A Narrative Review of Heavy Metals and Sperm Quality: The Interplay with Antioxidant Imbalance and Reactive Oxygen Species
Abstract
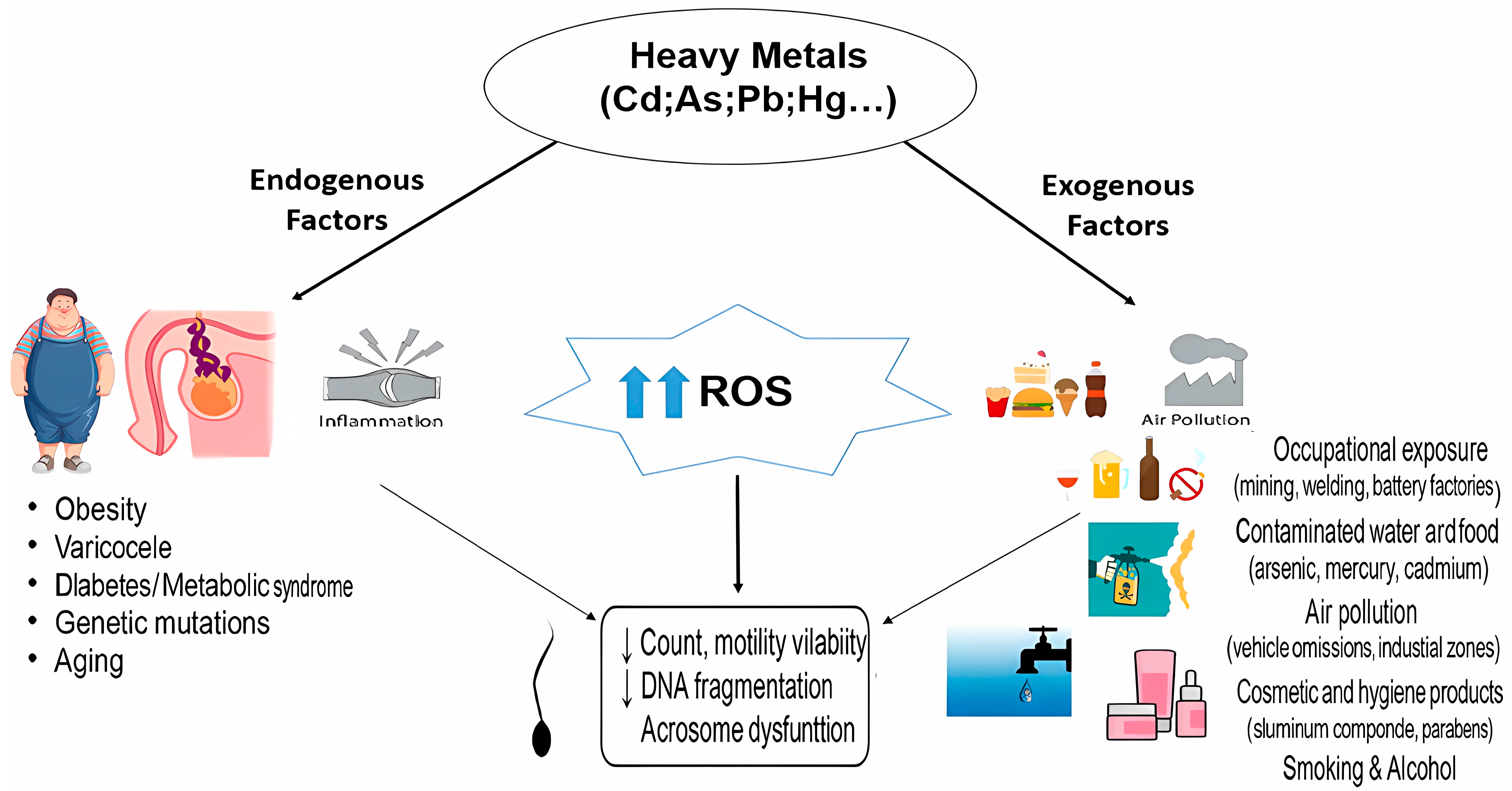
1. Introduction
2. Heavy Metals
2.1. Cadmium (Cd)
2.2. Lead (Pb)
2.3. Mercury (Hg)
2.4. Arsenic (As)
2.5. Cobalt (Co)
2.6. Aluminum (Al)
3. Oxidative Stress
4. Antioxidants
4.1. Zinc (Zn)
4.2. Selenium (Se)
4.3. Vitamin E
4.4. Vitamin C
4.5. Vitamin B12
4.6. Coenzyme Q10
4.7. L-Acetylcarnitine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dwyer, A.A.; Quinton, R. Anatomy and Physiology of the Hypothalamic-Pituitary-Gonadal (HPG) Axis. In Advanced Practice in Endocrinology Nursing; Springer: Berlin/Heidelberg, Germany, 2019; pp. 839–852. [Google Scholar]
- Oyola, M.G.; Handa, R.J. Hypothalamic–Pituitary–Adrenal and Hypothalamic–Pituitary–Gonadal Axes: Sex Differences in Regulation of Stress Responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Corradi, P.F.; Corradi, R.B.; Greene, L.W. Physiology of the Hypothalamic Pituitary Gonadal Axis in the Male. Urol. Clin. 2016, 43, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.; Jaffery, F.N. Biological Markers for Metal Toxicity. Environ. Toxicol. Pharmacol. 2005, 19, 335–349. [Google Scholar] [CrossRef]
- Hari Priya, P.; Reddy, P.S. Effect of Restraint Stress on Lead-induced Male Reproductive Toxicity in Rats. J. Exp. Zool. A Ecol. Genet. Physiol. 2012, 317, 455–465. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Mittal, M.; Saraf, P. Effective Attenuation of Glyphosate-induced Oxidative Stress and Granulosa Cell Apoptosis by Vitamins C and E in Caprines. Mol. Reprod. Dev. 2019, 86, 42–52. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A. Role of Antioxidants in Assisted Reproductive Techniques. World J. Men’s Health 2017, 35, 77–93. [Google Scholar] [CrossRef]
- Ramos-Trevino, J.; Bassol-Mayagoitia, S.; Hernández-Ibarra, J.A.; Ruiz-Flores, P.; Nava-Hernández, M.P. Toxic Effect of Cadmium, Lead, and Arsenic on the Sertoli Cell: Mechanisms of Damage Involved. DNA Cell Biol. 2018, 37, 600–608. [Google Scholar] [CrossRef]
- Atig, F.; Raffa, M.; Habib, B.-A.; Kerkeni, A.; Saad, A.; Ajina, M. Impact of Seminal Trace Element and Glutathione Levels on Semen Quality of Tunisian Infertile Men. BMC Urol. 2012, 12, 6. [Google Scholar] [CrossRef]
- Martinez, C.S.; Torres, J.G.D.; Peçanha, F.M.; Anselmo-Franci, J.A.; Vassallo, D.V.; Salaices, M.; Alonso, M.J.; Wiggers, G.A. 60-Day Chronic Exposure to Low Concentrations of HgCl2 Impairs Sperm Quality: Hormonal Imbalance and Oxidative Stress as Potential Routes for Reproductive Dysfunction in Rats. PLoS ONE 2014, 9, e111202. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Gao, X.; Tang, X.; Xu, H.; Wang, W.; Lei, X. Reproductive Toxicity of Cadmium Stress in Male Animals. Toxicology 2024, 504, 153787. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Gonçalves, A.; Rocha, E.; Sá, R.; Alves, A.; Silva, J.; Barros, A.; Pereira, M.L.; Sousa, M. Effect of in Vitro Exposure to Lead Chloride on Semen Quality and Sperm DNA Fragmentation. Zygote 2015, 23, 384–393. [Google Scholar] [CrossRef]
- Li, C.; Zhao, K.; Zhang, H.; Liu, L.; Xiong, F.; Wang, K.; Chen, B. Lead Exposure Reduces Sperm Quality and DNA Integrity in Mice. Environ. Toxicol. 2018, 33, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Tongesayi, T.; Fedick, P.; Lechner, L.; Brock, C.; Le Beau, A.; Bray, C. Daily Bioaccessible Levels of Selected Essential but Toxic Heavy Metals from the Consumption of Non-Dietary Food Sources. Food Chem. Toxicol. 2013, 62, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, R.; Hamza-Chaffai, A. Head and Neck Cancer Due to Heavy Metal Exposure via Tobacco Smoking and Professional Exposure: A Review. Toxicol. Appl. Pharmacol. 2010, 248, 71–88. [Google Scholar] [CrossRef]
- Marsh, G.M.; Youk, A.O.; Buchanich, J.M.; Erdal, S.; Esmen, N.A. Work in the Metal Industry and Nasopharyngeal Cancer Mortality among Formaldehyde-Exposed Workers. Regul. Toxicol. Pharmacol. 2007, 48, 308–319. [Google Scholar] [CrossRef]
- Hauptmann, M.; Lubin, J.H.; Stewart, P.A.; Hayes, R.B.; Blair, A. Mortality from Solid Cancers among Workers in Formaldehyde Industries. Am. J. Epidemiol. 2004, 159, 1117–1130. [Google Scholar] [CrossRef]
- Bouchala, F.; Boos, A.; Hamadouche, M.; Benboudiaf, S.; Azzouz, M. Évaluation de l’exposition Professionnelle Au Plomb, Cadmium et Arsenic Dans Une Usine de Fabrication et Recyclage de Batteries Acides Au Plomb. Toxicol. Anal. Et Clin. 2023, 35, S95. [Google Scholar] [CrossRef]
- Wang, G.; Yinglan, A.; Jiang, H.; Fu, Q.; Zheng, B. Modeling the Source Contribution of Heavy Metals in Surficial Sediment and Analysis of Their Historical Changes in the Vertical Sediments of a Drinking Water Reservoir. J. Hydrol. 2015, 520, 37–51. [Google Scholar] [CrossRef]
- Bretveld, R.; Brouwers, M.; Ebisch, I.; Roeleveld, N. Influence of Pesticides on Male Fertility. Scand. J. Work Environ. Health 2007, 33, 13–28. [Google Scholar] [CrossRef]
- Melgarejo, M.; Mendiola, J.; Koch, H.M.; Moñino-García, M.; Noguera-Velasco, J.A.; Torres-Cantero, A.M. Associations between Urinary Organophosphate Pesticide Metabolite Levels and Reproductive Parameters in Men from an Infertility Clinic. Environ. Res. 2015, 137, 292–298. [Google Scholar] [CrossRef]
- Witorsch, R.J.; Thomas, J.A. Personal Care Products and Endocrine Disruption: A Critical Review of the Literature. Crit. Rev. Toxicol. 2010, 40, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Alaee, S.; Talaiekhozani, A.; Rezaee, S.; Alaee, K.; Yousefian, E. Cadmium and Male Infertility. J. Infertil. Reprod. Biol. 2014, 2, 62–69. [Google Scholar]
- Al-Samman, T. Effect of Heavy Metal Impurities in Secondary Mg Alloys on the Microstructure and Mechanical Properties during Deformation. Mater. Des. 2015, 65, 983–988. [Google Scholar] [CrossRef]
- Ge, J.; Liu, L.-L.; Cui, Z.-G.; Talukder, M.; Lv, M.-W.; Li, J.-Y.; Li, J.-L. Comparative Study on Protective Effect of Different Selenium Sources against Cadmium-Induced Nephrotoxicity via Regulating the Transcriptions of Selenoproteome. Ecotoxicol. Environ. Saf. 2021, 215, 112135. [Google Scholar] [CrossRef]
- Cupertino, M.C.; Novaes, R.D.; Santos, E.C.; Neves, A.C.; Silva, E.; Oliveira, J.A.; Matta, S.L.P. Differential Susceptibility of Germ and Leydig Cells to Cadmium-Mediated Toxicity: Impact on Testis Structure, Adiponectin Levels, and Steroidogenesis. Oxidative Med. Cell. Longev. 2017, 2017, 3405089. [Google Scholar] [CrossRef]
- Mouro, V.G.S.; Siman, V.A.; da Silva, J.; Dias, F.C.R.; Damasceno, E.M.; do Carmo Cupertino, M.; de Melo, F.C.S.A.; da Matta, S.L.P. Cadmium-Induced Testicular Toxicity in Mice: Subacute and Subchronic Route-Dependent Effects. Biol. Trace Elem. Res. 2020, 193, 466–482. [Google Scholar] [CrossRef]
- Egbowon, B.F.; Harris, W.; Arnott, G.; Mills, C.L.; Hargreaves, A.J. Sub-Lethal Concentrations of CdCl2 Disrupt Cell Migration and Cytoskeletal Proteins in Cultured Mouse TM4 Sertoli Cells. Toxicol. Vitr. 2016, 32, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H. Testosterone Signaling and the Regulation of Spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef]
- Li, R.; Zhao, L.; Li, L.; Hou, Z.; Zhang, D.; Wan, L.; Wei, L.; Yang, Y.; Lv, J.; Ma, M. A Preliminary Study about the Potential Effects of Heavy Metals on the Human Male Reproductive Parameters in HIV-Infected Population in China. Biol. Trace Elem. Res. 2017, 180, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.A.; Huang, Y.-L.; Pan, C.-H.; Diawara, N. Role of Low Exposure to Metals as Male Reproductive Toxicants. Int. J. Environ. Health Res. 2015, 25, 405–417. [Google Scholar] [CrossRef]
- De Franciscis, P.; Ianniello, R.; Labriola, D.; Ambrosio, D.; Vagnetti, P.; Mainini, G.; Trotta, C.; Mele, D.; Campitiello, M.R.; Caprio, F. Environmental Pollution Due to Cadmium: Measure of Semen Quality as a Marker of Exposure and Correlation with Reproductive Potential. Clin. Exp. Obstet. Gynecol. 2015, 42, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Sukhn, C.; Awwad, J.; Ghantous, A.; Zaatari, G. Associations of Semen Quality with Non-Essential Heavy Metals in Blood and Seminal Fluid: Data from the Environment and Male Infertility (EMI) Study in Lebanon. J. Assist. Reprod. Genet. 2018, 35, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Rao, K.A.; Sudan, J.J.; Balasundaram, S. Cadmium Effects on Sperm Morphology and Semenogelin with Relates to Increased ROS in Infertile Smokers: An in Vitro and in Silico Approach. Reprod. Biol. 2018, 18, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Wang, P.; Feng, W.; Liu, C.; Yang, P.; Chen, Y.-J.; Sun, L.; Sun, Y.; Yue, J.; Gu, L.-J. Relationships between Seminal Plasma Metals/Metalloids and Semen Quality, Sperm Apoptosis and DNA Integrity. Environ. Pollut. 2017, 224, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.; Das, A.P. Lead Pollution: Impact on Environment and Human Health and Approach for a Sustainable Solution. Environ. Chem. Ecotoxicol. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, X.; Chen, J.; Wen, Y.; Liu, H.; Peng, Z.; Yeerken, R.; Wang, L.; Li, X. Lead-Mediated Inhibition of Lysine Acetylation and Succinylation Causes Reproductive Injury of the Mouse Testis during Development. Toxicol. Lett. 2020, 318, 30–43. [Google Scholar] [CrossRef]
- Marzec-Wróblewska, U.; Kamiński, P.; Łakota, P.; Szymański, M.; Wasilow, K.; Ludwikowski, G.; Jerzak, L.; Stuczyński, T.; Woźniak, A.; Buciński, A. Human Sperm Characteristics with Regard to Cobalt, Chromium, and Lead in Semen and Activity of Catalase in Seminal Plasma. Biol. Trace Elem. Res. 2019, 188, 251–260. [Google Scholar] [CrossRef]
- El-Magd, M.A.; Kahilo, K.A.; Nasr, N.E.; Kamal, T.; Shukry, M.; Saleh, A.A. A Potential Mechanism Associated with Lead-induced Testicular Toxicity in Rats. Andrologia 2017, 49, e12750. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.; Cao, L.; Ren, X.; Li, Y.; Shao, J.; Xu, L. Lead Acetate Induces Apoptosis in Leydig Cells by Activating PPARγ/Caspase-3/PARP Pathway. Int. J. Environ. Health Res. 2021, 31, 34–44. [Google Scholar] [CrossRef]
- Ommati, M.M.; Jamshidzadeh, A.; Heidari, R.; Sun, Z.; Zamiri, M.J.; Khodaei, F.; Mousapour, S.; Ahmadi, F.; Javanmard, N.; Shirazi Yeganeh, B. Carnosine and Histidine Supplementation Blunt Lead-Induced Reproductive Toxicity through Antioxidative and Mitochondria-Dependent Mechanisms. Biol. Trace Elem. Res. 2019, 187, 151–162. [Google Scholar] [CrossRef]
- Melnik, E. Determination of Human Exposure to Mercury. Chemosphere 2023, 312, 137314. [Google Scholar]
- Bank, M.S. Mercury in the Environment: Pattern and Process; University of California Press: Oakland, CA, USA, 2012; ISBN 0520271637. [Google Scholar]
- Capcarova, M.; Binkowski, L.J.; Stawarz, R.; Schwarczova, L.; Massanyi, P. Levels of Essential and Xenobiotic Elements and Their Relationships in Milk Available on the Slovak Market with the Estimation of Consumer Exposure. Biol. Trace Elem. Res. 2019, 188, 404–411. [Google Scholar] [CrossRef]
- Adelakun, S.A.; Ukwenya, V.O.; Akingbade, G.T.; Omotoso, O.D.; Aniah, J.A. Interventions of Aqueous Extract of Solanum Melongena Fruits (Garden Eggs) on Mercury Chloride Induced Testicular Toxicity in Adult Male Wistar Rats. Biomed. J. 2020, 43, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.; Moreno, J.M.; Roca, M.; Vergara-Juárez, N.; Martínez-García, M.J.; García-Sánchez, A.; Elvira-Rendueles, B.; Moreno-Grau, S.; López-Espín, J.J.; Ten, J. Relationships between Heavy Metal Concentrations in Three Different Body Fluids and Male Reproductive Parameters: A Pilot Study. Environ. Health 2011, 10, 6. [Google Scholar] [CrossRef]
- Gasparik, J.; Vladarova, D.; Capcarova, M.; Smehyl, P.; Slamecka, J.; Garaj, P.; Stawarz, R.; Massanyi, P. Concentration of Lead, Cadmium, Mercury and Arsenic in Leg Skeletal Muscles of Three Species of Wild Birds. J. Environ. Sci. Health Part A 2010, 45, 818–823. [Google Scholar] [CrossRef]
- Tan, S.W.; Meiller, J.C.; Mahaffey, K.R. The Endocrine Effects of Mercury in Humans and Wildlife. Crit. Rev. Toxicol. 2009, 39, 228–269. [Google Scholar] [CrossRef]
- Bjørklund, G.; Chirumbolo, S.; Dadar, M.; Pivina, L.; Lindh, U.; Butnariu, M.; Aaseth, J. Mercury Exposure and Its Effects on Fertility and Pregnancy Outcome. Basic Clin. Pharmacol. Toxicol. 2019, 125, 317–327. [Google Scholar] [CrossRef]
- Choy, C.M.Y.; Lam, C.W.K.; Cheung, L.T.F.; Briton-Jones, C.M.; Cheung, L.P.; Haines, C.J. Infertility, Blood Mercury Concentrations and Dietary Seafood Consumption: A Case–Control Study. BJOG 2002, 109, 1121–1125. [Google Scholar] [CrossRef]
- Mukherjee, S.; Gupte, T.; Jenifer, S.K.; Thomas, T.; Pradeep, T. Arsenic in Water: Speciation, Sources, Distribution, and Toxicology. In Encyclopedia of Water: Science, Technology, and Society; John Wiley and Sons: Hoboken, NJ, USA, 2019; pp. 1–17. [Google Scholar]
- Mandal, B.K.; Suzuki, K.T. Arsenic Round the World: A Review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Concessao, P.; Bairy, L.K.; Raghavendra, A.P. Effect of Aqueous Seed Extract of Mucuna Pruriens on Arsenic-Induced Testicular Toxicity in Mice. Asian Pac. J. Reprod. 2020, 9, 77–82. [Google Scholar] [CrossRef]
- Meng, P.; Zhang, S.; Jiang, X.; Cheng, S.; Zhang, J.; Cao, X.; Qin, X.; Zou, Z.; Chen, C. Arsenite Induces Testicular Oxidative Stress in Vivo and in Vitro Leading to Ferroptosis. Ecotoxicol. Environ. Saf. 2020, 194, 110360. [Google Scholar] [CrossRef]
- Wan, Z.-Z.; Chen, H.-G.; Lu, W.-Q.; Wang, Y.-X.; Pan, A. Metal/Metalloid Levels in Urine and Seminal Plasma in Relation to Computer-Aided Sperm Analysis Motion Parameters. Chemosphere 2019, 214, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.H.; Rumman, M. Cobalt Toxicity and Human Health. In Metal Toxicology Handbook; CRC Press: Boca Raton, FL, USA, 2020; pp. 273–285. [Google Scholar]
- Gál, J.; Hursthouse, A.; Tatner, P.; Stewart, F.; Welton, R. Cobalt and Secondary Poisoning in the Terrestrial Food Chain: Data Review and Research Gaps to Support Risk Assessment. Environ. Int. 2008, 34, 821–838. [Google Scholar] [CrossRef]
- Chen, Z.; Zuo, Q.; Song, F.; Fan, W.; Wang, Z.; Wu, D.; Cheng, W. Reproductive Toxicity in Adult Male Rats Following Intra-Articular Injection of Cobalt–Chromium Nanoparticles. J. Orthop. Sci. 2013, 18, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Elbetieha, A.; Aisha, S.; Rawdah, K.; Darmani, H.; Owais, W. Effects of Chronic Exposure to Cobalt Chloride on the Fertility and Testes in Mice. J. Appl. Biol. Sci. 2008, 2, 1–6. [Google Scholar]
- Yokel, R.A. Aluminum in Beverages and Foods: A Comprehensive Compilation of Regulations; Concentrations in Raw, Prepared, and Stored Beverages and Foods; and Intake. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70175. [Google Scholar] [CrossRef]
- ALMurshidi, M.M.H.; Raheem, S.A.; Razaq, R.A. Some Histological and Physiological Effects of Aluminum Chloride on Some Reproductive Organs of Male Albino Mice (Musmusculus). Ann. Rom. Soc. Cell Biol. 2021, 25, 5906–5918. [Google Scholar]
- Jamalan, M.; Ghaffari, M.A.; Hoseinzadeh, P.; Hashemitabar, M.; Zeinali, M. Human Sperm Quality and Metal Toxicants: Protective Effects of Some Flavonoids on Male Reproductive Function. Int. J. Fertil. Steril. 2016, 10, 215. [Google Scholar]
- Yousef, M.I.; Salama, A.F. Propolis Protection from Reproductive Toxicity Caused by Aluminium Chloride in Male Rats. Food Chem. Toxicol. 2009, 47, 1168–1175. [Google Scholar] [CrossRef]
- Yousef, M.I.; El-Morsy, A.M.A.; Hassan, M.S. Aluminium-Induced Deterioration in Reproductive Performance and Seminal Plasma Biochemistry of Male Rabbits: Protective Role of Ascorbic Acid. Toxicology 2005, 215, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Javorac, D.; Baralić, K.; Marić, Đ.; Mandić-Rajčević, S.; Đukić-Ćosić, D.; Bulat, Z.; Djordjevic, A.B. Exploring the Endocrine Disrupting Potential of Lead through Benchmark Modelling–Study in Humans. Environ. Pollut. 2023, 316, 120428. [Google Scholar] [CrossRef]
- Meligy, A.M.A.; Waheed, M.M.; El-Bahr, S.M. Effect of Heavy Metals Arsenic, Cadmium, and Lead on the Semen Variables of Dromedary Camels (Camelus Dromedarius). Anim. Reprod. Sci. 2019, 208, 106115. [Google Scholar] [CrossRef] [PubMed]
- Zargari, F.; Rahaman, M.S.; KazemPour, R.; Hajirostamlou, M. Arsenic, Oxidative Stress and Reproductive System. J. Xenobiotics 2022, 12, 214–222. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Onaolapo, M.C.; Oyowvi, M.O.; Akano, O.P.; Abidoye, M.O. The Cytotoxic Implication of Cobalt on Male Reproductive Functions: A Review. Toxin Rev. 2025, 44, 15–32. [Google Scholar] [CrossRef]
- Tiszler, M.; Olszak-Wąsik, K.; Machoń-Grecka, A.; Bellanti, F.; Dobrakowski, M.; Kasperczyk, S.; Olejek, A.; Kasperczyk, A. Cobalt’s Role in Modulating Antioxidant Systems and Semen Quality in Males. Reprod. Toxicol. 2024, 123, 108524. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive Oxygen Species and Male Reproductive Hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA Damage Caused by Oxidative Stress: Modifiable Clinical, Lifestyle and Nutritional Factors in Male Infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef]
- Ni, K.; Steger, K.; Yang, H.; Wang, H.; Hu, K.; Zhang, T.; Chen, B. A Comprehensive Investigation of Sperm DNA Damage and Oxidative Stress Injury in Infertile Patients with Subclinical, Normozoospermic, and Astheno/Oligozoospermic Clinical Varicocoele. Andrology 2016, 4, 816–824. [Google Scholar] [CrossRef]
- Rao, M.; Zhao, X.-L.; Yang, J.; Hu, S.-F.; Lei, H.; Xia, W.; Zhu, C.-H. Effect of Transient Scrotal Hyperthermia on Sperm Parameters, Seminal Plasma Biochemical Markers, and Oxidative Stress in Men. Asian J. Androl. 2015, 17, 668. [Google Scholar]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and Male Infertility: Mechanisms and Management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Oliveira, P.F.; Sousa, M.; Silva, B.M.; Alves, M.G. Role of Reactive Oxygen Species in Diabetes-Induced Male Reproductive Dysfunction. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–147. [Google Scholar]
- Saleh, R.A.; Agarwal, A.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J., Jr. Effect of Cigarette Smoking on Levels of Seminal Oxidative Stress in Infertile Men: A Prospective Study. Fertil. Steril. 2002, 78, 491–499. [Google Scholar] [CrossRef]
- Koch, H.; Meerkerk, G.-J.; Zaat, J.O.M.; Ham, M.F.; Scholten, R.J.P.M.; Assendelft, W.J.J. Accuracy of Carbohydrate-Deficient Transferrin in the Detection of Excessive Alcohol Consumption: A Systematic Review. Alcohol Alcohol. 2004, 39, 75–85. [Google Scholar] [CrossRef]
- Maneesh, M.; Jayalekshmi, H. Role of Reactive Oxygen Species and Antioxidants on Pathophysiology of Male Reproduction. Indian J. Clin. Biochem. 2006, 21, 80–89. [Google Scholar] [CrossRef]
- Singh, N.P.; Muller, C.H.; Berger, R.E. Effects of Age on DNA Double-Strand Breaks and Apoptosis in Human Sperm. Fertil. Steril. 2003, 80, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Impact of Oxidative Stress on Male and Female Germ Cells: Implications for Fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [PubMed]
- Abed, A.; Jarad, A. Significance of Some Trace Elements in Semen of Infertile Men. Ibnosina J. Med. Biomed. Sci. 2014, 6, 145–151. [Google Scholar] [CrossRef][Green Version]
- Dissanayake, D.; Wijesinghe, P.S.; Ratnasooriya, W.D.; Wimalasena, S. Relationship between Seminal Plasma Zinc and Semen Quality in a Subfertile Population. J. Hum. Reprod. Sci. 2010, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc Levels in Seminal Plasma Are Associated with Sperm Quality in Fertile and Infertile Men. Nutr. Res. 2009, 29, 82–88. [Google Scholar] [CrossRef]
- Henkel, R.; Maass, G.; Schuppe, H.-C.; Jung, A.; Schubert, J.; Schill, W.-B. Molecular Aspects of Declining Sperm Motility in Older Men. Fertil. Steril. 2005, 84, 1430–1437. [Google Scholar] [CrossRef]
- Pieczyńska, J.; Grajeta, H. The Role of Selenium in Human Conception and Pregnancy. J. Trace Elem. Med. Biol. 2015, 29, 31–38. [Google Scholar] [CrossRef]
- Mintziori, G.; Mousiolis, A.; Duntas, L.H.; Goulis, D.G. Evidence for a Manifold Role of Selenium in Infertility. Hormones 2020, 19, 55–59. [Google Scholar] [CrossRef]
- Foresta, C.; Flohé, L.; Garolla, A.; Roveri, A.; Ursini, F.; Maiorino, M. Male Fertility Is Linked to the Selenoprotein Phospholipid Hydroperoxide Glutathione Peroxidase. Biol. Reprod. 2002, 67, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Gralla, O.; Behrends, T.; Scharpf, M.; Endermann, T.; Rijntjes, E.; Pietschmann, N.; Hollenbach, B.; Schomburg, L. Selenoprotein P in Seminal Fluid Is a Novel Biomarker of Sperm Quality. Biochem. Biophys. Res. Commun. 2014, 443, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Pipkin, F.B.; Redman, C.W.G.; Poston, L. Selenium in Reproductive Health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef]
- Talebi, S.; Arab, A.; Sorraya, N. The Association between Dietary Antioxidants and Semen Parameters: A Cross-Sectional Study among Iranian Infertile Men. Biol. Trace Elem. Res. 2022, 200, 3957–3964. [Google Scholar] [CrossRef]
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Calogero, A.E.; Bagnara, V.; Aversa, A.; Greco, E.A.; Brunetti, A.; La Vignera, S. Effects of Selenium Supplementation on Sperm Parameters and DNA-Fragmentation Rate in Patients with Chronic Autoimmune Thyroiditis. J. Clin. Med. 2021, 10, 3755. [Google Scholar] [CrossRef]
- Ener, K.; Aldemir, M.; Işık, E.; Okulu, E.; Özcan, M.F.; Uğurlu, M.; Tangal, S.; Özayar, A. The Impact of Vitamin E Supplementation on Semen Parameters and Pregnancy Rates after Varicocelectomy: A Randomised Controlled Study. Andrologia 2016, 48, 829–834. [Google Scholar] [CrossRef]
- Rengaraj, D.; Hong, Y.H. Effects of Dietary Vitamin E on Fertility Functions in Poultry Species. Int. J. Mol. Sci. 2015, 16, 9910–9921. [Google Scholar] [CrossRef]
- Cyrus, A.; Kabir, A.; Goodarzi, D.; Moghimi, M. The Effect of Adjuvant Vitamin C after Varicocele Surgery on Sperm Quality and Quantity in Infertile Men: A Double-Blind Placebo Controlled Clinical Trial. Int. Braz. J. Urol. 2015, 41, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, T.; Soleymani, F.; Vatanchian, M. Protective Effect of Vitamin C and Zinc as an Antioxidant against Chemotherapy-Induced Male Reproductive Toxicity. J. Med. Life 2020, 13, 138. [Google Scholar] [CrossRef]
- Banihani, S.A. Vitamin B12 and Semen Quality. Biomolecules 2017, 7, 42. [Google Scholar] [CrossRef]
- Hosseinabadi, F.; Jenabi, M.; Ghafarizadeh, A.A.; Yazdanikhah, S. The Effect of Vitamin B12 Supplement on Post-thaw Motility, Viability and DNA Damage of Human Sperm. Andrologia 2020, 52, e13877. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, R.; González-Comadrán, M.; Solà, I.; López, G.; Brassesco, M.; Carreras, R.; Checa, M.A. Coenzyme Q10 and Male Infertility: A Meta-Analysis. J. Assist. Reprod. Genet. 2013, 30, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Derose, S.; Govindarajan, N.; Essa, M.M.; Qoronfleh, M.W.; Chidambaram, S.B.; Al-Bulushi, B. Fortification Methods of Coenzyme Q10 in Yogurt and Its Health Functionality—A Review. Front. Biosci. Sch. 2021, 13, 131–140. [Google Scholar] [CrossRef]
- Salvio, G.; Cutini, M.; Ciarloni, A.; Giovannini, L.; Perrone, M.; Balercia, G. Coenzyme Q10 and Male Infertility: A Systematic Review. Antioxidants 2021, 10, 874. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Singh, R.; Cannarella, R.; Sengupta, P.; Dutta, S. Coenzyme Q10, Oxidative Stress, and Male Infertility: A Review. Clin. Exp. Reprod. Med. 2021, 48, 97. [Google Scholar] [CrossRef]
- Nadjarzadeh, A.; Shidfar, F.; Amirjannati, N.; Vafa, M.R.; Motevalian, S.A.; Gohari, M.R.; Nazeri Kakhki, S.A.; Akhondi, M.M.; Sadeghi, M.R. Effect of Coenzyme Q10 Supplementation on Antioxidant Enzymes Activity and Oxidative Stress of Seminal Plasma: A Double-blind Randomised Clinical Trial. Andrologia 2014, 46, 177–183. [Google Scholar] [CrossRef]
- García-Díaz, E.C.; Gómez-Quiroz, L.E.; Arenas-Ríos, E.; Aragón-Martínez, A.; Ibarra-Arias, J.A.; del Socorro I Retana-Márquez, M. Oxidative Status in Testis and Epididymal Sperm Parameters after Acute and Chronic Stress by Cold-Water Immersion in the Adult Rat. Syst. Biol. Reprod. Med. 2015, 61, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.S.; Littarru, G.P.; Funahashi, I.; Painkara, U.S.; Dange, N.S.; Chauhan, P. Effect of Ubiquinol Therapy on Sperm Parameters and Serum Testosterone Levels in Oligoasthenozoospermic Infertile Men. J. Clin. Diagn. Res. 2015, 9, BC01. [Google Scholar] [CrossRef]
- Abdelrazik, H.; Sharma, R.; Mahfouz, R.; Agarwal, A. L-Carnitine Decreases DNA Damage and Improves the in Vitro Blastocyst Development Rate in Mouse Embryos. Fertil. Steril. 2009, 91, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Micic, S.; Lalic, N.; Djordjevic, D.; Bojanic, N.; Bogavac-Stanojevic, N.; Busetto, G.M.; Virmani, A.; Agarwal, A. Double-blind, Randomised, Placebo-controlled Trial on the Effect of L-carnitine and L-acetylcarnitine on Sperm Parameters in Men with Idiopathic Oligoasthenozoospermia. Andrologia 2019, 51, e13267. [Google Scholar] [CrossRef] [PubMed]
- Balercia, G.; Regoli, F.; Armeni, T.; Koverech, A.; Mantero, F.; Boscaro, M. Placebo-Controlled Double-Blind Randomized Trial on the Use of L-Carnitine, L-Acetylcarnitine, or Combined L-Carnitine and L-Acetylcarnitine in Men with Idiopathic Asthenozoospermia. Fertil. Steril. 2005, 84, 662–671. [Google Scholar] [CrossRef]
- Sigman, M.; Glass, S.; Campagnone, J.; Pryor, J.L. Carnitine for the Treatment of Idiopathic Asthenospermia: A Randomized, Double-Blind, Placebo-Controlled Trial. Fertil. Steril. 2006, 85, 1409–1414. [Google Scholar] [CrossRef] [PubMed]


| Heavy Metals | Effect on Male Fertility | Sperm Parameter Affected | Mechanisms of Toxicity | References |
|---|---|---|---|---|
| Cadmium (Cd) | Destruction of spermatogenesis | Increase in motility Decrease in sperm count Decrease in sperm morphology | Increase in ROS Apoptosis in Sertoli cells | [8] |
| Lead (Pb) | Decrease in testicular volume | Decrease in progressive motility Increase in ROS Decrease in antioxidants Decrease in reproductive hormones Decrease in viability | Increase in apoptosis Endocrine disruption | [65] |
| Mercury (Hg) | Decrease in testis weight Decrease in testis structure | Decrease in total and progressive motility Decrease in semen quality and quantity Decrease in sperm volume | Increase in DNA damage Increase in ROS Lipid peroxidation | [42] |
| Arsenic (As) | Testicular degeneration | Decrease in sperm count | Increase in lipid peroxidation Increase in DNA damage Increase in ROS | [67] |
| Aluminum (Al) | Increase in testicular tissue damage Decrease in testis volume | Decrease in sperm viability | Decrease in sex hormones Increase in ROS | [64] |
| Cobalt (Co) | Decrease in testicular weight Spermatogenetic arrest | Decrease in sperm motility Decrease in sperm viability | Increase in ROS | [68,69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azil, S.; Errafii, K.; Benkhalifa, M.; Louanjli, N.; Ghazi, B.; Hamdi, S. A Narrative Review of Heavy Metals and Sperm Quality: The Interplay with Antioxidant Imbalance and Reactive Oxygen Species. Curr. Issues Mol. Biol. 2025, 47, 650. https://doi.org/10.3390/cimb47080650
Azil S, Errafii K, Benkhalifa M, Louanjli N, Ghazi B, Hamdi S. A Narrative Review of Heavy Metals and Sperm Quality: The Interplay with Antioxidant Imbalance and Reactive Oxygen Species. Current Issues in Molecular Biology. 2025; 47(8):650. https://doi.org/10.3390/cimb47080650
Chicago/Turabian StyleAzil, Soukaina, Khaoula Errafii, Moncef Benkhalifa, Noureddine Louanjli, Bouchra Ghazi, and Salsabil Hamdi. 2025. "A Narrative Review of Heavy Metals and Sperm Quality: The Interplay with Antioxidant Imbalance and Reactive Oxygen Species" Current Issues in Molecular Biology 47, no. 8: 650. https://doi.org/10.3390/cimb47080650
APA StyleAzil, S., Errafii, K., Benkhalifa, M., Louanjli, N., Ghazi, B., & Hamdi, S. (2025). A Narrative Review of Heavy Metals and Sperm Quality: The Interplay with Antioxidant Imbalance and Reactive Oxygen Species. Current Issues in Molecular Biology, 47(8), 650. https://doi.org/10.3390/cimb47080650







