Molecular and Glycosylation Pathways in Osteosarcoma: Tumor Microenvironment and Emerging Strategies Toward Personalized Oncology
Abstract
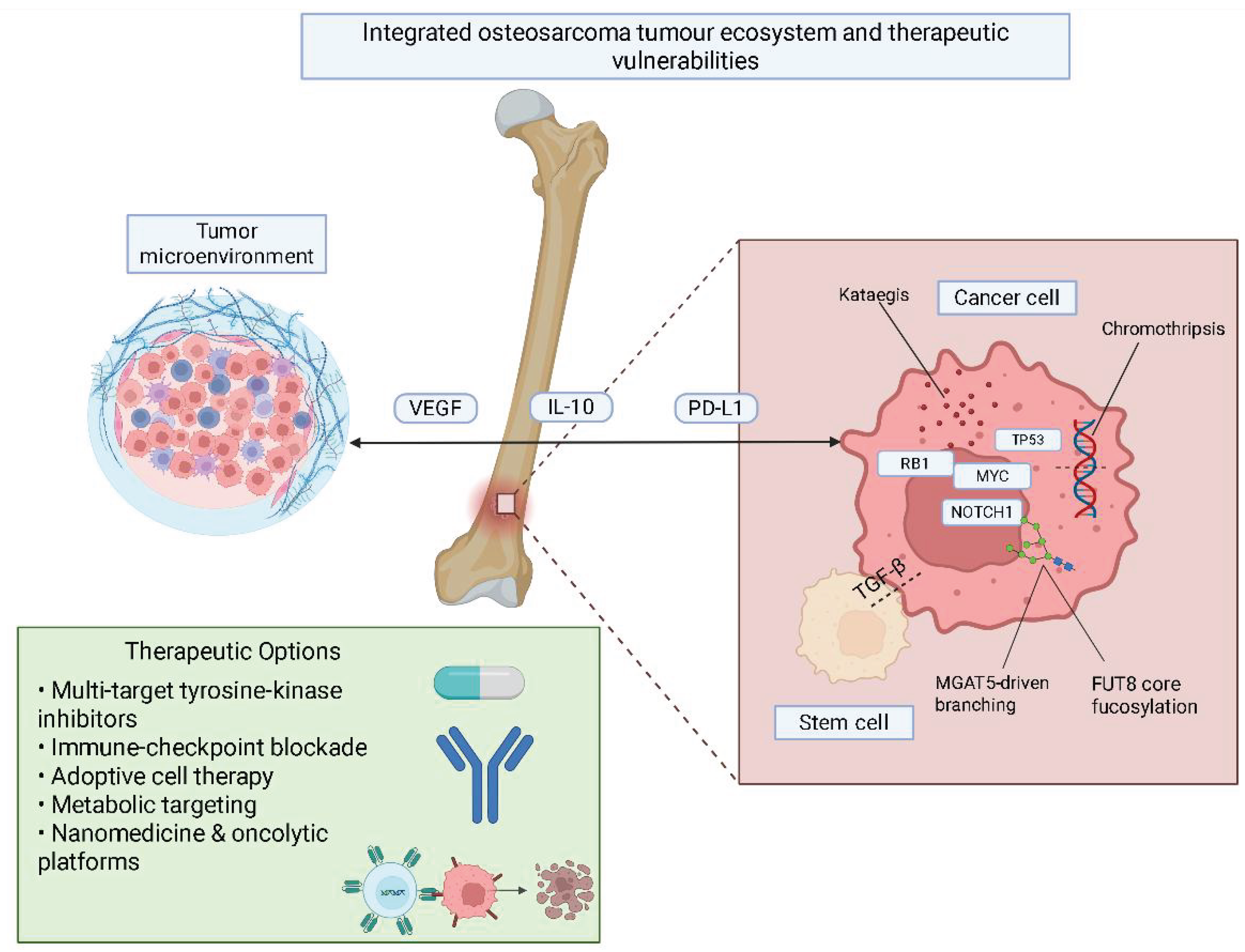
1. Introduction
2. Biology and Pathophysiology of Osteosarcoma
3. Glycosylation and Tumorigenesis
4. Genetic and Epigenetic Changes
5. Glycan Biomarkers
6. Therapeutic Implications and Challenges
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma Incidence and Survival Rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Harrison, D.J.; Geller, D.S.; Gill, J.D.; Lewis, V.O.; Gorlick, R. Current and Future Therapeutic Approaches for Osteosarcoma. Expert Rev. Anticancer. Ther. 2018, 18, 39–50. [Google Scholar] [CrossRef]
- Gaspar, N.; Occean, B.-V.; Pacquement, H.; Bompas, E.; Bouvier, C.; Brisse, H.J.; Castex, M.-P.; Cheurfa, N.; Corradini, N.; Delaye, J.; et al. Results of Methotrexate-Etoposide-Ifosfamide Based Regimen (M-EI) in Osteosarcoma Patients Included in the French OS2006/Sarcome-09 Study. Eur. J. Cancer 2018, 88, 57–66. [Google Scholar] [CrossRef]
- Robinson, M.J.; Davis, E.J. Neoadjuvant Chemotherapy for Adults with Osteogenic Sarcoma. Curr. Treat. Options Oncol. 2024, 25, 1366–1373. [Google Scholar] [CrossRef]
- Alfranca, A.; Martinez-Cruzado, L.; Tornin, J.; Abarrategi, A.; Amaral, T.; De Alava, E.; Menendez, P.; Garcia-Castro, J.; Rodriguez, R. Bone Microenvironment Signals in Osteosarcoma Development. Cell. Mol. Life Sci. 2015, 72, 3097–3113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Han, J.; Yang, L.; Cai, Z.; Sun, W.; Hua, Y.; Xu, J. Immune Microenvironment in Osteosarcoma: Components, Therapeutic Strategies and Clinical Applications. Front. Immunol. 2022, 13, 907550. [Google Scholar] [CrossRef] [PubMed]
- Pierrevelcin, M.; Flacher, V.; Mueller, C.G.; Vauchelles, R.; Guerin, E.; Lhermitte, B.; Pencreach, E.; Reisch, A.; Muller, Q.; Doumard, L.; et al. Engineering Novel 3D Models to Recreate High—Grade Osteosarcoma and Its Immune and Extracellular Matrix Microenvironment. Adv. Healthc. Mater. 2022, 11, 2200195. [Google Scholar] [CrossRef]
- Somaiah, N.; Conley, A.P.; Parra, E.R.; Lin, H.; Amini, B.; Solis Soto, L.; Salazar, R.; Barreto, C.; Chen, H.; Gite, S.; et al. Durvalumab plus Tremelimumab in Advanced or Metastatic Soft Tissue and Bone Sarcomas: A Single-Centre Phase 2 Trial. Lancet Oncol. 2022, 23, 1156–1166. [Google Scholar] [CrossRef]
- Xie, X.; Feng, Y.; Zhang, H.; Su, Q.; Song, T.; Yang, G.; Li, N.; Wei, X.; Li, T.; Qin, X.; et al. Remodeling Tumor Immunosuppressive Microenvironment via a Novel Bioactive Nanovaccines Potentiates the Efficacy of Cancer Immunotherapy. Bioact. Mater. 2022, 16, 107–119, Erratum in Bioact. Mater. 2022, 21, 239–240. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Chou, H.-Y.; Lien, H.-W.; Yeh, C.-A.; Wang, J.-R.; Chen, C.-H.; Fan, C.-C.; Hsu, C.-P.; Kao, T.-Y.; Ko, T.-M.; et al. A Fc-VEGF Chimeric Fusion Enhances PD-L1 Immunotherapy via Inducing Immune Reprogramming and Infiltration in the Immunosuppressive Tumor Microenvironment. Cancer Immunol. Immunother. 2023, 72, 351–369. [Google Scholar] [CrossRef]
- Le Cesne, A.; Marec-Berard, P.; Blay, J.-Y.; Gaspar, N.; Bertucci, F.; Penel, N.; Bompas, E.; Cousin, S.; Toulmonde, M.; Bessede, A.; et al. Programmed Cell Death 1 (PD-1) Targeting in Patients with Advanced Osteosarcomas: Results from the PEMBROSARC Study. Eur. J. Cancer 2019, 119, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.; Wahba, A.; Felix, K.; Cabrera, M.; Segura, M.G.; Kundra, V.; Ravoori, M.K.; Stewart, J.; Kleinerman, E.S.; Jensen, V.B.; et al. Bempegaldesleukin (BEMPEG.; NKTR-214) Efficacy as a Single Agent and in Combination with Checkpoint-Inhibitor Therapy in Mouse Models of Osteosarcoma. Int. J. Cancer 2021, 148, 1928–1937. [Google Scholar] [CrossRef]
- Miwa, S.; Shirai, T.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Current and Emerging Targets in Immunotherapy for Osteosarcoma. J. Oncol. 2019, 2019, 7035045. [Google Scholar] [CrossRef]
- Suehara, Y.; Alex, D.; Bowman, A.; Middha, S.; Zehir, A.; Chakravarty, D.; Wang, L.; Jour, G.; Nafa, K.; Hayashi, T.; et al. Clinical Genomic Sequencing of Pediatric and Adult Osteosarcoma Reveals Distinct Molecular Subsets with Potentially Targetable Alterations. Clin. Cancer Res. 2019, 25, 6346–6356. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; Guzmán, R.; Bonilla, P. Dedifferentiated Low-Grade Osteosarcoma, Outcome with or Without Chemotherapy: A Systematic Review. Orthop. Res. Rev. 2023, 15, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Sever, N.; Şimşek, F.; Onur, İ.D.; Arvas, H.; Guliyev, T.; Şakalar, T.; Çiçek, C.M.; Orman, S.; Çetin, E.B.; Kayaş, K.; et al. Prognostic Factors in High Grade Osteosarcoma Patients Who Received Neoadjuvant Therapy and Subsequently Underwent Surgery: Data from the Turkish Oncology Group. J. Clin. Med. 2025, 14, 2024. [Google Scholar] [CrossRef]
- Urlić, I.; Jovičić, M.Š.; Ostojić, K.; Ivković, A. Cellular and Genetic Background of Osteosarcoma. Curr. Issues Mol. Biol. 2023, 45, 4344–4358. [Google Scholar] [CrossRef]
- Zhou, W.-Y.; Zheng, H.; Du, X.-L.; Yang, J.-L.; Zhou, W.-Y.; Zheng, H.; Du, X.-L.; Yang, J.-L. Characterization of FGFR Signaling Pathway as Therapeutic Targets for Sarcoma Patients. Cancer Biol. Med. 2016, 13, 260–268. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Yang, Q.; Lv, X.; Huang, W.; Zhou, Z.; Wang, Y.; Zhang, Z.; Yuan, T.; Ding, X.; et al. Author Correction: Single-Cell RNA Landscape of Intratumoral Heterogeneity and Immunosuppressive Microenvironment in Advanced Osteosarcoma. Nat. Commun. 2021, 12, 2567. [Google Scholar] [CrossRef]
- Wu, R.; Dou, X.; Li, H.; Sun, Z.; Li, H.; Shen, Y.; Weng, W.; Min, J. Identification of Cell Subpopulations and Interactive Signaling Pathways From a Single-Cell RNA Sequencing Dataset in Osteosarcoma: A Comprehensive Bioinformatics Analysis. Front. Oncol. 2022, 12, 853979. [Google Scholar] [CrossRef]
- Verrecchia, F.; Rédini, F. Transforming Growth Factor-β Signaling Plays a Pivotal Role in the Interplay Between Osteosarcoma Cells and Their Microenvironment. Front. Oncol. 2018, 8, 133. [Google Scholar] [CrossRef]
- Ong, J.L.K.; Jalaludin, N.F.F.; Wong, M.K.; Tan, S.H.; Angelina, C.; Sukhatme, S.A.; Yeo, T.; Lim, C.T.; Lee, Y.T.; Soh, S.Y.; et al. Exosomal mRNA Cargo Are Biomarkers of Tumor and Immune Cell Populations in Pediatric Osteosarcoma. Transl. Oncol. 2024, 46, 102008. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Ciriano, I.; Lee, J.J.-K.; Xi, R.; Jain, D.; Jung, Y.L.; Yang, L.; Gordenin, D.; Klimczak, L.J.; Zhang, C.-Z.; Pellman, D.S.; et al. Comprehensive Analysis of Chromothripsis in 2,658 Human Cancers Using Whole-Genome Sequencing. Nat. Genet. 2020, 52, 331–341. [Google Scholar] [CrossRef]
- Perry, J.A.; Kiezun, A.; Tonzi, P.; Van Allen, E.M.; Carter, S.L.; Baca, S.C.; Cowley, G.S.; Bhatt, A.S.; Rheinbay, E.; Pedamallu, C.S.; et al. Complementary Genomic Approaches Highlight the PI3K/mTOR Pathway as a Common Vulnerability in Osteosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, E5564–E5573. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Jiang, M.-M.; Jiang, L.; Salvo, J.S.; Zeng, H.-C.; Dawson, B.; Bertin, T.K.; Rao, P.H.; Chen, R.; Donehower, L.A.; et al. Notch Activation as a Driver of Osteogenic Sarcoma. Cancer Cell 2014, 26, 390–401. [Google Scholar] [CrossRef]
- Kansara, M.; Tsang, M.; Kodjabachian, L.; Sims, N.A.; Trivett, M.K.; Ehrich, M.; Dobrovic, A.; Slavin, J.; Choong, P.F.M.; Simmons, P.J.; et al. Wnt Inhibitory Factor 1 Is Epigenetically Silenced in Human Osteosarcoma, and Targeted Disruption Accelerates Osteosarcomagenesis in Mice. J. Clin. Investig. 2009, 119, 837–851. [Google Scholar] [CrossRef]
- Ribeiro, C.; Rodrigues, C.; Moreira, R.; Santos, M. Chemical Variations on the P53 Reactivation Theme. Pharmaceuticals 2016, 9, 25. [Google Scholar] [CrossRef]
- Ho, X.D.; Phung, P.; Q Le, V.; H Nguyen, V.; Reimann, E.; Prans, E.; Kõks, G.; Maasalu, K.; Le, N.T.; H Trinh, L.; et al. Whole Transcriptome Analysis Identifies Differentially Regulated Networks between Osteosarcoma and Normal Bone Samples. Exp. Biol. Med. 2017, 242, 1802–1811. [Google Scholar] [CrossRef]
- Zandueta, C.; Ormazábal, C.; Perurena, N.; Martínez-Canarias, S.; Zalacaín, M.; Julián, M.S.; Grigoriadis, A.E.; Valencia, K.; Campos-Laborie, F.J.; Rivas, J.D.L.; et al. Matrix-Gla Protein Promotes Osteosarcoma Lung Metastasis and Associates with Poor Prognosis: Matrix-Gla Protein in Osteosarcoma Lung Metastases. J. Pathol. 2016, 239, 438–449. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, B.; Hu, J.; Zhou, Y.; Jiang, M.; Wu, M.; Qin, L.; Yang, X. miR-421 Is a Diagnostic and Prognostic Marker in Patients with Osteosarcoma. Tumor Biol. 2016, 37, 9001–9007. [Google Scholar] [CrossRef] [PubMed]
- Gemoll, T.; Epping, F.; Heinrich, L.; Fritzsche, B.; Roblick, U.J.; Szymczak, S.; Hartwig, S.; Depping, R.; Bruch, H.-P.; Thorns, C.; et al. Increased Cathepsin D Protein Expression Is a Biomarker for Osteosarcomas, Pulmonary Metastases and Other Bone Malignancies. Oncotarget 2015, 6, 16517–16526. [Google Scholar] [CrossRef]
- Lin, S.; Zhou, L.; Dong, Y.; Yang, Q.; Yang, Q.; Jin, H.; Yuan, T.; Zhou, S. Alpha-(1,6)-Fucosyltransferase (FUT8) Affects the Survival Strategy of Osteosarcoma by Remodeling TNF/NF-κB2 Signaling. Cell Death Dis. 2021, 12, 1124. [Google Scholar] [CrossRef]
- Mao, C.; Li, J.; Feng, L.; Gao, W. Beyond Antibody Fucosylation: α-(1,6)-Fucosyltransferase (Fut8) as a Potential New Therapeutic Target for Cancer Immunotherapy. Antib. Ther. 2023, 6, 87–96. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.-L.; Li, Z.-L.; Du, T.; Chen, Y.-H.; Wang, Y.; Ni, H.-H.; Zhang, K.-M.; Mai, J.; Hu, B.-X.; et al. FUT8-Mediated Aberrant N-Glycosylation of B7H3 Suppresses the Immune Response in Triple-Negative Breast Cancer. Nat. Commun. 2021, 12, 2672. [Google Scholar] [CrossRef]
- Li, J.; Guo, B.; Zhang, W.; Yue, S.; Huang, S.; Gao, S.; Ma, J.; Cipollo, J.F.; Yang, S. Recent Advances in Demystifying O-Glycosylation in Health and Disease. Proteomics 2022, 22, 2200156. [Google Scholar] [CrossRef] [PubMed]
- Bellis, S.L.; Reis, C.A.; Varki, A.; Kannagi, R.; Stanley, P. Glycosylation Changes in Cancer. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022; ISBN 978-1-62182-421-3. [Google Scholar]
- Brown, H.K.; Tellez-Gabriel, M.; Heymann, D. Cancer Stem Cells in Osteosarcoma. Cancer Lett. 2017, 386, 189–195. [Google Scholar] [CrossRef]
- Fujiwara, S.; Kawamoto, T.; Kawakami, Y.; Koterazawa, Y.; Hara, H.; Takemori, T.; Kitayama, K.; Yahiro, S.; Kakutani, K.; Matsumoto, T.; et al. Acquisition of Cancer Stem Cell Properties in Osteosarcoma Cells by Defined Factors. Stem Cell Res. Ther. 2020, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kang, L.; Luo, J.; Yang, M.; Wang, D.; Ye, J.; Yang, X.; Wan, W.; Wong, J.; Xiao, J. Targeting AKT as a Promising Strategy for SOX2-Positive, Chemoresistant Osteosarcoma. Bone Res. 2025, 13, 25. [Google Scholar] [CrossRef]
- Rahimkhoei, V.; Akbari, A.; Jassim, A.Y.; Hussein, U.A.-R.; Salavati-Niasari, M. Recent Advances in Targeting Cancer Stem Cells by Using Nanomaterials. Int. J. Pharm. 2025, 673, 125381. [Google Scholar] [CrossRef]
- Abarrategi, A.; Tornin, J.; Martinez-Cruzado, L.; Hamilton, A.; Martinez-Campos, E.; Rodrigo, J.P.; González, M.V.; Baldini, N.; Garcia-Castro, J.; Rodriguez, R. Osteosarcoma: Cells-of-Origin, Cancer Stem Cells, and Targeted Therapies. Stem Cells Int. 2016, 2016, 3631764. [Google Scholar] [CrossRef]
- Quist, T.; Jin, H.; Zhu, J.-F.; Smith-Fry, K.; Capecchi, M.R.; Jones, K.B. The Impact of Osteoblastic Differentiation on Osteosarcomagenesis in the Mouse. Oncogene 2015, 34, 4278–4284. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Wu, P.-K.; Chen, P.C.-H.; Lee, C.-W.; Chen, W.-M.; Hung, S.-C. Generation of Osteosarcomas from a Combination of Rb Silencing and C-Myc Overexpression in Human Mesenchymal Stem Cells. Stem Cells Transl. Med. 2017, 6, 512–526. [Google Scholar] [CrossRef]
- Chang, X.; Ma, Z.; Zhu, G.; Lu, Y.; Yang, J. New Perspective into Mesenchymal Stem Cells: Molecular Mechanisms Regulating Osteosarcoma. J. Bone Oncol. 2021, 29, 100372. [Google Scholar] [CrossRef] [PubMed]
- Le Nail, L.-R.; Brennan, M.; Rosset, P.; Deschaseaux, F.; Piloquet, P.; Pichon, O.; Le Caignec, C.; Crenn, V.; Layrolle, P.; Hérault, O.; et al. Comparison of Tumor- and Bone Marrow-Derived Mesenchymal Stromal/Stem Cells from Patients with High-Grade Osteosarcoma. Int. J. Mol. Sci. 2018, 19, 707. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, G.; Chen, R.; Hua, Y.; Cai, Z. Mesenchymal Stem Cells in the Osteosarcoma Microenvironment: Their Biological Properties, Influence on Tumor Growth, and Therapeutic Implications. Stem Cell Res. Ther. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, S.T.; Gallego, B.; Murillo, D.; Rodríguez, A.; Rodríguez, R. Cancer Stem Cells as a Source of Drug Resistance in Bone Sarcomas. J. Clin. Med. 2021, 10, 2621. [Google Scholar] [CrossRef]
- Arnold, C.R.; Mangesius, J.; Skvortsova, I.-I.; Ganswindt, U. The Role of Cancer Stem Cells in Radiation Resistance. Front. Oncol. 2020, 10, 164. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G.; Lee, S.-H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and Their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef]
- Wang, M.-L.; Xu, N.-Y.; Tang, R.-Z.; Liu, X.-Q. A 3D-Printed Scaffold-Based Osteosarcoma Model Allows to Investigate Tumor Phenotypes and Pathogenesis in an in Vitro Bone-Mimicking Niche. Mater. Today Bio. 2022, 15, 100295. [Google Scholar] [CrossRef]
- Bassi, G.; Panseri, S.; Dozio, S.M.; Sandri, M.; Campodoni, E.; Dapporto, M.; Sprio, S.; Tampieri, A.; Montesi, M. Scaffold-Based 3D Cellular Models Mimicking the Heterogeneity of Osteosarcoma Stem Cell Niche. Sci. Rep. 2020, 10, 22294. [Google Scholar] [CrossRef]
- Yu, S.; Yao, X. Advances on Immunotherapy for Osteosarcoma. Mol. Cancer 2024, 23, 192. [Google Scholar] [CrossRef]
- Lacinski, R.A.; Dziadowicz, S.A.; Melemai, V.K.; Fitzpatrick, B.; Pisquiy, J.J.; Heim, T.; Lohse, I.; Schoedel, K.E.; Llosa, N.J.; Weiss, K.R.; et al. Spatial Multiplexed Immunofluorescence Analysis Reveals Coordinated Cellular Networks Associated with Overall Survival in Metastatic Osteosarcoma. Bone Res. 2024, 12, 55. [Google Scholar] [CrossRef]
- Liu, W.; Hu, H.; Shao, Z.; Lv, X.; Zhang, Z.; Deng, X.; Song, Q.; Han, Y.; Guo, T.; Xiong, L.; et al. Characterizing the Tumor Microenvironment at the Single-Cell Level Reveals a Novel Immune Evasion Mechanism in Osteosarcoma. Bone Res. 2023, 11, 4. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, S.; Lv, J.; Feng, W.; Yu, Y.; Zhao, H. Osteosarcoma Immune Microenvironment: Cellular Struggle and Novel Therapeutic Insights. Front. Immunol. 2025, 16, 1584450. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S.; Haase, K.; Ye, H.; Young, M.D.; Alexandrov, L.B.; Farndon, S.J.; Collord, G.; Wedge, D.C.; Martincorena, I.; et al. Recurrent Mutation of IGF Signalling Genes and Distinct Patterns of Genomic Rearrangement in Osteosarcoma. Nat. Commun. 2017, 8, 15936. [Google Scholar] [CrossRef]
- Espejo Valle-Inclan, J.; De Noon, S.; Trevers, K.; Elrick, H.; Van Belzen, I.A.E.M.; Zumalave, S.; Sauer, C.M.; Tanguy, M.; Butters, T.; Muyas, F.; et al. Ongoing Chromothripsis Underpins Osteosarcoma Genome Complexity and Clonal Evolution. Cell 2025, 188, 352–370.e22. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.E.; Yang, T.; Wimberly, C.E.; Parmar, K.V.; Hansen, H.M.; De Smith, A.J.; Morimoto, L.M.; Metayer, C.; Ostrom, Q.T.; Eward, W.C.; et al. Genetic Variation near GRB10 Associated with Bone Growth and Osteosarcoma Risk in Canine and Human Populations. Cancer Epidemiol. 2024, 92, 102599. [Google Scholar] [CrossRef] [PubMed]
- Twenhafel, L.; Moreno, D.; Punt, T.; Kinney, M.; Ryznar, R. Epigenetic Changes Associated with Osteosarcoma: A Comprehensive Review. Cells 2023, 12, 1595. [Google Scholar] [CrossRef] [PubMed]
- Karimpour, M.; Ravanbakhsh, R.; Maydanchi, M.; Rajabi, A.; Azizi, F.; Saber, A. Cancer Driver Gene and Non-Coding RNA Alterations as Biomarkers of Brain Metastasis in Lung Cancer: A Review of the Literature. Biomed. Pharmacother. 2021, 143, 112190. [Google Scholar] [CrossRef]
- Liu, D.-Y.; Li, Z.; Zhang, K.; Jiao, N.; Lu, D.-G.; Zhou, D.-W.; Meng, Y.-B.; Sun, L. Circular RNA CircMTO1 Suppressed Proliferation and Metastasis of Osteosarcoma through miR-630/KLF6 Axis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 86–93. [Google Scholar] [CrossRef]
- Prasse, N.; Wessolowski, C.; Müller, I.; Cornils, K.; Franke, A.-K. Glycan Structures in Osteosarcoma as Targets for Lectin-Based Chimeric Antigen Receptor Immunotherapy. Int. J. Mol. Sci. 2024, 25, 5344. [Google Scholar] [CrossRef]
- Saldova, R.; Reuben, J.M.; Abd Hamid, U.M.; Rudd, P.M.; Cristofanilli, M. Levels of Specific Serum N-Glycans Identify Breast Cancer Patients with Higher Circulating Tumor Cell Counts. Ann. Oncol. 2011, 22, 1113–1119. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Liu, H. Biological Function, Regulatory Mechanism, and Clinical Application of Mannose in Cancer. Biochim. Biophys. Acta BBA—Rev. Cancer 2023, 1878, 188970. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Miyamoto, S.; Lebrilla, C.B. Developments in the Identification of Glycan Biomarkers for the Detection of Cancer. Mol. Cell. Proteom. 2013, 12, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Gutierrez Reyes, C.D.; Gautam, S.; Yu, A.; Cho, B.G.; Goli, M.; Donohoo, K.; Mondello, S.; Kobeissy, F.; Mechref, Y. MS-Based Glycomics and Glycoproteomics Methods Enabling Isomeric Characterization. Mass Spectrom. Rev. 2023, 42, 577–616. [Google Scholar] [CrossRef]
- Chang, D.; Zaia, J. Methods to Improve Quantitative Glycoprotein Coverage from Bottom-up LC-MS Data. Mass Spectrom. Rev. 2022, 41, 922–937. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jia, W.; Yang, J.; Zhan, X. Cancer Glycomics Offers Potential Biomarkers and Therapeutic Targets in the Framework of 3P Medicine. Front. Endocrinol. 2022, 13, 970489. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.F.; Koufos, A.; Gallie, B.L.; Phillips, R.A.; Fodstad, O.; Brøgger, A.; Gedde-Dahl, T.; Cavenee, W.K. Osteosarcoma and Retinoblastoma: A Shared Chromosomal Mechanism Revealing Recessive Predisposition. Proc. Natl. Acad. Sci. USA 1985, 82, 6216–6220. [Google Scholar] [CrossRef]
- Ren, W.; Gu, G. Prognostic Implications of RB1 Tumour Suppressor Gene Alterations in the Clinical Outcome of Human Osteosarcoma: A Meta-Analysis. Eur. J. Cancer Care 2017, 26, e12401. [Google Scholar] [CrossRef]
- Synoradzki, K.J.; Bartnik, E.; Czarnecka, A.M.; Fiedorowicz, M.; Firlej, W.; Brodziak, A.; Stasinska, A.; Rutkowski, P.; Grieb, P. TP53 in Biology and Treatment of Osteosarcoma. Cancers 2021, 13, 4284. [Google Scholar] [CrossRef]
- Zhou, C.; Balmer, L.; Song, M.; Mahara, G.; Wu, K.; Wang, W.; Wang, H. Identification of circRNA Biomarkers in Osteosarcoma: An Updated Systematic Review and Meta-Analysis. Non-Coding RNA Res. 2024, 9, 341–349. [Google Scholar] [CrossRef]
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A Comprehensive Review. SICOT-J. 2018, 4, 12. [Google Scholar] [CrossRef]
- Sheng, G.; Gao, Y.; Yang, Y.; Wu, H. Osteosarcoma and Metastasis. Front. Oncol. 2021, 11, 780264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Z.; Liu, G.; Li, D.; Gu, Z.; Zhang, L.; Pan, Y.; Cui, X.; Wang, L.; Liu, G.; et al. B7-H3 Targeted CAR-T Cells Show Highly Efficient Anti-Tumor Function against Osteosarcoma Both in Vitro and in Vivo. BMC Cancer 2022, 22, 1124. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, L.; Somovilla-Crespo, B.; Garcia-Rodriguez, P.; Morales-Molina, A.; Rodriguez-Milla, M.A.; Garcia-Castro, J. Switchable CAR T Cell Strategy against Osteosarcoma. Cancer Immunol. Immunother. CII 2023, 72, 2623–2633. [Google Scholar] [CrossRef]
- Liao, S.; Li, J.; Gao, S.; Han, Y.; Han, X.; Wu, Y.; Bi, J.; Xu, M.; Bi, W. Sulfatinib, a Novel Multi-Targeted Tyrosine Kinase Inhibitor of FGFR1, CSF1R, and VEGFR1–3, Suppresses Osteosarcoma Proliferation and Invasion via Dual Role in Tumor Cells and Tumor Microenvironment. Front. Oncol. 2023, 13, 1158857. [Google Scholar] [CrossRef]
- Proença, C.; Rufino, A.T.; Santos, I.; Albuquerque, H.M.T.; Silva, A.M.S.; Fernandes, E.; Ferreira De Oliveira, J.M.P. Gossypetin Is a Novel Modulator of Inflammatory Cytokine Production and a Suppressor of Osteosarcoma Cell Growth. Antioxidants 2023, 12, 1744. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, P.; Zhang, Y.; Han, S.; Wang, G.; Wang, H.; Song, H.; Li, S. Dynamic Nanoassemblies Derived from Small-Molecule Homodimeric Prodrugs for in Situ Drug Activation and Safe Osteosarcoma Treatment. iScience 2023, 26, 107409. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, X.; Yuan, B.; Jiang, S.; Yan, R.; Dong, X.; Yao, Q.; Liang, H. Soy Isoflavones Induces Mitophagy to Inhibit the Progression of Osteosarcoma by Blocking the AKT/mTOR Signaling Pathway. Mol. Med. 2024, 30, 5. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, Z.; Mi, Z.; Tao, W.; Liu, D.; Fu, J.; Fan, H. Deciphering the Heterogeneity and Immunosuppressive Function of Regulatory T Cells in Osteosarcoma Using Single-Cell RNA Transcriptome. Comput. Biol. Med. 2023, 165, 107417. [Google Scholar] [CrossRef]
- Wu, W.; Jing, D.; Meng, Z.; Hu, B.; Zhong, B.; Deng, X.; Jin, X.; Shao, Z. FGD1 Promotes Tumor Progression and Regulates Tumor Immune Response in Osteosarcoma via Inhibiting PTEN Activity. Theranostics 2020, 10, 2859–2871. [Google Scholar] [CrossRef]
- Higuchi, T.; Yamamoto, J.; Sugisawa, N.; Tashiro, Y.; Nishino, H.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; Igarashi, K.; et al. PPARγ Agonist Pioglitazone in Combination With Cisplatinum Arrests a Chemotherapy-Resistant Osteosarcoma PDOX Model. Cancer Genom. Proteom. 2020, 17, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, N.; Venkatramani, R.; Hecker-Nolting, S.; Melcon, S.G.; Locatelli, F.; Bautista, F.; Longhi, A.; Lervat, C.; Entz-Werle, N.; Casanova, M.; et al. Lenvatinib with Etoposide plus Ifosfamide in Patients with Refractory or Relapsed Osteosarcoma (ITCC-050): A Multicentre, Open-Label, Multicohort, Phase 1/2 Study. Lancet Oncol. 2021, 22, 1312–1321. [Google Scholar] [CrossRef]
- Rathore, R.; Van Tine, B.A. Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies. J. Clin. Med. 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Caldwell, K.E.; Schutt, C.; Brashears, C.B.; Prudner, B.C.; Ehrhardt, W.R.; Leung, C.H.; Lin, H.; Daw, N.C.; Beird, H.C.; et al. Metabolic Compensation Activates Pro-Survival mTORC1 Signaling upon 3-Phosphoglycerate Dehydrogenase Inhibition in Osteosarcoma. Cell Rep. 2021, 34, 108678. [Google Scholar] [CrossRef]
- Newman, A.C.; Maddocks, O.D.K. Serine and Functional Metabolites in Cancer. Trends Cell Biol. 2017, 27, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, M.F.; Wagner, L.M.; Cripe, T.P. Immunotherapy for Osteosarcoma: Where Do We Go from Here? Pediatr. Blood Cancer 2018, 65, e27227. [Google Scholar] [CrossRef]
- Wang, J.; Hu, F.; Yu, P.; Wang, J.; Liu, Z.; Bao, Q.; Zhang, W.; Wen, J. Sorafenib Inhibits Doxorubicin-Induced PD-L1 Upregulation to Improve Immunosuppressive Microenvironment in Osteosarcoma. J. Cancer Res. Clin. Oncol. 2023, 149, 5127–5138. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Li, Y. Effects of Microenvironment in Osteosarcoma on Chemoresistance and the Promise of Immunotherapy as an Osteosarcoma Therapeutic Modality. Front. Immunol. 2022, 13, 871076. [Google Scholar] [CrossRef]
- Liu, Z.; Wen, J.; Hu, F.; Wang, J.; Hu, C.; Zhang, W. Thrombospondin-1 Induced Programmed Death-ligand 1-mediated Immunosuppression by Activating the STAT3 Pathway in Osteosarcoma. Cancer Sci. 2022, 113, 432–445. [Google Scholar] [CrossRef]
- Xiang, D.; Han, X.; Li, J.; Zhang, J.; Xiao, H.; Li, T.; Zhao, X.; Xiong, H.; Xu, M.; Bi, W. Combination of IDO Inhibitors and Platinum(IV) Prodrugs Reverses Low Immune Responses to Enhance Cancer Chemotherapy and Immunotherapy for Osteosarcoma. Mater. Today Bio. 2023, 20, 100675. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Tazawa, H.; Demiya, K.; Kure, M.; Kondo, H.; Komatsubara, T.; Sugiu, K.; Hasei, J.; Yoshida, A.; Kunisada, T.; et al. Telomerase-Specific Oncolytic Immunotherapy for Promoting Efficacy of PD-1 Blockade in Osteosarcoma. Cancer Immunol. Immunother. 2021, 70, 1405–1417. [Google Scholar] [CrossRef]
- Shi, X.; Li, X.; Wang, H.; Yu, Z.; Zhu, Y.; Gao, Y. Specific Inhibition of PI3Kδ/γ Enhances the Efficacy of Anti-PD1 against Osteosarcoma Cancer. J. Bone Oncol. 2019, 16, 100206. [Google Scholar] [CrossRef]
- Chen, S.; Guenther, L.M.; Aronhalt, A.; Cardillo, L.; Janeway, K.A.; Church, A.J. PD-1 and PD-L1 Expression in Osteosarcoma: Which Specimen to Evaluate? J. Pediatr. Hematol. Oncol. 2020, 42, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.-X.; Zhang, T.-W.; Zhou, L.; Ding, W.; Liang, H.-F.; Hu, Z.-C.; Chen, Q.; Dong, J.; Xue, F.-F.; Yin, X.-F.; et al. Enhancement of Anti-PD-1/PD-L1 Immunotherapy for Osteosarcoma Using an Intelligent Autophagy-Controlling Metal Organic Framework. Biomaterials 2022, 282, 121407. [Google Scholar] [CrossRef]
- Tian, H.; Cao, J.; Li, B.; Nice, E.C.; Mao, H.; Zhang, Y.; Huang, C. Managing the Immune Microenvironment of Osteosarcoma: The Outlook for Osteosarcoma Treatment. Bone Res. 2023, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Sorkhabi, A.D.; Sarkesh, A.; Fotouhi, A.; Saeedi, H.; Aghebati-Maleki, L. Cancer Combination Therapies by Silencing of CTLA-4, PD-L1, and TIM3 in Osteosarcoma. IUBMB Life 2022, 74, 908–917. [Google Scholar] [CrossRef]
- Seidensaal, K.; Mattke, M.; Haufe, S.; Rathke, H.; Haberkorn, U.; Bougatf, N.; Kudak, A.; Blattmann, C.; Oertel, S.; Kirchner, M.; et al. The Role of Combined Ion-Beam Radiotherapy (CIBRT) with Protons and Carbon Ions in a Multimodal Treatment Strategy of Inoperable Osteosarcoma. Radiother. Oncol. 2021, 159, 8–16. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Y.; Wang, R.; Wang, T. Molecular Mechanisms of Tumor Resistance to Radiotherapy. Mol. Cancer 2023, 22, 96. [Google Scholar] [CrossRef]
- Astl, J.; Belsan, T.; Michnova, L.; Kubeš, J.; Filipovsky, T.; Blecha, J.; Holy, R. Highly Aggressive Osteosarcoma of the Ethmoids and Maxillary Sinus-A Case of Successful Surgery and Proton Beam Radiotherapy in a 65-Year-Old Man. Medicina 2022, 58, 1141. [Google Scholar] [CrossRef]
- Ioakeim-Ioannidou, M.; Rose, M.; Chen, Y.-L.; MacDonald, S.M. The Use of Proton and Carbon Ion Radiation Therapy for Sarcomas. Semin. Radiat. Oncol. 2024, 34, 207–217. [Google Scholar] [CrossRef]
- Bertho, A.; Graeff, C.; Ortiz, R.; Giorgi, M.; Schuy, C.; Juchaux, M.; Gilbert, C.; Espenon, J.; Oppermann, J.; Sokol, O.; et al. Carbon Minibeam Radiation Therapy Results in Tumor Growth Delay in an Osteosarcoma Murine Model. Sci. Rep. 2025, 15, 7305. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Y.; Hu, X.; Du, G.; Tang, X.; Min, L. Advances of Osteosarcoma Models for Drug Discovery and Precision Medicine. Biomolecules 2023, 13, 1362. [Google Scholar] [CrossRef]
- Petrescu, D.I.; Yustein, J.T.; Dasgupta, A. Preclinical Models for the Study of Pediatric Solid Tumors: Focus on Bone Sarcomas. Front. Oncol. 2024, 14, 1388484. [Google Scholar] [CrossRef]
- Khanna, C.; Fan, T.M.; Gorlick, R.; Helman, L.J.; Kleinerman, E.S.; Adamson, P.C.; Houghton, P.J.; Tap, W.D.; Welch, D.R.; Steeg, P.S.; et al. Toward a Drug Development Path That Targets Metastatic Progression in Osteosarcoma. Clin. Cancer Res. 2014, 20, 4200–4209. [Google Scholar] [CrossRef]
- Han, Z.; Chen, G.; Wang, D. Emerging Immunotherapies in Osteosarcoma: From Checkpoint Blockade to Cellular Therapies. Front. Immunol. 2025, 16, 1579822. [Google Scholar] [CrossRef]
- Zhra, M.; Akhund, S.A.; Mohammad, K.S. Advancements in Osteosarcoma Therapy: Overcoming Chemotherapy Resistance and Exploring Novel Pharmacological Strategies. Pharmaceuticals 2025, 18, 520. [Google Scholar] [CrossRef]
| Epigenetic Mechanism | Main Molecular Players (Examples) | Functional Consequence in Osteosarcoma | Clinical/Therapeutic Outlook |
|---|---|---|---|
| DNA-methylation gain | DNMT1/3A/3B; hypermethylation of miR-149, miR-195, CXCL12 [57] | Silencing of tumor suppressors and immune genes → promotes proliferation, invasion, and immune evasion [57] | DNMT inhibitors (decitabine, guadecitabine) [57] |
| Histone-mark loss (H3K27me3) | KDM6A/B erase H3K27me3 marks [57] | Enhances metastatic capacity and chemoresistance [57] | EZH2 agonists or KDM6A/B inhibitors under preclinical investigation [57] |
| m6A RNA methylation | METTL3 overexpression stabilizes DRG1, ATAD2 transcripts [57] | Drives glycolysis and cell-cycle progression [57] | METTL3 inhibitor (STM-2457) shows efficacy in preclinical OS models [57] |
| lncRNA-guided chromatin remodelling | lncRNAs THAP9-AS1, HOTAIR recruit DNMTs to gene promoters [58] | Fuels proliferation and migration [58] | Antisense oligonucleotides or CRISPR-based silencing in development [58] |
| circRNA networks | circECE1 (oncogenic); circMTO1 (tumor-suppressive) [59] | Regulate aerobic glycolysis, metastasis, and tumour suppression [59] | Potential use in liquid-biopsy diagnostics due to stability [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacobescu, G.L.; Corlatescu, A.-D.; Costin, H.P.; Spiridonica, R.; Popa, M.-I.-G.; Cirstoiu, C. Molecular and Glycosylation Pathways in Osteosarcoma: Tumor Microenvironment and Emerging Strategies Toward Personalized Oncology. Curr. Issues Mol. Biol. 2025, 47, 629. https://doi.org/10.3390/cimb47080629
Iacobescu GL, Corlatescu A-D, Costin HP, Spiridonica R, Popa M-I-G, Cirstoiu C. Molecular and Glycosylation Pathways in Osteosarcoma: Tumor Microenvironment and Emerging Strategies Toward Personalized Oncology. Current Issues in Molecular Biology. 2025; 47(8):629. https://doi.org/10.3390/cimb47080629
Chicago/Turabian StyleIacobescu, Georgian Longin, Antonio-Daniel Corlatescu, Horia Petre Costin, Razvan Spiridonica, Mihnea-Ioan-Gabriel Popa, and Catalin Cirstoiu. 2025. "Molecular and Glycosylation Pathways in Osteosarcoma: Tumor Microenvironment and Emerging Strategies Toward Personalized Oncology" Current Issues in Molecular Biology 47, no. 8: 629. https://doi.org/10.3390/cimb47080629
APA StyleIacobescu, G. L., Corlatescu, A.-D., Costin, H. P., Spiridonica, R., Popa, M.-I.-G., & Cirstoiu, C. (2025). Molecular and Glycosylation Pathways in Osteosarcoma: Tumor Microenvironment and Emerging Strategies Toward Personalized Oncology. Current Issues in Molecular Biology, 47(8), 629. https://doi.org/10.3390/cimb47080629







