An Integrated Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and Experimental Validation Study to Investigate the Potential Mechanism of Isoliquiritigenin in the Treatment of Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Network Pharmacology Analysis
2.3. Molecular Docking
2.4. MD Simulation
2.5. Cell Culture and Treatment
2.6. Cytotoxicity Evaluation of ISL
2.7. OGD/R Model Establishment
2.8. RT-qPCR Assay
2.9. Western Blotting (WB)
2.10. Statistical Analysis
3. Results
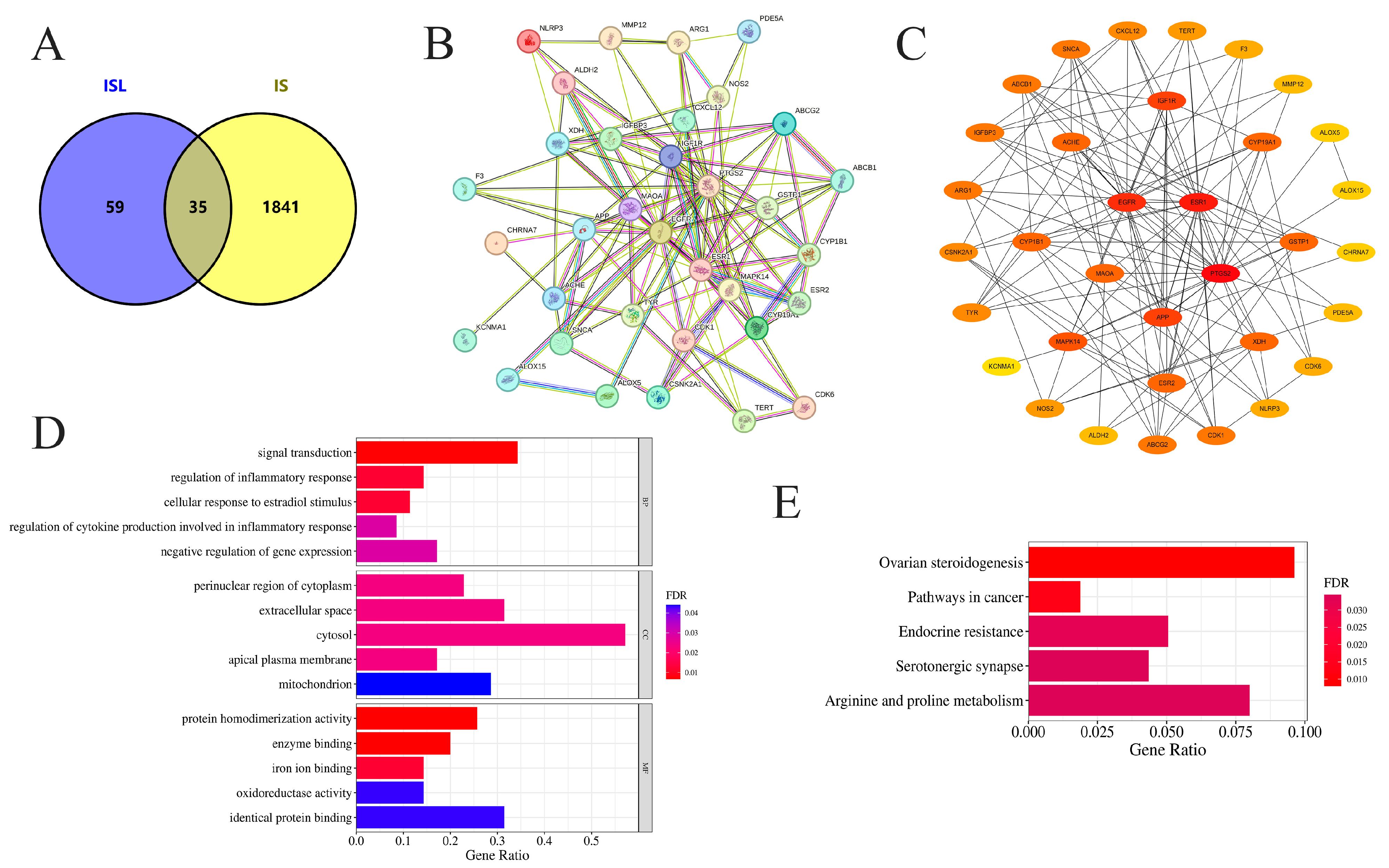
3.1. Potential Targets of ISL in the Treatment of IS
3.2. PPI Network Analysis and Screening of Key Targets
3.3. GO and KEGG Enrichment Analyses
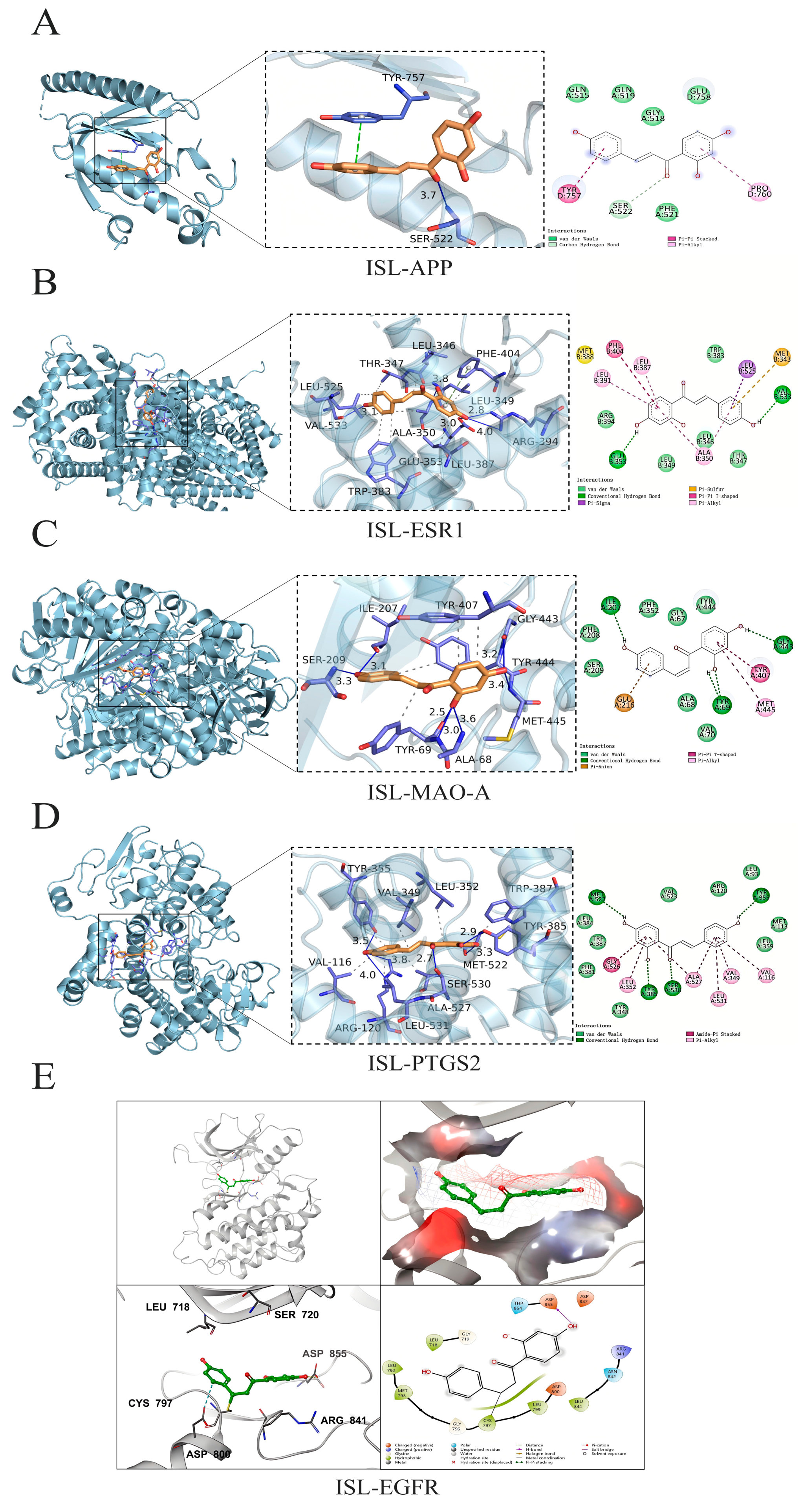
3.4. Molecular Docking Analysis
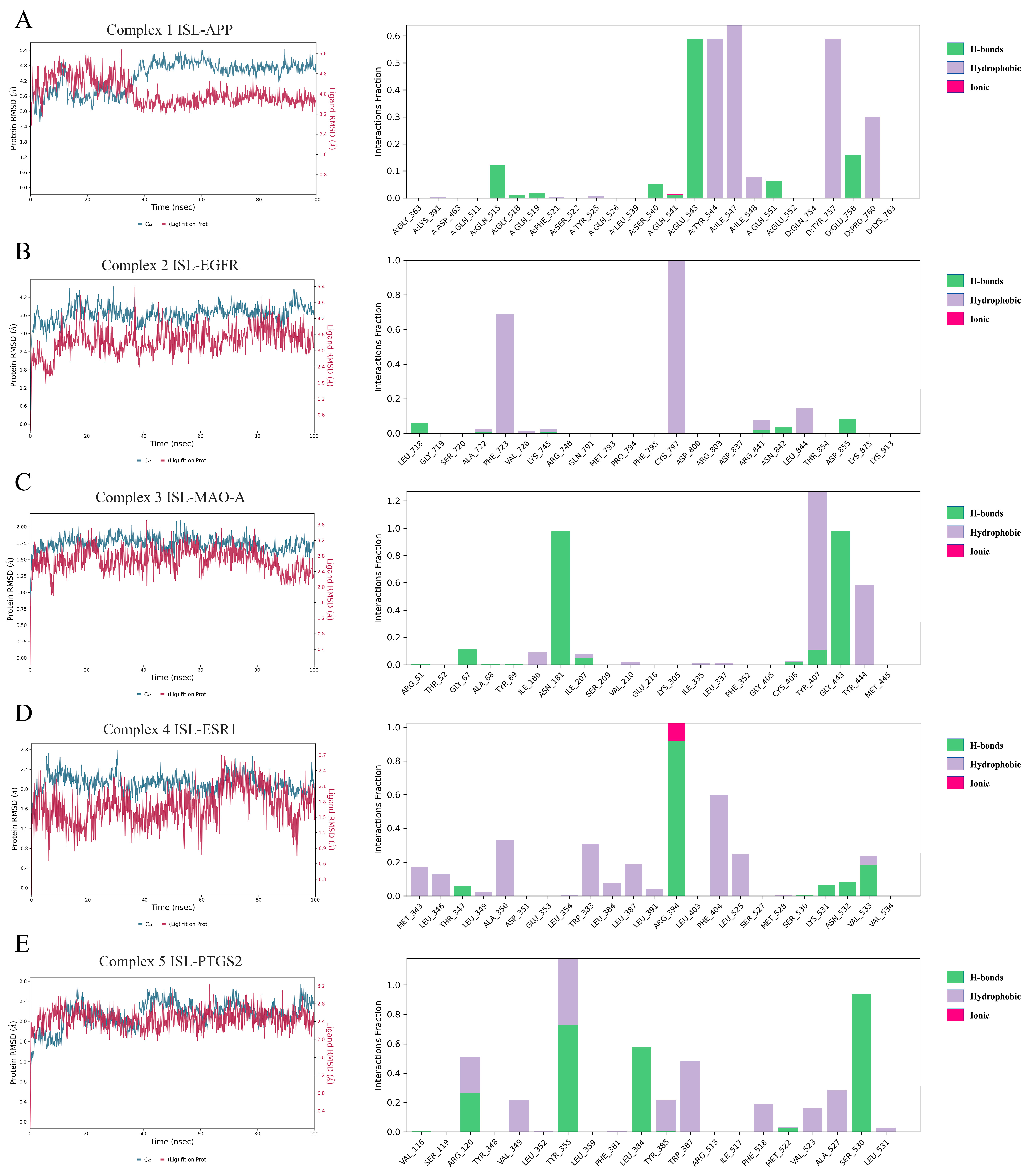
3.5. MD Simulations Results
3.6. Effects of ISL on OGD/R-Induced HT22 Cells
3.7. The Effect of ISL on the mRNA Expression of APP, ESR1, MAO-A, PTGS2, and EGFR
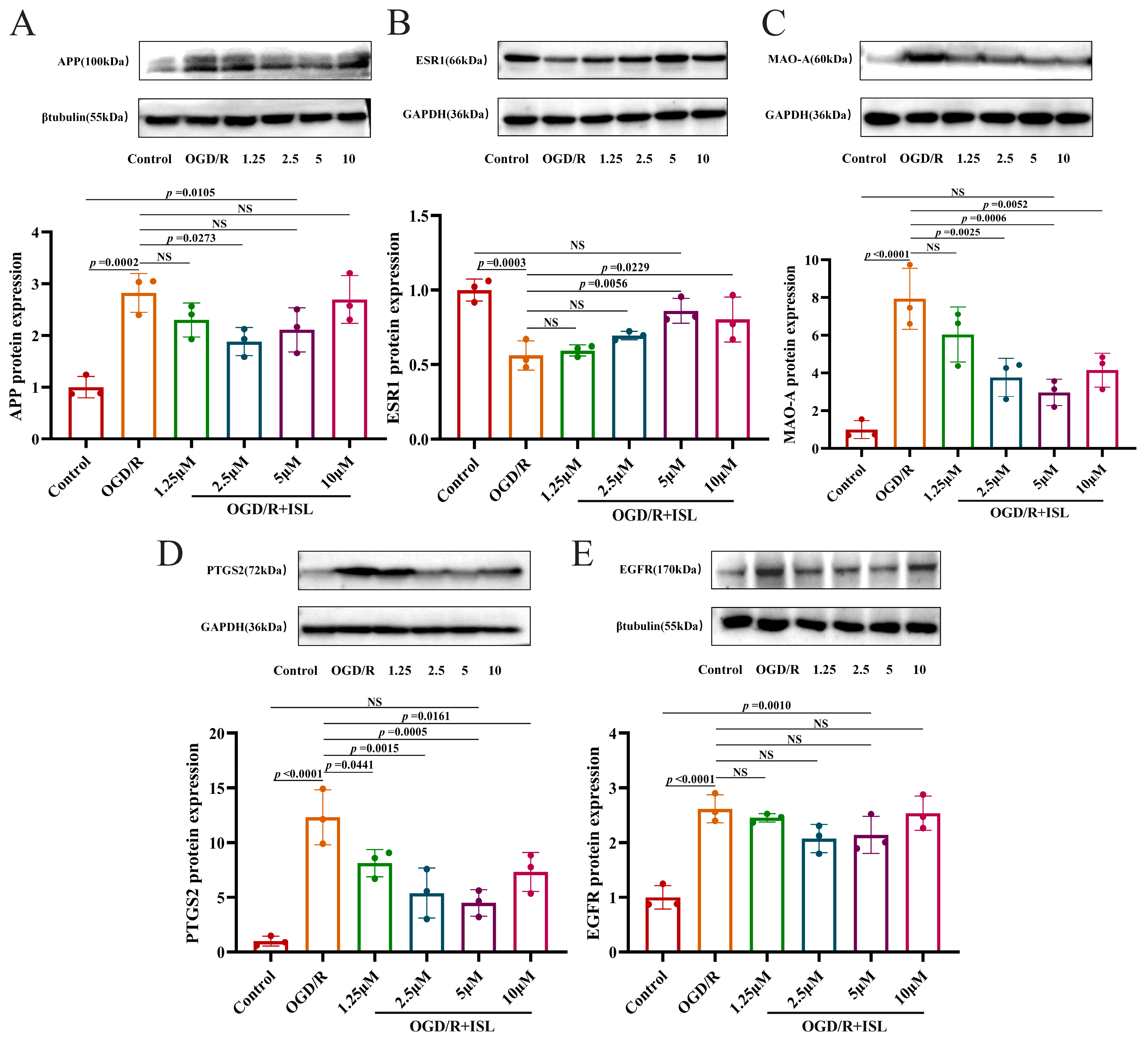
3.8. The Effect of ISL on the Protein Expression of APP, ESR1, MAO-A, PTGS2, and EGFR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Lin, Y.Q.; Wang, W.S.; Wang, X.Q. Excessive nNOS/NO/AMPK signaling activation mediated by the blockage of the CBS/H2S system contributes to oxygen-glucose deprivation-induced endoplasmic reticulum stress in PC12 cells. Int. J. Mol. Med. 2017, 40, 549–557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, R.-H.; Wang, H.; Long, C.-L.; Wang, H. Brain protection against ischemic stroke using choline as a new molecular bypass treatment. Acta Pharmacol. Sin. 2015, 36, 1416–1425. [Google Scholar] [CrossRef]
- Soria, F.N.; Pérez-Samartín, A.; Martin, A.; Gona, K.B.; Llop, J.; Szczupak, B.; Chara, J.C.; Matute, C.; Domercq, M. Extrasynaptic glutamate release through cystine/glutamate antiporter contributes to ischemic damage. J. Clin. Investig. 2014, 124, 3645–3655. [Google Scholar] [CrossRef]
- Bindal, P.; Kumar, V.; Kapil, L.; Singh, C.; Singh, A. Therapeutic management of ischemic stroke. N-S Arch. Pharmacol. 2024, 397, 2651–2679. [Google Scholar] [CrossRef]
- Samkaria, S.; Kumari, P. Wild Edible Flowers of Indian Himalayan Region, Their Traditional Uses and Potential Health Benefits: A Way Forward for Food and Nutritional Security. Plant Food Hum. Nutr. 2025, 80, 60. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J.; Chen, X.; Zhang, K.; Zhao, X.; Zhang, Y. Antidiabetic and antioxidant molecules of Ficus tikoua and their combined effect: Bioassay-guided isolation, in vitro and in silico analysis. Plant Food Hum. Nutr. 2025, 80, 87. [Google Scholar] [CrossRef]
- Hong, Z.; Cao, J.; Liu, D.; Liu, M.; Chen, M.; Zeng, F.; Qin, Z.; Wang, J.; Tao, T. Celastrol targeting Nedd4 reduces Nrf2-mediated oxidative stress in astrocytes after ischemic stroke. J. Pharm. Anal. 2023, 13, 156–169. [Google Scholar] [CrossRef]
- Younis, N.S.; Ghanim, A.M.H. The Protective Role of Celastrol in Renal Ischemia-Reperfusion Injury by Activating Nrf2/HO-1, PI3K/AKT Signaling Pathways, Modulating NF-κb Signaling Pathways, and Inhibiting ERK Phosphorylation. Cell Biochem. Biophys. 2022, 80, 191–202. [Google Scholar] [CrossRef]
- Subedi, L.; Gaire, B.P. Phytochemicals as regulators of microglia/macrophages activation in cerebral ischemia. Pharmacol. Res. 2021, 165, 105419. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, F.; Zhang, W.; Feng, Z.; Yang, Y.; Mei, Z. Novel insight into the therapeutical potential of flavonoids from traditional Chinese medicine against cerebral ischemia/reperfusion injury. Front. Pharmacol. 2024, 15, 1352760. [Google Scholar] [CrossRef]
- Lan, X.; Wang, Q.; Liu, Y.; You, Q.; Wei, W.; Zhu, C.; Hai, D.; Cai, Z.; Yu, J.; Zhang, J.; et al. Isoliquiritigenin alleviates cerebral ischemia-reperfusion injury by reducing oxidative stress and ameliorating mitochondrial dysfunction via activating the Nrf2 pathway. Redox Biol. 2024, 77, 103406. [Google Scholar] [CrossRef]
- Cui, L.; Ma, Z.; Li, W.; Ma, H.; Guo, S.; Wang, D.; Niu, Y. Inhibitory activity of flavonoids fraction from Astragalus membranaceus Fisch. ex Bunge stems and leaves on Bacillus cereus and its separation and purification. Front. Pharmacol. 2023, 14, 1183393. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, X.; Wang, R.; Pang, H.; Wang, J.; Liu, L. Determining the chemical profile of Caragana jubata (Pall.) Poir. by UPLC–QTOF–MS analysis and evaluating its anti-ischemic stroke effects. J. Ethnopharmacol. 2023, 309, 116275. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.M. Chemical Constituents of Traditional Tibetan Medicine Caragana changduensis. Master’s Thesis, Tianjin University, Tianjin, China, 2009. [Google Scholar]
- Mei, X.; Zhang, Q.; Shao, W.; Zheng, J.; Zhao, P.; Li, J. Targeted Inhibition of Aldose Reductase by Isoliquiritigenin Suppresses Fatty Acid Synthesis to Inhibit Macrophage M2 Polarization and Alleviate Pulmonary Fibrosis. J. Agric. Food Chem. 2025, 73, 18252–18267. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, T.; Zhou, L.; Lin, P.; Chen, Q.; Li, X.; He, R.; Yang, S.; Liu, Y.; Qi, F. Isoliquiritigenin alleviates abnormal sarcomere contraction and inflammation in myofascial trigger points via the IL-17RA/Act1/p38 pathway in rats. Phytomedicine 2025, 145, 156993. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Yang, J. Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Pharmacol. Res. 2006, 53, 303–309. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhu, P.Y.; Ma, J.R.; Wang, X.Y.; Wei, Q.; Gao, Y.M.; Zhang, D.Y.; Yuan, W.J. Antioxidant activity and mechanism of Centranthera grandiflora Benth roots based on network pharmacology and in vitro experiments. Nat. Prod. Res. Dev. 2024, 36, 155–166. [Google Scholar]
- Tao, L.; Ke, Z.P.; Wang, T.J.; Wang, Z.Z.; Cao, L.; Xiao, W. Frontier technologies and development trends of network pharmacology: A patent bibliometric analysis. China J. Chin. Mater. Med. 2025, 50, 3070–3078. [Google Scholar]
- Surovi, S.; Manobjyoti, B. Molecular Docking: Challenges, Advances and its Use in Drug Discovery Perspective. Curr. Drug Targets 2019, 20, 501–521. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, S.; He, R.; Liang, G. Application of Molecular Simulation Methods in Food Science: Status and Prospects. J. Agric. Food Chem. 2023, 71, 2684–2703. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.-L.; Nastou, K.-C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.-T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Otasek, D.; Morris, J.-H.; Bouças, J.; Pico, A.-R.; Demchak, B. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G., Jr.; Sherman, B.-T.; Hosack, D.-A.; Yang, J.; Gao, W.; Lane, H.-C.; Lempicki, R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ouyang, G.; Zhu, Y.; Ouyang, Z. Investigation of Scutellaria Barbata’s immunological mechanism against thyroid cancer using network pharmacology and experimental validation. Sci. Rep. 2025, 15, 2490. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.-H.; Zhang, H.; Pang, X.-W.; Chen, L.; Zhou, L.-Q.; Chen, M.; Tian, D.-S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- He, D.; Wang, P.; Liao, F.; Yu, L.; Gan, B. Cell membrane-coated biomimetic magnetic nanoparticles for the bio-specific extraction of components from Gualou Guizhi decoction exhibiting activities against oxygen-glucose deprivation/reperfusion injury. J. Pharm. Biomed. Anal. 2022, 209, 114528. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, W. Isoliquiritigenin regulates microglial M1/M2 polarisation by mediating the P38/MAPK pathway in cerebral stroke. J. Pharm. Pharmacol. 2023, 75, 828–836. [Google Scholar] [CrossRef]
- Song, W.; Bai, L.; Yang, Y.; Wang, Y.; Xu, P.; Zhao, Y.; Zhou, X.; Li, X.; Xue, M. Long-Circulation and Brain Targeted Isoliquiritigenin Micelle Nanoparticles: Formation, Characterization, Tissue Distribution, Pharmacokinetics and Effects for Ischemic Stroke. Int. J. Nanomed. 2022, 17, 3655–3670. [Google Scholar] [CrossRef]
- Shi, J.; Yang, S.-H.; Stubley, L.; Day, A.-L.; Simpkins, J.-W. Hypoperfusion induces overexpression of β-amyloid precursor protein mRNA in a focal ischemic rodent model. Brain Res. 2000, 853, 1–4. [Google Scholar] [CrossRef]
- Nihashi, T.; Inao, S.; Kajita, Y.; Kawai, T.; Sugimoto, T.; Niwa, M.; Kabeya, R.; Hata, N.; Hayashi, S.; Yoshida, J. Expression and Distribution of Beta Amyloid Precursor Protein and Beta Amyloid Peptide in Reactive Astrocytes After Transient Middle Cerebral Artery Occlusion. Acta Neurochir. 2001, 143, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-L.; Lin, K.-J.; Ho, M.-Y.; Chang, Y.-J.; Chang, C.-H.; Wey, S.-P.; Hsieh, C.-J.; Yen, T.-C.; Hsiao, I.-T.; Lee, T.-H. Amyloid deposition after cerebral hypoperfusion: Evidenced on [18F]AV-45 positron emission tomography. J. Neurol. Sci. 2012, 319, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Eckman, C.; Younkin, S.; Hsiao, K.-K.; Iadecola, C. Increased susceptibility to ischemic brain damage in transgenic mice overexpressing the amyloid precursor protein. J. Neurosci. 1997, 17, 7655–7661. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-L.; Tang, N.-L.-S.; Zhang, Y.-P.; Ji, L.-d.; Tam, C.-W.-C.; Lui, V.-W.-C.; Chiu, H.-F.-K.; Lam, L.-C.-W. Association of prostaglandin-endoperoxide synthase 2 (PTGS2) polymorphisms and Alzheimer’s disease in Chinese. Neurobiol. Aging 2008, 29, 856–860. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, Y.-L.; Ma, Y.-H.; Yu, H.-Y.; Zhao, X.-Z.; Zhang, L.-H.; Ge, S.-Q.; Zhang, G.-W.; Qin, X.-D. A network pharmacology approach to investigate the mechanism of Shuxuening injection in the treatment of ischemic stroke. J. Ethnopharmacol. 2020, 257, 112891. [Google Scholar] [CrossRef]
- Nakayama, M.; Uchimura, K.; Zhu, R.-L.; Nagayama, T.; Rose, M.-E.; Stetler, R.-A.; Isakson, P.-C.; Chen, J.; Graham, S.-H. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc. Natl. Acad. Sci. USA 1998, 95, 10954–10959. [Google Scholar] [CrossRef]
- Nogawa, S.; Zhang, F.; Ross, M.-E.; Iadecola, C. Cyclooxygenase-2 gene expression in neurons contributes to ischemic brain damage. J. Neurosci. 1997, 17, 2746–2755. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Y.; DeMars, K.-M.; Rosenberg, G.-A.; Candelario-Jalil, E. Genetic Deletion or Pharmacological Inhibition of Cyclooxygenase-2 Reduces Blood-Brain Barrier Damage in Experimental Ischemic Stroke. Front. Neurol. 2020, 11, 887. [Google Scholar] [CrossRef]
- Shih, J.-C.; Chen, K. Regulation of MAO-A and MAO-B Gene Expression. Curr. Med. Chem. 2004, 11, 1995–2005. [Google Scholar] [CrossRef]
- Wang, C.-C.; Borchert, A.; Ugun-Klusek, A.; Tang, L.-Y.; Lui, W.-T.; Chu, C.-Y.; Billett, E.; Kuhn, H.; Ufer, C. Monoamine Oxidase A Expression Is Vital for Embryonic Brain Development by Modulating Developmental Apoptosis. J. Biol. Chem. 2011, 286, 28322–28330. [Google Scholar] [CrossRef]
- Tong, J.; Meyer, J.-H.; Furukawa, Y.; Boileau, I.; Chang, L.-J.; Wilson, A.-A.; Houle, S. Distribution of Monoamine Oxidase Proteins in Human Brain: Implications for Brain Imaging Studies. J. Cereb. Blood Flow Metab. 2013, 33, 863–871. [Google Scholar] [CrossRef]
- Deshwal, S.; Di, S.-M.; Di, L.-F.; Kaludercic, N. Emerging role of monoamine oxidase as a therapeutic target for cardiovascular disease. Curr. Opin. Pharmacol. 2017, 33, 64–69. [Google Scholar] [CrossRef]
- He, P.; Wang, Z.; Yang, J.; Pan, P.; Shi, T.; Xu, S.; Lan, J.; Hao, Z.; Yang, A.; Chen, L.; et al. Mechanism of Ligusticum wallichii—Borneol in the Treatment of Cerebral Ischemic Stroke in Rats Based On Network Pharmacology, Molecular Docking, and Experimental Verification. Chem. Biodivers. 2025, 22, e202401893. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, X.; Qi, X.; Wang, G.; Ma, Y. Edaravone ameliorates inflammation in ischemic stroke mouse by regulating the CYP1A1 pathway through gut microbiota. Exp. Neurol. 2025, 390, 115263. [Google Scholar] [CrossRef] [PubMed]
- Crago, E.A.; Thampatty, B.P.; Sherwood, P.R.; Kuo, C.-W.J.; Bender, C.; Balzer, J.; Horowitz, M.; Poloyac, S.M. Cerebrospinal Fluid 20-HETE Is Associated with Delayed Cerebral Ischemia and Poor Outcomes After Aneurysmal Subarachnoid Hemorrhage. Stroke 2011, 42, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, W.; Hu, X.; Shen, Y.; Cao, J.; Wei, Z.; Luan, Y.; He, L.; Jiang, F.; Tao, Y. A role for ErbB signaling in the induction of reactive astrogliosis. Cell Discov. 2017, 3, 17044. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.-L.; Zhang, C.-X.; Zheng, Y.-X.; Chen, C.-A.; Dan, C.; Lan, X.; Yan, X.; Liu, Y.; He, Y.-H.; Cheng, J.-L.; et al. Refined qingkailing attenuates reactive astrocytes and glial scar formation after ischemia stroke via the EGFR/PLCγ pathway. Phytomedicine 2025, 142, 156696. [Google Scholar] [CrossRef]
- Luo, T.-T.; Lu, Y.; Yan, S.-K.; Xiao, X.; Rong, X.-L.; Guo, J. Network Pharmacology in Research of Chinese Medicine Formula: Methodology, Application and Prospective. Chin. J. Integr. Med. 2020, 26, 72–80. [Google Scholar] [CrossRef]
- Sun, R.; Liu, C.; Liu, J.; Yin, S.; Song, R.; Ma, J.; Cao, G.; Lu, Y.; Zhang, G.; Wu, Z.; et al. Integrated network pharmacology and experimental validation to explore the mechanisms underlying naringenin treatment of chronic wounds. Sci. Rep. 2023, 13, 132. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Q.; Yang, Q.; Wei, Q.; Man, N.; Adu-Frimpong, M.; Toreniyazov, E.; Ji, H.; Yu, J.; Xu, X. Enhancement of Oral Bioavailability and Anti-hyperuricemic Activity of Isoliquiritigenin via Self-Microemulsifying Drug Delivery System. AAPS PharmSciTech 2019, 20, 218. [Google Scholar] [CrossRef]


| Gene | Forward | Reverse |
|---|---|---|
| APP | TCCGAGAGGTGTGCTCTGAA | CCACATCCGCCGTAAAAGAATG |
| ESR1 | AAGACGCTCTTGAACCAGCA | AGGCTTTGGTGTGAAGGGTC |
| MAO-A | GCCCAGTATCACAGGCCAC | CGGGCTTCCAGAACCAAGA |
| PTGS2 | TGCACTATGGTTACAAAAGCTGG | TCAGGAAGCTCCTTATTTCCCTT |
| EGFR | CCGAAACTACGTGGTGACAGAT | TGCCATTACAAACTTTGCGAC |
| GAPDH | CACTCACGGCAAATTCAACGGCACA | GACTCCACGACATACTCAGCAC |
| Gene Name | Degree | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|
| PTGS2 | 24 | 320.2112364 | 28.5 |
| ESR1 | 22 | 218.4691729 | 27.5 |
| EGFR | 19 | 119.9617377 | 26 |
| APP | 11 | 114.8404762 | 22 |
| IGF1R | 11 | 18.46190476 | 21.83333333 |
| MAPK14 | 9 | 19.00417711 | 20.66666667 |
| MAOA | 8 | 26.03433584 | 20.5 |
| XDH | 8 | 33.59444444 | 20.33333333 |
| ACHE | 8 | 27.98145363 | 20.16666667 |
| GSTP1 | 8 | 24.95873016 | 20.16666667 |
| Target Protein | Binding Affinity (kcal·mol−1) | RMSD (Å) |
|---|---|---|
| APP | −5.80 | / |
| ESR1 | −8.60 | 1.019 |
| MAO-A | −8.80 | 1.275 |
| PTGS2 | −8.40 | 1.799 |
| EGFR | −7.90 | 1.677 |
| Protein | ΔGbind (MM-GBSA) |
|---|---|
| APP | −41.3 ± 3.4 |
| EGFR | −40.9 ± 5.1 |
| MAO-A | −46.9 ± 6.2 |
| ESR1 | −31.7 ± 3.7 |
| PTGS2 | −26.4 ± 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Hou, Y.; Jiao, Y.; Lu, X.; Liu, L. An Integrated Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and Experimental Validation Study to Investigate the Potential Mechanism of Isoliquiritigenin in the Treatment of Ischemic Stroke. Curr. Issues Mol. Biol. 2025, 47, 627. https://doi.org/10.3390/cimb47080627
Yuan H, Hou Y, Jiao Y, Lu X, Liu L. An Integrated Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and Experimental Validation Study to Investigate the Potential Mechanism of Isoliquiritigenin in the Treatment of Ischemic Stroke. Current Issues in Molecular Biology. 2025; 47(8):627. https://doi.org/10.3390/cimb47080627
Chicago/Turabian StyleYuan, Hang, Yuting Hou, Yuan Jiao, Xin Lu, and Liang Liu. 2025. "An Integrated Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and Experimental Validation Study to Investigate the Potential Mechanism of Isoliquiritigenin in the Treatment of Ischemic Stroke" Current Issues in Molecular Biology 47, no. 8: 627. https://doi.org/10.3390/cimb47080627
APA StyleYuan, H., Hou, Y., Jiao, Y., Lu, X., & Liu, L. (2025). An Integrated Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and Experimental Validation Study to Investigate the Potential Mechanism of Isoliquiritigenin in the Treatment of Ischemic Stroke. Current Issues in Molecular Biology, 47(8), 627. https://doi.org/10.3390/cimb47080627







