Smart Red Blood Cell Carriers: A Nanotechnological Approach to Cancer Drug Delivery
Abstract
1. Introduction
2. Methods
3. Results
3.1. Nanotechnology and Nanoparticles
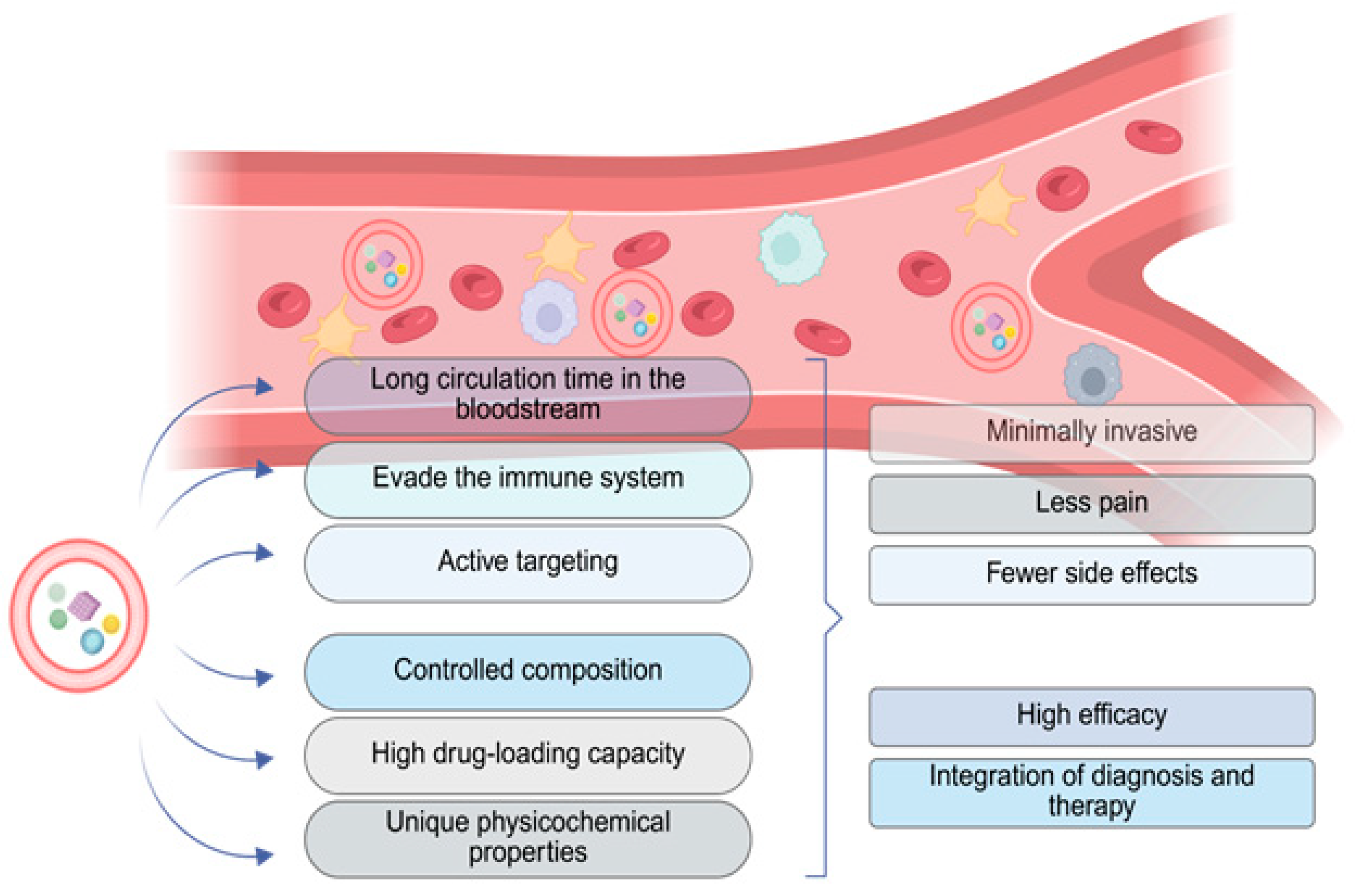
3.2. Advantages of Loading Erythrocytes with Nanoparticles
3.3. Hemocompatibility of Nanoparticles
3.4. Interaction with Erythrocyte Membranes and Laboratory Techniques to Identify the Modifications
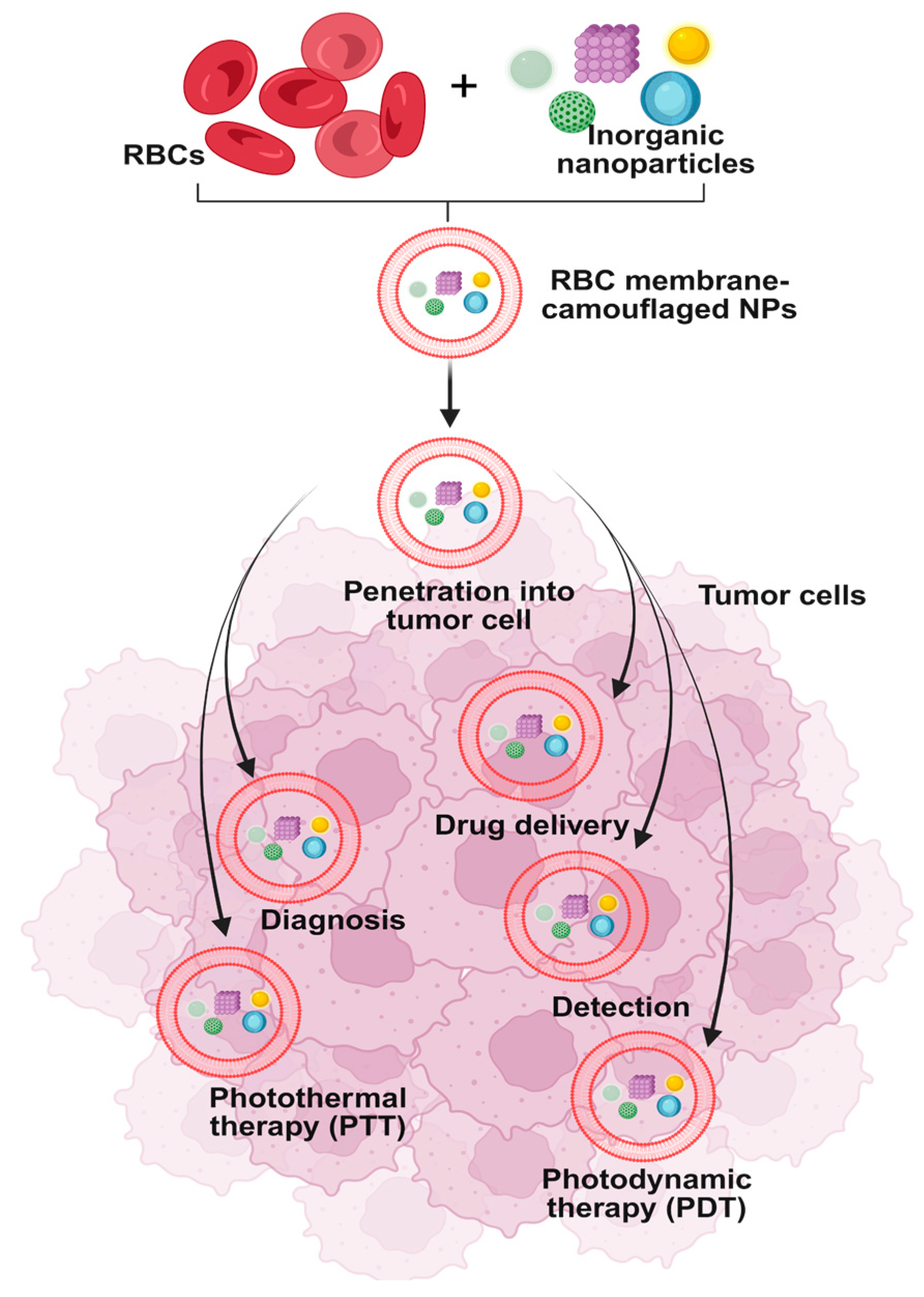
3.5. Clinical Application in Anticancer Therapy
4. Discussion
| Nanoparticle Composition (Drug Carrier Type) | Production Method | Results | Final Application | References |
|---|---|---|---|---|
| Polystyrene nanoparticles | Physical adsorption onto erythrocyte surface | Prolonged circulation time depending on NP size; RBC morphology largely maintained | Long circulating drug delivery system | [31] |
| Polymer–protein conjugate carriers | PEG-coated liposomes | Improved solubility; reduced solvent-associated toxicity; enhanced tumor penetration | Clinically approved for metastatic breast cancer, pancreatic cancer | [32] |
| Biodegradable polymeric nanoparticles (e.g., PLGA core) coated with natural erythrocyte membrane | Top-down biomimetic coating: hypotonic lysis of RBCs, continously → membrane vesicle preparation and then→ fusion (e.g., extrusion) onto polymeric nanoparticle | Significantly prolonged circulation half-life in mice; high retention at 72 h post-injection compared with PEGylated control particles | Long-circulating drug delivery platform that evades immune clearance and improves systemic retention | [60] |
| Polymer–drug conjugates | Covalent conjugation: Attaching drugs or proteins to polymers through stable covalent bonds. | Enhanced solubility and stability: conjugation improves the solubility and stability of poorly water-soluble drugs and proteins. | Cancer therapy: polymer–drug conjugates are utilized for targeted chemotherapy, reducing systemic toxicity. | [75] |
| Cell membrane coated nanoparticles | Extrusion | Immune evasion; prolonged circulation | Targeted cancer therapy | [70] |
| Erythrocyte-inspired functional materials (e.g., RBC membrane-coated nanoparticles, erythrocyte-mimicking carriers) | Biomimetic engineering approaches: coating synthetic nanoparticles with RBC membranes, constructing erythrocyte-mimetic systems | Prolonged circulation time, immune evasion, improved biocompatibility, targeted drug delivery | Drug delivery, biosensing, detoxification, and other biomedical applications | [68] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Xia, F.; Lin, R. Global Burden of Cancer and Associated Risk Factors in 204 Countries and Territories, 1980–2021: A Systematic Analysis for the GBD 2021. J. Hematol. Oncol. 2024, 17, 119. [Google Scholar] [CrossRef]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Cooper, G.M. The Development and Causes of Cancer. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2000; p. 743. Available online: http://www.ncbi.nlm.nih.gov/books/NBK9963/ (accessed on 15 May 2025).
- Popovich, J.R. Sarcoma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- De Voeght, A.; Jaspers, A.; Beguin, Y.; Baron, F.; De Prijck, B. Overview of the General Management of Acute Leukemia for Adults. Rev. Med. Liege 2021, 76, 470–475. [Google Scholar] [PubMed]
- Perincheri, S. Tumor Microenvironment of Lymphomas and Plasma Cell Neoplasms: Broad Overview and Impact on Evaluation for Immune Based Therapies. Front. Oncol. 2021, 11, 719140. [Google Scholar] [CrossRef]
- Tadman, M.; Roberts, D. Central Nervous System Cancer. In Oxford Handbook of Cancer Nursing; Oxford Academic: Oxford, UK, 2007; pp. 331–342. [Google Scholar] [CrossRef]
- Chun, Y.K. Neuroendocrine Tumors of the Female Reproductive Tract: A Literature Review. J. Pathol. Transl. Med. 2015, 49, 450–461. [Google Scholar] [CrossRef]
- Bamodu, O.A.; Chung, C.-C. Cancer Care Disparities: Overcoming Barriers to Cancer Control in Low- and Middle-Income Countries. JCO Glob. Oncol. 2024, 10, e2300439. [Google Scholar] [CrossRef]
- Chow, A.Y. Cell Cycle Control by Oncogenes and Tumor Suppressors: Driving the Transformation of Normal Cells into Cancerous Cells. Nat. Educ. 2010, 3, 7. [Google Scholar]
- Parsa, N. Environmental Factors Inducing Human Cancers. Iran. J. Public Health 2012, 41, 1. [Google Scholar]
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; Jiang, W.G. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2000; pp. 45–55. [Google Scholar]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor Biomarkers for Diagnosis, Prognosis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Feng, Y.; Zhang, J.; Swinnen, J.; Li, Y.; Ni, Y. A Review on Curability of Cancers: More Efforts for Novel Therapeutic Options Are Needed. Cancers 2019, 11, 1782. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Barros, S.M.; Whitaker, S.K.; Sukthankar, P.; Avila, L.A.; Gudlur, S.; Warner, M.; Beltrão, E.I.C.; Tomich, J.M. A Review of Solute Encapsulating Nanoparticles Used as Delivery Systems with Emphasis on Branched Amphipathic Peptide Capsules. Arch. Biochem. Biophys. 2016, 596, 22–42. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Estanqueiro, M.; Amaral, M.H.; Conceição, J.; Sousa Lobo, J.M. Nanotechnological Carriers for Cancer Chemotherapy: The State of the Art. Colloids Surf. B Biointerfaces 2015, 126, 631–648. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Barjesteh, T.; Mansur, S.; Bao, Y. Inorganic Nanoparticle-Loaded Exosomes for Biomedical Applications. Molecules 2021, 26, 1135. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2017, 12, 908–931. [Google Scholar] [CrossRef]
- Aschner, M. Nanoparticles: Transport across the Olfactory Epithelium and Application to the Assessment of Brain Function in Health and Disease. Prog. Brain Res. 2009, 180, 141–152. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Kiio, T.M.; Park, S. Physical Properties of Nanoparticles Do Matter. J. Pharm. Investig. 2021, 51, 35–51. [Google Scholar] [CrossRef]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Yadav, S.S.; Chauhan, V.; Shukla, S.; Vishnolia, K.K. Applications of Nanoparticles in Various Fields. Diagn. Appl. Health Intell. Surveill. Syst. 2021, 2021, 221–236. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, Q.; Zhang, Y.; Pan, J.; Zhang, L.; Zhang, Z.; Liu, Z. Surface Loading of Nanoparticles on Engineered or Natural Erythrocytes for Prolonged Circulation Time: Strategies and Applications. Acta Pharmacol. Sin. 2021, 42, 1040–1054. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nano-Micro Lett. 2019, 11, 100. [Google Scholar] [CrossRef]
- Shen, H.; Ouyang, Y.; Zhang, L.; Li, J.; Wang, S. Blood Cell Membrane-Coated Nanomaterials as a Versatile Biomimetic Nanoplatform for Antitumor Applications. Nanomaterials 2024, 14, 1757. [Google Scholar] [CrossRef]
- Fortis, S.P.; Batrinou, A.; Georgatzakou, H.T.; Tsamesidis, I.; Albanidis, G.; Papageorgiou, E.G.; Stamoulis, K.; Gkiliopoulos, D.; Pouroutzidou, G.K.; Theocharidou, A.; et al. Effect of Silica-Based Mesoporous Nanomaterials on Human Blood Cells. Chem. Biol. Interact. 2024, 387, 110784. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Bhatt, A.; Nair, R.P.; Rashmi, R.; Raju, R.; Geeverghese, R.; Lekshmi, P. Product Evaluation: Blood Compatibility Studies. Biomed. Prod. Mater. Eval. Stand. Ethics 2022, 13, 435–459. [Google Scholar] [CrossRef]
- Luu, C.H.; Nguyen, N.T.; Ta, H.T. Unravelling Surface Modification Strategies for Preventing Medical Device-Induced Thrombosis. Adv. Healthc. Mater. 2024, 13, 2301039. [Google Scholar] [CrossRef]
- Al-Zyoud, W.; Haddadin, D.; Hasan, S.A.; Jaradat, H.; Kanoun, O. Biocompatibility Testing for Implants: A Novel Tool for Selection and Characterization. Materials 2023, 16, 6881. [Google Scholar] [CrossRef]
- Desai, N.; Tambe, V.; Pofali, P.; Vora, L.K. Cell Membrane-Coated Nanoparticles: A New Frontier in Immunomodulation. Adv. NanoBiomed Res. 2024, 4, 2400012. [Google Scholar] [CrossRef]
- De Harpe, K.M.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; Toit, L.C.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell-Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Pouroutzidou, G.K.; Lymperaki, E.; Kazeli, K.; Lioutas, C.B.; Christodoulou, E.; Perio, P.; Reybier, K.; Pantaleo, A.; Kontonasaki, E. Effect of Ion Doping in Silica-Based Nanoparticles on the Hemolytic and Oxidative Activity in Contact with Human Erythrocytes. Chem. Biol. Interact. 2020, 318, 108974. [Google Scholar] [CrossRef]
- Ioannou, M.E.; Pouroutzidou, G.K.; Chatzimentor, I.; Tsamesidis, I.; Florini, N.; Tsiaoussis, I.; Lymperaki, E.; Komninou, P.; Kontonasaki, E. Synthesis and Characterization of Cerium Oxide Nanoparticles: Effect of Cerium Precursor to Gelatin Ratio. Appl. Sci. 2023, 13, 2676. [Google Scholar] [CrossRef]
- Yedgar, S.; Barshtein, G.; Gural, A. Hemolytic Activity of Nanoparticles as a Marker of Their Hemocompatibility. Micromachines 2022, 13, 2091. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Leng, Y.; He, C.; Li, X.; Zhao, L.; Qu, Y.; Wu, Y. Red Blood Cells: A Potential Delivery System. J. Nanobiotechnol. 2023, 21, 288. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Kazeli, K.; Lymperaki, E.; Pouroutzidou, G.K.; Oikonomou, I.M.; Komninou, P.; Zachariadis, G.; Reybier, K.; Pantaleo, A.; Kontonasaki, E. Effect of Sintering Temperature of Bioactive Glass Nanoceramics on the Hemolytic Activity and Oxidative Stress Biomarkers in Erythrocytes. Cell. Mol. Bioeng. 2020, 13, 201–218. [Google Scholar] [CrossRef]
- Akhter, M.H.; Khalilullah, H.; Gupta, M.; Alfaleh, M.A.; Alhakamy, N.A.; Riadi, Y.; Md, S. Impact of Protein Corona on the Biological Identity of Nanomedicine: Understanding the Fate of Nanomaterials in the Biological Milieu. Biomedicines 2021, 9, 496. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red Blood Cell Membrane-Camouflaged Nanoparticles: A Novel Drug Delivery System for Antitumor Application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Mohandas, N.; Gallagher, P.G. Red Cell Membrane: Past, Present, and Future. Blood 2008, 112, 3939–3948. [Google Scholar] [CrossRef]
- Nazemidashtarjandi, S.; Vahedi, A.; Farnoud, A.M. Lipid Chemical Structure Modulates the Disruptive Effects of Nanomaterials on Membrane Models. Langmuir 2020, 36, 4923–4932. [Google Scholar] [CrossRef] [PubMed]
- Walter, S. Structure of the Plasma Membrane: An Electron-Microscope Study. Circulation 1962, 26, 1066–1069. [Google Scholar]
- Chang, S.H.; Low, P.S. Regulation of the Glycophorin C-Protein 4.1 Membrane-to-Skeleton Bridge and Evaluation of Its Contribution to Erythrocyte Membrane Stability. J. Biol. Chem. 2001, 276, 22223–22230. [Google Scholar] [CrossRef] [PubMed]
- Kodippili, G.C.; Spector, J.; Hale, J.; Giger, K.; Hughes, M.R.; McNagny, K.M.; Birkenmeier, C.; Peters, L.; Ritchie, K.; Low, P.S. Analysis of the Mobilities of Band 3 Populations Associated with Ankyrin Protein and Junctional Complexes in Intact Murine Erythrocytes. J. Biol. Chem. 2012, 287, 4129–4138. [Google Scholar] [CrossRef]
- Midya, J.; Auth, T.; Gompper, G. Membrane-Mediated Interactions Between Nonspherical Elastic Particles. ACS Nano 2023, 17, 1935–1945. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, Z.; Dong, Y.; Wang, X.; Zhan, L. Current Advances in Nanomaterials Affecting Morphology, Structure, and Function of Erythrocytes. RSC Adv. 2021, 11, 6958–6971. [Google Scholar] [CrossRef] [PubMed]
- Handschuh-Wang, S.; Wang, T.; Zhou, X. Recent Advances in Hybrid Measurement Methods Based on Atomic Force Microscopy and Surface Sensitive Measurement Techniques. RSC Adv. 2017, 7, 47464–47499. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nanomater. Neoplasms 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Djayanti, K.; Maharjan, P.; Cho, K.H.; Jeong, S.; Kim, M.S.; Shin, M.C.; Min, K.A. Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations. Int. J. Mol. Sci. 2023, 24, 6349. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, L. Coating Nanoparticles with Cell Membranes for Targeted Drug Delivery. J. Drug Target. 2015, 23, 619–626. [Google Scholar] [CrossRef]
- Hu, C.M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef]
- Zhai, Y.; Su, J.; Ran, W.; Zhang, P.; Yin, Q.; Zhang, Z.; Yu, H.; Li, Y. Preparation and Application of Cell Membrane-Camouflaged Nanoparticles for Cancer Therapy. Theranostics 2017, 7, 2575–2592. [Google Scholar] [CrossRef]
- Nguyen, P.H.D.; Jayasinghe, M.K.; Le, A.H.; Peng, B.; Le, M.T.N. Advances in Drug Delivery Systems Based on Red Blood Cells and Their Membrane-Derived Nanoparticles. ACS Nano 2023, 17, 5187–5210. [Google Scholar] [CrossRef] [PubMed]
- Vincy, A.; Mazumder, S.; Amrita; Banerjee, I.; Hwang, K.C.; Vankayala, R. Recent Progress in Red Blood Cells-Derived Particles as Novel Bioinspired Drug Delivery Systems: Challenges and Strategies for Clinical Translation. Front. Chem. 2022, 10, 905256. [Google Scholar] [CrossRef]
- Kou, Q.; Huang, Y.; Su, Y.; Lu, L.; Li, X.; Jiang, H.; Huang, R.; Li, J.; Nie, X. Erythrocyte Membrane-Camouflaged DNA-Functionalized Upconversion Nanoparticles for Tumor-Targeted Chemotherapy and Immunotherapy. Nanoscale 2023, 15, 9457–9476. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Correia, I.J.; Moreira, A.F. Red Blood Cell Membrane-Camouflaged Gold-Core Silica Shell Nanorods for Cancer Drug Delivery and Photothermal Therapy. Int. J. Pharm. 2024, 655, 124007. [Google Scholar] [CrossRef]
- Hou, K.; Zhang, Y.; Bao, M.; Xin, C.; Wei, Z.; Lin, G.; Wang, Z. A Multifunctional Magnetic Red Blood Cell-Mimetic Micromotor for Drug Delivery and Image-Guided Therapy. ACS Appl. Mater. Interfaces 2022, 14, 3825–3837. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, G.; Tao, X.; Dong, D.; Liu, J. Targeted Mitochondrial Therapy for Pancreatic Cancer. Transl. Oncol. 2025, 54, 102340. [Google Scholar] [CrossRef]
- Luo, Z.; Sun, L.; Bian, F.; Wang, Y.; Yu, Y.; Gu, Z.; Zhao, Y. Erythrocyte-Inspired Functional Materials for Biomedical Applications. Adv. Sci. 2023, 10, 2206150. [Google Scholar] [CrossRef]
- Li, Z.; Lan, J.; Wu, Y.; Ding, Y.; Zhang, T. Homotypic Cell Membrane-Camouflaged Biomimetic PLGA Nanoparticle Loading Triptolide for the Treatment of Hepatocellular Carcinoma. Drug Deliv. 2024, 31, 2354687. [Google Scholar] [CrossRef]
- Beh, C.Y.; Prajnamitra, R.P.; Chen, L.L.; Hsieh, P.C.H. Advances in Biomimetic Nanoparticles for Targeted Cancer Therapy and Diagnosis. Molecules 2021, 26, 5052. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Raza, F.; Liu, Y.; Wei, Y.; Rong, R.; Zheng, M.; Yuan, W.; Su, J.; Qiu, M.; Li, Y.; et al. Clinical Progress and Advanced Research of Red Blood Cells Based Drug Delivery System. Biomaterials 2021, 279, 121202. [Google Scholar] [CrossRef]
- Samir, A.; Elgamal, B.M.; Gabr, H.; Sabaawy, H.E. Nanotechnology Applications in Hematological Malignancies. Oncol. Rep. 2015, 34, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current Approaches of Nanomedicines in the Market and Various Stage of Clinical Translation. Acta Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Mi, P. Polymer–Drug and Polymer–Protein Conjugated Nanocarriers: Design, Drug Delivery, Imaging, Therapy, and Clinical Applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1988. [Google Scholar] [CrossRef]
- Deivayanai, V.C.; Thamarai, P.; Karishma, S.; Saravanan, A.; Yaashikaa, P.R.; Vickram, A.S.; Hemavathy, R.V.; Kumar, R.R.; Rishikesavan, S.; Shruthi, S. A Comprehensive Review on Advances in Nanoparticle-Mediated Cancer Therapeutics: Current Research and Future Perspectives. Cancer Pathog. Ther. 2024, 3, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Glassman, P.M.; Muzykantov, V.R. Pharmacokinetic and Pharmacodynamic Properties of Drug Delivery Systems. J. Pharmacol. Exp. Ther. 2019, 370, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 2008. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsamesidis, I.; Dryllis, G.; Fortis, S.P.; Sphicas, A.; Konstantinidou, V.; Chatzidimitriou, M.; Mitka, S.; Trapali, M.; Skepastianos, P.; Kriebardis, A.G.; et al. Smart Red Blood Cell Carriers: A Nanotechnological Approach to Cancer Drug Delivery. Curr. Issues Mol. Biol. 2025, 47, 711. https://doi.org/10.3390/cimb47090711
Tsamesidis I, Dryllis G, Fortis SP, Sphicas A, Konstantinidou V, Chatzidimitriou M, Mitka S, Trapali M, Skepastianos P, Kriebardis AG, et al. Smart Red Blood Cell Carriers: A Nanotechnological Approach to Cancer Drug Delivery. Current Issues in Molecular Biology. 2025; 47(9):711. https://doi.org/10.3390/cimb47090711
Chicago/Turabian StyleTsamesidis, Ioannis, Georgios Dryllis, Sotirios P. Fortis, Andreas Sphicas, Vasiliki Konstantinidou, Maria Chatzidimitriou, Stella Mitka, Maria Trapali, Petros Skepastianos, Anastasios G. Kriebardis, and et al. 2025. "Smart Red Blood Cell Carriers: A Nanotechnological Approach to Cancer Drug Delivery" Current Issues in Molecular Biology 47, no. 9: 711. https://doi.org/10.3390/cimb47090711
APA StyleTsamesidis, I., Dryllis, G., Fortis, S. P., Sphicas, A., Konstantinidou, V., Chatzidimitriou, M., Mitka, S., Trapali, M., Skepastianos, P., Kriebardis, A. G., & Pessach, I. (2025). Smart Red Blood Cell Carriers: A Nanotechnological Approach to Cancer Drug Delivery. Current Issues in Molecular Biology, 47(9), 711. https://doi.org/10.3390/cimb47090711










