K-562 Extracellular Vesicles Partially Protect Intact Cells from Oxidative Stress and Provide Limited Resistance to Imatinib
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. EVs Isolation
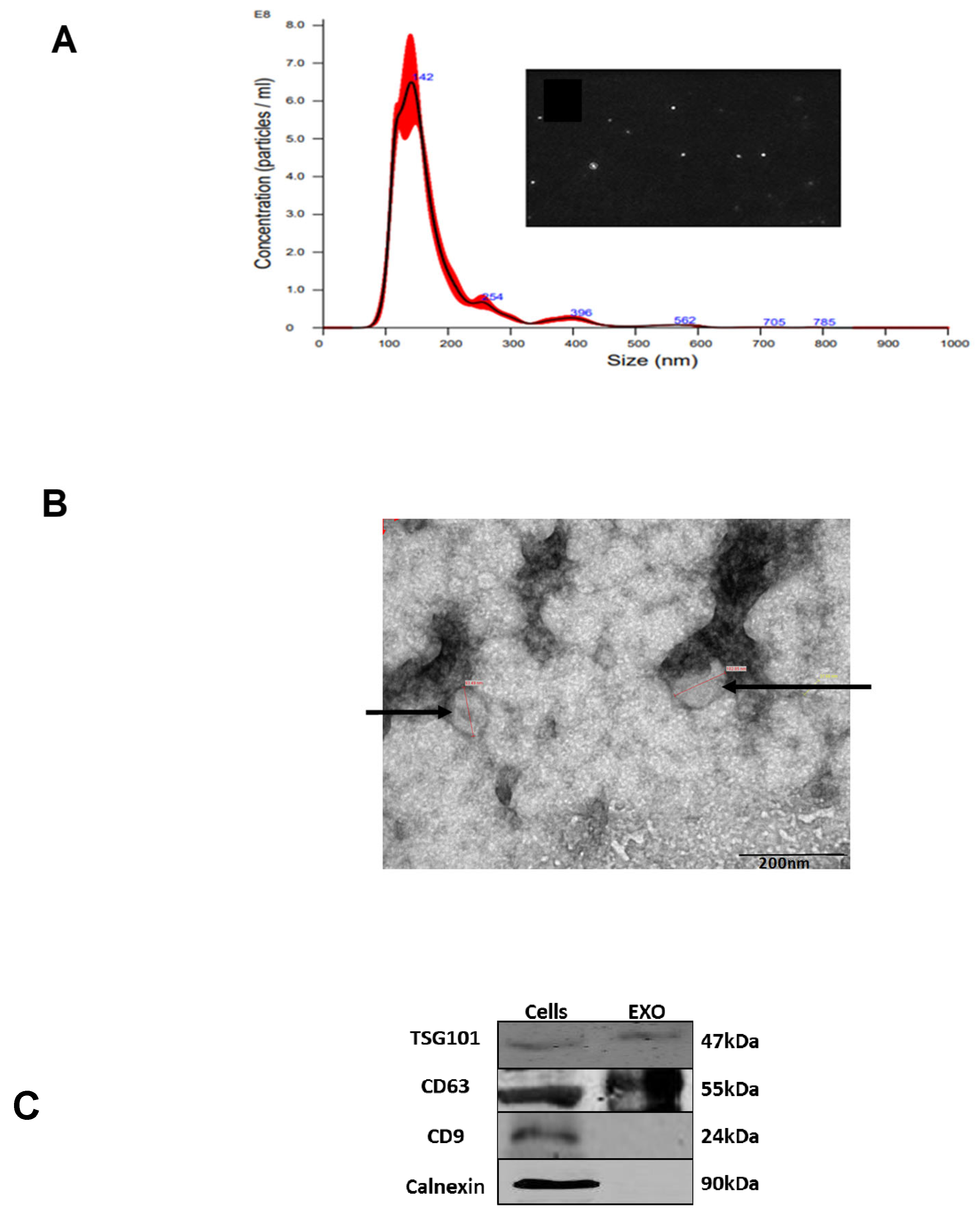
2.3. Characterization of EVs
2.4. Nano-Sight Tracking Analysis (NTA)
2.5. Transmission Electron Microscopy (TEM) Analysis
2.6. Western Immunoblotting Analysis
2.7. Proliferation Assay
2.8. RNA Extraction
2.9. Cell Viability
2.10. Exposure of Cells to Proliferative Stresses
2.11. Exposure of the Cells to Oxidative Stress
2.12. Evaluation of EVs Uptake
2.13. Apoptotic Cell Death Assay
2.14. Cell Cycle Analysis by Flow Cytometry
2.15. Measurement of Reactive Oxygen Species (ROS) by Flow Cytometry
2.16. EVs and Cell Co-Cultivation
2.17. Statistical Analysis
3. Results
3.1. Isolation and Characterization of K-562 Derived EVs
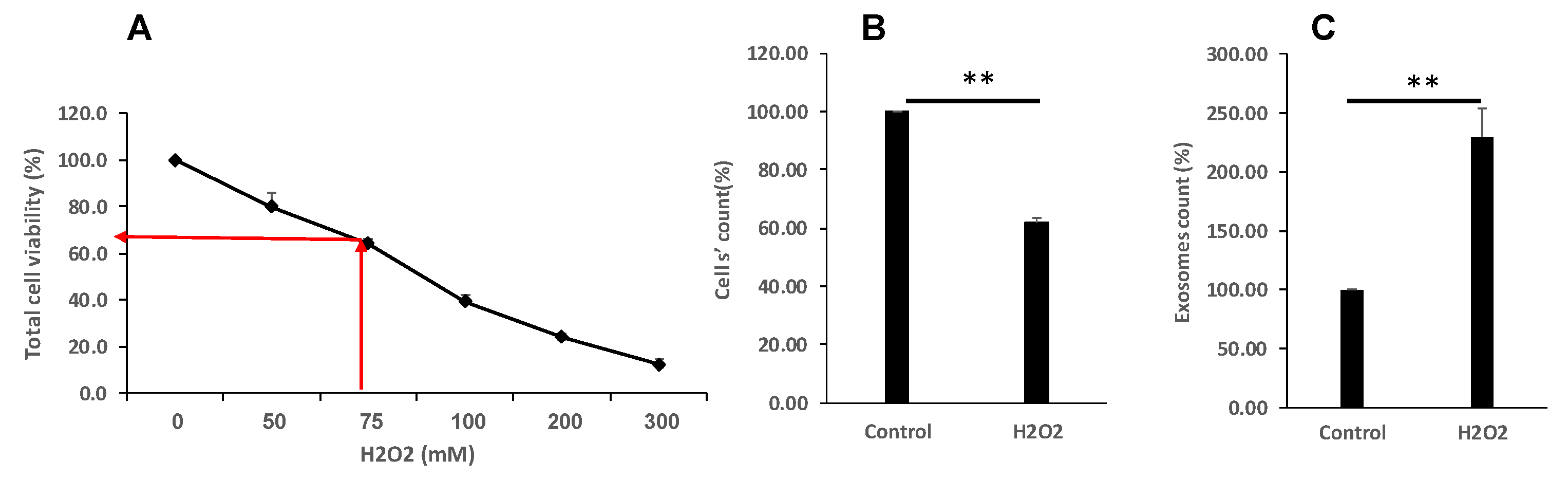
3.2. Oxidative Stress Reduced Cell Proliferation and Viability and Increase EVs Secretion
3.3. Proliferative Stress Reduced Cell Proliferation and Viability and Increase EVs Secretion

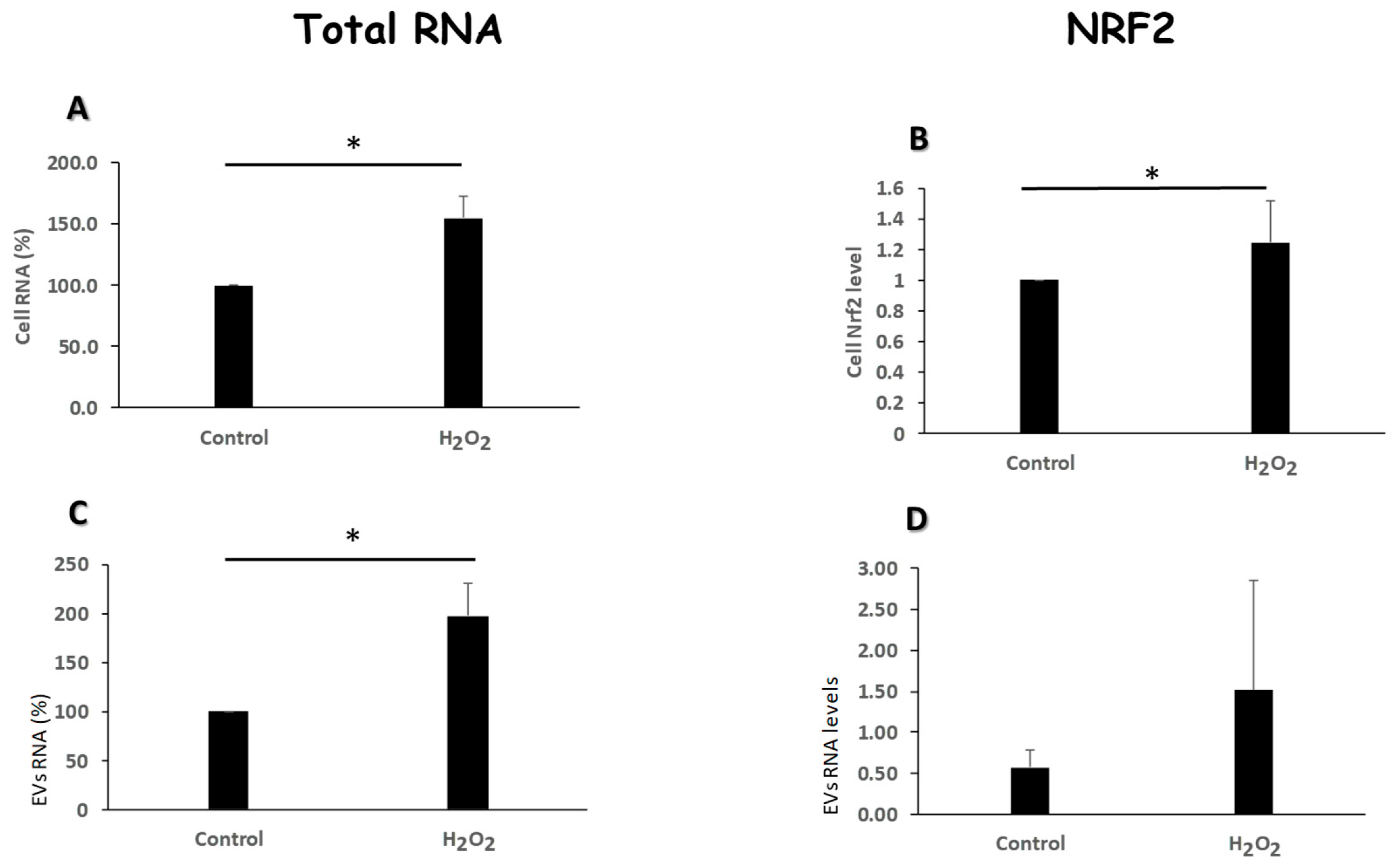
3.4. Oxidative Stress Modulates the Amount of RNA Content and Nrf2 Levels of Evs
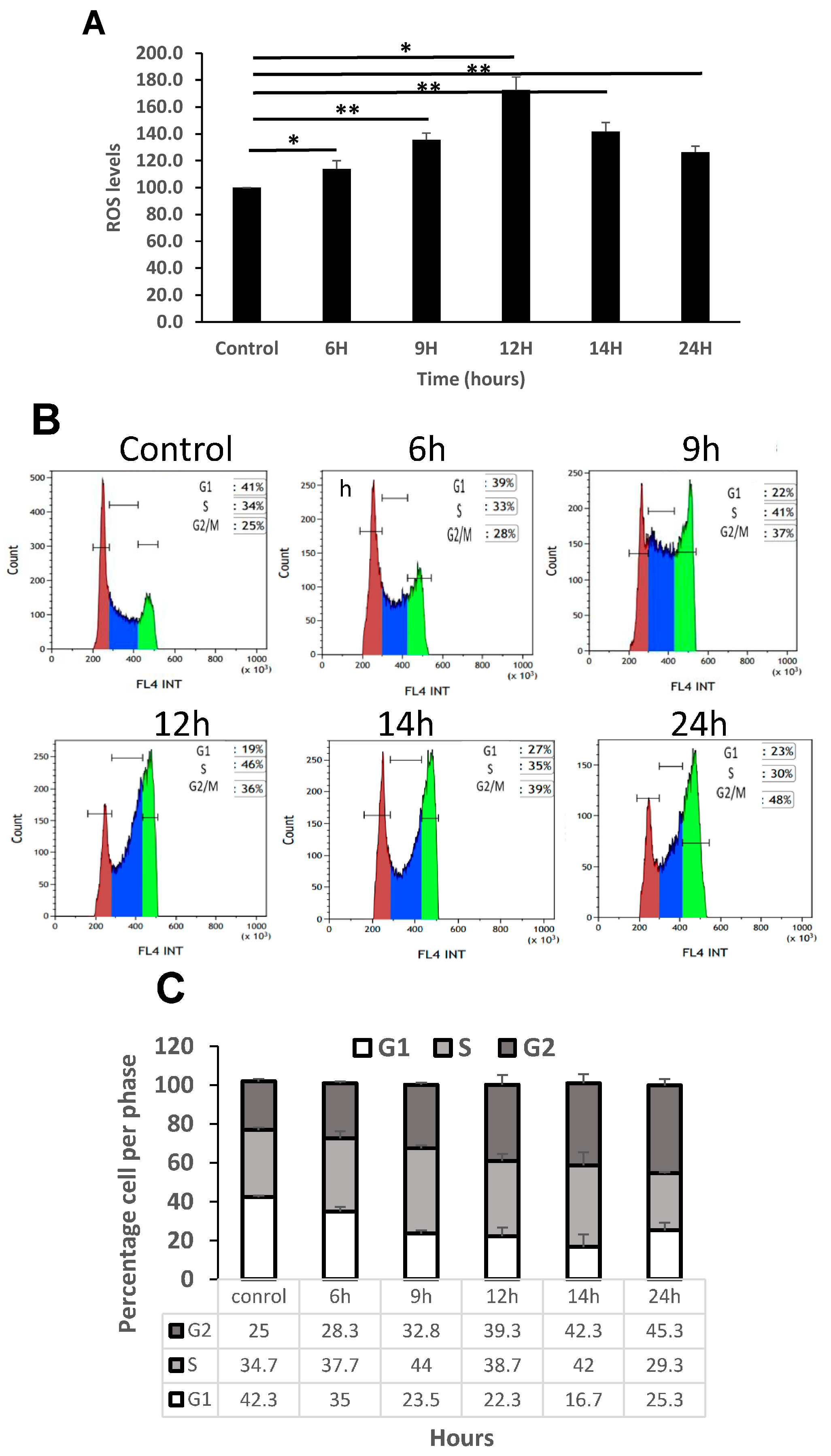
3.5. Increase in ROS Levels Arrest K562 Cells in G2 Phase of the Cell Cycle
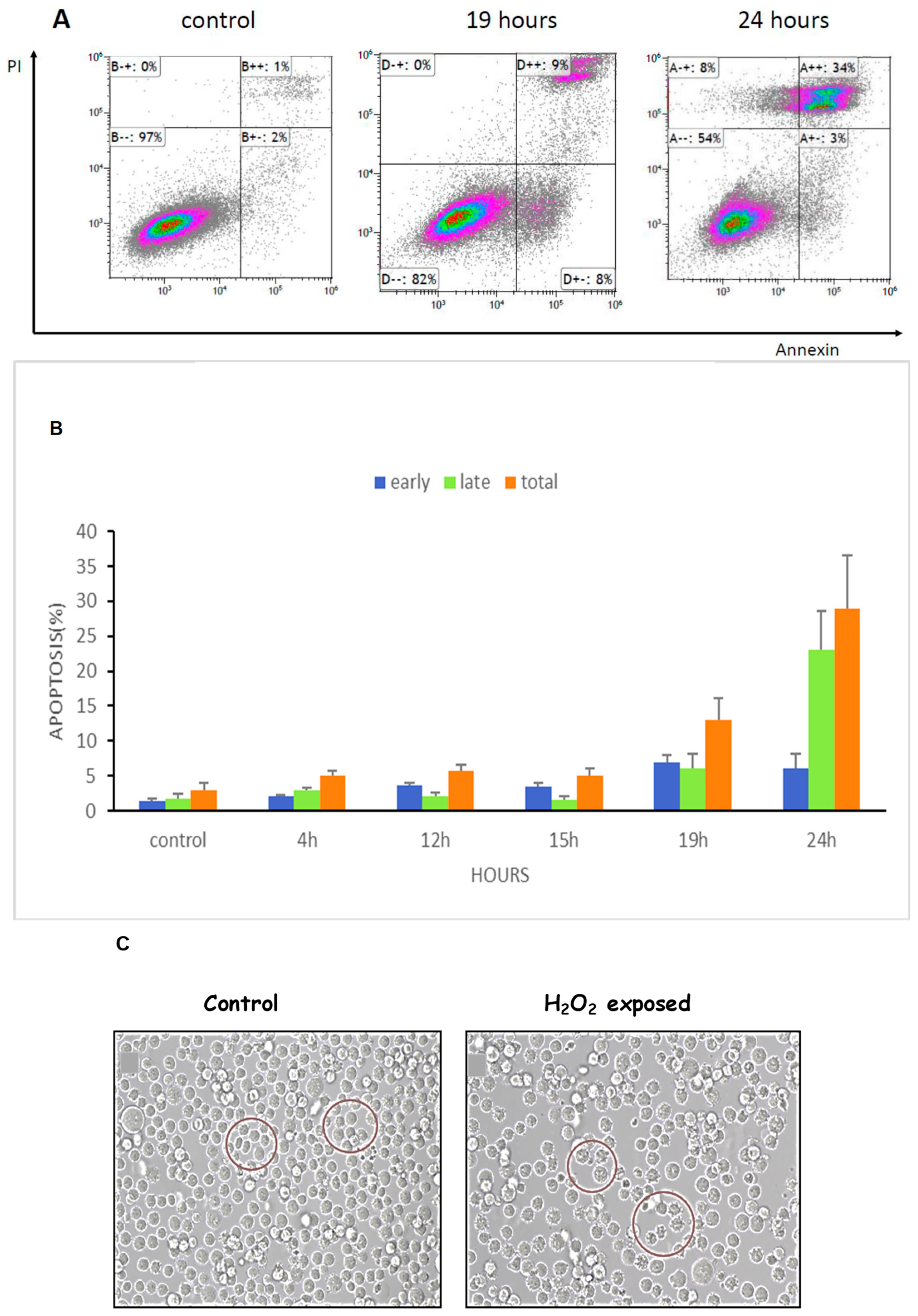
3.6. ROS Increased Apoptosis and Shrinkage of K562 Cells
3.7. EVs Are Taken up by K562 Cells in a Time and a Dose-Dependent Manner

3.8. EVs Derived from Oxidative Stress Exposed Cells (EVs-S) Increase the Proliferation of K562 Cells

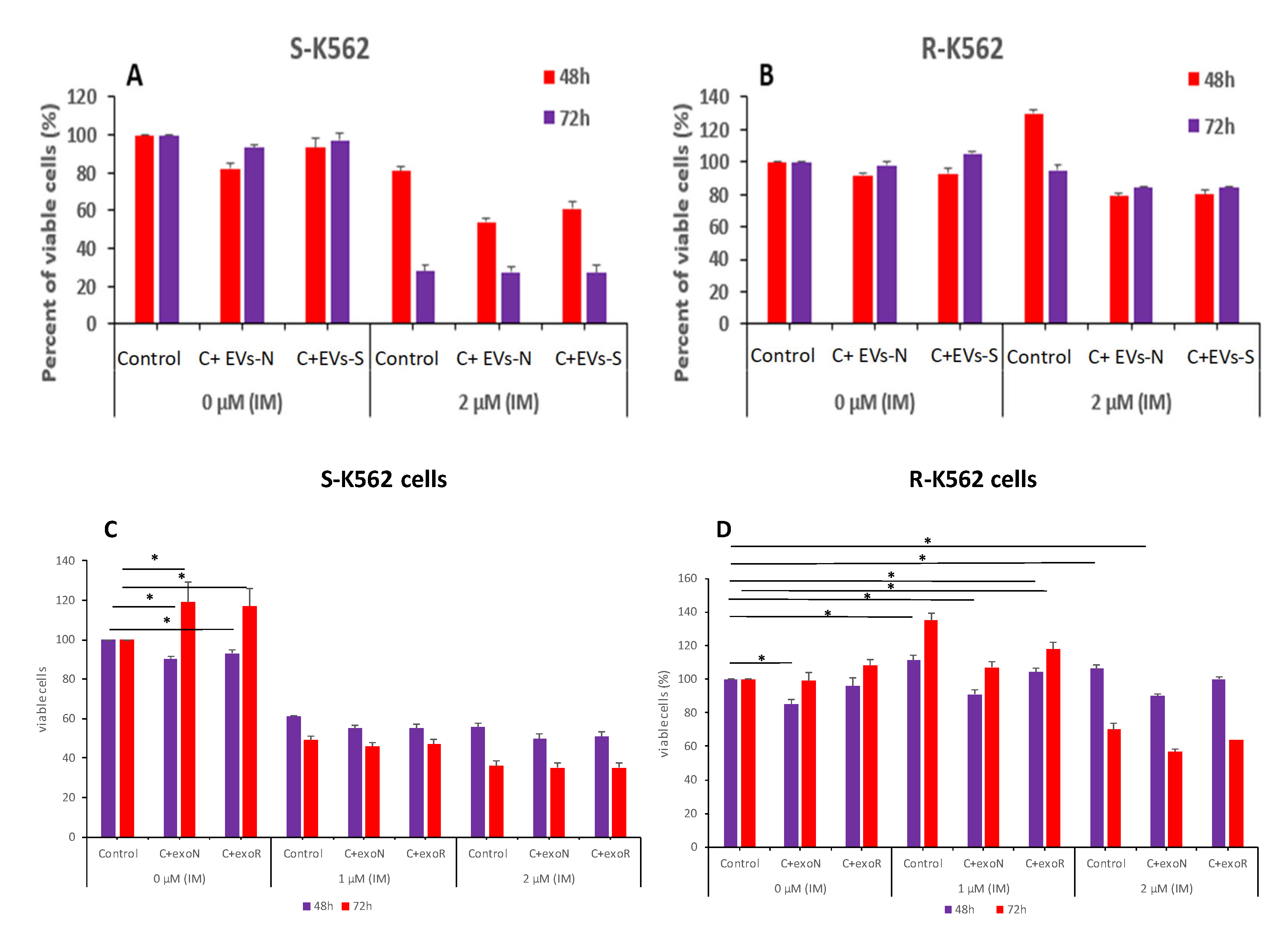
3.9. Cells That Are Resistant to IM Secrete Different Number of EVs Compared with IM Sensitive Cells
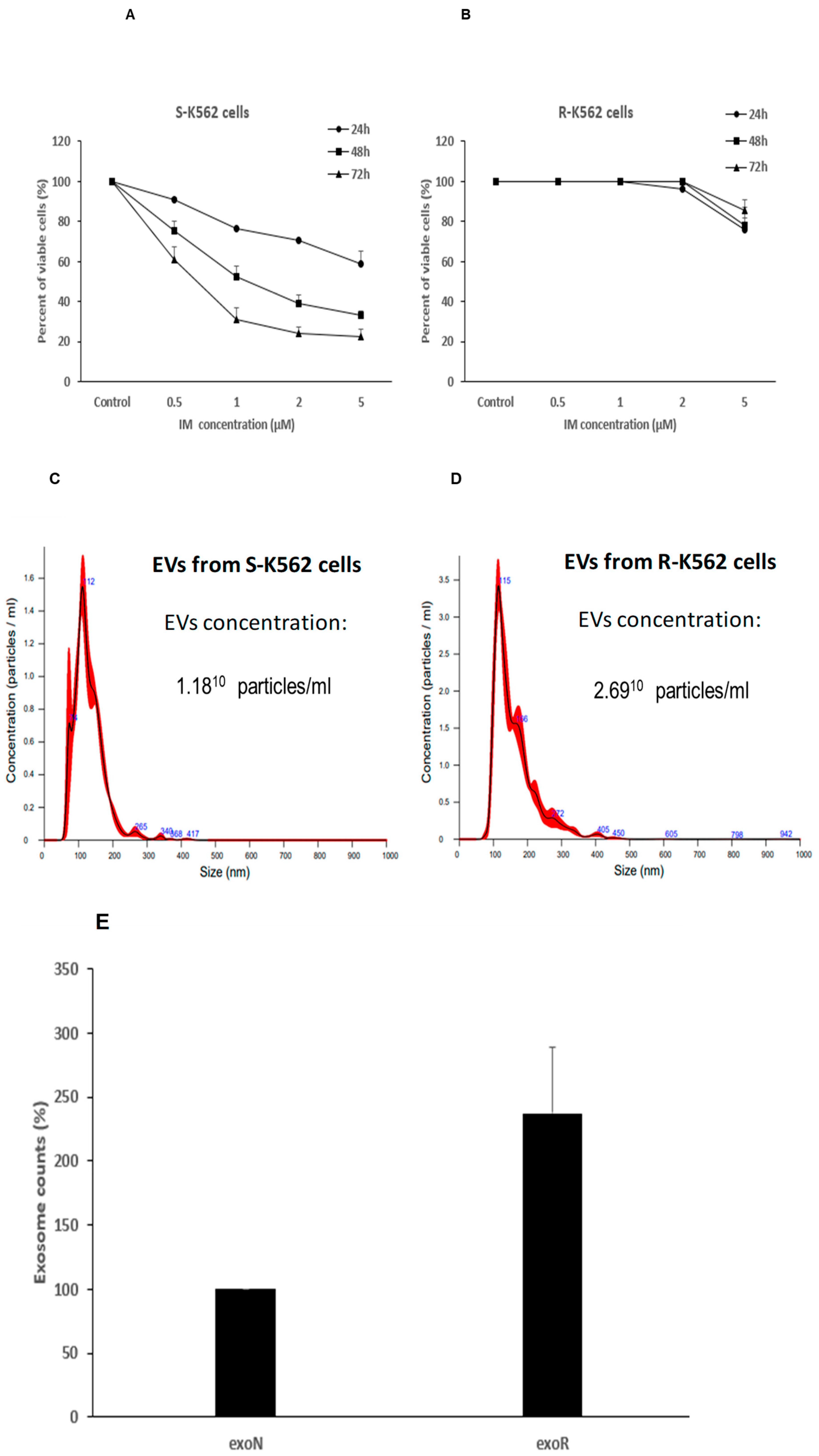
3.10. K562-R EVs Confer Partial Protection from Oxidative Stress and IM
- -
- The S-K562 EVs-treated (EVs-N and EVs-S) cells presented a decrease in cell proliferation (Figure 10A), compared with S-K562 cells without EVs treatment (control), suggesting that S-K562 cell-derived EVs do not confer drug resistance phenotype to S-K562 cells.
- -
- EVs from S-K562 cells (EVs-N and EVs-S) decreased cell viability of R-K562 cells in the presence of IM as shown in Figure 10B compared to control non-EVs exposed cells, although this did not reach statistically significance.
- -
- EVs from R-K562 cells (EVs-R) increased the survival of R-K562 cells in the presence of 1, 2 μM IM compared to EVs from S-K562 (EVs-N), as shown in Figure 10D.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CML | Chronic Myeloid Leukemia |
| S-K562 | Sensitive Cell Line |
| R-K562 | Resistant Cell Line |
| MtDNA | Mitochondrial DNA |
| ROS | Reactive Oxygen Species |
| EVs | Extracellular Vesicles |
| IM | Imatinib Mesylate |
| TKIs | Tyrosine Kinase Inhibitor |
| FBS | Fetal Bovine Serum |
| Nrf2 | The nuclear factor erythroid 2—related factor 2 |
References
- Quintás-Cardama, A.; Cortes, J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood 2009, 113, 1619–1630. [Google Scholar] [CrossRef]
- Samimi, A.; Kalantari, H.; Lorestani, M.Z.; Shirzad, R.; Saki, N. Oxidative stress in normal hematopoietic stem cells and leukemia. APMIS 2018, 126, 284–294. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Rana, A.; Datoguia, T.S.; Hamerschlak, N.; Brumatti, G. BCR-ABL1 Tyrosine Kinase Complex Signaling Transduction: Challenges to Overcome Resistance in Chronic Myeloid Leukemia. Pharmaceutics 2022, 14, 215. [Google Scholar] [CrossRef]
- Trela, E.; Glowacki, S.; Błasiak, J. Therapy of Chronic Myeloid Leukemia: Twilight of the Imatinib Era? ISRN Oncol. 2014, 2014, 596483. [Google Scholar] [CrossRef]
- Gainor, J.F.; Shaw, A.T. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J. Clin. Oncol. 2013, 31, 3987–3996. [Google Scholar] [CrossRef]
- Soverini, S.; Martinelli, G.; Rosti, G.; Iacobucci, I.; Baccarani, M. Advances in treatment of chronic myeloid leukemia with tyrosine kinase inhibitors: The evolving role of Bcr-Abl mutations and mutational analysis. Pharmacogenomics 2012, 13, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Eiring, A.M.; Khorashad, J.S.; Anderson, D.J.; Yu, F.; Redwine, H.M.; Mason, C.C.; Reynolds, K.R.; Clair, P.M.; Gantz, K.C.; Zhang, T.Y.; et al. β-Catenin is required for intrinsic but not extrinsic BCR-ABL1 kinase-independent resistance to tyrosine kinase inhibitors in chronic myeloid leukemia. Leukemia 2015, 29, 2328–2337. [Google Scholar] [CrossRef]
- Eiring, A.M.; Page, B.D.G.; Kraft, I.L.; Mason, C.C.; Vellore, N.A.; Resetca, D.; Zabriskie, M.S.; Zhang, T.Y.; Khorashad, J.S.; Engar, A.J.; et al. Combined STAT3 and BCR-ABL1 inhibition induces synthetic lethality in therapy-resistant chronic myeloid leukemia. Leukemia 2015, 29, 586–597, Erratum in Leukemia 2017, 31, 1253–1254. [Google Scholar] [CrossRef]
- Allegra, A.; Mirabile, G.; Caserta, S.; Stagno, F.; Russo, S.; Pioggia, G.; Gangemi, S. Oxidative Stress and Chronic Myeloid Leukemia: A Balance between ROS-Mediated Pro- and Anti-Apoptotic Effects of Tyrosine Kinase Inhibitors. Antioxidants 2024, 13, 461. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, N.; Safari, Z.; Pornour, M.; Amirizadeh, N.; Forouzandeh Moghadam, M.; Sadeghizadeh, M. Alteration of cellular and immune-related properties of bone marrow mesenchymal stem cells and macrophages by K562 chronic myeloid leukemia cell derived exosomes. J. Cell. Physiol. 2019, 234, 3697–3710. [Google Scholar] [CrossRef]
- Taverna, S.; Flugy, A.; Saieva, L.; Kohn, E.C.; Santoro, A.; Meraviglia, S.; De Leo, G.; Alessandro, R. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int. J. Cancer 2012, 130, 2033–2043. [Google Scholar] [CrossRef]
- Mineo, M.; Garfield, S.H.; Taverna, S.; Flugy, A.; De Leo, G.; Alessandro, R.; Kohn, E.C. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis 2012, 15, 33–45. [Google Scholar] [CrossRef]
- Ateeq, M.; Broadwin, M.; Sellke, F.W.; Abid, M.R. Extracellular Vesicles’ Role in Angiogenesis and Altering Angiogenic Signaling. Med. Sci. 2024, 12, 4. [Google Scholar] [CrossRef]
- Sattler, M.; Verma, S.; Shrikhande, G.; Byrne, C.H.; Pride, Y.B.; Winkler, T.; Greenfield, E.A.; Salgia, R.; Griffin, J.D. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J. Biol. Chem. 2000, 275, 24273–24278. [Google Scholar] [CrossRef]
- Koptyra, M.; Falinski, R.; Nowicki, M.O.; Stoklosa, T.; Majsterek, I.; Nieborowska-Skorska, M.; Blasiak, J.; Skorski, T. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood 2006, 108, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, E.; Hoser, G.; Wojcik, K.; Pawlowska, E.; Skorski, T.; Błasiak, J. UV differentially induces oxidative stress, DNA damage and apoptosis in BCR-ABL1-positive cells sensitive and resistant to imatinib. Int. J. Mol. Sci. 2015, 16, 18111–18128. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Oparka, M.; Walczak, J.; Malinska, D.; van Oppen, L.M.P.E.; Szczepanowska, J.; Koopman, W.J.H.; Wieckowski, M.R. Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods 2016, 109, 311. [Google Scholar] [CrossRef]
- Meshkini, A.; Yazdanparast, R. Involvement of oxidative stress in taxol-induced apoptosis in chronic myelogenous leukemia K562 cells. Exp. Toxicol. Pathol. 2012, 64, 357–365. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Dudas, J.; Skvortsov, S.; Ganswindt, U.; Riechelmann, H.; Skvortsova, I.I. Therapy resistance mediated by exosomes. Mol. Cancer 2019, 18, 58–69. [Google Scholar] [CrossRef]
- Nehrbas, J.; Butler, J.T.; Chen, D.W.; Kurre, P. Extracellular vesicles and chemotherapy resistance in the AML microenvironment. Front. Oncol. 2020, 10, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.H.; Wang, X.Z.; Zhang, J.; Chen, Q.G.; Li, S.Q.; Liu, X.Q.; Li, J.; Liu, J.; Yang, W.M.; Jiang, Y.H.; et al. Exosomes derived from imatinib-resistant chronic myeloid leukemia cells mediate a horizontal transfer of drug-resistant trait by delivering miR365. Exp. Cell Res. 2018, 362, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Mardani, R.; Jafari Najaf Abadi, M.H.; Motieian, M.; Taghizadeh-Boroujeni, S.; Bayat, A.; Farsinezhad, A.; Gheibi Hayat, S.M.; Motieian, M.; Pourghadamyari, H. MicroRNA in leukemia: Tumor suppressors and oncogenes with prognostic potential. J. Cell. Physiol. 2019, 234, 8465–8486. [Google Scholar] [CrossRef] [PubMed]
- Park, I.J.; Hwang, J.; Kim, Y.M.; Ha, J.; Park, O.J. Differential modulation of AMPK signaling pathways by low or high levels of exogenous reactive oxygen species in colon cancer cells. Ann. N.Y. Acad. Sci. 2006, 1091, 102–109. [Google Scholar] [CrossRef]
- Ammar, M.; Ben Mahmoud, L.; Medhaffar, M.; Ghozzi, H.; Sahnoun, Z.; Hakim, A.; Mseddi, M.; Elloumi, M.; Zeghal, K. Relationship of oxidative stress in the resistance to imatinib in Tunisian patients with chronic myeloid leukemia: A retrospective study. J. Clin. Lab. Anal. 2020, 34, e23050. [Google Scholar] [CrossRef]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular Vesicles under Oxidative Stress Conditions: Biological Properties and Physiological Roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef]
- Ilawadi, S.; Wang, X.; Gu, H.; Fan, G.C. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim. Biophys. Acta. 2015, 1852, 1–11. [Google Scholar] [CrossRef]
- Hrdinova, T.; Toman, O.; Dresler, J.; Klimentova, J.; Salovska, B.; Pajer, P.; Bartos, O.; Polivkova, V.; Linhartova, J.; Machova Polakova, K.; et al. Exosomes released by imatinib resistant K562 cells contain specific membrane markers, IFITM3, CD146 and CD36 and increase the survival of imatinib sensitive cells in the presence of imatinib. Int. J. Oncol. 2021, 58, 238–250. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, Y.; Yu, L.; Xue, T.M.; Xiao, Z.J.; Tin, P.C.; Fung, H.L.; Ma, H.T.; Yun, J.P.; Yam, J.W.P. NHE7 upregulation potentiates the uptake of small extracellular vesicles by enhancing maturation of macropinosomes in hepatocellular carcinoma. Cancer Commun. (Lond.) 2024, 44, 251–272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lerner, N.; Chen, I.; Schreiber-Avissar, S.; Beit-Yannai, E. Extracellular Vesicles Mediate Anti-Oxidative Response—In Vitro Study in the Ocular Drainage System. Int. J. Mol. Sci. 2020, 21, 6105. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, S.; Ren, Y.; Hu, C.; Zhang, A.; Lin, Y. Proteomics analysis reveals the correlation of programmed ROS-autophagy loop and dysregulated G1/S checkpoint with imatinib resistance in chronic myeloid leukemia cells. Proteomics 2022, 22, e2100094. [Google Scholar] [CrossRef] [PubMed]
- Brem, R.; Li, F.; Montaner, B.; Reelfs, O.; Karran, P. DNA breakage and cell cycle checkpoint abrogation induced by a therapeutic thiopurine and UVA radiation. Oncogene 2010, 29, 3953–3963. [Google Scholar] [CrossRef] [PubMed]
- Mutschelknaus, L.; Peters, C.; Winkler, K.; Yentrapalli, R.; Heider, T.; Atkinson, M.J.; Moertl, S. Exosomes derived from squamous head and neck cancer promote cell survival after ionizing radiation. PLoS ONE 2016, 11, e0152213. [Google Scholar] [CrossRef]
- de Jong, O.G.; Verhaar, M.C.; Chen, Y.; Vader, P.; Gremmels, H.; Posthuma, G.; Schiffelers, R.M.; Gucek, M.; van Balkom, B.W. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles 2012, 1, 18396. [Google Scholar] [CrossRef]
- Raimondo, S.; Saieva, L.; Corrado, C.; Fontana, S.; Flugy, A.; Rizzo, A.; De Leo, G.; Alessandro, R. Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Commun. Signal. 2015, 13, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Yousefpour, P.; Chilkoti, A. Co-opting biology to deliver drugs. Biotechnol. Bioeng. 2014, 111, 1699–1716. [Google Scholar] [CrossRef]
- Braccioli, L.; van Velthoven, C.; Heijnen, C.J. Exosomes: A new weapon to treat the central nervous system. Mol. Neurobiol. 2014, 49, 113–119. [Google Scholar] [CrossRef]
- Bourgeais, J.; Ishac, N.; Medrzycki, M.; Brachet-Botineau, M.; Desbourdes, L.; Gouilleux-Gruart, V.; Pecnard, E.; Rouleux-Bonnin, F.; Gyan, E.; Domenech, J.; et al. Oncogenic STAT5 signaling promotes oxidative stress in chronic myeloid leukemia cells by repressing antioxidant defenses. Oncotarget 2017, 8, 41876–41889. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Taverna, S.; Amodeo, V.; Saieva, L.; Russo, A.; Giallombardo, M.; De Leo, G.; Alessandro, R. Exosomal shuttling of miR-126 in endothelial cells modulates adhesive and migratory abilities of chronic myelogenous leukemia cells. Mol. Cancer 2014, 13, 169. [Google Scholar] [CrossRef]
- Li, M.Y.; Zhao, C.; Chen, L.; Yao, F.Y.; Zhong, F.M.; Chen, Y.; Xu, S.; Jiang, J.Y.; Yang, Y.L.; Min, Q.H.; et al. Quantitative Proteomic Analysis of Plasma Exosomes to Identify the Candidate Biomarker of Imatinib Resistance in Chronic Myeloid Leukemia Patients. Front. Oncol. 2021, 11, 779567. [Google Scholar] [CrossRef]
- Lakshmi, S.; Hughes, T.A.; Priya, S. Exosomes and exosomal RNAs in breast cancer: A status update. Eur. J. Cancer 2021, 144, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Liu, A.; Wang, J.; Li, L.; Chen, X.; Gao, X.; Xue, Y.; Zhang, X.; Liu, Y. Distinct Dasatinib-Induced Mechanisms of Apoptotic Response and Exosome Release in Imatinib-Resistant Human Chronic Myeloid Leukemia Cells. Int. J. Mol. Sci. 2016, 17, 531. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, H.; Li, P.; Chen, Y.; Zhang, M.; Yuan, Z.; Zhang, Y.; Xu, Z.; Luo, G.; Fang, Y.; et al. Exosomes: A New Pathway for Cancer Drug Resistance. Front. Oncol. 2021, 11, 743556. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Yang, Y.; Chen, H.; Tu, H.; Li, J. Exosomes from Bone Marrow Microenvironment-Derived Mesenchymal Stem Cells Affect CML Cells Growth and Promote Drug Resistance to Tyrosine Kinase Inhibitors. Stem Cells Int. 2020, 2020, 8890201. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.; Raimondo, S.; Calabrese, G.; Forte, S.; Cristaldi, M.; Patinella, A.; Memeo, L.; Manno, M.; Raccosta, S.; Diana, P.; et al. Interleukin 3-receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics 2017, 7, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Wuxiao, Z.; Wang, H.; Su, Q.; Zhou, H.; Hu, M.; Tao, S.; Xu, L.; Chen, Y.; Hao, X. MicroRNA 145 promotes the apoptosis of leukemic stem cells and enhances drug resistant K562/ADM cell sensitivity to adriamycin via the regulation of ABCE1. Int. J. Mol. Med. 2020, 46, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.Q.; Huang, B.; Li, J.; Liu, J.; Chen, X.M.; Xu, Y.M.; Chen, X.; Zhang, H.B.; Hu, L.H.; Wang, X.Z. Selective miRNA expression profile in chronic myeloid leukemia K562 cell-derived exosomes. Asian Pac. J. Cancer Prev. 2013, 14, 7501–7508. [Google Scholar] [CrossRef]
- Bennett, P.M. Plasmid encoded antibiotic resistance: Acquisition and transfer of antibiotic resistance genes in bacteria. Br. J. Pharmacol. 2008, 153 (Suppl. S1), S347–S357. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, W.L.; Ye, Y.Y.; Bai, X.C.; Liu, R.Q.; Chang, L.F.; Zhou, Q.; Sui, S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010, 11, 675–687. [Google Scholar] [CrossRef]
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbiet, J.; Beery, E.; Sarsor, Z.; Raanani, P.; Uziel, O. K-562 Extracellular Vesicles Partially Protect Intact Cells from Oxidative Stress and Provide Limited Resistance to Imatinib. Curr. Issues Mol. Biol. 2025, 47, 666. https://doi.org/10.3390/cimb47080666
Sbiet J, Beery E, Sarsor Z, Raanani P, Uziel O. K-562 Extracellular Vesicles Partially Protect Intact Cells from Oxidative Stress and Provide Limited Resistance to Imatinib. Current Issues in Molecular Biology. 2025; 47(8):666. https://doi.org/10.3390/cimb47080666
Chicago/Turabian StyleSbiet, Jiana, Einat Beery, Zinab Sarsor, Pia Raanani, and Orit Uziel. 2025. "K-562 Extracellular Vesicles Partially Protect Intact Cells from Oxidative Stress and Provide Limited Resistance to Imatinib" Current Issues in Molecular Biology 47, no. 8: 666. https://doi.org/10.3390/cimb47080666
APA StyleSbiet, J., Beery, E., Sarsor, Z., Raanani, P., & Uziel, O. (2025). K-562 Extracellular Vesicles Partially Protect Intact Cells from Oxidative Stress and Provide Limited Resistance to Imatinib. Current Issues in Molecular Biology, 47(8), 666. https://doi.org/10.3390/cimb47080666




