Physical Activity and Metabolic Disorders—What Does Gut Microbiota Have to Do with It?
Abstract
1. Introduction
2. Obesity
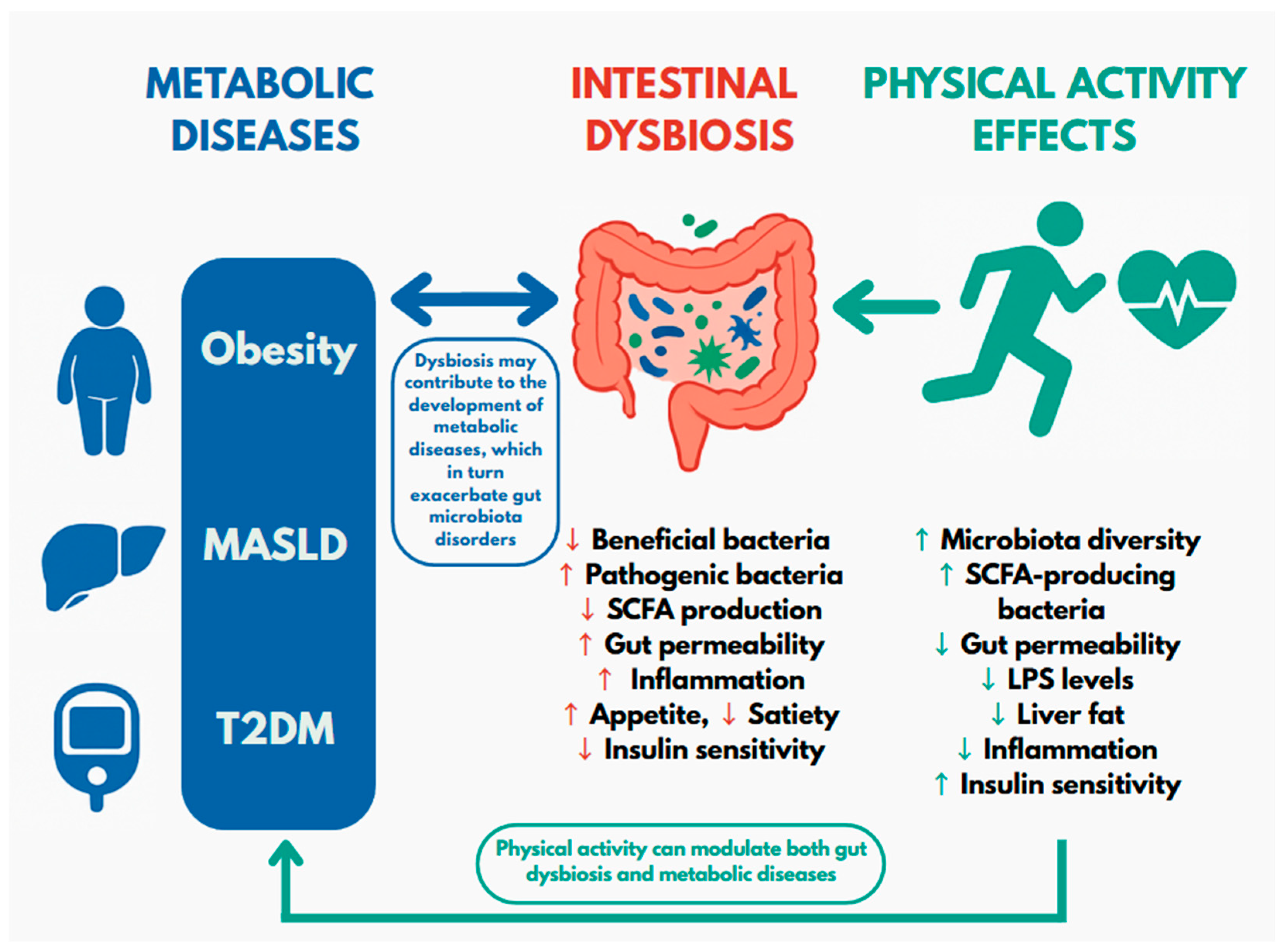
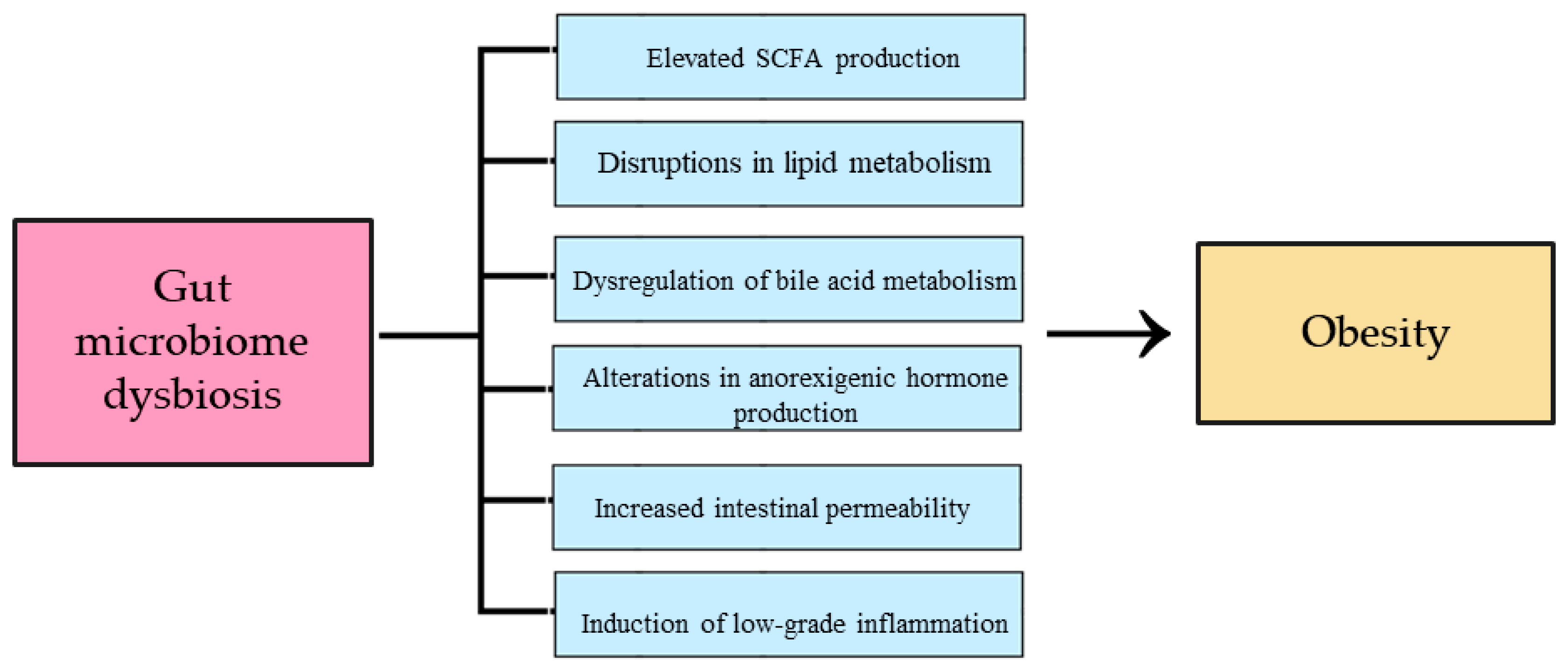
2.1. The Influence of Intestinal Dysbiosis on the Development of Obesity
2.2. The Influence of Physical Activity on the Gut Microbiota and Its Impact on Obesity
3. Diabetes Mellitus
Diabetes Mellitus, Gut Microbiota, and Physical Activity
4. Metabolic Dysfunction-Associated Steatotic Liver Disease
4.1. The Role of the Gut Microbiota in the Pathogenesis of MASLD
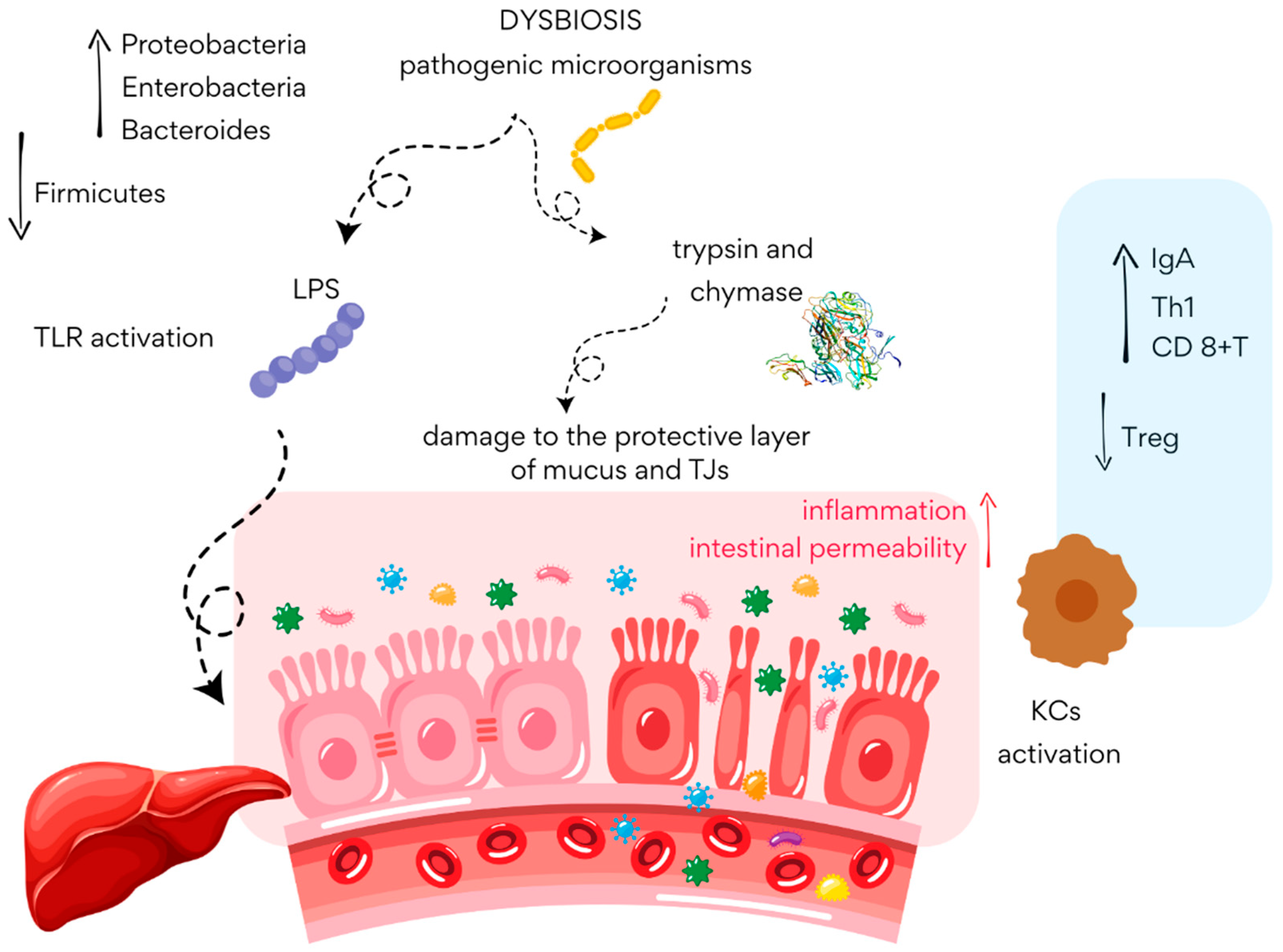
4.2. Non-Pharmacological Treatment of MASLD
4.3. The Role of Physical Activity in Modulating Gut Microbiota and the Risk of Developing MASLD
4.4. The Influence of Different Types of Physical Activity on the Microbiota in MASLD
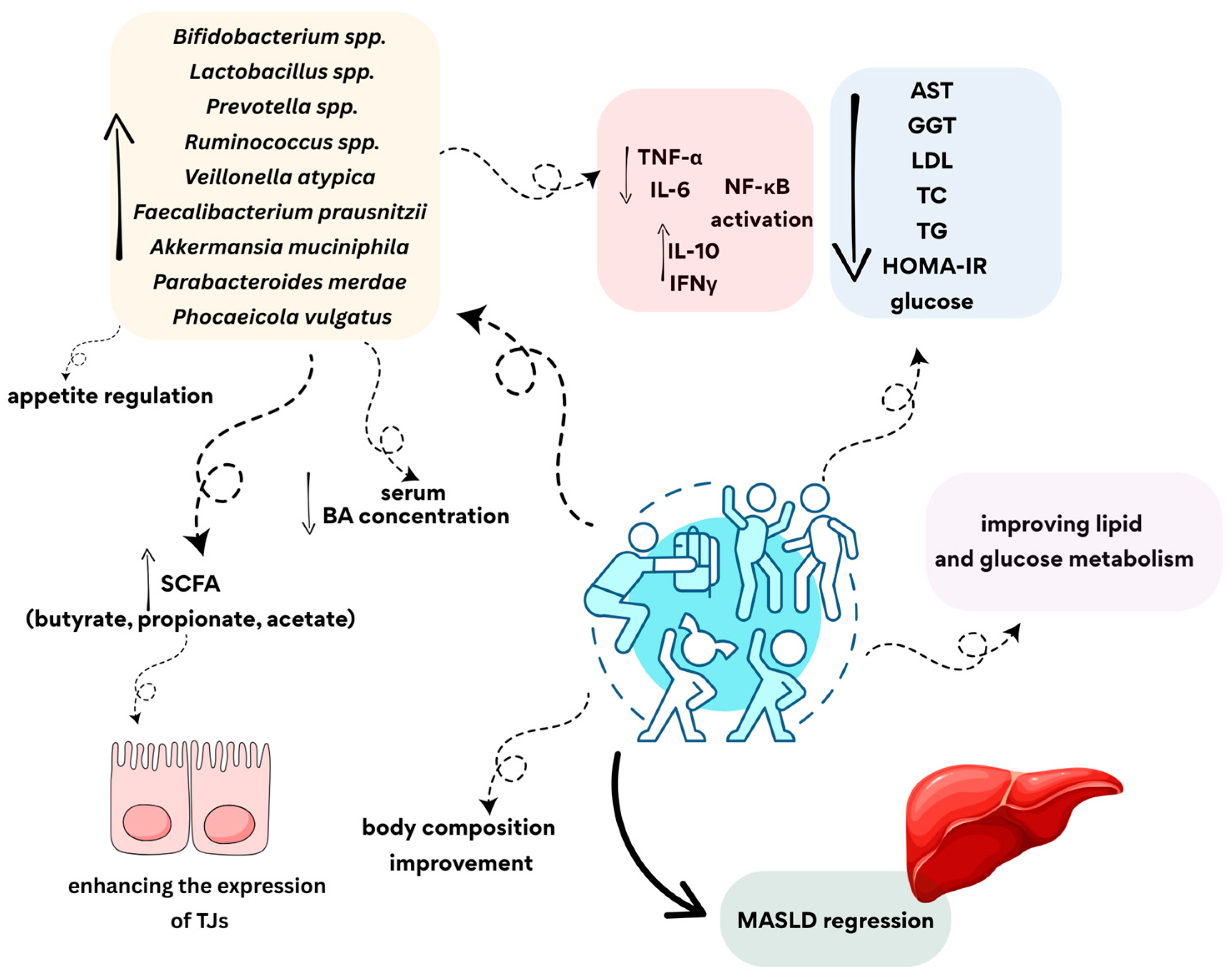
5. Key Findings from the Review
6. Limitation of Available Scientific Data
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 April 2025).
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar] [CrossRef]
- Młynarska, E.; Czarnik, W.; Dzieża, N.; Jędraszak, W.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Type 2 Diabetes Mellitus: New Pathogenetic Mechanisms, Treatment and the Most Important Complications. Int. J. Mol. Sci. 2025, 26, 1094. [Google Scholar] [CrossRef]
- Scarpellini, E.; Scarcella, M.; Tack, J.F.; Scarlata, G.G.M.; Zanetti, M.; Abenavoli, L. Gut Microbiota and Metabolic Dysfunction-Associated Steatotic Liver Disease. Antioxidants 2024, 13, 1386. [Google Scholar] [CrossRef]
- Abeltino, A.; Hatem, D.; Serantoni, C.; Riente, A.; De Giulio, M.M.; De Spirito, M.; De Maio, F.; Maulucci, G. Unraveling the Gut Microbiota: Implications for Precision Nutrition and Personalized Medicine. Nutrients 2024, 16, 3806. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Román, A.; Pagán-Zayas, N.; Velázquez-Rivera, L.I.; Torres-Ventura, A.C.; Godoy-Vitorino, F. Insights into Gut Dysbiosis: Inflammatory Diseases, Obesity, and Restoration Approaches. Int. J. Mol. Sci. 2024, 25, 9715. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Farhadipour, M.; Depoortere, I. The Function of Gastrointestinal Hormones in Obesity—Implications for the Regulation of Energy Intake. Nutrients 2021, 13, 1839. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Maslennikov, R.; Poluektova, E.; Zolnikova, O.; Sedova, A.; Kurbatova, A.; Shulpekova, Y.; Dzhakhaya, N.; Kardasheva, S.; Nadinskaia, M.; Bueverova, E.; et al. Gut Microbiota and Bacterial Translocation in the Pathogenesis of Liver Fibrosis. Int. J. Mol. Sci. 2023, 24, 16502. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Rao, S.; Khattak, A.; Zamir, F.; Chaari, A. Physical Exercise and the Gut Microbiome: A Bidirectional Relationship Influencing Health and Performance. Nutrients 2024, 16, 3663. [Google Scholar] [CrossRef]
- Silva, J.S.C.; Seguro, C.S.; Naves, M.M.V. Gut microbiota and physical exercise in obesity and diabetes—A systematic review. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 863–877. [Google Scholar] [CrossRef]
- Babu, A.F.; Csader, S.; Männistö, V.; Tauriainen, M.M.; Pentikäinen, H.; Savonen, K.; Klåvus, A.; Koistinen, V.; Hanhineva, K.; Schwab, U. Effects of exercise on NAFLD using non-targeted metabolomics in adipose tissue, plasma, urine, and stool. Sci. Rep. 2022, 12, 6485. [Google Scholar] [CrossRef]
- Li, R.Y.; Guo, L. Exercise in Diabetic Nephropathy: Protective Effects and Molecular Mechanism. Int. J. Mol. Sci. 2024, 25, 3605. [Google Scholar] [CrossRef]
- World Health Organization. Controlling the Global Obesity Epidemic. Available online: https://www.who.int/activities/controlling-the-global-obesity-epidemic (accessed on 10 April 2025).
- Ali, H.; Rasheed, W.; Moond, V.; Dahiya, D.S.; Swaiti, A.; Gangwani, M.K.; Hayat, U.; Advani, R. Obesity Related Mortality in the Next Generation: Projections Based on Machine Learning for Young Americans (1999–2035). Obes. Sci. Pract. 2025, 11, e70062. [Google Scholar] [CrossRef]
- GBD 2021 US Obesity Forecasting Collaborators. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990-2021, and forecasts up to 2050. Lancet 2024, 404, 2278–2298. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Ding, Y.; Cui, B.; Li, R.-r.; Qu, X.-r.; Zhou, H.; Au, K.-h.; Fan, X.-d.; Xie, J.; Huang, Y.; et al. Navigating obesity: A comprehensive review of epidemiology, pathophysiology, complications and management strategies. Innov. Med. 2024, 2, 100090. [Google Scholar] [CrossRef]
- Kadouh, H.C.; Acosta, A. Current paradigms in the etiology of obesity. Tech. Gastrointest. Endosc. 2017, 19, 2–11. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.T.; Minh Nguyet, N.T.; Nga, V.T.; Thai Lien, N.V.; Vo, D.D.; Lien, N.; Nhu Ngoc, V.T.; Son, L.H.; Le, D.H.; Nga, V.B.; et al. An update on obesity: Mental consequences and psychological interventions. Diabetes Metab. Syndr. 2019, 13, 155–160. [Google Scholar] [CrossRef]
- Ruban, A.; Stoenchev, K.; Ashrafian, H.; Teare, J. Current treatments for obesity. Clin. Med. 2019, 19, 205–212. [Google Scholar] [CrossRef]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metabolism 2019, 92, 163–169. [Google Scholar] [CrossRef]
- Patel, D. Pharmacotherapy for the management of obesity. Metabolism 2015, 64, 1376–1385. [Google Scholar] [CrossRef]
- Bouter, K.E.; van Raalte, D.H.; Groen, A.K.; Nieuwdorp, M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology 2017, 152, 1671–1678. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Al-Assal, K.; Martinez, A.C.; Torrinhas, R.S.; Cardinelli, C.; Waitzberg, D. Gut microbiota and obesity. Clin. Nutr. Exp. 2018, 20, 60–64. [Google Scholar] [CrossRef]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Afkhami, H.; Javadi, A.; Kashfi, M. Understanding the complex function of gut microbiota: Its impact on the pathogenesis of obesity and beyond: A comprehensive review. Diabetol. Metab. Syndr. 2024, 16, 308. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Howard, A.G.; Meyer, K.A.; Tsilimigras, M.C.B.; Avery, C.L.; Sha, W.; Sun, S.; Zhang, J.; Su, C.; et al. Circulating Short-Chain Fatty Acids Are Positively Associated with Adiposity Measures in Chinese Adults. Nutrients 2020, 12, 2127. [Google Scholar] [CrossRef]
- Sutoyo, D.; Atmaka, D.; Sidabutar, L. Dietary Factors Affecting Firmicutes and Bacteroidetes Ratio in Solving Obesity Problem: A Literature Review. Media Gizi Indones. 2020, 15, 94. [Google Scholar] [CrossRef]
- Tang, R.; Li, L. Modulation of Short-Chain Fatty Acids as Potential Therapy Method for Type 2 Diabetes Mellitus. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6632266. [Google Scholar] [CrossRef]
- Lee, C.J.; Sears, C.L.; Maruthur, N. Gut microbiome and its role in obesity and insulin resistance. Ann. N. Y. Acad. Sci. 2020, 1461, 37–52. [Google Scholar] [CrossRef]
- Rumyantsev, K.A.; Polyakova, V.V.; Sorokina, I.V.; Feoktistova, P.S.; Khatkov, I.E.; Bodunova, N.A.; Zhukova, L.G. The Gut Microbiota Impacts Gastrointestinal Cancers Through Obesity, Diabetes, and Chronic Inflammation. Life 2024, 14, 1219. [Google Scholar] [CrossRef]
- Sun, L.; Ma, L.; Ma, Y.; Zhang, F.; Zhao, C.; Nie, Y. Insights into the Role of Gut Microbiota in Obesity: Pathogenesis, Mechanisms, and Therapeutic Perspectives. Protein Cell 2018, 9, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Gerasimidis, K.; Edwards, C.A.; Shaikh, M.G. Role of Gut Microbiota in the Aetiology of Obesity: Proposed Mechanisms and Review of the Literature. J. Obes. 2016, 2016, 7353642. [Google Scholar] [CrossRef]
- Alhabeeb, H.; AlFaiz, A.; Kutbi, E.; AlShahrani, D.; Alsuhail, A.; AlRajhi, S.; Alotaibi, N.; Alotaibi, K.; AlAmri, S.; Alghamdi, S.; et al. Gut Hormones in Health and Obesity: The Upcoming Role of Short Chain Fatty Acids. Nutrients 2021, 13, 481. [Google Scholar] [CrossRef]
- Hersoug, L.G.; Møller, P.; Loft, S. Gut Microbiota-Derived Lipopolysaccharide Uptake and Trafficking to Adipose Tissue: Implications for Inflammation and Obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, X.; Qiu, J.; Sun, T.; Qu, K.; Din, A.U.; Yan, W.; Li, T.; Chen, Y.; Gu, W.; et al. Desulfovibrio desulfuricans Aggravates Atherosclerosis by Enhancing Intestinal Permeability and Endothelial TLR4/NF-κB Pathway in Apoe−/− Mice. Genes Dis. 2021, 10, 239–253. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. CMLS 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Mika, A.; Van Treuren, W.; González, A.; Herrera, J.J.; Knight, R.; Fleshner, M. Exercise Is More Effective at Altering Gut Microbial Composition and Producing Stable Changes in Lean Mass in Juvenile versus Adult Male F344 Rats. PLoS ONE 2015, 10, e0125889. [Google Scholar] [CrossRef]
- Munukka, E.; Ahtiainen, J.P.; Puigbó, P.; Jalkanen, S.; Pahkala, K.; Keskitalo, A.; Kujala, U.M.; Pietilä, S.; Hollmén, M.; Elo, L.; et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is Not Reflected in Systemic Metabolism in Over-Weight Women. Front. Microbiol. 2018, 9, 2323. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise Training Modulates the Gut Microbiota Profile and Impairs Inflammatory Signaling Pathways in Obese Children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Porras, D.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Juarez-Fernández, M.; Cuevas, M.J.; Mauriz, J.L.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Beneficial Effects of Exercise on Gut Microbiota Functionality and Barrier Integrity, and Gut-Liver Crosstalk in an In Vivo Model of Early Obesity and Non-Alcoholic Fatty Liver Disease. Dis. Model. Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.U.; Yassine, H.M.; Sohail, A.; Thani, A.A.A. Impact of Physical Exercise on Gut Microbiome, Inflammation, and the Pathobiology of Metabolic Disorders. Rev. Diabet. Stud. 2019, 15, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef]
- Soltani, N.; Esmaeil, N.; Marandi, S.M.; Hovsepian, V.; Momen, T.; Shahsanai, A.; Kelishadi, R. Assessment of the Effect of Short-Term Combined High-Intensity Interval Training on TLR4, NF-κB and IRF3 Expression in Young Overweight and Obese Girls. Public Health Genom. 2020, 23, 26–36. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Bonilla, D.A.; Gómez-Miranda, L.M.; Calleja-Núñez, J.J.; Arias, N.; Martínez-Guardado, I. Examining the Interaction between Exercise, Gut Microbiota, and Neurodegeneration: Future Research Directions. Biomedicines 2023, 11, 2267. [Google Scholar] [CrossRef]
- Kopczyńska, J.; Kowalczyk, M. The Potential of Short-Chain Fatty Acid Epigenetic Regulation in Chronic Low-Grade Inflammation and Obesity. Front. Immunol. 2024, 15, 1380476. [Google Scholar] [CrossRef] [PubMed]
- Abuqwider, J.N.; Mauriello, G.; Altamimi, M. Akkermansia muciniphila, a New Generation of Beneficial Microbiota in Modulating Obesity: A Systematic Review. Microorganisms 2021, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-Fat-Diet-Induced Obesity in Mice by Regulating G Protein-Coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural Products for the Treatment of Type 2 Diabetes Mellitus: Pharmacology and Mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 Diabetes Mellitus in Adults: Pathogenesis, Prevention and Therapy. Signal Transduct. Target Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 Diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, J.; Hao, W.; Zhang, J.; Li, H.; Yang, C.; Yang, J.; Chen, X.; Wang, H. Gut Microbiota and Type 2 Diabetes Mellitus: Association, Mechanism, and Translational Applications. Mediat. Inflamm. 2021, 2021, 5110276. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pérez, A.M.; Muñoz-Garach, A.; Lasserrot-Cuadrado, A.; Moreno-Indias, I.; Tinahones, F.J. Microbiota Transplantation in Individuals with Type 2 Diabetes and a High Degree of Insulin Resistance. Nutrients 2024, 16, 3491. [Google Scholar] [CrossRef] [PubMed]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut Microbiota Dysbiosis Associated with Glucose Metabolism Disorders and the Metabolic Syndrome in Older Adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wei, J.; Liu, P.; Zhang, Q.; Tian, Y.; Hou, G.; Meng, L.; Xin, Y.; Jiang, X. Role of the Gut Microbiota in Type 2 Diabetes and Related Diseases. Metabolism 2021, 117, 154712. [Google Scholar] [CrossRef]
- Saad, M.J.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, G.; Abdullah, N.; Marlini, M.; Baharom, N.; Lawley, B.; Omar, M.R.; Mohideen, F.B.S.; Addnan, F.H.; Nur Fariha, M.M.; Ismail, Z.; et al. Gut Microbiota Composition in Prediabetes and Newly Diagnosed Type 2 Diabetes: A Systematic Review of Observational Studies. Front. Cell. Infect. Microbiol. 2022, 12, 943427. [Google Scholar] [CrossRef]
- Palau-Rodriguez, M.; Tulipani, S.; Queipo-Ortuño, M.I.; Urpi-Sarda, M.; Tinahones, F.J.; Andres-Lacueva, C. Metabolomic Insights into the Intricate Gut Microbial-Host Interaction in the Development of Obesity and Type 2 Diabetes. Front. Microbiol. 2015, 6, 1151. [Google Scholar] [CrossRef]
- Camargo, A.; Jimenez-Lucena, R.; Alcala-Diaz, J.F.; Rangel-Zuñiga, O.A.; Garcia-Carpintero, S.; Lopez-Moreno, J.; Blanco-Rojo, R.; Delgado-Lista, J.; Perez-Martinez, P.; van Ommen, B.; et al. Postprandial Endotoxemia May Influence the Development of Type 2 Diabetes Mellitus: From the CORDIOPREV Study. Clin. Nutr. 2019, 38, 529–538. [Google Scholar] [CrossRef]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased Circulatory Levels of Lipopolysaccharide (LPS) and Zonulin Signify Novel Biomarkers of Proinflammation in Patients with Type 2 Diabetes. Mol. Cell. Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef]
- Zhai, L.; Wu, J.; Lam, Y.Y.; Kwan, H.Y.; Bian, Z.X.; Wong, H.L.X. Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 12846. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Maranduca, M.A.; Lacatusu, C.M.; Floria, M.; et al. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients 2020, 12, 3719. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, J.; Cheng, Y.; Zhu, M.; Xiao, Z.; Ruan, G.; Wei, Y. Gut microbiota: A new target for T2DM prevention and treatment. Front. Endocrinol. 2022, 13, 958218. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Huang, J.; Xing, Y.; Wong, F.S.; Suo, J.; Wen, L. Gut microbiota and therapy for obesity and type 2 diabetes. Front. Endocrinol. 2024, 15, 1333778. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Maina, J.G.; Balkhiyarova, Z.; Nouwen, A.; Pupko, I.; Ulrich, A.; Boissel, M.; Bonnefond, A.; Froguel, P.; Khamis, A.; Prokopenko, I.; et al. Bidirectional Mendelian Randomization and Multiphenotype GWAS Show Causality and Shared Pathophysiology Between Depression and Type 2 Diabetes. Diabetes Care 2023, 46, 1707–1714. [Google Scholar] [CrossRef]
- Li, S.; Yang, D.; Zhou, X.; Chen, L.; Liu, L.; Lin, R.; Li, X.; Liu, Y.; Qiu, H.; Cao, H.; et al. Neurological and Metabolic Related Pathophysiologies and Treatment of Comorbid Diabetes with Depression. CNS Neurosci. Ther. 2024, 30, e14497. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut Microbiota and Its Metabolites in Depression: From Pathogenesis to Treatment. EBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, B.; Ren, L.; Du, H.; Fei, C.; Qian, C.; Li, B.; Zhang, R.; Liu, H.; Li, Z.; et al. High-Fiber Diet Ameliorates Gut Microbiota, Serum Metabolism and Emotional Mood in Type 2 Diabetes Patients. Front. Cell Infect. Microbiol. 2023, 13, 1069954. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020, 31, 77–91. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B.; American College of Sports Medicine; et al. Exercise and Type 2 Diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint Position Statement Executive Summary. Diabetes Care 2010, 33, 2692–2696. [Google Scholar] [CrossRef]
- Lee, J.H.; Budanov, A.V.; Talukdar, S.; Park, E.J.; Park, H.L.; Park, H.W.; Bandyopadhyay, G.; Li, N.; Aghajan, M.; Jang, I.; et al. Maintenance of Metabolic Homeostasis by Sestrin2 and Sestrin3. Cell Metab. 2012, 16, 311–321. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Gervasi, M.; Bartolacci, A.; Ferrini, F.; Patti, A.; Sestili, P.; Stocchi, V.; Agostini, D. Targeting the Gut Microbiota for Prevention and Management of Type 2 Diabetes. Nutrients 2024, 16, 3951. [Google Scholar] [CrossRef] [PubMed]
- Codella, R.; Luzi, L.; Terruzzi, I. Exercise has the guts: How physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig. Liver Dis. 2018, 50, 331–341. [Google Scholar] [CrossRef]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. Breaking the barriers: The role of gut homeostasis in Metabolic-Associated Steatotic Liver Disease (MASLD). Gut Microbes 2024, 16, 2331460. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Le, P.; Tatar, M.; Dasarathy, S.; Alkhouri, N.; Herman, W.H.; Taksler, G.B.; Deshpande, A.; Ye, W.; Adekunle, O.A.; McCullough, A.; et al. Estimated Burden of Metabolic Dysfunction-Associated Steatotic Liver Disease in US Adults, 2020 to 2050. JAMA Netw. Open 2025, 8, e2454707. [Google Scholar] [CrossRef]
- Hamamah, S.; Iatcu, O.C.; Covasa, M. Dietary Influences on Gut Microbiota and Their Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Nutrients 2024, 17, 143. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Shao, C.; Zhong, B. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: A systematic review and Meta-analysis. Lipids Health Dis. 2021, 20, 22. [Google Scholar] [CrossRef]
- Zazueta, A.; Valenzuela-Pérez, L.; Ortiz-López, N.; Pinto-León, A.; Torres, V.; Guiñez, D.; Aliaga, N.; Merino, P.; Sandoval, A.; Covarrubias, N.; et al. Alteration of Gut Microbiota Composition in the Progression of Liver Damage in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Int. J. Mol. Sci. 2024, 25, 4387. [Google Scholar] [CrossRef]
- Li, Z.; Ni, M.; Yu, H.; Wang, L.; Zhou, X.; Chen, T.; Liu, G.; Gao, Y. Gut Microbiota and Liver Fibrosis: One Potential Biomarker for Predicting Liver Fibrosis. Biomed. Res. Int. 2020, 2020, 3905130. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, B.; Damman, C.J.; Carr, R.M. Metabolic dysfunction-associated steatotic liver disease and the gut microbiome: Pathogenic insights and therapeutic innovations. J. Clin. Investig. 2025, 135, e186423. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.F.; Varady, K.A.; Wang, X.D.; Targher, G.; Byrne, C.D.; Tayyem, R.; Latella, G.; Bergheim, I.; Valenzuela, R.; George, J.; et al. The role of dietary modification in the prevention and management of metabolic dysfunction-associated fatty liver disease: An international multidisciplinary expert consensus. Metabolism 2024, 161, 156028. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Chivese, T.; Elshaikh, U.; Sendall, M. Efficacy of the Mediterranean diet in treating metabolic dysfunction-associated steatotic liver disease (MASLD) in children and adolescents: A systematic review and meta-analysis. BMC Public Health 2024, 24, 2701. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Maciejewska-Markiewicz, D.; Sykulski, M.; Gruszczyńska, A.; Herman-Iżycka, J.; Wyleżoł, M.; Petriczko, K.K.; Palma, J.; Jakubczyk, K.; Janda-Milczarek, K.; et al. Gut Microbiome—How Does Two-Month Consumption of Fiber-Enriched Rolls Change Microbiome in Patients Suffering from MASLD? Nutrients 2024, 16, 1173. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Mambrini, S.P.; Grillo, A.; Colosimo, S.; Zarpellon, F.; Pozzi, G.; Furlan, D.; Amodeo, G.; Bertoli, S. Diet and physical exercise as key players to tackle MASLD through improvement of insulin resistance and metabolic flexibility. Front. Nutr. 2024, 11, 1426551. [Google Scholar] [CrossRef] [PubMed]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef]
- Houttu, V.; Boulund, U.; Grefhorst, A.; Soeters, M.R.; Pinto-Sietsma, S.J.; Nieuwdorp, M.; Holleboom, A.G. The Role of the Gut Microbiome and Exercise in Non-Alcoholic Fatty Liver Disease. Therap. Adv. Gastroenterol. 2020, 13, 1756284820941745. [Google Scholar] [CrossRef]
- Aya, V.; Flórez, A.; Perez, L.; Ramírez, J.D. Association between Physical Activity and Changes in Intestinal Microbiota Composition: A Systematic Review. PLoS ONE 2021, 16, e0247039. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics Analysis of Elite Athletes Identifies a Performance-Enhancing Microbe That Functions via Lactate Metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Aguiar, S.S.; Ribeiro, F.M.; Sousa Neto, I.V.; Franco, O.L.; Petriz, B. Effects of Physical Exercise on Akkermansia muciniphila: A Systematic Review of Human and Animal Studies. Benef. Microbes 2024, 15, 565–587. [Google Scholar] [CrossRef]
- Münte, E.; Viebahn, G.; Khurana, A.; Fujiki, J.; Nakamura, T.; Lang, S.; Demir, M.; Schnabl, B.; Hartmann, P. Faecalibacterium prausnitzii Is Associated with Disease Severity in MASLD but Its Supplementation Does Not Improve Diet-Induced Steatohepatitis in Mice. Microorganisms 2025, 13, 675. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.H.; Shin, J.H.; Lee, D.; Lee, S.N.; Seo, J.G.; Shin, J.H.; Nam, Y.D.; Kim, H.; Sun, X.; et al. Pharmaceutical efficacy of novel human-origin Faecalibacterium prausnitzii strains on high-fat-diet-induced obesity and associated metabolic disorders in mice. Front. Endocrinol. 2023, 14, 1220044. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Alonso-Peña, M.; Argos Vélez, P.; Crespo, J.; Iruzubieta, P. Unraveling MASLD: The Role of Gut Microbiota, Dietary Modulation, and AI-Driven Lifestyle Interventions. Nutrients 2025, 17, 1580. [Google Scholar] [CrossRef] [PubMed]
- Hizo, G.H.; Rampelotto, P.H. The Role of Bifidobacterium in Liver Diseases: A Systematic Review of Next-Generation Sequencing Studies. Microorganisms 2023, 11, 2999. [Google Scholar] [CrossRef] [PubMed]
- Olotu, T.; Ferrell, J.M. Lactobacillus sp. for the Attenuation of Metabolic Dysfunction-Associated Steatotic Liver Disease in Mice. Microorganisms 2024, 12, 2488. [Google Scholar] [CrossRef]
- Csader, S.; Chen, X.; Leung, H.; Männistö, V.; Pentikäinen, H.; Tauriainen, M.-M.; Savonen, K.; El-Nezami, H.; Schwab, U.; Panagiotou, G. Gut ecological networks reveal associations between bacteria, exercise, and clinical profile in non-alcoholic fatty liver disease patients. mSystems 2023, 8, e0022423. [Google Scholar] [CrossRef]
- Lai, J.; Luo, L.; Zhou, T.; Feng, X.; Ye, J.; Zhong, B. Alterations in Circulating Bile Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Systematic Review and Meta-Analysis. Biomolecules 2023, 13, 1356. [Google Scholar] [CrossRef]
- Nechalová, L.; Bielik, V.; Hric, I.; Babicová, M.; Baranovičová, E.; Grendár, M.; Koška, J.; Penesová, A. Gut Microbiota and Metabolic Responses to a 12-Week Caloric Restriction Combined with Strength and HIIT Training in Patients with Obesity: A Randomized Trial. BMC Sports Sci. Med. Rehabil. 2024, 16, 239. [Google Scholar] [CrossRef]
- Kazeminasab, F.; Miraghajani, M.; Mokhtari, K.; Karimi, B.; Rosenkranz, S.K.; Santos, H.O. The Effects of Probiotic Supplementation and Exercise Training on Liver Enzymes and Cardiometabolic Markers in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutr. Metab. 2024, 21, 59. [Google Scholar] [CrossRef]
- Kovynev, A.; Ying, Z.; Zhang, S.; Olgiati, E.; Lambooij, J.M.; Visentin, C.; Guigas, B.; Ducarmon, Q.R.; Rensen, P.C.N.; Schönke, M.; et al. Timing Matters: Late, but Not Early, Exercise Training Ameliorates MASLD in Part by Modulating the Gut-Liver Axis in Mice. J. Pineal Res. 2024, 76, e70003. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Wang, L.; Le, S.; Yang, Y.; Zhao, C.; Zhang, X.; Yang, X.; Xu, T.; Xu, L.; Wiklund, P.; et al. A Randomized Controlled Trial for Response of Microbiome Network to Exercise and Diet Intervention in Patients with Nonalcoholic Fatty Liver Disease. Nat. Commun. 2022, 13, 2555. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.M.; Disciglio, V.; Franco, I.; Sorino, P.; Bonfiglio, C.; Bianco, A.; Campanella, A.; Lippolis, T.; Pesole, P.L.; Polignano, M.; et al. A Low Glycemic Index Mediterranean Diet Combined with Aerobic Physical Activity Rearranges the Gut Microbiota Signature in NAFLD Patients. Nutrients 2022, 14, 1773. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Ni, Y.; Liu, Y.; Gao, X.; Tse, M.A.; Panagiotou, G.; Xu, A. Exercise-Changed Gut Mycobiome as a Potential Contributor to Metabolic Benefits in Diabetes Prevention: An Integrative Multi-Omics Study. Gut Microbes 2024, 16, 2416928. [Google Scholar] [CrossRef]
- Torquati, L.; Gajanand, T.; Cox, E.R.; Willis, C.R.G.; Zaugg, J.; Keating, S.E.; Coombes, J.S. Effects of Exercise Intensity on Gut Microbiome Composition and Function in People with Type 2 Diabetes. Eur. J. Sport Sci. 2023, 23, 530–541. [Google Scholar] [CrossRef] [PubMed]
| Disease/Condition | Altered Gut Microbiota Taxa | Type of Physical Activity Studied | Observed Clinical Outcomes |
|---|---|---|---|
| Obesity | ↑ Bacteroidetes, ↓ Firmicutes [46] | Aerobic activity |
|
| ↑ Akkermansia, ↓ Proteobacteria [47] | Aerobic activity |
| |
| ↓ Proteobacteria, Betaproteobacteria, Gammaproteobacteria, ↑ Actinobacteria, Clostridia, Flavobacteriia, Blautia, Dialister, Roseburia [48] | Combined aerobic and resistance training |
| |
| ↑ Parabacteroides, Bacteroides, Flavobacterium genera ↓ Blautia, Dysgonomonas, Porphyromonas [49] | Combined aerobic and resistance training |
| |
| ↑ Bacteroides, Collinsella, Lachnospira spp. ↓ Faecalibacterium spp. [56] | Resistance training |
| |
| Type 2 Diabetes | ↑ Verticillium [122] | 1.5-fold increase in physical activity |
|
| ↑ Akkermansia, Roseburia, Faecalibacterium [89] | Regular physical activity for 3 weeks |
| |
| ↑ Lachnospirales (Eubacteriales), Enterococcus spp., Clostridium Cluster IV [123] | combined aerobic and resistance moderate intensity continuous training |
| |
| ↑ Oscillospirales (R. bromii) [123] | combined aerobic and resistance high-intensity interval training |
| |
| MASLD (metabolic dysfunction-associated steatotic liver disease) | ↑ Faecalibacterium prausnitzii [111] | Aerobic activity |
|
| ↑ Bifidobacterium [113] | Aerobic activity |
| |
| ↑ Lactobacillus spp. [114] | Aerobic activity |
| |
| ↑ Akkermansia muciniphila, Parabacteroides merdae, Phocaeicola vulgatus [117] | High-intensity interval training (HIIT) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokal-Dembowska, A.; Polak-Szczybyło, E.; Helma, K.; Musz, P.; Setlik, M.; Fic, W.; Wachowiak, D.; Jarmakiewicz-Czaja, S. Physical Activity and Metabolic Disorders—What Does Gut Microbiota Have to Do with It? Curr. Issues Mol. Biol. 2025, 47, 630. https://doi.org/10.3390/cimb47080630
Sokal-Dembowska A, Polak-Szczybyło E, Helma K, Musz P, Setlik M, Fic W, Wachowiak D, Jarmakiewicz-Czaja S. Physical Activity and Metabolic Disorders—What Does Gut Microbiota Have to Do with It? Current Issues in Molecular Biology. 2025; 47(8):630. https://doi.org/10.3390/cimb47080630
Chicago/Turabian StyleSokal-Dembowska, Aneta, Ewelina Polak-Szczybyło, Kacper Helma, Patrycja Musz, Maciej Setlik, Weronika Fic, Dawid Wachowiak, and Sara Jarmakiewicz-Czaja. 2025. "Physical Activity and Metabolic Disorders—What Does Gut Microbiota Have to Do with It?" Current Issues in Molecular Biology 47, no. 8: 630. https://doi.org/10.3390/cimb47080630
APA StyleSokal-Dembowska, A., Polak-Szczybyło, E., Helma, K., Musz, P., Setlik, M., Fic, W., Wachowiak, D., & Jarmakiewicz-Czaja, S. (2025). Physical Activity and Metabolic Disorders—What Does Gut Microbiota Have to Do with It? Current Issues in Molecular Biology, 47(8), 630. https://doi.org/10.3390/cimb47080630





