Tumor Immune Microenvironment and Checkpoint Inhibition in Clear Cell Ovarian Carcinoma: Bridging Tumor Biology and Clinical Application in Immunotherapy
Abstract
1. Introduction
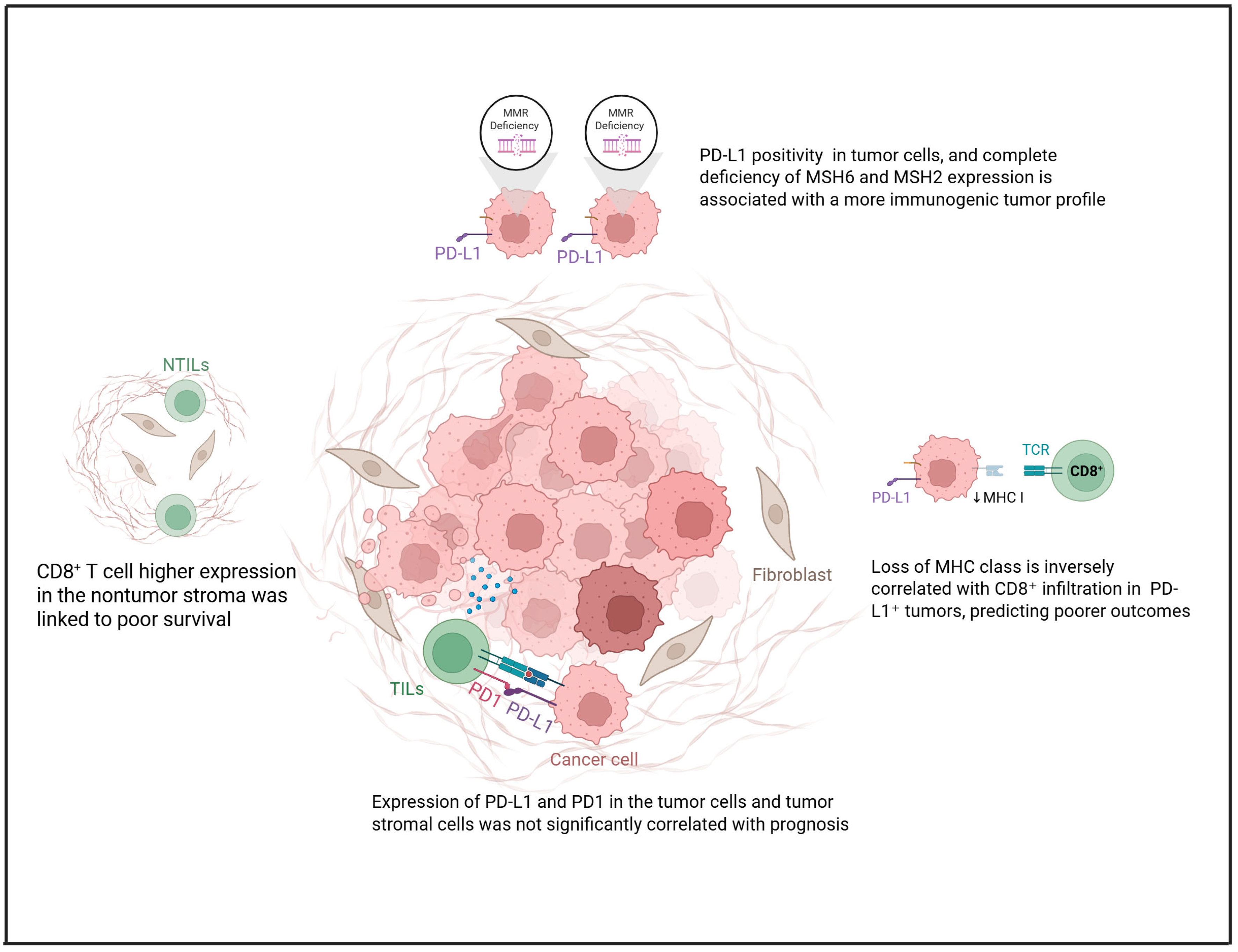
2. PD-L1 Expression in Clear Cell Ovarian Carcinoma
3. Genes and Pathways Related to Tumor Immune Microenvironment
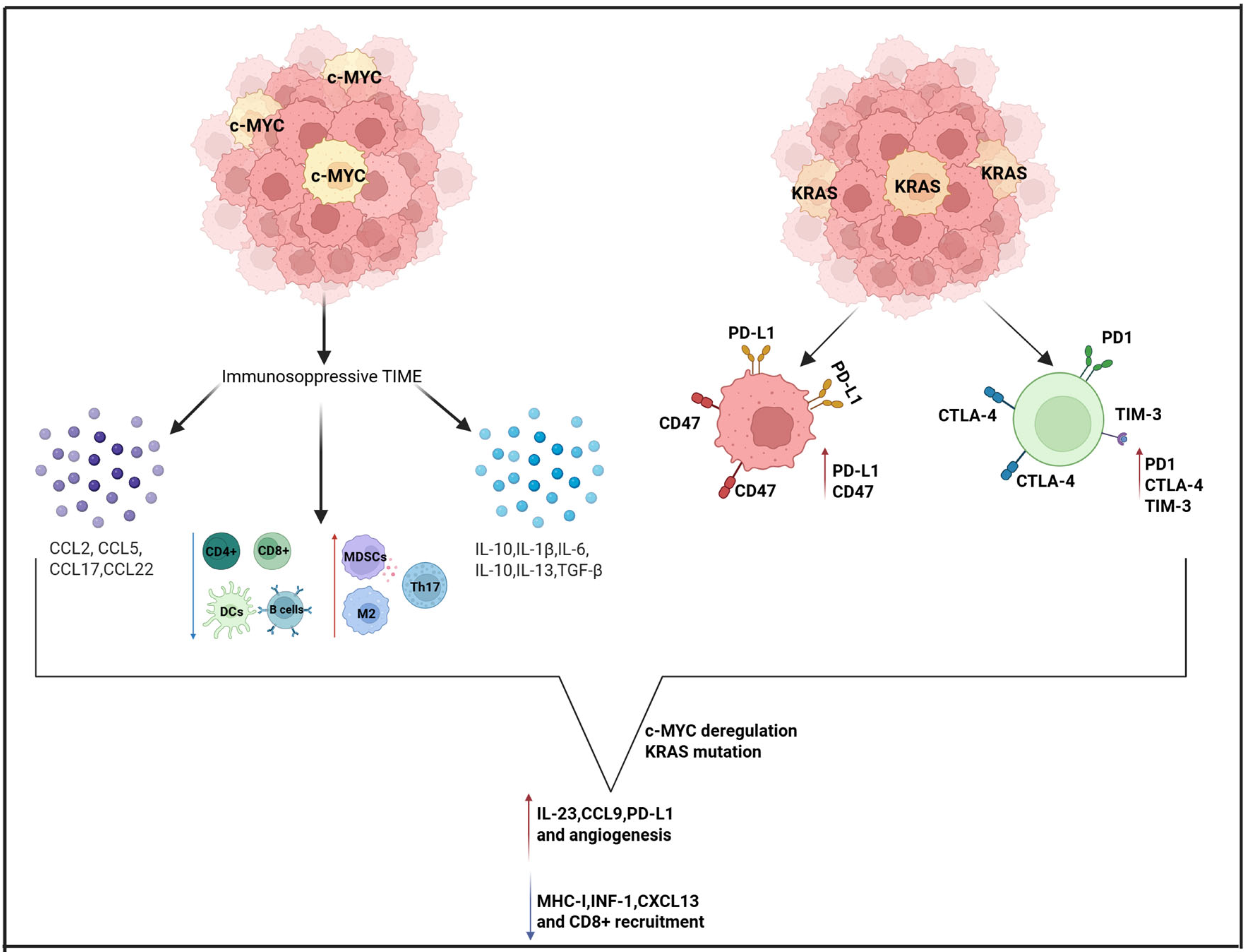
3.1. KRAS and MYC
3.2. PI3K
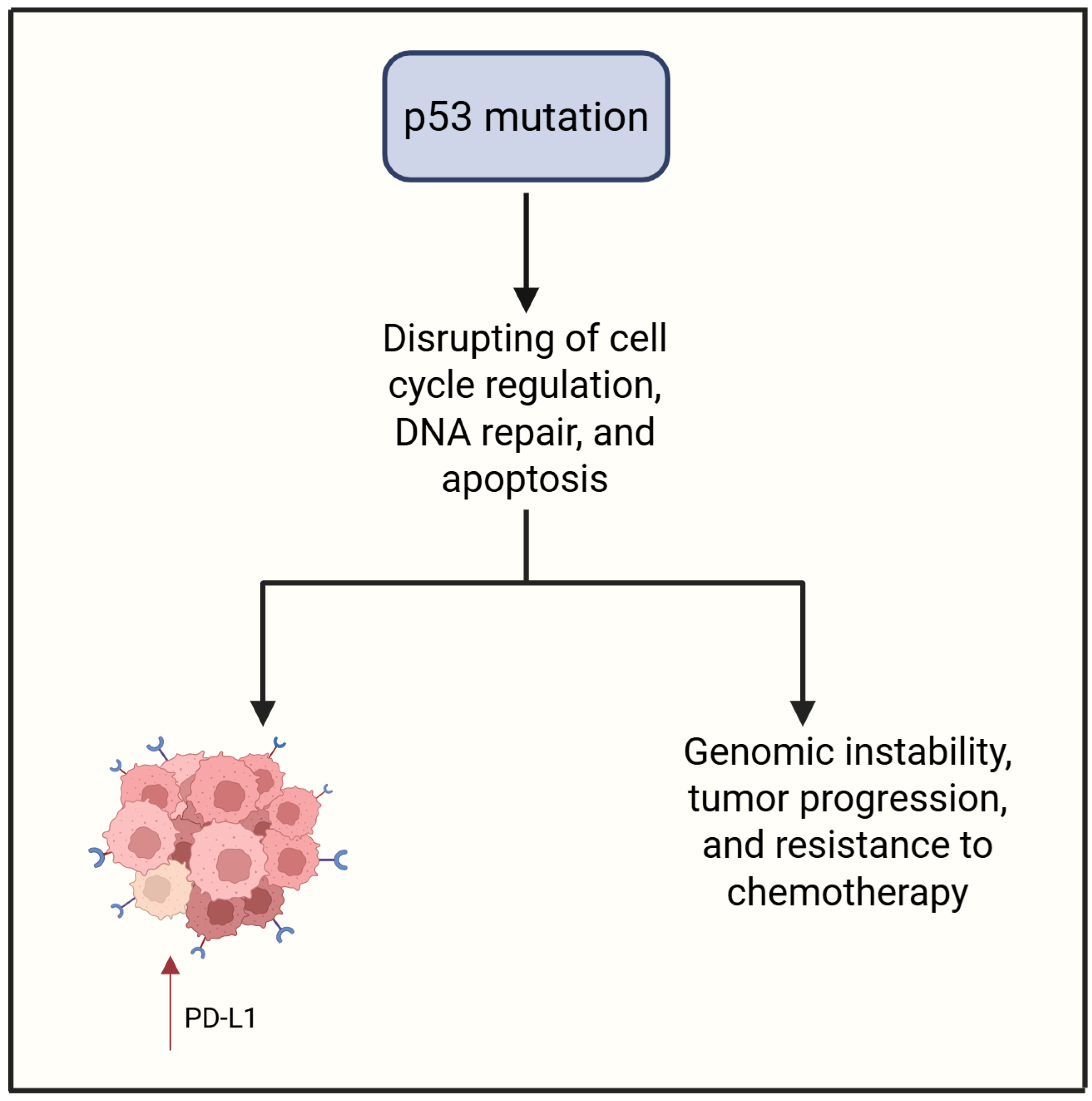
3.3. P53
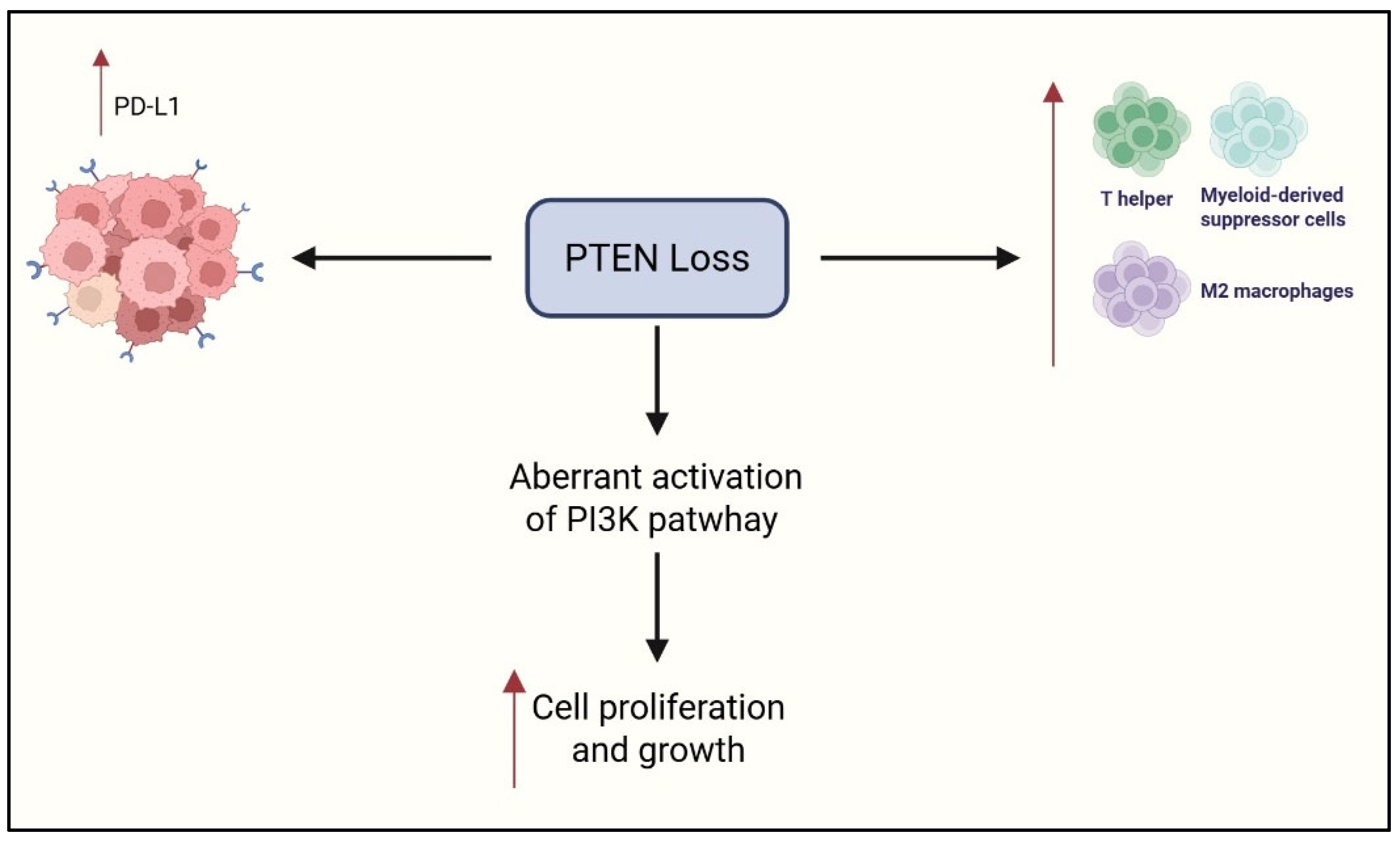
3.4. PTEN
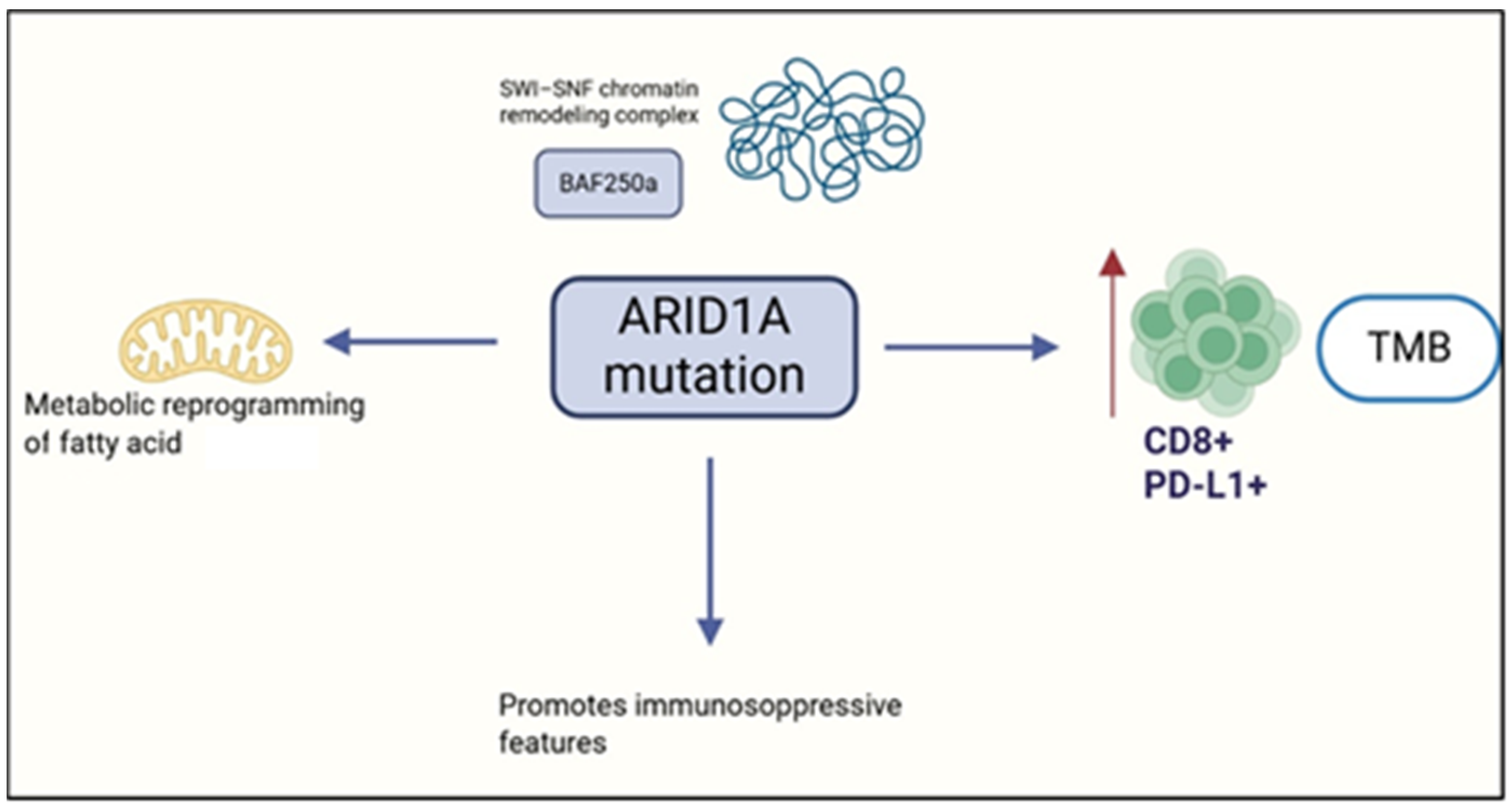
3.5. ARID1A
4. Natural Killer Cell Impairment
5. Immune Checkpoint Inhibitors in Ovarian Clear Cell Carcinoma
Combination Between ICI and Other Target Therapies
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Okamoto, A.; Glasspool, R.M.; Mabuchi, S.; Nomura, H. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the ovary. Int. J. Gynecol. Cancer 2014, 24, S20–S25, Erratum in Int. J. Gynecol. Cancer 2015, 25, 721. [Google Scholar] [CrossRef]
- Fuh, K.C.; Java, J.J.; Chan, J.K.; Kapp, D.S.; Monk, B.J.; Burger, R.A.; Young, R.C.; Alberts, D.S.; McGuire, W.P.; Markman, M.; et al. Differences in presentation and survival of Asians compared to Caucasians with ovarian cancer: An NRG Oncology/GOG Ancillary study of 7914 patients. Gynecol. Oncol. 2019, 154, 420–425. [Google Scholar] [CrossRef]
- Fujiwara, K.; Shintani, D.; Nishikawa, T. Clear-cell carcinoma of the ovary. Ann. Oncol. 2016, 27, i50–i52. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Chan, J.K.; Teoh, D.; Hu, J.M.; Shin, J.Y.; Osann, K.; Kapp, D.S. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol. Oncol. 2008, 109, 370–376. [Google Scholar] [CrossRef]
- Pozzati, F.; Moro, F.; Pasciuto, T.; Gallo, C.; Ciccarone, F.; Franchi, D.; Mancari, R.; Giunchi, S.; Timmerman, D.; Landolfo, C.; et al. Imaging in gynecological disease (14): Clinical and ultrasound characteristics of ovarian clear cell carcinoma. Ultrasound Obstet. Gynecol. 2018, 52, 792–800. [Google Scholar] [CrossRef]
- Rosso, R.; Turinetto, M.; Borella, F.; Chopin, N.; Meeus, P.; Lainè, A.; Ray-Coquard, I.; Le Saux, O.; Ferraioli, D. Ovarian clear cell carcinoma: Open questions on the management and treatment algorithm. Oncologist 2025, 30, oyae325. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Durand, F.; Ngoi, N.; Lim, D.; Ray-Coquard, I.; Tan, D.S. Clearer Horizons: The latest advances in clear cell ovarian cancer treatment. Cancer Treat. Rev. 2025, 138, 102977. [Google Scholar] [CrossRef] [PubMed]
- Drew, Y.; Bookman, M.A. Clearing the Path for Immunologic Targeting of Gynecologic Clear Cell Carcinomas. JAMA Oncol. 2025, online ahead of print. [Google Scholar] [CrossRef]
- Borella, F.; Ghisoni, E.; Giannone, G.; Cosma, S.; Benedetto, C.; Valabrega, G.; Katsaros, D. Immune Checkpoint Inhibitors in Epithelial Ovarian Cancer: An Overview on Efficacy and Future Perspectives. Diagnostics 2020, 10, 146. [Google Scholar] [CrossRef]
- Turinetto, M.; Valsecchi, A.A.; Tuninetti, V.; Scotto, G.; Borella, F.; Valabrega, G. Immunotherapy for Cervical Cancer: Are We Ready for Prime Time? Int. J. Mol. Sci. 2022, 23, 3559. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory Mechanisms of PD-1/PD-L1 in Cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Pavelescu, L.A.; Enache, R.M.; Roşu, O.A.; Profir, M.; Creţoiu, S.M.; Gaspar, B.S. Predictive Biomarkers and Resistance Mechanisms of Checkpoint Inhibitors in Malignant Solid Tumors. Int. J. Mol. Sci. 2024, 25, 9659. [Google Scholar] [CrossRef]
- Aden, D.; Zaheer, S.; Sureka, N.; Trisal, M.; Chaurasia, J.K.; Zaheer, S. Exploring Immune Checkpoint Inhibitors: Focus on PD-1/PD-L1 Axis and Beyond. Pathol. Res. Pract. 2025, 269, 155864. [Google Scholar] [CrossRef]
- Sue-A-Quan, R.; Patel, P.G.; Shakfa, N.; Nyi, M.N.; Afriyie-Asante, A.; Kang, E.Y.; Köbel, M.; Koti, M. Prognostic Significance of T Cells, PD-L1 Immune Checkpoint and Tumour Associated Macrophages in Clear Cell Carcinoma of the Ovary. Gynecol. Oncol. 2021, 162, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Miyamoto, M.; Hada, T.; Ishibashi, H.; Iwahashi, H.; Kakimoto, S.; Suzuki, R.; Ito, T.; Suminokura, J.; Tsuda, H.; et al. The Worsening Impact of Programmed Cell Death Ligand 1 in Ovarian Clear Cell Carcinomas. Arch. Gynecol. Obstet. 2022, 306, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Pan, B.; Jia, H.; Zhang, Y.; Wang, S.; Wang, Y.; Zhang, S.; Li, M.; Wang, A.; Wang, X.; et al. PD-L1 Expression in Ovarian Clear Cell Carcinoma Using the 22C3 PharmDx Assay. Diagn. Pathol. 2024, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhou, H.; Yang, J.; Cao, D.; Yuan, Z. Infiltration of CD8+ Cytotoxic T-Cells and Expression of PD-1 and PD-L1 in Ovarian Clear Cell Carcinoma. Sci. Rep. 2025, 15, 4716. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Hang, J.-F.; Lai, C.-R.; Chan, I.-S.; Shih, Y.-C.; Jiang, L.-Y.; Chang, Y.-H.; Chen, Y.-J. Loss of Major Histocompatibility Complex Class I, CD8+ Tumor-Infiltrating Lymphocytes, and PD-L1 Expression in Ovarian Clear Cell Carcinoma. Am. J. Surg. Pathol. 2023, 47, 124–130. [Google Scholar] [CrossRef]
- Alldredge, J.; Serna-Gallegos, T.; Gallegos, N.; VanLeer, J.P.; Chang, J.; Ziogas, A.; Goreal, W.; Randall, L. Evaluation of clear cell subtypes of ovarian and uterine malignancies with anti-PD-L1 and anti-PD1 immunohistochemical expression and their association with stage and survival. Gynecol. Oncol. 2019, 155, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, D.; Ameli, F.; Nili, F.; Edjtemaei, R.; Sheikhhasani, S. Immunohistochemical Expression of PD-L1 and Its Correlation with Microsatellite Status in Endometrial and Ovarian Clear Cell Carcinomas: A Cross-Sectional Study. BMC Cancer 2022, 22, 1362. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Strickland, K.C.; Sholl, L.M.; Rodig, S.; Ritterhouse, L.L.; Chowdhury, D.; D’Andrea, A.D.; Matulonis, U.A.; Konstantinopoulos, P.A. Clear Cell Ovarian Cancers with Microsatellite Instability: A Unique Subset of Ovarian Cancers with Increased Tumor-Infiltrating Lymphocytes and PD-1/PD-L1 Expression. OncoImmunology 2017, 6, e1277308. [Google Scholar] [CrossRef]
- Oda, K.; Hamanishi, J.; Matsuo, K.; Hasegawa, K. Genomics to Immunotherapy of Ovarian Clear Cell Carcinoma: Unique Opportunities for Management. Gynecol. Oncol. 2018, 151, 381–389. [Google Scholar] [CrossRef]
- Casacuberta-Serra, S.; González-Larreategui, Í.; Capitán-Leo, D.; Soucek, L. MYC and KRAS Cooperation: From Historical Challenges to Therapeutic Opportunities in Cancer. Signal Transduct. Target. Ther. 2024, 9, 205. [Google Scholar] [CrossRef]
- Therachiyil, L.; Anand, A.; Azmi, A.; Bhat, A.; Korashy, H.M.; Uddin, S. Role of RAS Signaling in Ovarian Cancer. F1000Research 2022, 11, 1253. [Google Scholar] [CrossRef]
- Plisiecka-Hałasa, J.; Karpińska, G.; Szymańska, T.; Ziółkowska, I.; Madry, R.; Timorek, A.; Dębniak, J.; Ułańska, M.; Jędryka, M.; Chudecka-Głaz, A.; et al. P21WAF1, P27KIP1, TP53 and C-MYC Analysis in 204 Ovarian Carcinomas Treated with Platinum-Based Regimens. Ann. Oncol. 2003, 14, 1078–1085. [Google Scholar] [CrossRef]
- Reyes-González, J.M.; Vivas-Mejía, P.E. c-MYC and Epithelial Ovarian Cancer. Front. Oncol. 2021, 11, 601512. [Google Scholar] [CrossRef]
- Miranda, A.; Pattnaik, S.; Hamilton, P.T.; Fuss, M.A.; Kalaria, S.; Laumont, C.M.; Smazynski, J.; Mesa, M.; Banville, A.; Jiang, X.; et al. N-MYC Impairs Innate Immune Signaling in High-Grade Serous Ovarian Carcinoma. Sci. Adv. 2024, 10, eadj5428. [Google Scholar] [CrossRef]
- Yoshida, M.; Taguchi, A.; Kawana, K.; Adachi, K.; Kawata, A.; Ogishima, J.; Nakamura, H.; Fujimoto, A.; Sato, M.; Inoue, T.; et al. Modification of the Tumor Microenvironment in KRAS or C-MYC-Induced Ovarian Cancer-Associated Peritonitis. PLoS ONE 2016, 11, e0160330. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Massi, D.; Teng, M.W.L.; Mandala, M. PI3K-AKT-mTOR Inhibition in Cancer Immunotherapy, Redux. Semin. Cancer Biol. 2018, 48, 91–103. [Google Scholar] [CrossRef]
- Collins, N.B.; Al Abosy, R.; Miller, B.C.; Bi, K.; Zhao, Q.; Quigley, M.; Ishizuka, J.J.; Yates, K.B.; Pope, H.W.; Manguso, R.T.; et al. PI3K Activation Allows Immune Evasion by Promoting an Inhibitory Myeloid Tumor Microenvironment. J. Immuno Ther. Cancer 2022, 10, e003402. [Google Scholar] [CrossRef]
- Zhan, J.; Jücker, M. The Role of PI3K/AKT/mTOR Signaling in Tumor Radioresistance and Advances in Inhibitor Research. Int. J. Mol. Sci. 2025, 26, 6853. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Luo, Z.; Zhang, X.; Yu, X.; Yuan, G.; Li, X.; Xie, F.; Jiang, O. Targeting PI3K Signaling to Overcome Tumor Immunosuppression: Synergistic Strategies to Enhance Cancer Vaccine Efficacy. Vaccines 2025, 13, 292. [Google Scholar] [CrossRef]
- Xu, H.; Russell, S.N.; Steiner, K.; O’Neill, E.; Jones, K.I. Targeting PI3K-Gamma in Myeloid Driven Tumour Immune Suppression: A Systematic Review and Meta-Analysis of the Preclinical Literature. Cancer Immunol. Immunother. 2024, 73, 204. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Yoshino, K.; Ueda, Y.; Matsuzaki, S.; Kakuda, M.; Okazawa, A.; Egawa-Takata, T.; Kobayashi, E.; Kimura, T. Potential Targets for Ovarian Clear Cell Carcinoma: A Review of Updates and Future Perspectives. Cancer Cell Int. 2015, 15, 117. [Google Scholar] [CrossRef]
- Mabuchi, S.; Kuroda, H.; Takahashi, R.; Sasano, T. The PI3K/AKT/mTOR Pathway as a Therapeutic Target in Ovarian Cancer. Gynecol. Oncol. 2015, 137, 173–179. [Google Scholar] [CrossRef]
- Zheng, K.; Jin, G.; Cao, R.; Gao, Y.; Xu, J.; Chai, R.; Kang, Y. Targeting on the PI3K/mTOR: A Potential Treatment Strategy for Clear Cell Ovarian Carcinoma. Cancer Chemother. Pharmacol. 2025, 95, 21. [Google Scholar] [CrossRef] [PubMed]
- Rzepecka, I.K.; Tysarowski, A.; Konopka, B.; Dansonka-Mieszkowska, A.; Kupryjanczyk, J. High Frequency of PIK3R1 Alterations in Ovarian Cancers: Clinicopathological and Molecular Associations. Cancers 2025, 17, 269. [Google Scholar] [CrossRef]
- Deng, J.; Bai, X.; Feng, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Inhibition of PI3K/Akt/mTOR Signaling Pathway Alleviates Ovarian Cancer Chemoresistance through Reversing Epithelial-Mesenchymal Transition and Decreasing Cancer Stem Cell Marker Expression. BMC Cancer 2019, 19, 618. [Google Scholar] [CrossRef]
- Rinne, N.; Christie, E.L.; Ardasheva, A.; Kwok, C.H.; Demchenko, N.; Low, C.; Tralau-Stewart, C.; Fotopoulou, C.; Cunnea, P. Targeting the PI3K/AKT/mTOR Pathway in Epithelial Ovarian Cancer: Therapeutic Treatment Options for Platinum-Resistant Ovarian Cancer. Cancer Drug Resist. 2021, 4, 573–595. [Google Scholar] [CrossRef]
- Gaillard, H.; Garcia-Muse, T.; Aguilera, A. Replication stress and cancer. Nat. Rev. Cancer 2015, 15, 276–289. [Google Scholar] [CrossRef]
- Adriaens, C.; Standaert, L.; Barra, J.; Latil, M.; Verfaillie, A.; Kalev, P.; Boeckx, B.; Wijnhoven, P.W.G.; Radaelli, E.; Vermi, W.; et al. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat. Med. 2016, 22, 861–868, Correction in Nat. Med. 2024, 30, 1506. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, M.S.; Bartek, J.; Maya-Mendoza, A. p53 at the crossroad of DNA replication and ribosome biogenesis stress pathways. Cell Death Differ. 2022, 29, 972–982. [Google Scholar] [CrossRef]
- Hafner, A.; Bulyk, M.L.; Jambhekar, A.; Lahav, G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 2019, 20, 199–210. [Google Scholar] [CrossRef]
- Boutelle, A.M.; Attardi, L.D. p53 and tumor suppression: It takes a network. Trends Cell Biol. 2021, 31, 298–310. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 2018, 25, 114–132. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, W. The complexity of p53-mediated metabolic regulation in tumor suppression. Semin. Cancer Biol. 2021, 85, 4–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Leng, S.; Eisenstat, D.D.; Sergi, C.; Leng, R. Targeting p53 for immune modulation: Exploring its functions in tumor immunity and inflammation. Cancer Lett. 2025, 617, 217614. [Google Scholar] [CrossRef]
- Liu, S.; Liu, T.; Jiang, J.; Guo, H.; Yang, R. p53 mutation and deletion contribute to tumor immune evasion. Front. Genet. 2023, 14, 1088455. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, L.; Zhang, S.; Tian, X.; De La Cruz, A.; George, A.; Arnoff, T.E.; El-Deiry, W.S. The role of p53 in anti-tumor immunity and response to immunotherapy. Front. Mol. Biosci. 2023, 10, 1148389. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tan, J.Y.M.; Chitkara, N.; Bhatt, S. TP53 Mutation-Mediated Immune Evasion in Cancer: Mechanisms and Therapeutic Implications. Cancers 2024, 16, 3069. [Google Scholar] [CrossRef]
- Park, E.; Han, H.; Choi, S.-E.; Park, H.; Woo, H.-Y.; Jang, M.; Shim, H.-S.; Hwang, S.; Kang, H.; Cho, N.-H. p53 Immunohistochemistry and Mutation Types Mismatching in High-Grade Serous Ovarian Cancer. Diagnostics 2022, 12, 579. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Shimizu, M.; Nikaido, T.; Toki, T.; Shiozawa, T.; Fujii, S. Clear cell carcinoma has an expression pattern of cell cycle regulatory molecules that is unique among ovarian adenocarcinomas. Cancer 1999, 85, 669–677. [Google Scholar] [CrossRef]
- Mabuchi, S.; Sugiyama, T.; Kimura, T. Clear cell carcinoma of the ovary: Molecular insights and future therapeutic perspectives. J. Gynecol. Oncol. 2016, 27, e31. [Google Scholar] [CrossRef] [PubMed]
- Similä-Maarala, J.; Soovares, P.; Pasanen, A.; Ahvenainen, T.; Vahteristo, P.; Bützow, R.; Lassus, H. TCGA molecular classification in endometriosis-associated ovarian carcinomas: Novel data on clear cell carcinoma. Gynecol. Oncol. 2022, 165, 577–584. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L.; Li, Q.; Zhou, S.; Hou, T.; Yang, H.; Ye, S. Molecular subtype of ovarian clear cell carcinoma: An analysis of 80 Chinese patients using the TCGA molecular classification of endometrial cancer. BMC Cancer 2025, 25, 90. [Google Scholar] [CrossRef]
- Ho, E.S.; Lai, C.R.; Hsieh, Y.T.; Chen, J.T.; Lin, A.J.; Hung, M.H.; Liu, F.S. p53 mutation is infrequent in clear cell carcinoma of the ovary. Gynecol. Oncol. 2001, 80, 189–193. [Google Scholar] [CrossRef]
- Köbel, M.; Kang, E.Y.; Weir, A.; Rambau, P.F.; Lee, C.H.; Nelson, G.S.; Ghatage, P.; Meagher, N.S.; Riggan, M.J.; Alsop, J.; et al. p53 and ovarian carcinoma survival: An Ovarian Tumor Tissue Analysis consortium study. J. Pathol. Clin. Res. 2023, 9, 208–222. [Google Scholar] [CrossRef]
- Wilkins, R.; Lin, L.H.; Xia, R.; Shiomi, T.; Zamuco, R.D.; Shukla, P.S. Clinical Outcome and Morphology-Based Analysis of p53 Aberrant and Mismatch Repair Protein-Deficient Ovarian Clear Cell Carcinoma and Their Association With p16, HER2, and PD-L1 Expression. Am. J. Clin. Pathol. 2023, 160, 466–476. [Google Scholar]
- Millis, S.Z.; Ikeda, S.; Reddy, S.; Gatalica, Z.; Kurzrock, R. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19,784 diverse solid tumors. JAMA Oncol. 2016, 2, 1565–1573. [Google Scholar]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef]
- Yehia, L.; Ngeow, J.; Eng, C. PTEN-opathies: From biological insights to evidence-based precision medicine. J. Clin. Investig. 2019, 129, 452–464. [Google Scholar] [CrossRef]
- Nowak, D.G.; Cho, H.; Herzka, T.; Watrud, K.; DeMarco, D.V.; Wang, V.M.Y.; Senturk, S.; Fellmann, C.; Ding, D.; Beinortas, T.; et al. MYC drives Pten/Trp53-deficient proliferation and metastasis due to IL6 secretion and AKT suppression via PHLPP2. Cancer Discov. 2015, 5, 636–651. [Google Scholar] [CrossRef]
- Li, S.; Zhu, M.; Pan, R.; Fang, T.; Cao, Y.Y.; Chen, S.; Zhao, X.; Lei, C.-Q.; Guo, L.; Chen, Y.; et al. The tumor suppressor PTEN has a critical role in antiviral innate immunity. Nat. Immunol. 2016, 17, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, D. The functions of tumor suppressor PTEN in innate and adaptive immunity. Cell. Mol. Immunol. 2017, 14, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, H.; Yang, L.; Zhang, Z.; Li, C.C.M.; Yuan, X.; Bu, L.; Chen, L.; Chen, Y.; Li, C.-M.; et al. PTEN-L promotes type I interferon responses and antiviral immunity. Cell. Mol. Immunol. 2018, 15, 48–57. [Google Scholar]
- Horton, B.L.; Williams, J.B.; Cabanov, A.; Spranger, S.; Gajewski, T.F. Intratumoral CD8+ T-cell apoptosis is a major component of T-cell dysfunction and impedes antitumor immunity. Cancer Immunol. Res. 2018, 6, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Vidotto, T.; Melo, C.M.; Castelli, E.; Koti, M.; dos Reis, R.B.; Squire, J.A. Emerging role of PTEN loss in evasion of the immune response to tumours. Br. J. Cancer 2020, 122, 1732–1743. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, L.; Li, S.L.; Gu, J.; Cui, X.; Zhou, Y. PTEN loss correlates with T cell exclusion across human cancers. BMC Cancer 2021, 21, 429. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Tsuda, H.; Inoue, T.; Berkowitz, R.S.; Mok, S.C. PTEN expression in clear cell adenocarcinoma of the ovary. Gynecol. Oncol. 2006, 101, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Tinker, A.; Clarke, B.; Ghatage, P.; Welch, S.; Weberpals, J.I.; Dhani, N.C.; Butler, M.O.; Tonkin, K.; Tan, Q.; et al. A Clinical and Molecular Phase II Trial of Oral ENMD-2076 in Ovarian Clear Cell Carcinoma (OCCC): A Study of the Princess Margaret Phase II Consortium. Clin. Cancer Res. 2018, 24, 6168–6174. [Google Scholar] [CrossRef]
- Nero, C.; Ciccarone, F.; Pietragalla, A.; Scambia, G. PTEN and Gynecological Cancers. Cancers 2019, 11, 1458. [Google Scholar] [CrossRef]
- Martins, F.C.; Couturier, D.L.; Paterson, A.; Karnezis, A.N.; Chow, C.; Nazeran, T.M.; Odunsi, A.; Gentry-Maharaj, A.; Vrvilo, A.; Hein, A.; et al. Clinical and pathological associations of PTEN expression in ovarian cancer: A multicentre study from the Ovarian Tumour Tissue Analysis Consortium. Br. J. Cancer 2020, 123, 793–802. [Google Scholar] [CrossRef]
- Wu, C.-H.; Lin, H.; Ou, Y.-C.; Fu, H.-C.; Yang, M.-Y.; Huang, C.-C. Molecular Interplay Between PTEN, ARID1A, PD-L1, and MMR in Asian Ovarian Clear Cell Carcinoma: Implications for Immunotherapy Response and Patient Stratification. Int. J. Mol. Sci. 2025, 26, 4915. [Google Scholar] [CrossRef]
- Reisman, D.; Glaros, S.; Thompson, E.A. The SWI/SNF complex and cancer. Oncogene 2009, 28, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hoang, L.; Ji, J.X.; Huntsman, D.G. SWI/SNF complex mutations in gynecologic cancers: Molecular mechanisms and models. Annu. Rev. Pathol. 2020, 15, 467–492. [Google Scholar] [CrossRef]
- Centore, R.C.; Sandoval, G.J.; Soares, L.M.M.; Kadoch, C.; Chan, H.M. Mammalian SWI/SNF chromatin remodeling complexes: Emerging mechanisms and therapeutic strategies. Trends Genet. 2020, 36, 936–950. [Google Scholar] [CrossRef]
- Takeda, T.; Banno, K.; Okawa, R.; Yanokura, M.; Iijima, M.; Irie-Kunitomi, H.; Nakamura, K.; Iida, M.; Adachi, M.; Umene, K.; et al. ARID1A gene mutation in ovarian and endometrial cancers (review). Oncol. Rep. 2016, 35, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Saotome, K.; Chiyoda, T.; Aimono, E.; Nakamura, K.; Tanishima, S.; Nohara, S.; Okada, C.; Hayashi, H.; Kuroda, Y.; Nomura, H.; et al. Clinical implications of next-generation sequencing-based panel tests for malignant ovarian tumors. Cancer Med. 2020, 9, 7407–7417. [Google Scholar] [CrossRef]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef]
- Kuroda, Y.; Chiyoda, T.; Kawaida, M.; Nakamura, K.; Aimono, E.; Yoshimura, T.; Takahashi, M.; Saotome, K.; Yoshihama, T.; Iwasa, N.; et al. ARID1A mutation/ARID1A loss is associated with a high immunogenic profile in clear cell ovarian cancer. Gynecol. Oncol. 2021, 162, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Chen, L.; Yu, M.; Li, J.; Chen, J.; Xu, F.; Ni, M.; Liu, C.; Wu, X.; Chen, X.; et al. Genetic and therapeutic heterogeneity shape the baseline and longitudinal immune ecosystem of ovarian clear cell carcinoma. J. Immunother. Cancer 2024, 12, e010069. [Google Scholar] [CrossRef]
- Devlin, M.J.; Miller, R.; Laforets, F.; Kotantaki, P.; Garsed, D.W.; Kristeleit, R.; Bowtell, D.D.; McDermott, J.; Maniati, E.; Balkwill, F.R. The Tumor Microenvironment of Clear-Cell Ovarian Cancer. Cancer Immunol. Res. 2022, 10, 1326–1339. [Google Scholar] [CrossRef]
- Patrizi, O.; Rampinelli, F.; Coltrini, D.; Pesce, S.; Carlomagno, S.; Sivori, S.; Pascale, A.; Marcenaro, E.; Parolini, S.; Tabellini, G. Natural killer cell impairment in ovarian clear cell carcinoma. J. Leukoc. Biol. 2020, 108, 1425–1434. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and Antitumor Activity of Anti–PD-1 Antibody, Nivolumab, in Patients with Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor Activity and Safety of Pembrolizumab in Patients with Advanced Recurrent Ovarian Cancer: Results from the Phase II KEYNOTE-100 Study. Ann. Oncol. 2019, 30, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Fujiwara, K.; Ledermann, J.A.; Oza, A.M.; Kristeleit, R.; Ray-Coquard, I.-L.; Richardson, G.E.; Sessa, C.; Yonemori, K.; Banerjee, S.; et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): An open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021, 22, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Kristeleit, R.; Devlin, M.J.; Clamp, A.; Gourley, C.; Roux, R.; Hall, M.; Nirsimloo, R.; Kounnis, V.; Sage, L.; Narayanan, P.; et al. Pembrolizumab in Patients With Advanced Clear Cell Gynecological Cancer: A Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2025, 11, 377–385. [Google Scholar] [CrossRef]
- Ngoi, N.Y.L.; Choi, C.H.; Zhu, J.; Lim, D.; Tan, T.Z.; Sun, H.; Heong, V.; Ow, S.G.W.; Chay, W.Y.; Kim, H.S.; et al. Durvalumab versus physician’s choice chemotherapy in recurrent ovarian clear cell adenocarcinoma (MOCCA/APGOT-OV2/GCGS-OV3): A multicenter, randomized, phase 2 trial. Clin. Cancer Res. 2025, 24, OF1–OF9. [Google Scholar] [CrossRef]
- Dizon, D.S.; Mathews, C.A.; MacLaughlan David, S.; Machan, J.T.; Hadfield, M.J.; Marks, E.I.; Bansal, R.; McGinn, C.; Hassinger, F.; Luppe, D.; et al. Final Results of BrUOG 354: A Randomized Phase II Trial of Nivolumab Alone or in Combination with Ipilimumab for People with Ovarian and Other Extra-Renal Clear Cell Carcinomas. J. Clin. Oncol. 2024, 42 (Suppl. S17). [Google Scholar] [CrossRef]
- Chae, Y.K.; Ryan, C.W.; Aung, N.; Robinson, W.R.; Othus, M.; Sharon, E.; O’Malley, D.M.; Backes, F.J.; Blanke, C.D.; Eskander, R.N.; et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) SWOG S1609: The Clear Cell Ovarian, Endometrial, Cervical Cancer Cohorts. Cancer Res. 2023, 83 (Suppl. S8), CT162. [Google Scholar] [CrossRef]
- Klein, O.; Carlino, M.S.; Michael, M.; Underhill, C.R.; So, J.; Kee, D.; Antill, Y.; Lam, W.-S.; Chan, H.; Harrup, R.A.; et al. Nivolumab and Ipilimumab Combination Treatment in Advanced Gynaecological Clear Cell Cancers: Results from the Phase II MoST-CIRCUIT Trial. Ann. Oncol. 2024, 35, S546–S547. [Google Scholar] [CrossRef]
- Peng, Z.; Li, H.; Gao, Y.; Sun, L.; Jiang, J.; Xia, B.; Huang, Y.; Zhang, Y.; Xia, Y.; Zhang, Y.; et al. Sintilimab Combined with Bevacizumab in Relapsed or Persistent Ovarian Clear Cell Carcinoma (INOVA): A Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2024, 25, 1288–1297. [Google Scholar] [CrossRef]
- Gien, L.T.; Enserro, D.M.; Block, M.S.; Waggoner, S.; Duska, L.R.; Wahner-Hendrickson, A.E.; Thaker, P.H.; Backes, F.; Kidd, M.; Muller, C.Y.; et al. Phase II trial of pembrolizumab and epacadostat in recurrent clear cell carcinoma of the ovary: An NRG oncology study GY016. Gynecol. Oncol. 2024, 186, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, N.; Lee, J.-Y.; Lim, D.; Thian, Y.L.; Lim, Y.W.; Chan, J.J.; Chay, W.Y.; Zhang, Z.; Gopinathan, A.; Lim, S.E.; et al. Pembrolizumab plus Lenvatinib (PL) in Recurrent Clear Cell Gynecological Cancer (CCGC): Phase II LARA Trial (GCGS-OV4/APGOT-OV3). Int. J. Gynecol. Cancer 2024, 35 (Suppl. S2), S589. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tan, D.; Ray-Coquard, I.; Lee, J.B.; Kim, B.G.; Van Nieuwenhuysen, E.; Huang, R.Y.; Tse, K.Y.; González-Martin, A.; Scott, C.; et al. Phase II Randomized Study of Dostarlimab Alone or with Bevacizumab versus Non-Platinum Chemotherapy in Recurrent Gynecological Clear Cell Carcinoma (DOVE/APGOT-OV7/ENGOT-ov80). J. Gynecol. Oncol. 2025, 36, e51. [Google Scholar] [CrossRef] [PubMed]

| Reference | Drug | Study Design | Clinical Setting | Number of Patients | Biomarkers | Main Results |
|---|---|---|---|---|---|---|
| Hamanishi et al. [86] | Nivolumab | Phase II | Platinum-resistant EOC | 26 EOC (2 OCCC) | / | 1 CR out of 2 cases with OCCC |
| KEYNOTE-100 [88] | Pemrbolizumab | Phase II | Recurrent, heavily pretreated EOC | 285 EOC (2 OCCC) | / | 1 CR out of 2 cases with OCCC |
| JAVELIN Ovarian 200 [89] | Avelumab or avelumab + PLD or PLD | Phase III | Platinum-resistant or refractory EOC | 566 EOC (73 OCCC) | / | PFS: 2.8 months (HR: 79; 95% CI 0.41–1.54), OS: 17.7 months (HR: 0.89; CI: 0.38–2.06) |
| PEACCOC trial [90] | Pembrolizumab | Phase II | Advanced CCGC | 48 CCGC (41 OCCC) | MMR, ARID1A, p53, PD-1, PD-L1 (none predictive of treatment response) | ORR: 25%. PFS 2.7 months, OS: 14.8 months |
| MOCCA trial [91] | Durvalumab vs. CHT | Phase II | Recurrent OCCC | 48 OCCC | ARID1A, PIK3CA, KRAS, TERT, PD-L1, PI3KCA (associated with long time to progression), ERBB2 (associated with worse survival) | PFS: 7.6 weeks (95% CI 7.0–16.0), OS: 37.9 weeks (95% CI 21.7–143.0) |
| BrUOG 354 [92] | Nivolumab or nivolumab + ipilimumab | Phase II | Recurrent extra-renal CCC | 44 CCC (36 OCCC) | / | ORR of 33% for nivolumab/ipilimumab versus 14.3% with nivolumab alone. PFS: 5.6 months for nivolumab/ipilimumab and 2.2 months with N; OS: 24.6 months for nivolumab/ipilimumab and 17 months for nivolumab |
| SWOG S1609 trial [93] | Nivolumab + imiplimab | Phase II | CCGC | 19 OCCC | / | ORR: 21.1%. Median PFS: 3.7 months. Median OS: 21.7 months |
| MoST-CIRCUIT trial [94] | Nivolumab or nivolumab + ipilimumab | Phase II | Advanced OCCC or UCCC | 24 OCCC; 4 UCCC | PIK3A, TMB (predictive of response), ARID1A (wild-type predictive of response) | ORR: 50%. PFS rate at 6 months: 52%. Median OS: 9.9 months |
| Inova trial [95] | Sintilimab + bevacizumab | Phase II | Recurrent/persistent OCCC | 41 OCCC | TMB, MMR, PD-L1, TILs, ARID1A (none predictive of treatment response) | ORR: 40.5%. Median PFS: 6.9 months (95% CI 5.3–8.1); median OS: 28.2 months (95% CI 23.8–NR) |
| NRG-GY016 trial [96] | Pembrolizumab + epacadostat | Phase II | Recurrent OCCC | 14 OCCC | / | ORR: 21%. PFS: 4.8 months (95% CI: 1.9–9.6) |
| LARA trial [97] | Pembrolizumab + lenvatinib | Phase II | 25 OCCC | ORR: 44.0%. PFS: 23.4 weeks (95% CI: 4.4–42.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borella, F.; Capella, G.; Cosma, S.; Gallio, N.; Gavello, F.; Revelli, A.; Ferraioli, D.; Cusato, J.; Castellano, I.; Cassoni, P.; et al. Tumor Immune Microenvironment and Checkpoint Inhibition in Clear Cell Ovarian Carcinoma: Bridging Tumor Biology and Clinical Application in Immunotherapy. Curr. Issues Mol. Biol. 2025, 47, 726. https://doi.org/10.3390/cimb47090726
Borella F, Capella G, Cosma S, Gallio N, Gavello F, Revelli A, Ferraioli D, Cusato J, Castellano I, Cassoni P, et al. Tumor Immune Microenvironment and Checkpoint Inhibition in Clear Cell Ovarian Carcinoma: Bridging Tumor Biology and Clinical Application in Immunotherapy. Current Issues in Molecular Biology. 2025; 47(9):726. https://doi.org/10.3390/cimb47090726
Chicago/Turabian StyleBorella, Fulvio, Giulia Capella, Stefano Cosma, Niccolò Gallio, Federica Gavello, Alberto Revelli, Domenico Ferraioli, Jessica Cusato, Isabella Castellano, Paola Cassoni, and et al. 2025. "Tumor Immune Microenvironment and Checkpoint Inhibition in Clear Cell Ovarian Carcinoma: Bridging Tumor Biology and Clinical Application in Immunotherapy" Current Issues in Molecular Biology 47, no. 9: 726. https://doi.org/10.3390/cimb47090726
APA StyleBorella, F., Capella, G., Cosma, S., Gallio, N., Gavello, F., Revelli, A., Ferraioli, D., Cusato, J., Castellano, I., Cassoni, P., & Bertero, L. (2025). Tumor Immune Microenvironment and Checkpoint Inhibition in Clear Cell Ovarian Carcinoma: Bridging Tumor Biology and Clinical Application in Immunotherapy. Current Issues in Molecular Biology, 47(9), 726. https://doi.org/10.3390/cimb47090726











