Serum Immunoglobulin Changes in Multiple Myeloma Patients Treated with CAR T-Cell Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Data
2.2. Paraprotein Analysis
2.3. Minimal Residual Disease
2.4. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics of the MM Patient Cohort
3.2. Association of Abnormal Protein Bands and Clinical Relapse
3.3. Characteristics of Abnormal Protein Bands in Relapsed vs. Non-Relapsed Patients
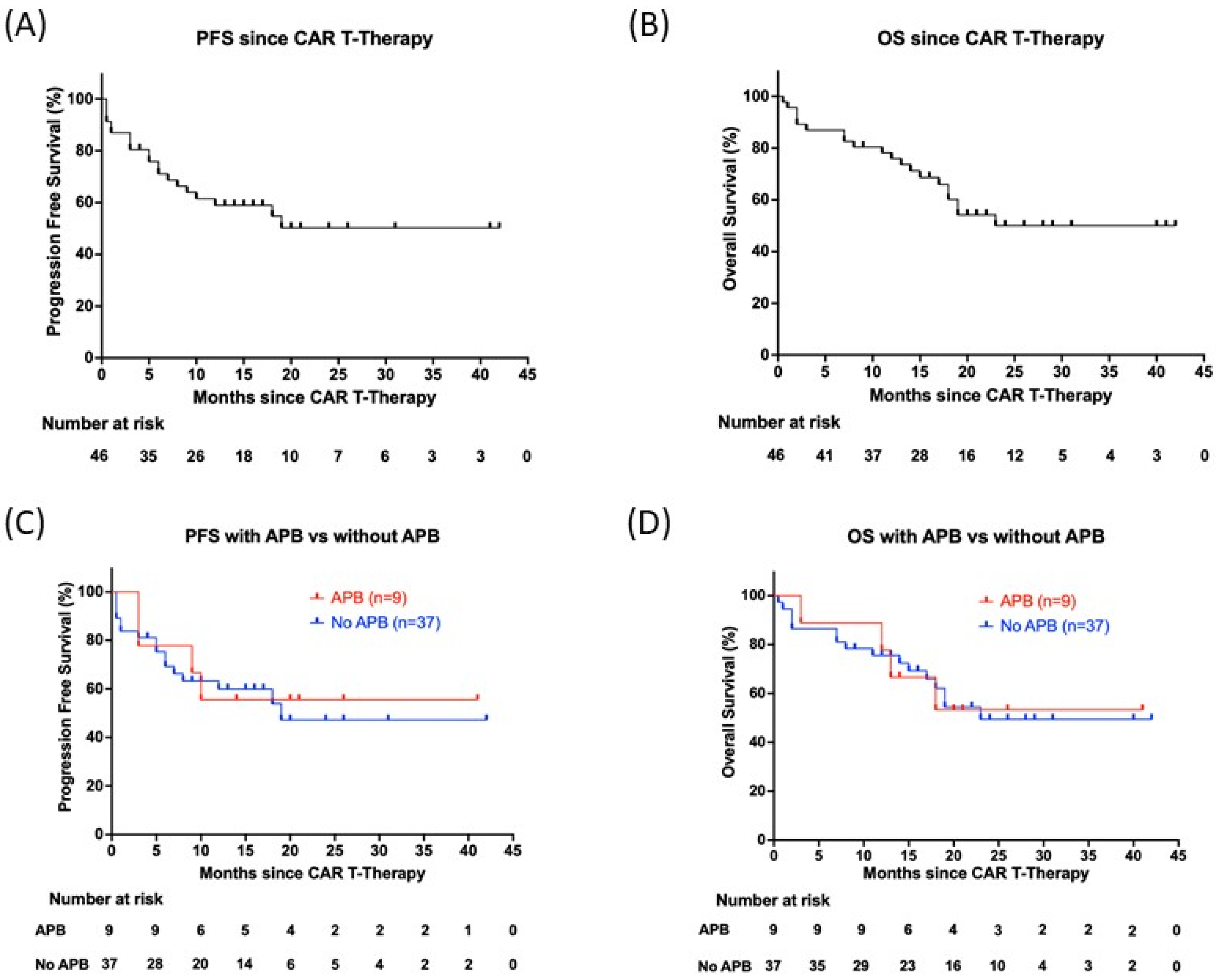
3.4. No Association of Abnormal Protein Bands and Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, M.; Chu, B.; Wang, Y.; Shi, L.; Gao, S.; Fang, L.; Xiang, Q.; Liu, X.; Ding, Y.; Chen, Y.; et al. Clinical Characteristics and Prognostic Significance of Immunoglobulin Isotype Switch in Patients with Multiple Myeloma. Cancer Pathog. Ther. 2023, 1, 149–153. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy Bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, L.-J.; Yang, S.-S.; Sun, Y.; Wu, W.; Liu, Y.-F.; Xu, J.; Zhuang, Y.; Zhang, W.; Weng, X.-Q.; et al. Exploratory Trial of a Biepitopic CAR T-Targeting B Cell Maturation Antigen in Relapsed/Refractory Multiple Myeloma. Proc. Natl. Acad. Sci. USA 2019, 116, 9543–9551. [Google Scholar] [CrossRef] [PubMed]
- Mailankody, S.; Ghosh, A.; Staehr, M.; Purdon, T.J.; Roshal, M.; Halton, E.; Diamonte, C.; Pineda, J.; Anant, P.; Bernal, Y.; et al. Clinical Responses and Pharmacokinetics of MCARH171, a Human-Derived Bcma Targeted CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma: Final Results of a Phase I Clinical Trial. Blood 2018, 132, 959. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Ahmadieh-Yazdi, A.; Vicidomini, R.; Poondla, N.; Tanzadehpanah, H.; Dirbaziyan, A.; Mahaki, H.; Manoochehri, H.; Kalhor, N.; Dama, P. CAR T Therapies in Multiple Myeloma: Unleashing the Future. Cancer Gene Ther. 2024, 31, 667–686. [Google Scholar] [CrossRef]
- Maisnar, V.; Tichý, M.; Smolej, L.; Zák, P.; Radocha, J.; Palicka, V.; Malý, J.; Bláha, V. Isotype Class Switching after Transplantation in Multiple Myeloma. Neoplasma 2007, 54, 225–228. [Google Scholar]
- Hall, S.L.; Tate, J.; Gill, D.; Mollee, P. Significance of Abnormal Protein Bands in Patients with Multiple Myeloma Following Autologous Stem Cell Transplantation. Clin. Biochem. Rev. 2009, 30, 113–118. [Google Scholar]
- Fernández de Larrea, C.; Tovar, N.; Cibeira, M.T.; Aróstegui, J.I.; Rosiñol, L.; Elena, M.; Filella, X.; Yagüe, J.; Bladé, J. Emergence of Oligoclonal Bands in Patients with Multiple Myeloma in Complete Remission after Induction Chemotherapy: Association with the Use of Novel Agents. Haematologica 2011, 96, 171–173. [Google Scholar] [CrossRef]
- Mark, T.; Jayabalan, D.; Coleman, M.; Pearse, R.N.; Wang, Y.L.; Lent, R.; Christos, P.J.; Lee, J.W.; Agrawal, Y.P.; Matthew, S.; et al. Atypical Serum Immunofixation Patterns Frequently Emerge in Immunomodulatory Therapy and Are Associated with a High Degree of Response in Multiple Myeloma. Br. J. Haematol. 2008, 143, 654–660. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Deng, J.; Xu, J.; Kang, Y.; Luo, W.; Chen, L.; Hu, Y.; Mei, H. Multiple Immunoglobulin Isotype Switch after Bispecific CAR-T Cell Therapy in Multiple Myeloma-A Case Report. Br. J. Haematol. 2023, 200, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Li, P.; Kang, L.; Zhou, L.; Xu, Y.; Ye, S.; Du, J.; Li, B.; Wang, Y.; Yu, L.; et al. Immunoglobulin Isotype Switch after Anti-BCMA CAR T-Cell Therapy for Relapsed or Refractory Multiple Myeloma. Blood Adv. 2022, 6, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Kündgen, L.J.; Akhoundova, D.; Hoffmann, M.; Legros, M.; Shaforostova, I.; Seipel, K.; Bacher, U.; Pabst, T. Prognostic Value of Post-Transplant MRD Negativity in Standard Versus High- and Ultra-High-Risk Multiple Myeloma Patients. Cancers 2025, 17, 1565. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yin, H.; Zhao, X.; Jin, D.; Liang, Y.; Xiong, T.; Li, L.; Tang, W.; Zhang, J.; Liu, M.; et al. High Efficacy and Safety of CD38 and BCMA Bispecific CAR-T in Relapsed or Refractory Multiple Myeloma. J. Exp. Clin. Cancer Res. 2022, 41, 2. [Google Scholar] [CrossRef]
- Kalariya, N.M.; Lee, H.C.; Qazilbash, M.H.; Patel, K.K. Secondary Monoclonal Gammopathy of Unknown Significance with Isotype Switching after CAR T-Cell Therapy for Multiple Myeloma: A Case Report. Curr. Probl. Cancer Case Rep. 2024, 13, 100276. [Google Scholar] [CrossRef]
- Merz, M.; Albici, A.; Von Tresckow, B.; Rathje, K.; Fenk, R.; Holderried, T.; Müller, F.; Tovar, N.; Oliver-Cáldes, A.; Vucinic, V.; et al. Idecabtagene Vicleucel or Ciltacabtagene Autoleucel for Relapsed or Refractory Multiple Myeloma: An International Multicenter Study. HemaSphere 2025, 9, e70070. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Roex, G.; Timmers, M.; Wouters, K.; Campillo-Davo, D.; Flumens, D.; Schroyens, W.; Chu, Y.; Berneman, Z.N.; Lion, E.; Luo, F.; et al. Safety and Clinical Efficacy of BCMA CAR-T-Cell Therapy in Multiple Myeloma. J. Hematol. Oncol. 2020, 13, 164. [Google Scholar] [CrossRef]
- Ye, R.; Kundrapu, S.; Gerson, S.L.; Driscoll, J.J.; Beck, R.; Ali, N.; Landgren, O.; VanHeeckeren, W.; Luo, G.; Kroger, N.; et al. Immune Signatures Associated with Clonal Isotype Switch After Autologous Stem Cell Transplantation for Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, e213–e220. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Park, Y.; Lee, B.-H.; Yang, M.; Kim, Y.; Kim, J.-A. The Presence of Clonal Isotype Switch after Autologous Stem Cell Transplantation as a Prognostic Biomarker for Long-Term Survival in Patients with Multiple Myeloma. Leuk. Res. 2025, 149, 107641. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gao, G.; Chen, H.; Dong, R.; Zhang, W.; Zhao, W.; Liu, J.; Wang, J.; Lei, B.; Wang, B.; et al. The Incidence and Clinical Significance of Monoclonal and Oligoclonal Protein Bands in Multiple Myeloma Patients after BCMA–CAR-T Cell Therapy: A Retrospective Study Based on LEGEND-2. HemaSphere 2024, 8, e70054. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.J.; Kriangkum, J.; Pittman, J.A.; Mant, M.J.; Reiman, T.; Belch, A.R.; Pilarski, L.M. Analysis of Clonotypic Switch Junctions Reveals Multiple Myeloma Originates from a Single Class Switch Event with Ongoing Mutation in the Isotype-Switched Progeny. Blood 2008, 112, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Reiman, T.; Seeberger, K.; Taylor, B.J.; Szczepek, A.J.; Hanson, J.; Mant, M.J.; Coupland, R.W.; Belch, A.R.; Pilarski, L.M. Persistent Preswitch Clonotypic Myeloma Cells Correlate with Decreased Survival: Evidence for Isotype Switching within the Myeloma Clone. Blood 2001, 98, 2791–2799. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zent, C.S.; Wilson, C.S.; Tricot, G.; Jagannath, S.; Siegel, D.; Desikan, K.R.; Munshi, N.; Bracy, D.; Barlogie, B.; Butch, A.W. Oligoclonal Protein Bands and Ig Isotype Switching in Multiple Myeloma Treated with High-Dose Therapy and Hematopoietic Cell Transplantation. Blood 1998, 91, 3518–3523. [Google Scholar] [CrossRef]
- Mitus, A.J.; Stein, R.; Rappeport, J.M.; Antin, J.H.; Weinstein, H.J.; Alper, C.A.; Smith, B.R. Monoclonal and Oligoclonal Gammopathy after Bone Marrow Transplantation. Blood 1989, 74, 2764–2768. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Xia, J.; Li, P.; Cao, J.; Pan, B.; Tan, X.; Li, H.; Qi, K.; Wang, X.; et al. Humoral Immune Reconstitution after Anti-BCMA CAR T-Cell Therapy in Relapsed/Refractory Multiple Myeloma. Blood Adv. 2021, 5, 5290–5299. [Google Scholar] [CrossRef]
| Parameter | All Pts (n = 46) | Pts w APBs (n = 9) | Pts w/o APBs (n = 37) | p * |
|---|---|---|---|---|
| Median age, years (range) | 60 (40–78) | 59 (44–75) | 60 (40–78) | |
| Males/females, n (ratio) | 32/14 (2.3) | 8/1 (8) | 24/13 (1.8) | |
| M-protein subtype, n (%) | ||||
| Kappa light chain | 15 (32.6%) | 1 (11.1%) | 6 (16.2%) | 1 |
| Lambda light chain | 23 (50.0%) | 0 (0%) | 6 (16.2%) | 0.327 |
| IgG | 27 (58.7%) | 5 (55.6%) | 22 (59.5%) | 1 |
| IgA | 11 (23.9%) | 5 (55.6%) | 6 (16.2%) | 0.025 |
| IgM | 1 (2.2%) | 0 (0.0%) | 1 (2.7%) | 1 |
| Light chain only | 8 (17.4%) | 0 (0.0%) | 8 (21.6%) | 0.324 |
| R-ISS stage, n (%) | ||||
| Available | 45 (97.8%) | 9 (100%) | 36 (97.3%) | |
| I | 12 (26.1%) | 1 (11.1%) | 11 (29.7%) | 0.409 |
| II | 20 (43.5%) | 6 (66.7%) | 14 (37.8%) | 0.149 |
| III | 13 (28.3%) | 2 (22.2%) | 11 (29.7%) | 1 |
| MM diagnostic criteria | ||||
| Anemia (Hb, f < 121 g/L, m < 135 g/L), n (%) | 41 (89.1%) | 8 (88.9%) | 33 (89.2%) | 1 |
| Hypercalcemia (>2.55 mmol/L), n (%) | 14 (30.4%) | 3 (33.3%) | 11 (29.7%) | 1 |
| Creatinine (f > 84 mmol/L, m > 104 mmol/L) | 19 (41.3%) | 3 (33.3%) | 16 (43.2%) | 0.716 |
| Osteolytic lesions, n (%) | 36 (78.3%) | 8 (88.9%) | 28 (75.7%) | 0.659 |
| Extramedullary disease, n (%) | 5 (8.7%) | 2 (22.2%) | 3 (8.1%) | 0.248 |
| Cytogenetics | ||||
| Available, n (%) | 40 (86.9%) | 8 (88.9%) | 32 (86.5%) | |
| t(4;14) translocation, n (%) | 6 (13.0%) | 3 (33.3%) | 3 (8.1%) | 0.079 |
| Del(17p), n (%) | 9 (19.6%) | 1 (11.1%) | 8 (21.6%) | 0.664 |
| Gain(1q), n (%) | 10 (21.7%) | 2 (22.2%) | 8 (21.6%) | 1 |
| No high-risk cytogenetics, n (%) | 15 (32.6%) | 3 (33.3%) | 12 (32.4%) | 1 |
| Parameter | All Pts (n = 46) | Pts w APBs (n = 9) | Pts w/o APBs (n = 37) | p * |
|---|---|---|---|---|
| Time ID to CAR T, months, median (range) | 62 (11–205) | 86 (21–200) | 61 (11–205) | |
| Remission status | 1 | |||
| Progressive disease, n (%) | 22 (47.8%) | 4 (44.4%) | 18 (48.6%) | |
| Stable disease, n (%) | 11 (23.9%) | 2 (22.2%) | 9 (24.3%) | |
| Partial response, n (%) | 11 (23.9%) | 2 (22.2%) | 9 (24.3%) | |
| Very good partial response, n (%) | 1 (2.2%) | 0 (0.0%) | 1 (2.7%) | |
| Complete response, n (%) | 1 (2.2%) | 1 (11.1%) | 0 (0.0%) | |
| Prior lines of therapy, median (range) | 5 (2–11) | 6 (2–7) | 5 (2–11) | 1 |
| Previous HDCT/ASCT | 1 | |||
| Yes, n (%) | 38 (82.6%) | 8 (88.9%) | 30 (81.1%) | |
| No, n (%) | 8 (17.4%) | 1 (11.1%) | 7 (18.9%) | |
| Clinical Relapse after CAR T-cell therapy | 0.472 | |||
| Yes, n (%) | 26 (56.5%) | 4 (44.4%) | 22 (59.5%) | |
| No, n (%) | 20 (43.5%) | 5 (55.6%) | 15 (40.5%) |
| Parameter | All Pts (n = 46) | Pts w Relapse (n = 26) | Pts w/o Relapse (n = 20) | p * |
|---|---|---|---|---|
| Abnormal Protein Bands | 9 (19.6%) | 4 (15.4%) | 5 (25%) | 0.472 |
| New APB without initial M-protein | 2 (4.3%) | 0 (0%) | 2 (10%) | 0.183 |
| Light chain escape | 1 (2.2%) | 1 (3.8%) | 0 (0%) | 1.0 |
| New APB addition alongside initial M-protein | 4 (8.7%) | 1 (3.8%) | 3 (15%) | 0.303 |
| Loss of one intact M-protein from biclonal | 2 (4.3%) | 2 (7.7%) | 0 (0%) | 0.497 |
| No Abnormal Protein Bands | 37 (80.4%) | 22 (84.6%) | 15 (75%) | 0.472 |
| No more M-protein in IFE | 15 (32.6%) | 3 (11.5%) | 12 (60%) | 0.0011 |
| Same isotype as before | 22 (47.8%) | 19 (73%) | 3 (15%) | 0.0001 |
| # | Age at ID | Sex | Primary Paraprotein | ISS Stage | FISH | BR Pre CART | Relapse | Paraprotein APB | BR Post CART | PFS (mo) | OS (mo) | FLC-Ratio | ΔFLC mg/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | m | IgG Kappa, IgA Kappa | II | t(4;14) | PD | No | IgM Kappa, IgG Lambda | CR | 41 | 41 | 1 | 0.1 |
| 2 | 44 | m | IgG Kappa | II | t(4;14), gain1q | PD | Yes | LC Kappa | PR | 3 | 3 | 3840 | 1920 |
| 3 | 45 | m | IgG Kappa, LC Lambda | II | del(17p) | SD | Yes | LC Lambda | CR | 3 | 18 | <0.01 | 332 |
| 4 | 49 | m | IgG Lambda | I | normal | PD | No | IgG Lambda, IgG Kappa | VGPR | 26 | 26 | 2 | 0.5 |
| 5 | 65 | m | IgA Lambda | II | gain 1q | CR | Yes | IgA Lambda, IgG Lambda | CR | 9 | 13 | 0.04 | 47.8 |
| 6 | 62 | m | IgG Lambda | III | normal | PR | No | IgG Kappa | CR | 21 | 21 | 1 | 0.5 |
| 7 | 67 | m | IgG Kappa | II | t(4:14) | SD | No | IgG Kappa, IgG Lambda | CR | 20 | 20 | 1.2 | 1.9 |
| 8 | 75 | m | IgA Kappa, IgG Kappa | III | n.d. | PD | Yes | IgA Kappa | CR | 10 | 12 | 18.3 | 58.9 |
| 9 | 60 | f | LC Lambda | II | normal | PR | No | IgG Lambda, IgG Kappa | CR | 14 | 14 | 0.17 | 92 |
| Author(s) | Year | Ref. | Study Type | Synopsis/Key Findings | Impact of APBs on Outcome |
|---|---|---|---|---|---|
| Lu et al. | 2023 | [1] | Retrospective (various therapies) | Isotype switch in 3.5% of relapsed patients (complete switching, light chain escape, non-secretory relapse) | No significant difference in prognosis of patients |
| Maisnar et al. | 2007 | [8] | Retrospective (HDCT/ASCT) | APBs in 43% of patients post ASCT, most likely due to immune reconstitution, not disease relapse | Associated with improved outcome |
| Hall et al. | 2009 | [9] | Retrospective (HDCT/ASCT) | APBs in 73% of patients, representing regeneration of a limited immune response | Favorable event-free survival |
| Fernández de Larrea et al. | 2011 | [10] | Retrospective (conventional and novel therapies) | APBs in 33.3% of patients, and in 60% of patients with novel agents | No assessment of impact on outcome |
| Mark et al. | 2008 | [11] | Retrospective (BiRD regimen) | Atypical IFE patterns in 33% likely reflect robust antitumor response and immune reconstitution | Significantly greater OR and CR |
| Ye et al. | 2019 | [20] | Retrospective (HDCT/ASCT) | Clonal isotype switching in 22% of ASCT patients, suggestive of more profound T-cell immune reconstitution | Improved PFS and OS |
| Liang et al. | 2022 | [13] | Case series (CAR T-cell, n = 12) | APBs in 33% likely reflect immune reconstitution | Favorable prognosis |
| Cho et al. | 2025 | [21] | Prospective (HDCT/ASCT) | Clonal isotype switch in 31.7% of patients, reflecting robust immune reconstitution | Favorable outcomes |
| Liu et al. (LEGEND-2) | 2024 | [22] | Retrospective (CAR T-cell) | 48.9% developed APBs post-CAR T, indicating robust immune reconstitution | Longer PFS and OS, higher response rates |
| Zent et al. | 1998 | [25] | Retrospective (HDCT/ASCT) | APBs in 10% of patients likely reflect immune recovery | Significantly higher CR, longer OS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burger, A.; Bacher, U.; Hoffmann, M.; Seipel, K.; Schild, C.; Shaforostova, I.; Pabst, T. Serum Immunoglobulin Changes in Multiple Myeloma Patients Treated with CAR T-Cell Therapy. Curr. Issues Mol. Biol. 2025, 47, 640. https://doi.org/10.3390/cimb47080640
Burger A, Bacher U, Hoffmann M, Seipel K, Schild C, Shaforostova I, Pabst T. Serum Immunoglobulin Changes in Multiple Myeloma Patients Treated with CAR T-Cell Therapy. Current Issues in Molecular Biology. 2025; 47(8):640. https://doi.org/10.3390/cimb47080640
Chicago/Turabian StyleBurger, Alexa, Ulrike Bacher, Michele Hoffmann, Katja Seipel, Christof Schild, Inna Shaforostova, and Thomas Pabst. 2025. "Serum Immunoglobulin Changes in Multiple Myeloma Patients Treated with CAR T-Cell Therapy" Current Issues in Molecular Biology 47, no. 8: 640. https://doi.org/10.3390/cimb47080640
APA StyleBurger, A., Bacher, U., Hoffmann, M., Seipel, K., Schild, C., Shaforostova, I., & Pabst, T. (2025). Serum Immunoglobulin Changes in Multiple Myeloma Patients Treated with CAR T-Cell Therapy. Current Issues in Molecular Biology, 47(8), 640. https://doi.org/10.3390/cimb47080640







