Evaluation of the Safety and Antiproliferative Activity of Bulgarian Rose Essential Oil: An In Vitro and In Silico Model of Colorectal Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Storage of Plant Material
2.2. Quantitative Determination of Rose Oil
2.3. Gas Chromatographic (GC) Analysis
2.4. Cell Lines, Maintenance and Treatment
2.5. In Vitro Safety Test
2.6. In Vitro Antiproliferative Activity
2.7. Clonogenic Assay
2.8. Statistical Analysis
2.9. Acridine Orange/Ethidium Bromide Live/Dead Staining
2.10. Nuclear Morphology Analysis
2.11. In Silico Molecular Docking Simulations
3. Results
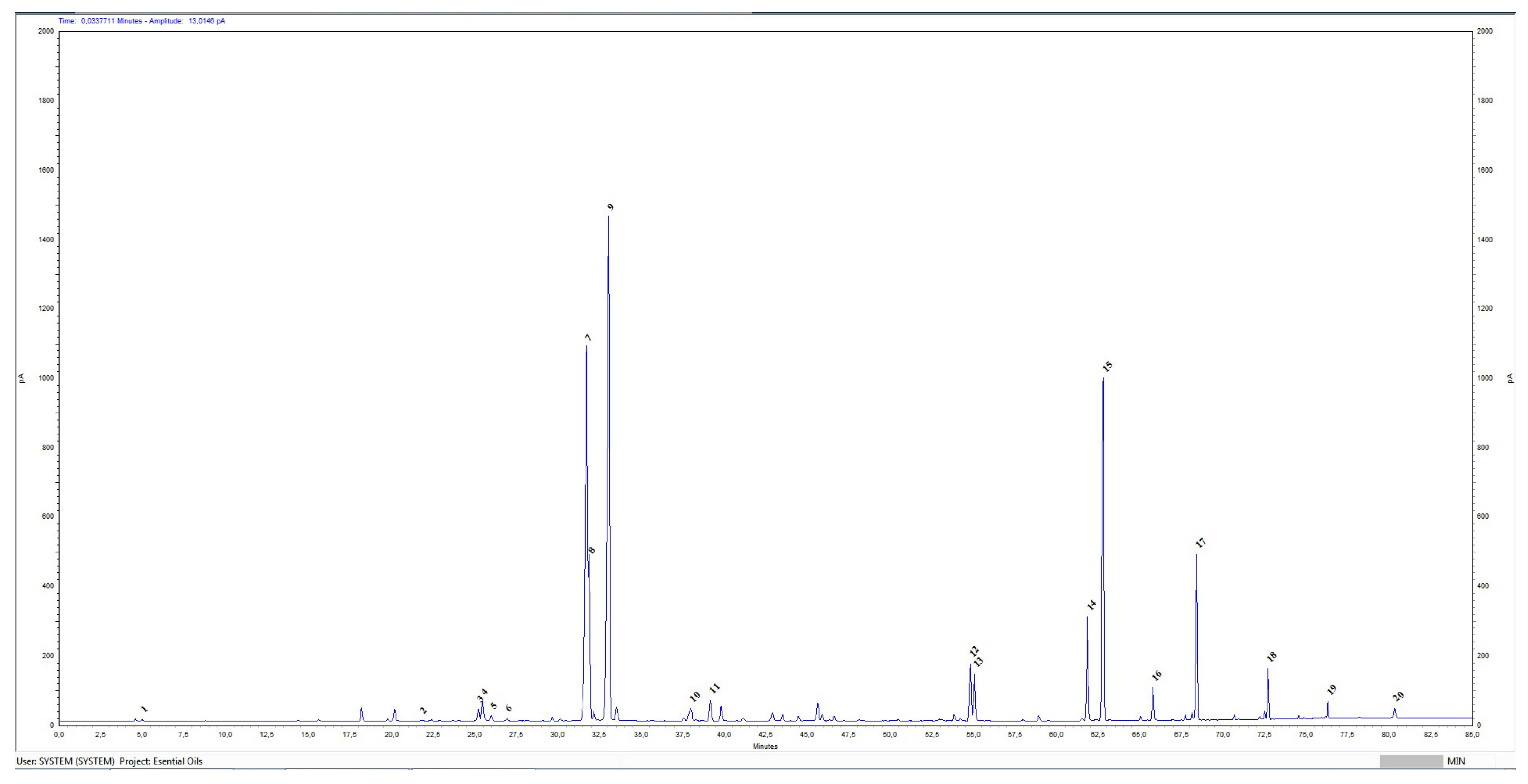
3.1. Gas Chromatographic Analysis of BREO
3.2. Safety Test
3.3. Antiproliferative Activity
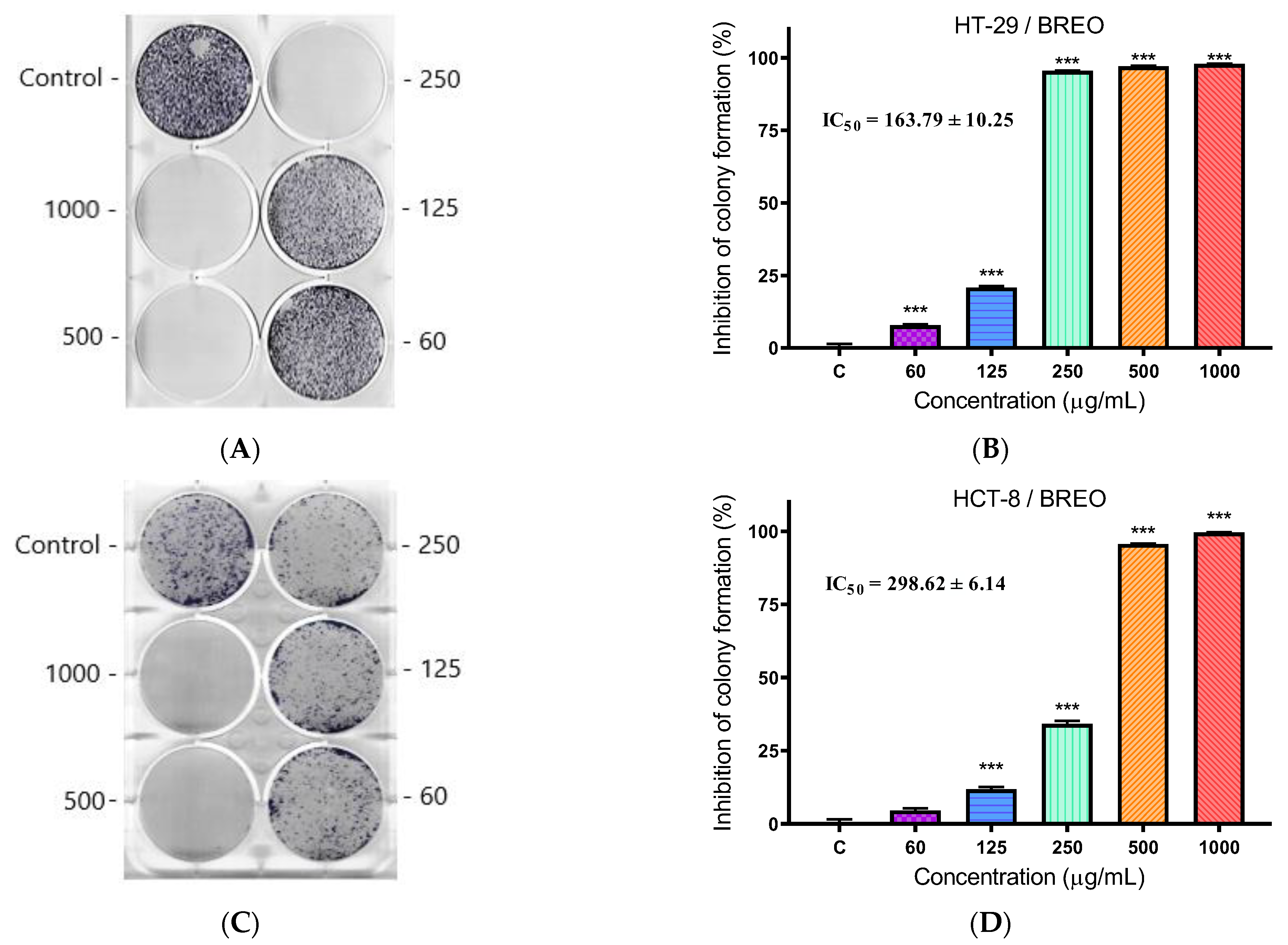
3.4. Clonogenic Assay
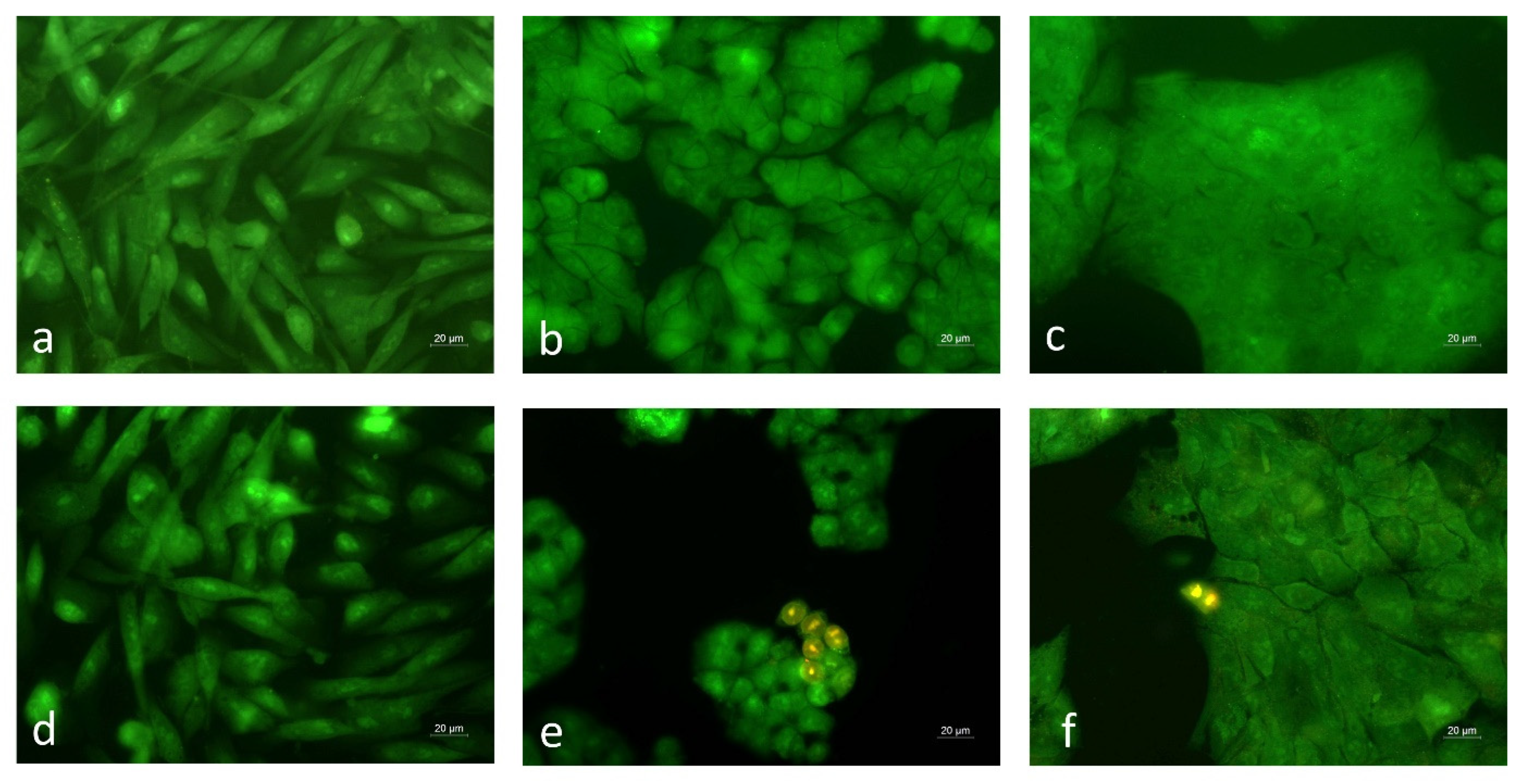
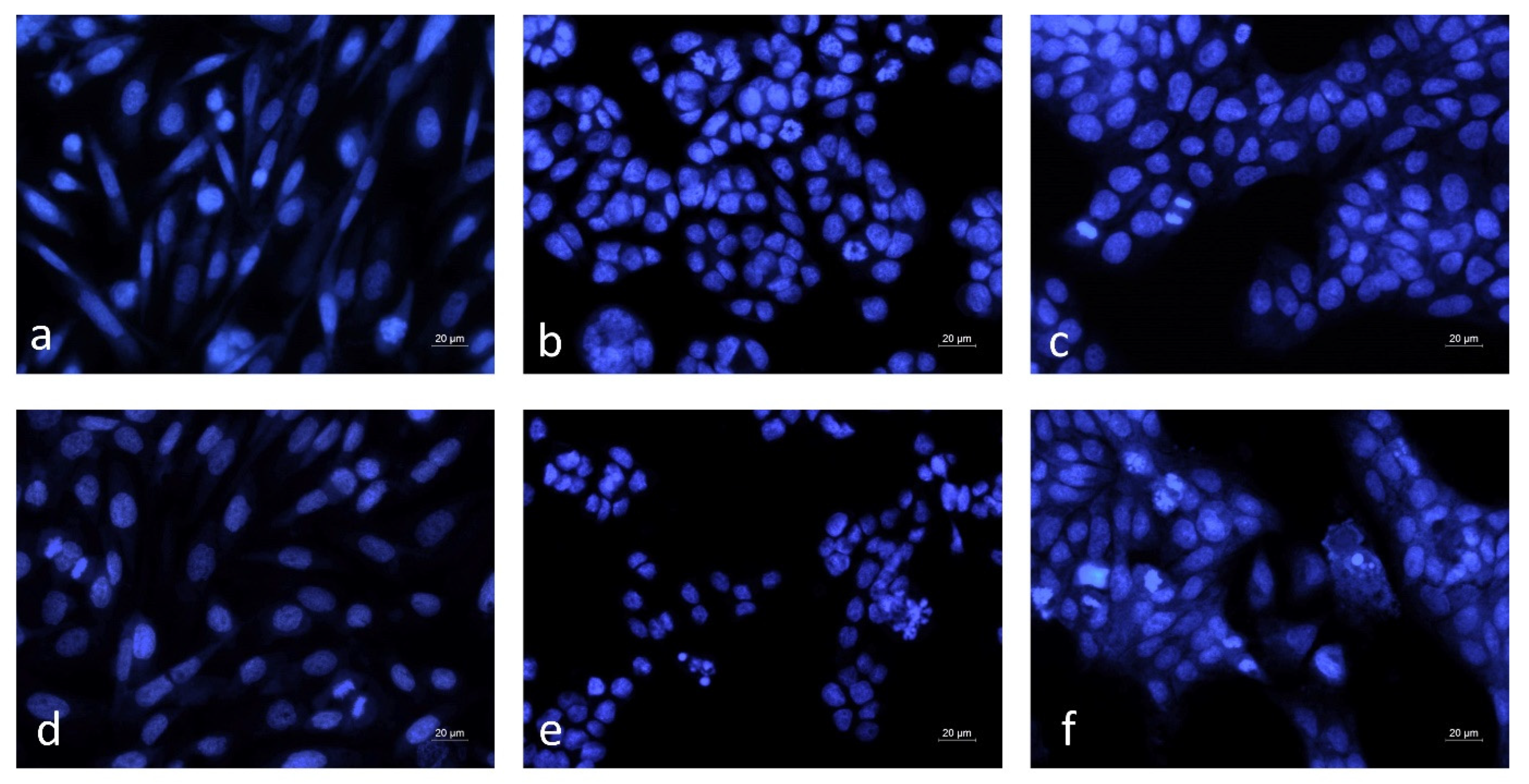
3.5. Microscopic Analysis
3.6. In Silico Molecular Docking Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | gas chromatographic |
| FID | flame ionization detector |
| NRU | neutral red uptake |
| MTT | (3-(4,5-diMethylThiazolyl-2)-2,5-diphenylTetrazolium bromide) |
| CC50 | 50% Cytotoxicity Concentration |
| PIF | photo-irritation factor |
| IC50 | 50% inhibitory concentration |
References
- Raeber, J.; Steuer, C. Exploring new dimensions: Single and multi-block analysis of essential oils using DBDI-MS and FT-IR for enhanced authenticity control. Anal. Chim. Acta. 2023, 1277, 341657. [Google Scholar] [CrossRef] [PubMed]
- Maleev, A.; Stoianov, S.; Neshev, G.; Antonov, P.; Tomov, T. Rosanol- Pharmacological and clinical investigations. Med. Biol. Inform. 1974, 3, 3–10. [Google Scholar]
- Maleev, A.; Neshev, G.; Stoianov, S.; Sheikov, N. Ulceroprotective and spasmolytic effect of the Bulgarian Rose oil. Exp. Med. Morph. 1972, 2, 55–60. [Google Scholar]
- Maleev, A.; Neshev, G.; Stoianov, S.; Kushev, V. Rose oil pharmacological investigations of its therapeutic properties. Med. Biol. Inform. 1970, 4, 26. [Google Scholar]
- Maleev, A.; Todorov, S.; Atanassova, E.; Radomirov, R.; Petkov, V.; Venkova, K.; Noeva, A.; Gachilova, S.; Neshev, G. On the mechanism of the smooth-muscle action of rosanol. Acta Physiol. Pharmacol. Bulg. 1987, 13, 3–10. [Google Scholar]
- Kirov, M.; Vankov, S. Rose, rose oil, gyrosital—Antisclerotic, antispasmodic, hepatoprotective, general stimulating drug. Publ. House Med. Phys. Educ. 1986, 121. [Google Scholar]
- Boyanova, L.; Neshev, G. Inhibitory effect of rose oil products on Helicobacter pylori growth in vitro: Preliminary report. J. Med. Microbiol. 1999, 48, 705–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neshev, G. Bulgarian rose oil—Pharmacological and Clinical Studies. Doctoral Thesis, Medical University-Sofia, Sofia, Bulgaria, 1990. [Google Scholar]
- Garzoli, S.; Alarcón-Zapata, P.; Seitimova, G.; Alarcón-Zapata, B.; Martorell, M.; Sharopov, F.; Fokou, P.; Dize, D.; Yamthe, L.; Les, F.; et al. Natural essential oils as a new therapeutic tool in colorectal cancer. Canc. Cell Intern. 2022, 22, 407. [Google Scholar] [CrossRef]
- Rezaie-Tavirani, M.; Fayazfar, S.; Heydari-Keshel, S.; Rezaee, M.B.; Zamanian-Azodi, M.; Rezaei-Tavirani, M.; Khodarahmi, R. Effect of essential oil of Rosa Damascena on human colon cancer cell line SW742. Gastroenterol. Hepatol. Bed. Bench. 2013, 6, 25–31. [Google Scholar] [PubMed]
- Fearon, E. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. 2011, 6, 479–507. [Google Scholar] [CrossRef]
- Barnum, K.; O’Connell, M. Cell Cycle Regulation by Checkpoints. Methods Mol. Biol. 2014, 1170, 29–40. [Google Scholar] [PubMed]
- Zhao, Y.; Ye, X.; Xiong, Z.; Ihsan, A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.; Anadón, A.; Wang, X.; et al. Cancer metabolism: The role of ROS in DNA damage and induction of apoptosis in cancer cells. Metabolites 2023, 13, 796. [Google Scholar] [CrossRef]
- Dolado, I.; Swat, A.; Ajenjo, N.; De Vita, G.; Cuadrado, A.; Nebreda, A.R. P38alpha MAP Kinase as a Sensor of Reactive Oxygen Species in Tumorigenesis. Cancer Cell. 2007, 11, 191–205. [Google Scholar] [CrossRef]
- García-Hernández, L.; García-Ortega, M.B.; Ruiz-Alcalá, G.; Carrillo, E.; Marchal, J.A.; García, M. The P38 MAPK Components and Modulators as Biomarkers and Molecular Targets in Cancer. Int. J. Mol. Sci. 2021, 23, 370. [Google Scholar] [CrossRef]
- Kennedy, N.J.; Cellurale, C.; Davis, R.J. A Radical Role for P38 MAPK in Tumor Initiation. Cancer Cell. 2007, 11, 101–103. [Google Scholar] [CrossRef]
- Aggarwal, S.; Bhadana, K.; Singh, B.; Rawat, M.; Mohammad, T.; Al-Keridis, L.A.; Alshammari, N.; Hassan, M.D.; Das, S.N. Cinnamomum zeylanicum Extract and its Bioactive Component Cinnamaldehyde Show Anti-Tumor Effects via Inhibition of Multiple Cellular Pathways. Front. Pharmacol. 2022, 13, 918479. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Argüelles, S. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef] [PubMed]
- Zeyen, L.; Seternes, O.M.; Mikkola, I. Crosstalk between p38 MARK and GR signaling. Int. J. Mol. Sci. 2022, 23, 3322. [Google Scholar] [CrossRef] [PubMed]
- Vilasco, M.; Communal, L.; Mourra, N.; Courtin, A.; Forgez, P.; Gompel, A. Glucocorticoid receptor and breast cancer. Breast Cancer Res. Treat. 2011, 130, 1–10. [Google Scholar] [CrossRef]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 2013, 1072, 1–13. [Google Scholar] [CrossRef]
- Minaiyan, M.; Ghannadi, A.R.; Afsharipour, M.; Mahzouni, P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res. Pharm. Sci. 2011, 6, 13–21. [Google Scholar]
- Thapa, D.; Richardson, A.J.; Zweifel, B.; Wallace, R.J.; Gratz, S.W. Genoprotective Effects of Essential Oil Compounds Against Oxidative and Methylated DNA Damage in Human Colon Cancer Cells. J. Food Sci. 2019, 84, 1979–1985. [Google Scholar] [CrossRef]
- Coates, E.; Popa, G.; Gill, C.; McCann, M.; McDougall, G.; Stewart, D.; Rowland, I. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer. J. Carcinogen. 2007, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Chel-Guerrero, L.; Scampicchio, M.; Ferrentino, G.; Rodríguez-Buenfil, I.; Fragoso-Serrano, M. In vitro assessment of antiproliferative activity and cytotoxicity modulation of capsicum chinense by-product extracts. Appl. Sci. 2022, 12, 5818. [Google Scholar] [CrossRef]
- Singh, J.; Meena, A. Exploration of chemopreventive potential of linalool in targeting lung cancer biomarkers. Endocr. Metab. Immune Disord. Drug Targets (Former. Curr. Drug Targets Immune Endocr. Metab. Disord.) 2022, 22, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Staykov, V.; Decheva, R.; Balinova-Tsvetkova, A. Studies on the composition of rose oil obtained from the flowers in different stages of their development. Riv. Ital. 1975, 57, 192–195. [Google Scholar]
- Balinova-Cvetkova, A.; Dyakov, D. Improved apparatus for microdistillation of oil-bearing rose. Plant Sci. 1974, 11, 79–86. [Google Scholar]
- Clevenger, J. Apparatus for the determination of volatile oil. J. Am. Pharm. Assoc. 1928, 17, 345–349. [Google Scholar] [CrossRef]
- Angelova, N.; Iliev, I.; Nemska, V.; Naydenova, E. Design, Synthesis, and Biological Evaluation of New Analogs of Aurein 1.2 Containing Non-Proteinogenic Amino Acids. Molecules 2025, 30, 2050. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, D.; Nemska, V.; Foteva, T.; Iliev, I.; Georgieva, N.; Danalev, D. Synthesis and Biological Studies of New Temporin A Analogs Containing Unnatural Amino Acids in Position 7. Pharmaceutics 2024, 16, 716. [Google Scholar] [CrossRef]
- Georgieva, A.; Sulikovska, I.; Toshkova-Yotova, T.; Djeliova, V.; Tsonevski, N.; Petkova-Kirova, P.; Tasheva, K. Antitumor Activity of Whole-Plant Extracts from In Vitro Cultured and Wild-Growing Clinopodium vulgare Plants on a Panel of Human Tumor Cell Lines. Appl. Sci. 2025, 15, 925. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Agu, C.; Afiukwa, A.; Orji, U.; Ezeh, M.; Ofoke, H.; Ogbu, O.; Ugwuja, I.; Aja, M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef] [PubMed]
- ISO 9842:2024; Essential oil of Rose (Rosa x damascena Miller). International Organization for Standardization: Geneva, Switzerland, 2024. Available online: https://www.iso.org/standard/86897.html (accessed on 4 December 2024).
- Raeber, J.; Favrod, S.; Steuer, C. Determination of major, minor and chiral components as quality and authenticity markers of Rosa damascena oil by GC-FID. Plants 2023, 12, 506. [Google Scholar] [CrossRef]
- Tursun, A. Impact of soil types on chemical composition of essential oil of purple basil. Saudi J. Biol. Sci. 2022, 29, 103314. [Google Scholar] [CrossRef]
- Kovacheva, N.; Rusanov, K.; Atanassov, I. Industrial cultivation of oil bearing rose and rose oil production in Bulgaria during 21st century, directions and challenges. Biotechnol. Biotechnol. Equip. 2010, 24, 1793–1798. [Google Scholar] [CrossRef]
- Adeosun, W.; Bodede, O.; Prinsloo, G. Effect of Different Climatic Regions and Seasonal Variation on the Antibacterial and Antifungal Activity, and Chemical Profile of Helichrysum aureonitens Sch. Bip. Metabolites 2022, 12, 758. [Google Scholar] [CrossRef]
- Gochev, V.; Jirovetz, L.; Wlcek, K.; Buchbauer, G.; Schmidt, E.; Stoyanova, A.; Dobreva, A. Chemical Composition and Antimicrobial Activity of Historical Rose Oil from Bulgaria. J. Essent. Oil-Bear. Plants 2009, 12, 1–6. [Google Scholar] [CrossRef]
- Katsukawa, M.; Nakata, R.; Koeji, S.; Hori, K.; Takahashi, S.; Inoue, H. Citronellol and geraniol, components of rose oil, activate peroxisome proliferator-activated receptor α and γ and suppress cyclooxygenase-2 expression. Biosc. Biotechnol. Biochem. 2011, 75, 1010–1012. [Google Scholar] [CrossRef]
- Kladniew, B.; Polo, M.; Villegas, S.; Galle, M.; Crespo, R.; De Bravo, M. Synergistic antiproliferative and anticholesterogenic effects of linalool,1,8-cineole, and simvastatin on human cell lines. Chem. Biol. Interact. 2014, 214, 57–68. [Google Scholar] [CrossRef]
- Sun, X.; Wang, S.; Li, T.; Yang, Y. Anticancer activity of linalool terpenoid: Apoptosis induction and cell cycle arrest in prostate cancer cells. Trop. J. Pharm. Res. 2015, 14, 619–625. [Google Scholar] [CrossRef]
- Gu, Y.; Ting, Z.; Qiu, X.; Zhang, X.; Gan, X.; Fang, Y.; Xu, X.; Xu, R. Linalool preferentially induces robust apoptosis of a variety of leukemia cells via upregulating p53 and cyclin-dependent kinase inhibitors. Toxicology 2010, 268, 19–24. [Google Scholar] [CrossRef]
- Chang, M.; Shieh, D.; Chen, C.; Yeh, C.; Dong, H. Linalool induces cell cycle arrest and apoptosis in leukemia cells and cervical cancer cells through CDKIs. Int. J. Mol. Sci. 2015, 16, 28169–28179. [Google Scholar] [CrossRef]
- Ravizza, R.; Gariboldi, M.; Molteni, R.; Monti, E. Linalool, a plant-derived monoterpene alcohol, reverses doxorubicin resistance in human breast adenocarcinoma cells. Oncol. Rep. 2008, 20, 625–630. [Google Scholar] [CrossRef]
- Tsvetelina Gerasimova, T.; Gateva, S.; Topashka-Ancheva, M.; Jovtchev, J.; Angelova, T.; Dobreva, A.; Mileva, M. Rosa damascena Mill. essential oil: Analysis of in vitro and in vivo genotoxic and cytotoxic potentials by employing three cytogenetic Endpoints. Molecules 2025, 30, 78. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Habibi, E.; Modanloo, M. Cytotoxic and genotoxic studies of essential oil from Rosa damascene Mill., Kashan, Iran. Med. Glas. 2017, 14, 152–157. [Google Scholar] [CrossRef]
- Vejselova, S.; İzgördü, H.; İşcan, G.; İşcan, A.; Kutlu, H. Cytotoxic and antimicrobial activities of Turkish rose oil and its major metabolite s-(-)-citronellol. J. Res. Pharm. 2024, 28, 899–911. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Schneider, Y.; Ceraline, J.; Duranton, B.; Gosse, F.; Seiler, N.; Raul, F. Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells. J. Pharmacol. Exp. Ther. 2001, 298, 197–200. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Cui, J.; Hao, Z. Apoptosis-Mediated anticancer activity of geraniol inhibits NF-κB, MAPK, and JAK-STAT-3 signaling pathways in human thyroid cancer cells. Pharmacog. Mag. 2022, 18, 1183–1189. [Google Scholar]
- Fatima, K.; Wani, Z.; Meena, A.; Luqman, S. Geraniol exerts its antiproliferative action by modulating molecular targets in lung and skin carcinoma cells. Phytother. Res. 2021, 35, 3861–3874. [Google Scholar] [CrossRef]
- Ho, Y.; Suphrom, N.; Daowtak, K.; Potup, P.; Thongsri, Y.; Usuwanthim, K. Anticancer effect of citrus hystrix DC. leaf extractand its bioactive constituents citronellol and, citronellal on the triple negative breast cancer MDA-MB-231 cell line. Pharmaceut. 2020, 13, 476. [Google Scholar] [CrossRef]
- Rajendran, J.; Pachaiappan, P.; Thangarasu, R. Citronellol, an acyclic monoterpene induces mitochondrial-mediated apoptosis through activation of proapoptotic factors in MCF-7 and MDA-MB-231 human mammary tumor cells. Nutr. Cancer 2021, 73, 1448–1458. [Google Scholar] [CrossRef]
- Inaba, M.; Tanaka, T.; Sawada, H. Increased Sensitivity to Long-term 5-Fluorouracil Exposure of Human Colon Cancer HT-29 Cells Resistant to Short-term Exposure. Jpn. J. Cancer Res. 1998, 89, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, G.; Sun, Z.; Handschumacher, R. Aberrant cell cycle inhibition pattern in human colon carcinoma cell lines after exposure to 5-fluorouracil. Biochem. Pharmacol. 1995, 49, 553–557. [Google Scholar] [CrossRef]
- Azevedo, R.; van Zeeland, M.; Raaijmakers, H.; Kazemier, B.; Jacob de Vlieg, W.; Oubrie, A. X-ray structure of p38a bound to TAK-715: Comparison with three classic inhibitors. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 1041–1050. [Google Scholar] [CrossRef]
- Karamalakova, Y.; Nikolova, G.; Kovacheva, N.; Zheleva, A.; Gadjeva, V. Study of the radical-scavenging activities and radioprotective properties of Bulgarian essential rose oil from Rosa Damascena Mill. Bulg. Chem. Commun. 2019, 51, 101–107. [Google Scholar]
- Todorova, M.; Dobreva, A.; Petkova, N.; Grozeva, N.; Gerdzhikova, M.; Veleva, P. Organic vs conventional farming of oil-bearing rose: Effect on essential oil and antioxidant activity. BioRisk 2022, 17, 271–285. [Google Scholar] [CrossRef]
- Schoch, G.; D’Arcy, B.; Stihle, M.; Burger, D.; Bär, D.; Benz, J.; Thoma, R.; Ruf, A. Molecular Switch in the Glucocorticoid Receptor: Active and Passive Antagonist Conformations. J. Mol. Biol. 2010, 395, 568–577. [Google Scholar] [CrossRef]
- Biggadike, K.; Bledsoe, R.; Coe, D.; Cooper, T.; House, D.; Iannone, M.; Macdonald, S.; Madauss, K.; McLay, I.; Shipley, T.; et al. Design and X-ray Crystal Structures of High-Potency Nonsteroidal Glucocorticoid Agonists Exploiting a Novel Binding Site on the Receptor. Proc. Natl. Acad. Sci. USA 2009, 106, 18114–18119. [Google Scholar] [CrossRef]
- Seitz, T.; Thoma, R.; Schoch, G.; Stihle, M.; Benz, J.; D’Arcy, B.; Wiget, A.; Ruf, A.; Hennig, M.; Sterner, R. Enhancing the Stability and Solubility of the Glucocorticoid Receptor Ligand-Binding Domain by High-Throughput Library Screening. J. Mol. Biol. 2010, 403, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Scheschowitsh, K.; Leite, J.A.; Assreuy, J. New insights in Glucocorticoid receptor signaling – more than just a ligand binding receptor. Front. Endocrinol. 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, R.; Montana, V.; Stanley, T.; Delves, C.; Apolito, C.; McKee, D.; Consler, T.; Parks, D.; Stewart, E.; Willson, T.; et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 2002, 110, 93–105. [Google Scholar] [CrossRef] [PubMed]


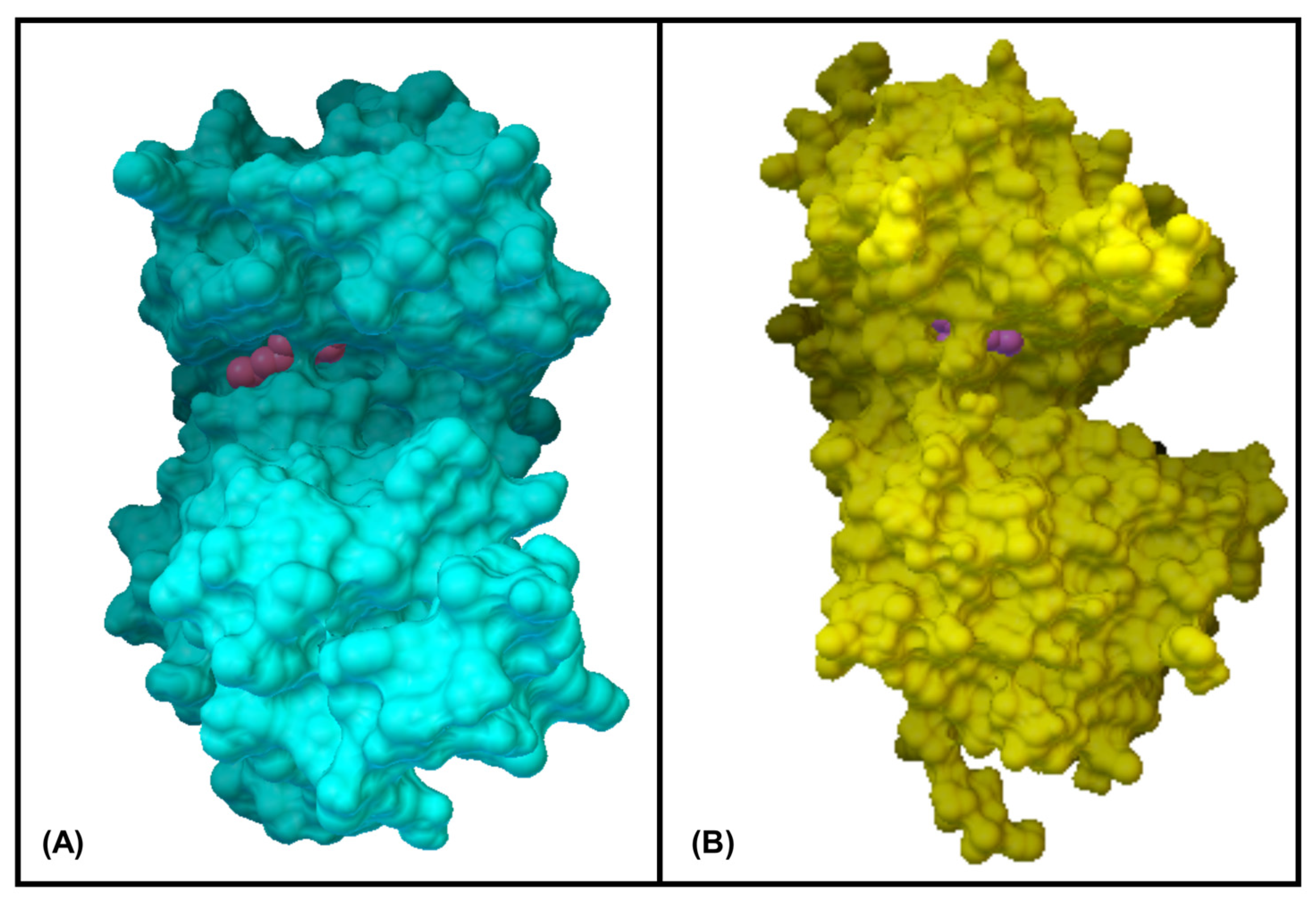
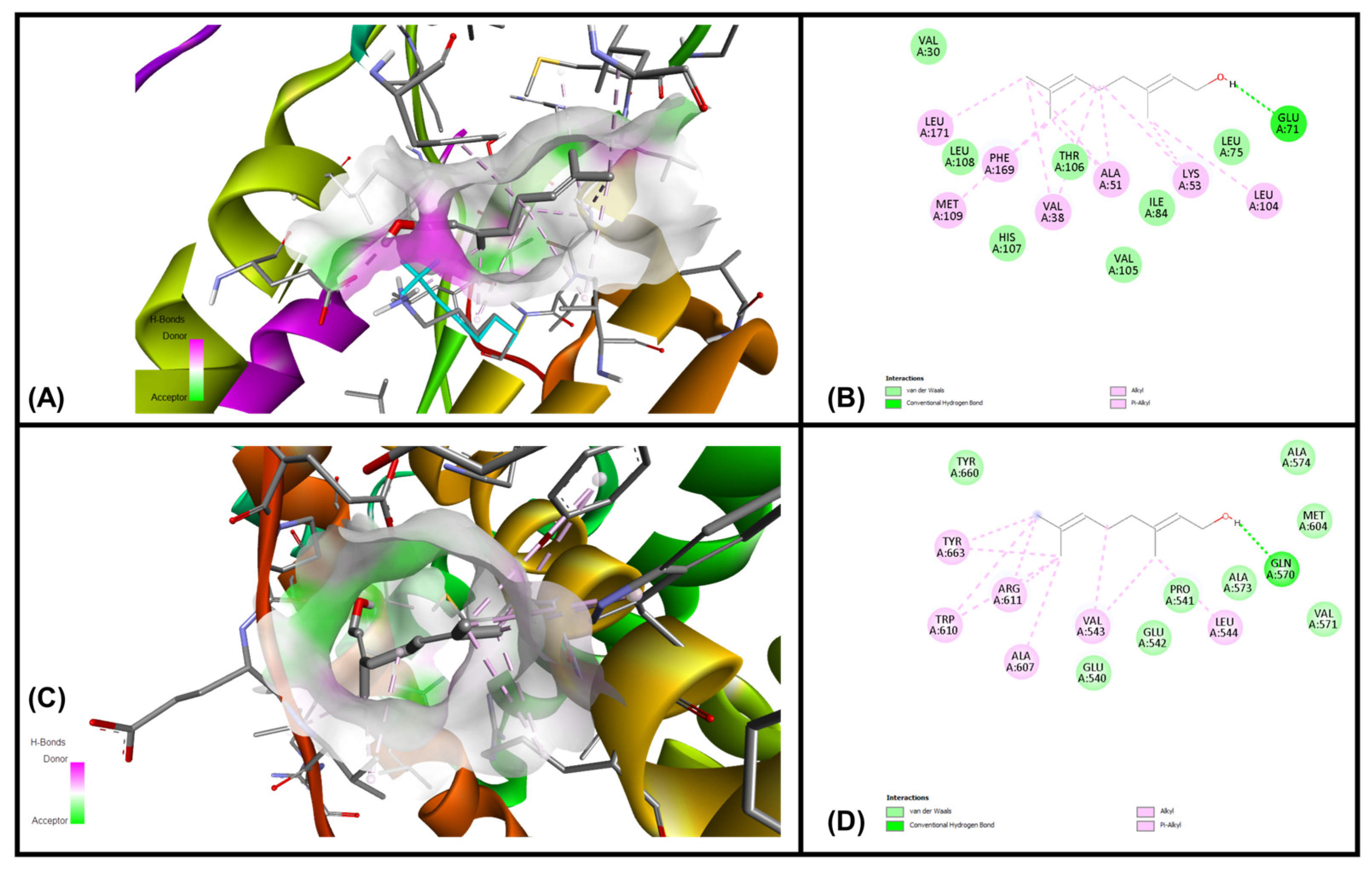
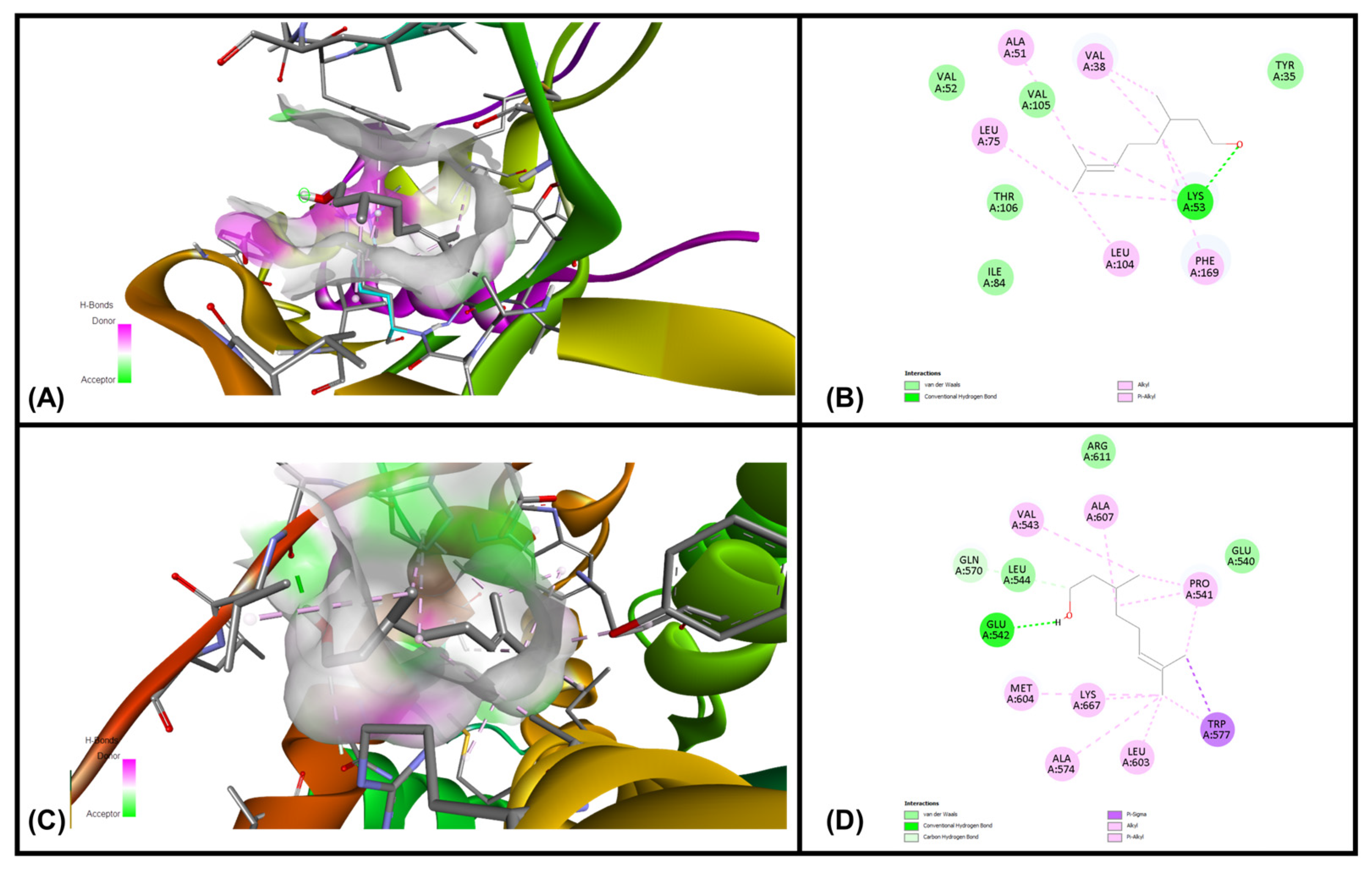
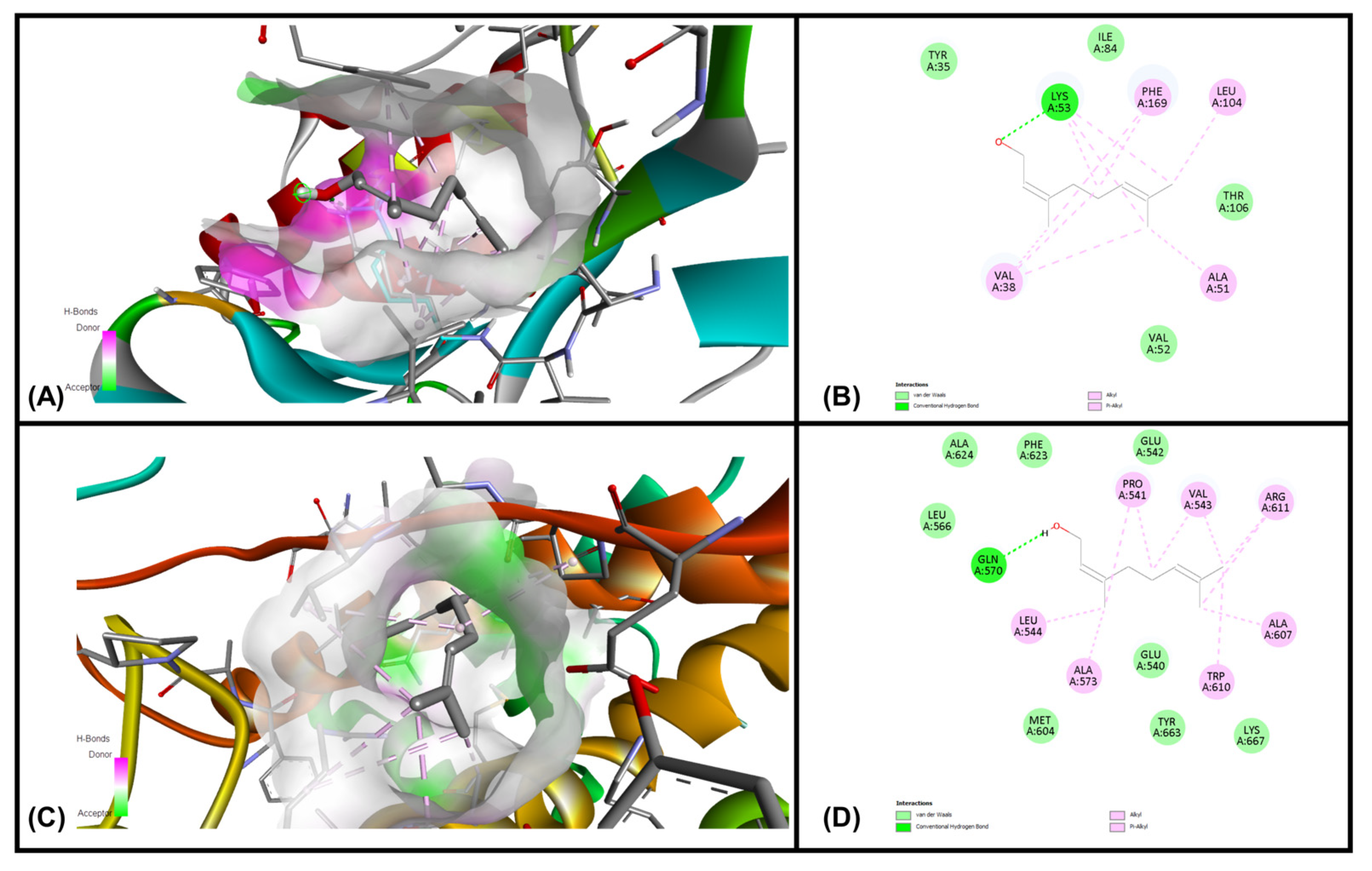
| Sample | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Ratio | 1:4 | 1:4 | 1:4 | 1:4 | 1:4 | 1:4 |
| Volume (mL) | 0.11 | 0.10 | 0.10 | 0.12 | 0.10 | 0.11 |
| Yield (%) | 0.0475 | 0.043 | 0.043 | 0.0518 | 0.043 | 0.0475 |
| № | Components | Molecular Formula | Retention Time (RT) | Relative % | Reference Values: ISO 9842:2024 |
|---|---|---|---|---|---|
| 1 | Ethanol | C2H6O | 4.996 | 0.06 | ≤3.0% |
| 2 | Limonene | C10H16 | 21.796 | 0.05 | |
| 3 | Linalool | C10H18O | 25.216 | 0.53 | |
| 4 | Phenylethanol | C8H10O | 25.462 | 0.96 | ≤2.5% |
| 5 | Cis-rose oxide | C10H18O | 25.987 | 0.23 | |
| 6 | Trans- rose oxide | C10H18O | 26.935 | 0.13 | |
| 7 | Citronellol | C10H20O | 31.726 | 21.50 | 20.0–34.0% |
| 8 | Nerol | C10H18O | 31.871 | 5.51 | 5.0–12.0% |
| 9 | Geraniol | C10H18O | 33.064 | 28.73 | 14.0–22.0% |
| 10 | Eugenol | C10H12O | 37.987 | 0.85 | |
| 11 | Methyl eugenol | C11H14O2 | 39.811 | 0.70 | 0.8–3.0% |
| 12 | Heptadecane | C17H36 | 54.805 | 2.29 | 1.0–2.5% |
| 13 | Farnesol | C15H26O | 55.049 | 2.11 | |
| 14 | Nonadecene | C19H38 | 61.846 | 3.93 | 1.5–4.0% |
| 15 | Nonadecane | C19H40 | 62.787 | 13.13 | 8.0–15.0% |
| 16 | Eicosane | C20H42 | 65.789 | 1.01 | |
| 17 | Heneicosane | C21H44 | 68.410 | 4.87 | 3.0–5.5% |
| 18 | Tricosane | C23H48 | 72.698 | 1.29 | |
| 19 | Pentacosane | C25H25 | 76.302 | 0.46 | |
| 20 | Heptacosane | C27H56 | 80.324 | 0.39 |
| Compounds | Mean CC50 ± SD (µg/mL) | PIF * | |
|---|---|---|---|
| −Irr | +Irr ** | ||
| BREO | 629.72 ± 22.38 | 682.99 ± 14.39 | 0.92 |
| Chlorpromazine *** | 12.74 ± 0.82 | 1.31 ± 0.07 | 9.73 |
| Compounds | Mean IC50 ± SD (µg/mL) | SI * | |||
|---|---|---|---|---|---|
| MCF-12F | HT-29 | HCT-8 | HT-29 | HCT-8 | |
| BREO | 383.90 ± 34.75 | 290.45 ± 10.79 | 363.67 ± 12.43 | 1.32 | 1.06 |
| Cisplatin ** | 22.8 ± 1.5 | 8.2 ± 0.4 | 13.2 ± 1.2 | 2.78 | 1.73 |
| Ligands | MAPKP38α (Kcal/mol) | GR-LBD (Kcal/mol) |
|---|---|---|
| Geraniol | −7.07 | −6.34 |
| Citronellol | −6.01 | −5.28 |
| Nerol | −5.75 | −5.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nenova, R.; Kalinov, K.; Nedeva, D.; Dobreva, A.; Vilhelmova-Ilieva, N.; Georgieva, A.; Iliev, I. Evaluation of the Safety and Antiproliferative Activity of Bulgarian Rose Essential Oil: An In Vitro and In Silico Model of Colorectal Adenocarcinoma. Curr. Issues Mol. Biol. 2025, 47, 649. https://doi.org/10.3390/cimb47080649
Nenova R, Kalinov K, Nedeva D, Dobreva A, Vilhelmova-Ilieva N, Georgieva A, Iliev I. Evaluation of the Safety and Antiproliferative Activity of Bulgarian Rose Essential Oil: An In Vitro and In Silico Model of Colorectal Adenocarcinoma. Current Issues in Molecular Biology. 2025; 47(8):649. https://doi.org/10.3390/cimb47080649
Chicago/Turabian StyleNenova, Rayna, Kalin Kalinov, Deyana Nedeva, Ana Dobreva, Neli Vilhelmova-Ilieva, Ani Georgieva, and Ivan Iliev. 2025. "Evaluation of the Safety and Antiproliferative Activity of Bulgarian Rose Essential Oil: An In Vitro and In Silico Model of Colorectal Adenocarcinoma" Current Issues in Molecular Biology 47, no. 8: 649. https://doi.org/10.3390/cimb47080649
APA StyleNenova, R., Kalinov, K., Nedeva, D., Dobreva, A., Vilhelmova-Ilieva, N., Georgieva, A., & Iliev, I. (2025). Evaluation of the Safety and Antiproliferative Activity of Bulgarian Rose Essential Oil: An In Vitro and In Silico Model of Colorectal Adenocarcinoma. Current Issues in Molecular Biology, 47(8), 649. https://doi.org/10.3390/cimb47080649








