Exploring CCND1 as a Key Target of Acorus calamus Against RSV Infection: Network Pharmacology, Molecular Docking, and Bioinformatics Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening for Active Compounds and Their Corresponding Targets in Acorus calamus
2.2. Gathering of RSV-Related Targets
2.3. Shared Targets Were Determined from the Intersection of Active Compound Targets and RSV-Associated Targets
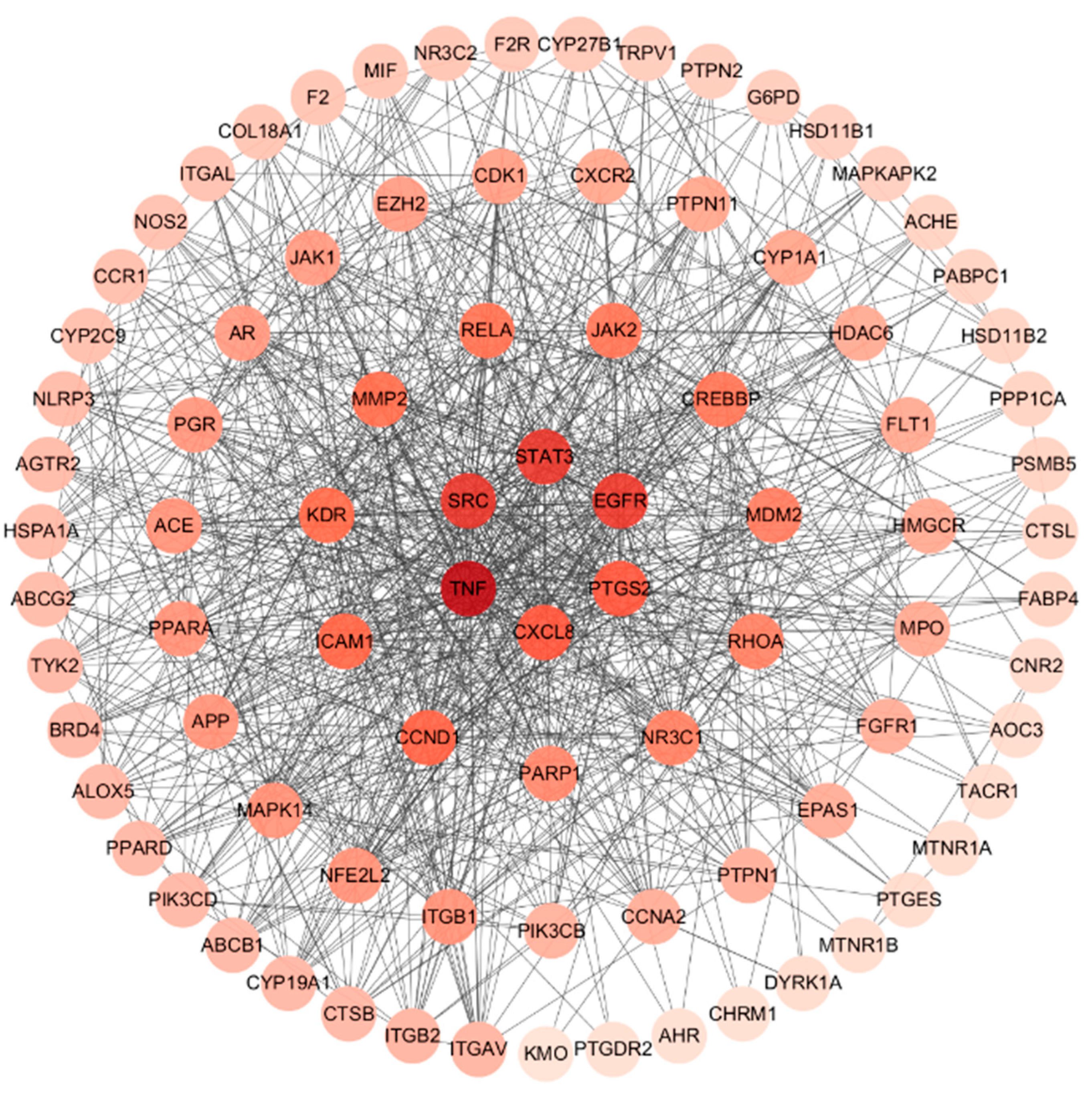
2.4. Constructing a Network to Visualize PPI
2.5. DO, GO, and KEGG Enrichment Analyses
2.6. Molecular Docking Verification
2.7. RSV Datasets Acquisition and Target Validation
3. Results
3.1. The Candidate Targets of Acorus calamus and Their Corresponding Active Compounds
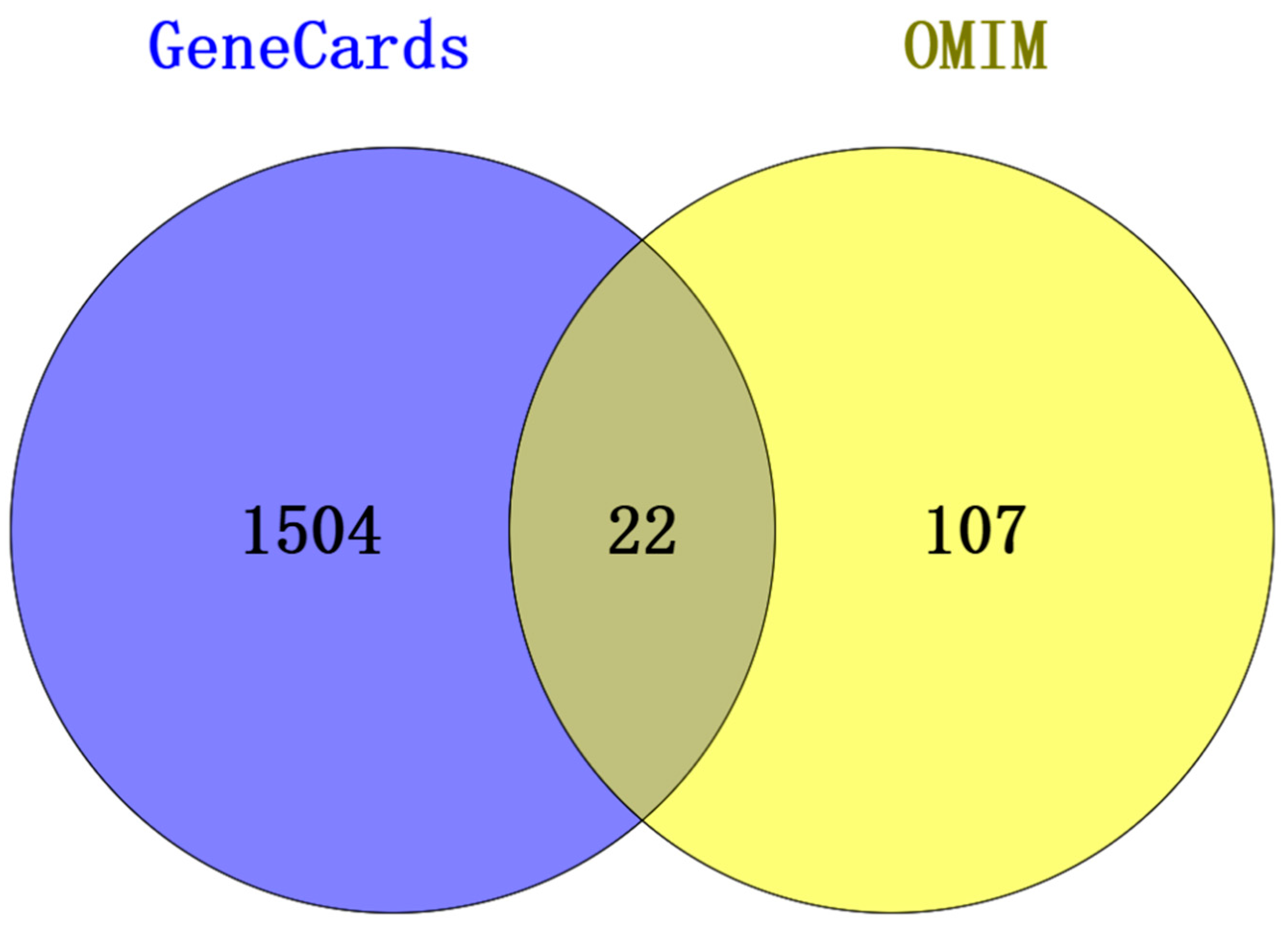
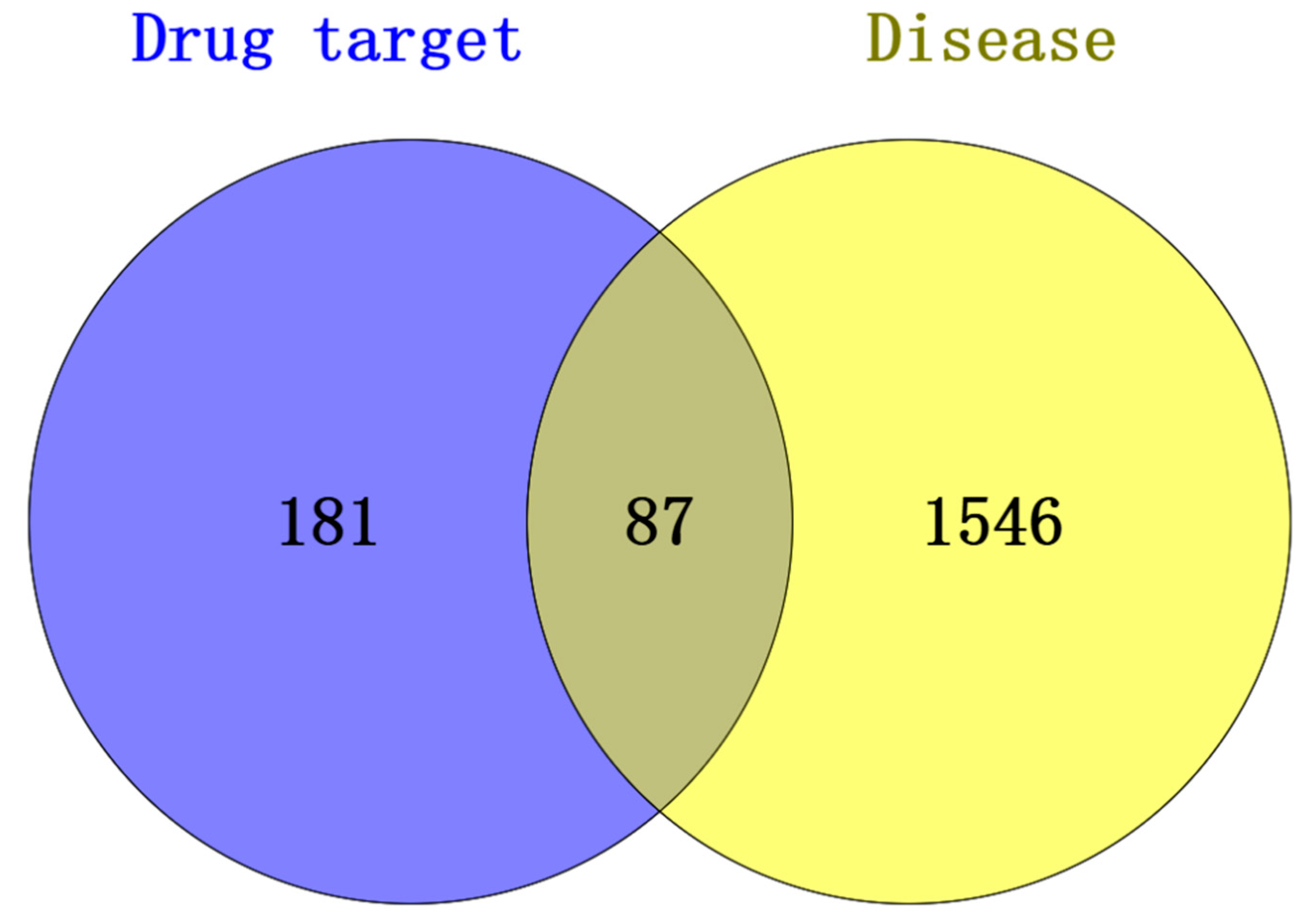
3.2. RSV-Related Targets
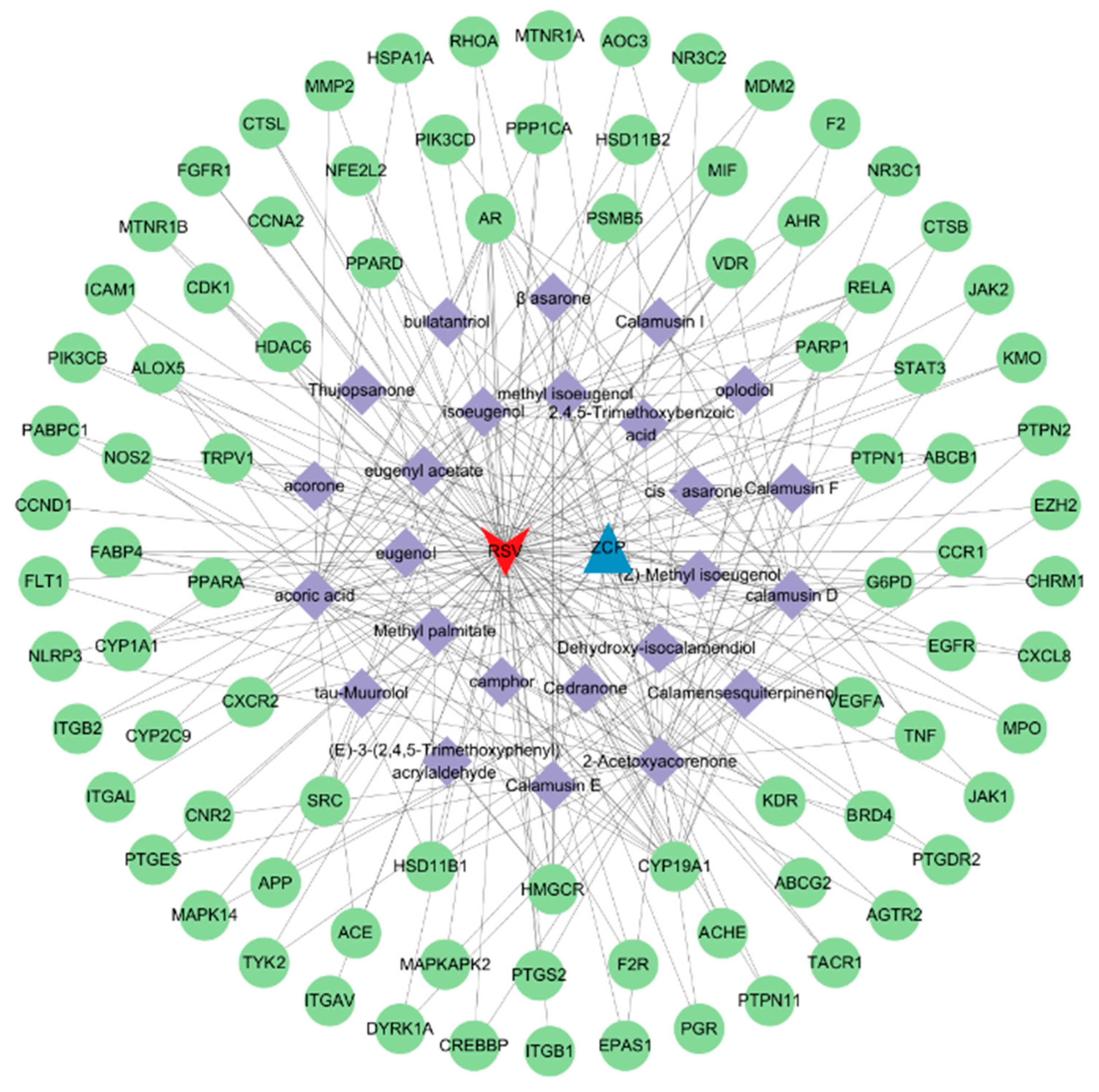
3.3. Construction of Herb–Compound–Target Network
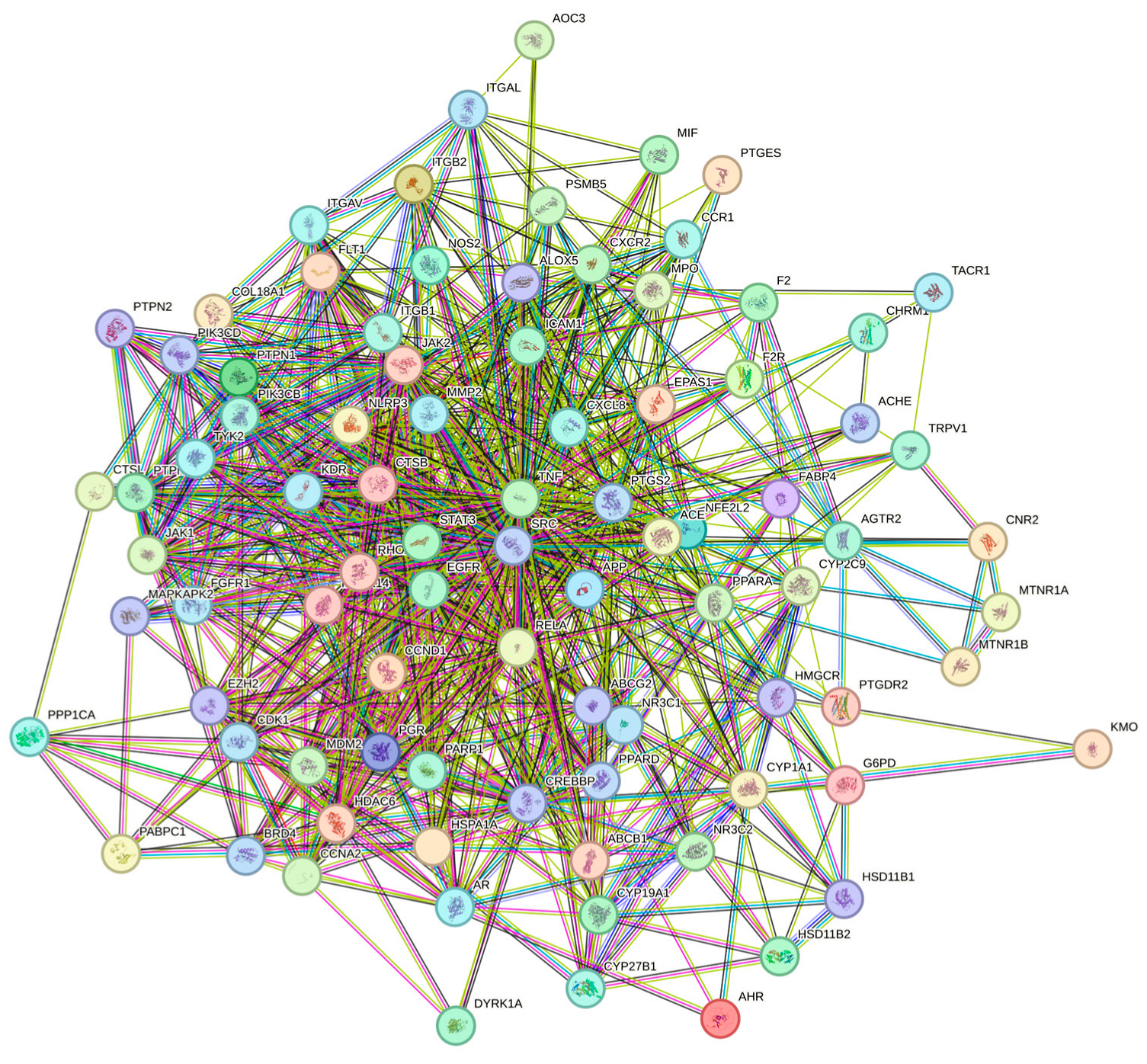
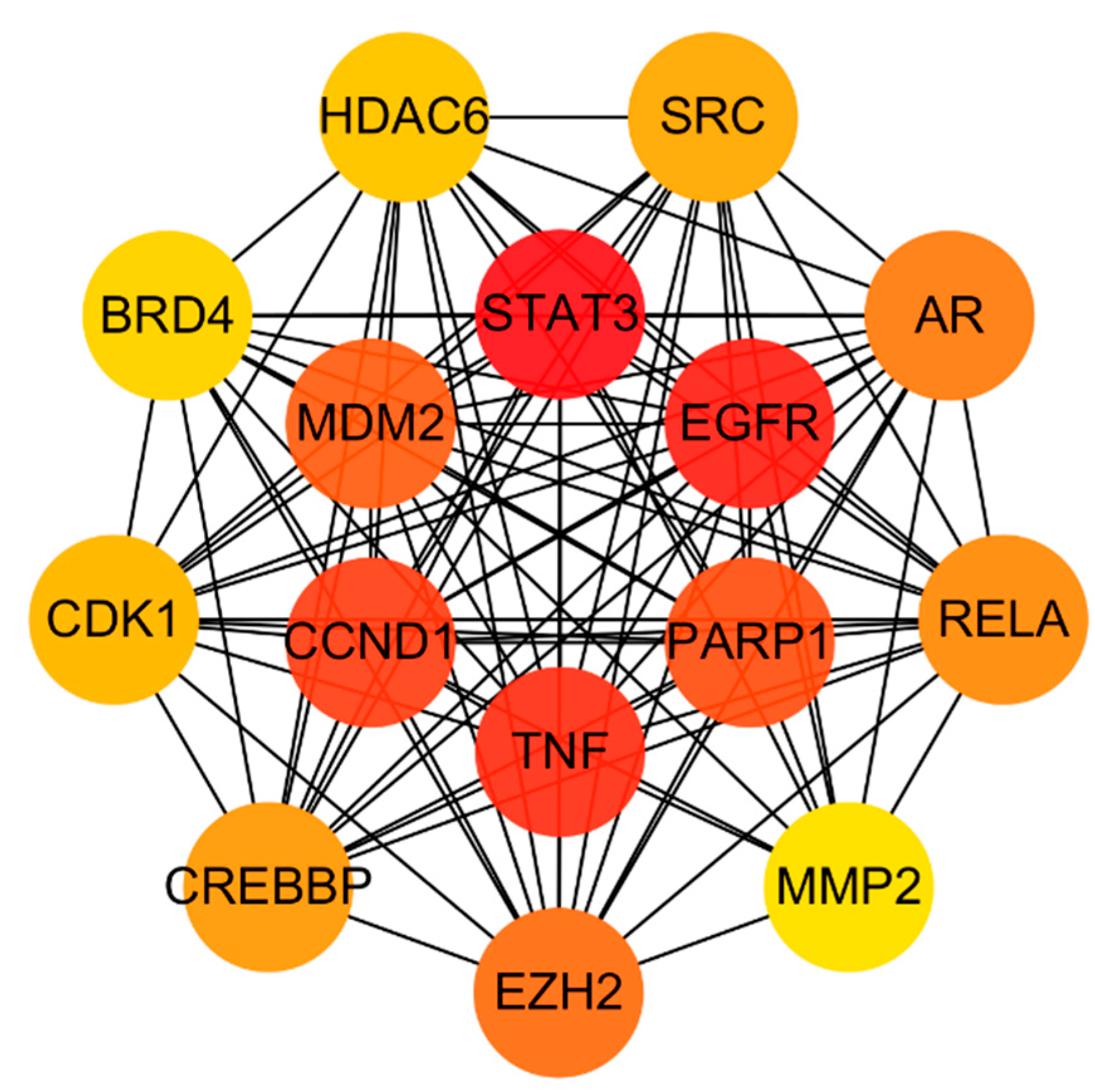
3.4. Analysis of Herb–Compound–Target Network and Construction of PPI Network for Common Targets
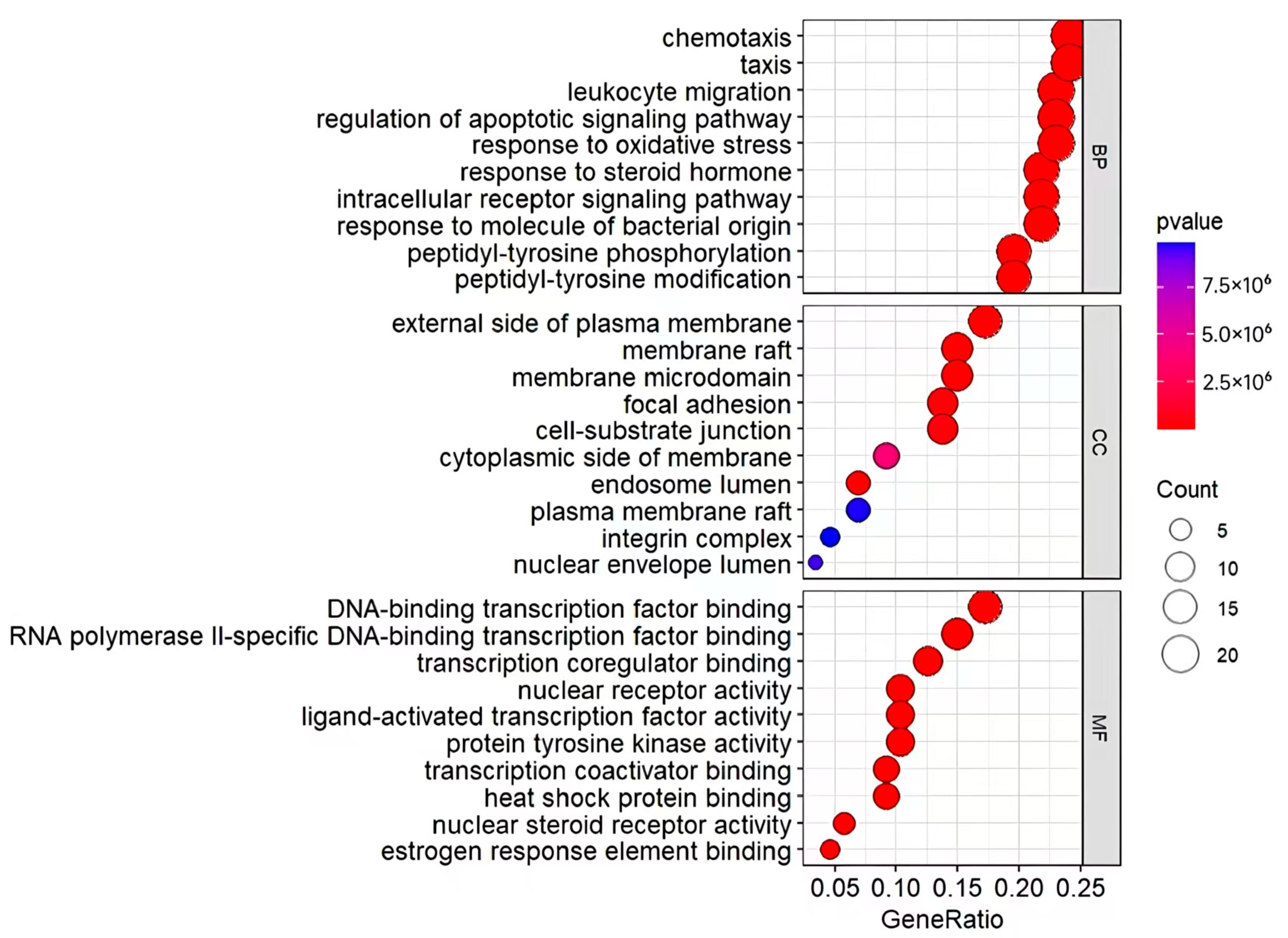
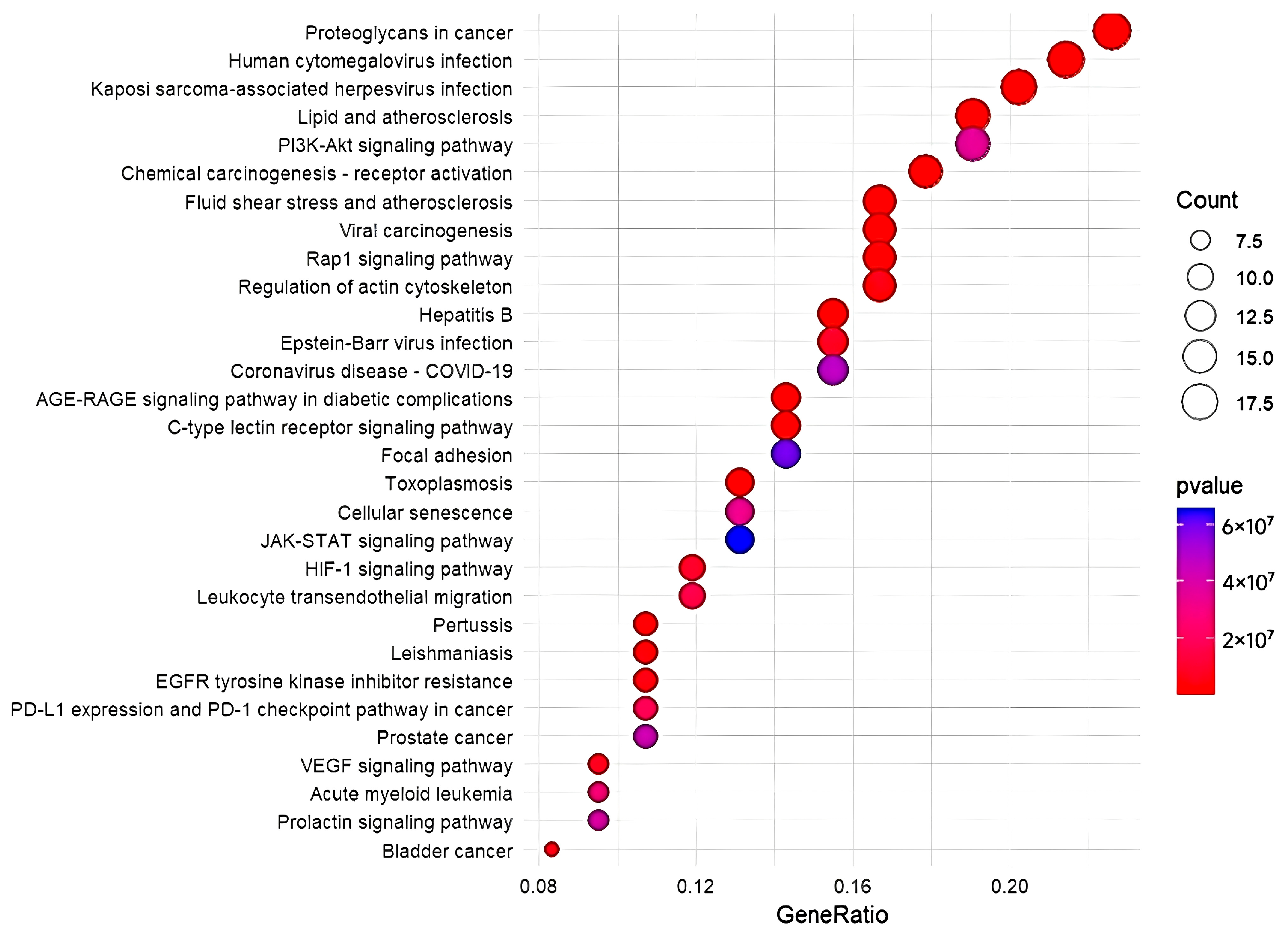
3.5. DO, GO, and KEGG Enrichment Analysis
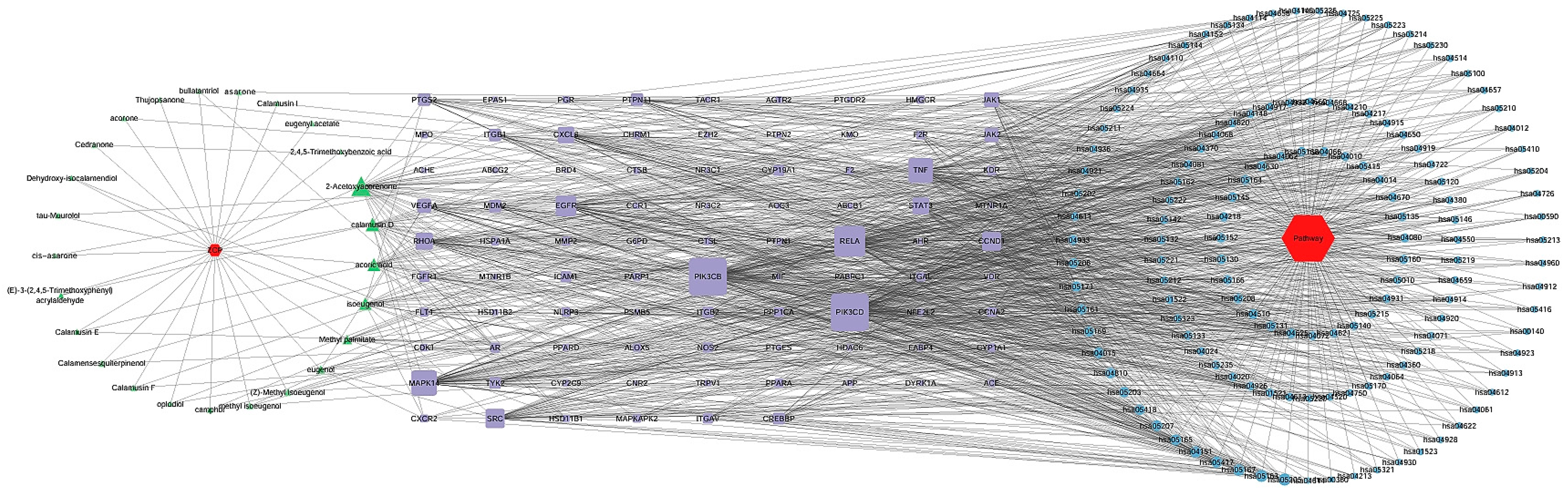
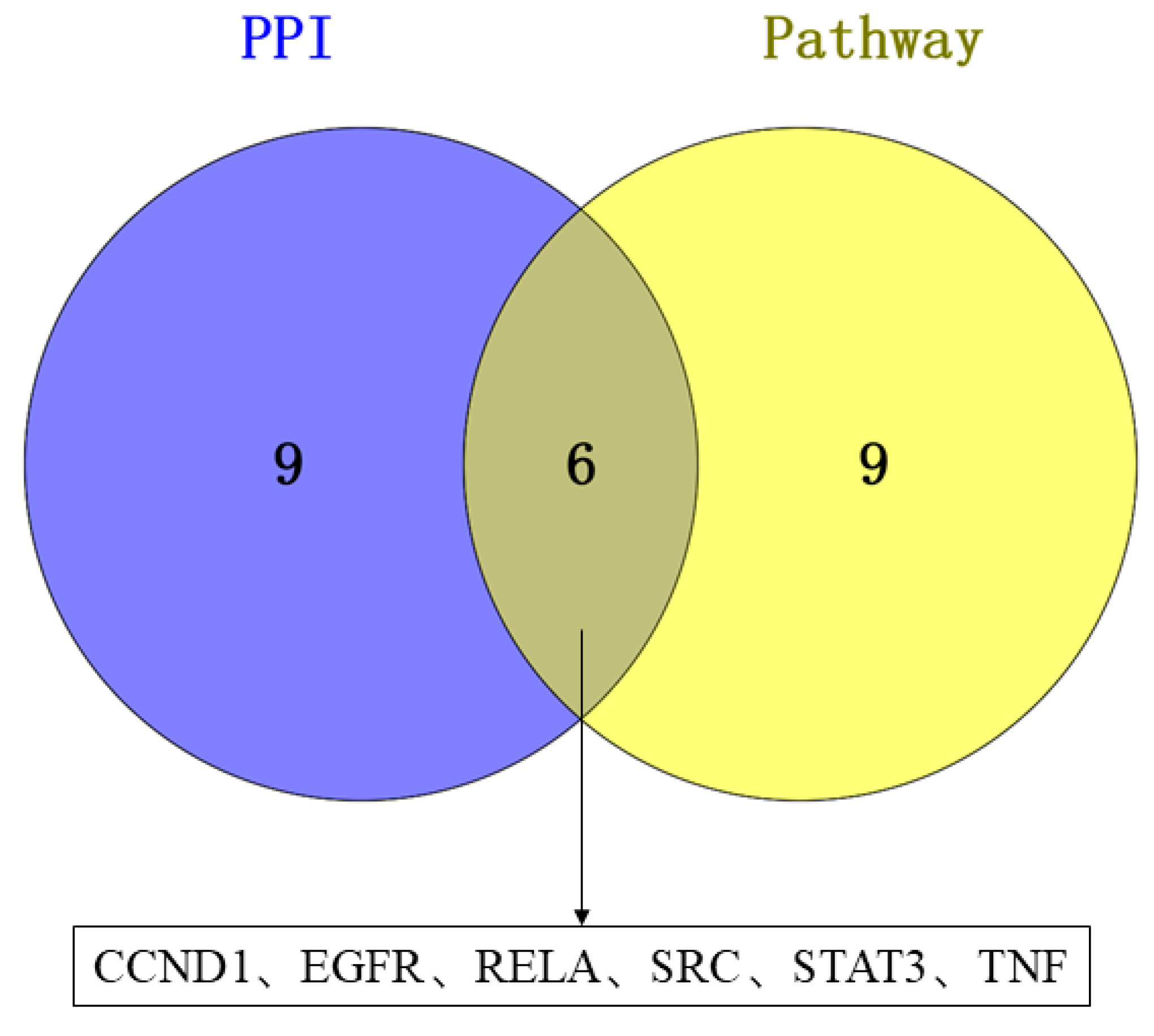
3.6. Construction of Compound–Target–Pathway Network and Identification of Core Targets for RSV
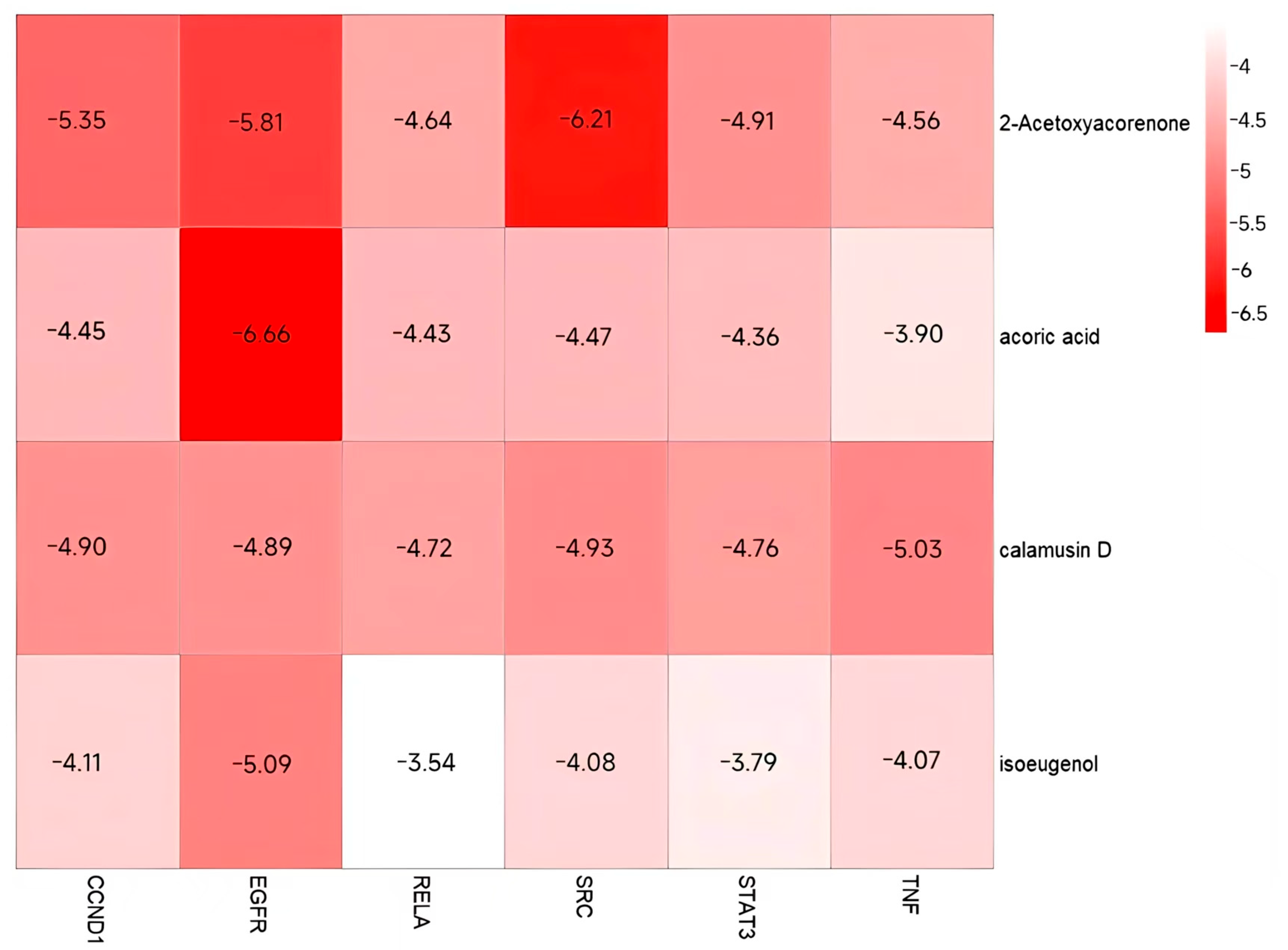
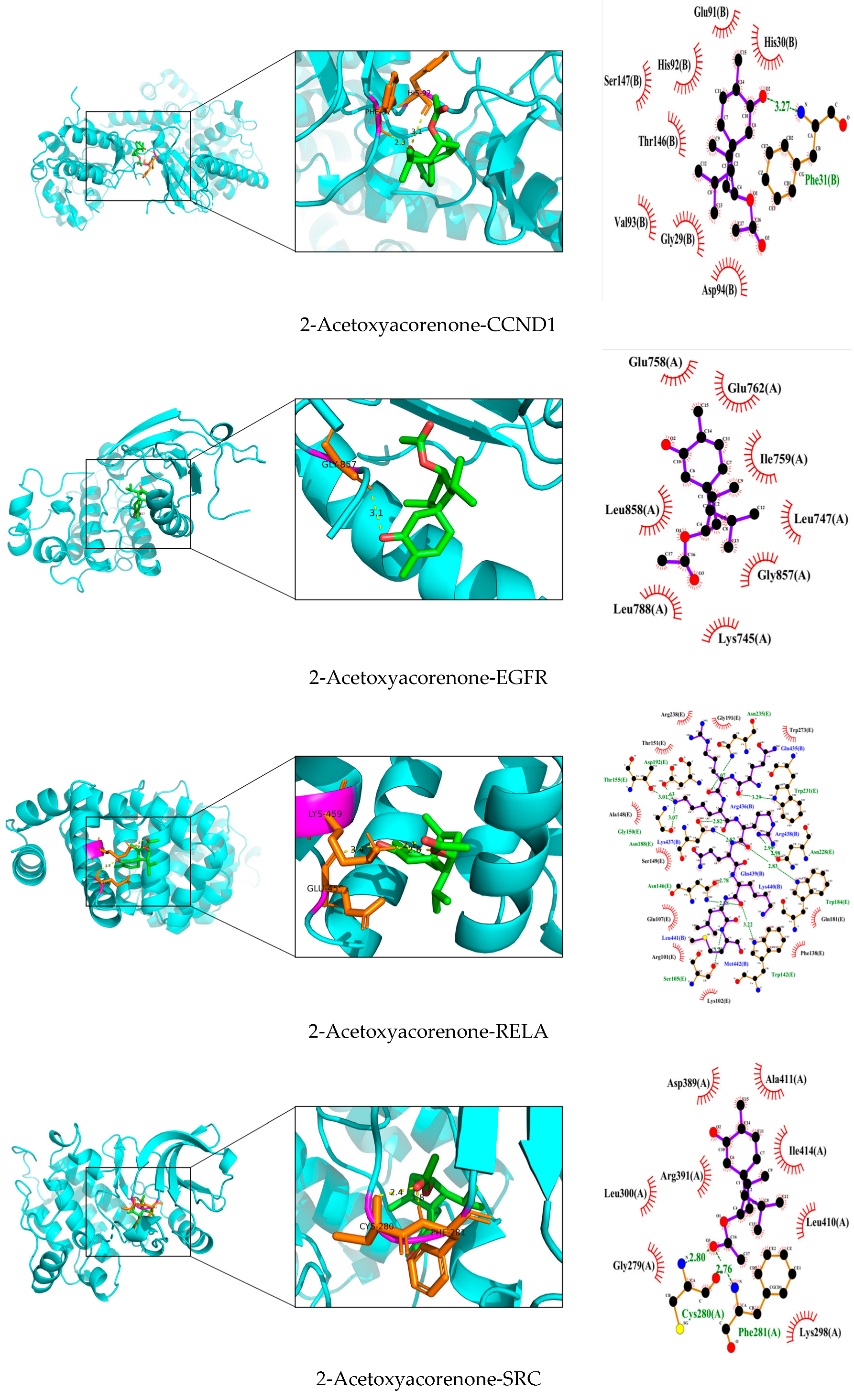
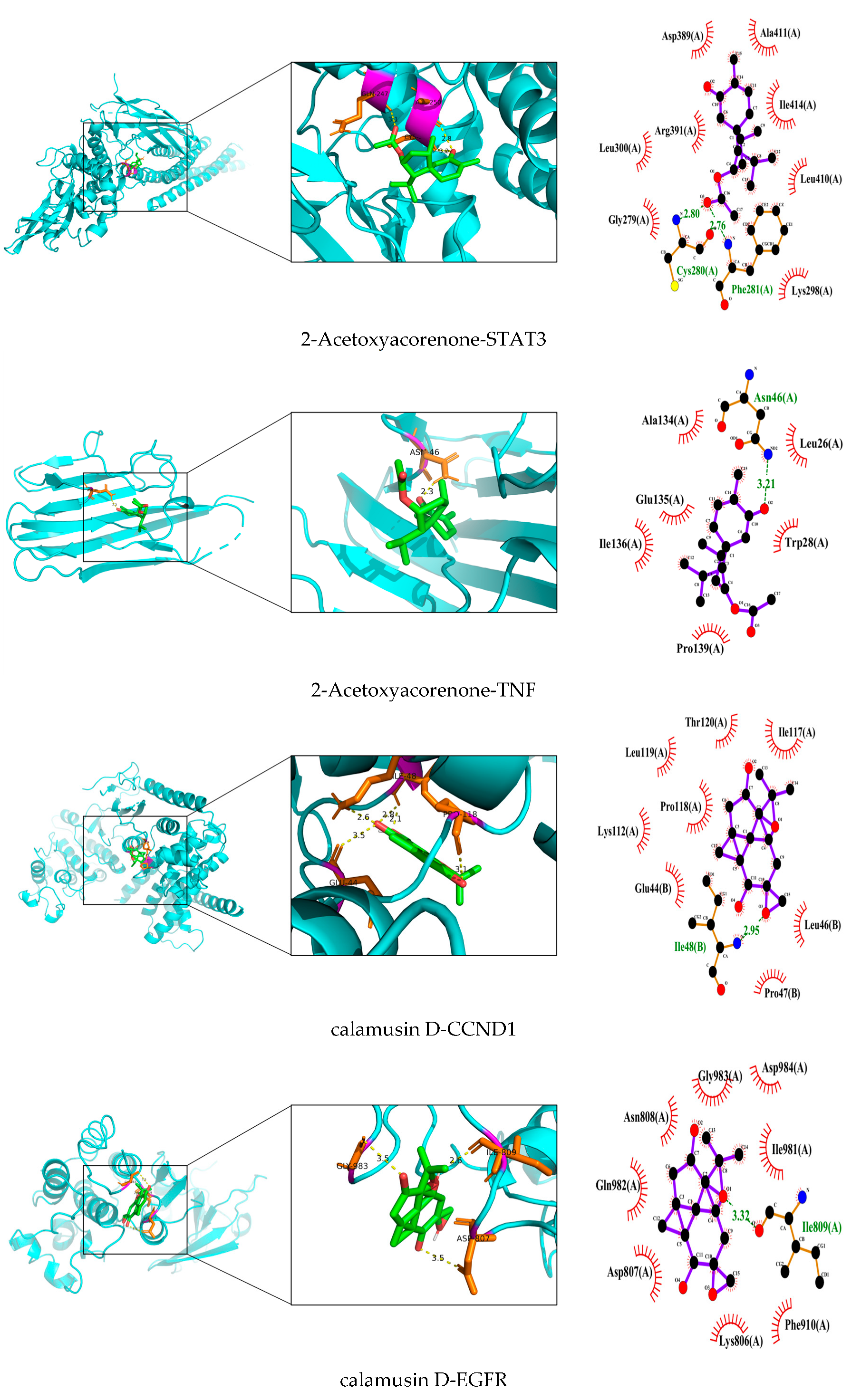
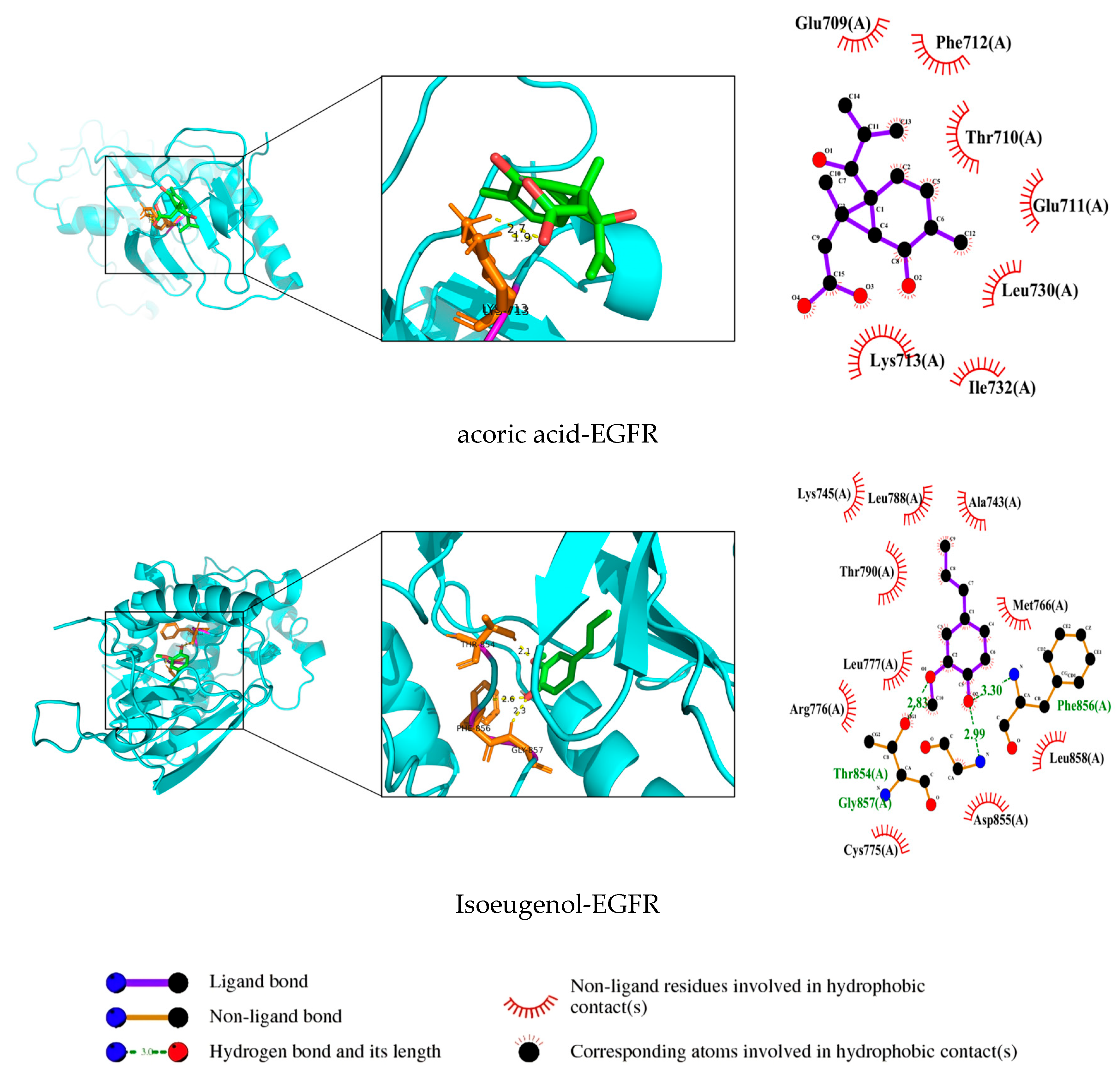
3.7. Validation and Graphical Representation of Molecular Docking Outcomes
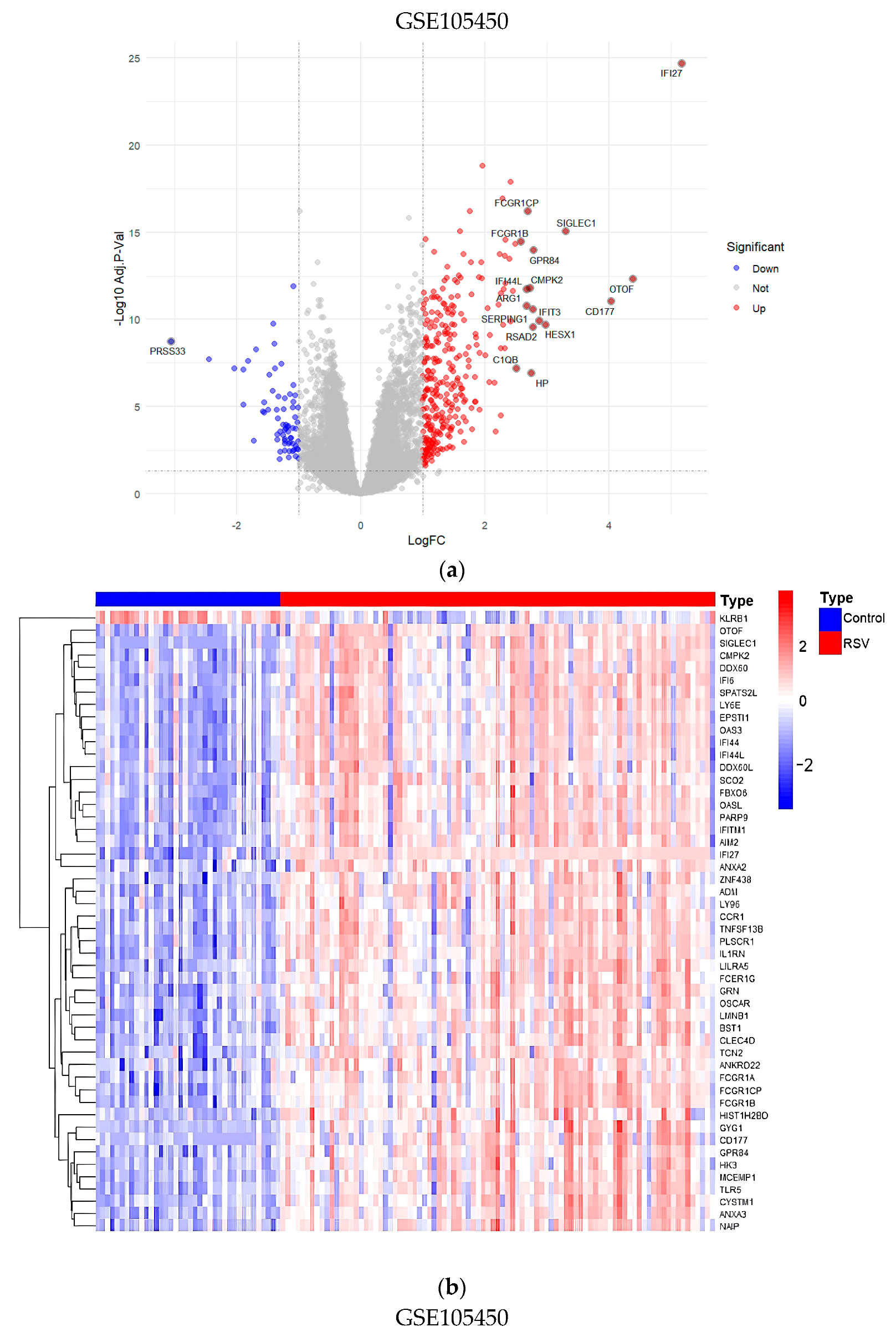
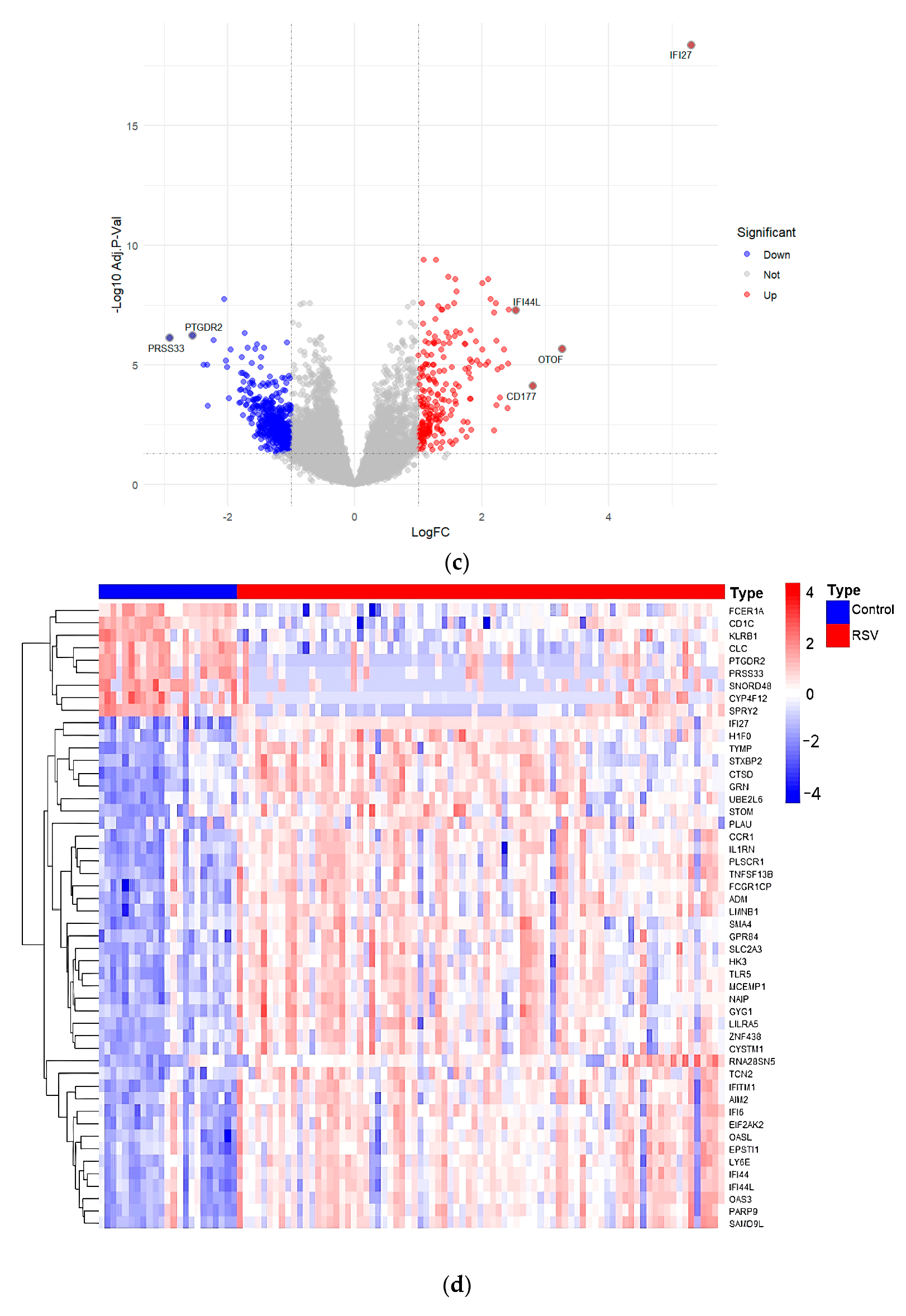
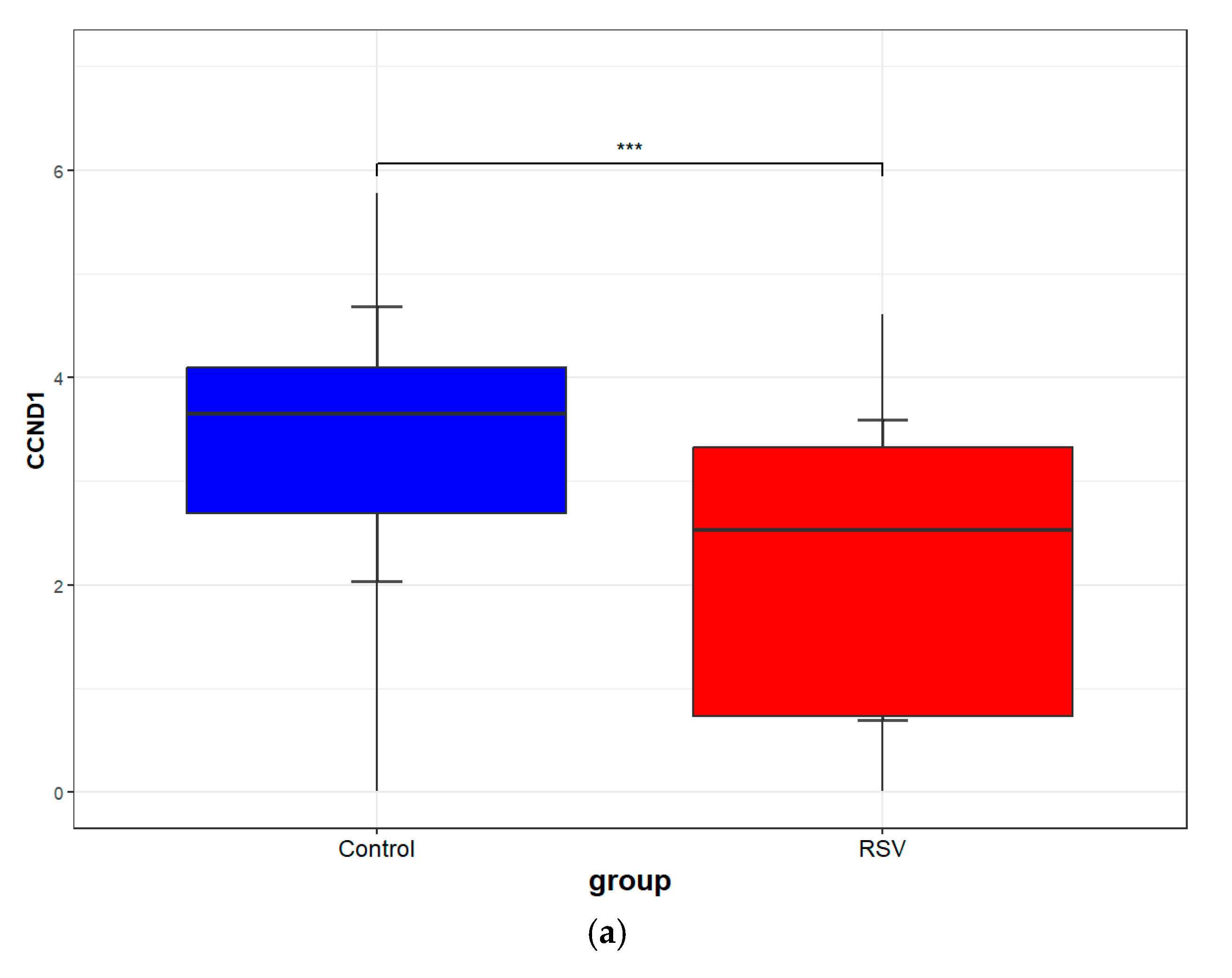
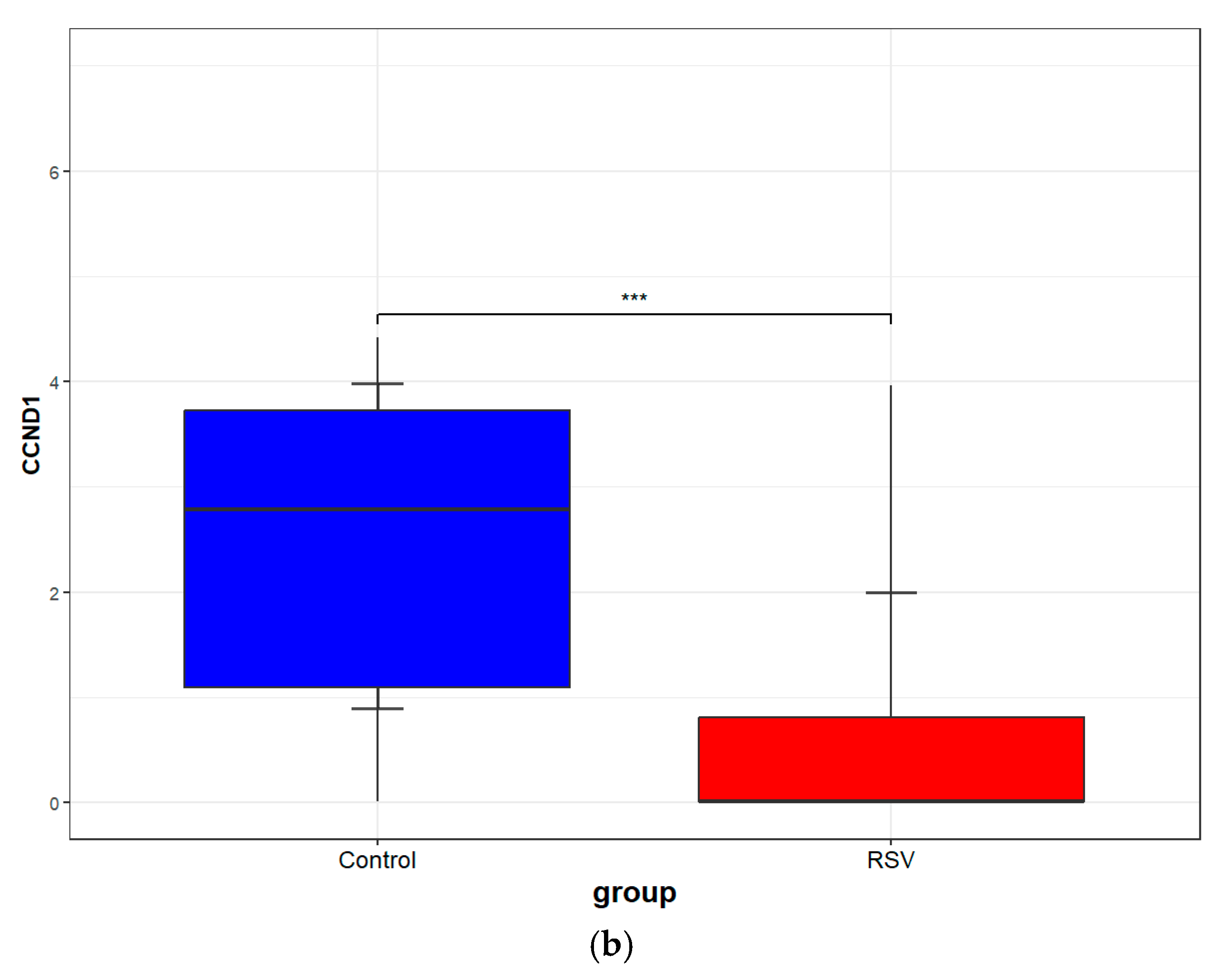
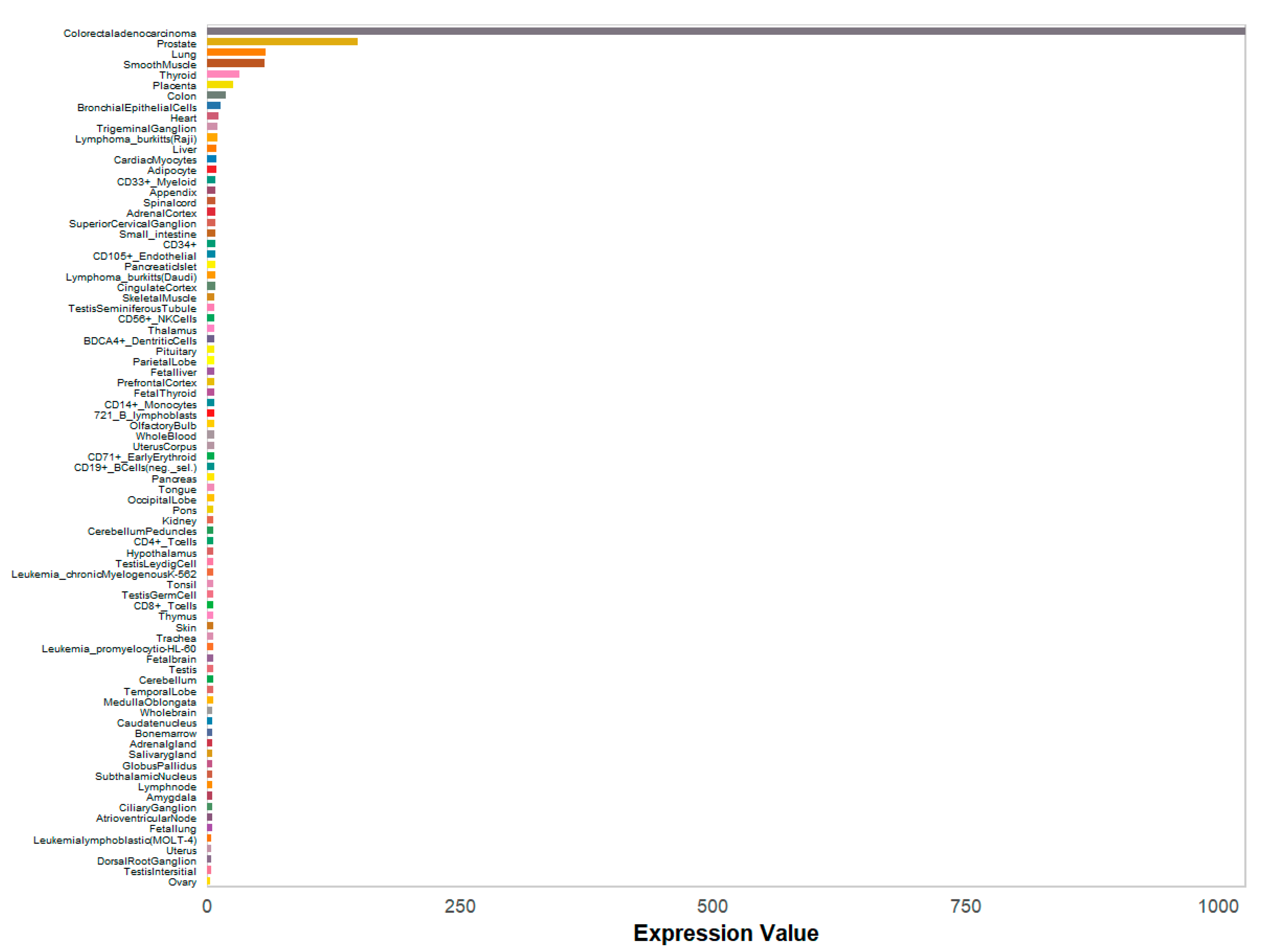
3.8. Validation of Core Targets by RSV Datasets from GEO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verwey, C.; Madhi, S.A. Review and Update of Active and Passive Immunization Against Respiratory Syncytial Virus. BioDrugs 2023, 37, 295–309. [Google Scholar] [CrossRef] [PubMed]
- McMorrow, M.L.; Moline, H.L.; Toepfer, A.P.; Halasa, N.B.; Schuster, J.E.; Staat, M.A.; Williams, J.V.; Klein, E.J.; Weinberg, G.A.; Clopper, B.R.; et al. Respiratory Syncytial Virus-Associated Hospitalizations in Children < 5 Years: 2016–2022. Pediatrics 2024, 154, e2023065623. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhang, H.Y.; Ren, L.L.; Lu, Q.B.; Ren, X.; Zhang, C.H.; Wang, Y.F.; Lin, S.H.; Zhang, X.A.; Li, J.; et al. Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun. 2021, 12, 5026. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 389, 1053. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- Carvajal, J.J.; Avellaneda, A.M.; Salazar-Ardiles, C.; Maya, J.E.; Kalergis, A.M.; Lay, M.K. Host Components Contributing to Respiratory Syncytial Virus Pathogenesis. Front. Immunol. 2019, 10, 2152. [Google Scholar] [CrossRef]
- Verwey, C.; Nunes, M.C.; Dangor, Z.; Madhi, S.A. Pulmonary function sequelae after respiratory syncytial virus lower respiratory tract infection in children: A systematic review. Pediatr. Pulmonol. 2020, 55, 1567–1583. [Google Scholar] [CrossRef]
- Sevindik, M.; Bal, C.; Eraslan, E.C.; Uysal, I.; Mohammed, F.S. Medicinal mushrooms: A comprehensive study on their antiviral potential. Prospect. Pharm. Sci. 2023, 21, 42–56. [Google Scholar] [CrossRef]
- Fu, K.; Xu, M.; Zhou, Y.; Li, X.; Wang, Z.; Liu, X.; Meng, X.; Zeng, Y.; Zhang, H. The Status quo and way forwards on the development of Tibetan medicine and the pharmacological research of tibetan materia Medica. Pharmacol. Res. 2020, 155, 104688. [Google Scholar] [CrossRef]
- Si, M.M.; Lou, J.S.; Zhou, C.X.; Shen, J.N.; Wu, H.H.; Yang, B.; He, Q.J.; Wu, H.S. Insulin releasing and alpha-glucosidase inhibitory activity of ethyl acetate fraction of Acorus calamus in vitro and in vivo. J. Ethnopharmacol. 2010, 128, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, L.; Peng, J.; Sangji, K.; Luo, Y.; Zeng, Y.; Zeweng, Y.; Fan, G. Traditional Tibetan medicine to fight against COVID-19: Basic theory and therapeutic drugs. Front. Pharmacol. 2023, 14, 1098253. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.; Rojmala, J.V.; Chotai, N.M.; Waghela, B.N.; Thakor, P. Virtual screening and identification of potent phytoconstituents from Acorus calamus L. as inhibitors of Monkeypox virus infection. J. Genet. Eng. Biotechnol. 2025, 23, 100487. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, M.H.; Wang, F.; Chen, P.Y.; Ke, X.G.; Yu, B.; Yang, Y.F.; You, P.T.; Wu, H.Z. Network pharmacology and molecular docking reveal the mechanism of Scopoletin against non-small cell lung cancer. Life Sci. 2021, 270, 119105. [Google Scholar] [CrossRef]
- Assaggaf, H.; Jeddi, M.; Mrabti, H.N.; Ez-Zoubi, A.; Qasem, A.; Attar, A.; Goh, B.H.; Tan, S.L.; Bouyahya, A.; Goh, K.W.; et al. Design of three-component essential oil extract mixture from Cymbopogon flexuosus, Carum carvi, and Acorus calamus with enhanced antioxidant activity. Sci. Rep. 2024, 14, 9195. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Ma, Y.; Wu, Q.; Kong, J.; Zhao, L.; Li, S.; Li, J. Screening for Active Compounds of Acorus calamus against SARS-CoV-2 Viral Protease and Mechanism Prediction. Pharmaceuticals 2024, 17, 325. [Google Scholar] [CrossRef]
- Gupta, H.; Deeksha; Urvashi; Reddy, S.G.E. Insecticidal and Detoxification Enzyme Inhibition Activities of Essential Oils for the Control of Pulse Beetle, Callosobruchus maculatus (F.) and Callosobruchus chinensis (L.) (Coleoptera: Bruchidae). Molecules 2023, 28, 492. [Google Scholar] [CrossRef] [PubMed]
- Shalini, K.; Guleria, S.; Salaria, D.; Rolta, R.; Fadare, O.A.; Mehta, J.; Awofisayo, O.; Mandyal, P.; Shandilya, P.; Kaushik, N.; et al. Antimicrobial potential of phytocompounds of Acorus calamus: In silico approach. J. Biomol. Struct. Dyn. 2024, 42, 2726–2737. [Google Scholar] [CrossRef]
- Bai, D.; Li, X.; Wang, S.; Zhang, T.; Wei, Y.; Wang, Q.; Dong, W.; Song, J.; Gao, P.; Li, Y.; et al. Advances in extraction methods, chemical constituents, pharmacological activities, molecular targets and toxicology of volatile oil from Acorus calamus var. angustatus Besser. Front. Pharmacol. 2022, 13, 1004529. [Google Scholar] [CrossRef]
- Kumar, C.; Akhter, S.; Satti, N.K.; Gupta, V.K.; Meena, S.R.; Vishwakarma, R.; Hassan, Q.P.; Verma, M.K. Dereplication approach for the first time isolation of tatarinowin a and pentadecanoic acid from Acorus calamus L. by using GC-MS. Nat. Prod. Res. 2023, 37, 2632–2637. [Google Scholar] [CrossRef]
- Islamoglu, F. Molecular docking, bioactivity, ADME, toxicity risks, and quantum mechanical parameters of some 1,2dihydroquinoline derivatives were calculated theoretically for investigation of its use as a pharmaceutical active ingredient in the treatment of multiple sclerosis (MS). Prospect. Pharm. Sci. 2024, 22, 168–187. [Google Scholar] [CrossRef]
- Shang, Z.; Tan, S.; Ma, D. Respiratory syncytial virus: From pathogenesis to potential therapeutic strategies. Int. J. Biol. Sci. 2021, 17, 4073–4091. [Google Scholar] [CrossRef]
- Labrie, L.; McVea, R.C.; Karkout, R.; Aldossary, H.; Gaudreault, V.; Ward, B.J.; Fixman, E.D. Early-life RSV infection modulates innate immune events, preferentially enhancing allergen-induced type 2 lung inflammation in females. PLoS Pathog. 2025, 21, e1013340. [Google Scholar] [CrossRef]
- Yanik, S.D.; Jamal Jameel, K.; Rohde, S.; Bürger, P.; Bülthoff, E.; Grunwald, T.; Kronsbein, J.; Koch, A.; Edwards, M.R.; Tenbusch, M.; et al. Cytokine production of mononuclear leukocytes in response to respiratory syncytial virus is increased in COPD but suppressed in non-COPD tobacco smokers. Mol. Med. 2025, 31, 237. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Liu, M.L.; Lv, J.N.; Chen, R.L.; Ding, K.; He, J.Q. Polysaccharides from Platycodonis Radix ameliorated respiratory syncytial virus-induced epithelial cell apoptosis and inflammation through activation of miR-181a-mediated Hippo and SIRT1 pathways. Int. Immunopharmacol. 2022, 104, 108510. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Nie, Y.; Zhan, F.; Zhu, B. Oxidative stress and ROS-mediated cellular events in RSV infection: Potential protective roles of antioxidants. Virol. J. 2023, 20, 224. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.; Herbert, J.A.; Palor, M.; Ren, L.; Larken, I.; Patel, A.; Moulding, D.; Cortina-Borja, M.; Smyth, R.L.; Smith, C.M. Trans-epithelial migration is essential for neutrophil activation during RSV infection. J. Leukoc. Biol. 2023, 113, 354–364. [Google Scholar] [CrossRef]
- Stegmann, F.; Lepenies, B. Myeloid C-type lectin receptors in host-pathogen interactions and glycan-based targeting. Curr. Opin. Chem. Biol. 2024, 82, 102521. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Xu, W.; Chen, J.; Tang, Y.; Xiong, S.; Li, Y.; Zhang, H.; Li, M.; Liu, Z. Cholesterol-rich lysosomes induced by respiratory syncytial virus promote viral replication by blocking autophagy flux. Nat. Commun. 2024, 15, 6311. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Liu, Q.; Wang, Y. Network pharmacology, molecular docking, and untargeted metabolomics reveal molecular mechanisms of multi-targets effects of Qingfei Tongluo Plaster improving respiratory syncytial virus pneumonia. Chin. Herb. Med. 2024, 16, 638–655. [Google Scholar] [CrossRef]
- Efstathiou, C.; Zhang, Y.; Kandwal, S.; Fayne, D.; Molloy, E.J.; Stevenson, N.J. Respiratory syncytial virus NS1 inhibits anti-viral Interferon-α-induced JAK/STAT signaling, by limiting the nuclear translocation of STAT1. Front. Immunol. 2024, 15, 1395809. [Google Scholar] [CrossRef]
- Li, L.; Xu, W.; Luo, Y.; Lao, C.; Tong, X.; Du, J.; Huang, B.; Li, D.; Chen, J.; Ye, H.; et al. Aloe polymeric acemannan inhibits the cytokine storm in mouse pneumonia models by modulating macrophage metabolism. Carbohydr. Polym. 2022, 297, 120032. [Google Scholar] [CrossRef]
- Mazarakis, N.; Higgins, R.A.; Anderson, J.; Toh, Z.Q.; Luwor, R.B.; Snibson, K.J.; Karagiannis, T.C.; Do, L.A.H.; Licciardi, P.V. The effects of the dietary compound L-sulforaphane against respiratory pathogens. Int. J. Antimicrob. Agents 2021, 58, 106460. [Google Scholar] [CrossRef]
- Noh, S.S.; Shin, H.J. RSV Induces Activation of Intracellular EGFR on the Mitochondrial Membrane for Virus Propagation. Int. J. Mol. Sci. 2023, 24, 17431. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qiao, D.; Pan, L.; Boldogh, I.; Zhao, Y.; Brasier, A.R. RELA∙8-Oxoguanine DNA Glycosylase1 Is an Epigenetic Regulatory Complex Coordinating the Hexosamine Biosynthetic Pathway in RSV Infection. Cells 2022, 11, 2210. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Z.; Ye, Y.; Shao, Y.; Zhu, P.; Li, X.; Ma, Y.; Xu, F.; Zhou, J.; Wu, M.; et al. DTX3L Enhances Type I Interferon Antiviral Response by Promoting the Ubiquitination and Phosphorylation of TBK1. J. Virol. 2023, 97, e0068723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Bai, Y.; Wang, W.; Amonkar, G.M.; Mou, H.; Olejnik, J.; Hume, A.J.; Mühlberger, E.; Lukacs, N.W.; Fearns, R.; et al. Activation of STAT3-mediated ciliated cell survival protects against severe infection by respiratory syncytial virus. J. Clin. Invest. 2024, 134, e183978. [Google Scholar] [CrossRef]
- Santos, L.D.; Antunes, K.H.; Muraro, S.P.; de Souza, G.F.; da Silva, A.G.; Felipe, J.S.; Zanetti, L.C.; Czepielewski, R.S.; Magnus, K.; Scotta, M.; et al. TNF-mediated alveolar macrophage necroptosis drives disease pathogenesis during respiratory syncytial virus infection. Eur. Respir. J. 2021, 57, 2003764. [Google Scholar] [CrossRef]
- Lingemann, M.; McCarty, T.; Liu, X.; Buchholz, U.J.; Surman, S.; Martin, S.E.; Collins, P.L.; Munir, S. The alpha-1 subunit of the Na+,K+-ATPase (ATP1A1) is required for macropinocytic entry of respiratory syncytial virus (RSV) in human respiratory epithelial cells. PLoS Pathog. 2019, 15, e1007963. [Google Scholar] [CrossRef]
- Gibbs, J.D.; Ornoff, D.M.; Igo, H.A.; Zeng, J.Y.; Imani, F. Cell cycle arrest by transforming growth factor beta1 enhances replication of respiratory syncytial virus in lung epithelial cells. J. Virol. 2009, 83, 12424–12431. [Google Scholar] [CrossRef]
- Mohapatra, S.; Park, S.J.; Boyapalle, S.; Pastey, M.K.; Graham, B.S.; Blanck, G. Human respiratory syncytial virus reduces the number of cells in S-phase and increases GADD153 expression in HEp-2 cells. Acta Virol. 2009, 53, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Fu, H.; Liu, Q.; Tie, Y.; Zhu, J.; Xing, R.; Sun, Z.; Zheng, X. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008, 582, 1564–1568. [Google Scholar] [CrossRef]
- Bakre, A.; Mitchell, P.; Coleman, J.K.; Jones, L.P.; Saavedra, G.; Teng, M.; Tompkins, S.M.; Tripp, R.A. Respiratory syncytial virus modifies microRNAs regulating host genes that affect virus replication. J. Gen. Virol. 2012, 93, 2346–2356. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, Z.; Cheng, Y.; Ma, C.; Zhong, Y.; Xiao, Y.; Gao, X.; Li, Z. M2 macrophage-derived exosomal microRNAs inhibit cell migration and invasion in gliomas through PI3K/AKT/mTOR signaling pathway. J. Transl. Med. 2021, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Yang, Y.; Yang, D. Cyclin D1 mediated by the nuclear translocation of nuclear factor kappa B exerts an oncogenic role in lung cancer. Bioengineered 2022, 13, 6866–6879. [Google Scholar] [CrossRef]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Shtro, A.A.; Petukhova, G.D.; Romanova, A.S. Protein and Peptide Substances in the Treatment of Respiratory Syncytial Infection: Current State. Molecules 2022, 27, 2263. [Google Scholar] [CrossRef]
- Mammas, I.N.; Drysdale, S.B.; Rath, B.; Theodoridou, M.; Papaioannou, G.; Papatheodoropoulou, A.; Koutsounaki, E.; Koutsaftiki, C.; Kozanidou, E.; Achtsidis, V.; et al. Update on current views and advances on RSV infection (Review). Int. J. Mol. Med. 2020, 46, 509–520. [Google Scholar] [CrossRef]
- Hu, M.; Bogoyevitch, M.A.; Jans, D.A. Impact of Respiratory Syncytial Virus Infection on Host Functions: Implications for Antiviral Strategies. Physiol. Rev. 2020, 100, 1527–1594. [Google Scholar] [CrossRef]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to combat viral infections: Development strategies and progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef] [PubMed]
- Nawamaki, K.; Kuroyanagi, M. Sesquiterpenoids from Acorus calamus as germination inhibitors. Phytochemistry 1996, 43, 1175–1182. [Google Scholar] [CrossRef]



| NO. | Compound Name | PubChem CID | Structure |
|---|---|---|---|
| 1 | 2-Acetoxyacorenone | 10850234 | 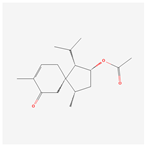 |
| 2 | calamusin D | 60156053 |  |
| 3 | acoric acid | 15558301 |  |
| 4 | isoeugenol | 853433 |  |
| 5 | Methyl palmitate | 8181 |  |
| 6 | eugenol | 3314 |  |
| 7 | (Z)-Methyl isoeugenol | 1549045 |  |
| 8 | methyl isoeugenol | 637776 |  |
| 9 | Calamensesquiterpinenol | 75250012 |  |
| 10 | Calamusin F | 60156148 |  |
| 11 | camphor | 2537 |  |
| 12 | Calamusin E | 60156054 |  |
| 13 | eugenyl acetate | 7136 |  |
| 14 | bullatantriol | 71430886 |  |
| 15 | 2,4,5-Trimethoxybenzoic acid | 10276 |  |
| 16 | Cedranone | 111402 |  |
| 17 | Dehydroxy-isocalamendiol | 535379 |  |
| 18 | cis –asarone | 636822 |  |
| 19 | Calamusin I | 60156151 |  |
| 20 | Isoacoramone | 3083746 |  |
| 21 | β asarone | 5281758 |  |
| 22 | acorone | 5316254 |  |
| 23 | tau-Muurolol | 6432221 |  |
| 24 | (E)-3-(2,4,5-Trimethoxyphenyl)acrylaldehyde | 9813266 |  |
| 25 | oplodiol | 12313756 |  |
| 26 | Thujopsanone | 13893399 |  |
| NO. | Target | NO. | Target | NO. | Target | NO. | Target |
|---|---|---|---|---|---|---|---|
| 1 | ABCB1 | 23 | CYP19A1 | 45 | ITGB2 | 67 | PIK3CD |
| 2 | ABCG2 | 24 | CYP1A1 | 46 | JAK1 | 68 | PPARA |
| 3 | ACE | 25 | CYP2C9 | 47 | JAK2 | 69 | PPARD |
| 4 | ACHE | 26 | DYRK1A | 48 | KDR | 70 | PPP1CA |
| 5 | AGTR2 | 27 | EGFR | 49 | KMO | 71 | PSMB5 |
| 6 | AHR | 28 | EPAS1 | 50 | MAPK14 | 72 | PTGDR2 |
| 7 | ALOX5 | 29 | EZH2 | 51 | MAPKAPK2 | 73 | PTGES |
| 8 | AOC3 | 30 | F2 | 52 | MDM2 | 74 | PTGS2 |
| 9 | APP | 31 | F2R | 53 | MIF | 75 | PTPN1 |
| 10 | AR | 32 | FABP4 | 54 | MMP2 | 76 | PTPN11 |
| 11 | BRD4 | 33 | FGFR1 | 55 | MPO | 77 | PTPN2 |
| 12 | CCNA2 | 34 | FLT1 | 56 | MTNR1A | 78 | RELA |
| 13 | CCND1 | 35 | G6PD | 57 | MTNR1B | 79 | RHOA |
| 14 | CCR1 | 36 | HDAC6 | 58 | NFE2L2 | 80 | SRC |
| 15 | CDK1 | 37 | HMGCR | 59 | NLRP3 | 81 | STAT3 |
| 16 | CHRM1 | 38 | HSD11B1 | 60 | NOS2 | 82 | TACR1 |
| 17 | CNR2 | 39 | HSD11B2 | 61 | NR3C1 | 83 | TNF |
| 18 | CREBBP | 40 | HSPA1A | 62 | NR3C2 | 84 | TRPV1 |
| 19 | CTSB | 41 | ICAM1 | 63 | PABPC1 | 85 | TYK2 |
| 20 | CTSL | 42 | ITGAL | 64 | PARP1 | 86 | VDR |
| 21 | CXCL8 | 43 | ITGAV | 65 | PGR | 87 | VEGFA |
| 22 | CXCR2 | 44 | ITGB1 | 66 | PIK3CB |
| Rank | Compound Name | PubChem CID | Degree |
|---|---|---|---|
| 1 | 2-Acetoxyacorenone | 10850234 | 41 |
| 2 | calamusin D | 60156053 | 24 |
| 3 | acoric acid | 15558301 | 22 |
| 4 | isoeugenol | 853433 | 21 |
| 5 | Methyl palmitate | 8181 | 13 |
| 6 | eugenol | 3314 | 9 |
| 7 | (Z)-Methyl isoeugenol | 1549045 | 4 |
| 8 | methyl isoeugenol | 637776 | 4 |
| 9 | Calamensesquiterpinenol | 75250012 | 3 |
| 10 | Calamusin F | 60156148 | 3 |
| Rank | Gene Name | Score |
|---|---|---|
| 1 | STAT3 | 1.66 × 109 |
| 2 | EGFR | 1.66 × 109 |
| 3 | TNF | 1.66 × 109 |
| 4 | CCND1 | 1.63 × 109 |
| 5 | PARP1 | 1.39 × 109 |
| 6 | MDM2 | 1.38 × 109 |
| 7 | EZH2 | 1.22 × 109 |
| 8 | AR | 1.21 × 109 |
| 9 | RELA | 1.17 × 109 |
| 10 | CREBBP | 1.10 × 109 |
| 11 | SRC | 1.06 × 109 |
| 12 | CDK1 | 1.04 × 109 |
| 13 | HDAC6 | 1.00 × 109 |
| 14 | BRD4 | 5.19 × 108 |
| 15 | MMP2 | 5.07 × 108 |
| Rank | Gene Name | Degree | CC | BC |
|---|---|---|---|---|
| 1 | PIK3CB | 92 | 0.498998 | 0.080407 |
| 2 | PIK3CD | 92 | 0.498998 | 0.080407 |
| 3 | RELA | 72 | 0.447038 | 0.061346 |
| 4 | MAPK14 | 57 | 0.424191 | 0.031357 |
| 5 | TNF | 55 | 0.434555 | 0.045415 |
| 6 | EGFR | 44 | 0.399679 | 0.020541 |
| 7 | SRC | 40 | 0.395866 | 0.019708 |
| 8 | CCND1 | 39 | 0.375566 | 0.011924 |
| 9 | RHOA | 34 | 0.403566 | 0.016991 |
| 10 | STAT3 | 33 | 0.387247 | 0.011039 |
| 11 | CXCL8 | 32 | 0.381317 | 0.013256 |
| 12 | JAK1 | 28 | 0.378995 | 0.007265 |
| 13 | JAK2 | 26 | 0.377845 | 0.007811 |
| 14 | VEGFA | 25 | 0.374436 | 0.008855 |
| 15 | PTGS2 | 21 | 0.384853 | 0.011878 |
| Gene Name | PDB ID |
|---|---|
| CCND1 | 6p8e |
| EGFR | 8a27 |
| RELA | 7let |
| SRC | 7ng7 |
| STAT3 | 6njs |
| TNF | 5uui |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.; Shao, L.; Tao, K.; Chen, X.; Liao, H.; Liao, W.; Xue, B.; Wang, S. Exploring CCND1 as a Key Target of Acorus calamus Against RSV Infection: Network Pharmacology, Molecular Docking, and Bioinformatics Analysis. Curr. Issues Mol. Biol. 2025, 47, 695. https://doi.org/10.3390/cimb47090695
Chang H, Shao L, Tao K, Chen X, Liao H, Liao W, Xue B, Wang S. Exploring CCND1 as a Key Target of Acorus calamus Against RSV Infection: Network Pharmacology, Molecular Docking, and Bioinformatics Analysis. Current Issues in Molecular Biology. 2025; 47(9):695. https://doi.org/10.3390/cimb47090695
Chicago/Turabian StyleChang, Haojing, Li Shao, Ke Tao, Xiangjun Chen, Hehe Liao, Wang Liao, Bei Xue, and Shaokang Wang. 2025. "Exploring CCND1 as a Key Target of Acorus calamus Against RSV Infection: Network Pharmacology, Molecular Docking, and Bioinformatics Analysis" Current Issues in Molecular Biology 47, no. 9: 695. https://doi.org/10.3390/cimb47090695
APA StyleChang, H., Shao, L., Tao, K., Chen, X., Liao, H., Liao, W., Xue, B., & Wang, S. (2025). Exploring CCND1 as a Key Target of Acorus calamus Against RSV Infection: Network Pharmacology, Molecular Docking, and Bioinformatics Analysis. Current Issues in Molecular Biology, 47(9), 695. https://doi.org/10.3390/cimb47090695








