Multifunctional Dermatological Effects of Whole-Plant Bassia scoparia Extract: Skin Repair and Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of WPBS Extracts
2.2. LC-QTOF-MS Analyses
2.3. Cell Line and Cell Culture (Keratinocyte HaCaT)
2.3.1. Cell Viability Assay
2.3.2. Moisturizing Model and Treatment
2.3.3. Inflammatory Model and Treatment
2.4. Fibroblast (Hs68) Cell Culture for Treatment
2.4.1. UVB-Induced Photoaging Model and Treatment
2.4.2. Wound-Healing (Migration) Assay
2.4.3. Anti-Angiogenic Model and Treatment
2.5. RNA Extraction and Real-Time PCR
2.6. RNA Sequencing and Bioinformatic Analysis
2.6.1. Sample Preparation for RNA-Seq
2.6.2. Library Construction and Sequencing
2.6.3. Differential Gene Expression and Pathway Analysis
2.7. Statistical Analysis
3. Results
3.1. Identification of Bioactive Compounds in WPBS Extract
3.2. Effect of WPBS on Cell Viability
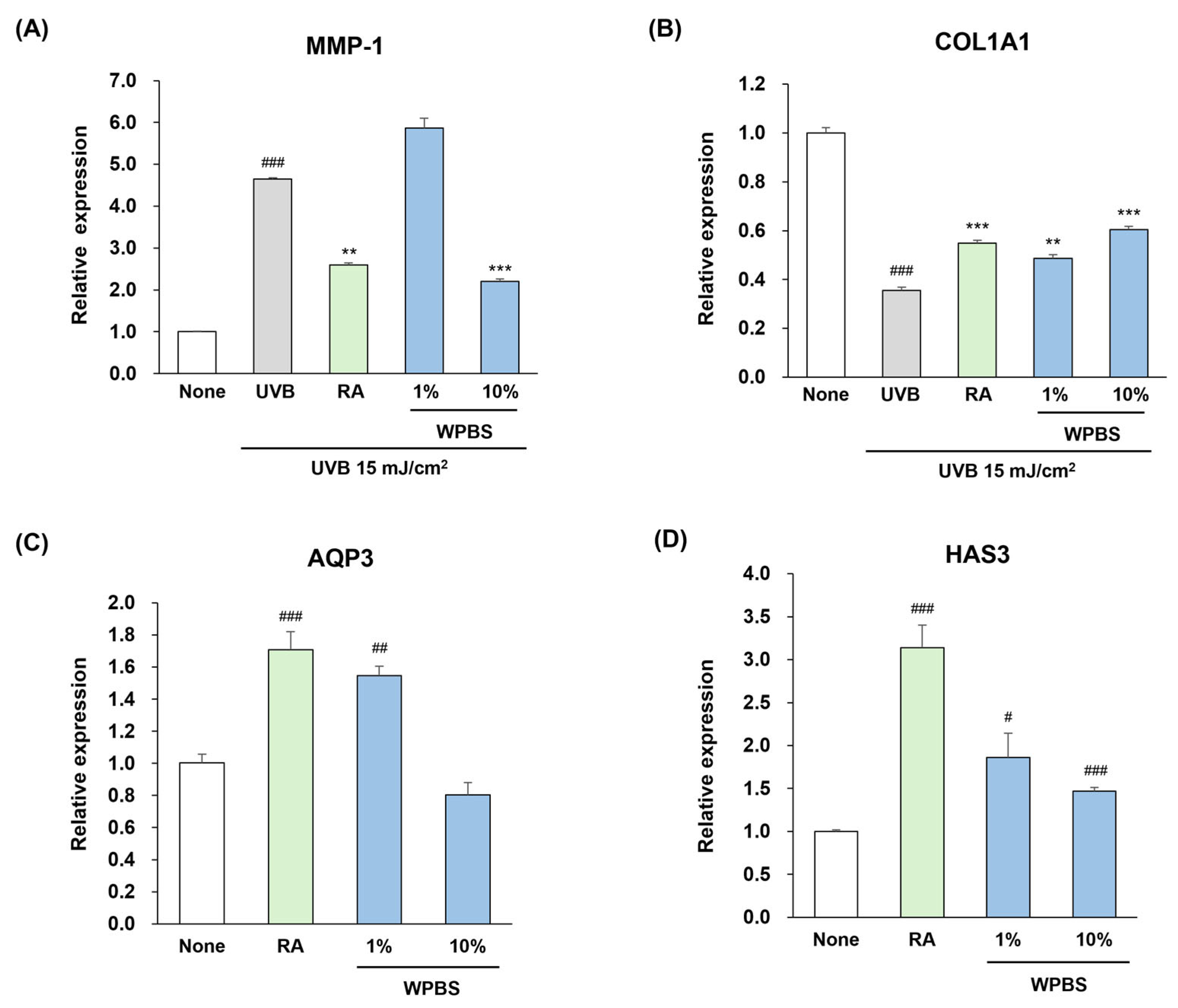
3.3. Anti-Photoaging Effects of WPBS
3.4. Moisturizing Effects of WPBS
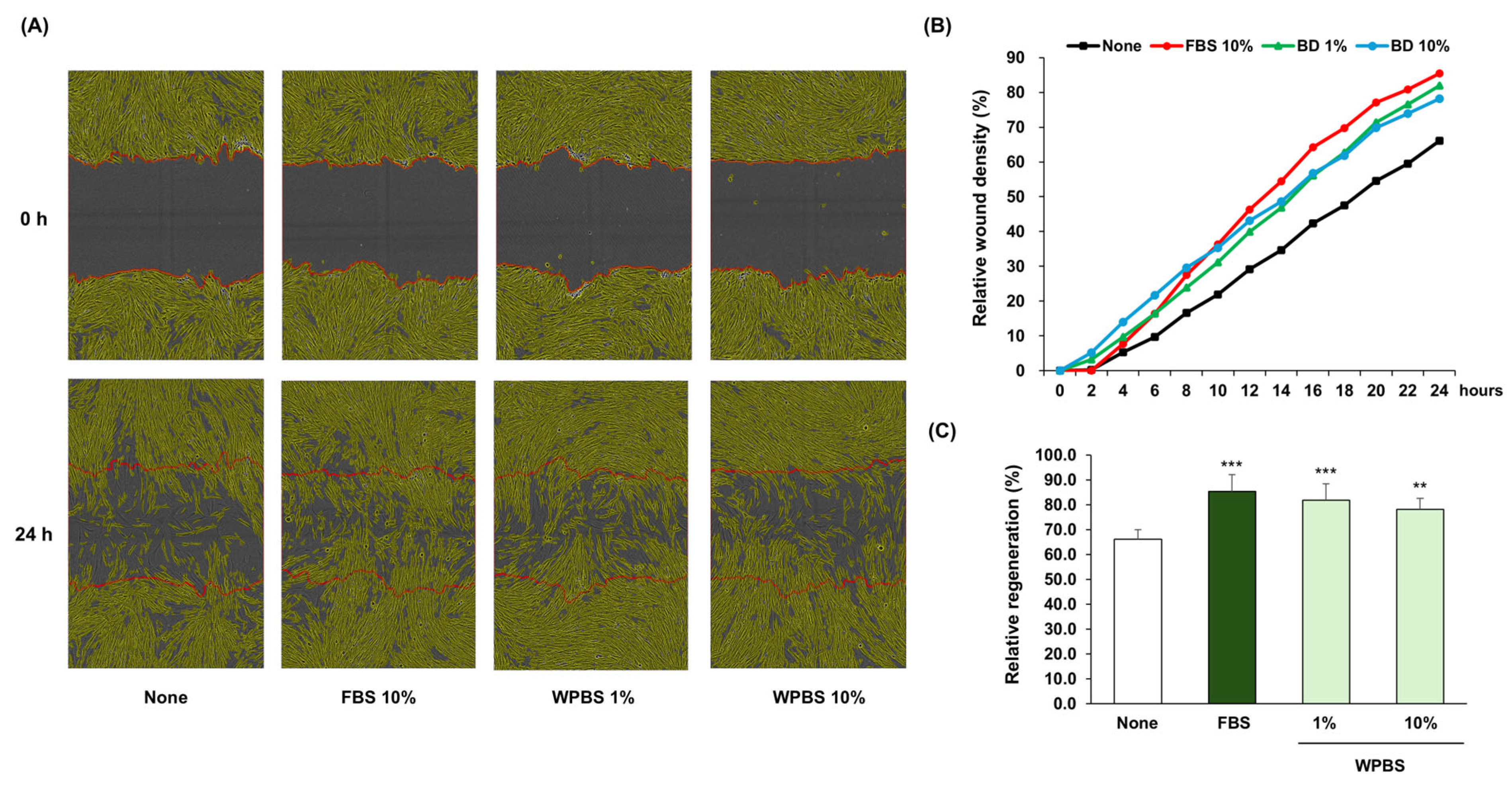
3.5. Wound-Healing Effects of WPBS
3.6. Anti-Inflammatory and Anti-Angiogenic Effects of WPBS
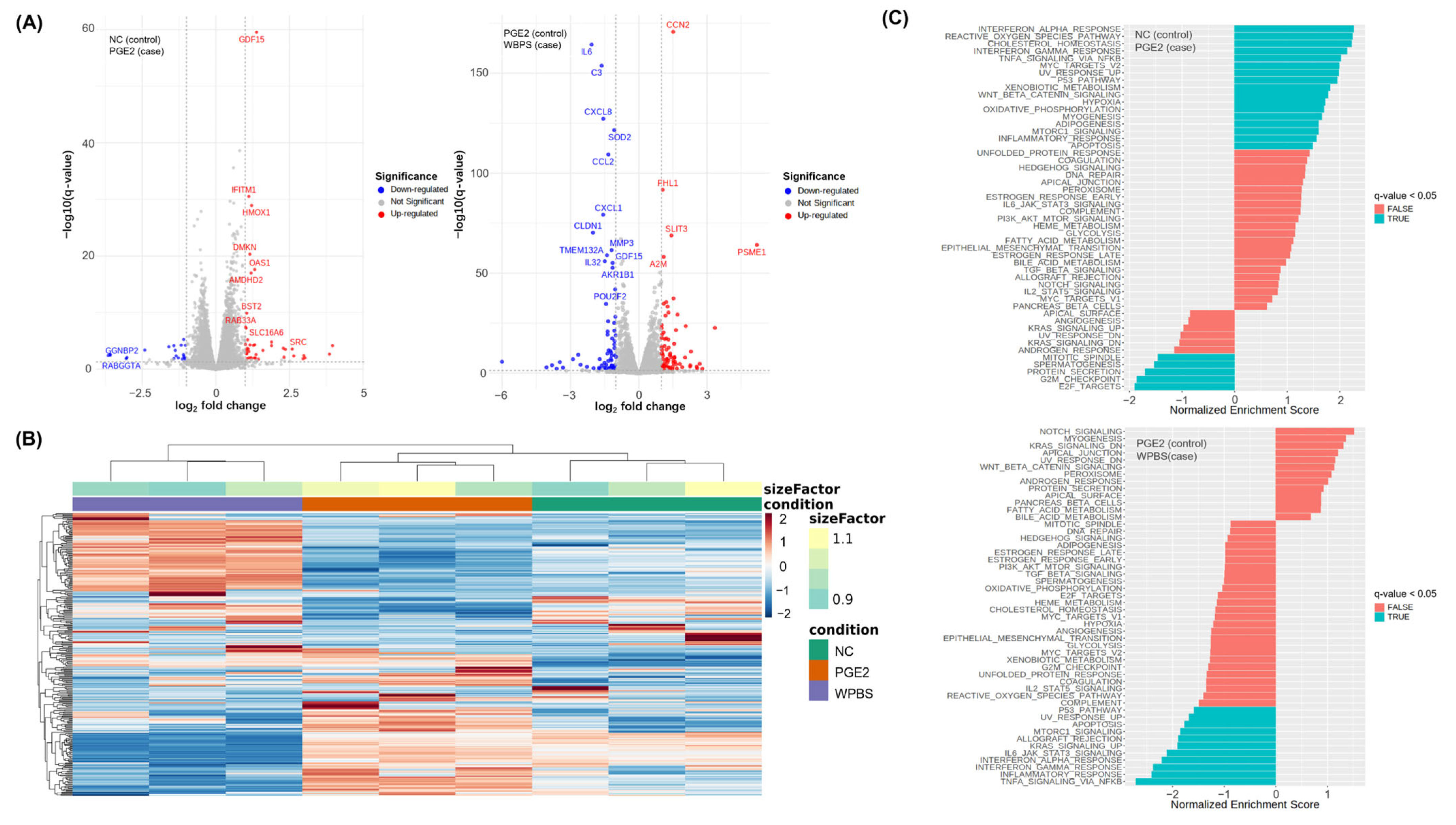
3.7. RNA-Seq-Based Transcriptomic Analysis of WPBS Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| AQP3 | Aquaporin-3 |
| C3 | Complement C3 |
| cDNA | Complementary DNA |
| COL1A1 | collagen type I alpha 1 |
| COX-2 | cyclooxygenase-2 |
| CXCL8 | Interleukin-8 |
| DEGs | differentially expressed genes |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| FBS | fetal bovine serum |
| GABA | gamma-aminobutyric acid |
| GDF15 | Growth Differentiation Factor 15 |
| GSEA | gene set enrichment analysis |
| HaCaT | human adult low calcium temperature keratinocytes |
| HMOX1 | Heme Oxygenase 1 |
| HAS3 | hyaluronan synthase-3 |
| Hs68 | human foreskin fibroblasts |
| IFITM1 | Interferon-Induced Transmembrane Protein 1 |
| IL6 | Interleukin 6 |
| iNOS | inducible NO synthase |
| KF | Kochiae Fructus |
| LC-QTOF-MS | Liquid Chromatography—Quadrupole Time-of-Flight—Mass Spectrometry |
| LPS | lipopolysaccharide |
| NC | untreated control |
| NO | nitric oxide |
| MMP-1 | matrix metalloproteinase-1 |
| MMP-13 | matrix metalloproteinase-13 |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide |
| PABA | 4-aminobenzoic acid |
| PGE2 | prostaglandin E2 |
| poly I:C | polyinosinic-polycytidylic acid |
| qPCR | quantitative real-time PCR |
| RA | retinoic acid |
| TGF-β | transforming growth factor-beta |
| TLR3 | toll-like receptor 3 |
| TNF- α | tumor necrosis factor-α |
| UV | ultraviolet |
| UVB | ultraviolet B |
| VEGF | vascular endothelial growth factor |
| WPBS | whole-plant Bassia scoparia |
References
- Kim, N.-Y.; Lee, M.-K.; Park, M.-J.; Kim, S.-J.; Park, H.-J.; Choi, J.-W.; Kim, S.-H.; Cho, S.-Y.; Lee, J.-S. Momordin Ic and oleanolic acid from Kochiae Fructus reduce carbon tetrachloride-induced hepatotoxicity in rats. J. Med. Food 2005, 8, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, K.-T.; Jung, H.-J.; Park, H.-S.; Park, H.-J. Anti-rheumatoid arthritis effect of the Kochia scoparia fruits and activity comparison of momordin Ic, its prosapogenin and sapogenin. Arch. Pharmacal Res. 2002, 25, 336–342. [Google Scholar] [CrossRef]
- Sukhorukov, A.P.; Wen, Z.; Krinitsina, A.A.; Fedorova, A.V.; Verloove, F.; Kushunina, M.; Léger, J.-F.; Chambouleyron, M.; Tanji, A.; Sennikov, A.N. A Revised Taxonomy of the Bassia scoparia Complex (Camphorosmoideae, Amaranthaceae sl) with an Updated Distribution of B. indica in the Mediterranean Region. Plants 2025, 14, 398. [Google Scholar] [CrossRef]
- Zou, W.; Tang, Z.; Long, Y.; Xiao, Z.; Ouyang, B.; Liu, M. Kochiae fructus, the fruit of common potherb Kochia scoparia (L.) Schrad: A review on phytochemistry, pharmacology, toxicology, quality control, and pharmacokinetics. Evid.-Based Complement. Altern. Med. 2021, 2021, 5382684. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, D.H.; Nho, Y.-H.; Park, J.-E.; Kim, S.-N.; Choi, E.H. A mixture of extracts of Kochia scoparia and Rosa multiflora with PPAR α/γ dual agonistic effects prevents photoaging in hairless mice. Int. J. Mol. Sci. 2016, 17, 1919. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Photoaging. J. Investig. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef]
- Chen, C.-L.; Liou, S.-F.; Chen, S.-J.; Shih, M.-F. Protective effects of Chlorella-derived peptide on UVB-induced production of MMP-1 and degradation of procollagen genes in human skin fibroblasts. Regul. Toxicol. Pharmacol. 2011, 60, 112–119. [Google Scholar] [CrossRef]
- Jo, S.; Ryu, J.; Han, H.-Y.; Lee, G.; Ryu, M.H.; Kim, H. Anti-inflammatory activity of Kochia scoparia fruit on contact dermatitis in mice. Mol. Med. Rep. 2016, 13, 1695–1700. [Google Scholar] [CrossRef]
- Shin, K.-M.; Kim, Y.-H.; Park, W.-S.; Kang, I.; Ha, J.; Choi, J.-W.; Park, H.-J.; Lee, K.-T. Inhibition of methanol extract from the fruits of Kochia scoparia on lipopolysaccharide-induced nitric oxide, prostagladin E2, and tumor necrosis factor-α production from murine macrophage RAW 264.7 cells. Biol. Pharm. Bull. 2004, 27, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-D.; Kim, J.-H.; Park, J.-K.; Hong, S.-M.; Kim, D.-H.; Seo, K.-I. Kochia scoparia seed extract suppresses VEGF-induced angiogenesis via modulating VEGF receptor 2 and PI3K/AKT/mTOR pathways. Pharm. Biol. 2019, 57, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Ge, J.-Y.; Huang, X.-Y.; Liu, X.-M.; Liao, Y.; Zhang, S.-J.; Wu, L.; Chen, X.-F.; Yu, B. Isosilybin A exhibits anti-inflammatory properties in rosacea by inhibiting MAPK pathway and M1 macrophage polarization. Int. Immunopharmacol. 2024, 143, 113323. [Google Scholar] [CrossRef]
- Farag, M.A.; Baky, M.H.; Morgan, I.; Khalifa, M.R.; Rennert, R.; Mohamed, O.G.; El-Sayed, M.M.; Porzel, A.; Wessjohann, L.A.; Ramadan, N.S. Comparison of Balanites aegyptiaca parts: Metabolome providing insights into plant health benefits and valorization purposes as analyzed using multiplex GC-MS, LC-MS, NMR-based metabolomics, and molecular networking. RSC Adv. 2023, 13, 21471–21493. [Google Scholar] [CrossRef]
- Liu, J.; Mu, X.; Liang, J.; Zhang, J.; Qiang, T.; Li, H.; Li, B.; Liu, H.; Zhang, B. Metabolic profiling on the analysis of different parts of Schisandra chinensis based on UPLC-QTOF-MS with comparative bioactivity assays. Front. Plant Sci. 2022, 13, 970535. [Google Scholar] [CrossRef] [PubMed]
- Kanodia, L.; Das, S. A comparative study of analgesic property of whole plant and fruit extracts of Fragaria vesca in experimental animal models. Bangladesh J. Pharmacol. 2009, 4, 35–38. [Google Scholar] [CrossRef]
- Nantia, A.; Soh, D.; Choumessi, T.; Ngum, N.; Chi, H.; Kenfack, A. In vitro antioxidant property of the methanol extracts of the whole plant and fruit of Momordica foetida (Cucurbitaceae). Pharm. Chem. J. 2018, 5, 117–125. [Google Scholar]
- Chaachouay, N. Synergy, Additive Effects, and Antagonism of Drugs with Plant Bioactive Compounds. Drugs Drug Candidates 2025, 4, 4. [Google Scholar] [CrossRef]
- Costa, E.F.; Magalhães, W.V.; Di Stasi, L.C. Recent advances in herbal-derived products with skin anti-aging properties and cosmetic applications. Molecules 2022, 27, 7518. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Cui, H.; Liu, G.; Zhao, X.; Li, W.; Piao, G. How can synergism of traditional medicines benefit from network pharmacology? Molecules 2017, 22, 1135. [Google Scholar] [CrossRef]
- Sayo, T.; Sakai, S.; Inoue, S. Synergistic effect of N-acetylglucosamine and retinoids on hyaluronan production in human keratinocytes. Ski. Pharmacol. Physiol. 2004, 17, 77–83. [Google Scholar] [CrossRef]
- Bellemere, G.; Von Stetten, O.; Oddos, T. Retinoic acid increases aquaporin 3 expression in normal human skin. J. Investig. Dermatol. 2008, 128, 542–548. [Google Scholar] [CrossRef]
- Choi, E.; Kang, Y.-G.; Hwang, S.-H.; Kim, J.K.; Hong, Y.D.; Park, W.-S.; Kim, D.; Kim, E.; Cho, J.Y. In vitro effects of dehydrotrametenolic acid on skin barrier function. Molecules 2019, 24, 4583. [Google Scholar] [CrossRef]
- Jahid, M.; Chawla, D.; Avasthi, R.; Ahmed, R.S. Association of polymorphic variants in IL1B gene with secretion of IL-1β protein and inflammatory markers in north Indian rheumatoid arthritis patients. Gene 2018, 641, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, M.B.; Xie, S.; Khorasani, N.M.; Kon, O.M.; Stanbridge, R.; Issa, R.; Chung, K.F. Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J. Allergy Clin. Immunol. 2006, 118, 641–648. [Google Scholar] [CrossRef]
- Chouinard, N.; Rouabhia, M. Effects of all-trans retinoic acid on UVB-irradiated human skin substitute. J. Cell. Physiol. 1999, 181, 14–23. [Google Scholar] [CrossRef]
- Kim, K.; Kim, C.-E.; Baek, D.-J.; Park, E.-Y.; Oh, Y.S. Prevention of UVB-Induced Photoaging by an Ethyl Acetate Fraction from Allomyrina dichotoma Larvae and Its Potential Mechanisms in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2024, 25, 7850. [Google Scholar] [CrossRef]
- Mehra, V.C.; Jackson, E.; Zhang, X.M.; Jiang, X.-C.; Dobrucki, L.W.; Yu, J.; Bernatchez, P.; Sinusas, A.J.; Shulman, G.I.; Sessa, W.C. Ceramide-activated phosphatase mediates fatty acid–induced endothelial VEGF resistance and impaired angiogenesis. Am. J. Pathol. 2014, 184, 1562–1576. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Korotkevich, G.; Sukhov, V.; Budin, N.; Shpak, B.; Artyomov, M.N.; Sergushichev, A. Fast gene set enrichment analysis. bioRxiv 2016. bioRxiv:060012. [Google Scholar]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The molecular signatures database hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Han, D.; Kim, H.-Y.; Lee, H.-J.; Shim, I.; Hahm, D.-H. Wound healing activity of gamma-aminobutyric Acid (GABA) in rats. J. Microbiol. Biotechnol. 2007, 17, 1661–1669. [Google Scholar] [PubMed]
- Sigruener, A.; Tarabin, V.; Paragh, G.; Liebisch, G.; Koehler, T.; Farwick, M.; Schmitz, G. Effects of sphingoid bases on the sphingolipidome in early keratinocyte differentiation. Exp. Dermatol. 2013, 22, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, H.-Y.; Jeong, T.-S. Pheophorbide a derivatives exert antiwrinkle effects on UVB-induced skin aging in human fibroblasts. Life 2021, 11, 147. [Google Scholar] [CrossRef]
- Tiwari, R.; Pathak, K. Local drug delivery strategies towards wound healing. Pharmaceutics 2023, 15, 634. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-Y.; Shin, J.-C.; Park, S.-M.; Kim, N.-R.; Kwak, W.; Choi, B.-H. Pinus densiflora extract protects human skin fibroblasts against UVB-induced photoaging by inhibiting the expression of MMPs and increasing type I procollagen expression. Toxicol. Rep. 2014, 1, 658–666. [Google Scholar] [CrossRef]
- Bl, Z.-G. UVB-irradiated human keratinocytes and interleukin-1α indirectly increase MAP kinase/AP-1 activation and MMP-1 production in UVA-irradiated dermal fibroblasts. Chin. Med. J. 2006, 119, 827–831. [Google Scholar]
- Karadeniz, F.; Oh, J.H.; Kim, H.R.; Ko, J.; Kong, C.-S. Camellioside A, isolated from Camellia japonica flowers, attenuates UVA-induced production of MMP-1 in HaCaT keratinocytes via suppression of MAPK activation. Exp. Ther. Med. 2021, 21, 16. [Google Scholar] [CrossRef]
- Schrader, A.; Siefken, W.; Kueper, T.; Breitenbach, U.; Gatermann, C.; Sperling, G.; Biernoth, T.; Scherner, C.; Stäb, F.; Wenck, H. Effects of glyceryl glucoside on AQP3 expression, barrier function and hydration of human skin. Ski. Pharmacol. Physiol. 2012, 25, 192–199. [Google Scholar] [CrossRef]
- Sayo, T.; Sugiyama, Y.; Takahashi, Y.; Ozawa, N.; Sakai, S.; Inoue, S.; Ishikawa, O.; Tamura, M. Hyaluronan synthase 3 regulates hyaluronan synthesis in cultured human keratinocytes. J. Investig. Dermatol. 2002, 118, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Sparavigna, A. The 24-hour skin hydration and barrier function effects of a hyaluronic 1%, glycerin 5%, and Centella asiatica stem cells extract moisturizing fluid: An intra-subject, randomized, assessor-blinded study. Clin. Cosmet. Investig. Dermatol. 2017, 10, 311–315. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Zhong, W.; Liang, F.; Guo, Y.; Wang, Y.; Wang, Z. Moisturizing and antioxidant effects of Artemisia argyi essence liquid in HaCaT keratinocytes. Int. J. Mol. Sci. 2023, 24, 6809. [Google Scholar] [CrossRef]
- Sugimoto, M.; Arai, I.; Futaki, N.; Hashimoto, Y.; Honma, Y.; Nakaike, S. Role of COX-1 and COX-2 on skin PGs biosynthesis by mechanical scratching in mice. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 1–8. [Google Scholar] [CrossRef]
- Kopp, K.L.M.; Kauczok, C.S.; Lauenborg, B.; Krejsgaard, T.; Eriksen, K.W.; Zhang, Q.; Wasik, M.; Geisler, C.; Ralfkiaer, E.; Becker, J. COX-2-dependent PGE2 acts as a growth factor in mycosis fungoides (MF). Leukemia 2010, 24, 1179–1185. [Google Scholar] [CrossRef]
- Neisius, U.; Olssonb, R.; Rukwied, R.; Lischetzki, G.; Schmelz, M. Prostaglandin E2 induces vasodilation and pruritus, but no protein extravasation in atopic dermatitis and controls. J. Am. Acad. Dermatol. 2002, 47, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Giblin, M.J.; Smith, T.E.; Winkler, G.; Pendergrass, H.A.; Kim, M.J.; Capozzi, M.E.; Yang, R.; McCollum, G.W.; Penn, J.S. Nuclear factor of activated T-cells (NFAT) regulation of IL-1β-induced retinal vascular inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166238. [Google Scholar] [CrossRef]
- Kajiya, K.; Hirakawa, S.; Detmar, M. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am. J. Pathol. 2006, 169, 1496–1503. [Google Scholar] [CrossRef]
- Lephart, E.D.; Naftolin, F. Menopause and the skin: Old favorites and new innovations in cosmeceuticals for estrogen-deficient skin. Dermatol. Ther. 2021, 11, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. A role for estrogen in skin ageing and dermal biomechanics. Mech. Ageing Dev. 2021, 197, 111513. [Google Scholar] [CrossRef]
- Koussounadis, A.; Langdon, S.P.; Um, I.H.; Harrison, D.J.; Smith, V.A. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci. Rep. 2015, 5, 10775. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Kim, M.H.; Lee, J.Y.; Hong, J.; Kim, S.-H.; Yang, W.M. Topical application of Kochia scoparia inhibits the development of contact dermatitis in mice. J. Ethnopharmacol. 2014, 154, 380–385. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Kim, H.-B.; Lee, D.-G.; Park, E.; Kyung, S.; Kang, S.; Roo, D.; Moh, S.H.; Jang, S.J.; Jang, J.; et al. Multifunctional Dermatological Effects of Whole-Plant Bassia scoparia Extract: Skin Repair and Protection. Curr. Issues Mol. Biol. 2025, 47, 617. https://doi.org/10.3390/cimb47080617
Jeong S, Kim H-B, Lee D-G, Park E, Kyung S, Kang S, Roo D, Moh SH, Jang SJ, Jang J, et al. Multifunctional Dermatological Effects of Whole-Plant Bassia scoparia Extract: Skin Repair and Protection. Current Issues in Molecular Biology. 2025; 47(8):617. https://doi.org/10.3390/cimb47080617
Chicago/Turabian StyleJeong, Seogyun, Hye-Been Kim, Dong-Geol Lee, Eunjin Park, Seoyeon Kyung, Seunghyun Kang, Dayeon Roo, Sang Hyun Moh, Sung Joo Jang, Jihyeon Jang, and et al. 2025. "Multifunctional Dermatological Effects of Whole-Plant Bassia scoparia Extract: Skin Repair and Protection" Current Issues in Molecular Biology 47, no. 8: 617. https://doi.org/10.3390/cimb47080617
APA StyleJeong, S., Kim, H.-B., Lee, D.-G., Park, E., Kyung, S., Kang, S., Roo, D., Moh, S. H., Jang, S. J., Jang, J., Jo, H., & Lee, S. (2025). Multifunctional Dermatological Effects of Whole-Plant Bassia scoparia Extract: Skin Repair and Protection. Current Issues in Molecular Biology, 47(8), 617. https://doi.org/10.3390/cimb47080617








