Oxytocin, Vasopressin and Stress: A Hormetic Perspective
Abstract
1. Purpose and Historical Background
1.1. Oxytocin and Vasopressin
1.2. Time Matters
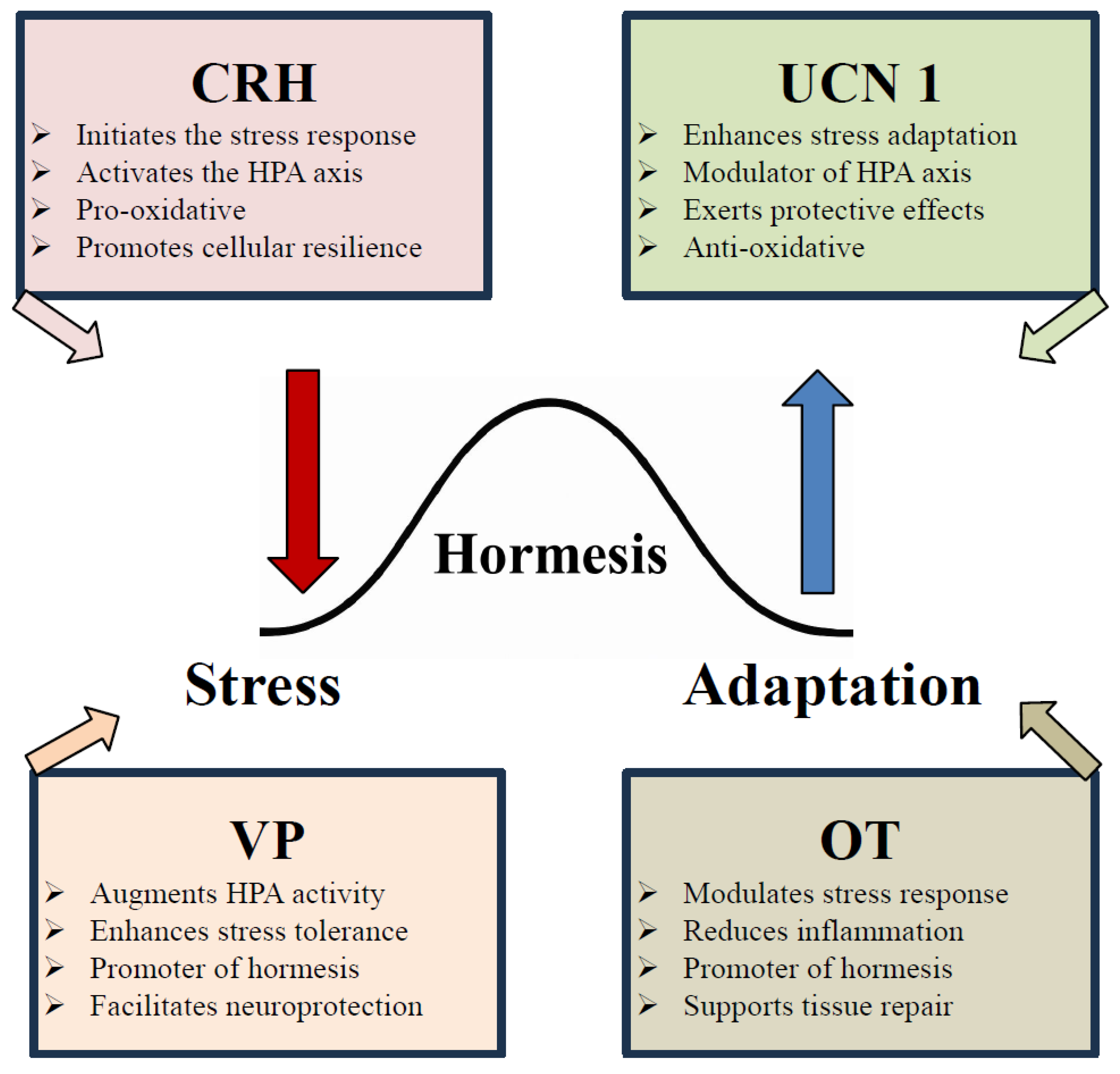
2. Hormesis
2.1. The Role of Peptides in the Hormetic Hypothesis
2.2. Classical Definitions of Hormesis
| Domain | Example Stressors/Interventions | Mechanisms Catabolic–Anabolic Cycling | Key Outcomes | Ref. |
|---|---|---|---|---|
| Aging & Geroscience |
|
|
| [32,33] |
| Cardiovascular Resilience |
|
|
| [32,34] |
| Neuropsychiatric Health |
|
|
| [33,35,36] |
2.3. Ancient Peptides and Hormesis
2.4. Hormesis: Conceptual Foundations
2.4.1. Oxidative Stress
2.4.2. HPA Axis Hormones and Hormesis
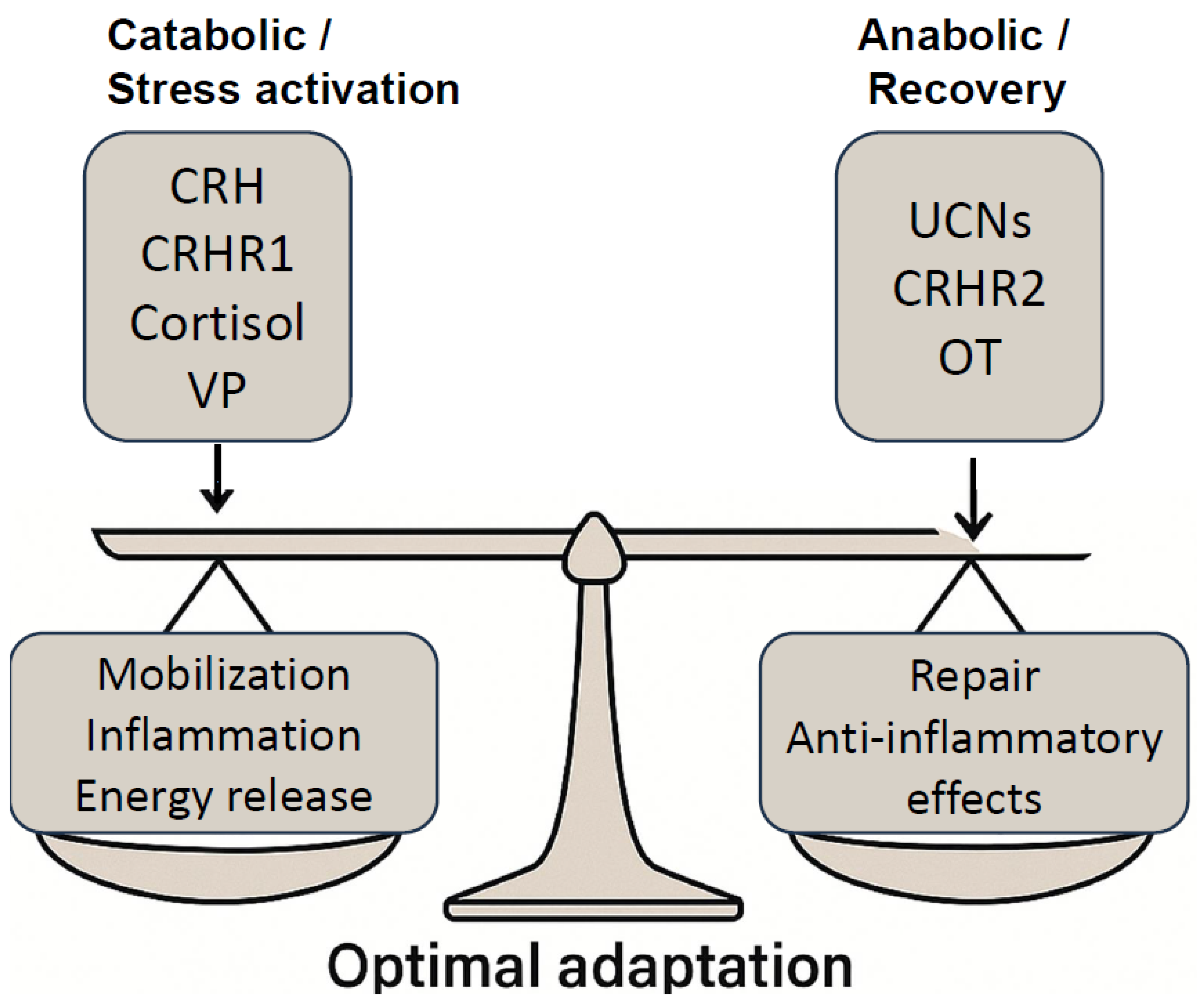
3. Catabolic–Anabolic Cycling Hormesis Model: A Dynamic Framework for Adaptive Health
3.1. Theoretical Foundations and Examples
3.2. CRH, Urocortins, and the Neuroendocrine Modulation of CACH
4. Vasopressin and Oxytocin as Endocrine Drivers of CACH
5. Stress
5.1. Neuroendocrine Architecture of the Stress Response
5.2. Vasopressin and Oxytocin in the Central Stress Network
6. Hormetic Stress: Cellular Adaptation and Systems Resilience
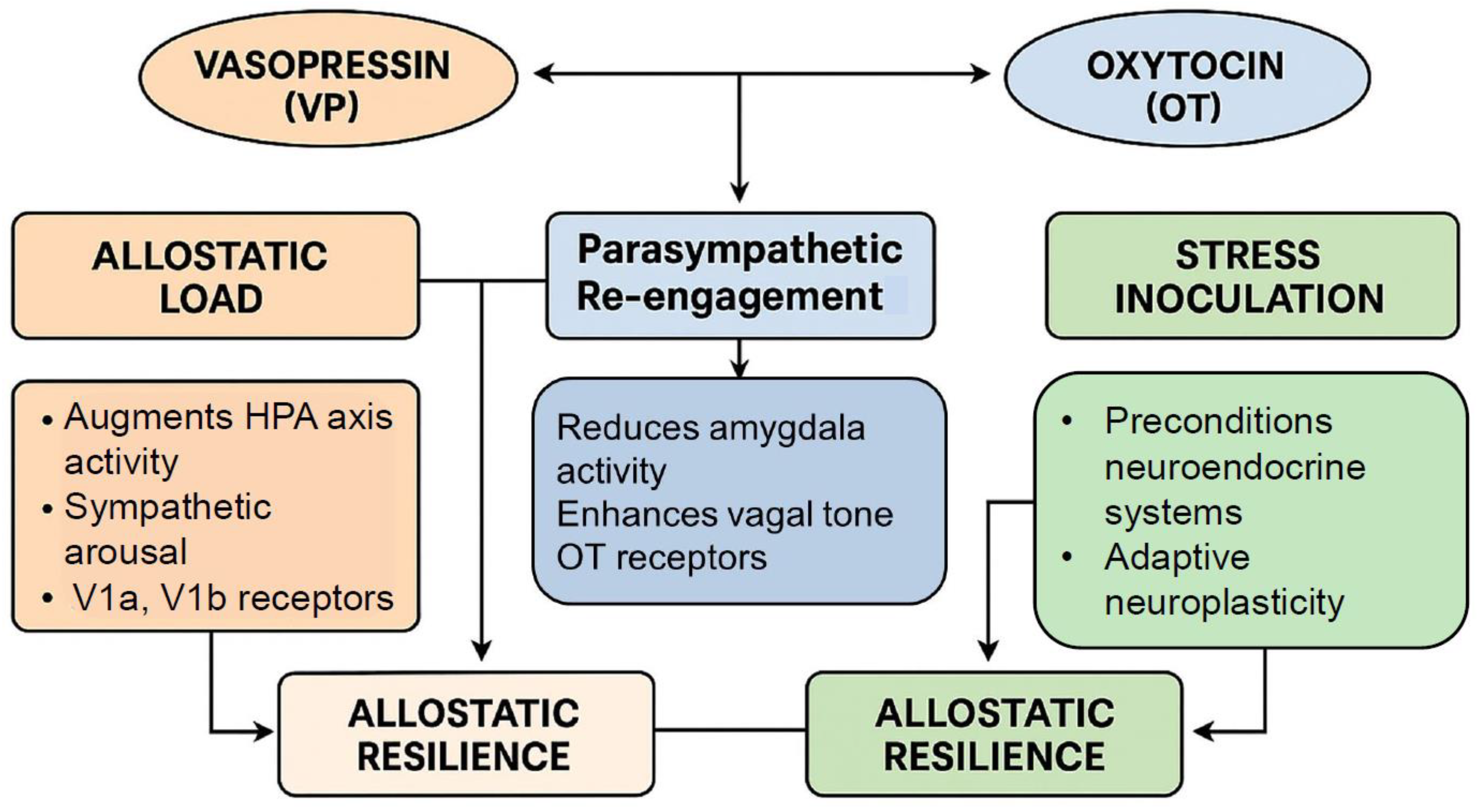
6.1. Chronic Stress, Allostatic Load, and Pathophysiological Transition
6.2. Integration into the Hormetic Framework: Oxytocin and Vasopressin as Bidirectional Modulators of Allostatic Flexibility
6.3. Vasopressin and Oxytocin as Hormetic Modulators
6.4. Vasopressin’s Role in Neuroendocrine Plasticity
6.4.1. Structural and Functional Plasticity of Vasopressin Neurons
6.4.2. Epigenetic Regulation and Early-Life Programming
6.4.3. Receptor-Specific Signaling and Behavioral Adaptation
6.4.4. Integration of Vasopressin into Hormetic Stress Networks
6.5. Oxytocin’s Behavioral and Cellular Roles in Hormetic Regulation
6.5.1. Context-Dependent Modulation of Stress and Behavior
6.5.2. Cellular Mechanisms of Oxytocin-Mediated Hormesis
6.5.3. Social Hormesis: Affiliative Behavior as Adaptive Stressor
7. Therapeutical Implications and Translational Opportunities
7.1. Precision Neuropeptide Modulation
7.2. Hormetic Interventions and Lifestyle Medicine
8. CRH, Urocortins, and the Hormetic Stress Response—Setting the Stage for Oxytocin and Vasopressin
8.1. CRH as a Hormetic Initiator
8.1.1. Catabolic Phase Activation: Metabolic and Circadian Modulation
8.1.2. Epigenetic Reprogramming and Intergenerational Stress Signatures
8.2. CRH as a Conditioning Signal
8.2.1. Immediate Response Calibration
8.2.2. Neuroplastic Priming
9. CRH, Vasopressin and Oxytocin Interact in Developmental and Behavioral Conditioning
9.1. CRH as the Gateway to Neuroendocrine Hormesis
9.2. Urocortins in Hormetic Stress Modulation—Bridging Catabolic Initiation and Adaptive Recovery
9.2.1. Urocortin Subtypes and Functional Roles
9.2.2. Hormetic Mechanisms of Urocortins Action
9.2.3. Urocortins and the Transition to Anabolic Recovery
9.3. Integration into the Catabolic-Anabolic Cycling Hormesis Model
9.4. Urocortins as Bidirectional Stress Regulators
10. Preparing the Stage for Oxytocin and Vasopressin
10.1. Recovery Adaptation Modulators
10.2. Integration of Oxytocin and Vasopressin Function
10.3. Dynamic OT-VP Interplay
10.4. Targeted Clinical Applications of OT–VP Modulation
10.5. Hormesis Blueprint for Resilience
11. Mechanistic Foundations
11.1. Hormetic Biphasic Signaling of Vasopressin and Oxytocin
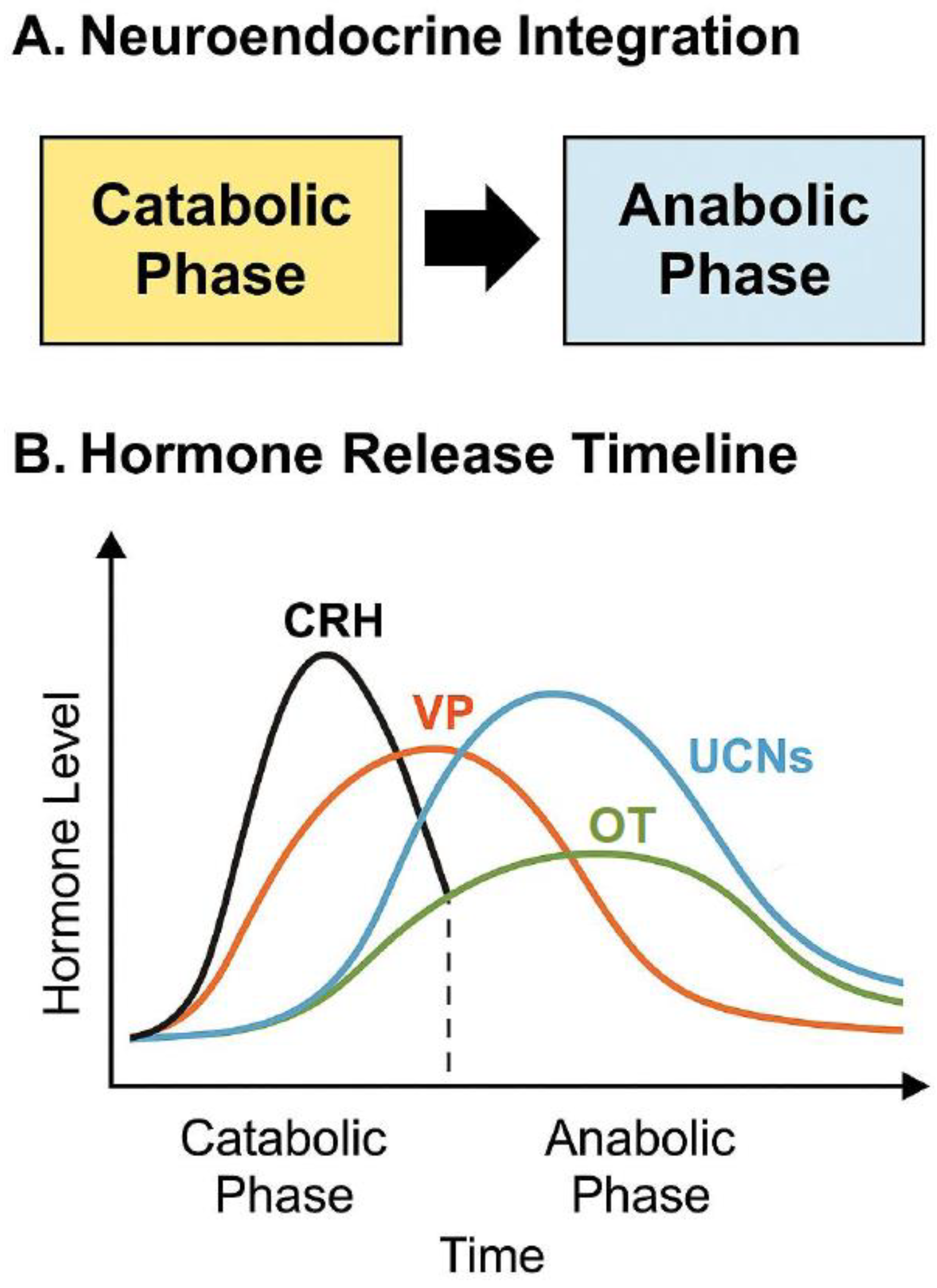
11.2. Neuroendocrine Integration
11.3. The Temporal Role of Each Peptide in Initiating or Resolving Stress
12. Hormesis and Allostasis
Autonomic Effects of Vasopressin and Oxytocin
13. Translational and Clinical Potential
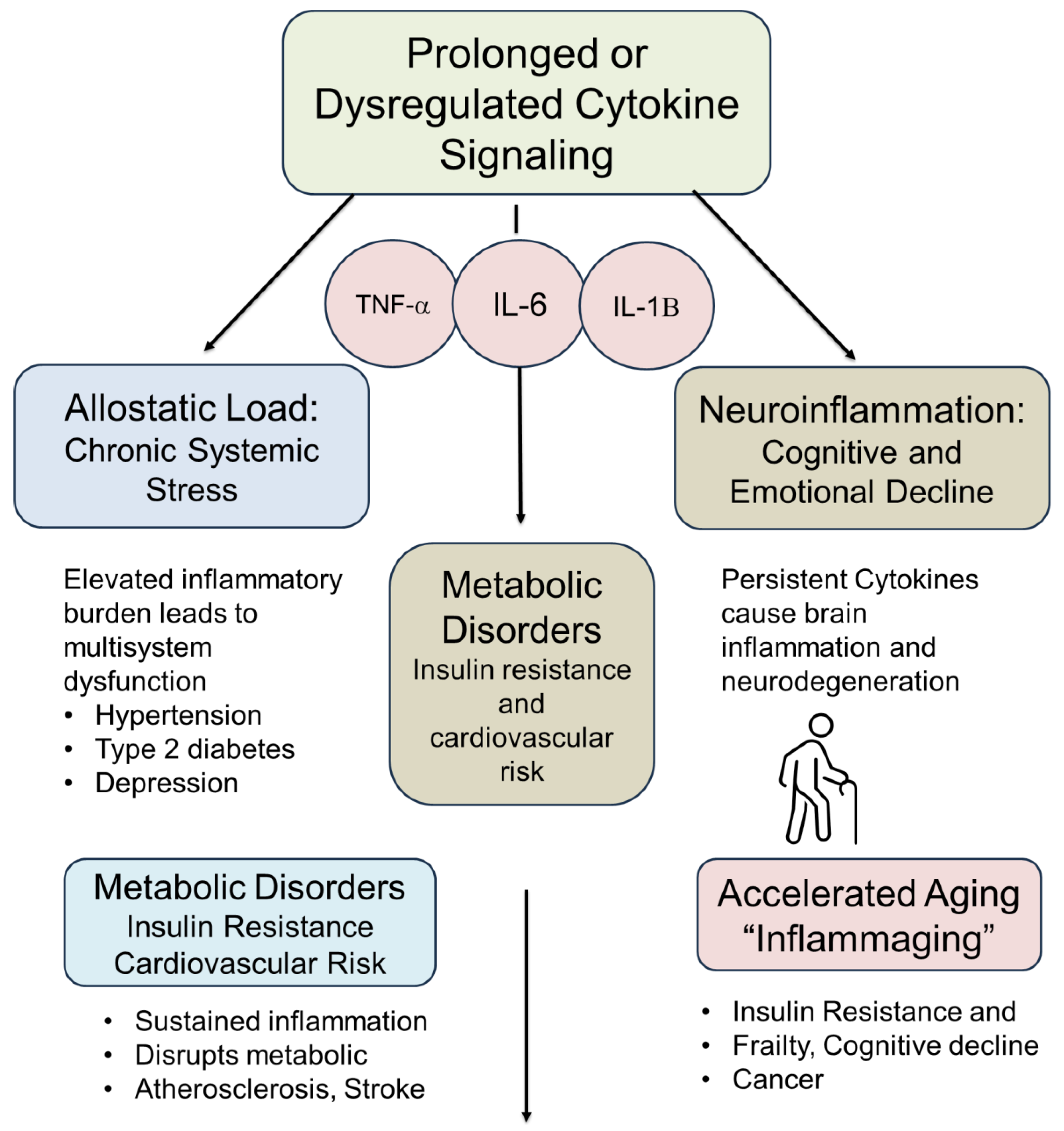
14. Cytokines as Dynamic Modulators of Hormetic Responses
14.1. Pro-Inflammatory Cytokine Surge: The “Alarm Phase” of Hormesis
14.2. Transition Phase: Activation of Cytoprotective and Repair Programs
14.3. Resolution Phase: Anti-Inflammatory and Anabolic Signaling
14.4. Failure of Resolution: When Hormesis Becomes Pathology
14.5. Biological and Clinical Implications of Cytokine Surges
14.6. Neuroimmune Interactions: OT, VP, and Cytokine Modulation
14.6.1. Oxytocin: A Neuroimmune Brake on Inflammation
14.6.2. Neuroprotection via Microglial Modulation
15. Integrated Neuroimmune Crosstalk
16. Future Directions and Research Priorities
17. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calabrese, E.J.; Mattson, M.P. The Catabolic–Anabolic Cycling Hormesis Model of Health and Resilience. Ageing Res. Rev. 2024, 102, 102588. [Google Scholar] [CrossRef]
- Du Vigneaud, V.; Ressler, C.; Trippett, S. The Sequence of Amino Acids in Oxytocin, with a Proposal for the Structure of Oxytocin. J. Biol. Chem. 1953, 205, 949–957. [Google Scholar] [CrossRef]
- Murphy, M.R.; Seckl, J.R.; Burton, S.; Checkley, S.A.; Lightman, S.L. Changes in Oxytocin and Vasopressin Secretion during Sexual Activity in Men. J. Clin. Endocrinol. Metab. 1987, 65, 738–741. [Google Scholar] [CrossRef]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is Oxytocin “Nature’s Medicine”? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S. The Psychedelic-Peptide Paradox: A Hormetic Hypothesis. Compr. Psychoneuroendocrinology 2025, 23, 100303. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S. Close encounters with oxytocin. Compr. Psychoneuroendocrinology 2023, 15, 100189. [Google Scholar] [CrossRef] [PubMed]
- Uvnäs-Moberg, K. The Physiology and Pharmacology of Oxytocin in Labor and in the Peripartum Period. Am. J. Obstet. Gynecol. 2024, 230, S740–S758. [Google Scholar] [CrossRef]
- Onaka, T.; Takayanagi, Y. Role of Oxytocin in the Control of Stress and Food Intake. J. Neuroendocrinol. 2019, 31, e12700. [Google Scholar] [CrossRef]
- Landgraf, R.; Neumann, I.D. Vasopressin and Oxytocin Release Within the Brain: A Dynamic Concept of Multiple and Variable Modes of Neuropeptide Communication. Front. Neuroendocrinol. 2004, 25, 150–176. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K. Oxytocin May Mediate the Benefits of Positive Social Interaction and Emotions. Psychoneuroendocrinology 1998, 23, 819–835. [Google Scholar] [CrossRef]
- Horn, A.J.; Carter, C.S. Love and Longevity: A Social Dependency Hypothesis. Compr. Psychoneuroendocrinology 2021, 8, 100088. [Google Scholar] [CrossRef]
- Szeto, A.; Nation, D.A.; Mendez, A.J.; Dominguez-Bendala, J.; Brooks, L.G.; Schneiderman, N.; McCabe, P.M. Oxytocin Attenuates NADPH-Dependent Superoxide Activity and IL-6 Secretion in Macrophages and Vascular Cells. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1495–E1501. [Google Scholar] [CrossRef] [PubMed]
- Uvnäs-Moberg, K.; Julius, H.; Handlin, L.; Petersson, M. Editorial: Sensory Stimulation and Oxytocin: Their Roles in Social Interaction and Health Promotion. Front. Psychol. 2022, 13, 929741. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Kingsbury, M.A. Oxytocin and Oxygen: The Evolution of a Solution to the ‘Stress of Life’. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 1–14. [Google Scholar] [CrossRef]
- Theofanopoulou, C.; Gedman, G.; Cahill, J.A.; Boeckx, C.; Jarvis, E.D. Universal Nomenclature for Oxytocin–Vasotocin Ligand and Receptor Families. Nature 2021, 592, 747–755. [Google Scholar] [CrossRef]
- Albers, H.E. The Regulation of Social Recognition, Social Communication and Aggression: Vasopressin in the Social Behavior Neural Network. Horm. Behav. 2012, 61, 283–292. [Google Scholar] [CrossRef]
- Antoni, F.A. Magnocellular Vasopressin and the Mechanism of “Glucocorticoid Escape”. Front. Endocrinol. 2019, 10, 422. [Google Scholar] [CrossRef]
- Horn, A.J.; Cole, S.; Nazarloo, N.P.; Nazarloo, P.; Davis, J.M.; Carrier, D.; Bryan, C.; Carter, C.S. Severe PTSD is Marked by Reduced Oxytocin and Elevated Vasopressin. Compr. Psychoneuroendocrinology 2024, 19, 100236. [Google Scholar] [CrossRef]
- Carter, C.S. Neuroendocrine Perspectives on Social Attachment and Love. Psychoneuroendocrinology 1998, 23, 779–818. [Google Scholar] [CrossRef]
- Carter, C.S. Sex, Love and Oxytocin: Two Metaphors and a Molecule. Neurosci. Biobehav. Rev. 2022, 143, 104948. [Google Scholar] [CrossRef]
- Salehi, M.S.; Neumann, I.D.; Jurek, B.; Pandamooz, S. Co-Stimulation of Oxytocin and Arginine-Vasopressin Receptors Affect Hypothalamic Neurospheroid Size. Int. J. Mol. Sci. 2021, 22, 8464. [Google Scholar] [CrossRef]
- Ellis, B.J.; Horn, A.J.; Carter, C.S.; van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J. Developmental Programming of Oxytocin through Variation in Early-Life Stress: Four Meta-Analyses and a Theoretical Reinterpretation. Clin. Psychol. Rev. 2021, 86, 101985. [Google Scholar] [CrossRef]
- Danoff, J.S.; Connelly, J.J.; Morris, J.P.; Perkeybile, A.M. An Epigenetic Rheostat of Experience: DNA Methylation of OXTR as a Mechanism of Early-Life Allostasis. Compr. Psychoneuroendocrinology 2021, 8, 100098. [Google Scholar] [CrossRef]
- Bartz, J.A.; Zaki, J.; Bolger, N.; Ochsner, K.N. Social Effects of Oxytocin in Humans: Context and Person Matter. Trends Cogn. Sci. 2011, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The Dose–Response Revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Hormesis Defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis and Medicine. Br. J. Clin. Pharmacol. 2008, 66, 594–617. [Google Scholar] [CrossRef]
- Neumann, I.D.; Landgraf, R. Balance of Brain Oxytocin and Vasopressin: Implications for Anxiety, Depression, and Social Behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Osakabe, N.; Di Paola, R.; Siracusa, R.; Fusco, R.; D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Fritsch, T.; Abdelhameed, A.S.; et al. Hormesis Defines the Limits of Lifespan. Ageing Res. Rev. 2023, 91, 102074. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Evolution of Hormesis Research: A Bibliometric Analysis. Arch. Toxicol. 2024, 98, 577–578. [Google Scholar] [CrossRef]
- Rattan, S.I.S.; Kyriazis, M. (Eds.) The Science of Hormesis in Health and Longevity; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128142530. [Google Scholar]
- Martucci, K.T.; MacNiven, K.H.; Borg, N.; Knutson, B.; Mackey, S. Altered Prefrontal Correlates of Monetary Anticipation and Outcome in Chronic Pain. Pain. 2018, 159, 1494–1507. [Google Scholar] [CrossRef]
- López-Lluch, G.; Navas, P. Coenzyme Q at the Hinge of Health and Metabolic Diseases. Antioxidants 2021, 10, 1785. [Google Scholar] [CrossRef] [PubMed]
- Giona, L.; Musillo, C.; De Cristofaro, G.; Ristow, M.; Zarse, K.; Siems, K.; Tait, S.; Cirulli, F.; Berry, A. Western Diet-Induced Cognitive and Metabolic Dysfunctions in Aged Mice Are Prevented by Rosmarinic Acid in a Sex-Dependent Fashion. Clin. Nutr. 2024, 43, 2236–2248. [Google Scholar] [CrossRef] [PubMed]
- Loth, M.K.; Schmidt, J.C.; Gonzalez, C.A.; Brusman, L.E.; Sadino, J.M.; Winther, K.E.; Protter, D.S.W.; Donaldson, Z.R. Oxytocin and Dopamine Receptor Expression: Cellular Level Implications for Pair Bonding. bioRxiv 2025, preprint. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the Brain: From Adaptation to Disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Mattson, M.P. The Cyclic Metabolic Switching Theory of Intermittent Fasting. Nat. Metab. 2025, 7, 665–678. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Hormesis: Transforming Disciplines That Rely on the Dose Response. IUBMB Life 2021, 73, 1197–1203. [Google Scholar] [CrossRef]
- Radak, Z.; Rattan, S.I.S. Exercise, Hormesis and Ageing: A New Section in Biogerontology. Biogerontology 2024, 26, 26. [Google Scholar] [CrossRef]
- López-Otín, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef]
- Knobloch, H.S.; Grinevich, V. Evolution of Oxytocin Pathways in the Brain of Vertebrates. Front. Behav. Neurosci. 2014, 8, 31. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Petrulis, A. Editorial: The Vasopressin System and Behavior. Front. Endocrinol. 2018, 9, 438. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Psychoneuroendocrinology 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Larauche, M.; Moussaoui, N.; Biraud, M.; Bae, W.K.; Duboc, H.; Million, M.; Taché, Y. Brain Corticotropin-Releasing Factor Signaling: Involvement in Acute Stress-Induced Visceral Analgesia in Male Rats. Neurogastroenterol. Motil. 2019, 31, e13489. [Google Scholar] [CrossRef] [PubMed]
- Danhof, H.A.; Lee, J.; Thapa, A.; Britton, R.A.; Di Rienzi, S.C. Microbial Stimulation of Oxytocin Release from the Intestinal Epithelium via Secretin Signaling. Gut Microbes 2023, 15, 2256043. [Google Scholar] [CrossRef] [PubMed]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Heinrichs, M.; von Dawans, B.; Domes, G. Oxytocin, Vasopressin, and Human Social Behavior. Front. Neuroendocrinol. 2009, 30, 548–557. [Google Scholar] [CrossRef]
- Carter, C.S. Oxytocin Pathways and the Evolution of Human Behavior. Annu. Rev. Psychol. 2014, 65, 17–39. [Google Scholar] [CrossRef]
- Smith, A.S.; Wang, Z. Hypothalamic Oxytocin Mediates Social Buffering of the Stress Response. Biol. Psychiatry 2014, 76, 281–288. [Google Scholar] [CrossRef]
- Guarente, L.; Sinclair, D.A.; Kroemer, G. Human Trials Exploring Anti-Aging Medicines. Cell Metab. 2024, 36, 354–376. [Google Scholar] [CrossRef]
- Longo, S.K.; Guo, M.G.; Ji, A.L.; Khavari, P.A. Integrating Single-Cell and Spatial Transcriptomics to Elucidate Intercellular Tissue Dynamics. Nat. Rev. Genet. 2021, 22, 627–644. [Google Scholar] [CrossRef]
- Pillon, N.J.; Gabriel, B.M.; Dollet, L.; Smith, J.A.B.; Sardón Puig, L.; Botella, J.; Bishop, D.J.; Krook, A.; Zierath, J.R. Transcriptomic Profiling of Skeletal Muscle Adaptations to Exercise and Inactivity. Nat. Commun. 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, Oxidants, and Antioxidants Change the Shape of the Bell-Shaped Hormesis Curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of Intermittent Fasting on Health and Disease Processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G. HPA Axis Responsiveness to Stress: Implications for Healthy Aging. Exp. Gerontol. 2011, 46, 90–95. [Google Scholar] [CrossRef]
- Zalachoras, I.; Houtzager, S.W.J.; Servaes, S.; Reul, J.M.H.M. CRH Receptors and Their Role in Neuroendocrine Adaptation to Stress: Implications for Mental Health. Curr. Opin. Neurobiol. 2022, 72, 134–141. [Google Scholar] [CrossRef]
- Fischer, L.; Paschke, B.; Gareis, F.; Schumacher, M.; Liere, P.; Hiergeist, A.; Gessner, A.; Rupprecht, R.; Neumann, I.D.; Bosch, O.J. The Translocator Protein 18 kDa (TSPO) Ligand Etifoxine in an Animal Model of Anxiety: Line- and Sex-Dependent Effects on Emotionality, Stress Reactivity, Spine Density, Oxytocin Receptors, Steroids, and Microbiome Composition. Neuropharmacology 2025, 266, 110282. [Google Scholar] [CrossRef]
- Vetter, C.; Scheer, F.A.J.L.; Shea, S.A. Circadian Biology: Uncoupling Human Body Clocks by Food Timing. Curr. Biol. 2017, 27, R656–R658. [Google Scholar] [CrossRef]
- Brar, B.; Bayoumy, M.; Salama, A.; Henry, A.; Chigurupati, R. A Survey Assessing the Early Effects of the COVID-19 Pandemic on Oral and Maxillofacial Surgery Training Programs. Oral Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2021, 131, 27–42. [Google Scholar] [CrossRef]
- Winter, J.; Meyer, M.; Berger, I.; Royer, M.; Bianchi, M.; Kuffner, K.; Peters, S.; Stang, S.; Langgartner, D.; Hartmann, F.; et al. Chronic Oxytocin-Driven Alternative Splicing of Crfr2α Induces Anxiety. Mol. Psychiatry 2023, 28, 4742–4755. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Saleh, M.S.M.; Elsabahy, H.E.; Abdelhamid, Z.N. Information Technology as an Opportunity for Improving Nurses’ Work Outcomes in Egypt. Biosci. Res. 2021, 18, 345–358. [Google Scholar]
- Jankowski, P.J.; Sandage, S.J.; Bell, C.A.; Davis, D.E.; Porter, E.; Jessen, M.; Motzny, C.L.; Ross, K.V.; Owen, J. Virtue, Flourishing, and Positive Psychology in Psychotherapy: An Overview and Research Prospectus. Psychotherapy 2020, 57, 291–309. [Google Scholar] [CrossRef]
- Szeto, A.C.H.; Ferreira, A.C.F.; Mannion, J.; Clark, P.A.; Sivasubramaniam, M.; Heycock, M.W.D.; Crisp, A.; Jolin, H.E.; Kozik, P.; Knolle, M.D.; et al. An αvβ3 Integrin Checkpoint Is Critical for Efficient TH2 Cell Cytokine Polarization and Potentiation of Antigen-Specific Immunity. Nat. Immunol. 2023, 24, 123–135. [Google Scholar] [CrossRef]
- Moghadam, M.R.S.; Ghasemnia Arabi, N.; Khoshsima, G. A Review of Case Study Method in Operations Management Research. Int. J. Qual. Methods 2021, 20, 1–11. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Iavicoli, I. Hormesis: Wound Healing and Fibroblasts. Pharmacol. Res. 2022, 182, 106304. [Google Scholar] [CrossRef] [PubMed]
- Lods, M.; Mortessagne, P.; Pacary, E.; Terral, G.; Farrugia, F.; Mazier, W.; Masachs, N.; Charrier, V.; Cota, D.; Ferreira, G.; et al. Chemogenetic Stimulation of Adult Neurogenesis, and Not Neonatal Neurogenesis, Is Sufficient to Improve Long-Term Memory Accuracy. Prog. Neurobiol. 2022, 219, 102364. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Dias, G.P. Hormetic Mechanisms in Neuroplasticity: Stress Resilience and Cognitive Adaptation. Neurosci. Biobehav. Rev. 2021, 124, 304–315. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M.; Liang, H. FOXO Transcription Factors in Cellular Stress Responses and Aging: Regulation by AMPK and SIRT1. Mech. Ageing Dev. 2023, 211, 111742. [Google Scholar] [CrossRef]
- Perrelli, M.; Goparaju, P.; Postolache, T.T.; Del Bosque-Plata, L.; Gragnoli, C. Stress and the CRH System, Norepinephrine, Depression, and Type 2 Diabetes. Biomedicines 2024, 12, 1187. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis and Health: The Integration of Stress Physiology Across Systems. Trends Endocrinol. Metab. 2023, 34, 9–22. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Kozumbo, W.J. The Hormetic Dose-Response Mechanism: Nrf2 Activation. Pharmacol. Res. 2021, 167, 105526. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Kopin, I.J. Linking Stress, Catecholamine Autotoxicity, and Allostatic Load with Neurodegenerative Diseases: A Focused Review in Memory of Richard Kvetnansky. Cell Mol. Neurobiol. 2018, 38, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Slattery, D.A. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol. Psychiatry 2016, 79, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Olff, M.; Frijling, J.L.; Kubzansky, L.D.; Bradley, B.; Ellenbogen, M.A.; Cardoso, C.; van Zuiden, M. The Role of Oxytocin in Social Bonding, Stress Regulation and Mental Health: An Update on the Moderating Effects of Context and Interindividual Differences. Psychoneuroendocrinology 2013, 38, 1883–1894. [Google Scholar] [CrossRef]
- Wang, P.; Yang, H.P.; Tian, S.; Wang, L.; Wang, S.C.; Zhang, F.; Wang, X. Oxytocin-Secreting System: A Major Part of the Neuroendocrine Center Regulating Social Behavior. J. Neuroendocrinol. 2019, 31, e12792. [Google Scholar] [CrossRef]
- Rigney, N.; de Vries, G.J.; Petrulis, A.; Young, L.J. Oxytocin, Vasopressin, and Social Behavior: From Neural Circuits to Clinical Opportunities. Endocrinology 2022, 163, bqac111. [Google Scholar] [CrossRef]
- Giano, Z.; Wheeler, D.L.; Hubach, R.D. Intersectionality and Adverse Childhood Experiences: Racial/Ethnic Differences in the Prevalence of ACEs in a Nationally Representative Sample. Am. J. Prev. Med. 2024, 67, 328–338. [Google Scholar] [CrossRef]
- Szczepańska-Sadowska, E. Interplay of Angiotensin Peptides, Vasopressin, and Insulin in the Heart: Experimental and Clinical Evidence of Altered Interactions in Obesity and Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 1310. [Google Scholar] [CrossRef]
- Moslemi, M.; Motamedi, F.; Asadi, S.; Khodagholi, F. Peroxisomal Malfunction Caused by Mitochondrial Toxin 3-NP: Protective Role of Oxytocin. Iran. J. Pharm. Res. 2019, 18, 296–307. [Google Scholar] [CrossRef]
- Carter, C.S.; Porges, S.W. The Biochemistry of Love: An Oxytocin Hypothesis. EMBO Rep. 2016, 17, 69–75. [Google Scholar] [CrossRef]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef]
- Volpi, S.; Rabadan-Diehl, C.; Aguilera, G. Vasopressinergic Regulation of the Hypothalamic–Pituitary–Adrenal Axis and Stress Adaptation. Stress 2004, 7, 75–83. [Google Scholar] [CrossRef]
- Murgatroyd, C.; Spengler, D. Oxytocin and Stress-Related Disorders: Insights from Epigenetics. Biol. Psychiatry 2021, 89, 309–317. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How Does Hormesis Impact Biology, Toxicology, and Medicine? NPJ Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, M.; Ebner, K.; Landgraf, R. Oxytocin and Stress: An Update on the Modulatory Role of Oxytocin in Stress Responses and Its Relevance for Stress-Related Disorders. Front. Neuroendocrinol. 2021, 61, 100873. [Google Scholar] [CrossRef]
- Martins, D.; Brodmann, K.; Veronese, M.; Dipasquale, O.; Mazibuko, N.; Schuschnig, U.; Zelaya, F.; Paloyelis, Y. “Less Is More”: A Dose–Response Account of Intranasal Oxytocin Pharmacodynamics in the Human Brain. Prog. Neurobiol. 2022, 211, 102239. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Zhuang, Q.; Xu, D.; Yao, S.; Zhao, W.; Becker, B. Oro-Mucosal Administration of Oxytocin Using Medicated Lollipops Alters Social Attention, Similar to Intranasal and Lingual Routes: Implications for Therapeutic Use. Front. Neurosci. 2022, 16, 1022101. [Google Scholar] [CrossRef]
- Carter, C.S. The Oxytocin–Vasopressin Pathway in the Context of Love and Fear. Front. Endocrinol. 2017, 8, 356. [Google Scholar] [CrossRef]
- Leng, G.; Ludwig, M. Oxytocin, A Social Peptide? Deconstructing the Evidence. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20210055. [Google Scholar] [CrossRef]
- Carter, C.S. Oxytocin and love: Myths, metaphors and mysteries. Compr. Psychoneuroendocrinology 2021, 9, 100107. [Google Scholar] [CrossRef]
- Kageyama, K.; Iwasaki, Y.; Daimon, M. Influence of Arginine Vasopressin on the Ultradian Dynamics of the Hypothalamic-Pituitary-Adrenal Axis. Front. Endocrinol. 2022, 13, 976323. [Google Scholar] [CrossRef]
- Carter, C.S.; Perkeybile, A.M. The Monogamy Paradox: What Do Love and Sex Have to Do with It? Front. Ecol. Evol. 2018, 6, 202. [Google Scholar] [CrossRef] [PubMed]
- Sofer, Y.; Zilkha, N.; Gimpel, E.; Wagner, S.; Chuartzman, S.G.; Kimchi, T. Sexually Dimorphic Oxytocin Circuits Drive Intragroup Social Conflict and Aggression in Wild House Mice. Nat. Neurosci. 2024, 27, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Steinman, M.Q.; Duque-Wilckens, N.; Greenberg, G.D.; Hao, R.; Campi, K.L.; Laredo, S.A.; Trainor, B.C. Sex-Specific Effects of Stress on Oxytocin Neurons Correspond with Responses to Intranasal Oxytocin. Biol. Psychiatry 2016, 80, 406–414. [Google Scholar] [CrossRef]
- Yamasue, H.; Okada, T.; Munesue, T.; Kuroda, M.; Fujioka, T.; Uno, Y.; Matsumoto, K.; Kuwabara, H.; Mori, D.; Okamoto, Y.; et al. Effect of Intranasal Oxytocin on the Core Social Symptoms of Autism Spectrum Disorder: A Randomized Clinical Trial. Mol. Psychiatry 2020, 25, 1849–1858. [Google Scholar] [CrossRef]
- Ohbuchi, T.; Haam, J.; Tasker, J.G. Regulation of Neuronal Activity in Hypothalamic Vasopressin Neurons. Interdiscip. Inf. Sci. 2015, 21, 225–234. [Google Scholar] [CrossRef]
- Bains, R.S.; Bhatti, J.S. Oxytocin and Stress Resilience: Neuroprotective Roles, Mechanisms, and Therapeutic Potential. Neurochem. Int. 2022, 157, 105356. [Google Scholar] [CrossRef]
- Duarte-Guterman, P.; Lieblich, S.E.; Qiu, W.; Splinter, J.E.J.; Go, K.A.; Casanueva-Reimon, L.; Galea, L.A.M. Oxytocin Has Sex-Specific Effects on Social Behaviour and Hypothalamic Oxytocin Immunoreactive Cells but Not Hippocampal Neurogenesis in Adult Rats. Horm. Behav. 2020, 122, 104734. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Lee, H.J.; Macbeth, A.H.; Young, W.S. Vasopressin: Behavioral Roles of an “Original” Neuropeptide. Prog. Neurobiol. 2008, 84, 1–24. [Google Scholar] [CrossRef]
- Carter, C.S.; Grippo, A.J.; Pournajafi-Nazarloo, H.; Ruscio, M.G.; Porges, S.W. Oxytocin, Vasopressin and Sociality. Prog. Brain Res. 2008, 170, 331–336. [Google Scholar] [CrossRef]
- Griebel, G.; Holsboer, F. Neuropeptide Receptor Antagonists as Therapeutic Agents: The Vasopressin V1b Receptor Example. Pharmacol. Ther. 2021, 219, 107709. [Google Scholar] [CrossRef]
- Packard, K.; Opendak, M. Rodent Models of Early Adversity: Impacts on Developing Social Behavior Circuitry and Clinical Implications. Front. Behav. Neurosci. 2022, 16, 918862. [Google Scholar] [CrossRef] [PubMed]
- Stribley, J.M.; Carter, C.S. Developmental Exposure to Vasopressin Increases Aggression in Adult Prairie Voles. Proc. Natl. Acad. Sci. USA 1999, 96, 12601–12604. [Google Scholar] [CrossRef]
- Zelena, D.; Pinter, O.; Balazsfi, D.G.; Langnaese, K.; Richter, K.; Landgraf, R.; Makara, G.B.; Engelmann, M. Vasopressin Signaling at Brain Level Controls Stress Hormone Release: The Vasopressin-Deficient Brattleboro Rat as a Model. Amino Acids 2015, 47, 2245–2253. [Google Scholar] [CrossRef]
- Fineberg, S.K.; Ross, D.A. Oxytocin and the Social Brain. Biol. Psychiatry 2017, 81, e19–e21. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. Hormesis and Adaptive Plasticity: Implications for Resilience in Neurobiology and Medicine. Trends Neurosci. 2023, 46, 14–26. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.G.; Abu-Akel, A. The Social Salience Hypothesis of Oxytocin. Biol. Psychiatry 2016, 79, 194–202. [Google Scholar] [CrossRef]
- Kovács, Z.; Szabó, É. Effects of Oxytocin Administration on Oxidative Markers in the Temporal Lobe of Aged Rats. Neurosci. Behav. Physiol. 2019, 49, 456–462. [Google Scholar] [CrossRef]
- Numakawa, T.; Kajihara, R. Involvement of Brain-Derived Neurotrophic Factor Signaling in the Pathogenesis of Stress-Related Brain Diseases. Front. Mol. Neurosci. 2023, 16, 1247422. [Google Scholar] [CrossRef]
- Bordt, E.A.; Smith, C.J.; Demarest, T.G.; Bilbo, S.D.; Kingsbury, M.A. Mitochondria, Oxytocin, and Vasopressin: Unfolding the Inflammatory Protein Response. Neurotox. Res. 2019, 36, 239–256. [Google Scholar] [CrossRef]
- Gebauer, L.; Witek, M.A.G.; Hansen, N.C.; Thomas, J.; Konvalinka, I.; Vuust, P. Oxytocin improves synchronisation in leader-follower interaction. Sci. Rep. 2016, 6, 38416. [Google Scholar] [CrossRef] [PubMed]
- Griebel, G.; Holsboer, F.; Neumann, I.D. Oxytocin and Vasopressin: Signalling, Behavioural Modulation and Potential Therapeutic Targets. Br. J. Pharmacol. 2021, 178, 1269–1283. [Google Scholar] [CrossRef]
- MacDonald, K.; Feifel, D. Oxytocin’s role in anxiety: A critical appraisal. Brain Res. 2014, 1580, 22–56. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.H.; Chung, C.; Kim, J.J.; Choi, S.Y.; Han, J.S. Oxytocin Protects Hippocampal Memory and Plasticity from Uncontrollable Stress. Sci. Rep. 2015, 5, 18540. [Google Scholar] [CrossRef]
- Morrison, S.F.; Madden, C.J.; Tupone, D. Central Neural Regulation of Brown Adipose Tissue Thermogenesis and Energy Expenditure. Cell Metab. 2014, 19, 741–756. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Stress-Induced Activation of Brown Adipose Tissue Prevents Obesity in Mice. Cell Rep. 2015, 13, 1523–1530. [Google Scholar] [CrossRef]
- Labbé, S.M.; Caron, A.; Bakan, I.; Laplante, M.; Lecomte, R.; Côté, C.H.; Richard, D.; Carpentier, A.C. In Vivo Measurement of Energy Substrate Contribution to Cold-Induced Brown Adipose Tissue Thermogenesis. FASEB J. 2015, 29, 2046–2058. [Google Scholar] [CrossRef]
- Jones, J.R.; Simon, T.; Lones, L.; Herzog, E.D. SCN VIP Neurons Are Essential for Normal Light-Mediated Resetting of Circadian Rhythms and Glucocorticoid Release. Nat. Commun. 2021, 12, 5280. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Nelson, R.J. Influence of the Modern Light Environment on Mood. Mol. Psychiatry 2013, 18, 751–757. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention Against Diverse Nutritional Challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Mehta, M.; Liu, Y.; Zhang, P. PGC-1α Controls Mitochondrial Biogenesis and Dynamics in Lead-Induced Neurotoxicity. Aging 2015, 7, 629–647. [Google Scholar] [CrossRef]
- Davidson, R.J.; McEwen, B.S. Social Influences on Neuroplasticity: Stress and Interventions to Promote Well-Being. Nat. Neurosci. 2012, 15, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.J.; Chen, M.C.; Gotlib, I.H. Variant in Oxytocin Receptor Gene Is Associated with Amygdala Volume. Psychoneuroendocrinology 2011, 36, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Perkeybile, A.M.; Carter, C.S.; Wroblewski, K.L.; Puglia, M.H.; Kenkel, W.M.; Lillard, T.S.; Karaoli, T.; Gregory, S.G.; Mohammadi, N.; Epstein, L.; et al. Early Nurture Epigenetically Tunes the Oxytocin Receptor. Psychoneuroendocrinology 2019, 99, 128–136. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 Threatens Human Nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Joëls, M.; Baram, T.Z. The Neuro-Symphony of Stress. Nat. Rev. Neurosci. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Chen, Y.; Andres, A.L.; Frotscher, M.; Baram, T.Z. Tuning Synaptic Transmission in the Hippocampus by Stress: The CRH System. Front. Cell. Neurosci. 2012, 6, 13. [Google Scholar] [CrossRef]
- Walker, C.M.; Gopnik, A. Discriminating Relational and Perceptual Judgments: Evidence from Human Toddlers. Cognition 2017, 166, 23–27. [Google Scholar] [CrossRef][Green Version]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef]
- Jamieson, J.P.; Nock, M.K.; Mendes, W.B. Improving Acute Stress Responses: The Power of Reappraisal. Curr. Dir. Psychol. Sci. 2013, 22, 51–56. [Google Scholar] [CrossRef]
- Bale, T.L.; Vale, W.W. CRF and CRF Receptors: Role in Stress Responsivity and Other Behaviors. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 525–557. [Google Scholar] [CrossRef] [PubMed]
- Vandael, D.; Wierda, K.; Vints, K.; Baatsen, P.; de Groef, L.; Moons, L.; Rybakin, V.; Gounko, N.V. Corticotropin-releasing factor induces functional and structural synaptic remodeling in acute stress. Transl. Psychiatry 2021, 11, 378. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Onaka, T. The Oxytocin System and Early-Life Experience-Dependent Plastic Changes. J. Neuroendocrinol. 2021, 33, e13049. [Google Scholar] [CrossRef]
- Kuperman, Y.; Weiss, M.; Dine, J.; Staikin, K.; Golani, O.; Ramot, A.; Nahum, T.; Kühne, C.; Shemesh, Y.; Wurst, W.; et al. CRFR1 in AgRP Neurons Modulates Sympathetic Nervous System Activity to Adapt to Cold Stress and Fasting. Cell Metab. 2016, 23, 1185–1199. [Google Scholar] [CrossRef]
- Li, Y.; Hassett, A.L.; Seng, J.S. Exploring the Mutual Regulation Between Oxytocin and Cortisol as a Marker of Resilience. Arch. Psychiatr. Nurs. 2019, 33, 164–173. [Google Scholar] [CrossRef]
- Roustit, M.M.; Vaughan, J.M.; Jamieson, P.M.; Cleasby, M.E. Urocortin 3 Activates AMPK and AKT Pathways and Enhances Glucose Disposal in Rat Skeletal Muscle. J. Endocrinol. 2014, 223, 143–154. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Wang, Y. Chronic UCN2 Treatment Desensitizes CRHR2 and Improves Insulin Sensitivity in Skeletal Muscle. Nat. Commun. 2023, 14, 3953. [Google Scholar] [CrossRef]
- Yamada, A.; Chen, X.; Martinez, J.M.; Thompson, B.T. Neural Stimulation Suppresses mTORC1-Mediated Protein Synthesis During Muscle Inactivity. Sci. Adv. 2025, 11, eadt4955. [Google Scholar] [CrossRef]
- Kirsch, P.; Esslinger, C.; Chen, Q.; Mier, D.; Lis, S.; Siddhanti, S.; Gruppe, H.; Mattay, V.S.; Gallhofer, B.; Meyer-Lindenberg, A. Oxytocin Modulates Neural Circuitry for Social Cognition and Fear in Humans. J. Neurosci. 2005, 25, 11489–11493. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Tost, H. Vasopressin and Post-Traumatic Stress Disorder. Stress 2020, 23, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Bosch, O.J.; Neumann, I.D. Both Oxytocin and Vasopressin Are Mediators of Maternal Care and Aggression in Rodents: From Central Release to Sites of Action. Horm. Behav. 2012, 61, 293–303. [Google Scholar] [CrossRef]
- Spengler, F.B.; Schultz, J.; Scheele, D.; Essel, M.; Maier, W.; Heinrichs, M.; Hurlemann, R. Kinetics and Dose Dependency of Intranasal Oxytocin Effects on Amygdala Reactivity. Biol. Psychiatry 2017, 82, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Slater, M.J.; Turner, M.J. Coach Identity Leadership Behaviours Are Positively Associated with Athlete Resource Appraisals: The Mediating Roles of Relational and Group Identification. Psychol. Sport. Exerc. 2020, 51, 101755. [Google Scholar] [CrossRef]
- Bales, K.L.; Carter, C.S. Developmental Exposure to Oxytocin Facilitates Partner Preference in Male Prairie Voles. Behav. Neurosci. 2003, 117, 854–859. [Google Scholar] [CrossRef]
- Stoop, R. Neuromodulation by Oxytocin and Vasopressin in the Social Brain. Nat. Rev. Neurosci. 2022, 23, 519–533. [Google Scholar] [CrossRef]
- Rigney, N.; de Vries, G.J.; Petrulis, A. Modulation of Social Behavior by Distinct Vasopressin Sources. Front. Endocrinol. 2023, 14, 1127792. [Google Scholar] [CrossRef]
- Yang, X.J.; Jiang, H.M.; Hou, X.H.; Song, J. Anxiety and Depression in Patients with Gastroesophageal Reflux Disease and Their Effect on Quality of Life. World J. Gastroenterol. 2021, 27, 2985–2995. [Google Scholar] [CrossRef]
- Ibayashi, Y.; Hasuzawa, N.; Nomura, S.; Kabashima, M.; Nagayama, A.; Iwata, S.; Kitamura, M.; Ashida, K.; Moriyama, Y.; Yamamoto, K.; et al. Mitochondrial Fission Is Required for Thermogenesis in Brown Adipose Tissue. PLoS ONE 2024, 19, e0312352. [Google Scholar] [CrossRef]
- Szeto, A.; Sun-Suslow, N.; Mendez, A.J.; Hernandez, R.I.; Wagner, K.V.; McCabe, P.M. Regulation of the Macrophage Oxytocin Receptor in Response to Inflammation. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E183–E189. [Google Scholar] [CrossRef]
- O’Malley, D.; Julio-Pieper, M.; Gibney, S.M.; Dinan, T.G.; Cryan, J.F. Oxytocin Inhibits Visceral Hypersensitivity and Colonic Barrier Dysfunction in a Stress-Sensitive Rat Strain. Neurogastroenterol. Motil. 2020, 32, e13715. [Google Scholar] [CrossRef]
- Peters, S.; Slattery, D.A.; Usherwood, T.; Rehn, L. Oxytocin Enhances Resting-State Connectivity Between Amygdala and Medial Frontal Cortex. Int. J. Neuropsychopharmacol. 2017, 20, 803–809. [Google Scholar] [CrossRef]
- Smith, A.S.; Wang, Z. Oxytocin Facilitates Adaptive Fear and Attenuates Anxiety Responses. Front. Behav. Neurosci. 2016, 10, 92. [Google Scholar] [CrossRef]
- Ebner, N.C.; Maura, G.M.; Macdonald, K.; Westberg, L.; Fischer, H. Oxytocin and Socioemotional Aging: Current Knowledge and Future Trends. Front. Hum. Neurosci. 2018, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Steinman, M.Q.; Laredo, S.A.; Lopez, E.M.; Manning, C.E.; Hao, R.C.; Doig, I.E.; Campi, K.L.; Flowers, A.E.; Knight, J.K.; Trainor, B.C. Hypothalamic Vasopressin Systems Are More Sensitive to the Long-Term Effects of Social Defeat in Males versus Females. Psychoneuroendocrinology 2015, 51, 122–134. [Google Scholar] [CrossRef]
- Rigney, N.; Campos-Lira, E.; Kirchner, M.K.; Wei, W.; Belkasim, S.; Beaumont, R.; Singh, S.; de Vries, G.J.; Petrulis, A. A Vasopressin Circuit That Modulates Sex-Specific Social Interest and Anxiety-Like Behavior in Mice. bioRxiv 2023. [Google Scholar] [CrossRef]
- Carter, C.S.; Altemus, M. Integrative Functions of Lactational Hormones in Social Behavior and Stress Management. Ann. N. Y. Acad. Sci. 1997, 807, 164–174. [Google Scholar] [CrossRef]
- Ramos, L.; Hicks, C.; Caminer, A.; Narlawar, R.; Kassiou, M.; McGregor, I.S. Oxytocin and Vasopressin Receptor Antagonists as a Novel Pharmacotherapy for Alcohol and Drug Dependence: A Translational Review. Neurosci. Biobehav. Rev. 2019, 106, 46–68. [Google Scholar] [CrossRef]
- Difede, J.; McAleavey, A.A.; Emrich, M.; Jick, A.; Ovalles, A.; Wyka, K.; Spielman, L.; Olden, M.; Peskin, M.; Becket-Davenport, C.; et al. A Proof-of-Concept Randomized Crossover Clinical Trial of a First-in-Class Vasopressin 1a Receptor Antagonist for PTSD. Contemp. Clin. Trials Commun. 2023, 33, 101116. [Google Scholar] [CrossRef]
- Ding, C.; Leow, M.K.-S.; Magkos, F. Oxytocin in Metabolic Homeostasis: Implications for Obesity and Diabetes Management. Obes. Rev. 2019, 20, 22–40. [Google Scholar] [CrossRef]
- Perisic, M.; Woolcock, K.; Hering, A.; Mendel, H.; Muttenthaler, M. Oxytocin and Vasopressin Signaling in Health and Disease. Trends Biochem. Sci. 2024, 49, 361–377. [Google Scholar] [CrossRef]
- Guzmán, Y.F.; Tronson, N.C.; Jovasevic, V.; Sato, K.; Guedea, A.L.; Mizukami, H.; Nishimori, K.; Radulovic, J. Fear-Enhancing Effects of Septal Oxytocin Receptors. Nat. Neurosci. 2013, 16, 1185–1187. [Google Scholar] [CrossRef] [PubMed]
- Chini, B.; Verhage, M.; Grinevich, V. The Action Radius of Oxytocin Release in the Mammalian CNS: From Single Vesicles to Behavior. Trends Pharmacol. Sci. 2021, 42, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Wirobski, G.; Range, F.; Schaebs, F.S.; Palme, R.; Deschner, T.; Marshall-Pescini, S. Life Experience Rather Than Domestication Accounts for Dogs’ Increased Oxytocin Release During Social Contact with Humans. Sci. Rep. 2021, 11, 14423. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.B.J.; van Zuiden, M.; Nawijn, L.; Frijling, J.L.; Veltman, D.J.; Olff, M. Intranasal Oxytocin Administration Dampens Amygdala Reactivity Toward Emotional Faces in PTSD Patients. Neuropsychopharmacology 2016, 41, 1495–1504. [Google Scholar] [CrossRef]
- Lefevre, A.; Sirigu, A. The Two-Fold Role of Oxytocin in Social Developmental Disorders: A Cause and a Remedy? Neurosci. Biobehav. Rev. 2016, 63, 168–176. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, J.; Zhang, H.; Jin, J. CRH Neurons and the Neuroendocrine Regulation of Stress. Trends Endocrinol. Metab. 2023, 34, 91–106. [Google Scholar] [CrossRef]
- Russell, G.M.; Lightman, S.L. The Human Stress Response: Sympathetic Nervous System and HPA Axis. Neurosci. Lett. 2019, 702, 134–143. [Google Scholar] [CrossRef]
- Kageyama, K.; Hanada, K.; Moriyama, T.; Imaizumi, T.; Satoh, K.; Suda, T. Differential Regulation of CREB and ERK Phosphorylation Through Corticotropin-Releasing Factor Receptors Type 1 and 2 in AtT-20 and A7r5 Cells. Mol. Cell. Endocrinol. 2007, 263, 90–102. [Google Scholar] [CrossRef]
- Pérez-García, S.; Juarranz, Y.; Carrión, M.; Gutiérrez-Cañas, I.; Margioris, A.; Pablos, J.L.; Tsatsanis, C.; Gomariz, R.P. Mapping the CRF-Urocortins System in Human Osteoarthritic and Rheumatoid Synovial Fibroblasts: Effect of Vasoactive Intestinal Peptide. J. Cell. Physiol. 2011, 226, 3261–3269. [Google Scholar] [CrossRef]
- Triana-Del Rio, R.; Ranade, S.; Guardado, J.; LeDoux, J.; Klann, E.; Shrestha, P. The Modulation of Emotional and Social Behaviors by Oxytocin Signaling in Limbic Network. Front. Mol. Neurosci. 2022, 15, 1002846. [Google Scholar] [CrossRef] [PubMed]
- Sobota, R.; Mihara, T.; Forrest, A.; Featherstone, R.E.; Siegel, S.J. Oxytocin Reduces Amygdala Activity, Increases Social Interactions, and Reduces Anxiety-Like Behavior Irrespective of NMDAR Antagonism. Behav. Neurosci. 2015, 129, 389–398. [Google Scholar] [CrossRef]
- Barberis, C.; Tribollet, E. Vasopressin and Oxytocin Receptors in the Central Nervous System. Crit. Rev. Neurobiol. 1996, 10, 119–154. [Google Scholar] [CrossRef]
- Tregeagle, D.; Doherty, C.; Callis, T.; Kassiou, M. Non-Peptidic Natural Products That Target and Modulate Oxytocin and Arginine Vasopressin Receptors: Opportunities and Challenges. J. Pharmacol. Sci. 2025, 158, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Albers, H.E. Cross-Talk Among Oxytocin and Vasopressin Receptors: Relevance for Basic and Clinical Studies of the Brain. Front. Neuroendocrinol. 2018, 51, 14–24. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Porges, S.W.; Porges, S. Our Polyvagal World: How Safety and Trauma Change Us; W.W. Norton & Company: New York, NY, USA, 2023. [Google Scholar]
- Branchi, I. The Double-Edged Sword of Early-Life Adversity: Mechanisms of Adaptive Versus Maladaptive Plasticity. Dev. Psychobiol. 2021, 63, e22164. [Google Scholar] [CrossRef]
- Karelina, K.; Stuller, K.A.; Jarrett, B.; Zhang, N.; Wells, J.; DeVries, A.C. Oxytocin Promotes Resilience to Stress and Inflammation in Rats. Psychoneuroendocrinology 2011, 36, 1082–1093. [Google Scholar] [CrossRef]
- Szabó, P.; Bonet, S.; Hetényi, R.; Hanna, D.; Kovács, Z.; Prisztóka, G.; Križalkovičová, Z.; Szentpéteri, J. Systematic Review: Pain, Cognition, and Cardioprotection—Unpacking Oxytocin’s Contributions in a Sport Context. Front. Physiol. 2024, 15, 1393497. [Google Scholar] [CrossRef]
- Quintana, D.S.; Alvares, G.A.; Heathers, J.A.J. Guidelines for Reporting HRV in Research on the Effects of Oxytocin. Biol. Psychol. 2016, 116, 146–151. [Google Scholar] [CrossRef]
- Flanagan, J.C.; Mitchell, J.M.; Baker, N.L.; Woolley, J.; Wangelin, B.; Back, S.E.; McQuaid, J.R.; Neylan, T.C.; Wolfe, W.R.; Brady, K.T. Enhancing Prolonged Exposure Therapy for PTSD among Veterans with Oxytocin: Design of a Multisite Randomized Controlled Trial. Contemp. Clin. Trials 2020, 95, 106074. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.H.; Rubin, D.B.; Veenstra-VanderWeele, J.; Carter, C.S.; Sweeney, J.A. Sex Differences and Vasopressin System Dysfunction in Schizophrenia and Related Disorders. Biol. Psychiatry 2023, 94, 183–192. [Google Scholar] [CrossRef]
- Mahableshwarkar, A.R.; Jacobsen, P.L.; Chen, Y.; Serenko, M. A Randomized, Placebo-Controlled, Phase 2 Trial of ABT-436, a Novel V1B Receptor Antagonist, for Major Depressive Disorder. Neuropsychopharmacology 2015, 40, 415–422. [Google Scholar] [CrossRef]
- Kemp, A.H.; Quintana, D.S.; Kuhnert, R.L.; Griffiths, K.; Hickie, I.B.; Guastella, A.J. Impact of Oxytocin on Heart Rate Variability and Neurocardiac Function. Biol. Psychol. 2012, 90, 243–249. [Google Scholar] [CrossRef]
- MacLean, E.L.; Wilson, S.R.; Martin, W.L.; Davis, J.M.; Nazarloo, H.P.; Carter, C.S. Challenges for measuring oxytocin: The blind men and the elephant? Psychoneuroendocrinology 2019, 107, 225–231. [Google Scholar] [CrossRef]
- Aulinas, A.; Lawson, E.A. The Oxytocin System and Implications for Oxytocin Deficiency in Hypothalamic-Pituitary Disease. Endocr. Rev. 2025, 46, bnaf008. [Google Scholar] [CrossRef]
- Giovanna, G.; Damiani, S.; Fusar-Poli, L.; Rocchetti, M.; Brondino, N.; de Cagna, F.; Mori, A.; Politi, P. Intranasal Oxytocin as a Potential Therapeutic Strategy in Post-Traumatic Stress Disorder: A Systematic Review. Psychoneuroendocrinology 2020, 115, 104605. [Google Scholar] [CrossRef]
- Calabrese, V.; Wenzel, U.; Piccoli, T.; Jacob, U.M.; Nicolosi, L.; Fazzolari, G.; Failla, G.; Fritsch, T.; Osakabe, N.; Calabrese, E.J. Investigating Hormesis, Aging, and Neurodegeneration: From Bench to Clinics. Open Med. 2024, 19, 20240986. [Google Scholar] [CrossRef]
- Wang, J.; Hodes, G.E.; Zhang, H.; Zhang, S.; Zhao, W.; Golden, S.A.; Bi, W.; Menard, C.; Kana, V.; Leboeuf, M.; et al. Epigenetic Modulation of Inflammation and Synaptic Plasticity Promotes Resilience Against Stress in Mice. Nat. Commun. 2018, 9, 477. [Google Scholar] [CrossRef]
- Xu, J.; Wakai, M.; Xiong, K.; Yang, Y.; Prabakaran, A.; Wu, S.; Ahrens, D.; Molina-Portela, M.D.P.; Ni, M.; Bai, Y.; et al. The Pro-Inflammatory Cytokine IL6 Suppresses Mitochondrial Function via the gp130-JAK1/STAT1/3-HIF1α/ERRα Axis. Cell Rep. 2025, 44, 115403. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, H.J.; Kim, J.W.; Yu, B.P. Cytokines as Regulators of the Hormetic Stress Response. Ageing Res. Rev. 2023, 88, 101927. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Witkowski, J.M. Immunosenescence and Inflammaging in Healthy Aging: Myth or Reality? Curr. Opin. Immunol. 2023, 83, 102300. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, B.; Xu, X.; Guo, Z. Hormetic Remodeling of Mitochondrial Function and Immune Signaling: A Future Therapeutic Direction. Trends Mol. Med. 2024, 30, 31–47. [Google Scholar] [CrossRef]
- Liberale, L.; Montecucco, F.; Tardif, J.-C.; Libby, P.; Camici, G.G. Inflamm-Ageing: The Role of Inflammation in Age-Dependent Cardiovascular Disease. Eur. Heart J. 2020, 41, 2974–2982. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A New Immune–Metabolic Viewpoint for Age-Related Diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Sarvas, J.L.; Khaper, N.; Lees, S.J. The IL-6 Paradox: Context Dependent Interplay of SOCS3 and AMPK. J. Diabetes Metab. 2013, S13, 003. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 Regulatory Network Provides an Interface between Redox and Intermediary Metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Weavers, H. Biological Resilience in Health and Disease. Dis. Model. Mech. 2024, 17, dmm050799. [Google Scholar] [CrossRef]
- Zeytinoğlu, M.; Çınaroğlu, O.S.; Bora, E.S.; Erbaş, O. Healing with Love: Oxytocin Accelerates Oral Ulcer Recovery by Reducing Inflammation. J. Clin. Med. 2025, 14, 2667. [Google Scholar] [CrossRef]

| Conceptual Domains | Unresolved Research Questions |
|---|---|
| Mechanistic Foundations |
|
| Neuroendocrine Integration |
|
| Hormesis and Allostasis |
|
| Translational and Clinical Potential |
|
| System | Catabolic Phase (“Challenge”) | Anabolic Phase (“Restoration”) | Primary References |
|---|---|---|---|
| Skeletal muscle physiology |
|
| [53] |
| Metabolic regulation |
|
| [51] |
| Cognitive performance/brain |
|
| [61] |
| Phase | Key Signaling Nodes/Mediators | Core Functions | Primary References |
| Catabolic (Energy-Mobilizing) |
| Maintain vital function under challenge; initiate cellular “clean-up” | [51,53,69] |
| Anabolic (Recovery/Growth) |
| Restore and enhance structure/function; build resilience to future stressors | [32,33,64] |
| Aspect | Oxytocin (OT) | Vasopressin (VP) | References |
|---|---|---|---|
| Primary Role in Stress Response | Promotes recovery, emotional regulation, social bonding, resilience | Initiates acute stress response, vigilance, energy mobilization | [6,46,48,49,81,91,142] |
| Timing of Activation | Activated during post-stress recovery (delayed response) | Activated immediately during stress (early response) | [46,74,135] |
| Interaction with CRH | Inhibits CRH-induced amygdala activation; suppresses CRH, ACTH | Potentiates CRH activity and ACTH release | [46,57,141] |
| HPA Axis Effects | Negative feedback on HPA axis; enhances glucocorticoid receptor sensitivity | Stimulates HPA axis and increases glucocorticoid output | [46,47,57,67,135] |
| Social Behavior Modulation | Enhances prosocial behavior, trust, and social bonding | Supports dominance, aggression, territorial behavior | [48,81,135,142] |
| Cognitive Effects | Improves cognitive flexibility, prevents hippocampal atrophy | Enhances memory consolidation, but excessive levels cause anxiety and PTSD | [49,116] |
| Autonomic Regulation | Enhances parasympathetic tone, reduces sympathetic arousal | Increases sympathetic arousal; may disrupt recovery | [49,81,135] |
| Gastrointestinal Function | Restores vagal motility, reduces inflammation, supports gut integrity | Regulates motility (via V1a/V1b); excess leads to GI dysfunction | [44,46,152,153] |
| Immune Modulation | Suppresses pro-inflammatory cytokines; activates T-regs and M2 macrophages | Amplifies inflammation under chronic stress | [135,150,151] |
| Neuroendocrine Plasticity | Programs stress resilience, especially during development | Modulates HPA tone, stress coping (context-dependent) | [88,101,143] |
| Sex Differences | Stronger response in females; enhances cardiovascular and social resilience | Stronger in males; linked to aggression and prolonged HPA activation | [81,114,135,157] |
| Therapeutic Potential | Used in PTSD, depression, GI disorders, aging, and metabolic recovery | Targeted in PTSD, anxiety, schizophrenia; VP antagonists under study | [44,45,81,135,161,164,165,166] |
| Hormone | Onset Time | Peak Time | Duration | Phase | Functional Role |
|---|---|---|---|---|---|
| CRH | Onset within seconds to 2 min after stress | Peaks at 10–20 min | Returns to baseline by ~60–90 min | Early Catabolic | Initiates HPA axis, stimulates ACTH, mobilizes energy |
| VP | Onset: 2–10 min | Peaks at 20–40 min | May persist up to 2–4 h, with chronic stress | Mid-to-Late Catabolic | Prolongs ACTH release, enhances cardiovascular and metabolic drive |
| UCNs (esp. UCN2/UCN3) | Onset: ~30–60 min post-stressor | Peaks at 1–3 h | Sustained up to 6 h | Transition to Anabolic | Dampens HPA activation, promotes neuroprotection and tissue repair |
| OT | Onset: ~20–60 min post-stressor (delayed) | Peaks at 1–4 h | Sustained release up to 12–24 h (especially in recovery-promoting contexts) | Anabolic | Supports parasympathetic tone, social behavior, anti-inflammation, and regenerative recovery |
| Axis | Oxytocin | Vasopressin |
|---|---|---|
| Cytokine Modulation | Suppression TNT-a, IL-b, IL-6 | Amplifies early pro-inflammatory signals |
| Parasympathetic/ Sympathetic | Enhances vagal tone, recovery | Boosts sympathetic drive, mobilization |
| Microglial Activity | Inhibits activation, promotes neuroplasticity | Sustains glial activation if prolonged |
| Stress Phase | Dominates resolution and recovery phase | Dominates catabolic and alarm phases |
| Priority | Future Direction | Focus Area | Potential Impact | Notes |
|---|---|---|---|---|
| 1 | Multi-omic profiling to map individual stress-response signatures | Epigenomics, transcriptomics, proteomics | Personalized hormesis models; identification of hormetic thresholds and maladaptive tipping points | Critical for precision medicine and individualized resilience protocols |
| 2 | Neuroadaptive technologies for real-time modulation of resilience circuits | Real-time fMRI, tDCS/TMS, closed-loop OT/VP delivery | Enables state-contingent intervention; rapid feedback-based enhancement of stress recovery and cognitive performance | High innovation; bridges neuroscience and technology |
| 3 | Integrated lifestyle-based hormetic interventions | Combined use of CR, exercise, social bonding, cognitive stress, and neuropeptide enhancement | Scalable, low-cost strategies to increase population-level resilience and healthspan | High feasibility and translatability to clinical and public health settings |
| 4 | Longitudinal developmental studies on neuropeptide plasticity | Developmental biology, early life stress, OT/VP programming | Insights into critical windows, reversibility of early adversity, and preventive strategies | Long timeline; foundational for understanding life-course effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarloo, H.P.; Kingsbury, M.A.; Lamont, H.; Dale, C.V.; Nazarloo, P.; Davis, J.M.; Porges, E.C.; Cuffe, S.P.; Carter, C.S. Oxytocin, Vasopressin and Stress: A Hormetic Perspective. Curr. Issues Mol. Biol. 2025, 47, 632. https://doi.org/10.3390/cimb47080632
Nazarloo HP, Kingsbury MA, Lamont H, Dale CV, Nazarloo P, Davis JM, Porges EC, Cuffe SP, Carter CS. Oxytocin, Vasopressin and Stress: A Hormetic Perspective. Current Issues in Molecular Biology. 2025; 47(8):632. https://doi.org/10.3390/cimb47080632
Chicago/Turabian StyleNazarloo, Hans P., Marcy A. Kingsbury, Hannah Lamont, Caitlin V. Dale, Parmida Nazarloo, John M. Davis, Eric C. Porges, Steven P. Cuffe, and C. Sue Carter. 2025. "Oxytocin, Vasopressin and Stress: A Hormetic Perspective" Current Issues in Molecular Biology 47, no. 8: 632. https://doi.org/10.3390/cimb47080632
APA StyleNazarloo, H. P., Kingsbury, M. A., Lamont, H., Dale, C. V., Nazarloo, P., Davis, J. M., Porges, E. C., Cuffe, S. P., & Carter, C. S. (2025). Oxytocin, Vasopressin and Stress: A Hormetic Perspective. Current Issues in Molecular Biology, 47(8), 632. https://doi.org/10.3390/cimb47080632






