Feminization of the Blood–Brain Barrier Changes the Brain Transcriptome of Drosophila melanogaster Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Stocks
2.2. Whole Brain Dissection and RNA Sequencing
2.3. Sequence Analysis
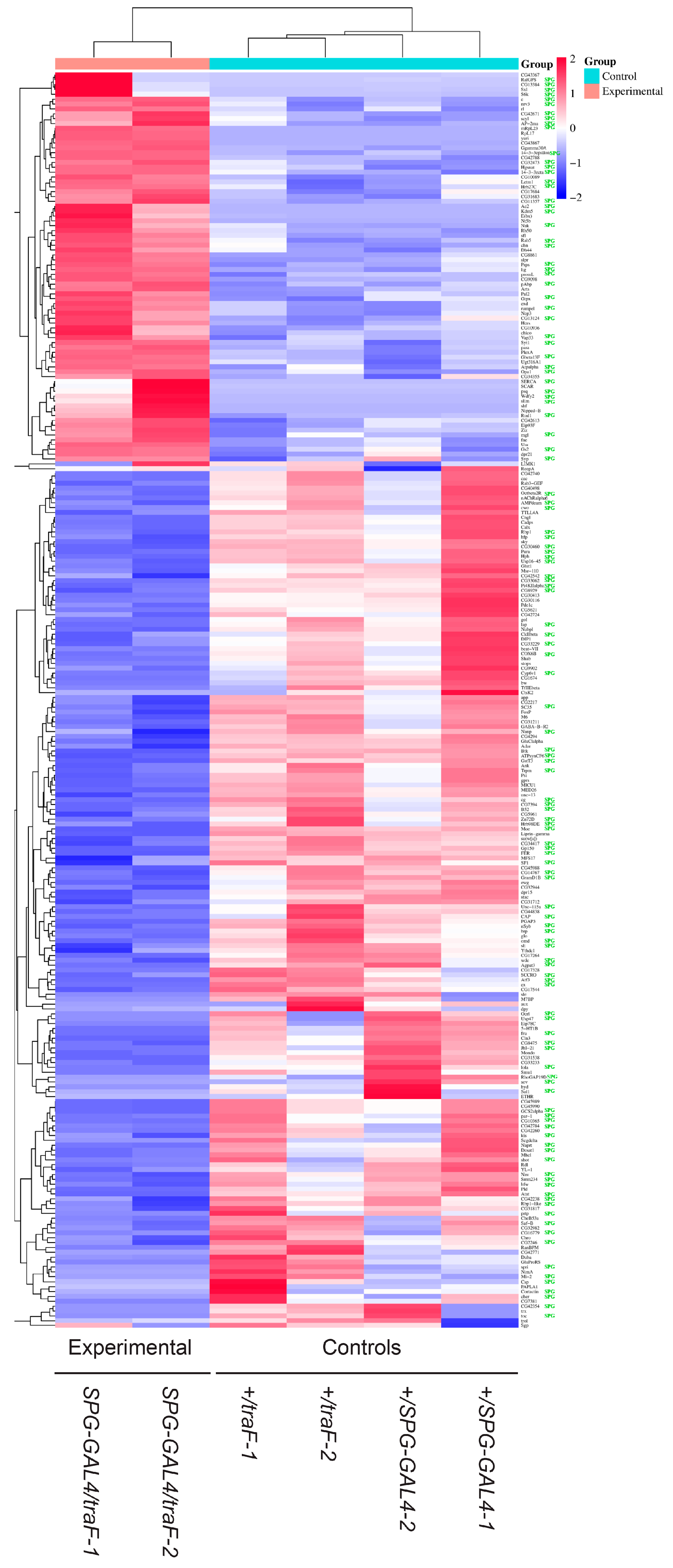
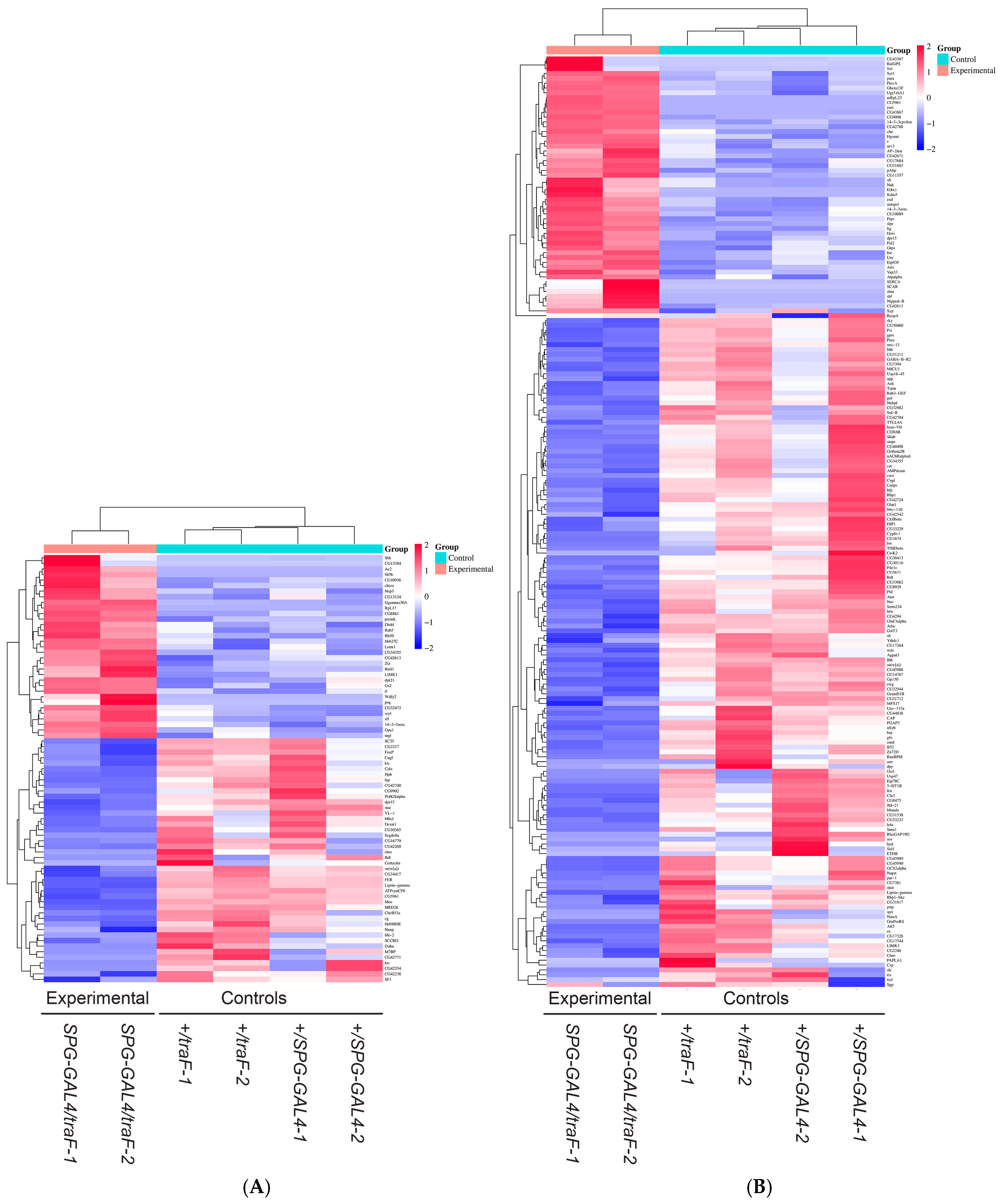
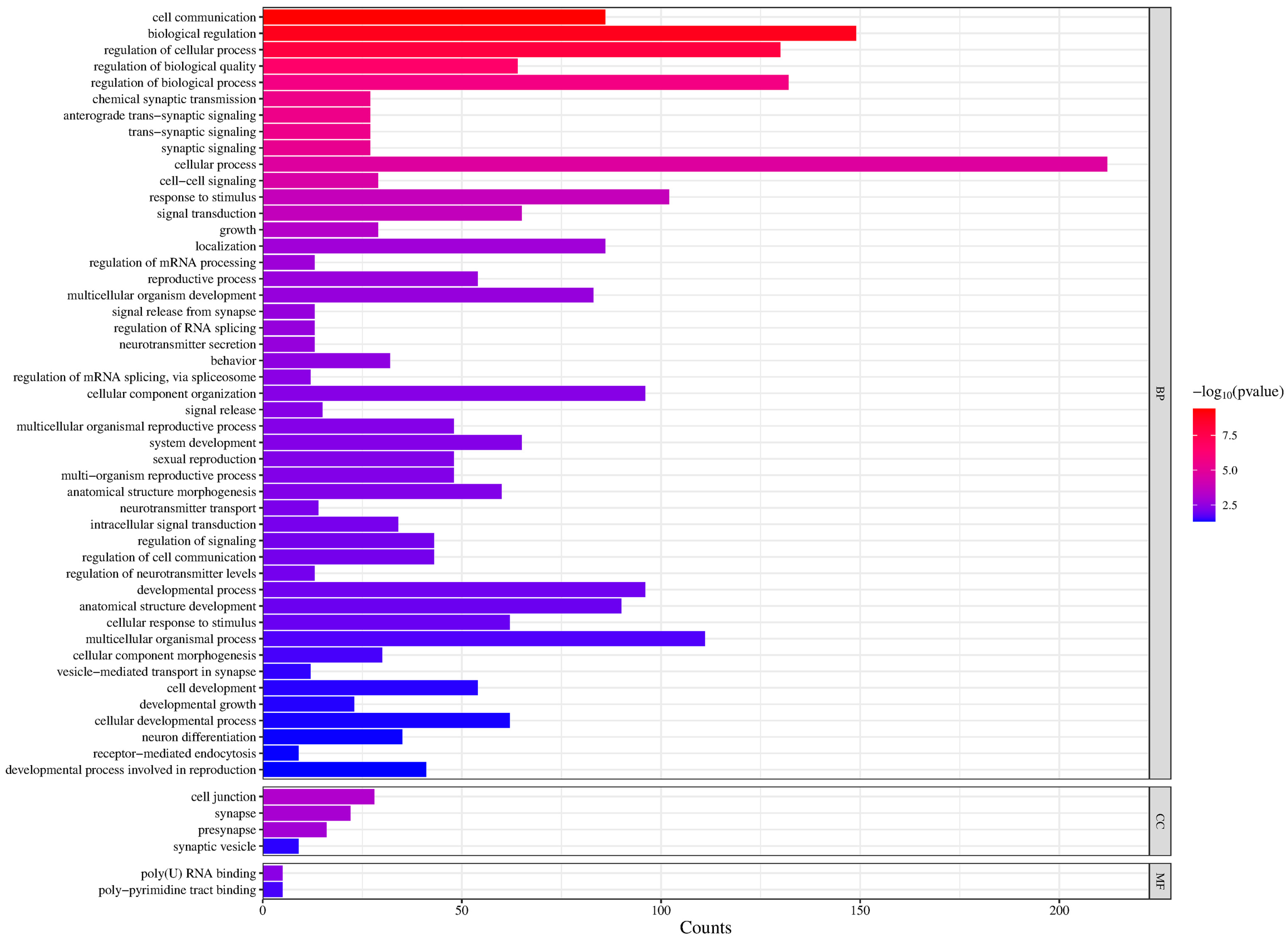
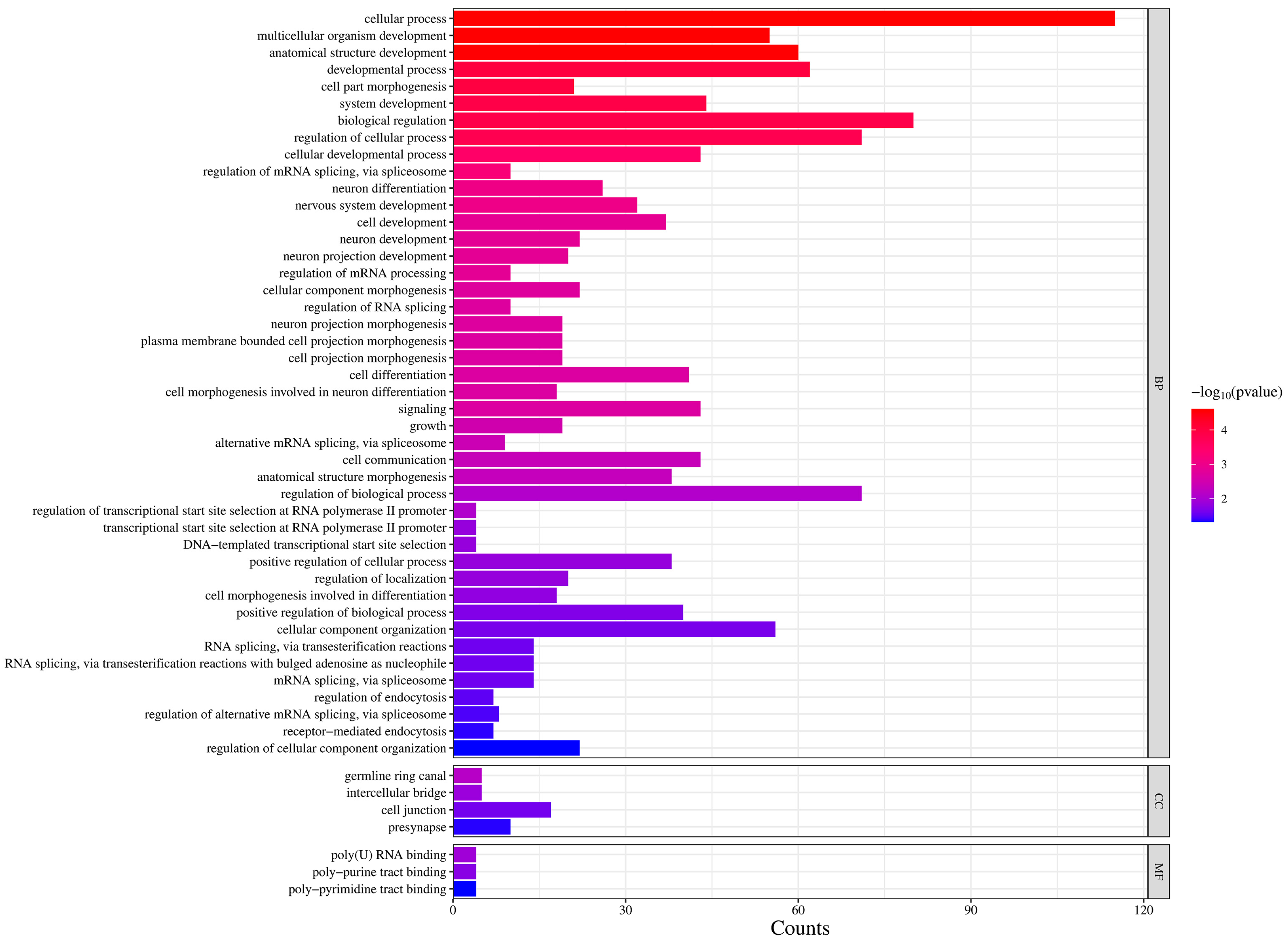
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, B.S.; Ridge, K.A. Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster. Genetics 1980, 94, 383–423. [Google Scholar] [CrossRef]
- Salz, H.K. Sex determination in insects: A binary decision based on alternative splicing. Curr. Opin. Genet. Dev. 2011, 21, 395–400. [Google Scholar] [CrossRef]
- Dauwalder, B. The roles of fruitless and doublesex in the control of male courtship. Int. Rev. Neurobiol. 2011, 99, 87–105. [Google Scholar] [CrossRef]
- Kimura, K.; Hachiya, T.; Koganezawa, M.; Tazawa, T.; Yamamoto, D. Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 2008, 59, 759–769. [Google Scholar] [CrossRef]
- Villella, A.; Hall, J.C. Courtship anomalies caused by doublesex mutations in Drosophila melanogaster. Genetics 1996, 143, 331–344. [Google Scholar] [CrossRef]
- Shirangi, T.R.; Taylor, B.J.; McKeown, M. A double-switch system regulates male courtship behavior in male and female Drosophila melanogaster. Nat. Genet. 2006, 38, 1435–1439. [Google Scholar] [CrossRef]
- Demir, E.; Dickson, B.J. fruitless splicing specifies male courtship behavior in Drosophila. Cell 2005, 121, 785–794. [Google Scholar] [CrossRef]
- Ito, H.; Fujitani, K.; Usui, K.; Shimizu-Nishikawa, K.; Tanaka, S.; Yamamoto, D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl. Acad. Sci. USA 1996, 93, 9687–9692. [Google Scholar] [CrossRef]
- Manoli, D.S.; Foss, M.; Villella, A.; Taylor, B.J.; Hall, J.C.; Baker, B.S. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 2005, 436, 395–400. [Google Scholar] [CrossRef]
- Rideout, E.J.; Dornan, A.J.; Neville, M.C.; Eadie, S.; Goodwin, S.F. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 2010, 13, 458–466. [Google Scholar] [CrossRef]
- Ryner, L.C.; Goodwin, S.F.; Castrillon, D.H.; Anand, A.; Villella, A.; Baker, B.S.; Hall, J.C.; Taylor, B.J.; Wasserman, S.A. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 1996, 87, 1079–1089. [Google Scholar] [CrossRef]
- Stockinger, P.; Kvitsiani, D.; Rotkopf, S.; Tirian, L.; Dickson, B.J. Neural circuitry that governs Drosophila male courtship behavior. Cell 2005, 121, 795–807. [Google Scholar] [CrossRef]
- Dauwalder, B.; Tsujimoto, S.; Moss, J.; Mattox, W. The Drosophila takeout gene is regulated by the somatic sex-determination pathway and affects male courtship behavior. Genes. Dev. 2002, 16, 2879–2892. [Google Scholar] [CrossRef]
- Fujii, S.; Amrein, H. Genes expressed in the Drosophila head reveal a role for fat cells in sex-specific physiology. EMBO J. 2002, 21, 5353–5363. [Google Scholar] [CrossRef]
- Lazareva, A.A.; Roman, G.; Mattox, W.; Hardin, P.E.; Dauwalder, B. A role for the adult fat body in Drosophila male courtship behavior. PLoS Genet. 2007, 3, e16. [Google Scholar] [CrossRef]
- Bainton, R.J.; Tsai, L.T.; Schwabe, T.; DeSalvo, M.; Gaul, U.; Heberlein, U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell 2005, 123, 145–156. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, A.M.; Paik, R.; Li, J.; Bhat, M.A. Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila. J. Neurosci. 2006, 26, 3319–3329. [Google Scholar] [CrossRef]
- Baumgartner, S.; Littleton, J.T.; Broadie, K.; Bhat, M.A.; Harbecke, R.; Lengyel, J.A.; Chiquet-Ehrismann, R.; Prokop, A.; Bellen, H.J. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell 1996, 87, 1059–1068. [Google Scholar] [CrossRef]
- Blauth, K.; Banerjee, S.; Bhat, M.A. Axonal ensheathment and intercellular barrier formation in Drosophila. Int. Rev. Cell Mol. Biol. 2010, 283, 93–128. [Google Scholar] [CrossRef]
- DeSalvo, M.K.; Hindle, S.J.; Rusan, Z.M.; Orng, S.; Eddison, M.; Halliwill, K.; Bainton, R.J. The Drosophila surface glia transcriptome: Evolutionary conserved blood-brain barrier processes. Front. Neurosci. 2014, 8, 346. [Google Scholar] [CrossRef]
- DeSalvo, M.K.; Mayer, N.; Mayer, F.; Bainton, R.J. Physiologic and anatomic characterization of the brain surface glia barrier of Drosophila. Glia 2011, 59, 1322–1340. [Google Scholar] [CrossRef]
- Faivre-Sarrailh, C.; Banerjee, S.; Li, J.; Hortsch, M.; Laval, M.; Bhat, M.A. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development 2004, 131, 4931–4942. [Google Scholar] [CrossRef]
- Schwabe, T.; Bainton, R.J.; Fetter, R.D.; Heberlein, U.; Gaul, U. GPCR signaling is required for blood-brain barrier formation in drosophila. Cell 2005, 123, 133–144. [Google Scholar]
- Stork, T.; Engelen, D.; Krudewig, A.; Silies, M.; Bainton, R.J.; Klambt, C. Organization and function of the blood-brain barrier in Drosophila. J. Neurosci. 2008, 28, 587–597. [Google Scholar] [CrossRef]
- Contreras, E.G.; Glavic, A.; Brand, A.H.; Sierralta, J.A. The Serine Protease Homolog, Scarface, Is Sensitive to Nutrient Availability and Modulates the Development of the Drosophila Blood-Brain Barrier. J. Neurosci. 2021, 41, 6430–6448. [Google Scholar] [CrossRef]
- Speder, P.; Brand, A.H. Gap junction proteins in the blood-brain barrier control nutrient-dependent reactivation of Drosophila neural stem cells. Dev. Cell 2014, 30, 309–321. [Google Scholar] [CrossRef]
- Liu, J.; Speder, P.; Brand, A.H. Control of brain development and homeostasis by local and systemic insulin signalling. Diabetes Obes. Metab. 2014, 16 (Suppl. 1), 16–20. [Google Scholar] [CrossRef]
- Axelrod, S.; Li, X.; Sun, Y.; Lincoln, S.; Terceros, A.; O’Neil, J.; Wang, Z.; Nguyen, A.; Vora, A.; Spicer, C.; et al. The Drosophila blood-brain barrier regulates sleep via Moody G protein-coupled receptor signaling. Proc. Natl. Acad. Sci. USA 2023, 120, e2309331120. [Google Scholar] [CrossRef]
- Li, F.; Artiushin, G.; Sehgal, A. Modulation of sleep by trafficking of lipids through the Drosophila blood-brain barrier. Elife 2023, 12, e86336. [Google Scholar] [CrossRef]
- Li, H.; Aboudhiaf, S.; Parrot, S.; Scote-Blachon, C.; Benetollo, C.; Lin, J.S.; Seugnet, L. Pallidin function in Drosophila surface glia regulates sleep and is dependent on amino acid availability. Cell Rep. 2023, 42, 113025. [Google Scholar] [CrossRef]
- Hoxha, V.; Lama, C.; Chang, P.L.; Saurabh, S.; Patel, N.; Olate, N.; Dauwalder, B. Sex-specific signaling in the blood-brain barrier is required for male courtship in Drosophila. PLoS Genet. 2013, 9, e1003217. [Google Scholar] [CrossRef]
- Lama, C.; Love, C.R.; Le, H.N.; Waqar, M.; Reeve, J.L.; Lama, J.; Dauwalder, B. The nuclear receptor Hr46/Hr3 is required in the blood brain barrier of mature males for courtship. PLoS Genet. 2022, 18, e1009519. [Google Scholar] [CrossRef]
- Ferveur, J.F.; Stortkuhl, K.F.; Stocker, R.F.; Greenspan, R.J. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science 1995, 267, 902–905. [Google Scholar]
- McKeown, M.; Belote, J.M.; Boggs, R.T. Ectopic expression of the female transformer gene product leads to female differentiation of chromosomally male Drosophila. Cell 1988, 53, 887–895. [Google Scholar]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Chang, P.L.; Dunham, J.P.; Nuzhdin, S.V.; Arbeitman, M.N. Somatic sex-specific transcriptome differences in Drosophila revealed by whole transcriptome sequencing. BMC Genom. 2011, 12, 364. [Google Scholar] [CrossRef]
- Hatan, M.; Shinder, V.; Israeli, D.; Schnorrer, F.; Volk, T. The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures. J. Cell Biol. 2011, 192, 307–319. [Google Scholar] [CrossRef]
- Love, C.R.; Gautam, S.; Lama, C.; Le, N.H.; Dauwalder, B. The Drosophila dopamine 2-like receptor D2R (Dop2R) is required in the blood brain barrier for male courtship. Genes. Brain Behav. 2023, 22, e12836. [Google Scholar] [CrossRef]
- Wijesekera, T.P.; Saurabh, S.; Dauwalder, B. Juvenile Hormone Is Required in Adult Males for Drosophila Courtship. PLoS ONE 2016, 11, e0151912. [Google Scholar] [CrossRef]
- Meiselman, M.; Lee, S.S.; Tran, R.T.; Dai, H.; Ding, Y.; Rivera-Perez, C.; Wijesekera, T.P.; Dauwalder, B.; Noriega, F.G.; Adams, M.E. Endocrine network essential for reproductive success in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2017, 114, E3849–E3858. [Google Scholar] [CrossRef]
- Hindle, S.J.; Munji, R.N.; Dolghih, E.; Gaskins, G.; Orng, S.; Ishimoto, H.; Soung, A.; DeSalvo, M.; Kitamoto, T.; Keiser, M.J.; et al. Evolutionarily Conserved Roles for Blood-Brain Barrier Xenobiotic Transporters in Endogenous Steroid Partitioning and Behavior. Cell Rep. 2017, 21, 1304–1316. [Google Scholar] [CrossRef]
- Dalton, J.E.; Lebo, M.S.; Sanders, L.E.; Sun, F.; Arbeitman, M.N. Ecdysone receptor acts in fruitless- expressing neurons to mediate drosophila courtship behaviors. Curr. Biol. 2009, 19, 1447–1452. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, D.S.; Hashem, W.; Lama, C.; Reeve, J.L.; Dauwalder, B. Feminization of the Blood–Brain Barrier Changes the Brain Transcriptome of Drosophila melanogaster Males. Curr. Issues Mol. Biol. 2025, 47, 626. https://doi.org/10.3390/cimb47080626
Davis DS, Hashem W, Lama C, Reeve JL, Dauwalder B. Feminization of the Blood–Brain Barrier Changes the Brain Transcriptome of Drosophila melanogaster Males. Current Issues in Molecular Biology. 2025; 47(8):626. https://doi.org/10.3390/cimb47080626
Chicago/Turabian StyleDavis, Danyel S., Warda Hashem, Chamala Lama, Joseph L. Reeve, and Brigitte Dauwalder. 2025. "Feminization of the Blood–Brain Barrier Changes the Brain Transcriptome of Drosophila melanogaster Males" Current Issues in Molecular Biology 47, no. 8: 626. https://doi.org/10.3390/cimb47080626
APA StyleDavis, D. S., Hashem, W., Lama, C., Reeve, J. L., & Dauwalder, B. (2025). Feminization of the Blood–Brain Barrier Changes the Brain Transcriptome of Drosophila melanogaster Males. Current Issues in Molecular Biology, 47(8), 626. https://doi.org/10.3390/cimb47080626





