BCG Impact on PD-1/PD-L1 Expression in Peripheral Immunocytes of Cancer Patients—A Potential Explanation for Its Activity in Preventing Alzheimer’s Disease
Abstract
1. Introduction
1.1. Justification of Using Vaccines to Study Alzheimer’s Disease
1.2. Peripheral Immunocytes Involvement in AD
1.3. Repurposing of PBMCs Under BCG Vaccine Treatment for Studying Molecules Involved in AD
1.4. PD-L1 Signaling in PD-1 Target Cells
1.5. Aims of Studying PD-1/PD-L1 in PBMCS Under BCG Concerning Alzheimer’s Disease
2. Materials and Methods
2.1. Treatment Protocol
2.2. PBMC Sampling
2.3. Thawing of PBMC and Protein Extraction
2.4. Preparation of Immune-Electrophoresis Samples
2.5. Antibodies
2.6. Analysis of the Results
2.7. Presentation of the Results
2.7.1. Bar Graphs of Mean Values Compared by T-Test
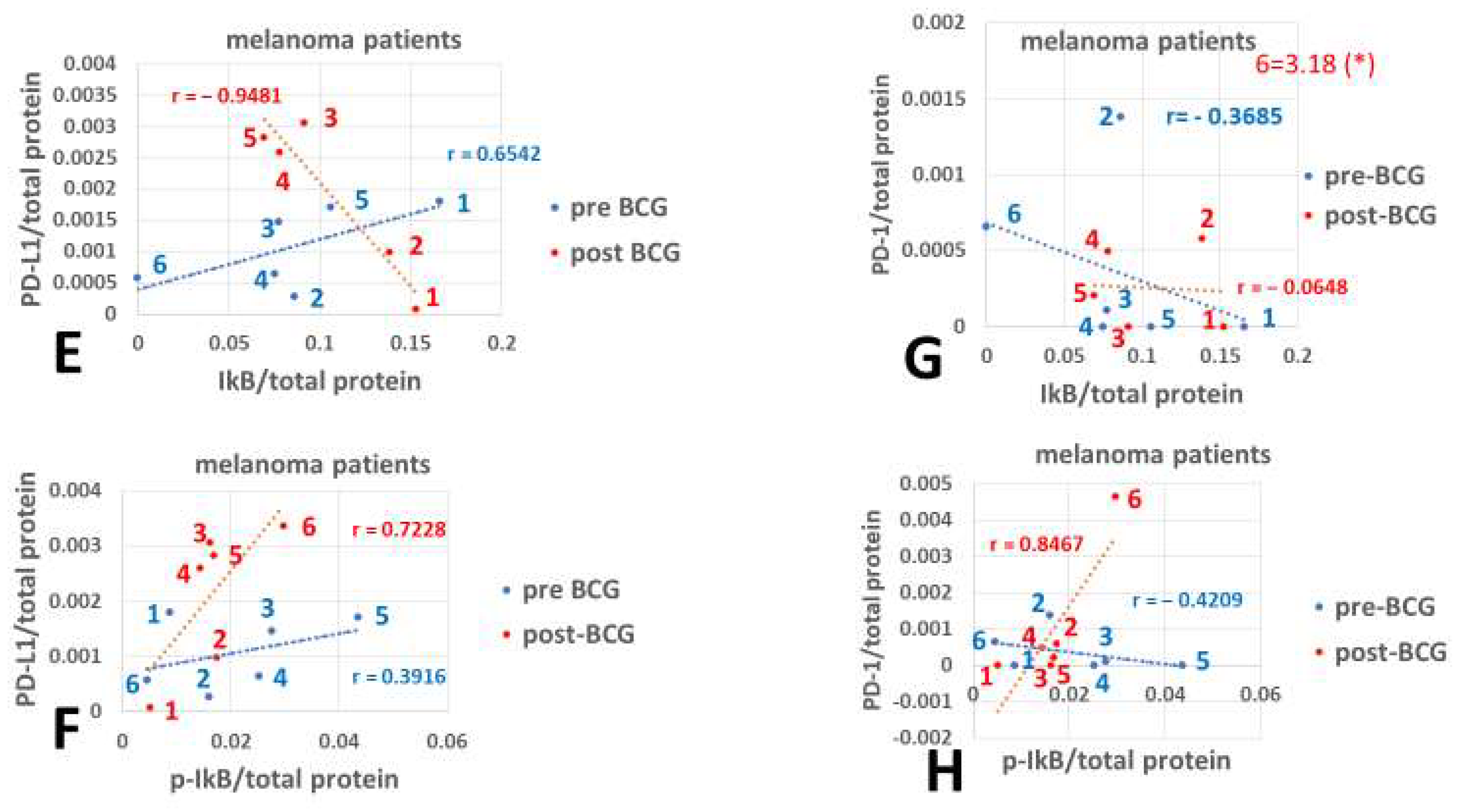
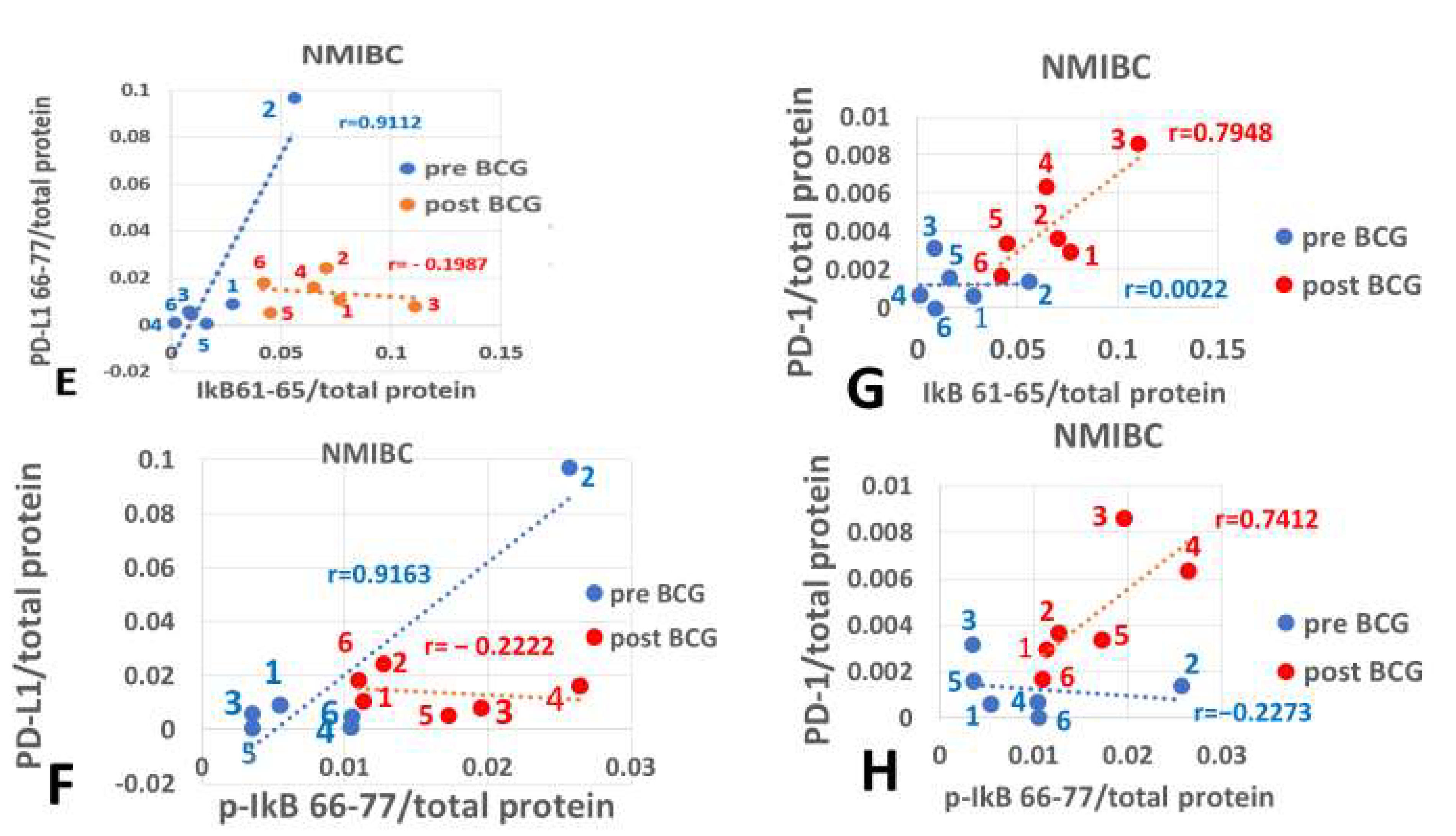
2.7.2. Correlation Coefficients of Pre- and Post-BCG Values
2.7.3. Comparison Between Pearson Correlation Coefficients
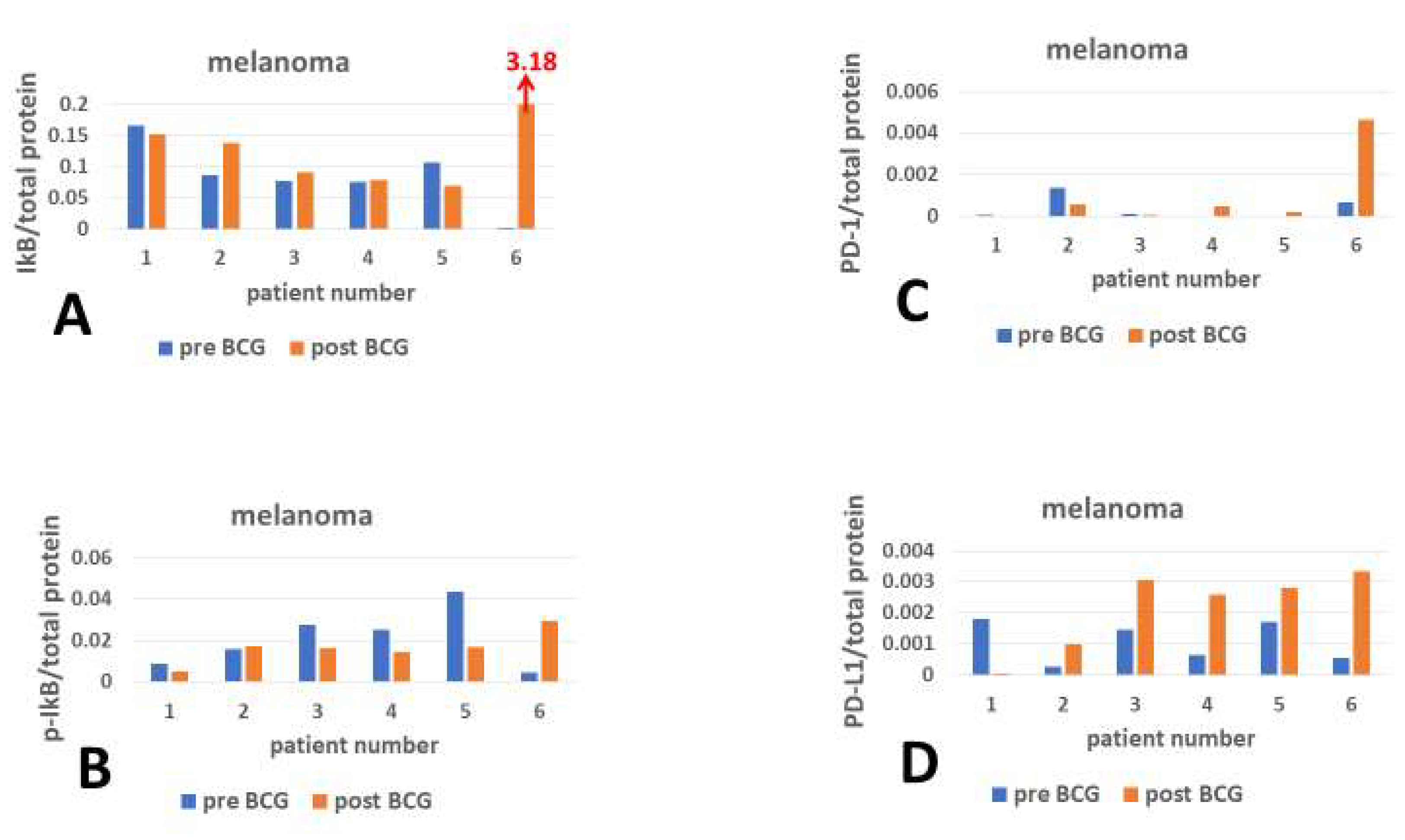
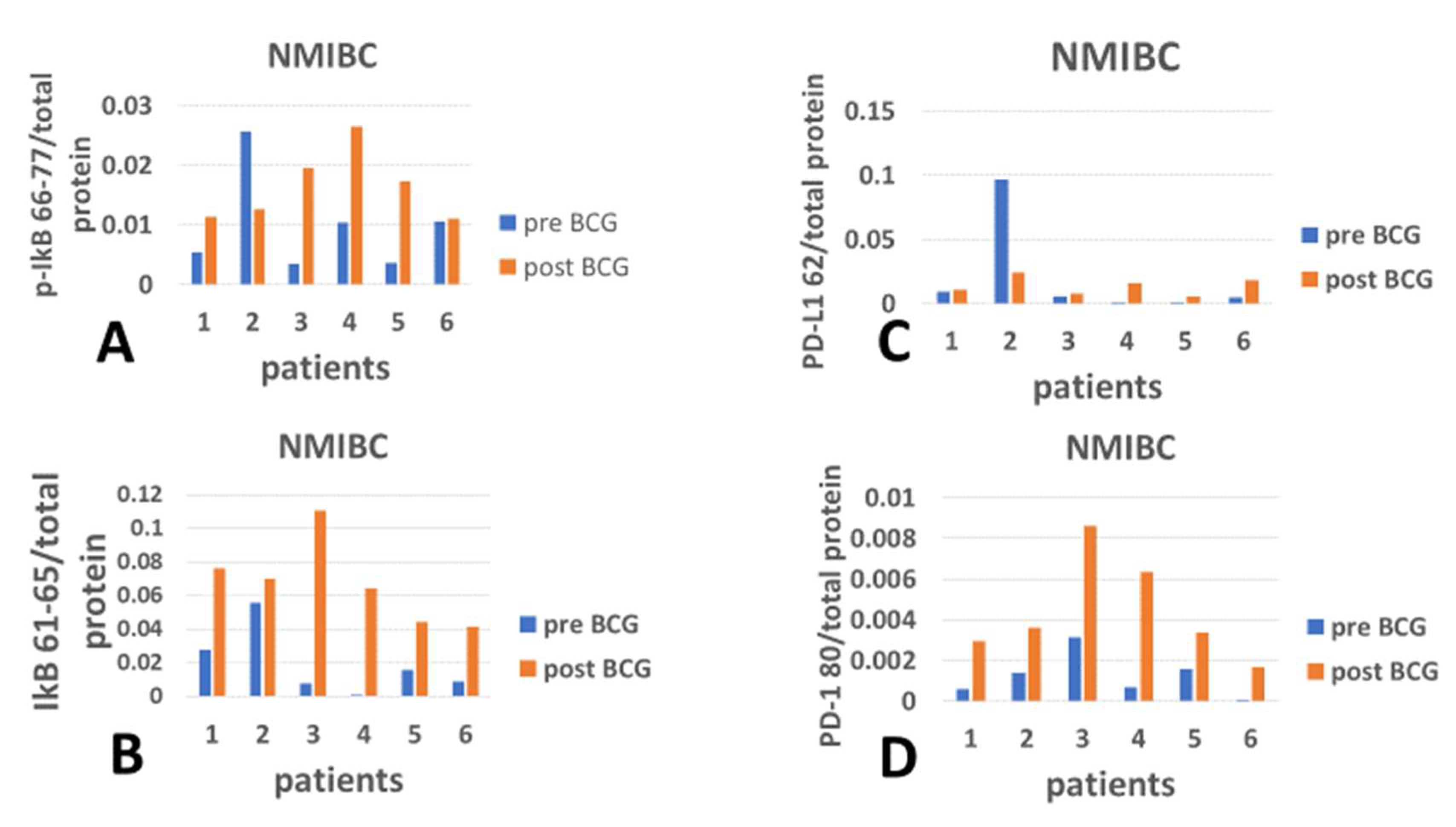
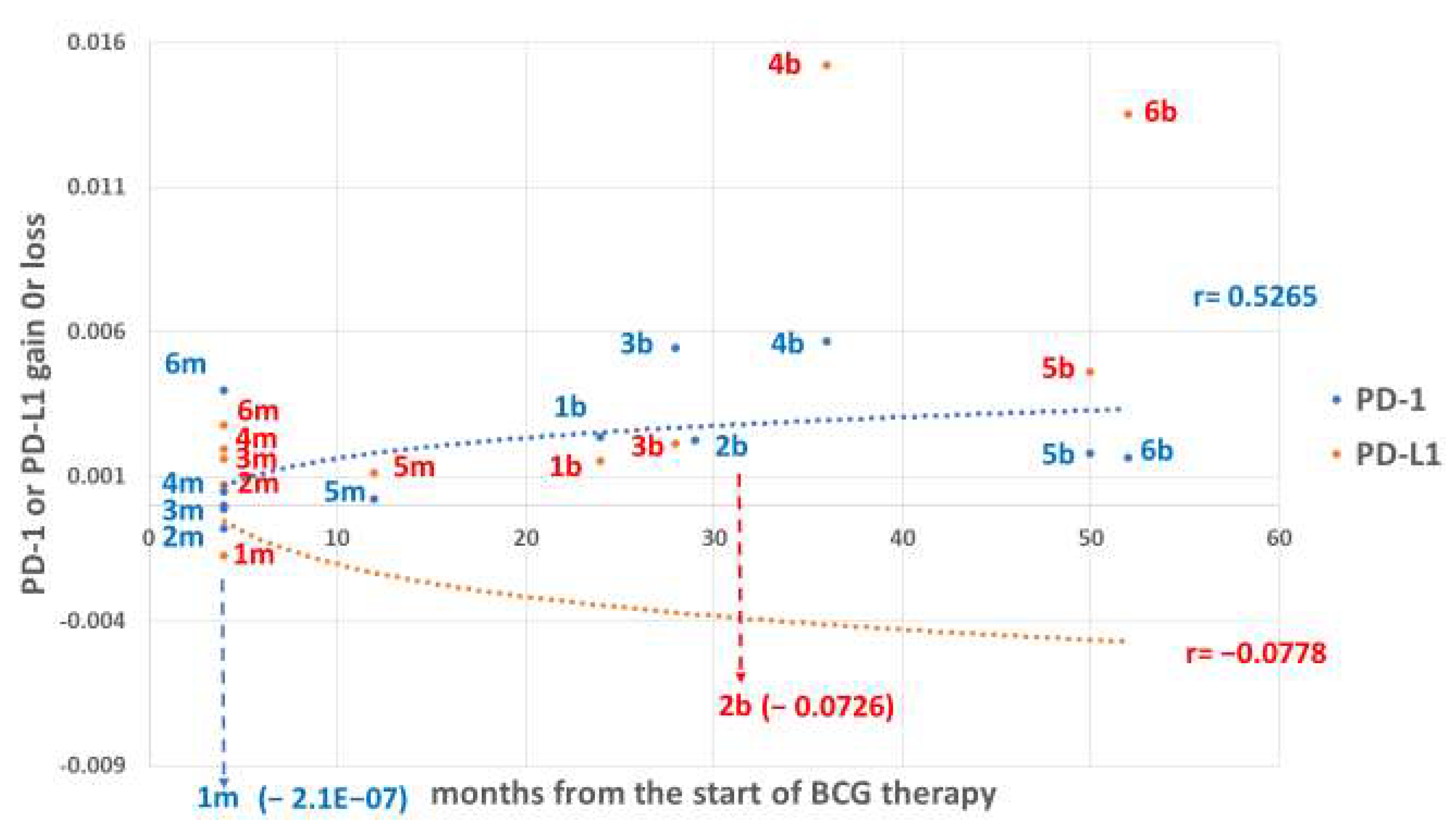
2.8. Presentation of the Gains and Losses of Checkpoint Proteins
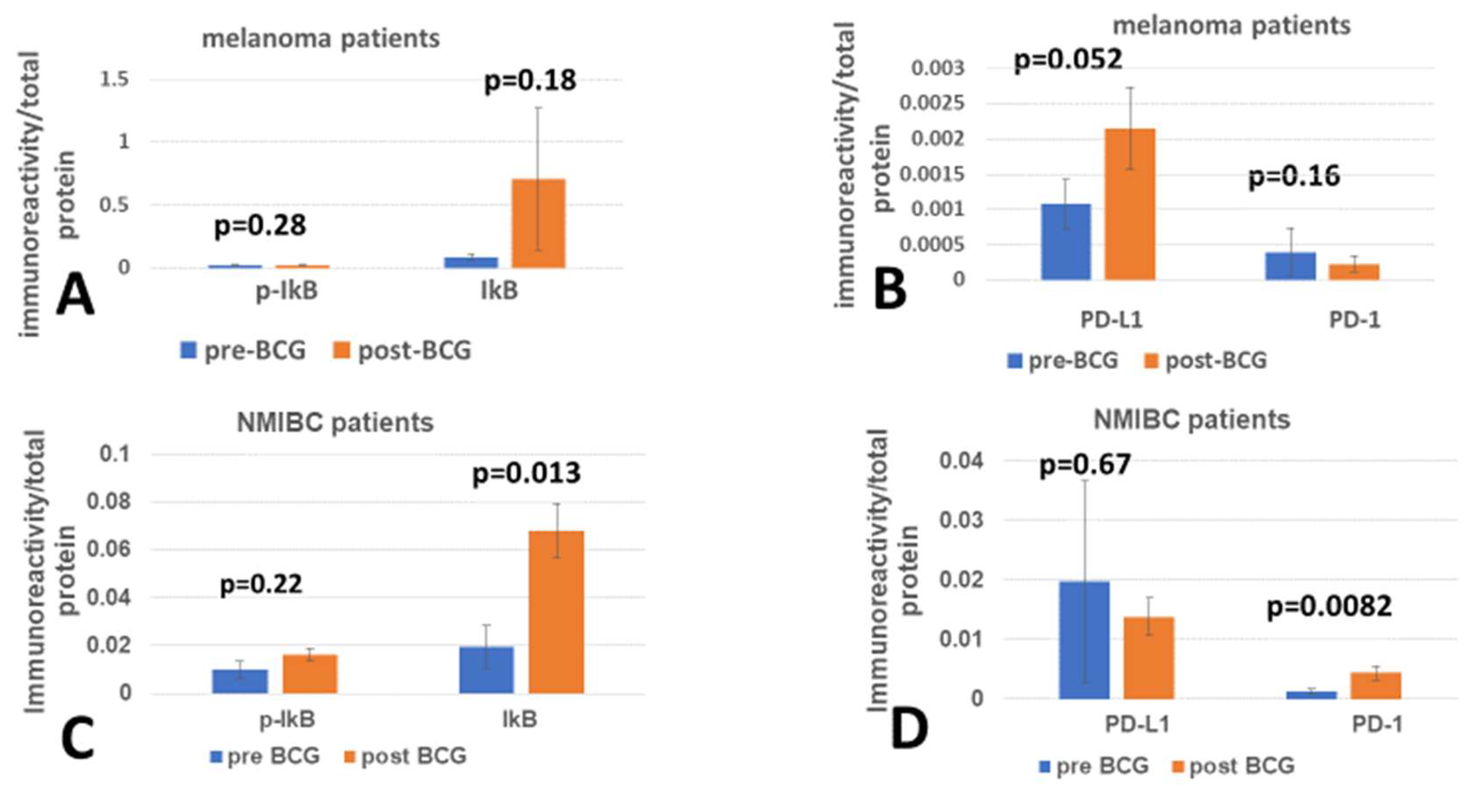
3. Results
4. Discussion
4.1. Analysis of PBMCs Justified Based on Previous Studies
4.2. Checkpoint Proteins’ Relation to Inflammation Inhibition
4.2.1. PD-L1 Versus Inflammation Inhibitor
4.2.2. PD-1 Versus Inflammation Inhibitor
4.3. Temporal Requirement for BCG to Change PD-1/PD-L1 Expression Levels
4.4. Hypothetical Mechanism by Which PD-1/PD-L1 Protects Against AD
4.5. Limitations of This Study
4.6. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Mao, C.; Hou, Y.; Luo, Y.; Binder, J.L.; Zhou, Y.; Bekris, L.M.; Shin, J.; Hu, M.; Wang, F.; et al. Interpretable deep learning translation of GWAS and multi-omics findings to identify pathobiology and drug repurposing in Alzheimer’s disease. Cell Rep. 2022, 41, 111717. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Klein, B.Y.; Cohen, I.R.; Ben-Hur, T.; Greenblatt, C.L.; Bercovier, H. Bacillus Calmette-Guerin (BCG) therapy lowers the incidence of Alzheimer’s disease in bladder cancer patients. PLoS ONE 2019, 14, e0224433. [Google Scholar] [CrossRef]
- Scherrer, J.F.; Salas, J.; Wiemken, T.L.; Jacobs, C.; Morley, J.E.; Hoft, D.F. Lower Risk for Dementia Following Adult Tetanus, Diphtheria, and Pertussis (Tdap) Vaccination. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1436–1443. [Google Scholar] [CrossRef]
- Poynton-Smith, E.; Orrell, M.; Morley, J.E.; Scherrer, J.F. Could routine vaccinations prevent dementia? Int. J. Geriatr. Psychiatry 2023, 38, e5978. [Google Scholar] [CrossRef]
- Netea, M.G. Training innate immunity: The changing concept of immunological memory in innate host defence. Eur. J. Clin. Investig. 2013, 43, 881–884. [Google Scholar] [CrossRef]
- Dias, H.F.; Kuhtreiber, W.M.; Nelson, K.J.; Ng, N.C.; Zheng, H.; Faustman, D.L. Epigenetic changes related to glucose metabolism in type 1 diabetes after BCG vaccinations: A vital role for KDM2B. Vaccine 2022, 40, 1540–1554. [Google Scholar] [CrossRef]
- Schwartz, M.; Moalem, G.; Leibowitz-Amit, R.; Cohen, I.R. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999, 22, 295–299. [Google Scholar] [CrossRef]
- Schwartz, M. Can immunotherapy treat neurodegeneration? Science 2017, 357, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Castellani, G.; Schwartz, M. Immunological Features of Non-neuronal Brain Cells: Implications for Alzheimer’s Disease Immunotherapy. Trends Immunol. 2020, 41, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Heavener, K.S.; Bradshaw, E.M. The aging immune system in Alzheimer’s and Parkinson’s diseases. Semin. Immunopathol. 2022, 44, 649–657. [Google Scholar] [CrossRef]
- Nguyen, J.N.; Chauhan, A. Bystanders or not? Microglia and lymphocytes in aging and stroke. Neural Regen. Res. 2023, 18, 1397–1403. [Google Scholar] [CrossRef]
- Kim, J.S.; Trzebanski, S.; Shin, S.H.; Schori, L.; Friedman, G.R.F.; Ilani, N.C.; Kadam, A.; Vicario, R.; Aust, O.; Bugaeva, P.; et al. Clonal hematopoiesis-associated motoric deficits caused by monocyte-derived microglia accumulating in aging mice. Cell Rep. 2025, 44, 115609. [Google Scholar] [CrossRef]
- Weimann, J.M.; Charlton, C.A.; Brazelton, T.R.; Hackman, R.C.; Blau, H.M. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc. Natl. Acad. Sci. USA 2003, 100, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Asheuer, M.; Pflumio, F.; Benhamida, S.; Dubart-Kupperschmitt, A.; Fouquet, F.; Imai, Y.; Aubourg, P.; Cartier, N. Human CD34 + cells differentiate into microglia and express recombinant therapeutic protein. Proc. Natl. Acad. Sci. USA 2004, 101, 3557–3562. [Google Scholar] [CrossRef]
- Massengale, M.; Wagers, A.J.; Vogel, H.; Weissman, I.L. Hematopoietic cells maintain hematopoietic fates upon entering the brain. J. Exp. Med. 2005, 201, 1579–1589. [Google Scholar] [CrossRef]
- Masliah, E.; Dumaop, W.; Galasko, D.; Desplats, P. Distinctive patterns of DNA methylation associated with Parkinson disease: Identification of concordant epigenetic changes in brain and peripheral blood leukocytes. Epigenetics 2013, 8, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.Y.; Gofrit, O.N.; Greenblatt, C.L. Testing Protein Stress Signals in Peripheral Immunocytes Under the Same Treatment Capable of Decreasing the Incidence of Alzheimer’s Disease in Bladder Cancer Patients. Curr. Issues Mol. Biol. 2025, 47, 392. [Google Scholar] [CrossRef]
- Klein, B.Y.; Ben-David, I.; Gofrit, O.N.; Greenblatt, C.L. Repurposing peripheral immunocytes of Bacillus Calmette Guerin-vaccinated melanoma patients to reveal preventive Alzheimer’s disease mechanisms, possibly via the unfolded protein response. J. Alzheimers Dis. Rep. 2025, 9, 25424823241309664. [Google Scholar] [CrossRef]
- Baruch, K.; Deczkowska, A.; Rosenzweig, N.; Tsitsou-Kampeli, A.; Sharif, A.M.; Matcovitch-Natan, O.; Kertser, A.; David, E.; Amit, I.; Schwartz, M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 2016, 22, 135–137. [Google Scholar] [CrossRef]
- Rosenzweig, N.; Dvir-Szternfeld, R.; Tsitsou-Kampeli, A.; Keren-Shaul, H.; Ben-Yehuda, H.; Weill-Raynal, P.; Cahalon, L.; Kertser, A.; Baruch, K.; Amit, I.; et al. PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat. Commun. 2019, 10, 465. [Google Scholar] [CrossRef]
- Dvir-Szternfeld, R.; Castellani, G.; Arad, M.; Cahalon, L.; Colaiuta, S.P.; Keren-Shaul, H.; Croese, T.; Burgaletto, C.; Baruch, K.; Ulland, T.; et al. Alzheimer’s disease modification mediated by bone marrow-derived macrophages via a TREM2-independent pathway in mouse model of amyloidosis. Nat. Aging 2022, 2, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Fousteri, G. Role of the PD-1/PD-L1 Dyad in the Maintenance of Pancreatic Immune Tolerance for Prevention of Type 1 Diabetes. Front. Endocrinol. 2020, 11, 569. [Google Scholar] [CrossRef]
- Chang, C.H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef]
- Gato-Canas, M.; Zuazo, M.; Arasanz, H.; Ibanez-Vea, M.; Lorenzo, L.; Fernandez-Hinojal, G.; Vera, R.; Smerdou, C.; Martisova, E.; Arozarena, I.; et al. PDL1 Signals through Conserved Sequence Motifs to Overcome Interferon-Mediated Cytotoxicity. Cell Rep. 2017, 20, 1818–1829. [Google Scholar] [CrossRef]
- Escors, D.; Gato-Canas, M.; Zuazo, M.; Arasanz, H.; Garcia-Granda, M.J.; Vera, R.; Kochan, G. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct. Target. Ther. 2018, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Lotem, M.; Peretz, T.; Drize, O.; Gimmon, Z.; Ad El, D.; Weitzen, R.; Goldberg, H.; Ben David, I.; Prus, D.; Hamburger, T.; et al. Autologous cell vaccine as a post operative adjuvant treatment for high-risk melanoma patients (AJCC stages III and IV). The new American Joint Committee on Cancer. Br. J. Cancer 2002, 86, 1534–1539. [Google Scholar] [CrossRef]
- Lotem, M.; Merims, S.; Frank, S.; Hamburger, T.; Nissan, A.; Kadouri, L.; Cohen, J.; Straussman, R.; Eisenberg, G.; Frankenburg, S.; et al. Adjuvant Autologous Melanoma Vaccine for Macroscopic Stage III Disease: Survival, Biomarkers, and Improved Response to CTLA-4 Blockade. J. Immunol. Res. 2016, 2016, 8121985. [Google Scholar] [CrossRef]
- Teng, F.; Meng, X.; Kong, L.; Yu, J. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: A systematic review. Cancer Lett. 2018, 414, 166–173. [Google Scholar] [CrossRef]
- Kayar, R.; Bastug, Y.; Tokuc, E.; Topaktas, R.; Akyurek, E.A.; Kayar, K.; Artuk, I.; Ozturk, M. Pan-immune-inflammation value as a prognostic tool for overall survival and disease-free survival in non-metastatic muscle-invasive bladder cancer. Int. Urol. Nephrol. 2024, 56, 509–518. [Google Scholar] [CrossRef]
- Halliday, G.; Robinson, S.R.; Shepherd, C.; Kril, J. Alzheimer’s disease and inflammation: A review of cellular and therapeutic mechanisms. Clin. Exp. Pharmacol. Physiol. 2000, 27, 1–8. [Google Scholar] [CrossRef]
- Kuhtreiber, W.M.; Tran, L.; Kim, T.; Dybala, M.; Nguyen, B.; Plager, S.; Huang, D.; Janes, S.; Defusco, A.; Baum, D.; et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: The value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines 2018, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kuhtreiber, W.M.; Keefe, R.C.; Lee, A.H.; Aristarkhova, A.; Dias, H.F.; Ng, N.; Nelson, K.J.; Bien, S.; Scheffey, D.; et al. BCG vaccinations drive epigenetic changes to the human T cell receptor: Restored expression in type 1 diabetes. Sci. Adv. 2022, 8, eabq7240. [Google Scholar] [CrossRef] [PubMed]
- Gofrit, O.N.; Greenblatt, C.L.; Klein, B.Y.; Ben-Hur, T.; Bercovier, H. Can immunization with BCG delay the onset of Alzheimer’s disease? Geriatr. Gerontol. Int. 2023, 23, 889–890. [Google Scholar] [CrossRef]
- Berry, K.; Farias-Itao, D.S.; Grinberg, L.T.; Plowey, E.D.; Schneider, J.A.; Rodriguez, R.D.; Suemoto, C.K.; Buckwalter, M.S. B and T Lymphocyte Densities Remain Stable With Age in Human Cortex. ASN Neuro 2021, 13, 17590914211018117. [Google Scholar] [CrossRef] [PubMed]
- Deczkowska, A.; Schwartz, M. Targeting neuro-immune communication in neurodegeneration: Challenges and opportunities. J. Exp. Med. 2018, 215, 2702–2704. [Google Scholar] [CrossRef]
- Croese, T.; Castellani, G.; Schwartz, M. Immune cell compartmentalization for brain surveillance and protection. Nat. Immunol. 2021, 22, 1083–1092. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Du, T.; Hou, Y.; Fan, W.; Wu, Q.; Yan, H. Enhanced Expression of PD-L1 on Microglia After Surgical Brain Injury Exerts Self-Protection from Inflammation and Promotes Neurological Repair. Neurochem. Res. 2019, 44, 2470–2481. [Google Scholar] [CrossRef]
- Schwartz, M.; Arad, M.; Ben-Yehuda, H. Potential immunotherapy for Alzheimer disease and age-related dementia. Dialogues Clin. Neurosci. 2019, 21, 21–25. [Google Scholar] [CrossRef]
- Munir, S.; Lundsager, M.T.; Jorgensen, M.A.; Hansen, M.; Petersen, T.H.; Bonefeld, C.M.; Friese, C.; Met, O.; Straten, P.T.; Andersen, M.H. Inflammation induced PD-L1-specific T cells. Cell Stress 2019, 3, 319–327. [Google Scholar] [CrossRef]
- Si, Z.; Zhang, S.; Yang, X.; Ding, N.; Xiang, M.; Zhu, Q.; Mao, Y.; Lv, Y.; Yu, L.; Shang, H.; et al. The Association Between the Incidence Risk of Peripheral Neuropathy and PD-1/PD-L1 Inhibitors in the Treatment for Solid Tumor Patients: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Keefe, R.C.; Takahashi, H.; Tran, L.; Nelson, K.; Ng, N.; Kuhtreiber, W.M.; Faustman, D.L. BCG therapy is associated with long-term, durable induction of Treg signature genes by epigenetic modulation. Sci. Rep. 2021, 11, 14933. [Google Scholar] [CrossRef]
- Kates, M.; Matoso, A.; Choi, W.; Baras, A.S.; Daniels, M.J.; Lombardo, K.; Brant, A.; Mikkilineni, N.; McConkey, D.J.; Kamat, A.M.; et al. Adaptive Immune Resistance to Intravesical BCG in Non-Muscle Invasive Bladder Cancer: Implications for Prospective BCG-Unresponsive Trials. Clin. Cancer Res. 2020, 26, 882–891. [Google Scholar] [CrossRef]
- Bedke, J.; Black, P.C.; Szabados, B.; Guerrero-Ramos, F.; Shariat, S.F.; Xylinas, E.; Brinkmann, J.; Blake-Haskins, J.A.; Cesari, R.; Redorta, J.P. Optimizing outcomes for high-risk, non-muscle-invasive bladder cancer: The evolving role of PD-(L)1 inhibition. Urol. Oncol. 2023, 41, 461–475. [Google Scholar] [CrossRef]
- Lotem, M.; Machlenkin, A.; Hamburger, T.; Nissan, A.; Kadouri, L.; Frankenburg, S.; Gimmon, Z.; Elias, O.; David, I.B.; Kuznetz, A.; et al. Autologous melanoma vaccine induces antitumor and self-reactive immune responses that affect patient survival and depend on MHC class II expression on vaccine cells. Clin. Cancer Res. 2009, 15, 4968–4977. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.Y.; Devens, B.; Deutsch, O.; Ahituv, A.; Frenkel, S.; Kobrin, B.J.; Naor, D. Isolation of immunogenic and suppressogenic determinants of the nonimmunogenic YAC tumor and the change in its immunogenic repertoire after in vitro cultivation. Transplant. Proc. 1981, 13, 790–797. [Google Scholar]
- Kobrin, B.J.; Naor, D.; Klein, B.Y. Immunogenicity of subcellular fractions and molecular species of MuLV-induced tumors. II. Stimulation of syngeneic antitumor cell-mediated immune responses by subcellular fractions and molecular species of the Rauscher-virus-induced RBL5 tumor. J. Immunol. 1981, 126, 1874–1882. [Google Scholar] [CrossRef]
- Munir, S.; Andersen, G.H.; Met, O.; Donia, M.; Frosig, T.M.; Larsen, S.K.; Klausen, T.W.; Svane, I.M.; Andersen, M.H. HLA-restricted CTL that are specific for the immune checkpoint ligand PD-L1 occur with high frequency in cancer patients. Cancer Res. 2013, 73, 1764–1776. [Google Scholar] [CrossRef]
- Minami, T.; Minami, T.; Shimizu, N.; Yamamoto, Y.; De Velasco, M.; Nozawa, M.; Yoshimura, K.; Harashima, N.; Harada, M.; Uemura, H. Identification of Programmed Death Ligand 1-derived Peptides Capable of Inducing Cancer-reactive Cytotoxic T Lymphocytes From HLA-A24+ Patients With Renal Cell Carcinoma. J. Immunother. 2015, 38, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Hirata-Nozaki, Y.; Ohkuri, T.; Ohara, K.; Kumai, T.; Nagata, M.; Harabuchi, S.; Kosaka, A.; Nagato, T.; Ishibashi, K.; Oikawa, K.; et al. PD-L1-specific helper T-cells exhibit effective antitumor responses: New strategy of cancer immunotherapy targeting PD-L1 in head and neck squamous cell carcinoma. J. Transl. Med. 2019, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, C.L.; Bercovier, H.; Klein, B.Y.; Gofrit, O.N. Intravesical Bacille Calmette-Guerin (BCG) Vaccine Affects Cognition. J. Alzheimers Dis. 2024, 100, 771–774. [Google Scholar] [CrossRef] [PubMed]
| Patient Number | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Melanoma | Gender | Male | Male | Male | Male | Female | Male |
| Age | 72 | 76 | 63 | 44 | 18 | 70 | |
| Months from the start of BCG | 4 | 4 | 4 | 4 | 12 | 4 | |
| Non-muscle invasive bladder cancer (NMIBC) | Gender | Male | Male | Male | Male | Male | Female |
| Age | 69 | 60 | 66 | 50 | 65 | 86 | |
| Months from the start of BCG | 24 | 29 | 28 | 36 | 50 | 52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, B.Y.; Gofrit, O.N.; Greenblatt, C.L. BCG Impact on PD-1/PD-L1 Expression in Peripheral Immunocytes of Cancer Patients—A Potential Explanation for Its Activity in Preventing Alzheimer’s Disease. Curr. Issues Mol. Biol. 2025, 47, 651. https://doi.org/10.3390/cimb47080651
Klein BY, Gofrit ON, Greenblatt CL. BCG Impact on PD-1/PD-L1 Expression in Peripheral Immunocytes of Cancer Patients—A Potential Explanation for Its Activity in Preventing Alzheimer’s Disease. Current Issues in Molecular Biology. 2025; 47(8):651. https://doi.org/10.3390/cimb47080651
Chicago/Turabian StyleKlein, Benjamin Y., Ofer N. Gofrit, and Charles L. Greenblatt. 2025. "BCG Impact on PD-1/PD-L1 Expression in Peripheral Immunocytes of Cancer Patients—A Potential Explanation for Its Activity in Preventing Alzheimer’s Disease" Current Issues in Molecular Biology 47, no. 8: 651. https://doi.org/10.3390/cimb47080651
APA StyleKlein, B. Y., Gofrit, O. N., & Greenblatt, C. L. (2025). BCG Impact on PD-1/PD-L1 Expression in Peripheral Immunocytes of Cancer Patients—A Potential Explanation for Its Activity in Preventing Alzheimer’s Disease. Current Issues in Molecular Biology, 47(8), 651. https://doi.org/10.3390/cimb47080651






