Hydroxytyrosol and Brain Tumors: Mechanisms of Action and Therapeutic Potential
Abstract
1. Introduction
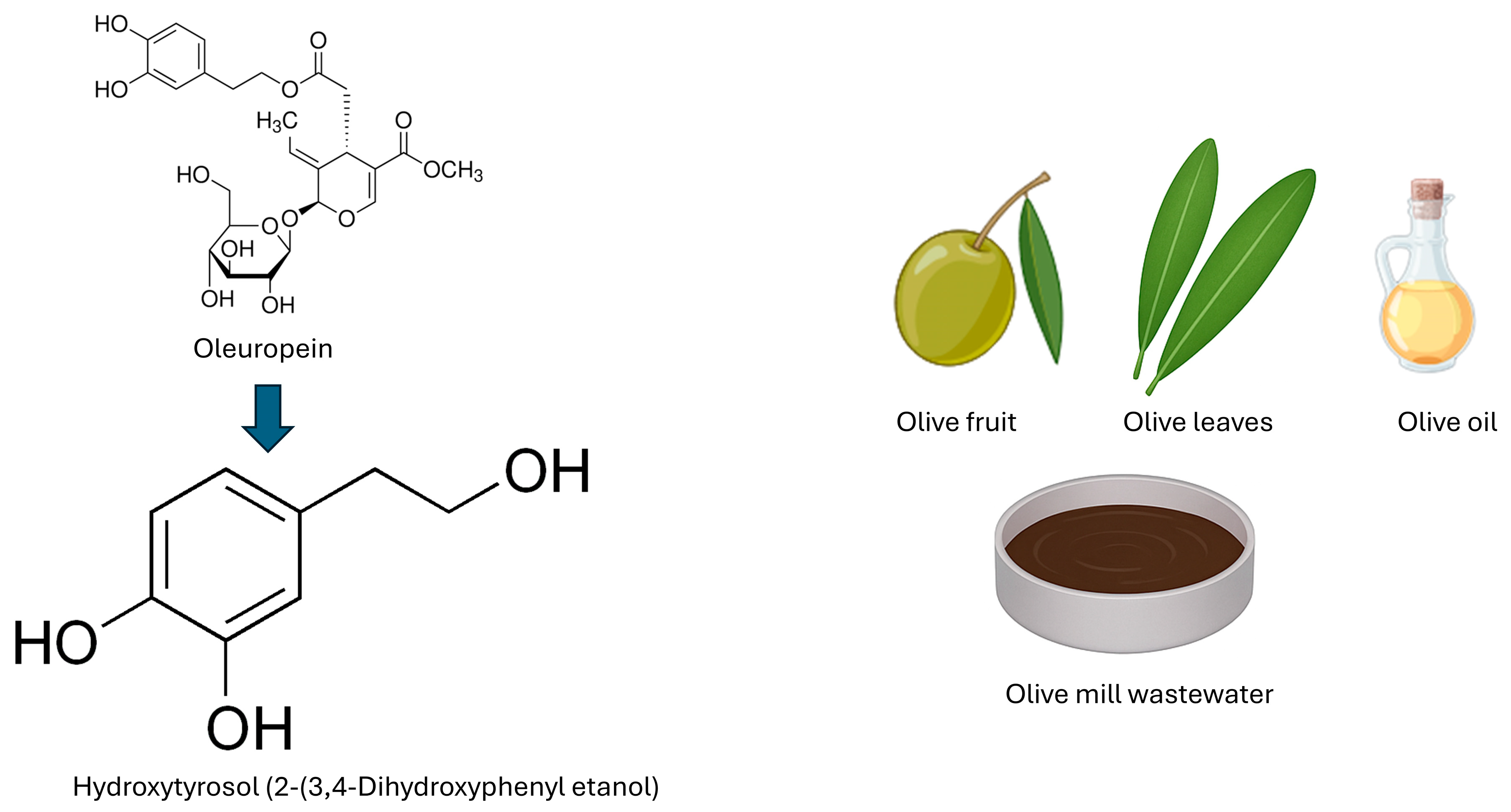
2. Chemical Characteristics of HTX
2.1. Synthesis and Metabolism of HTX
2.2. Bioavailability of Hydroxytyrosol
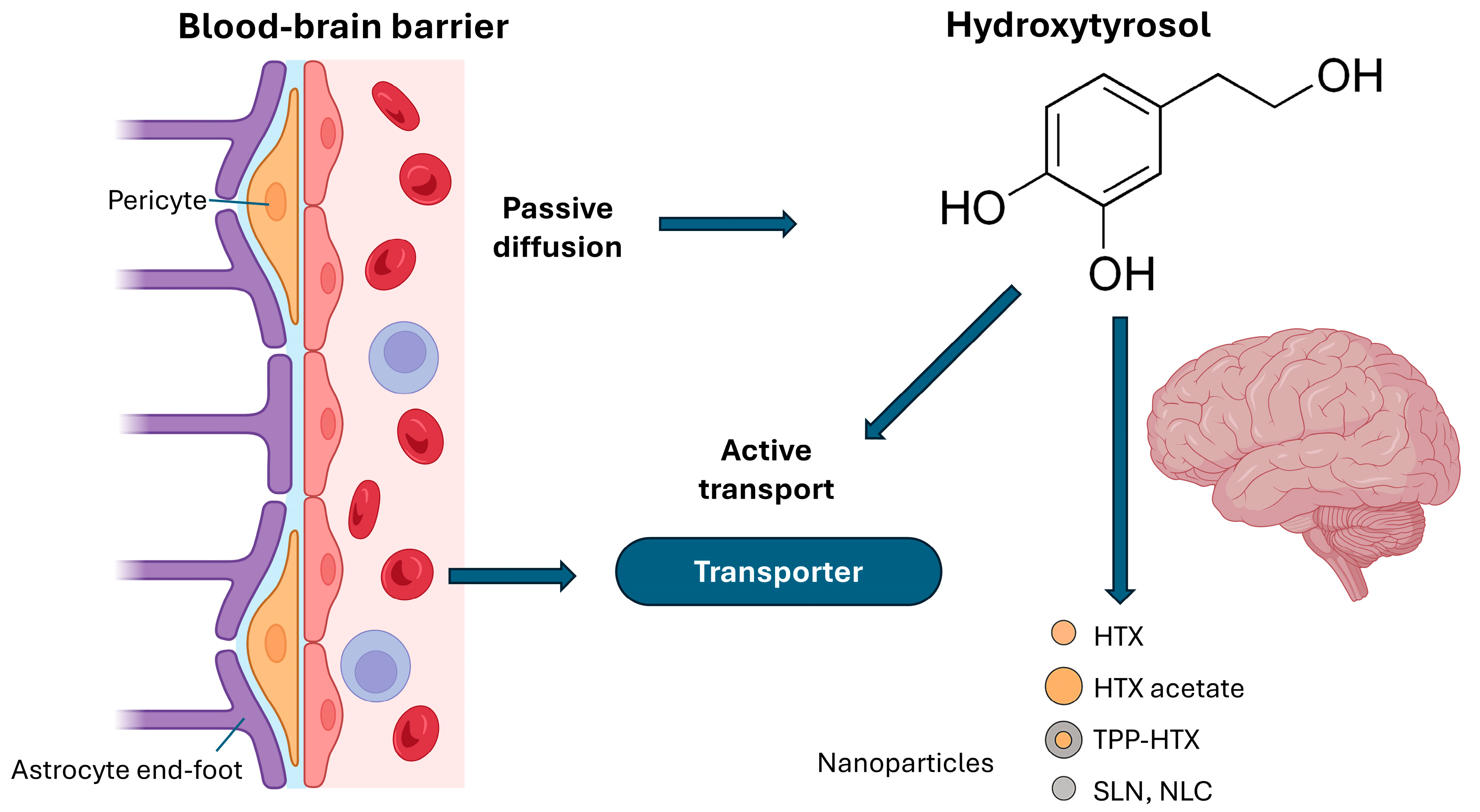
2.3. Hydroxytyrosol and the Blood–Brain Barrier
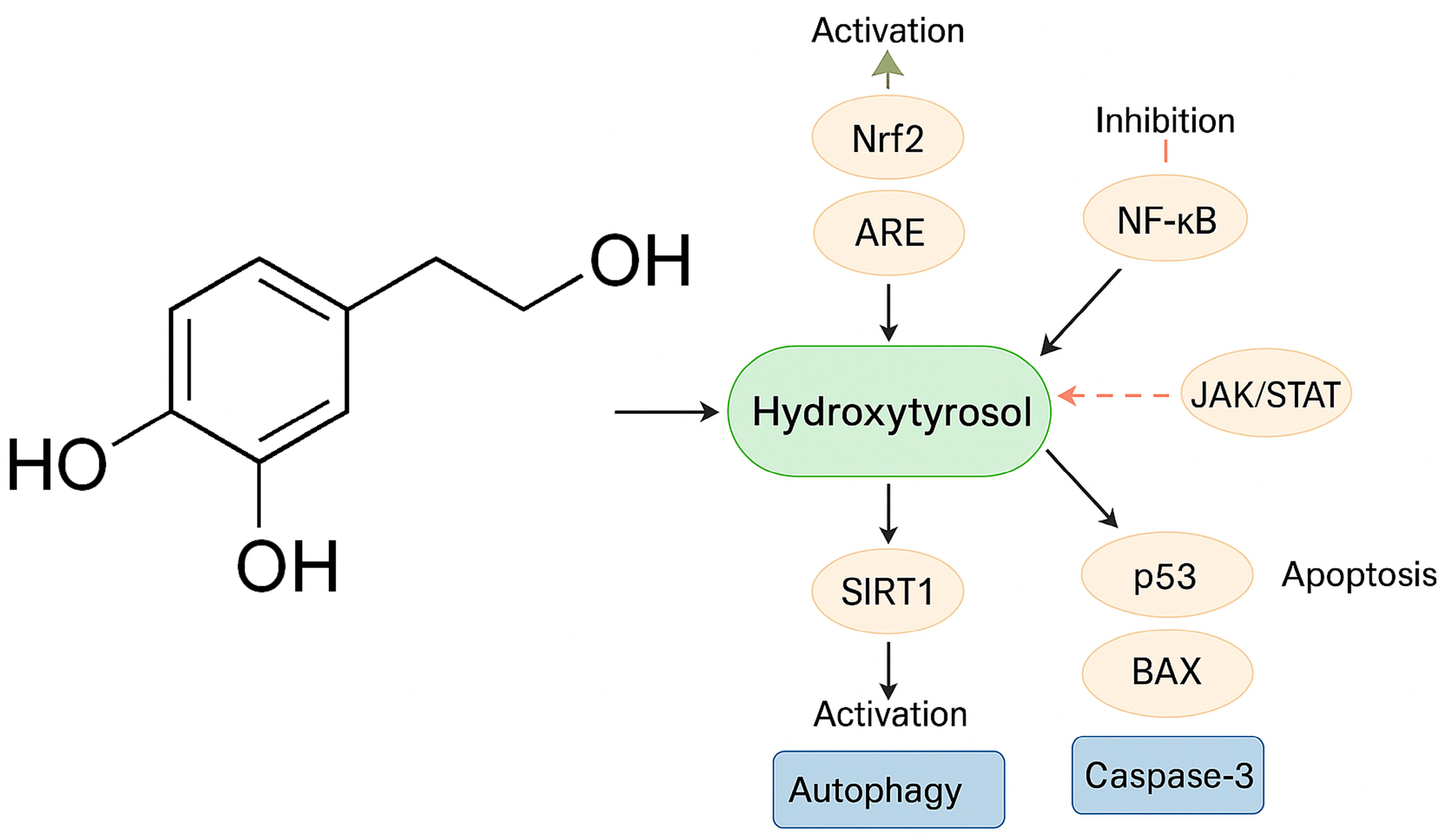
3. Mechanisms of Action of HTX
3.1. Antioxidant Properties and Modulation of Oxidative Stress
Signaling Pathways
3.2. Anti-Inflammatory Properties
3.3. Induction of Apoptosis and Antiproliferative Effects
3.4. Autophagy
3.5. Stress Response
3.6. Epigenetic Effects
3.7. Other Signaling Pathways
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| APP | Amyloid precursor protein |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BMI1 | B lymphoma Mo-MLV insertion region 1 homolog |
| bRAS | Brain renin–angiotensin system |
| CAT | Catalase |
| CD | Cluster of Differentiation |
| CNS | Central nervous system |
| CREB | cAMP response element-binding protein |
| ENU | N-nitroso-N-ethylurea |
| ER | Estrogen receptor |
| EVOO | Extra virgin olive oil |
| EZH2 | Enhancer of zeste homolog 2 |
| GCLC | Glutamate cysteine ligase |
| GPRC5A | G protein-coupled receptor class C group 5 member A |
| GPX | Glutathione peroxidase |
| GSCs | Glioma stem cells |
| GSH | Glutathione |
| GST | Glutathione S-transferase |
| HO-1 | Heme oxygenase-1 |
| HSF1 | Heat shock factor 1 |
| HTX | Hydroxytyrosol |
| IDH | Isocitrate dehydrogenase |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| LPS | Lipopolysaccharide |
| LSD1 | Histone demethylase 1 |
| MES | Mesenchymal |
| NLC | Nanostructured lipid carrier |
| NPC | Neural progenitor cell |
| NQO1 | NAD(P)H:quinone oxidoreductase 1 (NQO1) |
| NF-κB | Nuclear Factor Kappa B |
| Nrf2/ARE | Nuclear factor erythroid 2-related factor 2/antioxidant response element |
| OLE | Oleuropein |
| OLIG2 | Oligodendrocyte transcription factor 2 |
| OPC | Oligodendrocyte progenitor cell |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SIRT1 | Sirtuin 1 |
| SLN | Solid lipid nanoparticle |
| SOD | Superoxide dismutase |
| SOX2 | SRY-box transcription factor 2 |
| TAC | Total antioxidant capacity |
| TPP | Triphenylphosphine |
| TREM2 | Triggering Receptor Expressed on Myeloid cells 2 |
| Trx | Thioredoxin |
| TWIST1 | Twist family BHLH transcription factor 1 |
| VOO | Virgin olive oil |
| YKL40 | Chitinase-3-like protein 1 |
| γ-GCS | γ-glutamylcysteine synthetase |
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, P. Neurooncology: 2021 update. Free Neuropathol. 2021, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Bihn, J.R.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Neff, C.; Price, M.; Ostrom, Q.T.; Swinnerton, K.N.; Elbers, D.C.; Mooney, M.A.; et al. Brain tumors in United States military veterans. Neuro Oncol. 2024, 26, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.J.; Bauchet, L.; McKean-Cowdin, R.; Kruchko, C.; Lau, C.C.; Ostrom, Q.T.; Scheurer, M.E.; Villano, J.; Yuan, Y. Impact of environment on pediatric and adult brain tumors: The 2023 Brain Tumor Epidemiology Consortium meeting report. Clin. Neuropathol. 2024, 43, 29–35. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Francis, S.S.; Barnholtz-Sloan, J.S. Epidemiology of Brain and Other CNS Tumors. Curr. Neurol. Neurosci. Rep. 2021, 21, 68. [Google Scholar] [CrossRef]
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro Oncol. 2024, 26, vi1–vi85. [Google Scholar] [CrossRef]
- Price, M.; Neff, C.; Nagarajan, N.; Kruchko, C.; Waite, K.A.; Cioffi, G.; Cordeiro, B.B.; Willmarth, N.; Penas-Prado, M.; Gilbert, M.R.; et al. CBTRUS Statistical Report: American Brain Tumor Association & NCI Neuro-Oncology Branch Adolescent and Young Adult Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro Oncol. 2024, 26, iii1–iii53. [Google Scholar] [CrossRef]
- Thomas, G.; Rahman, R. Evolution of Preclinical Models for Glioblastoma Modelling and Drug Screening. Curr. Oncol. Rep. 2025, 27, 601–624. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Kaddoumi, A. Extra-Virgin Olive Oil in Alzheimer’s Disease: A Comprehensive Review of Cellular, Animal, and Clinical Studies. Int. J. Mol. Sci. 2024, 25, 1914. [Google Scholar] [CrossRef]
- Lee, B.; Hwang, S.; Bae, H.; Choi, K.H.; Suh, Y.L. Diagnostic utility of genetic alterations in distinguishing IDH-wildtype glioblastoma from lower-grade gliomas: Insight from next-generation sequencing analysis of 479 cases. Brain Pathol. 2024, 34, e13234. [Google Scholar] [CrossRef]
- Priesterbach-Ackley, L.P.; Cordier, F.; de Witt Hamer, P.; Snijders, T.J.; Robe, P.A.; Kusters, B.; de Leng, W.W.J.; den Dunnen, W.F.A.; Brandsma, D.; Jansen, C.; et al. Diffuse, IDH-wildtype gliomas in adults with minimal histological change and isolated TERT promoter mutation: Not simply CNS WHO grade 4. Acta Neuropathol. 2024, 148, 12. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.M.; Znaor, A.; Sunguc, C.; Zahwe, M.; Marcos-Gragera, R.; Figueroa, J.D.; Bray, F. Cancers of the brain and central nervous system: Global patterns and trends in incidence. J. Neurooncol. 2025, 172, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Sociedad Española de Oncología Médica. Las Cifras Del Cáncer en España 2025. Available online: https://seom.org/prensa/el-cancer-en-cifras (accessed on 30 June 2025).
- Bauchet, L.; Rigau, V.; Mathon, B.; Darlix, A.; Société Française de Neurochirurgie; Club de Neuro-oncologie of SFNC; Société Française de Neuropathologie; Association des Neuro-Oncologues d’Expression Française. Epidemiological analysis of adult-type diffuse lower-grade gliomas and incidence and prevalence estimates of diffuse IDH-mutant gliomas in France. Neurochirurgie 2025, 71, 101627. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, O.; Moiraghi, A.; Elia, A.; Tauziede-Espariat, A.; Roux, A.; Leclerc, A.; Planet, M.; Bedioui, A.; Simboli, G.A.; Dhermain, F.; et al. Long-term survivors in 976 supratentorial glioblastoma, IDH-wildtype patients. J. Neurosurg. 2025, 142, 174–186. [Google Scholar] [CrossRef]
- Goethe, E.A.; Deneen, B.; Noebels, J.; Rao, G. The Role of Hyperexcitability in Gliomagenesis. Int. J. Mol. Sci. 2023, 24, 749. [Google Scholar] [CrossRef]
- Huang, Q.; Lian, C.; Dong, Y.; Zeng, H.; Liu, B.; Xu, N.; He, Z.; Guo, H. SNAP25 Inhibits Glioma Progression by Regulating Synapse Plasticity via GLS-Mediated Glutaminolysis. Front. Oncol. 2021, 11, 698835. [Google Scholar] [CrossRef]
- Kumaria, A.; Ashkan, K. Novel therapeutic strategies in glioma targeting glutamatergic neurotransmission. Brain Res. 2023, 1818, 148515. [Google Scholar] [CrossRef]
- Rodriguez-Mendoza, B.; Figueroa-Gonzalez, A.; Cano-Herrera, G.; Gutierrez-Rosas, L.E.; Romero-Torres, C.I.; Victoria-Garcia, L.O.; Gonzalez-Castillo, P.; Guerrero-Cazares, H.; Ibarra, A. Glioblastoma and its interaction with neurogenesis. Rev. Neurol. 2024, 79, 279–287. [Google Scholar] [CrossRef]
- Srivastava, S.; Anbiaee, R.; Houshyari, M.; Laxmi; Sridhar, S.B.; Ashique, S.; Hussain, S.; Kumar, S.; Taj, T.; Akbarnejad, Z.; et al. Amino acid metabolism in glioblastoma pathogenesis, immune evasion, and treatment resistance. Cancer Cell Int. 2025, 25, 89. [Google Scholar] [CrossRef]
- Walbert, T.; Avila, E.K.; Boele, F.W.; Hertler, C.; Lu-Emerson, C.; van der Meer, P.B.; Peters, K.B.; Rooney, A.G.; Templer, J.W.; Koekkoek, J.A.F. Symptom management in isocitrate dehydrogenase mutant glioma. Neurooncol. Pract. 2025, 12, i38–i48. [Google Scholar] [CrossRef]
- Chen, W.; Wu, D.; Chen, X. Drug adverse events associated with temozolomide administration: A real-world pharmacovigilance study using the FAERS database from 2014 to 2024. J. Oncol. Pharm. Pract. 2025, 10781552251350618. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, Y.; Chen, Y.; Lv, J.; Qu, C.; Mei, T.; Zheng, Y.; Ye, C.; Li, F.; Ge, S.; et al. Overcoming temozolomide resistance in glioma: Recent advances and mechanistic insights. Acta Neuropathol. Commun. 2025, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Yuan, K.; Tao, J.; Qin, Y.; Li, Y.; Fu, J.; Li, Z.; Zhou, H.; Tang, Z.; Li, L.; et al. Glioblastoma multiforme: An updated overview of temozolomide resistance mechanisms and strategies to overcome resistance. Discov. Oncol. 2025, 16, 731. [Google Scholar] [CrossRef]
- Inggas, M.A.M.; Patel, U.; Wijaya, J.H.; Otinashvili, N.; Menon, V.R.; Iyer, A.K.; Turjman, T.; Dadwal, S.; Gadaevi, M.; Ismayilova, A.; et al. The role of temozolomide as adjuvant therapy in glioblastoma management: A systematic review and meta-analysis. BMC Cancer 2025, 25, 399. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Vorasidenib: First Approval. Drugs 2024, 84, 1325–1331. [Google Scholar] [CrossRef]
- Touat, M. INDIGO and Beyond: Approaching Vorasidenib With Cautious Optimism. Int. J. Radiat. Oncol. Biol. Phys. 2025, 122, 222–223. [Google Scholar] [CrossRef]
- Silvani, A.; Franceschi, E. A new era after a long wait: Vorasidenib, an inhibitor of mutant IDH1 and IDH2 enzymes in patients with IDH-mutant glioma. Tumori 2024, 110, 160–161. [Google Scholar] [CrossRef]
- Kresbach, C.; Neyazi, S.; Schuller, U. Updates in the classification of ependymal neoplasms: The 2021 WHO Classification and beyond. Brain Pathol. 2022, 32, e13068. [Google Scholar] [CrossRef]
- Choi, J.Y. Medulloblastoma: Current Perspectives and Recent Advances. Brain Tumor Res. Treat. 2023, 11, 28–38. [Google Scholar] [CrossRef]
- Pan, Z.; Bao, J.; Wei, S. Advancing medulloblastoma therapy: Strategies and survival insights. Clin. Exp. Med. 2025, 25, 119. [Google Scholar] [CrossRef]
- McLaughlin, M.P.; Marcus, R.B., Jr.; Buatti, J.M.; McCollough, W.M.; Mickle, J.P.; Kedar, A.; Maria, B.L.; Million, R.R. Ependymoma: Results, prognostic factors and treatment recommendations. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Vera-Bolanos, E.; Aldape, K.; Yuan, Y.; Wu, J.; Wani, K.; Necesito-Reyes, M.J.; Colman, H.; Dhall, G.; Lieberman, F.S.; Metellus, P.; et al. Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro Oncol. 2015, 17, 440–447. [Google Scholar] [CrossRef]
- Alboqami, M.N.; Khalid, S.A.A.; Bukhari, B.H.; Alkhaibary, A.; Alharbi, A.; Khairy, S.; Alassiri, A.H.; AlSufiani, F.; Alkhani, A.; Aloraidi, A. Craniopharyngioma: A comprehensive review of the clinical presentation, radiological findings, management, and future Perspective. Heliyon 2024, 10, e32112. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Liu, Z.; Yan, O.; He, W.; Jie, D.; Qie, Y.; Xu, J. Nomograms for Predicting Overall Survival Among Patients with Craniopharyngiomas at Initial Diagnosis: A SEER Population-Based Analysis. Int. J. Gen. Med. 2021, 14, 3517–3527. [Google Scholar] [CrossRef] [PubMed]
- Zacharia, B.E.; Bruce, S.S.; Goldstein, H.; Malone, H.R.; Neugut, A.I.; Bruce, J.N. Incidence, treatment and survival of patients with craniopharyngioma in the surveillance, epidemiology and end results program. Neuro Oncol. 2012, 14, 1070–1078. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Du, H.Z.; Lu, T.T.; Song, S.H.; Xu, R.; Jiang, Y.; Pan, H. Long-term outcome of childhood and adolescent patients with craniopharyngiomas: A single center retrospective experience. BMC Cancer 2024, 24, 1555. [Google Scholar] [CrossRef]
- Sin-Chan, P.; Li, B.K.; Ho, B.; Fonseca, A.; Huang, A. Molecular Classification and Management of Rare Pediatric Embryonal Brain Tumors. Curr. Oncol. Rep. 2018, 20, 69. [Google Scholar] [CrossRef]
- Carceller, F. Long-term survivors of diffuse intrinsic pontine glioma (DIPG): Myth or reality. Transl. Cancer Res. 2019, 8, 343–345. [Google Scholar] [CrossRef]
- Hoffman, L.M.; Veldhuijzen van Zanten, S.E.M.; Colditz, N.; Baugh, J.; Chaney, B.; Hoffmann, M.; Lane, A.; Fuller, C.; Miles, L.; Hawkins, C.; et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018, 36, 1963–1972. [Google Scholar] [CrossRef]
- Stock, A.; Brackmann, H.; Warmuth-Metz, M.; Miller, E.; Eyrich, M.; Kramm, C.M.; Schlegel, P.G.; Wiegering, V.A. DIPG-Very Long-Term Survivors—Are There Factors Which May Predict a Better Outcome? Klin. Padiatr. 2022, 234, 391–394. [Google Scholar] [CrossRef]
- Roschewski, M.; Hodson, D.J. Diffuse large B-cell lymphoma involving the central nervous system: Biologic rationale for targeted therapy. Haematologica 2024, 109, 388–400. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, D.; Zhu, X.; Zhang, Y.; Xiao, Y. Updates of primary central nervous system lymphoma. Ther. Adv. Hematol. 2024, 15, 20406207241259010. [Google Scholar] [CrossRef]
- Carrera-González, M.P.; Ramírez-Expósito, M.J.; Mayas, M.D.; Martínez-Martos, J.M. Protective role of oleuropein and its metabolite hydroxytyrosol on cancer. Trends Food Sci. Technol. 2013, 31, 92–99. [Google Scholar] [CrossRef]
- Ramirez-Exposito, M.J.; Martinez-Martos, J.M. The Delicate Equilibrium between Oxidants and Antioxidants in Brain Glioma. Curr. Neuropharmacol. 2019, 17, 342–351. [Google Scholar] [CrossRef]
- Hernandez-Garcia, S.; Garcia-Cano, B.; Martinez-Rodriguez, P.; Henarejos-Escudero, P.; Gandia-Herrero, F. Olive oil tyrosols reduce alpha-synuclein aggregation in vitro and in vivo after ingestion in a Caenorhabditis elegans Parkinson’s model. Food Funct. 2024, 15, 7214–7223. [Google Scholar] [CrossRef]
- Panara, A.; Biliraki, D.; Nussbaumer, M.; Filiou, M.D.; Thomaidis, N.S.; Kostakis, I.K.; Gikas, E. Liquid Chromatography-Tandem Mass Spectrometry Method Development and Validation for the Determination of a New Mitochondrial Antioxidant in Mouse Liver and Cerebellum, Employing Advanced Chemometrics. Molecules 2025, 30, 1900. [Google Scholar] [CrossRef]
- Leri, M.; Sun, D.; Svedruzic, Z.M.; Sulskis, D.; Smirnovas, V.; Stefani, M.; Morozova-Roche, L.; Bucciantini, M. Pro-inflammatory protein S100A9 targeted by a natural molecule to prevent neurodegeneration onset. Int. J. Biol. Macromol. 2024, 276, 133838. [Google Scholar] [CrossRef]
- Najafi, N.; Rezaee, R.; Hayes, A.W.; Karimi, G. A review of mechanisms underlying the protective effects of natural compounds against arsenic-induced neurotoxicity. Biometals 2023, 36, 799–813. [Google Scholar] [CrossRef]
- Micheli, L.; Bertini, L.; Bonato, A.; Villanova, N.; Caruso, C.; Caruso, M.; Bernini, R.; Tirone, F. Role of Hydroxytyrosol and Oleuropein in the Prevention of Aging and Related Disorders: Focus on Neurodegeneration, Skeletal Muscle Dysfunction and Gut Microbiota. Nutrients 2023, 15, 1767. [Google Scholar] [CrossRef]
- Barca, C.; Wiesmann, M.; Calahorra, J.; Wachsmuth, L.; Doring, C.; Foray, C.; Heiradi, A.; Hermann, S.; Peinado, M.A.; Siles, E.; et al. Impact of hydroxytyrosol on stroke: Tracking therapy response on neuroinflammation and cerebrovascular parameters using PET-MR imaging and on functional outcomes. Theranostics 2021, 11, 4030–4049. [Google Scholar] [CrossRef]
- Chen, S.; Cai, T.; Lu, J.; Le, J.; Zhang, J.; Yao, Q.; Chen, L. Hydroxytyrosol promotes random skin flap survival by activating SIRT1-mediated enhancement of autophagy. J. Funct. Foods 2024, 121, 106443. [Google Scholar] [CrossRef]
- Li, S.; Shao, H.; Sun, T.; Guo, X.; Zhang, X.; Zeng, Q.; Fang, S.; Liu, X.; Wang, F.; Liu, F.; et al. Anti-neuroinflammatory effect of hydroxytyrosol: A potential strategy for anti-depressant development. Front. Pharmacol. 2024, 15, 1366683. [Google Scholar] [CrossRef] [PubMed]
- Perez-Barron, G.; Montes, S.; Aguirre-Vidal, Y.; Santiago, M.; Gallardo, E.; Espartero, J.L.; Rios, C.; Monroy-Noyola, A. Antioxidant Effect of Hydroxytyrosol, Hydroxytyrosol Acetate and Nitrohydroxytyrosol in a Rat MPP+ Model of Parkinson’s Disease. Neurochem. Res. 2021, 46, 2923–2935. [Google Scholar] [CrossRef] [PubMed]
- Romaus-Sanjurjo, D.; Castanon-Apilanez, M.; Lopez-Arias, E.; Custodia, A.; Martin-Martin, C.; Ouro, A.; Lopez-Cancio, E.; Sobrino, T. Neuroprotection Afforded by an Enriched Mediterranean-like Diet Is Modified by Exercise in a Rat Male Model of Cerebral Ischemia. Antioxidants 2024, 13, 138. [Google Scholar] [CrossRef]
- Papadopoulou, P.; Polissidis, A.; Kythreoti, G.; Sagnou, M.; Stefanatou, A.; Theoharides, T.C. Anti-Inflammatory and Neuroprotective Polyphenols Derived from the European Olive Tree, Olea europaea L., in Long COVID and Other Conditions Involving Cognitive Impairment. Int. J. Mol. Sci. 2024, 25, 11040. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Zheng, M.; Xi, X.; Zhang, X.; Han, C. Structure Properties, Acquisition Protocols, and Biological Activities of Oleuropein Aglycone. Front. Chem. 2018, 6, 239. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertolini, A.; Silva, A.M.; Rodrigues, F.; Gabbia, D.; De Martin, S.; Saba, A.; Bertini, S.; Digiacomo, M.; Macchia, M. Comparative Analysis on Polyphenolic Composition of Different Olive Mill Wastewater and Related Extra Virgin Olive Oil Extracts and Evaluation of Nutraceutical Properties by Cell-Based Studies. Foods 2024, 13, 3312. [Google Scholar] [CrossRef]
- Gea-Gonzalez, A.; Hernandez-Garcia, S.; Henarejos-Escudero, P.; Martinez-Rodriguez, P.; Garcia-Carmona, F.; Gandia-Herrero, F. Polyphenols from traditional Chinese medicine and Mediterranean diet are effective against Abeta toxicity in vitro and in vivo in Caenorhabditis elegans. Food Funct. 2022, 13, 1206–1217. [Google Scholar] [CrossRef]
- Avila-Galvez, M.A.; Garay-Mayol, B.; Marin, A.; Brito, M.A.; Gimenez-Bastida, J.A.; Espin, J.C.; Gonzalez-Sarrias, A. Metabolic Profiling of a Mediterranean-Inspired (Poly) phenol-Rich Mixture in the Brain: Perfusion Effect and In Vitro Blood-Brain Barrier Transport Validation. J. Agric. Food Chem. 2025, 73, 11056–11066. [Google Scholar] [CrossRef]
- Ramirez-Exposito, M.J.; Carrera-Gonzalez, M.P.; Mayas, M.D.; Martinez-Martos, J.M. Gender differences in the antioxidant response of oral administration of hydroxytyrosol and oleuropein against N-ethyl-N-nitrosourea (ENU)-induced glioma. Food Res. Int. 2021, 140, 110023. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Vasarri, M.; Carnemolla, F.; Oriente, F.; Cabaro, S.; Stio, M.; Degl’Innocenti, D.; Stefani, M.; Bucciantini, M. EVOO Polyphenols Exert Anti-Inflammatory Effects on the Microglia Cell through TREM2 Signaling Pathway. Pharmaceuticals 2023, 16, 933. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Chamorro, L.; Fernandez-Prior, A.; Claro-Cala, C.M.; Del Rio-Vazquez, J.L.; Rivero-Pino, F.; la Paz, S.M.-D. Unveiling the neuroprotective impact of virgin olive oil ingestion via the microbiota-gut-brain axis. Food Funct. 2025, 16, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Jinsmaa, Y.; Sullivan, P.; Holmes, C.; Kopin, I.J.; Sharabi, Y. 3,4-Dihydroxyphenylethanol (Hydroxytyrosol) Mitigates the Increase in Spontaneous Oxidation of Dopamine During Monoamine Oxidase Inhibition in PC12 Cells. Neurochem. Res. 2016, 41, 2173–2178. [Google Scholar] [CrossRef]
- Aleman-Jimenez, C.; Dominguez-Perles, R.; Medina, S.; Prgomet, I.; Lopez-Gonzalez, I.; Simonelli-Munoz, A.; Campillo-Cano, M.; Aunon, D.; Ferreres, F.; Gil-Izquierdo, A. Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food matrix in humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef]
- Fan, L.; Peng, Y.; Li, X. Brain regional pharmacokinetics of hydroxytyrosol and its molecular mechanism against depression assessed by multi-omics approaches. Phytomedicine 2023, 112, 154712. [Google Scholar] [CrossRef]
- Kundisova, I.; Colom, H.; Juan, M.E.; Planas, J.M. Pharmacokinetics of Hydroxytyrosol and Its Sulfate and Glucuronide Metabolites after the Oral Administration of Table Olives to Sprague-Dawley Rats. J. Agric. Food Chem. 2024, 72, 2154–2164. [Google Scholar] [CrossRef]
- Di Renzo, L.; Smeriglio, A.; Ingegneri, M.; Gualtieri, P.; Trombetta, D. The Pharmaceutical Formulation Plays a Pivotal Role in Hydroxytyrosol Pharmacokinetics. Pharmaceutics 2023, 15, 743. [Google Scholar] [CrossRef]
- Lopez-Yerena, A.; Vallverdu-Queralt, A.; Jauregui, O.; Garcia-Sala, X.; Lamuela-Raventos, R.M.; Escribano-Ferrer, E. Tissue Distribution of Oleocanthal and Its Metabolites after Oral Ingestion in Rats. Antioxidants 2021, 10, 688. [Google Scholar] [CrossRef]
- Lopez-Yerena, A.; Vallverdu-Queralt, A.; Lamuela-Raventos, R.M.; Escribano-Ferrer, E. LC-ESI-LTQ-Orbitrap-MS for Profiling the Distribution of Oleacein and Its Metabolites in Rat Tissues. Antioxidants 2021, 10, 1083. [Google Scholar] [CrossRef]
- De La Cruz Cortes, J.P.; Perez de Algaba, I.; Martin-Aurioles, E.; Arrebola, M.M.; Ortega-Hombrados, L.; Rodriguez-Perez, M.D.; Fernandez-Prior, M.A.; Bermudez-Oria, A.; Verdugo, C.; Gonzalez-Correa, J.A. Extra Virgin Oil Polyphenols Improve the Protective Effects of Hydroxytyrosol in an In Vitro Model of Hypoxia-Reoxygenation of Rat Brain. Brain Sci. 2021, 11, 1133. [Google Scholar] [CrossRef]
- Rodriguez-Perez, M.D.; Perez de Algaba, I.; Martin-Aurioles, E.; Arrebola, M.M.; Ortega-Hombrados, L.; Verdugo, C.; Fernandez-Prior, M.A.; Bermudez-Oria, A.; De La Cruz, J.P.; Gonzalez-Correa, J.A. Neuroprotective Effect of 3′,4′-Dihydroxyphenylglycol in Type-1-like Diabetic Rats-Influence of the Hydroxytyrosol/3′,4′-dihydroxyphenylglycol Ratio. Nutrients 2022, 14, 1146. [Google Scholar] [CrossRef]
- Bender, C.; Strassmann, S.; Golz, C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients 2023, 15, 325. [Google Scholar] [CrossRef] [PubMed]
- Dzidic-Krivic, A.; Fajkic, A.; Farhat, E.K.; Lekic, L.; Ejubovic, A.; Vukas, S.K.; Ejubovic, M.; Lepara, O.; Sher, E.K. Linking Metabolic Syndrome to Neurodegeneration Mechanisms and Potential Treatments. Mol. Neurobiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, D.; Narita, Y. The curse of blood-brain barrier and blood-tumor barrier in malignant brain tumor treatment. Int. J. Clin. Oncol. 2025, 30, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Kshirsagar, P.; Agrawal, P.; Murry, D.J. Crossing the Blood-Brain Barrier: Innovations in Receptor- and Transporter-Mediated Transcytosis Strategies. Pharmaceutics 2025, 17, 706. [Google Scholar] [CrossRef]
- Xiang, Y.; Gu, Q.; Liu, D. Brain Endothelial Cells in Blood-Brain Barrier Regulation and Neurological Therapy. Int. J. Mol. Sci. 2025, 26, 5843. [Google Scholar] [CrossRef]
- Fong, H.; Zhou, B.; Feng, H.; Luo, C.; Bai, B.; Zhang, J.; Wang, Y. Recapitulation of Structure-Function-Regulation of Blood-Brain Barrier under (Patho)Physiological Conditions. Cells 2024, 13, 260. [Google Scholar] [CrossRef]
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2019, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Tabanez, M.; Santos, I.R.; Ikebara, J.M.; Camargo, M.L.M.; Dos Santos, B.A.; Freire, B.M.; Batista, B.L.; Takada, S.H.; Squitti, R.; Kihara, A.H.; et al. The Impact of Hydroxytyrosol on the Metallomic-Profile in an Animal Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, H.; Han, T.; Shi, S.; Zhang, X.; Shi, X.; Zhang, H.; Zhang, B. A novel hydroxytyrosol derivative HT-3 enhances antioxidant and neuroprotective activity through efficient molecular conjugation. Bioorg. Chem. 2025, 161, 108484. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Fernandez, M.; Garcia, A.R.; Hornedo-Ortega, R.; Troncoso, A.M.; Garcia-Parrilla, M.C.; Brito, M.A. In vitro study of the blood-brain barrier transport of bioactives from Mediterranean foods. Food Funct. 2024, 15, 3420–3432. [Google Scholar] [CrossRef]
- Shi, L.; Gao, P.; Zhang, Y.; Liu, Q.; Hu, R.; Zhao, Z.; Hu, Y.; Xu, X.; Shen, Y.; Liu, J.; et al. 2-(3, 4-Dihydroxyphenyl) ethyl 3-hydroxybutanoate Ameliorates Cognitive Dysfunction and Inflammation Via Modulating Gut Microbiota in Aged Senescence-Accelerated Mouse Prone8 Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, 220. [Google Scholar] [CrossRef]
- Akpinar Adscheid, S.; Tureli, A.E.; Gunday-Tureli, N.; Schneider, M. Nanotechnological approaches for efficient N2B delivery: From small-molecule drugs to biopharmaceuticals. Beilstein J. Nanotechnol. 2024, 15, 1400–1414. [Google Scholar] [CrossRef]
- Correia, A.C.; Monteiro, A.R.; Silva, R.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: Crossing or circumventing the blood-brain barrier (BBB) to manage neurological disorders. Adv. Drug Deliv. Rev. 2022, 189, 114485. [Google Scholar] [CrossRef]
- Haqqani, A.S.; Bélanger, K.; Stanimirovic, D.B. Receptor-mediated transcytosis for brain delivery of therapeutics: Receptor classes and criteria. Front. Drug Deliv. 2024, 4, 1360302. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Li, Y.; Liu, R.; Zhao, Z. Targeting Brain Drug Delivery with Macromolecules Through Receptor-Mediated Transcytosis. Pharmaceutics 2025, 17, 109. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Surface-modified lipid nanocarriers for crossing the blood-brain barrier (BBB): A current overview of active targeting in brain diseases. Colloids Surf. B Biointerfaces 2023, 221, 112999. [Google Scholar] [CrossRef]
- Laghezza Masci, V.; Bernini, R.; Villanova, N.; Clemente, M.; Cicaloni, V.; Tinti, L.; Salvini, L.; Taddei, A.R.; Tiezzi, A.; Ovidi, E. In Vitro Anti-Proliferative and Apoptotic Effects of Hydroxytyrosyl Oleate on SH-SY5Y Human Neuroblastoma Cells. Int. J. Mol. Sci. 2022, 23, 12348. [Google Scholar] [CrossRef]
- Nardi, M.; Brocchini, S.; Somavarapu, S.; Procopio, A. Hydroxytyrosol oleate: A promising neuroprotective nanocarrier delivery system of oleuropein and derivatives. Int. J. Pharm. 2023, 631, 122498. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.K.; Srivastava, R. Drug Delivery With Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 3, 644564. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Sun, B.; Zhu, S.; Jia, Z.; Liu, L.; Liu, L. Regulation of Glycolysis by SMAD5 in Glioma Cells: Implications for Tumor Growth and Apoptosis. Neurochem. Res. 2025, 50, 101. [Google Scholar] [CrossRef]
- Cueto-Urena, C.; Ramirez-Exposito, M.J.; Mayas, M.D.; Carrera-Gonzalez, M.P.; Godoy-Hurtado, A.; Martinez-Martos, J.M. Glutathione Peroxidase gpx1 to gpx8 Genes Expression in Experimental Brain Tumors Reveals Gender-Dependent Patterns. Genes 2023, 14, 1674. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Exposito, M.J.; Mayas, M.D.; Carrera-Gonzalez, M.P.; Martinez-Martos, J.M. Gender Differences in the Antioxidant Response to Oxidative Stress in Experimental Brain Tumors. Curr. Cancer Drug Targets 2019, 19, 641–654. [Google Scholar] [CrossRef]
- Martínez-Martos, J.M.; Mayas, M.D.; Carrera, P.; Arias de Saavedra, J.M.; Sánchez-Agesta, R.; Arrazola, M.; Ramírez-Expósito, M.J. Phenolic compounds oleuropein and hydroxytyrosol exert differential effects on glioma development via antioxidant defense systems. J. Funct. Foods 2014, 11, 221–234. [Google Scholar] [CrossRef]
- Ramírez-Expósito, M.J.; Cueto-Ureña, C.; Carrera-González, M.P.; Martínez-Martos, J.M. Effects of oleuropein and hydroxytyrosol on brain renin-angiotensin system-regulating aminopeptidases in experimental glioma. Cancer Plus 2025, 7, 28–42. [Google Scholar] [CrossRef]
- Ramirez-Exposito, M.J.; Martinez-Martos, J.M. Anti-Inflammatory and Antitumor Effects of Hydroxytyrosol but Not Oleuropein on Experimental Glioma in Vivo. a Putative Role for the Renin-Angiotensin System. Biomedicines 2018, 6, 11. [Google Scholar] [CrossRef]
- Mayas, M.D.; Ramirez-Exposito, M.J.; Carrera, M.P.; Cobo, M.; Martinez-Martos, J.M. Renin-angiotensin system-regulating aminopeptidases in tumor growth of rat C6 gliomas implanted at the subcutaneous region. Anticancer. Res. 2012, 32, 3675–3682. [Google Scholar]
- Ramirez-Exposito, M.J.; Carrera-Gonzalez, M.P.; Martinez-Martos, J.M. Sex differences exist in brain renin-angiotensin system-regulating aminopeptidase activities in transplacental ethyl-nitrosourea-induced gliomas. Brain Res. Bull. 2021, 168, 1–7. [Google Scholar] [CrossRef]
- Romero-Marquez, J.M.; Forbes-Hernandez, T.Y.; Navarro-Hortal, M.D.; Quirantes-Pine, R.; Grosso, G.; Giampieri, F.; Lipari, V.; Sanchez-Gonzalez, C.; Battino, M.; Quiles, J.L. Molecular Mechanisms of the Protective Effects of Olive Leaf Polyphenols against Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhang, L.; Yin, H.; Shen, L.; Zheng, W.; Zhang, K.; Zeng, J.; Hu, C.; Liu, Y. Hydroxytyrosol alleviates oxidative stress and neuroinflammation and enhances hippocampal neurotrophic signaling to improve stress-induced depressive behaviors in mice. Food Funct. 2021, 12, 5478–5487. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Osakabe, N.; Siracusa, R.; Modafferi, S.; Di Paola, R.; Cuzzocrea, S.; Jacob, U.M.; Fritsch, T.; Abdelhameed, A.S.; Rashan, L.; et al. Transgenerational hormesis in healthy aging and antiaging medicine from bench to clinics: Role of food components. Mech. Ageing Dev. 2024, 220, 111960. [Google Scholar] [CrossRef]
- Reyes-Corral, M.; Gil-Gonzalez, L.; Gonzalez-Diaz, A.; Tovar-Luzon, J.; Ayuso, M.I.; Lao-Perez, M.; Montaner, J.; de la Puerta, R.; Fernandez-Torres, R.; Ybot-Gonzalez, P. Pretreatment with oleuropein protects the neonatal brain from hypoxia-ischemia by inhibiting apoptosis and neuroinflammation. J. Cereb. Blood Flow. Metab. 2025, 45, 717–734. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Wang, Y.; Zhang, Y.; Wang, Z.; Xu, X.; Zhang, T.; Zhang, T.; Zhang, S.; Hu, R.; et al. Sleep Deprivation Triggers Mitochondrial DNA Release in Microglia to Induce Neural Inflammation: Preventative Effect of Hydroxytyrosol Butyrate. Antioxidants 2024, 13, 833. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Huang, B.; Chen, A.; Li, X. Oleuropein inhibits the proliferation and invasion of glioma cells via suppression of the AKT signaling pathway. Oncol. Rep. 2016, 36, 2009–2016. [Google Scholar] [CrossRef]
- Gallardo-Fernandez, M.; Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Hydroxytyrosol and dopamine metabolites: Anti-aggregative effect and neuroprotective activity against alpha-synuclein-induced toxicity. Food Chem. Toxicol. 2023, 171, 113542. [Google Scholar] [CrossRef]
- Ercelik, M.; Tekin, C.; Tezcan, G.; Ak Aksoy, S.; Bekar, A.; Kocaeli, H.; Taskapilioglu, M.O.; Eser, P.; Tunca, B. Olea europaea Leaf Phenolics Oleuropein, Hydroxytyrosol, Tyrosol, and Rutin Induce Apoptosis and Additionally Affect Temozolomide against Glioblastoma: In Particular, Oleuropein Inhibits Spheroid Growth by Attenuating Stem-like Cell Phenotype. Life 2023, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Katsetos, C.D.; Reginato, M.J.; Baas, P.W.; D’Agostino, L.; Legido, A.; Tuszyn Ski, J.A.; Draberova, E.; Draber, P. Emerging microtubule targets in glioma therapy. Semin. Pediatr. Neurol. 2015, 22, 49–72. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Hitomi, M.; Gallagher, J.; Gadani, S.P.; Adkins, J.; Vasanji, A.; Liu, L.; Eyler, C.E.; Heddleston, J.M.; Wu, Q.; et al. Distribution of CD133 reveals glioma stem cells self-renew through symmetric and asymmetric cell divisions. Cell Death Dis. 2011, 2, e200. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Londono, A.P.; Robles-Flores, M. Functional Roles of CD133: More than Stemness Associated Factor Regulated by the Microenvironment. Stem Cell Rev. Rep. 2024, 20, 25–51. [Google Scholar] [CrossRef]
- Pecina-Slaus, N.; Hrascan, R. Glioma Stem Cells-Features for New Therapy Design. Cancers 2024, 16, 1557. [Google Scholar] [CrossRef]
- He, J.; Yan, X.; Hu, S. Glioma stem cells: Drivers of tumor progression and recurrence. Stem Cell Res. Ther. 2025, 16, 293. [Google Scholar] [CrossRef]
- Li, H.; Xu, X.; Cai, M.; Qu, Y.; Ren, Z.; Ye, C.; Shen, H. The combination of HT-ac and HBET improves the cognitive and learning abilities of heat-stressed mice by maintaining mitochondrial function through the PKA-CREB-BDNF pathway. Food Funct. 2022, 13, 6166–6179. [Google Scholar] [CrossRef]
- Liu, S.; Lu, Y.; Tian, D.; Zhang, T.; Zhang, C.; Hu, C.Y.; Chen, P.; Meng, Y. Hydroxytyrosol Alleviates Obesity-Induced Cognitive Decline by Modulating the Expression Levels of Brain-Derived Neurotrophic Factors and Inflammatory Factors in Mice. J. Agric. Food Chem. 2024, 72, 6250–6264. [Google Scholar] [CrossRef]
- Eren, E.; Das, J.; Tollefsbol, T.O. Polyphenols as Immunomodulators and Epigenetic Modulators: An Analysis of Their Role in the Treatment and Prevention of Breast Cancer. Nutrients 2024, 16, 4143. [Google Scholar] [CrossRef]
- Li, X.; Tian, X.; Liu, T.; Li, M.; Wang, W.; Wang, P.; Guo, Z. Hydroxytyrosol Alleviated Hypoxia-Mediated PC12 Cell Damage through Activating PI3K/AKT/mTOR-HIF-1alpha Signaling. Oxid. Med. Cell Longev. 2022, 2022, 8673728. [Google Scholar] [CrossRef]
- Scuto, M.; Trovato Salinaro, A.; Caligiuri, I.; Ontario, M.L.; Greco, V.; Sciuto, N.; Crea, R.; Calabrese, E.J.; Rizzolio, F.; Canzonieri, V.; et al. Redox modulation of vitagenes via plant polyphenols and vitamin D: Novel insights for chemoprevention and therapeutic interventions based on organoid technology. Mech. Ageing Dev. 2021, 199, 111551. [Google Scholar] [CrossRef]
- Karagiannis, T.C.; Ververis, K.; Liang, J.J.; Pitsillou, E.; Liu, S.; Bresnehan, S.M.; Xu, V.; Wijoyo, S.J.; Duan, X.; Ng, K.; et al. Identification and Evaluation of Olive Phenolics in the Context of Amine Oxidase Enzyme Inhibition and Depression: In Silico Modelling and In Vitro Validation. Molecules 2024, 29, 2446. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cueto-Ureña, C.; Ramírez-Expósito, M.J.; Carrera-González, M.P.; Martínez-Martos, J.M. Hydroxytyrosol and Brain Tumors: Mechanisms of Action and Therapeutic Potential. Curr. Issues Mol. Biol. 2025, 47, 667. https://doi.org/10.3390/cimb47080667
Cueto-Ureña C, Ramírez-Expósito MJ, Carrera-González MP, Martínez-Martos JM. Hydroxytyrosol and Brain Tumors: Mechanisms of Action and Therapeutic Potential. Current Issues in Molecular Biology. 2025; 47(8):667. https://doi.org/10.3390/cimb47080667
Chicago/Turabian StyleCueto-Ureña, Cristina, María Jesús Ramírez-Expósito, María Pilar Carrera-González, and José Manuel Martínez-Martos. 2025. "Hydroxytyrosol and Brain Tumors: Mechanisms of Action and Therapeutic Potential" Current Issues in Molecular Biology 47, no. 8: 667. https://doi.org/10.3390/cimb47080667
APA StyleCueto-Ureña, C., Ramírez-Expósito, M. J., Carrera-González, M. P., & Martínez-Martos, J. M. (2025). Hydroxytyrosol and Brain Tumors: Mechanisms of Action and Therapeutic Potential. Current Issues in Molecular Biology, 47(8), 667. https://doi.org/10.3390/cimb47080667






