Ketogenic Diet and Thyroid Function: A Delicate Metabolic Balancing Act
Abstract
1. Introduction
2. Pathways of Thyroid Regulation Under the Ketogenic Diet
2.1. The HPT Axis and Metabolic Regulation
2.2. Hormonal Modulators of Thyroid Function
2.3. Macronutrient Composition and Thyroid Hormone Conversion
3. Immune, Inflammatory and Oxidative Modulation Under Ketogenic Diet
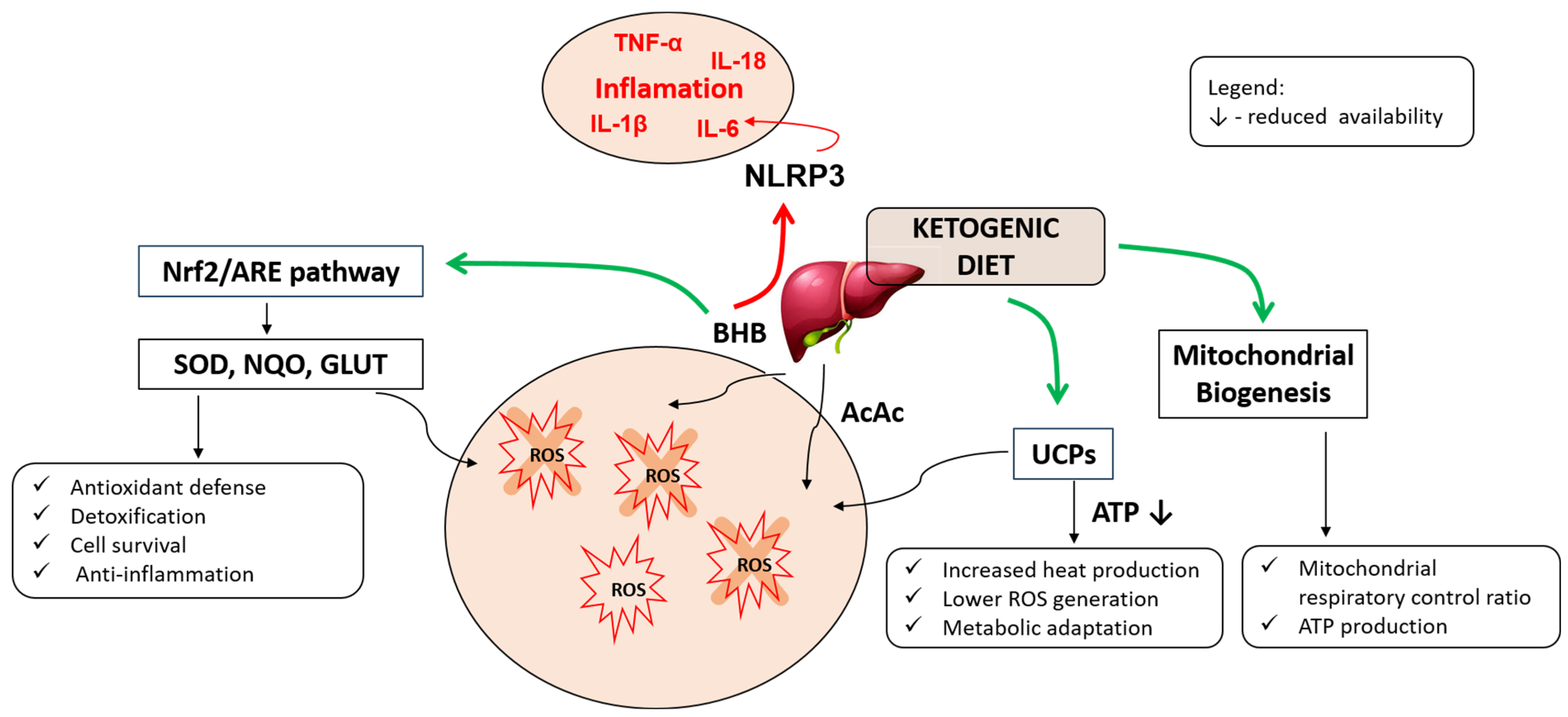
3.1. Oxidative Stress and Mitochondrial Function
3.2. Anti-Inflammatory Effects of Ketogenic Adaptation
3.3. Autoimmune Modulation and Regulatory T Cells
4. Genetic and Individual Factors Influencing Response to KD
4.1. Genetic Determinants
4.2. Sex-Based Differences and Reproductive Endocrinology
5. Gut Microbiota, Nutrient Absorption, and SCFA Deficiency
6. Clinical Impact and Population-Specific Adaptation of the Ketogenic Diet
6.1. Hypothyroidism and LT4 Dependence
6.2. Euthyroid Adaptation and Subclinical Risk
6.3. Hashimoto’s Thyroiditis
6.4. Hyperthyroidism and Graves’ Disease
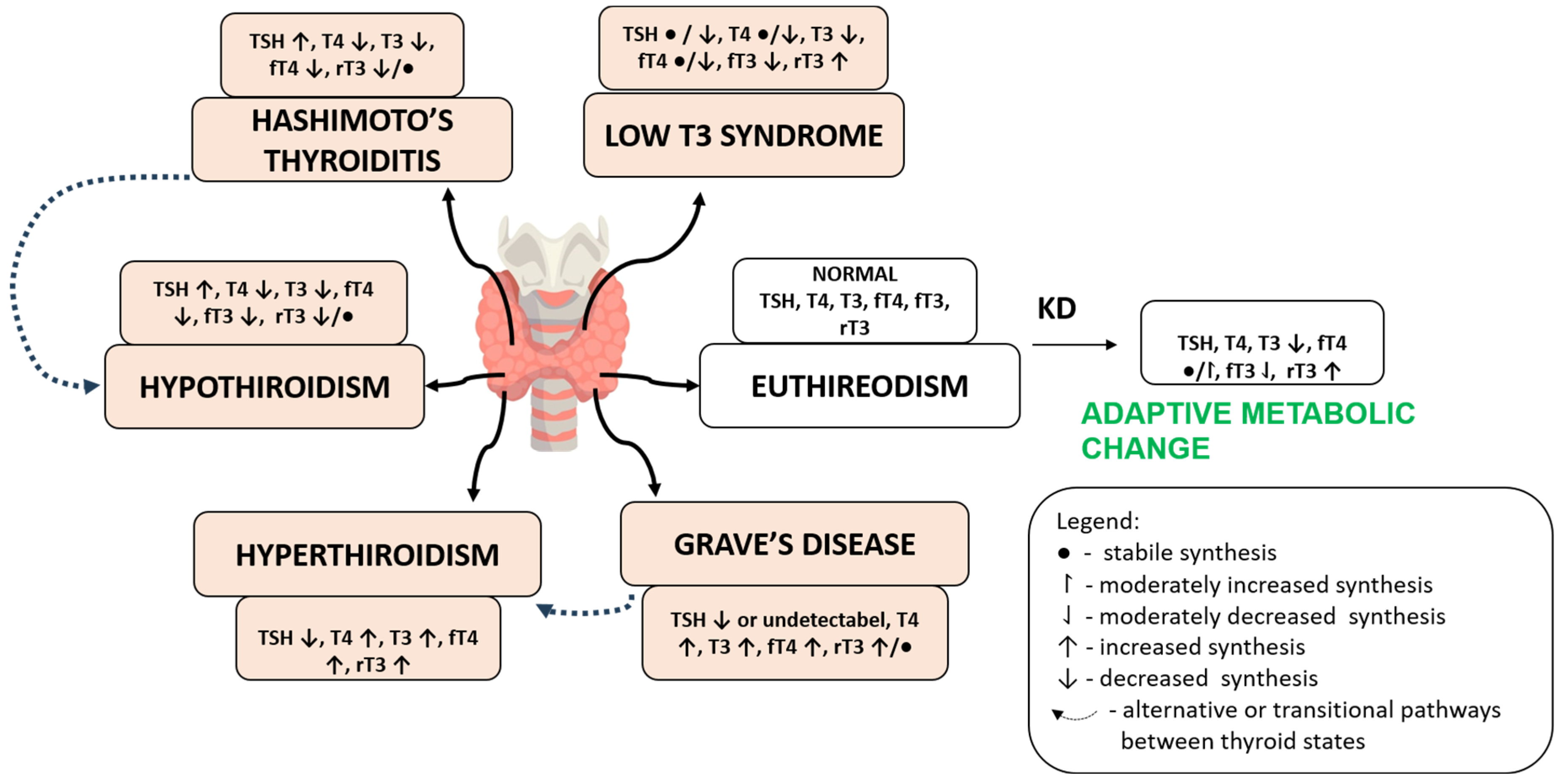
6.5. Thyroid Cancer
7. Risks, Monitoring, and Personalized Recommendations
8. Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| T4 | Thyroxine |
| T3 | Triiodothyronine |
| HPT | Hypothalamic–pituitary–thyroid axis |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid-stimulating hormone |
| BMR | Basal metabolic rate |
| KD | Ketogenic diet |
| BHB | Beta-hydroxybutyrate |
| AcAc | Acetoacetate |
| DIO | Deiodinase enzymes |
| rT3 | Reverse T3 |
| fT3 | Free T3 |
| fT4 | Free T4 |
| RMR | Resting metabolic rate |
| ROS | Reactive oxygen species |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| SOD | Superoxide dismutase |
| NLRP3 | NOD-like receptor protein 3 |
| IL-1β | Interleukin-1β |
| IL-18 | Interleukin-18 |
| TNF-α | Tumor necrosis factor-alpha |
| IFN-γ | Interferon-gamma |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| NF-κB | Nuclear factor kappa B |
| COX-2 | Cyclooxygenase-2 |
| Treg | Regulatory T cell |
| HDAC | Histone deacetylase |
| Th17 | T helper 17 cell |
| MHC | Major histocompatibility complex |
| AMPK | AMP-activated protein kinase |
| PD-L1 | Programmed cell death 1 ligand 1 |
| HPG | Hypothalamic–pituitary–gonadal axis |
| TBG | Thyroxine-binding globulin |
| SCFA | Short-chain fatty acids |
| GPCR | G-protein-coupled receptor |
| TPOAb | Thyroid peroxidase antibody |
| LT4 | Levothyroxine |
References
- Armstrong, M.; Asuka, E.; Fingeret, A. Physiology, Thyroid Function. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ilahi, A.; Muco, E.; Ilahi, T.B. Anatomy, Head and Neck, Parathyroid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Shahid, M.A.; Ashraf, M.A.; Sharma, S. Physiology, Thyroid Hormone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.K.; Gupta, S. Biochemistry, Ketogenesis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kim, J.-M. Ketogenic Diet: Old Treatment, New Beginning. Clin. Neurophysiol. Pract. 2017, 2, 161–162. [Google Scholar] [CrossRef]
- Bistrian, B.R.; Blackburn, G.L.; Flatt, J.P.; Sizer, J.; Scrimshaw, N.S.; Sherman, M. Nitrogen Metabolism and Insulin Requirements in Obese Diabetic Adults on a Protein-Sparing Modified Fast. Diabetes 1976, 25, 494–504. [Google Scholar] [CrossRef]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D.; et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther. 2018, 9, 583–612, Correction in Diabetes Ther. 2018, 9, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, M.; Afghan, M.; Tarmahi, V.; Behtari, M.; Rahimi Khamaneh, S.; Raeisi, S. Ketogenic Diet: Overview, Types, and Possible Anti-Seizure Mechanisms. Nutr. Neurosci. 2021, 24, 307–316. [Google Scholar] [CrossRef]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef] [PubMed]
- Majid, B.; Khan, M.A.I.; Maqsood, A.M.; Khalid, M.; Ahmad, Z.; Raza, S.S. Impact of Ketogenic Diet on Insulin and Thyroid Hormones in a Healthy Cohort. Int. J. Health Sci. 2022, 6, 3231–3238. [Google Scholar] [CrossRef]
- Iacovides, S.; Maloney, S.K.; Bhana, S.; Angamia, Z.; Meiring, R.M. Could the Ketogenic Diet Induce a Shift in Thyroid Function and Support a Metabolic Advantage in Healthy Participants? A Pilot Randomized-Controlled-Crossover Trial. PLoS ONE 2022, 17, e0269440. [Google Scholar] [CrossRef]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the Local Control of Thyroid Hormone Action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef]
- Bostock, E.C.S.; Kirkby, K.C.; Taylor, B.V.M. The Current Status of the Ketogenic Diet in Psychiatry. Front. Psychiatry 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, R.; Leone, A.; Lessa, C.; Foppiani, A.; Ravella, S.; Ravasenghi, S.; Trentani, C.; Ferraris, C.; Veggiotti, P.; De Giorgis, V.; et al. Long-Term Effects of a Classic Ketogenic Diet on Ghrelin and Leptin Concentration: A 12-Month Prospective Study in a Cohort of Italian Children and Adults with GLUT1-Deficiency Syndrome and Drug Resistant Epilepsy. Nutrients 2019, 11, 1716. [Google Scholar] [CrossRef]
- Hesch, R.D.; Brunner, G.; Söling, H.D. Conversion of Thyroxine (T4) and Triiodothyronine (T3) and the Subcellular Localisation of the Converting Enzyme. Clin. Chim. Acta 1975, 59, 209–213. [Google Scholar] [CrossRef]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, Cellular and Molecular Biology, and Physiological Roles of the Iodothyronine Selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef]
- Lartey, L.J.; Werneck-de-Castro, J.P.; O-Sullivan, I.; Unterman, T.G.; Bianco, A.C. Coupling between Nutrient Availability and Thyroid Hormone Activation. J. Biol. Chem. 2015, 290, 30551–30561. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.L.; Carvalho, D.P. Bioenergetic Impact of Tissue-Specific Regulation of Iodothyronine Deiodinases during Nutritional Imbalance. J. Bioenerg. Biomembr. 2011, 43, 59–65. [Google Scholar] [CrossRef]
- de Vries, E.M.; van Beeren, H.C.; Ackermans, M.T.; Kalsbeek, A.; Fliers, E.; Boelen, A. Differential Effects of Fasting vs Food Restriction on Liver Thyroid Hormone Metabolism in Male Rats. J. Endocrinol. 2015, 224, 25–35. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Boden, G.; Sargrad, K.; Homko, C.; Mozzoli, M.; Stein, T.P. Effect of a Low-Carbohydrate Diet on Appetite, Blood Glucose Levels, and Insulin Resistance in Obese Patients with Type 2 Diabetes. Ann. Intern. Med. 2005, 142, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Nillni, E.A.; Vaslet, C.; Harris, M.; Hollenberg, A.; Bjørbæk, C.; Flier, J.S. Leptin Regulates Prothyrotropin-Releasing Hormone Biosynthesis: Evidence for Direct and Indirect Pathways*. J. Biol. Chem. 2000, 275, 36124–36133. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Aschkenasi, C.; Elias, C.F.; Chandrankunnel, A.; Nillni, E.A.; Bjørbæk, C.; Elmquist, J.K.; Flier, J.S.; Hollenberg, A.N. Transcriptional Regulation of the Thyrotropin-Releasing Hormone Gene by Leptin and Melanocortin Signaling. J. Clin. Investig. 2001, 107, 111–120. [Google Scholar] [CrossRef]
- Kok, P.; Roelfsema, F.; Langendonk, J.G.; Frölich, M.; Burggraaf, J.; Meinders, A.E.; Pijl, H. High Circulating Thyrotropin Levels in Obese Women Are Reduced after Body Weight Loss Induced by Caloric Restriction. J. Clin. Endocrinol. Metab. 2005, 90, 4659–4663. [Google Scholar] [CrossRef]
- Ahima, R.S. Leptin and the Neuroendocrinology of Fasting. Front. Horm. Res. 2000, 26, 42–56. [Google Scholar] [CrossRef]
- Dyńka, D.; Rodzeń, Ł.; Rodzeń, M.; Pacholak-Klimas, A.; Ede, G.; Sethi, S.; Łojko, D.; Bartoń, K.; Berry, K.; Deptuła, A.; et al. Ketogenic Diets for Body Weight Loss: A Comparison with Other Diets. Nutrients 2025, 17, 965. [Google Scholar] [CrossRef]
- Martinez-deMena, R.; Calvo, R.-M.; Garcia, L.; Obregon, M.J. Effect of Glucocorticoids on the Activity, Expression and Proximal Promoter of Type II Deiodinase in Rat Brown Adipocytes. Mol. Cell. Endocrinol. 2016, 428, 58–67. [Google Scholar] [CrossRef]
- Köhrle, J. Local Activation and Inactivation of Thyroid Hormones: The Deiodinase Family. Mol. Cell. Endocrinol. 1999, 151, 103–119. [Google Scholar] [CrossRef]
- Elumalai, V.; Mahadevan, Y.; Balasubramanium, S.; Anton, M.C.; Jyothirmayi, B.; Sridevi, C. Evaluation of Thyroid Status in Type 2 Diabetes Mellitus with Reference to Insulin Resistance. Biomed. Pharmacol. J. 2024, 17, 2575–2584. [Google Scholar] [CrossRef]
- Kalra, S.; Aggarwal, S.; Khandelwal, D. Thyroid Dysfunction and Type 2 Diabetes Mellitus: Screening Strategies and Implications for Management. Diabetes Ther. 2019, 10, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Sharman, M.J.; Love, D.M.; Avery, N.G.; Gómez, A.L.; Scheett, T.P.; Kraemer, W.J. Body Composition and Hormonal Responses to a Carbohydrate-Restricted Diet. Metabolism 2002, 51, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Engel, F.L.; Engel, M.G.; Mcpherson, H.T. Ketogenic and Adipokinetic Activities of Pituitary Hormones. Endocrinology 1957, 61, 713–723. [Google Scholar] [CrossRef]
- Polito, R.; Messina, G.; Valenzano, A.; Scarinci, A.; Villano, I.; Monda, M.; Cibelli, G.; Porro, C.; Pisanelli, D.; Monda, V.; et al. The Role of Very Low Calorie Ketogenic Diet in Sympathetic Activation through Cortisol Secretion in Male Obese Population. J. Clin. Med. 2021, 10, 4230. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.; Riedl, S.; Uhr, M.; Schmidt, D.; Zhang, X.; Yassouridis, A.; Steiger, A. Ghrelin Affects the Hypothalamus–Pituitary–Thyroid Axis in Humans by Increasing Free Thyroxine and Decreasing TSH in Plasma. Eur. J. Endocrinol. 2010, 162, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, L.; Gherasim, A.; Niţă, O.; Ungureanu, M.C.; Pădureanu, S.S.; Gavril, R.S.; Arhire, L.I. Effects of Ghrelin in Energy Balance and Body Weight Homeostasis. Hormones 2016, 15, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Gardner, J.K.; Frye, B.R.; Snook, M.L.; Schuchert, M.K.; Richard, E.L.; Williams, N.I. Circulating Ghrelin Is Sensitive to Changes in Body Weight during a Diet and Exercise Program in Normal-Weight Young Women. J. Clin. Endocrinol. Metab. 2004, 89, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, S.W.; Chopra, I.J.; Sherwin, R.S.; Lyall, S.S. Effect of Caloric Restriction and Dietary Composition on Serum T3 and Reverse T3 in Man. J. Clin. Endocrinol. Metab. 1976, 42, 197–200. [Google Scholar] [CrossRef]
- Carvalho, D.P.; Dupuy, C. Thyroid Hormone Biosynthesis and Release. Mol. Cell. Endocrinol. 2017, 458, 6–15. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Shao, S.; Xu, G.; Song, Y.; Xu, C.; Gao, L.; Hu, C.; Zhao, J. A High-Fat Diet Rich in Saturated and Mono-Unsaturated Fatty Acids Induces Disturbance of Thyroid Lipid Profile and Hypothyroxinemia in Male Rats. Mol. Nutr. Food Res. 2018, 62, 1700599. [Google Scholar] [CrossRef]
- Zhao, W.; Peng, X.; Liu, Y.; Li, S.; Li, X.; Gao, Z.; Han, C.; Zhu, Z. The Association between Circulating Saturated Fatty Acids and Thyroid Function: Results from the NHANES 2011−2012. Front. Endocrinol. 2024, 15, 1405758. [Google Scholar] [CrossRef]
- Molteberg, E.; Thorsby, P.; Kverneland, M.; Iversen, P.; Selmer, K.; Nakken, K.; Taubøll, E. Effects of Modified Atkins Diet on Thyroid Function in Adult Patients with Pharmacoresistant Epilepsy. Epilepsy Behav. 2020, 111, 107285. [Google Scholar] [CrossRef]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s Thyroiditis: An Update on Pathogenic Mechanisms, Diagnostic Protocols, Therapeutic Strategies, and Potential Malignant Transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef]
- Greco, T.; Glenn, T.C.; Hovda, D.A.; Prins, M.L. Ketogenic Diet Decreases Oxidative Stress and Improves Mitochondrial Respiratory Complex Activity. J. Cereb. Blood Flow Metab. 2016, 36, 1603–1613. [Google Scholar] [CrossRef]
- Milder, J.; Patel, M.N. Modulation of Oxidative Stress and Mitochondrial Function by the Ketogenic Diet. Epilepsy Res. 2012, 100, 295–303. [Google Scholar] [CrossRef]
- Lin, J.; Ren, Q.; Zhang, F.; Gui, J.; Xiang, X.; Wan, Q. D-β-Hydroxybutyrate Dehydrogenase Mitigates Diabetes-Induced Atherosclerosis through the Activation of Nrf2. Thromb. Haemost. 2023, 123, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Keijer, J.; Adjobo-Hermans, M.J.W.; van de Wal, M.; Schirris, T.; van Karnebeek, C.; Pan, Y.; Koopman, W.J.H. The Ketogenic Diet as a Therapeutic Intervention Strategy in Mitochondrial Disease. Int. J. Biochem. Cell Biol. 2021, 138, 106050. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Roliński, J. Immune Disorders in Hashimoto’s Thyroiditis: What Do We Know So Far? J. Immunol. Res. 2015, 2015, 979167. [Google Scholar] [CrossRef]
- Yao, Z.; Guo, F.; Tan, Y.; Zhang, Y.; Geng, Y.; Yang, G.; Wang, S. Causal Relationship between Inflammatory Cytokines and Autoimmune Thyroid Disease: A Bidirectional Two-Sample Mendelian Randomization Analysis. Front. Immunol. 2024, 15, 1334772. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wu, Y.; Hou, Y.; Liu, Y.; Liu, T.; Zhang, H.; Fan, C.; Guan, H.; Li, Y.; Shan, Z.; et al. Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis. Front. Immunol. 2018, 9, 1197. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The Ketone Metabolite β-Hydroxybutyrate Blocks NLRP3 Inflammasome-Mediated Inflammatory Disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Link, V.M.; Subramanian, P.; Cheung, F.; Han, K.L.; Stacy, A.; Chi, L.; Sellers, B.A.; Koroleva, G.; Courville, A.B.; Mistry, S.; et al. Differential Peripheral Immune Signatures Elicited by Vegan versus Ketogenic Diets in Humans. Nat. Med. 2024, 30, 560–572. [Google Scholar] [CrossRef]
- Jeong, E.A.; Jeon, B.T.; Shin, H.J.; Kim, N.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Ketogenic Diet-Induced Peroxisome Proliferator-Activated Receptor-γ Activation Decreases Neuroinflammation in the Mouse Hippocampus after Kainic Acid-Induced Seizures. Exp. Neurol. 2011, 232, 195–202. [Google Scholar] [CrossRef]
- Ji, J.; Fotros, D.; Sohouli, M.H.; Velu, P.; Fatahi, S.; Liu, Y. The Effect of a Ketogenic Diet on Inflammation-Related Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2025, 83, 40–58. [Google Scholar] [CrossRef]
- Marazuela, M.; García-López, M.A.; Figueroa-Vega, N.; de la Fuente, H.; Alvarado-Sánchez, B.; Monsiváis-Urenda, A.; Sánchez-Madrid, F.; González-Amaro, R. Regulatory T Cells in Human Autoimmune Thyroid Disease. J. Clin. Endocrinol. Metab. 2006, 91, 3639–3646. [Google Scholar] [CrossRef]
- Glick, A.B.; Wodzinski, A.; Fu, P.; Levine, A.D.; Wald, D.N. Impairment of Regulatory T-Cell Function in Autoimmune Thyroid Disease. Thyroid® 2013, 23, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Li, C.; Liao, J.; Wang, G.; Lin, S.; Xia, Y.; Wen, J. The Effects of Ketogenic Diet on the Th17/Treg Cells Imbalance in Patients with Intractable Childhood Epilepsy. Seizure 2016, 38, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kalin, J.H.; Butler, K.V.; Akimova, T.; Hancock, W.W.; Kozikowski, A.P. Second-Generation Histone Deacetylase 6 Inhibitors Enhance the Immunosuppressive Effects of Foxp3+ T-Regulatory Cells. J. Med. Chem. 2012, 55, 639–651. [Google Scholar] [CrossRef]
- Duan, R.; Wang, T.; Li, Z.; Jiang, L.; Yu, X.; He, D.; Tao, T.; Liu, X.; Huang, Z.; Feng, L.; et al. Ketogenic Diet Modulates Immune Cell Transcriptional Landscape and Ameliorates Experimental Autoimmune Uveitis in Mice. J. Neuroinflamm. 2024, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Bu, X.; Gao, Y.; Guo, J.; Hu, J.; Jiang, C.; Zhang, Z.; Xu, K.; Duan, J.; He, S.; et al. Energy Status Dictates PD-L1 Protein Abundance and Anti-Tumor Immunity to Enable Checkpoint Blockade. Mol. Cell 2021, 81, 2317–2331.e6. [Google Scholar] [CrossRef]
- Murphy, S.; Rahmy, S.; Gan, D.; Liu, G.; Zhu, Y.; Manyak, M.; Duong, L.; He, J.; Schofield, J.H.; Schafer, Z.T.; et al. Ketogenic Diet Alters the Epigenetic and Immune Landscape of Prostate Cancer to Overcome Resistance to Immune Checkpoint Blockade Therapy. Cancer Res. 2024, 84, 1597–1612. [Google Scholar] [CrossRef]
- Peeters, R.P.; van Toor, H.; Klootwijk, W.; de Rijke, Y.B.; Kuiper, G.G.J.M.; Uitterlinden, A.G.; Visser, T.J. Polymorphisms in Thyroid Hormone Pathway Genes Are Associated with Plasma TSH and Iodothyronine Levels in Healthy Subjects. J. Clin. Endocrinol. Metab. 2003, 88, 2880–2888. [Google Scholar] [CrossRef]
- Deng, Y.; Han, Y.; Gao, S.; Dong, W.; Yu, Y. The Physiological Functions and Polymorphisms of Type II Deiodinase. Endocrinol. Metab. 2023, 38, 190–202. [Google Scholar] [CrossRef]
- de Almeida, G.G.; Bolin, A.; Batistuzzo, A.; Fonseca, T.L.; Ribero, M.O.; Bianco, A.C. Genetic Background Strongly Influences the Impact of Carrying the Thr92Ala-DIO2 Polymorphism in the Male Mouse. Endocrinology 2024, 165, bqae064. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kitagawa, K.; Kitado, H.; Kogishi, K.; Matsushita, T.; Hosokawa, M.; Higuchi, K. Regulation of the Metabolism of Plasma Lipoproteins by Apolipoprotein A-II. Biochim. Biophys. Acta 1997, 1345, 248–258. [Google Scholar] [CrossRef]
- Corella, D.; Peloso, G.; Arnett, D.K.; Demissie, S.; Cupples, L.A.; Tucker, K.; Lai, C.-Q.; Parnell, L.D.; Coltell, O.; Lee, Y.-C.; et al. APOA2, Dietary Fat, and Body Mass Index: Replication of a Gene-Diet Interaction in 3 Independent Populations. Arch. Intern. Med. 2009, 169, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Gardner, C.D.; Parnell, L.; Lai, C.; Ordovás, J. Differential Weight-Loss Responses of APOA2 Genotype Carriers to Low-Carbohydrate and Low-Fat Diets: The DIETFITS Trial. Obesity 2025, 33, 1048–1057. [Google Scholar] [CrossRef]
- Aoyama, T.; Peters, J.; Iritani, N.; Nakajima, T.; Furihata, K.; Hashimoto, T.; Gonzalez, F. Altered Constitutive Expression of Fatty Acid-Metabolizing Enzymes in Mice Lacking the Peroxisome Proliferator-Activated Receptor α (PPARα)*. J. Biol. Chem. 1998, 273, 5678–5684. [Google Scholar] [CrossRef]
- Kersten, S. Integrated Physiology and Systems Biology of PPARα. Mol. Metab. 2014, 3, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska-Skrendo, A.; Buryta, M.; Czarny, W.; Król, P.; Spieszny, M.; Šťastný, P.; Petr, M.; Safranow, K.; Sawczuk, M. The Polymorphisms of the Peroxisome-Proliferator Activated Receptors’ Alfa Gene Modify the Aerobic Training Induced Changes of Cholesterol and Glucose. J. Clin. Med. 2019, 8, 1043. [Google Scholar] [CrossRef]
- Paradis, A.-M.; Fontaine-Bisson, B.; Bossé, Y.; Robitaille, J.; Lemieux, S.; Jacques, H.; Lamarche, B.; Tchernof, A.; Couture, P.; Vohl, M. The Peroxisome Proliferator-Activated Receptor Alpha Leu162Val Polymorphism Influences the Metabolic Response to a Dietary Intervention Altering Fatty Acid Proportions in Healthy Men. Am. J. Clin. Nutr. 2005, 81, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Chen, X.; Liu, L.; Lu, Y.; Gao, M.; Wang, Q.; Chi, X.; Mo, Y. Sex Differences in Ketogenic Diet: Are Men More Likely than Women to Lose Weight? Front. Nutr. 2025, 12, 1600927. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, U. Thyroid and Leptin. Thyroid Off. J. Am. Thyroid Assoc. 2007, 17, 413–419. [Google Scholar] [CrossRef]
- Childs, G.V.; Odle, A.K.; MacNicol, M.C.; MacNicol, A.M. The Importance of Leptin to Reproduction. Endocrinology 2021, 162, bqaa204. [Google Scholar] [CrossRef]
- Chan, J.L.; Mantzoros, C.S. Role of Leptin in Energy-Deprivation States: Normal Human Physiology and Clinical Implications for Hypothalamic Amenorrhoea and Anorexia Nervosa. Lancet 2005, 366, 74–85. [Google Scholar] [CrossRef]
- Loucks, A.B. Energy Availability, Not Body Fatness, Regulates Reproductive Function in Women. Exerc. Sport Sci. Rev. 2003, 31, 144. [Google Scholar] [CrossRef]
- Vasudevan, N.; Ogawa, S.; Pfaff, D. Estrogen and Thyroid Hormone Receptor Interactions: Physiological Flexibility by Molecular Specificity. Physiol. Rev. 2002, 82, 923–944. [Google Scholar] [CrossRef] [PubMed]
- Léan, A.; Ferland, L.; Drouin, J.; Kelly, P.; Labrie, F. Modulation of Pituitary Thyrotropin Releasing Hormone Receptor Levels by Estrogens and Thyroid Hormones. Endocrinology 1977, 100, 1496–1504. [Google Scholar] [CrossRef]
- Zhu, Y.-S.; Yen, P.M.; Chin, W.W.; Pfaff, D.W. Estrogen and Thyroid Hormone Interaction on Regulation of Gene Expression. Proc. Natl. Acad. Sci. USA 1996, 93, 12587–12592. [Google Scholar] [CrossRef] [PubMed]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets with Whey, Vegetable or Animal Protein in Patients with Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, 2039–3949. [Google Scholar] [CrossRef] [PubMed]
- Chapela, S.P.; Simancas-Racines, A.; Ceriani, F.; Martinuzzi, A.L.N.; Russo, M.P.; Zambrano, A.K.; Simancas-Racines, D.; Verde, L.; Muscogiuri, G.; Katsanos, C.S.; et al. Obesity and Obesity-Related Thyroid Dysfunction: Any Potential Role for the Very Low-Calorie Ketogenic Diet (VLCKD)? Curr. Nutr. Rep. 2024, 13, 194–213. [Google Scholar] [CrossRef]
- Baker, F.C.; Siboza, F.; Fuller, A. Temperature Regulation in Women: Effects of the Menstrual Cycle. Temperature 2020, 7, 226–262. [Google Scholar] [CrossRef]
- Magagnini, M.C.; Condorelli, R.; Cimino, L.; Cannarella, R.; Aversa, A.; Calogero, A.; Vignera, S.L. Does the Ketogenic Diet Improve the Quality of Ovarian Function in Obese Women? Nutrients 2022, 14, 4147. [Google Scholar] [CrossRef]
- Sharifi, M.; Saber, A.M.; Moludi, J.; Salimi, Y.; Jahan-Mihan, A. The Effects of Portfolio Moderate-Carbohydrate and Ketogenic Diets on Anthropometric Indices, Metabolic Status, and Hormonal Levels in Overweight or Obese Women with Polycystic Ovary Syndrome: A Randomized Controlled Trial. Nutr. J. 2024, 23, 152. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.M.; Volek, J.S.; D’Agostino, D.P. Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Men. J. Strength Cond. Res. 2020, 34, 3463. [Google Scholar] [CrossRef] [PubMed]
- Mongioì, L.M.; Cimino, L.; Condorelli, R.A.; Magagnini, M.C.; Barbagallo, F.; Cannarella, R.; La Vignera, S.; Calogero, A.E. Effectiveness of a Very Low Calorie Ketogenic Diet on Testicular Function in Overweight/Obese Men. Nutrients 2020, 12, 2967. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.C.; Salas-Lucia, F.; Bianco, A.C. Deiodinases and the Metabolic Code for Thyroid Hormone Action. Endocrinology 2021, 162, bqab059. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Verde, L.; Frias-Toral, E.; Reytor-González, C.; Annunziata, G.; Proganò, M.; Savastano, S.; Simancas-Racines, D.; Colao, A.; Barrea, L. Weight Loss, Changes in Body Composition and Inflammatory Status after a Very Low-Energy Ketogenic Therapy (VLEKT): Does Gender Matter? J. Transl. Med. 2024, 22, 949. [Google Scholar] [CrossRef]
- de Freitas Cayres, L.C.; de Salis, L.V.V.; Rodrigues, G.S.P.; van Helvoort Lengert, A.; Biondi, A.P.C.; Sargentini, L.D.B.; Brisotti, J.L.; Gomes, E.; de Oliveira, G.L.V. Detection of Alterations in the Gut Microbiota and Intestinal Permeability in Patients With Hashimoto Thyroiditis. Front. Immunol. 2021, 12, 579140. [Google Scholar] [CrossRef]
- Christovich, A.; Luo, X. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front. Immunol. 2022, 13, 946248. [Google Scholar] [CrossRef]
- Santangelo, A.; Corsello, A.; Spolidoro, G.; Trovato, C.M.; Agostoni, C.; Orsini, A.; Milani, G.; Peroni, D. The Influence of Ketogenic Diet on Gut Microbiota: Potential Benefits, Risks and Indications. Nutrients 2023, 15, 3680. [Google Scholar] [CrossRef]
- Akansel, M.G.; Baş, M.; Gençalp, C.; Kahrıman, M.; Şahin, E.; Öztürk, H.; Gür, G.; Gür, C. Effects of the Ketogenic Diet on Microbiota Composition and Short-Chain Fatty Acids in Women with Overweight/Obesity. Nutrients 2024, 16, 4374. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Gruszczyński, D.; Zawalna, N.; Nijakowski, K.; Muller, I.; Karpiński, T.; Salvi, M.; Ruchała, M. Microbiota Alterations in Patients with Autoimmune Thyroid Diseases: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13450. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Luu, M.; Weigand, K.; Wedi, F.; Breidenbend, C.; Leister, H.; Pautz, S.; Adhikary, T.; Visekruna, A. Regulation of the Effector Function of CD8+ T Cells by Gut Microbiota-Derived Metabolite Butyrate. Sci. Rep. 2018, 8, 14430. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Meroni, E.; Casiraghi, M.C.; Tagliabue, A.; Giorgis, V.D.; Erba, D. One Month of Classic Therapeutic Ketogenic Diet Decreases Short Chain Fatty Acids Production in Epileptic Patients. Front. Nutr. 2021, 8, 613100. [Google Scholar] [CrossRef]
- Miyamoto, J.; Ohue-Kitano, R.; Mukouyama, H.; Nishida, A.; Watanabe, K.; Igarashi, M.; Irie, J.; Tsujimoto, G.; Satoh-Asahara, N.; Itoh, H.; et al. Ketone Body Receptor GPR43 Regulates Lipid Metabolism under Ketogenic Conditions. Proc. Natl. Acad. Sci. USA 2019, 116, 23813–23821. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; He, C.; Lin, Y.; Ma, L.; Xue, H. IL-9-Producing Th9 Cells Participate in the Occurrence and Development of Iodine-Induced Autoimmune Thyroiditis. Biol. Trace Elem. Res. 2023, 201, 5298–5308. [Google Scholar] [CrossRef]
- Teng, D.; Yang, W.; Shi, X.; Li, Y.; Ba, J.; Chen, B.; Du, J.; He, L.; Lai, X.; Li, Y.; et al. An Inverse Relationship between Iodine Intake and Thyroid Antibodies: A National Cross-Sectional Survey in Mainland China. Thyroid Off. J. Am. Thyroid Assoc. 2020, 30, 1656–1665. [Google Scholar] [CrossRef]
- Wang, B.; He, W.; Li, Q.; Jia, X.; Yao, Q.; Song, R.; Qin, Q.; Zhang, J. U-Shaped Relationship between Iodine Status and Thyroid Autoimmunity Risk in Adults. Eur. J. Endocrinol. 2019, 181, 255–266. [Google Scholar] [CrossRef]
- Masood, W.; Annamaraju, P.; Suheb, M.Z.K.; Uppaluri, K.R. Ketogenic Diet. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Schomburg, L. Selenium, Selenoproteins and the Thyroid Gland: Interactions in Health and Disease. Nat. Rev. Endocrinol. 2012, 8, 160–171. [Google Scholar] [CrossRef]
- Senyushkina, E.S.; Troshina, E. The Role of Zinc in the Synthesis and Metabolism of Thyroid Hormones. Clin. Exp. Thyroidol. 2021, 16, 25–30. [Google Scholar] [CrossRef]
- Shulhai, A.-M.; Rotondo, R.; Petraroli, M.; Patianna, V.; Predieri, B.; Iughetti, L.; Esposito, S.; Street, M.E. The Role of Nutrition on Thyroid Function. Nutrients 2024, 16, 2496. [Google Scholar] [CrossRef]
- Luo, Z.; Huang, Y.; Yong, K.; Wu, D.; Zheng, L.; Yao, X.; Shen, L.; Yu, S.; Wang, B.; Cao, S. Gut Microbiota Regulates Hepatic Ketogenesis and Lipid Accumulation in Ketogenic Diet-Induced Hyperketonemia by Disrupting Bile Acid Metabolism. Gut Microbes 2025, 17, 2496437. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Zhou, X.; Dai, C.; Kong, M.; Xie, L.; Liu, C.; Liu, Y.; Li, D.; Ma, X.; et al. Ketogenic Diet-Induced Bile Acids Protect against Obesity through Reduced Calorie Absorption. Nat. Metab. 2024, 6, 1397–1414. [Google Scholar] [CrossRef]
- Kose, E.; Guzel, O.; Demir, K.; Arslan, N. Changes of Thyroid Hormonal Status in Patients Receiving Ketogenic Diet Due to Intractable Epilepsy. J. Pediatr. Endocrinol. Metab. 2017, 30, 411–416. [Google Scholar] [CrossRef]
- Docter, R.; Krenning, E.; Jong, M.; Hennemann, G. The Sick Euthyroid Syndrome: Changes in Thyroid Hormone Serum Parameters and Hormone Metabolism. Clin. Endocrinol. 1993, 39, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hong, Y.; Wang, Z.; Li, Y. Analysis of the Incidence of Euthyroid Sick Syndrome in Comprehensive Intensive Care Units and Related Risk Factors. Front. Endocrinol. 2021, 12, 656641. [Google Scholar] [CrossRef]
- Panicker, V.; Saravanan, P.; Vaidya, B.; Evans, J.; Hattersley, A.T.; Frayling, T.M.; Dayan, C.M. Common Variation in the DIO2 Gene Predicts Baseline Psychological Well-Being and Response to Combination Thyroxine Plus Triiodothyronine Therapy in Hypothyroid Patients. J. Clin. Endocrinol. Metab. 2009, 94, 1623–1629. [Google Scholar] [CrossRef]
- Wiersinga, W.M. Paradigm Shifts in Thyroid Hormone Replacement Therapies for Hypothyroidism. Nat. Rev. Endocrinol. 2014, 10, 164–174. [Google Scholar] [CrossRef]
- Elmalky, H.A.; Zewain, S.K.E.-D.; Korany, M.A.E.R.; El-Hefnawy, S.M.I.; Hamed, M.B. Study of DIO2 Thr92Ala Genetic Polymorphism (Rs225014) in Hypothyroid Patients Who Achieved Biochemical Euthyroidism. Zagazig Univ. Med. J. 2025, 31, 1354–1363. [Google Scholar] [CrossRef]
- Midgley, J.E.M.; Toft, A.D.; Larisch, R.; Dietrich, J.W.; Hoermann, R. Time for a Reassessment of the Treatment of Hypothyroidism. BMC Endocr. Disord. 2019, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Juby, A.; Hanly, M.; Lukaczer, D. Clinical Challenges in Thyroid Disease: Time for a New Approach? Maturitas 2016, 87, 72–78. [Google Scholar] [CrossRef]
- Szewczykowski, M.; Dorobek, M.; Otręba, E.; Klinkosz, T.; Walukiewicz, A.; Ogończyk-Mąkowski, F. Supplementation of Iron, Selenium, Zinc and Iodine in Hypothyroidism. Arch. Euromedica 2025, 12, 867–878. [Google Scholar] [CrossRef]
- Miller, V.J.; LaFountain, R.A.; Barnhart, E.C.; Sapper, T.S.; Short, J.A.; Arnold, W.D.; Hyde, P.N.; Crabtree, C.D.; Kackley, M.L.; Kraemer, W.; et al. A Ketogenic Diet Combined with Exercise Alters Mitochondrial Function in Human Skeletal Muscle While Improving Metabolic Health. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E995–E1007. [Google Scholar] [CrossRef]
- Yılmaz, Ü.; Nalbantoğlu, Ö.; Güzin, Y.; Edizer, S.; Akışın, Z.; Pekuz, S.; Kırkgöz, H.H.; Yavuz, M.; Ünalp, A.; Özkan, B. The Effect of Ketogenic Diet on Thyroid Functions in Children with Drug-Resistant Epilepsy. Neurol. Sci. 2021, 42, 5261–5269. [Google Scholar] [CrossRef]
- Roti, E.; Minelli, R.; Salvi, M. Thyroid Hormone Metabolism in Obesity. Int. J. Obes. 2000, 24, S113–S115. [Google Scholar] [CrossRef] [PubMed]
- Burman, K.D.; Baker, J.R. Immune Mechanisms in Graves’ Disease. Endocr. Rev. 1985, 6, 183–232. [Google Scholar] [CrossRef]
- Diana, T.; Daiber, A.; Oelze, M.; Neumann, S.; Olivo, P.D.; Kanitz, M.; Stamm, P.; Kahaly, G. Stimulatory TSH-Receptor Antibodies and Oxidative Stress in Graves Disease. J. Clin. Endocrinol. Metab. 2018, 103, 509. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid® 2021, 31, 337–386. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Yuan, Z.; Barletta, J.A.; Lorch, J.H.; Nehs, M.A. Ketogenic Diet Combined with Antioxidant N-Acetylcysteine Inhibits Tumor Growth in a Mouse Model of Anaplastic Thyroid Cancer. Surgery 2020, 167, 87–93. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Kim, D.Y.; Hao, J.; Liu, R.; Turner, G.; Shi, F.-D.; Rho, J.M. Inflammation-Mediated Memory Dysfunction and Effects of a Ketogenic Diet in a Murine Model of Multiple Sclerosis. PLoS ONE 2012, 7, e35476. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Milder, J.B.; Liang, L.-P.; Patel, M. The Ketogenic Diet Increases Mitochondrial Glutathione Levels. J. Neurochem. 2008, 106, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, Ketogenic Diet and Food Intake Control: A Complex Relationship. Front. Psychol. 2015, 6, 27. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of Intermittent Fasting on Health and Disease Processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Virili, C.; Antonelli, A.; Santaguida, M.G.; Benvenga, S.; Centanni, M. Gastrointestinal Malabsorption of Thyroxine. Endocr. Rev. 2019, 40, 118–136. [Google Scholar] [CrossRef]
- Churuangsuk, C.; Kherouf, M.; Combet, E.; Lean, M. Low-Carbohydrate Diets for Overweight and Obesity: A Systematic Review of the Systematic Reviews. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018, 19, 1700–1718. [Google Scholar] [CrossRef]
- Paoli, A.; Bianco, A.; Grimaldi, K.A. The Ketogenic Diet and Sport: A Possible Marriage? Exerc. Sport Sci. Rev. 2015, 43, 153–162. [Google Scholar] [CrossRef]
- Fekete, C.; Lechan, R.M. Central Regulation of Hypothalamic-Pituitary-Thyroid Axis under Physiological and Pathophysiological Conditions. Endocr. Rev. 2014, 35, 159–194. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Thuma, J.R. Luteinizing Hormone Pulsatility Is Disrupted at a Threshold of Energy Availability in Regularly Menstruating Women. J. Clin. Endocrinol. Metab. 2003, 88, 20369. [Google Scholar] [CrossRef] [PubMed]

| Mechanism/Pathway | Key Mediators | Thyroid Effect | Clinical Implication |
|---|---|---|---|
| Reduced glucose availability | ↓ Insulin, ↓ Leptin | ↓ DIO2 activity, ↓ T3 | Lower peripheral T3, especially in LT4 monotherapy patients |
| Elevated ketone bodies | ↑ BHB | ↓ NLRP3, ↑ Tregs, HDAC inhibition | Reduced inflammation, possible autoimmunity modulation |
| Cortisol elevation (early KD) | ↑ Cortisol | ↑ rT3, ↓ T3 receptor activation | Functional hypothyroidism; fatigue, low body temperature |
| SCFA depletion (low fiber) | ↓ Butyrate, Acetate, Propionate | ↓ DIO2, ↑ permeability | Gut–thyroid axis disruption, impaired T4-to-T3 conversion |
| Bile acid dysregulation | Altered secondary bile acids | ↓ Hepatic deiodinase activity | Reduced hormone clearance, disrupted liver feedback loop |
| Iodine and selenium intake variability | ↓ Iodine, ↓ Selenium, ↓ Zinc | ↓ T4 synthesis, ↓ antioxidant enzymes | Hypothyroidism risk, immune activation in Hashimoto’s |
| Leptin suppression | ↓ Leptin | ↓ TRH, ↓ TSH, ↓ GnRH | Central suppression; amenorrhea or thyroid downregulation |
| Energy conservation adaptation | ↑ AMPK, ↑ SIRT1 | ↓ DIO1/2, ↑ mitochondrial efficiency | “Low T3 syndrome”; masked hypothyroidism despite normal TSH |
| Histone modification | HDAC inhibition by BHB | ↑ Treg differentiation, ↓ IL-6/TNF-α | Immunomodulation; reduced thyroid antibody titers in some cases |
| Genetic predisposition | DIO2 Thr92Ala, PPARα, APOA2 | Altered T3 conversion, lipid metabolism | Personalized response to KD; some genotypes requires T3 support |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vranjić, P.; Vuković, M.; Blažetić, S.; Viljetić, B. Ketogenic Diet and Thyroid Function: A Delicate Metabolic Balancing Act. Curr. Issues Mol. Biol. 2025, 47, 696. https://doi.org/10.3390/cimb47090696
Vranjić P, Vuković M, Blažetić S, Viljetić B. Ketogenic Diet and Thyroid Function: A Delicate Metabolic Balancing Act. Current Issues in Molecular Biology. 2025; 47(9):696. https://doi.org/10.3390/cimb47090696
Chicago/Turabian StyleVranjić, Petar, Mladen Vuković, Senka Blažetić, and Barbara Viljetić. 2025. "Ketogenic Diet and Thyroid Function: A Delicate Metabolic Balancing Act" Current Issues in Molecular Biology 47, no. 9: 696. https://doi.org/10.3390/cimb47090696
APA StyleVranjić, P., Vuković, M., Blažetić, S., & Viljetić, B. (2025). Ketogenic Diet and Thyroid Function: A Delicate Metabolic Balancing Act. Current Issues in Molecular Biology, 47(9), 696. https://doi.org/10.3390/cimb47090696






