The Central Nervous System Modulatory Activities of N-Acetylcysteine: A Synthesis of Two Decades of Evidence
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Mechanistic studies that explore NAC’s neuromodulatory effects—specifically its impact on oxidative stress, neurotransmission, and neuroinflammatory signaling—in both in vitro and in vivo models;
- (2)
- Clinical trials that evaluate the effectiveness of NAC in modulating central nervous system function in neuropsychiatric and neurodegenerative conditions.
3. Mechanisms of Central Nervous System Modulation
3.1. Antioxidant Properties
3.1.1. Glutathione-Dependent Pathways
- (1)
- De novo synthesis, where glutamate-cysteine ligase (GCL) and glutathione synthase sequentially synthesize GSH from cysteine, glutamate, and glycine;
- (2)
- Redox recycling, in which GPx oxidizes GSH to glutathione disulfide (GSSG), followed by NADPH-dependent reduction back to GSH via glutathione reductase;
- (3)
- Xenobiotic conjugation, facilitated by GSTs, which catalyze the covalent attachment of GSH to electrophilic substances [24].
- Preclinical Evidence for GSH replenishment
- Clinical Findings
3.1.2. Glutathione-Independent Pathways
- Direct Antioxidant Effects
- Neutralization of lipid peroxides
- Mitochondrial H2S/Sulfane Sulfur Production
- Nrf2-ARE Pathway Activation
- Repair of Oxidative DNA Damage
3.2. Anti-Neuroinflammatory Effects
3.2.1. Suppression of Cytokine Production and Inhibition of NF-κB and iNOS Activities
3.2.2. PPAR-α Activation
3.2.3. JAK/STAT Pathway Modulation
3.2.4. Microglial Modulation
3.3. Enhancement of Neuroprotection and Cellular Viability
3.3.1. Initiation of Pro-Survival Signaling
- ERK Pathway Activation
- Inhibition of MAPK Signaling and Tau Hyperphosphorylation
3.3.2. Mitochondrial Protection and Redox Balance
3.3.3. Modulation of Neurotrophic Factors
3.4. Modulation of Neurotransmission
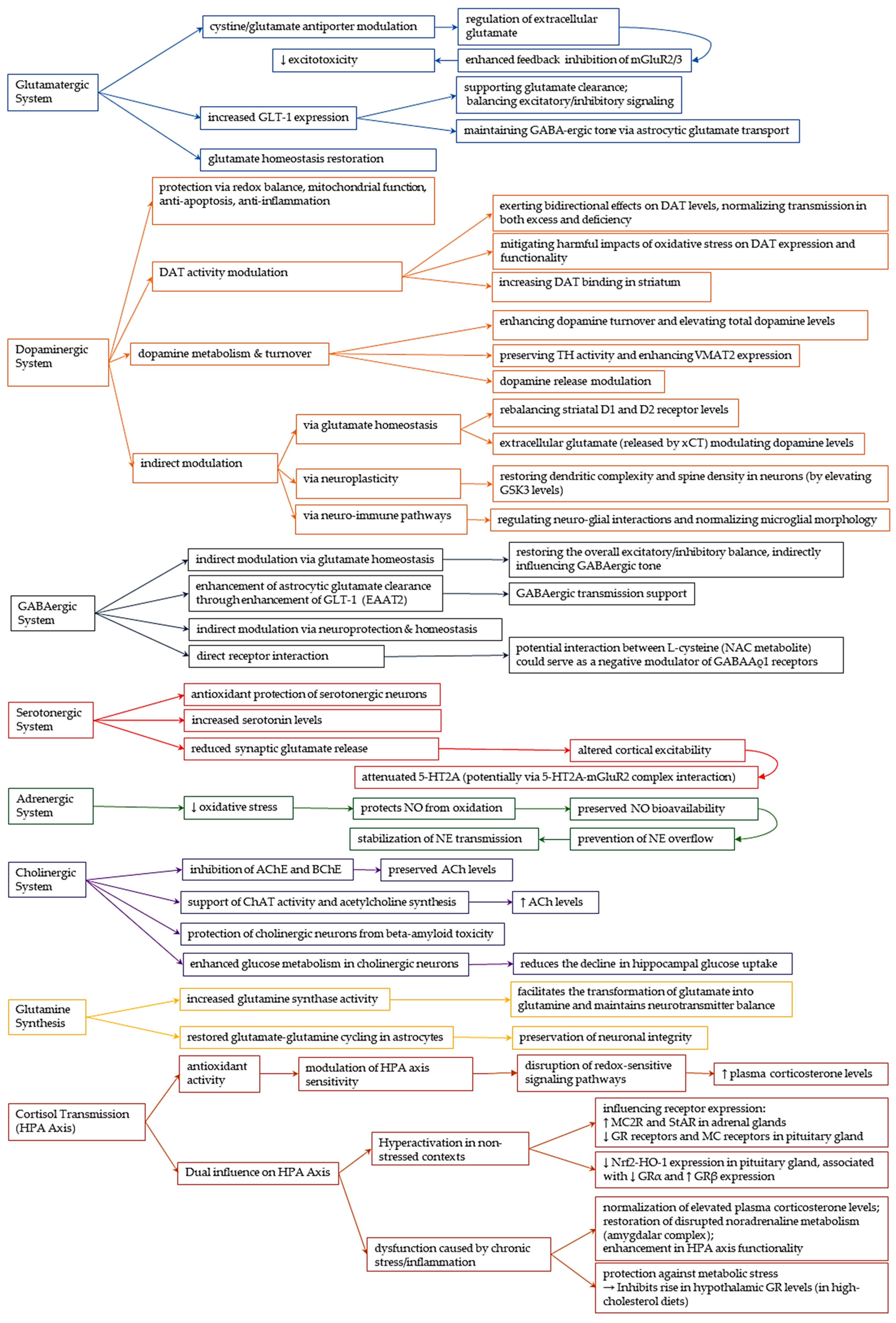
3.4.1. Glutamatergic System
- Cystine-Glutamate Antiporter (System xCT) Modulation
- Glutamate Transporter 1 (GLT-1) upregulation
- NMDA Receptor Modulation
3.4.2. Dopaminergic System
- Dopaminergic Protection: Redox Balance, Mitochondrial Function
- Cellular Evidence: Redox Restoration and Protection from Dopamine Toxicity
- Redox-based protection in various in vivo models
- Modulation of Dopamine Transporter Activity
- Influence on Dopamine Metabolism and Turnover
- Indirect Modulation via Glutamatergic, Neuroplastic, and Neuro-Immune Pathways
3.4.3. GABAergic System
- Astrocytic Glutamate Transport and Heterosynaptic Regulation
- GABA Levels and Receptor Interaction: Limited and Conflicting Evidence
3.4.4. Serotonergic System
- Serotonin Preservation through Antioxidant and Anti-inflammatory Action
- Modulation of Serotonin Levels and Receptor Function
3.4.5. Adrenergic System
3.4.6. Cholinergic System
- Restoration of Enzymatic Homeostasis in Acetylcholine Metabolism
- Protection against Neurotoxicity and Metabolic Support
3.4.7. Glutamine Synthesis
3.4.8. Cortisol Transmission
3.5. Modulation of Gene Expression
4. Discussion and Future Perspectives
5. Critical Interpretation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| ALOX5 | Arachidonate 5-lipoxygenase |
| ATP | Adenosine Triphosphate |
| BBB | Blood–Brain Barrier |
| BDNF | Brain-Derived Neurotrophic Factor |
| CAT | Catalase |
| ChAT | Choline Acetyltransferase |
| Cdk5 | Cyclin-dependent kinase 5 |
| CEF | Ceftriaxone |
| CIS | Cisplatin |
| CNS | Central Nervous System |
| DAT | Dopamine Transporter |
| DOI | (±)1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane |
| DOPAC | 3,4-Dihydroxyphenylacetic acid |
| ERK | Extracellular–Signal–Regulated kinase |
| GCL | Glutamate–Cysteine Ligase |
| GLT-1 | Glutamate transporter 1 |
| GPx | Glutathione Peroxidase |
| GS | Glutamine Synthase |
| GRIN2A | Glutamate Receptor Ionotropic N-methyl D-aspartate 2A |
| GRIN2B | Glutamate Receptor Ionotropic N-methyl D-aspartate 2B |
| GR | Glucocorticoid receptor |
| GSH | Glutathione |
| GSSG | Glutathione Disulfide |
| GST | Glutathione-S-Transferase |
| HO-1 | Heme Oxygenase-1 |
| HPA | Hypothalamic–Pituitary–Adrenal |
| ICH | Intracerebral Hemorrhage |
| IEG | Immediate Early Genes |
| iNOS | Inducible Nitric Oxide Synthase |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| JNK | C-Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-Activated Protein Kinase |
| MC | Mineralocorticoid |
| MCAO | Middle Cerebral artery occlusion |
| MDA | Malondialdehyde |
| MMP | Matrix Metalloproteinases |
| MST | 3-Mercaptopyruvate Sulfurtransferase |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NAC | N-acetylcysteine |
| NAc | Nucleus accumbens |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NMDAR | N-methyl D-aspartate receptor |
| NO | Nitric Oxide |
| Nrf2-ARE | Nuclear factor erythroid 2-related factor 2–Antioxidant Response Element |
| PD | Parkinson’s Disease |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| RGS4 | Regulator of G-protein Signaling 4 |
| ROS | Reactive Oxygen Species |
| SN | Substantia Nigra |
| SOD | Superoxide Dismutase |
| SQR | Sulfide: quinone oxidoreductase |
| SUD | Substance use disorder |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TH | Tyrosine Hydroxylase |
| TNF | Tumor Necrosis Factor |
| VMAT2 | Vesicular Monoamine Transporter 2 |
| xCT | Cystine–glutamate antiporter |
References
- Schwalfenberg, G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J. Nutr. Metab. 2021, 2021, 9949453. [Google Scholar] [CrossRef]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.D.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Deepmala; Slattery, J.; Kumar, N.; Delhey, L.; Berk, M.; Dean, O.; Spielholz, C.; Frye, R. Clinical Trials of N-Acetylcysteine in Psychiatry and Neurology: A Systematic Review. Neurosci. Biobehav. Rev. 2015, 55, 294–321. [Google Scholar] [CrossRef]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.-Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Berk, M. (Eds.) The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine; Springer: Singapore, 2019; ISBN 978-981-10-5310-8. [Google Scholar]
- Sahasrabudhe, S.A.; Terluk, M.R.; Kartha, R.V. N-Acetylcysteine Pharmacology and Applications in Rare Diseases—Repurposing an Old Antioxidant. Antioxidants 2023, 12, 1316. [Google Scholar] [CrossRef] [PubMed]
- Brivio, P.; Gallo, M.T.; Gruca, P.; Lason, M.; Litwa, E.; Fumagalli, F.; Papp, M.; Calabrese, F. Chronic N-Acetyl-Cysteine Treatment Enhances the Expression of the Immediate Early Gene Nr4a1 in Response to an Acute Challenge in Male Rats: Comparison with the Antidepressant Venlafaxine. Int. J. Mol. Sci. 2023, 24, 7321. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Malhi, G.S.; Gray, L.J.; Dean, O.M. The Promise of N-Acetylcysteine in Neuropsychiatry. Trends Pharmacol. Sci. 2013, 34, 167–177. [Google Scholar] [CrossRef]
- Santos, C.L.; Bobermin, L.D.; Souza, D.G.; Bellaver, B.; Bellaver, G.; Arús, B.A.; Souza, D.O.; Gonçalves, C.-A.; Quincozes-Santos, A. Lipoic Acid and N-Acetylcysteine Prevent Ammonia-Induced Inflammatory Response in C6 Astroglial Cells: The Putative Role of ERK and HO1 Signaling Pathways. Toxicol. In Vitro 2015, 29, 1350–1357. [Google Scholar] [CrossRef]
- Alam, R.T.; Imam, T.S.; Abo-Elmaaty, A.M.A.; Arisha, A.H. Amelioration of Fenitrothion Induced Oxidative DNA Damage and Inactivation of Caspase-3 in the Brain and Spleen Tissues of Male Rats by N-Acetylcysteine. Life Sci. 2019, 231, 116534. [Google Scholar] [CrossRef]
- Caruso, G.; Di Pietro, L.; Caraci, F. Gap Junctions and Connexins in Microglia-Related Oxidative Stress and Neuroinflammation: Perspectives for Drug Discovery. Biomolecules 2023, 13, 505. [Google Scholar] [CrossRef]
- Kupchik, Y.M.; Moussawi, K.; Tang, X.-C.; Wang, X.; Kalivas, B.C.; Kolokithas, R.; Ogburn, K.B.; Kalivas, P.W. The Effect of N-Acetylcysteine in the Nucleus Accumbens on Neurotransmission and Relapse to Cocaine. Biol. Psychiatry 2012, 71, 978–986. [Google Scholar] [CrossRef]
- Gere-Pászti, E.; Jakus, J. The Effect of N- Acetylcysteine on Amphetamine-mediated Dopamine Release in Rat Brain Striatal Slices by Ion-pair Reversed-phase High Performance Liquid Chromatography. Biomed. Chromatogr. 2009, 23, 658–664. [Google Scholar] [CrossRef]
- Virel, A.; Johansson, J.; Axelsson, J.; Ericsson, M.; Laterveer, R.; Ögren, M.; Orädd, G.; Jakobson Mo, S.; Af Bjerkén, S. N-Acetylcysteine Decreases Dopamine Transporter Availability in the Non-Lesioned Striatum of the 6-OHDA Hemiparkinsonian Rat. Neurosci. Lett. 2022, 770, 136420. [Google Scholar] [CrossRef] [PubMed]
- Caridade-Silva, R.; Araújo, B.; Martins-Macedo, J.; Teixeira, F.G. N-Acetylcysteine Treatment May Compensate Motor Impairments through Dopaminergic Transmission Modulation in a Striatal 6-Hydroxydopamine Parkinson’s Disease Rat Model. Antioxidants 2023, 12, 1257. [Google Scholar] [CrossRef]
- El-Habta, R.; Af Bjerkén, S.; Virel, A. N -Acetylcysteine Increases Dopamine Release and Prevents the Deleterious Effects of 6-OHDA on the Expression of VMAT2, α-Synuclein, and Tyrosine Hydroxylase. Neurol. Res. 2024, 46, 406–415. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Bavarsad Shahripour, R.; Harrigan, M.R.; Alexandrov, A.V. N-Acetylcysteine (NAC) in Neurological Disorders: Mechanisms of Action and Therapeutic Opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef]
- Ding, H.; Wang, X.; Wang, H.; Zhu, L.; Wang, Q.; Jia, Y.; Wei, W.; Zhou, C.; Wu, H.; Ding, K. Nrf2-ARE Signaling Provides Neuroprotection in Traumatic Brain Injury via Modulation of the Ubiquitin Proteasome System. Neurochem. Int. 2017, 111, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, H.; Zhou, X.; Fang, J.; Zhu, L.; Ding, K. N-Acetylcysteine Amide Provides Neuroprotection via Nrf2-ARE Pathway in a Mouse Model of Traumatic Brain Injury. Drug Des. Dev. Ther. 2018, 12, 4117–4127. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Alin, L.; Chen, Y.; Brand, D.; Bourassa, M.W.; Dietrich, K.; Wilkinson, C.M.; Nadeau, C.A.; Kumar, A.; Perry, S.; et al. N-Acetylcysteine Targets 5 Lipoxygenase-Derived, Toxic Lipids and Can Synergize with Prostaglandin E2 to Inhibit Ferroptosis and Improve Outcomes Following Hemorrhagic Stroke in Mice. Ann. Neurol. 2018, 84, 854–872. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakaki, T. Inhibition of GTRAP3-18 May Increase Neuroprotective Glutathione (GSH) Synthesis. Int. J. Mol. Sci. 2012, 13, 12017–12035. [Google Scholar] [CrossRef]
- Dwivedi, D.; Megha, K.; Mishra, R.; Mandal, P.K. Glutathione in Brain: Overview of Its Conformations, Functions, Biochemical Characteristics, Quantitation and Potential Therapeutic Role in Brain Disorders. Neurochem. Res. 2020, 45, 1461–1480. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in Brain Disorders and Aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Clore, E.L.; Zheng, K.; Adame, A.; Masliah, E.; Simon, D.K. Oral N-Acetyl-Cysteine Attenuates Loss of Dopaminergic Terminals in α-Synuclein Overexpressing Mice. PLoS ONE 2010, 5, e12333. [Google Scholar] [CrossRef] [PubMed]
- Mocelin, R.; Marcon, M.; D’ambros, S.; Mattos, J.; Sachett, A.; Siebel, A.M.; Herrmann, A.P.; Piato, A. N-Acetylcysteine Reverses Anxiety and Oxidative Damage Induced by Unpredictable Chronic Stress in Zebrafish. Mol. Neurobiol. 2019, 56, 1188–1195. [Google Scholar] [CrossRef]
- Hwang, O. Role of Oxidative Stress in Parkinson’s Disease. Exp. Neurobiol. 2013, 22, 11–17. [Google Scholar] [CrossRef]
- Khorchid, A.; Fragoso, G.; Shore, G.; Almazan, G. Catecholamine-Induced Oligodendrocyte Cell Death in Culture Is Developmentally Regulated and Involves Free Radical Generation and Differential Activation of Caspase-3. Glia 2002, 40, 283–299. [Google Scholar] [CrossRef]
- Wu, W.; Liu, B.; Xie, C.; Xia, X.; Zhang, Y. Neuroprotective Effects of N-Acetyl Cysteine on Primary Hippocampus Neurons against Hydrogen Peroxide-Induced Injury Are Mediated via Inhibition of Mitogen-Activated Protein Kinases Signal Transduction and Antioxidative Action. Mol. Med. Rep. 2018, 17, 6647–6654. [Google Scholar] [CrossRef]
- Olivieri, G.; Baysang, G.; Meier, F.; Mu, F.; Brack, C. N-Acetyl-l-Cysteine Protects SHSY5Y Neuroblastoma Cells from Oxidative Stress and Cell Cytotoxicity: Effects on b-Amyloid Secretion and Tau Phosphorylation. J. Neurochem. 2001, 76, 224–233. [Google Scholar] [CrossRef]
- Dean, O.M.; Van Den Buuse, M.; Berk, M.; Copolov, D.L.; Mavros, C.; Bush, A.I. N-Acetyl Cysteine Restores Brain Glutathione Loss in Combined 2-Cyclohexene-1-One and d-Amphetamine-Treated Rats: Relevance to Schizophrenia and Bipolar Disorder. Neurosci. Lett. 2011, 499, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Singh, S.; Singh, A.K.; Rizvi, S.I. N-Acetyl-l-Cysteine Attenuates Oxidative Damage and Neurodegeneration in Rat Brain during Aging. Can. J. Physiol. Pharmacol. 2018, 96, 1189–1196. [Google Scholar] [CrossRef]
- Abdel-Wahab, W.M.; Moussa, F.I. Neuroprotective Effect of N-Acetylcysteine against Cisplatin-Induced Toxicity in Rat Brain by Modulation of Oxidative Stress and Inflammation. Drug Des. Devel. Ther. 2019, 13, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Dorcas Aremu, T.; Ramírez Ortega, D.; Blanco Ayala, T.; González Esquivel, D.F.; Pineda, B.; Pérez de la Cruz, G.; Salazar, A.; Flores, I.; Meza-Sosa, K.F.; Sánchez Chapul, L.; et al. Modulation of Brain Kynurenic Acid by N-Acetylcysteine Prevents Cognitive Impairment and Muscular Weakness Induced by Cisplatin in Female Rats. Cells 2024, 13, 1989. [Google Scholar] [CrossRef]
- Holmay, M.J.; Terpstra, M.; Coles, L.D.; Mishra, U.; Ahlskog, M.; Öz, G.; Cloyd, J.C.; Tuite, P.J. N-Acetylcysteine Boosts Brain and Blood Glutathione in Gaucher and Parkinson’s Diseases. Clin. Neuropharmacol. 2013, 36, 103–106. [Google Scholar] [CrossRef]
- Coles, L.D.; Tuite, P.J.; Öz, G.; Mishra, U.R.; Kartha, R.V.; Sullivan, K.M.; Cloyd, J.C.; Terpstra, M. Repeated-Dose Oral N-Acetylcysteine in Parkinson Disease: Pharmacokinetics and Effect on Brain Glutathione and Oxidative Stress. J. Clin. Pharmacol. 2018, 58, 158–167. [Google Scholar] [CrossRef]
- Martinez-Banaclocha, M. N-Acetyl-Cysteine: Modulating the Cysteine Redox Proteome in Neurodegenerative Diseases. Antioxidants 2022, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an Antioxidant and Disulphide Breaking Agent: The Reasons Why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, G.F.; Megson, I.L. Existing and Potential Therapeutic Uses for N-Acetylcysteine: The Need for Conversion to Intracellular Glutathione for Antioxidant Benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef]
- Ezeriņa, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H2S and Sulfane Sulfur Production. Cell Chem. Biol. 2018, 25, 447–459.e4. [Google Scholar] [CrossRef]
- Morris, G.; Walker, A.J.; Walder, K.; Berk, M.; Marx, W.; Carvalho, A.F.; Maes, M.; Puri, B.K. Increasing Nrf2 Activity as a Treatment Approach in Neuropsychiatry. Mol. Neurobiol. 2021, 58, 2158–2182. [Google Scholar] [CrossRef]
- Habib, E.; Linher-Melville, K.; Lin, H.-X.; Singh, G. Expression of xCT and Activity of System Xc− Are Regulated by NRF2 in Human Breast Cancer Cells in Response to Oxidative Stress. Redox Biol. 2015, 5, 33–42. [Google Scholar] [CrossRef]
- Shih, A.Y.; Johnson, D.A.; Wong, G.; Kraft, A.D.; Jiang, L.; Erb, H.; Johnson, J.A.; Murphy, T.H. Coordinate Regulation of Glutathione Biosynthesis and Release by Nrf2-Expressing Glia Potently Protects Neurons from Oxidative Stress. J. Neurosci. 2003, 23, 3394–3406. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, M.E.; Ezquer, F.; Morales, P.; Ezquer, M.; Olivares, B.; Santapau, D.; Herrera-Marschitz, M.; Israel, Y. N-Acetylcysteine and Acetylsalicylic Acid Inhibit Alcohol Consumption by Different Mechanisms: Combined Protection. Front. Behav. Neurosci. 2020, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.E.; Chan, W.Y.; Brennan, A.M.; Reyes, R.C.; Adler, B.L.; Suh, S.W.; Kauppinen, T.M.; Edling, Y.; Swanson, R.A. N-Acetylcysteine Prevents Loss of Dopaminergic Neurons in the EAAC−/− Mouse. Ann. Neurol. 2011, 69, 509–520. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, G.; Li, H.; Zhang, Q. Antioxidant NAC and AMPA/KA Receptor Antagonist DNQX Inhibited JNK3 Activation Following Global Ischemia in Rat Hippocampus. Neurosci. Res. 2003, 46, 191–197. [Google Scholar] [CrossRef]
- Fan, C.; Long, Y.; Wang, L.; Liu, X.; Liu, Z.; Lan, T.; Li, Y.; Yu, S.Y. N-Acetylcysteine Rescues Hippocampal Oxidative Stress-Induced Neuronal Injury via Suppression of P38/JNK Signaling in Depressed Rats. Front. Cell. Neurosci. 2020, 14, 554613. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation Pathways: A General Review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Bettcher, B.M.; Kramer, J.H. Inflammation and Clinical Presentation in Neurodegenerative Disease: A Volatile Relationship. Neurocase 2013, 19, 182–200. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Khan, M.; Sekhon, B.; Jatana, M.; Giri, S.; Gilg, A.G.; Sekhon, C.; Singh, I.; Singh, A.K. Administration of N-acetylcysteine after Focal Cerebral Ischemia Protects Brain and Reduces Inflammation in a Rat Model of Experimental Stroke. J. Neurosci. Res. 2004, 76, 519–527. [Google Scholar] [CrossRef]
- Zhong, Y.; Guan, J.; Ma, Y.; Xu, M.; Cheng, Y.; Xu, L.; Lin, Y.; Zhang, X.; Wu, R. Role of Imaging Modalities and N-Acetylcysteine Treatment in Sepsis-Associated Encephalopathy. ACS Chem. Neurosci. 2023, 14, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-B.; Kim, Y.-J.; Lee, Y.-J.; Yoo, J.-Y.; Choi, Y.; Kim, E.-M.; Suh, S.W.; Woo, R.-S. N-Acetylcysteine Alleviates Depressive-Like Behaviors in Adolescent EAAC1-/- Mice and Early Life Stress Model Rats. Int. J. Biol. Sci. 2024, 20, 5450–5473. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Herrmann, A.P.; Benvenutti, R.; Noetzold, G.; Giongo, F.; Gama, C.S.; Piato, A.L.; Elisabetsky, E. Anxiolytic Properties of N -Acetylcysteine in Mice. Behav. Brain Res. 2017, 317, 461–469. [Google Scholar] [CrossRef]
- Paintlia, M.K.; Paintlia, A.S.; Khan, M.; Singh, I.; Singh, A.K. Modulation of Peroxisome Proliferator-Activated Receptor-α Activity by N-Acetyl Cysteine Attenuates Inhibition of Oligodendrocyte Development in Lipopolysaccharide Stimulated Mixed Glial Cultures. J. Neurochem. 2008, 105, 956–970. [Google Scholar] [CrossRef]
- Al-Samhari, M.M.; Al-Rasheed, N.M.; Al-Rejaie, S.; Al-Rasheed, N.M.; Hasan, I.H.; Mahmoud, A.M.; Dzimiri, N. Possible Involvement of the JAK/STAT Signaling Pathway in N-Acetylcysteine-Mediated Antidepressant-like Effects. Exp. Biol. Med. 2016, 241, 509–518. [Google Scholar] [CrossRef]
- Sakai, M.; Yu, Z.; Taniguchi, M.; Picotin, R.; Oyama, N.; Stellwagen, D.; Ono, C.; Kikuchi, Y.; Matsui, K.; Nakanishi, M.; et al. N-Acetylcysteine Suppresses Microglial Inflammation and Induces Mortality Dose-Dependently via Tumor Necrosis Factor-α Signaling. Int. J. Mol. Sci. 2023, 24, 3798. [Google Scholar] [CrossRef]
- Dwir, D.; Cabungcal, J.-H.; Xin, L.; Giangreco, B.; Parietti, E.; Cleusix, M.; Jenni, R.; Klauser, P.; Conus, P.; Cuénod, M.; et al. Timely N-Acetyl-Cysteine and Environmental Enrichment Rescue Oxidative Stress-Induced Parvalbumin Interneuron Impairments via MMP9/RAGE Pathway: A Translational Approach for Early Intervention in Psychosis. Schizophr. Bull. 2021, 47, 1782–1794. [Google Scholar] [CrossRef]
- Woo, M.; Park, J.; Choi, I.; Kim, W.; Kim, H. Inhibition of MMP-3 or -9 Suppresses Lipopolysaccharide-induced Expression of Proinflammatory Cytokines and iNOS in Microglia. J. Neurochem. 2008, 106, 770–780. [Google Scholar] [CrossRef]
- Sachdeva, S.; Pant, S.C.; Kushwaha, P.; Bhargava, R.; Flora, S.J.S. Sodium Tungstate Induced Neurological Alterations in Rat Brain Regions and Their Response to Antioxidants. Food Chem. Toxicol. 2015, 82, 64–71. [Google Scholar] [CrossRef]
- Hsiao, Y.; Chen, P.; Yeh, S.; Lin, C.; Gean, P. N-acetylcysteine Prevents Β-amyloid Toxicity by a Stimulatory Effect on P35/Cyclin-dependent Kinase 5 Activity in Cultured Cortical Neurons. J. Neurosci. Res. 2008, 86, 2685–2695. [Google Scholar] [CrossRef]
- Wang, S.H.; Lee, D.-S.; Kim, T.-H.; Kim, J.-E.; Kang, T.-C. Reciprocal Regulation of Oxidative Stress and Mitochondrial Fission Augments Parvalbumin Downregulation through CDK5-DRP1- and GPx1-NF-κB Signaling Pathways. Cell Death Dis. 2024, 15, 707. [Google Scholar] [CrossRef]
- Amouri, S.S.E.; Waker, C.A.; Huang, L.; Smith, C.L.; Mayes, D.A. N-Acetylcysteine Modulation of Mitochondrial Respiration and Blood Brain Barrier Permeability Is Time Dependent and Cell Type Specific. Int. J. Mol. Biol. 2018, 3, 266–276. [Google Scholar] [CrossRef][Green Version]
- Lee, C.-Y.; Su, C.-H.; Tsai, P.-K.; Yang, M.-L.; Ho, Y.-C.; Lee, S.-S.; Chen, C.-H.; Chen, W.-Y.; Lin, M.-L.; Chen, C.-J.; et al. Cadmium Nitrate-Induced Neuronal Apoptosis Is Protected by N-Acetyl-l-Cysteine via Reducing Reactive Oxygen Species Generation and Mitochondria Dysfunction. Biomed. Pharmacother. 2018, 108, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Syiem, R.P.; Wahlang, J.B.; Syiem, R.P.R.K.; Kalyan, P.B.; Nahakpam, D.; Langstieh, A.J. Exploring the Novel Therapeutic Potential of N-Acetylcysteine in Depression, Bipolar Disorders and Anxiety. J. Pharmacol. Pharmacother. 2024, 15, 133–141. [Google Scholar] [CrossRef]
- Willborn, R.J.; Hall, C.P.; Fuller, M.A. Recycling N-Acetylcysteine: A Review of Evidence for Adjunctive Therapy in Schizophrenia. Ment. Health Clin. 2019, 9, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Olive, M.F.; Cleva, R.M.; Kalivas, P.W.; Malcolm, R.J. Glutamatergic Medications for the Treatment of Drug and Behavioral Addictions. Pharmacol. Biochem. Behav. 2012, 100, 801–810. [Google Scholar] [CrossRef]
- Önder, A.; Adanır, A.; Çoban, Ö.; Bilaç, Ö.; Kara, A. The Efficiency and Safety of N-Acetylcysteine Augmentation in the Autistic Children with Severe Irritability and Aggression: Six Cases. Anatol. J. Psychiatry 2020, 21, 218–221. [Google Scholar] [CrossRef]
- Moran, M.M.; McFarland, K.; Melendez, R.I.; Kalivas, P.W.; Seamans, J.K. Cystine/Glutamate Exchange Regulates Metabotropic Glutamate Receptor Presynaptic Inhibition of Excitatory Transmission and Vulnerability to Cocaine Seeking. J. Neurosci. 2005, 25, 6389–6393. [Google Scholar] [CrossRef]
- Kau, K.S.; Madayag, A.; Mantsch, J.R.; Grier, M.D.; Abdulhameed, O.; Baker, D.A. Blunted Cystine–Glutamate Antiporter Function in the Nucleus Accumbens Promotes Cocaine-Induced Drug Seeking. Neuroscience 2008, 155, 530–537. [Google Scholar] [CrossRef]
- Moussawi, K.; Pacchioni, A.; Moran, M.; Olive, M.F.; Gass, J.T.; Lavin, A.; Kalivas, P.W. N-Acetylcysteine Reverses Cocaine Induced Metaplasticity. Nat. Neurosci. 2009, 12, 182–189. [Google Scholar] [CrossRef]
- Adil, M.; Amin, S.; Mohtashim, M. N-Acetylcysteine in Dermatology. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 652. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.A.; Xi, Z.-X.; Shen, H.; Swanson, C.J.; Kalivas, P.W. The Origin and Neuronal Function of In Vivo Nonsynaptic Glutamate. J. Neurosci. 2002, 22, 9134–9141. [Google Scholar] [CrossRef]
- Krzyżanowska, W.; Pomierny, B.; Bystrowska, B.; Pomierny-Chamioło, L.; Filip, M.; Budziszewska, B.; Pera, J. Ceftriaxone- and N-Acetylcysteine-Induced Brain Tolerance to Ischemia: Influence on Glutamate Levels in Focal Cerebral Ischemia. PLoS ONE 2017, 12, e0186243. [Google Scholar] [CrossRef]
- Kalivas, P.W. The Glutamate Homeostasis Hypothesis of Addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef]
- Roberts-Wolfe, D.J.; Kalivas, P.W. Glutamate Transporter GLT-1 as a Therapeutic Target for Substance Use Disorders. CNS Neurol. Disord. Drug Targets 2015, 14, 745–756. [Google Scholar] [CrossRef]
- Huang, M.-W.; Lin, Y.-J.; Chang, C.-W.; Lei, F.-J.; Ho, E.-P.; Liu, R.-S.; Shyu, W.-C.; Hsieh, C.-H. RGS4 Deficit in Prefrontal Cortex Contributes to the Behaviors Related to Schizophrenia via System Xc--Mediated Glutamatergic Dysfunction in Mice. Theranostics 2018, 8, 4781–4794. [Google Scholar] [CrossRef]
- Moro, F.; Giannotti, G.; Caffino, L.; Marzo, C.M.; Di Clemente, A.; Fumagalli, F.; Cervo, L. Lasting Reduction of Nicotine-seeking Behavior by Chronic N-acetylcysteine during Experimental Cue-exposure Therapy. Addict. Biol. 2020, 25, e12771. [Google Scholar] [CrossRef]
- Lau, B.K.; Murphy-Royal, C.; Kaur, M.; Qiao, M.; Bains, J.S.; Gordon, G.R.; Borgland, S.L. Obesity-Induced Astrocyte Dysfunction Impairs Heterosynaptic Plasticity in the Orbitofrontal Cortex. Cell Rep. 2021, 36, 109563. [Google Scholar] [CrossRef] [PubMed]
- Schmaal, L.; Veltman, D.J.; Nederveen, A.; Van Den Brink, W.; Goudriaan, A.E. N-Acetylcysteine Normalizes Glutamate Levels in Cocaine-Dependent Patients: A Randomized Crossover Magnetic Resonance Spectroscopy Study. Neuropsychopharmacology 2012, 37, 2143–2152. [Google Scholar] [CrossRef]
- Winterlind, E.L.; Malone, S.G.; Setzer, M.R.; Murphy, M.A.; Saunders, D.; Gray, J.C. N-acetylcysteine as a Treatment for Substance Use Cravings: A Meta-analysis. Addict. Biol. 2024, 29, e70001. [Google Scholar] [CrossRef] [PubMed]
- Duailibi, M.S.; Cordeiro, Q.; Brietzke, E.; Ribeiro, M.; LaRowe, S.; Berk, M.; Trevizol, A.P. N-acetylcysteine in the Treatment of Craving in Substance Use Disorders: Systematic Review and Meta-analysis. Am. J. Addict. 2017, 26, 660–666. [Google Scholar] [CrossRef]
- Chang, C.-T.; Hsieh, P.-J.; Lee, H.-C.; Lo, C.-H.; Tam, K.-W.; Loh, E.-W. Effectiveness of N-Acetylcysteine in Treating Clinical Symptoms of Substance Abuse and Dependence: A Meta-Analysis of Randomized Controlled Trials. Clin. Psychopharmacol. Neurosci. 2021, 19, 282–293. [Google Scholar] [CrossRef]
- Bagh, M.B.; Maiti, A.K.; Jana, S.; Banerjee, K.; Roy, A.; Chakrabarti, S. Quinone and Oxyradical Scavenging Properties of N-Acetylcysteine Prevent Dopamine Mediated Inhibition of Na+, K+ -ATPase and Mitochondrial Electron Transport Chain Activity in Rat Brain: Implications in the Neuroprotective Therapy of Parkinson’s Disease. Free Radic. Res. 2008, 42, 574–581. [Google Scholar] [CrossRef]
- Jana, S.; Sinha, M.; Chanda, D.; Roy, T.; Banerjee, K.; Munshi, S.; Patro, B.S.; Chakrabarti, S. Mitochondrial Dysfunction Mediated by Quinone Oxidation Products of Dopamine: Implications in Dopamine Cytotoxicity and Pathogenesis of Parkinson’s Disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2011, 1812, 663–673. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, J.; Han, Y.; Lau, A.S.Y.; Rong, J. Z-Ligustilide Potentiates the Cytotoxicity of Dopamine in Rat Dopaminergic PC12 Cells. Neurotox. Res. 2012, 22, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Kerk, S.Y.; Xiong, G.G.; Lim, T.M. Dopamine Auto-Oxidation Aggravates Non-Apoptotic Cell Death Induced by over-Expression of Human A53T Mutant Alpha-Synuclein in Dopaminergic PC12 Cells. J. Neurochem. 2009, 108, 601–610. [Google Scholar] [CrossRef]
- Lee, C.S.; Song, E.H.; Park, S.Y.; Han, E.S. Combined Effect of Dopamine and MPP+ on Membrane Permeability in Mitochondria and Cell Viability in PC12 Cells. Neurochem. Int. 2003, 43, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zafar, K.S.; Inayat-Hussain, S.H.; Ross, D. A Comparative Study of Proteasomal Inhibition and Apoptosis Induced in N27 Mesencephalic Cells by Dopamine and MG132. J. Neurochem. 2007, 102, 913–921. [Google Scholar] [CrossRef]
- Chandramani Shivalingappa, P.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A. N-Acetyl Cysteine Protects against Methamphetamine-Induced Dopaminergic Neurodegeneration via Modulation of Redox Status and Autophagy in Dopaminergic Cells. Park. Dis. 2012, 2012, 424285. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, S.; Tian, J.; Chen, L.; Zhang, W.; Zhao, J.; Tang, H.; Zhang, X.; Chen, J. Protective Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-Glucoside in the MPTP-Induced Mouse Model of Parkinson’s Disease: Involvement of Reactive Oxygen Species-Mediated JNK, P38 and Mitochondrial Pathways. Eur. J. Pharmacol. 2015, 767, 175–182. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, P.; Kumar, V.; Gill, K.D. Attenuation of 1-Methyl-4-Phenyl-1, 2,3,6-Tetrahydropyridine Induced Nigrostriatal Toxicity in Mice by N-Acetyl Cysteine. Cell. Mol. Biol. Noisy-Gd. Fr. 2007, 53, 48–55. [Google Scholar]
- Chen, C.; Yin, M.; Hsu, C.; Liu, T. Antioxidative and Anti-Inflammatory Effects of Four Cysteine-Containing Agents in Striatum of MPTP-Treated Mice. Nutrition 2007, 23, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Rahimmi, A.; Khosrobakhsh, F.; Izadpanah, E.; Moloudi, M.R.; Hassanzadeh, K. N-Acetylcysteine Prevents Rotenone-Induced Parkinson’s Disease in Rat: An Investigation into the Interaction of Parkin and Drp1 Proteins. Brain Res. Bull. 2015, 113, 34–40. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, B.K.; Ahmad, I.; Shukla, S.; Patel, D.K.; Srivastava, G.; Kumar, V.; Pandey, H.P.; Singh, C. Involvement of NADPH Oxidase and Glutathione in Zinc-Induced Dopaminergic Neurodegeneration in Rats: Similarity with Paraquat Neurotoxicity. Brain Res. 2012, 1438, 48–64. [Google Scholar] [CrossRef]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.-W.; Wintering, N.A.; Cai, J.; Wei, X.; Bazzan, A.J.; Zhong, L.; Bowen, B.; et al. N-Acetyl Cysteine May Support Dopamine Neurons in Parkinson’s Disease: Preliminary Clinical and Cell Line Data. PLoS ONE 2016, 11, e0157602. [Google Scholar] [CrossRef]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.; Wintering, N.A.; Bazzan, A.J.; Zhong, L.; Bowens, B.K.; Chervoneva, I.; Intenzo, C.; et al. N-Acetyl Cysteine Is Associated With Dopaminergic Improvement in Parkinson’s Disease. Clin. Pharmacol. Ther. 2019, 106, 884–890. [Google Scholar] [CrossRef]
- Bauzo, R.M.; Kimmel, H.L.; Howell, L.L. The Cystine–Glutamate Transporter Enhancer N-Acetyl-l-Cysteine Attenuates Cocaine-Induced Changes in Striatal Dopamine but Not Self-Administration in Squirrel Monkeys. Pharmacol. Biochem. Behav. 2012, 101, 288–296. [Google Scholar] [CrossRef][Green Version]
- Wan, F.-J.; Tung, C.-S.; Shiah, I.-S.; Lin, H.-C. Effects of α-Phenyl-N-Tert-Butyl Nitrone and N-Acetylcysteine on Hydroxyl Radical Formation and Dopamine Depletion in the Rat Striatum Produced by d-Amphetamine. Eur. Neuropsychopharmacol. 2006, 16, 147–153. [Google Scholar] [CrossRef]
- Laverde, C.F.; Morais-Silva, G.; Amaral, V.C.S.; Marin, M.T. Effects of N-Acetylcysteine Treatment on Ethanol’s Rewarding Properties and Dopaminergic Alterations in Mesocorticolimbic and Nigrostriatal Pathways. Behav. Pharmacol. 2021, 32, 239. [Google Scholar] [CrossRef] [PubMed]
- Dean, O.; Giorlando, F.; Berk, M. N-Acetylcysteine in Psychiatry: Current Therapeutic Evidence and Potential Mechanisms of Action. J. Psychiatry Neurosci. 2011, 36, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Baskaran, R.; Tsao, C.-Y.; Tuan, L.-H.; Siow, P.-F.; Palani, M.; Lee, L.J.-H.; Liu, C.-M.; Hwu, H.-G.; Lee, L.-J. Chronic N-Acetylcysteine Treatment Prevents Amphetamine-Induced Hyperactivity in Heterozygous Disc1 Mutant Mice, a Putative Prodromal Schizophrenia Animal Model. Int. J. Mol. Sci. 2022, 23, 9419. [Google Scholar] [CrossRef]
- Lyng, G.D.; Seegal, R.F. Polychlorinated biphenyl-induced oxidative stress in organotypic co-cultures: Experimental dopamine depletion prevents reductions in GABA. Neurotoxicology 2008, 29, 301–308. [Google Scholar] [CrossRef][Green Version]
- Knackstedt, L.A.; Melendez, R.I.; Kalivas, P.W. Ceftriaxone Restores Glutamate Homeostasis and Prevents Relapse to Cocaine Seeking. Biol. Psychiatry 2010, 67, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Reissner, K.J.; Brown, R.M.; Spencer, S.; Tran, P.K.; Thomas, C.A.; Kalivas, P.W. Chronic Administration of the Methylxanthine Propentofylline Impairs Reinstatement to Cocaine by a GLT-1-Dependent Mechanism. Neuropsychopharmacology 2014, 39, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Reissner, K.J.; Gipson, C.D.; Tran, P.K.; Knackstedt, L.A.; Scofield, M.D.; Kalivas, P.W. Glutamate Transporter GLT -1 Mediates N -acetylcysteine Inhibition of Cocaine Reinstatement. Addict. Biol. 2015, 20, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Durieux, A.M.S.; Fernandes, C.; Murphy, D.; Labouesse, M.A.; Giovanoli, S.; Meyer, U.; Li, Q.; So, P.-W.; McAlonan, G. Targeting Glia with N-Acetylcysteine Modulates Brain Glutamate and Behaviors Relevant to Neurodevelopmental Disorders in C57BL/6J Mice. Front. Behav. Neurosci. 2015, 9, 343. [Google Scholar] [CrossRef]
- Schulte, M.; Goudriaan, A.; Kaag, A.; Kooi, D.; Van Den Brink, W.; Wiers, R.; Schmaal, L. The Effect of N-Acetylcysteine on Brain Glutamate and Gamma-Aminobutyric Acid Concentrations and on Smoking Cessation: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Psychopharmacol. 2017, 31, 1377–1379. [Google Scholar] [CrossRef]
- Beltrán González, A.N.; Vicentini, F.; Calvo, D.J. Negative Modulation of the GABAA Ρ1 Receptor Function by l -cysteine. J. Neurochem. 2018, 144, 50–57. [Google Scholar] [CrossRef]
- Chopra, D.; Sharma, S.; Sharma, N.; Nehru, B. N-Acetylcysteine Ameliorates Neurotoxic Effects of Manganese Intoxication in Rats: A Biochemical and Behavioral Study. Neurochem. Res. 2021, 46, 1953–1969. [Google Scholar] [CrossRef]
- Smaga, I.; Frankowska, M.; Filip, M. N -acetylcysteine as a New Prominent Approach for Treating Psychiatric Disorders. Br. J. Pharmacol. 2021, 178, 2569–2594. [Google Scholar] [CrossRef]
- Aldbass, A.M.; Bhat, R.S.; El-Ansary, A. Protective and Therapeutic Potency of N-Acetyl-Cysteine on Propionic Acid-Induced Biochemical Autistic Features in Rats. J. Neuroinflamm. 2013, 10, 837. [Google Scholar] [CrossRef]
- Fernandes, J.; Gupta, G.L. N-Acetylcysteine Attenuates Neuroinflammation Associated Depressive Behavior Induced by Chronic Unpredictable Mild Stress in Rat. Behav. Brain Res. 2019, 364, 356–365. [Google Scholar] [CrossRef]
- Yawalkar, R.; Changotra, H.; Gupta, G.L. Protective Influences of N-Acetylcysteine against Alcohol Abstinence-Induced Depression by Regulating Biochemical and GRIN2A, GRIN2B Gene Expression of NMDA Receptor Signaling Pathway in Rats. Neurochem. Int. 2018, 118, 73–81. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Chiang, C.-C.; Chiu, H.-Y.; Chan, M.-H.; Chen, H.-H. N-Acetylcysteine Modulates Hallucinogenic 5-HT2A Receptor Agonist-Mediated Responses: Behavioral, Molecular, and Electrophysiological Studies. Neuropharmacology 2014, 81, 215–223. [Google Scholar] [CrossRef]
- Lafleur, D.L.; Pittenger, C.; Kelmendi, B.; Gardner, T.; Wasylink, S.; Malison, R.T.; Sanacora, G.; Krystal, J.H.; Coric, V. N-Acetylcysteine Augmentation in Serotonin Reuptake Inhibitor Refractory Obsessive-Compulsive Disorder. Psychopharmacology 2006, 184, 254–256. [Google Scholar] [CrossRef]
- Macarthur, H.; Westfall, T.C.; Wilken, G.H. Oxidative Stress Attenuates NO-Induced Modulation of Sympathetic Neurotransmission in the Mesenteric Arterial Bed of Spontaneously Hypertensive Rats. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, H183–H189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shahat, A.S.; Hassan, W.A.; El-Sayed, W.M. N-Acetylcysteine and Safranal Prevented the Brain Damage Induced by Hyperthyroidism in Adult Male Rats. Nutr. Neurosci. 2022, 25, 231–245. [Google Scholar] [CrossRef]
- Costa, M.; Bernardi, J.; Fiuza, T.; Costa, L.; Brandão, R.; Pereira, M.E. N-Acetylcysteine Protects Memory Decline Induced by Streptozotocin in Mice. Chem. Biol. Interact. 2016, 253, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.M.; Scaini, G.; Carvalho-Silva, M.; Gomes, M.L.; Malgarin, F.; Kist, L.W.; Bogo, M.R.; Rico, E.P.; Zugno, A.I.; Deroza, P.F.P.; et al. Antioxidants Reverse the Changes in the Cholinergic System Caused by L-Tyrosine Administration in Rats. Neurotox. Res. 2018, 34, 769–780. [Google Scholar] [CrossRef]
- Gonçalves, J.F.; Fiorenza, A.M.; Spanevello, R.M.; Mazzanti, C.M.; Bochi, G.V.; Antes, F.G.; Stefanello, N.; Rubin, M.A.; Dressler, V.L.; Morsch, V.M.; et al. N-Acetylcysteine Prevents Memory Deficits, the Decrease in Acetylcholinesterase Activity and Oxidative Stress in Rats Exposed to Cadmium. Chem. Biol. Interact. 2010, 186, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Moyano, P.; Flores, A.; San Juan, J.; García, J.; Anadón, M.J.; Plaza, J.C.; Naval, M.V.; Fernández, M.D.L.C.; Guerra-Menéndez, L.; Del Pino, J. Imidacloprid Unique and Repeated Treatment Produces Cholinergic Transmission Disruption and Apoptotic Cell Death in SN56 Cells. Food Chem. Toxicol. 2024, 193, 114988. [Google Scholar] [CrossRef]
- Walczewska, S.; Wawrzyniak, A. N-Acetylcysteine in Clinical Practice—Properties, Use and Adverse Effects. Pediatr. Med. Rodz. 2020, 16, 243–246. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.-E. Deletion of P2X7 Receptor Decreases Basal Glutathione Level by Changing Glutamate-Glutamine Cycle and Neutral Amino Acid Transporters. Cells 2020, 9, 995. [Google Scholar] [CrossRef]
- Visalli, V.; Muscoli, C.; Sacco, I.; Sculco, F.; Palma, E.; Costa, N.; Colica, C.; Rotiroti, D.; Mollace, V. N-Acetylcysteine Prevents HIV Gp 120-Related Damage of Human Cultured Astrocytes: Correlation with Glutamine Synthase Dysfunction. BMC Neurosci. 2007, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Prevatto, J.P.; Torres, R.C.; Diaz, B.L.; Silva, P.M.R.E.; Martins, M.A.; Carvalho, V.F. Antioxidant Treatment Induces Hyperactivation of the HPA Axis by Upregulating ACTH Receptor in the Adrenal and Downregulating Glucocorticoid Receptors in the Pituitary. Oxid. Med. Cell. Longev. 2017, 2017, 4156361. [Google Scholar] [CrossRef]
- Chaves, A.S.; Ventura, R.D.; Pacini, M.F.; Magalhães, N.S.; Silva, P.M.R.E.; Martins, M.A.; Pérez, A.R.; Carvalho, V.F. Activation of the Nrf2/HO-1 Pathway Restores N-Acetylcysteine-Induced Impairment of the Hypothalamus-Pituitary-Adrenal Axis Negative Feedback by up-Regulating GRα Expression and down-Regulating GRβ Expression into Pituitary Glands. Front. Endocrinol. 2025, 16, 1500630. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S. Chronic Stress, Neuroinflammation, and Depression: An Overview of Pathophysiological Mechanisms and Emerging Anti-Inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef]
- Korou, L.-M.; Agrogiannis, G.; Koros, C.; Kitraki, E.; Vlachos, I.S.; Tzanetakou, I.; Karatzas, T.; Pergialiotis, V.; Dimitroulis, D.; Perrea, D.N. Impact of N-Acetylcysteine and Sesame Oil on Lipid Metabolism and Hypothalamic–Pituitary–Adrenal Axis Homeostasis in Middle-Aged Hypercholesterolemic Mice. Sci. Rep. 2014, 4, 6806. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Tripathi, S.J.; Raju, T.R.; Shankaranarayana Rao, B.S. Mechanisms Underlying Remediation of Depression-Associated Anxiety by Chronic N-Acetyl Cysteine Treatment. Psychopharmacology 2020, 237, 2967–2981. [Google Scholar] [CrossRef]


| Mechanism | Key Effects/Outcomes | References | |
|---|---|---|---|
| GSH-Dependent | Enhances intracellular GSH synthesis via cysteine precursor | Restores redox balance; protects neurons in Parkinson’s disease | [4,18] |
| De novo GSH synthesis via GCL and GS | Maintains GSH levels | [30] | |
| Redox recycling via GPx | Detoxifying reactive peroxides | [4,21] | |
| Xenobiotic conjugation via GSTs | Detoxifies electrophilic compounds | [4,21] | |
| Modulates α-synuclein and NFκB signaling | Reduces neuroinflammation and protein aggregation | [25,45] | |
| Increases brain GSH (animal and some human studies) | Confers neuroprotection | [35] | |
| Protects against age-related and chemotherapy-induced oxidative damage | Preserves neuronal integrity | [32,33,34,45] | |
| GSH-Independent | Direct antioxidant effects via –SH group | Scavenges NO2, hypohalous acids; limited in vivo efficacy | [4,38] |
| Neutralization of lipid peroxides (via ALOX5 inhibition) | Prevents ferroptosis, reduces neuronal loss in ICH models | [21] | |
| Mitochondrial sulfane sulfur/H2S production via MST and SQR | Generates hydropersulfides; enhances mitochondrial redox balance | [40] | |
| Activation of Nrf2-ARE pathway | Upregulates antioxidant and detox genes; improves GSH levels and reduces inflammation | [19,41,42,43,44] | |
| DNA repair and anti-apoptotic gene modulation | Decreases 8OH2dG; regulates Bax, p53, and Bcl-2 expression | [10] | |
| Suppression of heme-oxygenase-1 (HO-1) | Limits dopamine-triggered oxidative stress in oligodendrocytes | [28] | |
| SOD and CAT activity up-regulation | Restores enzymatic antioxidant defenses after chronic stress or cisplatin | [26,33] | |
| Inhibition of oxidative stress–induced MAPK activation | Prevents tau hyperphosphorylation and aggregation Preserves neuronal structure and function | [29,46,47] | |
| Modulating the Cysteine Redox Proteome | Addresses dysregulation of cysteine residues, prevents protein aggregation | [37] |
| Mechanism | Pathway/Target | Effect of NAC | Key Findings/Studies |
|---|---|---|---|
| Suppression of Cytokine Production and NF-κB/iNOS Inhibition | TNF-α, IL-1β, IL-6, NF-κB, iNOS | ↓ Cytokines ↓ iNOS expression ↓ NF-κB activation ↑ GSH levels | |
| PPAR-α Activation | PPAR-α-Peroxisomal proteins | ↑ PPAR-α activity ↑ OL development ↑ Peroxisomal function |
|
| JAK/STAT Pathway Modulation | JAK1/2/3, Tyk2, STAT3, SOCS3 | ↓ STAT3 mRNA/protein ↑ SOCS3 expression ↓ Phosphorylation of STAT3 |
|
| Microglial Modulation | Connexins and Pannexins MMP-3/MMP-9 ROS, TNF-α | ↓ Pro-inflammatory cytokines ↓ ROS ↓ MMPs Modulates microglial activity | |
| Restoration of PVI Functionality | MMP9/RAGE-Perineuronal nets GABA signaling | ↑ PVI network integrity ↓ RAGE ↑ Prefrontal GABA Improved cognition |
|
| Submechanism | Pathway/Target | Effect of NAC | Key Findings/Studies |
|---|---|---|---|
| Initiation of Pro-survival Signaling | |||
| ERK Pathway Activation | Cdk5-p35 ERK Bcl-2 | ↑ Cdk5 and ERK ↑ Bcl-2 ↓ Aβ toxicity | |
| Inhibition of MAPK and Tau Phosphorylation | JNK, p38 Tau (Ser199, Ser202, Thr205, Ser396) | ↓ Tau phosphorylation ↓ JNK/p38 activity | |
| Mitochondrial Protection and Redox Balance | |||
| Mitochondrial Dynamics Regulation | CDK5-DRP1 GPx1-NF-κB Parvalbumin | ↓ DRP1 fission ↑ GPx1 ↑ ATP and mitochondrial integrity |
|
| Blood–Brain Barrier Modulation | Occludin, Claudin-5 nNOS Connexins 37/43 | Time-dependent BBB effects: 24 h ↑ Permeability 48 h ↑ Integrity |
|
| Redox Balance and Energy Metabolism | NADPH cycling Complex I | ↑ Mitochondrial respiration ↓ ROS (astrocytes) |
|
| Modulation of Neurotrophic Factors | |||
| BDNF TrkB PI3K/Akt, ERK, PLC-γ | ↑ BDNF expression ↑ ERK signaling ↓ Apoptosis |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherneva, D.I.; Kehayova, G.; Dimitrova, S.; Dragomanova, S. The Central Nervous System Modulatory Activities of N-Acetylcysteine: A Synthesis of Two Decades of Evidence. Curr. Issues Mol. Biol. 2025, 47, 710. https://doi.org/10.3390/cimb47090710
Cherneva DI, Kehayova G, Dimitrova S, Dragomanova S. The Central Nervous System Modulatory Activities of N-Acetylcysteine: A Synthesis of Two Decades of Evidence. Current Issues in Molecular Biology. 2025; 47(9):710. https://doi.org/10.3390/cimb47090710
Chicago/Turabian StyleCherneva, Desislava Ivanova, Gabriela Kehayova, Simeonka Dimitrova, and Stela Dragomanova. 2025. "The Central Nervous System Modulatory Activities of N-Acetylcysteine: A Synthesis of Two Decades of Evidence" Current Issues in Molecular Biology 47, no. 9: 710. https://doi.org/10.3390/cimb47090710
APA StyleCherneva, D. I., Kehayova, G., Dimitrova, S., & Dragomanova, S. (2025). The Central Nervous System Modulatory Activities of N-Acetylcysteine: A Synthesis of Two Decades of Evidence. Current Issues in Molecular Biology, 47(9), 710. https://doi.org/10.3390/cimb47090710









