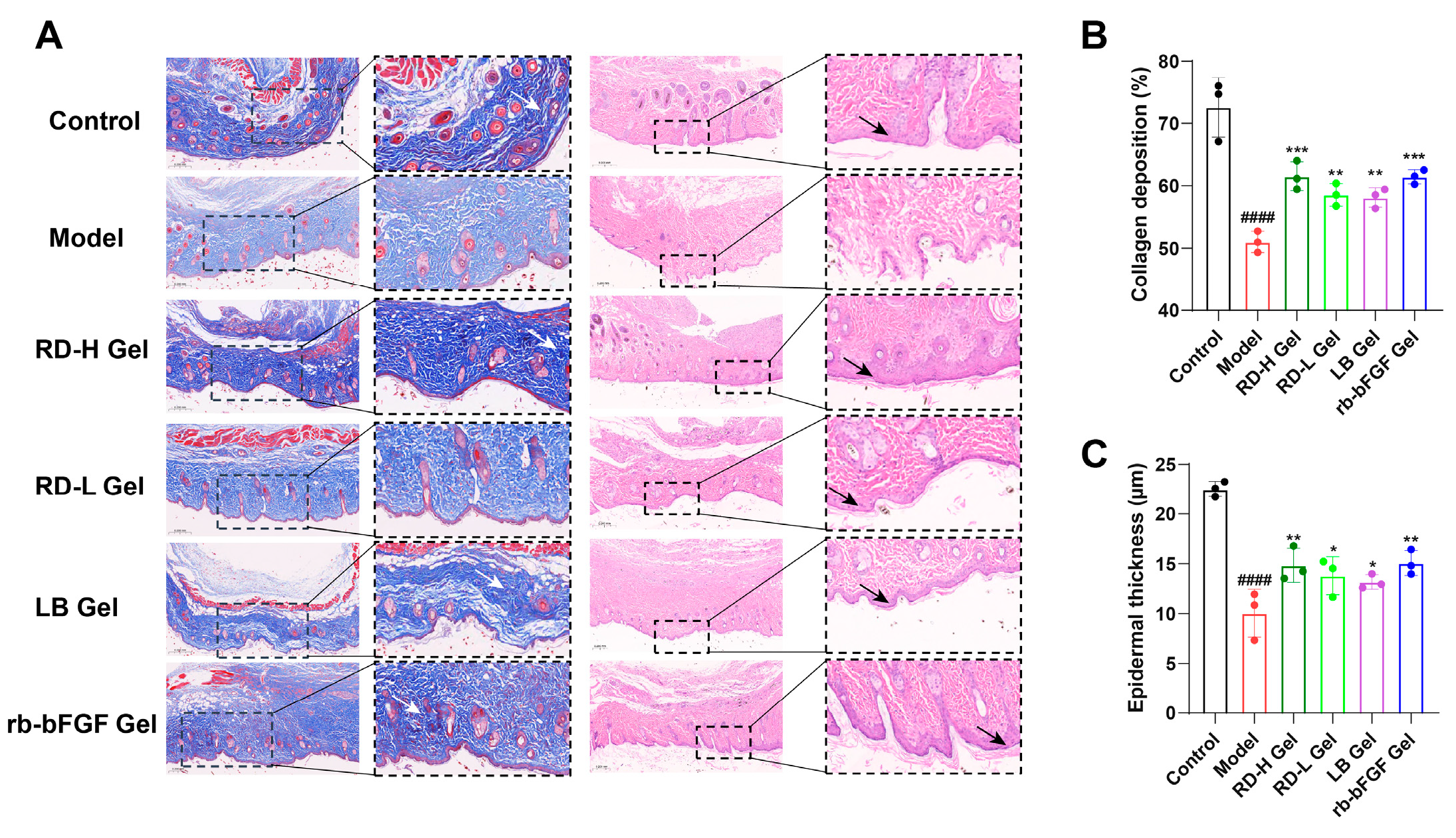
3.6. RD and LB Promote Wound Healing in Diabetic Mice During the Inflammatory Phase
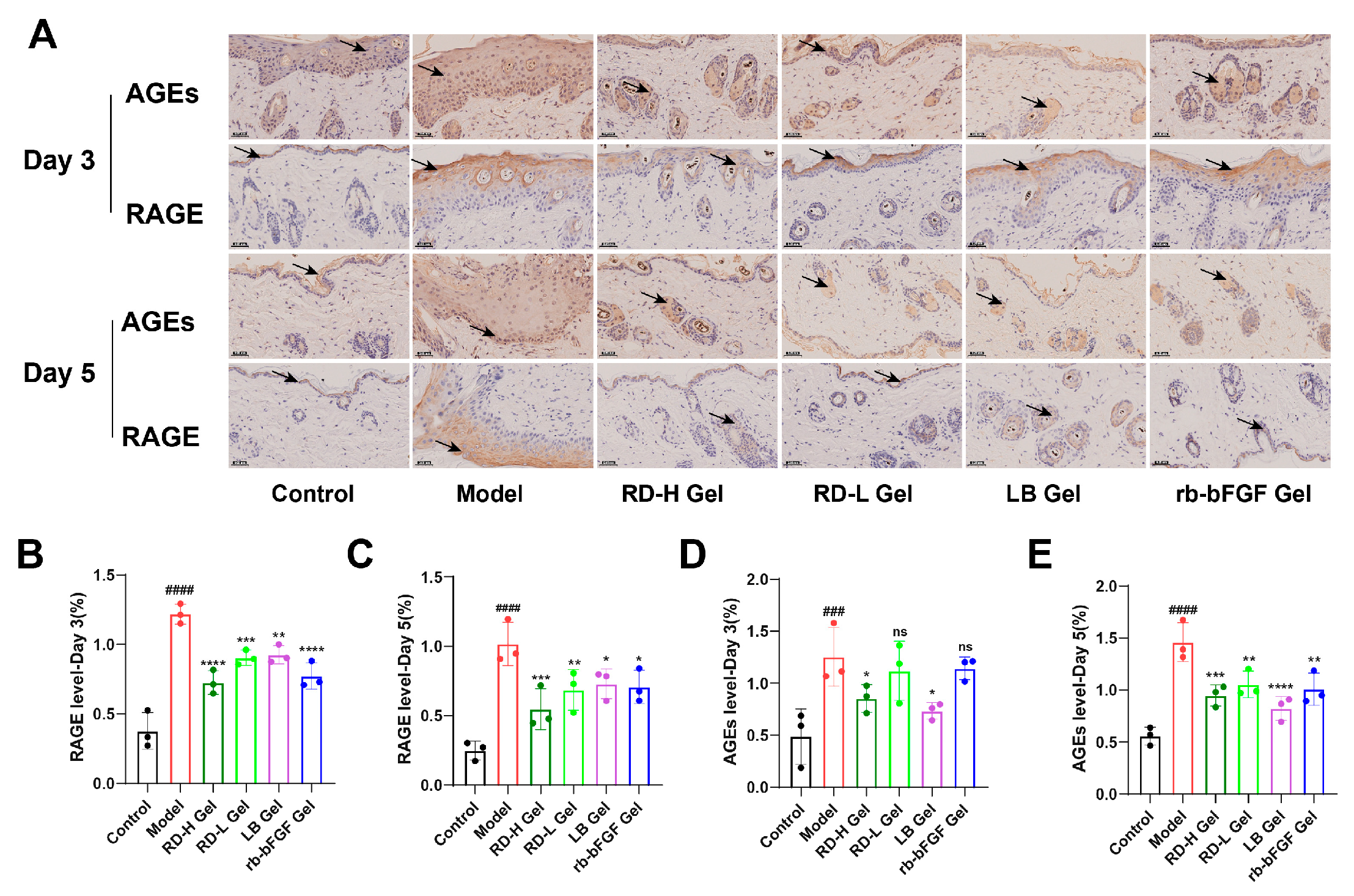
3.7. RD and Its Bioactive Components Significantly Downregulate AGE and RAGE Expression During the Inflammatory Phase
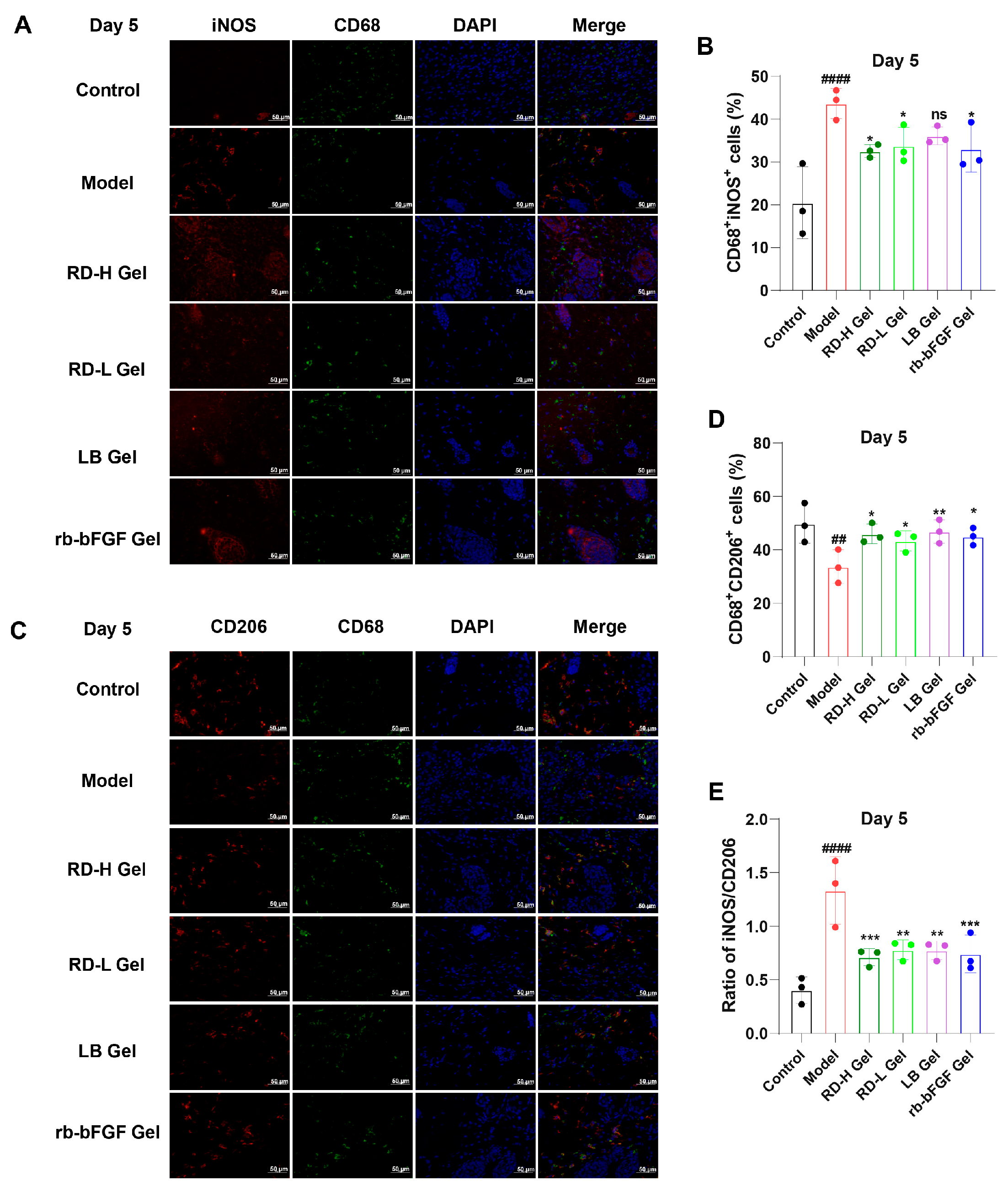
3.8. RD and Its Active Components Induce M2 Macrophage Polarization While Suppressing AGEs-Induced M1 Macrophage Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RD | Resina Draconis |
| AGEs | Advanced Glycation End Products |
| RAGE | Receptor for Advanced Glycation End Products |
| LA | Loureirin A |
| LB | Loureirin B |
| LC | Loureirin C |
| LD | Loureirin D |
| 7,4′-DHF | 7,4′-Dihydroxyflavone |
| RSV | Resveratrol |
| CCK-8 | Cell Counting Kit-8 |
| STZ | Streptozotocin |
| rb-bFGF | Recombinant Human Basic Fibroblast Growth Factor |
| ROS | Reactive Oxygen Species |
| PPI | Protein–Protein Interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor-α |
| COX-2 | Cyclooxygenase-2 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
References
- Luo, Y.L.; Luo, W.J.; Cao, Y.N.; Wang, Z.P. m6A demethylase FTO/ALKBH5 promotes diabetes-induced endothelial cell dysfunction by negatively regulating lncRNA H19. Exp. Mol. Pathol. 2025, 143, 104970. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.M.A. Unani treatment and leech therapy saved the diabetic foot of a patient from amputation. Int. Wound J. 2016, 13, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Raghav, A.; Khan, Z.A.; Labala, R.K.; Ahmad, J.; Noor, S.; Mishra, B.K. Financial burden of diabetic foot ulcers to world: A progressive topic to discuss always. Ther. Adv. Endocrinol. 2018, 9, 29–31. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkoli, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Koike, S.; Mitsuhashi, H.; Kishida, A.; Ogasawara, Y. Elucidating the Antiglycation Effect of Creatine on Methylglyoxal-Induced Carbonyl Stress In Vitro. Int. J. Mol. Sci. 2024, 25, 10880. [Google Scholar] [CrossRef]
- Aronson, D. Hyperglycemia and the pathobiology of diabetic complications. Adv. Cardiol. 2008, 45, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Hudson, B.I.; Moser, B.; Guo, J.C.; Rong, L.L.; Lu, Y.; Qu, W.; Lalla, E.; Lerner, S.; Chen, Y.L.; et al. Receptor for advanced glycation end products and its ligands—A journey from the complications of diabetes to its pathogenesis. Ann. N. Y. Acad. Sci. 2005, 1043, 553–561. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Darkwah, E.K. Advanced Glycation End Products in Health and Disease. Microorganisms 2022, 10, 1848. [Google Scholar] [CrossRef]
- Manigrasso, M.B.; Rabbani, P.; Egaña-Gorroño, L.; Quadri, N.; Frye, L.; Zhou, B.Y.; Reverdatto, S.; Ramirez, L.S.; Dansereau, S.; Pan, J.H.; et al. Small-molecule antagonism of the interaction of the RAGE cytoplasmic domain with DIAPH1 reduces diabetic complications in mice. Sci. Transl. Med. 2021, 13, eabf7084. [Google Scholar] [CrossRef]
- Lin, L. RAGE on the Toll Road? Cell Mol. Immunol. 2006, 3, 351–358. [Google Scholar]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Wang, J.; Mao, W.; Yang, Y.; He, F.; Li, J.; Wang, H.-H.; Long, J. Targeting the Receptor for Advanced Glycosylation End Products in Inflammation-Associated Diabetes Mellitus. J. Bio-X Res. 2024, 7, 196–204. [Google Scholar] [CrossRef]
- Sun, D.; Chang, Q.; Lu, F. Immunomodulation in diabetic wounds healing: The intersection of macrophage reprogramming and immunotherapeutic hydrogels. J. Tissue Eng. 2024, 15, 20417314241265202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, Y.L.; Xu, C.; Wang, J. Macrophages: Key players in diabetic wound healing. World J. Diabetes 2024, 15, 2177. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.Y.; Jiang, X.; Zhou, X.W.; Wang, O.R.; Wu, Q.Z.; Ren, L.; Zhu, J.X.; Zhu, S.S.; Tebon, P.; Sun, W.J.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Healthc. Mater. 2020, 9, 1901714. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.Y.; Nong, K.L.; Vandvik, P.O.; Guyatt, G.H.; Schnell, O.; Rydén, L.; Marx, N.; Brosius, F.I.I.I.; Mustafa, R.A.; Agarwal, A.; et al. Benefits and harms of drug treatment for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. Bmj-Brit Med. J. 2023, 381, e074068. [Google Scholar] [CrossRef]
- Baviera, M.; Foresta, A.; Colacioppo, P.; Macaluso, G.; Roncaglioni, M.C.; Tettamanti, M.; Fortino, I.; Genovese, S.; Caruso, I.; Giorgino, F. Effectiveness and safety of GLP-1 receptor agonists versus SGLT-2 inhibitors in type 2 diabetes: An Italian cohort study. Cardiovasc. Diabetol. 2022, 21, 162. [Google Scholar] [CrossRef]
- Norman, G.; Dumville, J.C.; Crosbie, E.J. Antiseptics and Antibiotics for Surgical Wounds Healing by Secondary Intention Summary of a Cochrane Review. JAMA Dermatol. 2016, 152, 1266–1268. [Google Scholar] [CrossRef]
- Schneider, I.; Calcagni, M.; Buschmann, J. Adipose-derived stem cells applied in skin diseases, wound healing and skin defects: A review. Cytotherapy 2023, 25, 105–119. [Google Scholar] [CrossRef]
- Fan, J.Y.; Yi, T.; Sze-To, C.M.; Zhu, L.; Peng, W.L.; Zhang, Y.Z.; Zhao, Z.Z.; Chen, H.B. A Systematic Review of the Botanical, Phytochemical and Pharmacological Profile of Dracaena cochinchinensis, a Plant Source of the Ethnomedicine “Dragon’s Blood”. Molecules 2014, 19, 10650–10669. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.S.; Yao, R.Y.; Li, C.J.; Zhang, Z.L.; Xu, Y.H.; Wei, J.H. Dragon’s Blood from Worldwide: Species, Traditional Uses, Phytochemistry and Pharmacology. Am. J. Chin. Med. 2021, 49, 1315–1367. [Google Scholar] [CrossRef]
- Al-Awthan, Y.S.; Bahattab, O.S. Phytochemistry and Pharmacological Activities of Dracaena cinnabari Resin. Biomed. Res. Int.-UK 2021, 2021, 8561696. [Google Scholar] [CrossRef]
- Gupta, D.; Bleakley, B.; Gupta, R.K. Dragon’s blood: Botany, chemistry and therapeutic uses. J. Ethnopharmacol. 2008, 115, 361–380. [Google Scholar] [CrossRef]
- Sun, S.L.; Wang, P.; Yue, K.F.; Yi, Q.P.; Xie, X.J.; Xie, X.M. Effect and Mechanism of Dragon’s Blood on Wound Healing of Patients with Stress Hand Injury. Evid.-Based Compl. Alt. Med. 2023, 2023, 6122331. [Google Scholar] [CrossRef]
- Gao, L.L.; Liu, T.M.; Cheng, X.; Li, N. Wound healing activity of a traditional Chinese medicine (Longxuejie) in capsule dosage form. Pak. J. Pharm. Sci. 2020, 33, 445–448. [Google Scholar] [CrossRef]
- Fan, W.J.; Qu, Y.; Yuan, X.; Shi, H.S.; Liu, G.B. Loureirin B Accelerates Diabetic Wound Healing by Promoting TGFβ/Smad-Dependent Macrophage M2 Polarization: A Concerted Analytical Approach Through Single-Cell RNA Sequencing and Experimental Verification. Phytother. Res. 2024. [Google Scholar] [CrossRef]
- Ning, S.; Dai, Z.; Feng, X. Chemical composition analysis and the determination of main components of Resina Draconis based on UPLC-QE-Orbitrap-MS. J. Shandong Univ. (Nat. Sci.) 2025, 60, 10–23. [Google Scholar]
- Li, Y.; Zang, J.; Wang, X.M.; Feng, X.C.; Qiu, F. Deciphering the underlying wound healing mechanisms of (Lour.) Merr. by integrating network pharmacology, transcriptomics, and experimental validation. J. Ethnopharmacol. 2023, 302, 115890. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, J.N.; Fan, B.; Chen, X.N.; Pang, D.R.; Zheng, J.A.; Zhang, Q.A.; Zhao, Y.F.; Xiao, W.; Tu, P.F.; et al. Phenolic constituents, pharmacological activities, quality control, and metabolism of species: A review. J. Ethnopharmacol. 2019, 244, 112138. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, M.; Lei, W.; Yang, R.; Fu, S.; Fan, Z.; Yang, Y.; Zhang, T. Advances in the role of STAT3 in macrophage polarization. Front. Immunol. 2023, 14, 1160719. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. Bcl-2-regulated apoptosis: Mechanism and therapeutic potential. Curr. Opin. Immunol. 2007, 19, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.-Y. Caspase-3 regulates the migration, invasion and metastasis of colon ca ncer cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Luciano, A.K.; Ackah, E.; Rodriguez-Vita, J.; Bancroft, T.A.; Eichmann, A.; Simons, M.; Kyriakides, T.R.; Morales-Ruiz, M.; Sessa, W.C. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple ang iogenic substrates. Proc. Natl. Acad. Sci. USA 2014, 111, 12865–12870. [Google Scholar] [CrossRef] [PubMed]
- Tito, C.; Masciarelli, S.; Colotti, G.; Fazi, F. EGF receptor in organ development, tissue homeostasis and regeneration. J. Biomed. Sci. 2025, 32, 24. [Google Scholar] [CrossRef]
- Leśniak, W.; Filipek, A. S100A6 Protein—Expression and Function in Norm and Pathology. Int. J. Mol. Sci. 2023, 24, 1341. [Google Scholar] [CrossRef]
- Borgo, C.; D’Amore, C.; Sarno, S.; Salvi, M.; Ruzzene, M. Protein kinase CK2: A potential therapeutic target for diverse human diseases. Signal Transduct. Target. Ther. 2021, 6, 183. [Google Scholar] [CrossRef]
- Naba, A. Mechanisms of assembly and remodelling of the extracellular matrix. Nat. Rev. Mol. Cell Biol. 2024, 25, 865–885. [Google Scholar] [CrossRef]
- Hashimoto, K.; Higashiyama, S.; Asada, H.; Hashimura, E.; Kobayashi, T.; Sudo, K.; Nakagawa, T.; Damm, D.; Yoshikawa, K.; Taniguchi, N. Heparin-binding epidermal growth factor-like growth factor is an autoc rine growth factor for human keratinocytes. J. Biol. Chem. 1994, 269, 20060–20066. [Google Scholar] [CrossRef]
- Banfi, C.; Amadio, P.; Zarà, M.; Brioschi, M.; Sandrini, L.; Barbieri, S.S. Prenylcysteine Oxidase 1 (PCYOX1), a New Player in Thrombosis. Int. J. Mol. Sci. 2022, 23, 2831. [Google Scholar] [CrossRef]
- Park, J.-J.; Lee, S.J.; Baek, M.; Lee, O.-J.; Nam, S.; Kim, J.; Kim, J.Y.; Shin, E.-Y.; Kim, E.-G. FRMD6 determines the cell fate towards senescence: Involvement of the Hippo-YAP-CCN3 axis. Cell Death Differ. 2024, 31, 1398–1409. [Google Scholar] [CrossRef]
- Nguyen, D.-H.T.; Gao, L.; Wong, A.; Chen, C.S. Cdc42 regulates branching in angiogenic sprouting in vitro. Microcirculation 2017, 24, e12372. [Google Scholar] [CrossRef]
- Sun, J.S.; Song, L.Y.; Zhou, Y.; Wu, K.Y.; Li, C.Y.; Han, B.Q.; Chang, J. Review: Advances in multifunctional hydrogels based on carbohydrate polymer and protein in the treatment of diabetic wounds. Int. J. Biol. Macromol. 2025, 309, 142693. [Google Scholar] [CrossRef] [PubMed]
- Pianissoli, L.G.; Silva, J.P.R.; Penteado, E.A.; Lopes, D.R.C.; Mengal, V.F. Oxidative Stress and Inflammation in Wound Repair: Molecular and Cellular Mechanisms. Faseb J. 2019, 33, 542.3. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Xia, X.; Chen, Y.; Mao, T.C.; Shi, X.H.; Zhang, Y.M.; Fan, D.L. Retrospective analysis of related factors affecting skin wound healing. Int. J. Clin. Exp. Med. 2018, 11, 8615–8621. [Google Scholar]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Biswas, S.; Shang, Y.L.; Collard, E.; Azad, A.; Kauh, C.; Bhasker, V.; Gordillo, G.M.; Sen, C.K.; Roy, S. Macrophage Dysfunction Impairs Resolution of Inflammation in the Wounds of Diabetic Mice. PLoS ONE 2010, 5, e9539. [Google Scholar] [CrossRef]
- Chawla, D.; Bansal, S.; Banerjee, B.D.; Madhu, S.V.; Kalra, O.P.; Tripathi, A.K. Role of advanced glycation end product (AGE)-induced receptor (RAGE) expression in diabetic vascular complications. Microvasc. Res. 2014, 95, 1–6. [Google Scholar] [CrossRef]
- Mata, R.; Yao, Y.; Cao, W.; Ding, J.; Zhou, T.; Zhai, Z.; Gao, C. The Dynamic Inflammatory Tissue Microenvironment: Signality and Disease Therapy by Biomaterials. Research 2021, 2021, 4189516. [Google Scholar] [CrossRef]
- Hudson, B.I.; Lippman, M.E. Targeting RAGE Signaling in Inflammatory Disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef]
- Kierdorf, K.; Fritz, G. RAGE regulation and signaling in inflammation and beyond. J. Leukocyte Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, Y.; Shi, L.; Li, L.; Zhang, D.; Gong, Z.; Wu, Q. Activation and modulation of the AGEs-RAGE axis: Implications for infl ammatory pathologies and therapeutic interventions—A review. Pharmacol. Res. 2024, 206, 107282. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| β-actin | CTATTGGCAACGAGCGGTTC | ACTGTGTTGGCATAGAGGTCTT |
| IL-1β | ATCTCGCAGCAGCACATCA | CCAGCAGGTTATCATCATCATCC |
| IL-6 | TTCCATCCAGTTGCCTTCTTG | AATTAGCCTCCGACTTGTGAA |
| TNF-α | CCACGTCGTAGCAAACCACC | GTGAGGAGCACGTAGTCGG |
| COX-2 | AGCCCATTGAACCTGGACTG | ACCCAATCAGCGTTTCTCGT |
| MCP-1 | AGCCAACTCTCACTGAAGCC | GCGTTAACTGCATCTGGCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Li, A.; Dai, Z.; Li, Y.; Feng, X.; Qiu, F. Resina Draconis Promotes Diabetic Wound Healing by Regulating the AGE-RAGE Pathway to Modulate Macrophage Polarization. Curr. Issues Mol. Biol. 2025, 47, 748. https://doi.org/10.3390/cimb47090748
Jin X, Li A, Dai Z, Li Y, Feng X, Qiu F. Resina Draconis Promotes Diabetic Wound Healing by Regulating the AGE-RAGE Pathway to Modulate Macrophage Polarization. Current Issues in Molecular Biology. 2025; 47(9):748. https://doi.org/10.3390/cimb47090748
Chicago/Turabian StyleJin, Xin, Ang Li, Zhaoyuan Dai, Yi Li, Xinchi Feng, and Feng Qiu. 2025. "Resina Draconis Promotes Diabetic Wound Healing by Regulating the AGE-RAGE Pathway to Modulate Macrophage Polarization" Current Issues in Molecular Biology 47, no. 9: 748. https://doi.org/10.3390/cimb47090748
APA StyleJin, X., Li, A., Dai, Z., Li, Y., Feng, X., & Qiu, F. (2025). Resina Draconis Promotes Diabetic Wound Healing by Regulating the AGE-RAGE Pathway to Modulate Macrophage Polarization. Current Issues in Molecular Biology, 47(9), 748. https://doi.org/10.3390/cimb47090748







