Molecular Targets for Pharmacotherapy of Head and Neck Squamous Cell Carcinomas
Abstract
1. Introduction
2. Chemotherapy
| Drug Name | Category | Subcategory | Mechanism of Action | Dose of Drug by Body Surface Area (BSA) | Primary Site of Drug Metabolism | References |
|---|---|---|---|---|---|---|
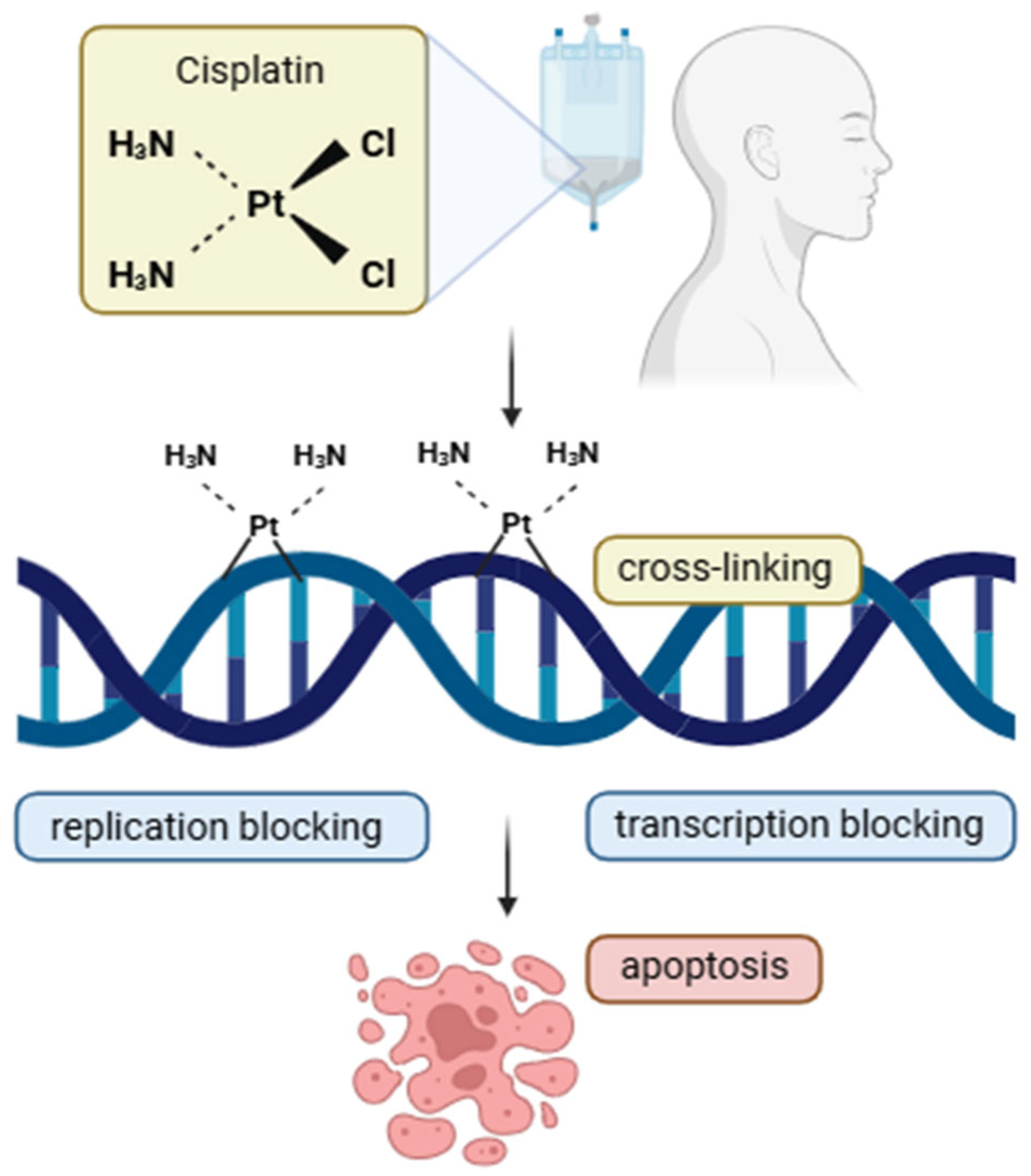
| Cisplatin | Cytotoxic antineoplastic agents | Platinum-based | Cisplatin is a platinum compound that forms covalent DNA crosslinks in cancer cells, blocking replication and transcription. This triggers repair mechanisms that, if overwhelmed, induce apoptosis. It acts through a classical alkylating agent mechanism with notable effectiveness in tumors. | 200 mg/m2—Cumulative dose of cisplatin (CRT schema) 100 mg/m2—Triweekly high dose of cisplatin (CRT schema) 40 mg/m2—Weekly dose of cisplatin (CRT schema) | Kidney | [8,37,38] |
| Carboplatin | Cytotoxic antineoplastic agents | Platinum-based | Carboplatin, a cisplatin analog, forms DNA crosslinks in cancer cells but has lower chemical reactivity. It causes less nephro- and neurotoxicity but greater myelosuppression. | 100 mg/m2—Weekly dose of carboplatin (CRT schema) | Kidney | [38,39] |
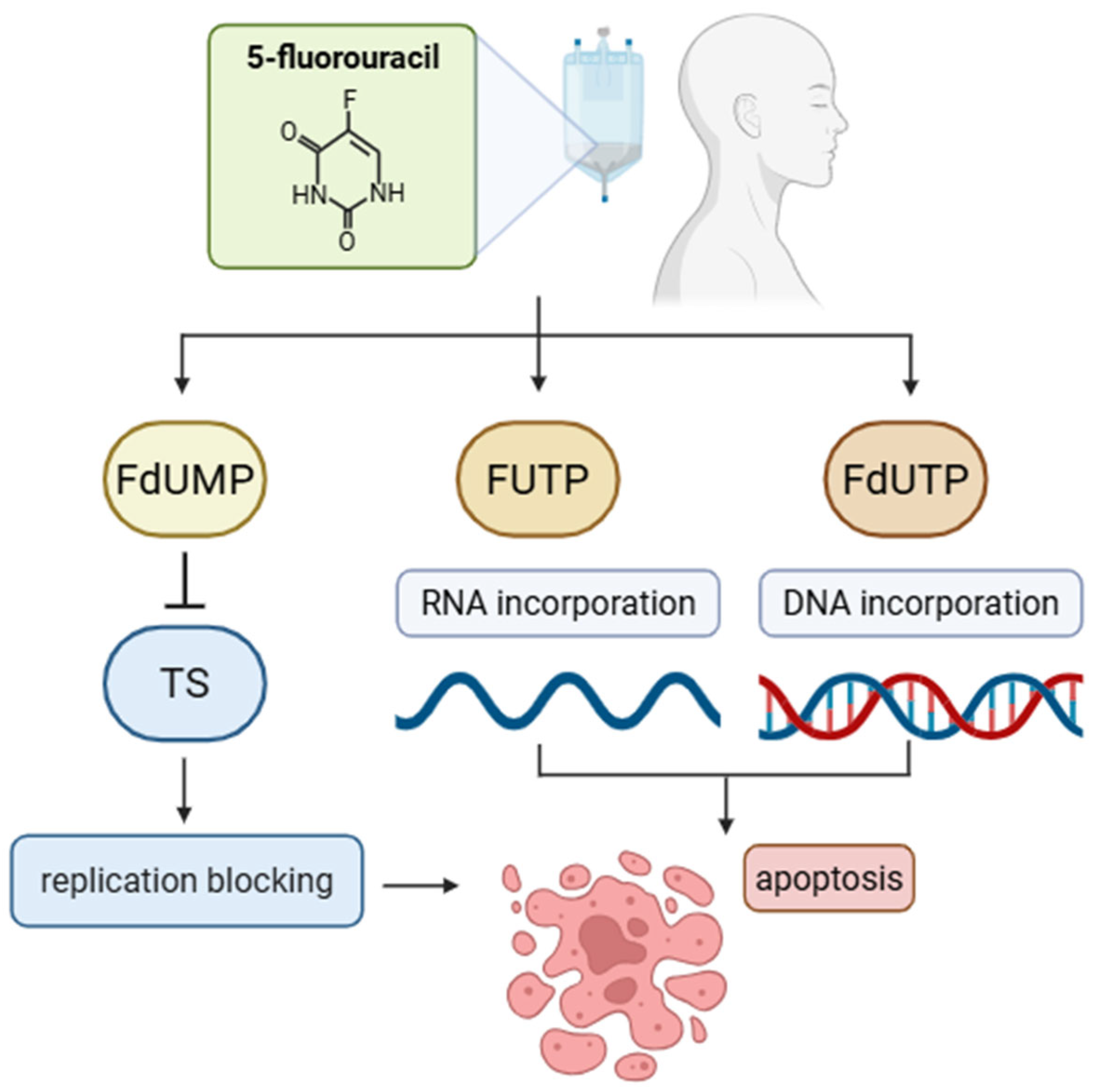
| 5-Fluorouracil | Antimetabolite | Pyrimidine analog | Inhibits thymidylate synthase, blocks DNA synthesis; incorporates into RNA, disrupts RNA function. | 250 mg/m2—earlier standard dose of 5-FU (per 5 days) 1000 mg/m2—standard dose of 5-FU (per 4 days) | Liver | [32,33,40,41] |
| Docetaxel | Antimitotic agent | Taxane | Stabilizes microtubules, prevents their depolymerization, and arrests cell division in mitosis. | 75 mg/m2—standard dose of docetaxel in combination chemotherapy (TPF) | Liver | [35,42] |
| Type of Combination Therapy | Drugs | Indication | References |
|---|---|---|---|
| DC | Cisplatin, docetaxel | First-line treatment of recurrent/metastatic HNSCC | [46] |
| PF | Cisplatin, 5-FU | Induction therapy | [47] |
| TPF | Cisplatin, 5-FU, docetaxel | Induction therapy | [44] |
| TPEx | Cisplatin, cetuximab, docetaxel | First-line treatment of recurrent/metastatic HNSCC | [48] |
| EXTREME | Cisplatin, 5-FU, cetuximab | First-line treatment of recurrent/metastatic HNSCC | [49] |
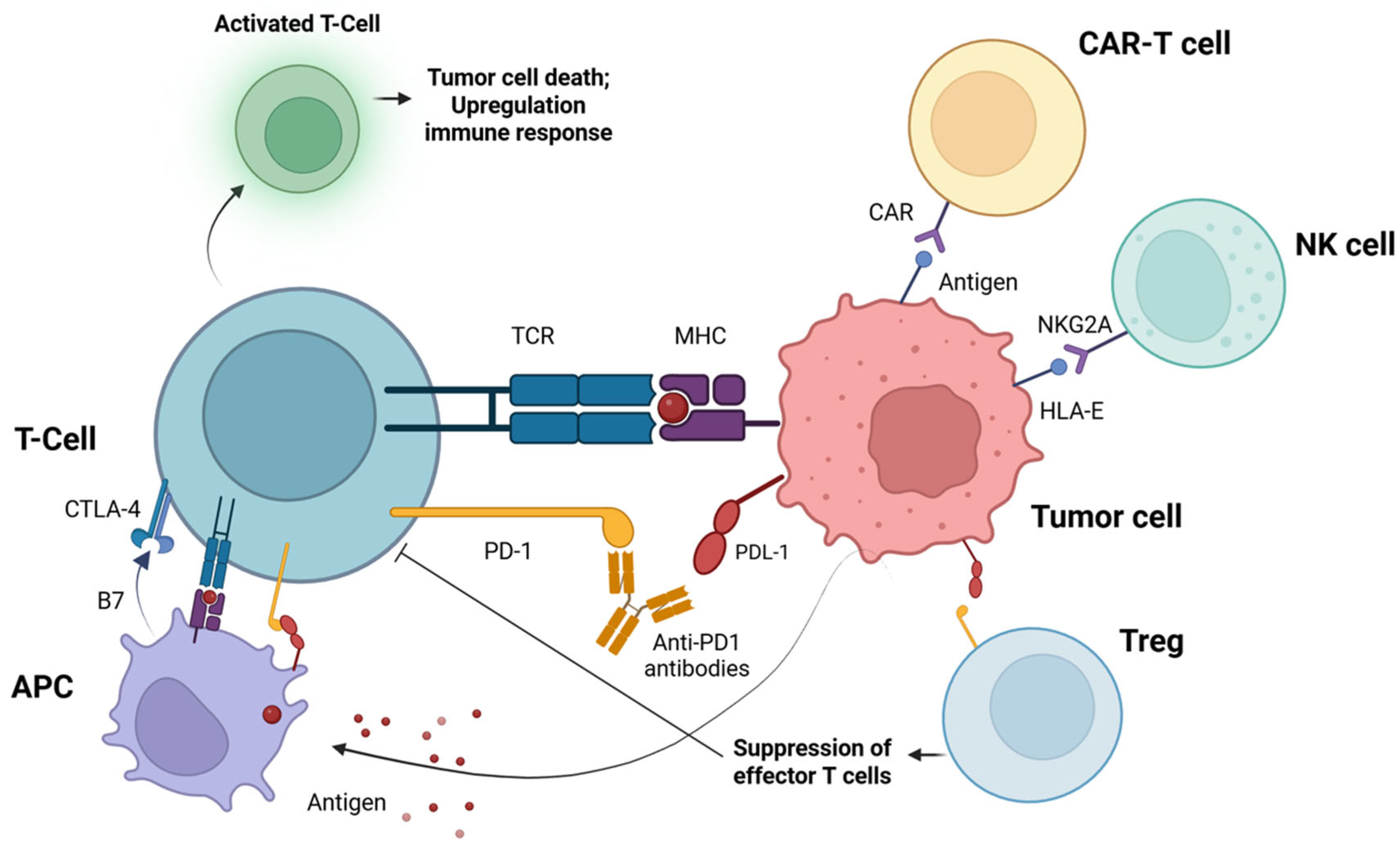
3. Immunotherapy
4. CDK Inhibitors
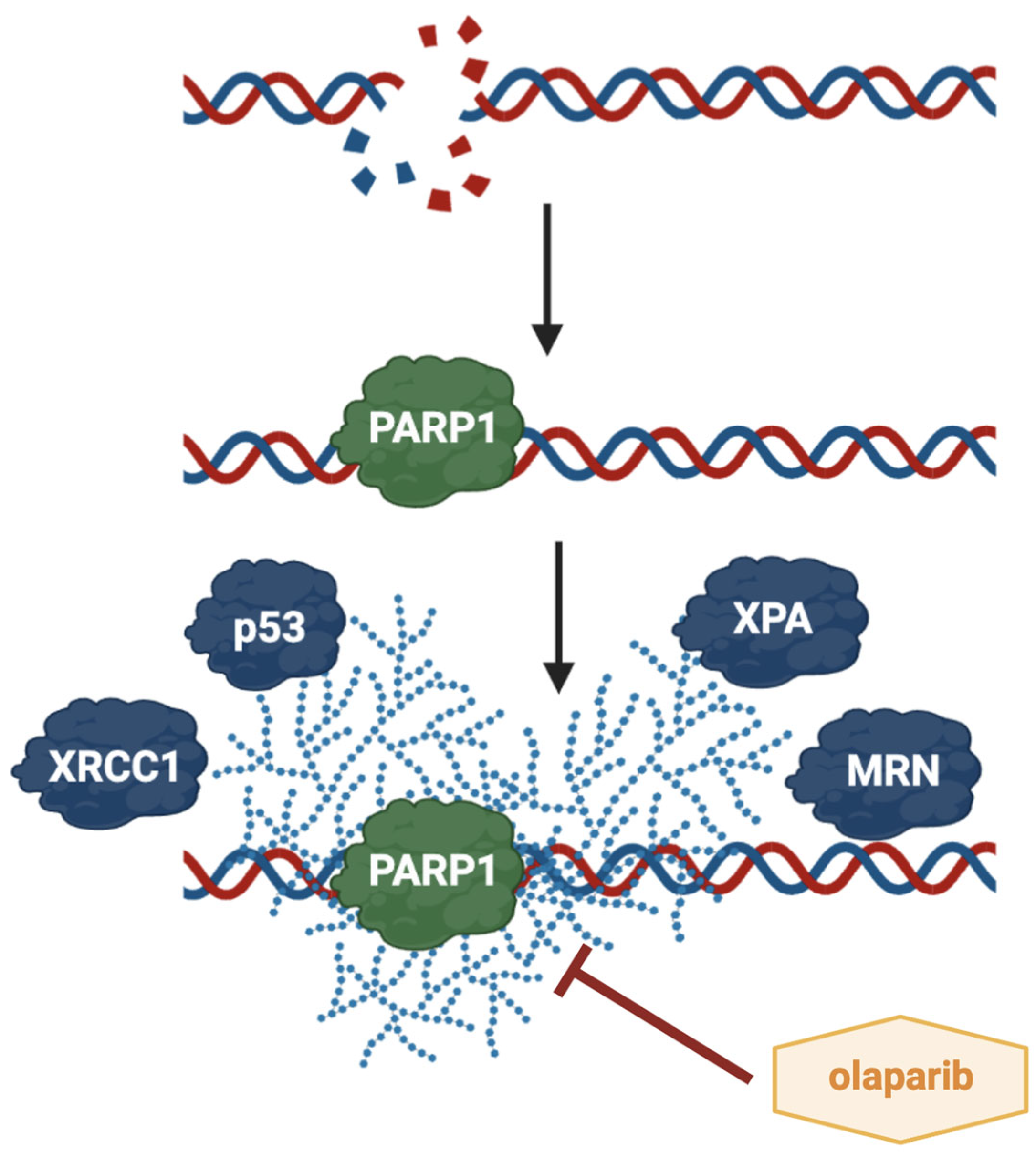
5. The Role of PARP1 and Its Inhibitor Olaparib in HNSCC
6. Potential Role of New Molecular Targets
7. Clinical Trials and Direction of Molecular Therapies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Z.; Li, K.; Ren, X.; Wang, X.; Yang, D.; Ma, S.; Zeng, X.; Zhang, P. The Role of the Tumor Microenvironment in HNSCC Resistance and Targeted Therapy. Front. Immunol. 2025, 16, 1554835. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Chen, S.M.Y.; Krinsky, A.L.; Woolaver, R.A.; Wang, X.; Chen, Z.; Wang, J.H. Tumor Immune Microenvironment in Head and Neck Cancers. Mol. Carcinog. 2020, 59, 766–774. [Google Scholar] [CrossRef]
- Strzelczyk, J.K.; Świętek, A.; Biernacki, K.; Gołąbek, K.; Gaździcka, J.; Miśkiewicz-Orczyk, K.; Ścierski, W.; Strzelczyk, J.; Fiolka, R.; Misiołek, M. PCR Detection of Epstein-Barr Virus (EBV) DNA in Patients with Head and Neck Squamous Cell Carcinoma, in Patients with Chronic Tonsillitis, and in Healthy Individuals. Biomed Res. Int. 2022, 2022, 8506242. [Google Scholar] [CrossRef]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Frontiers | Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Available online: https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2019.00827/full (accessed on 26 June 2025).
- Maghami, E.; Ismaila, N.; Alvarez, A.; Chernock, R.; Duvvuri, U.; Geiger, J.; Gross, N.; Haughey, B.; Paul, D.; Rodriguez, C.; et al. Diagnosis and Management of Squamous Cell Carcinoma of Unknown Primary in the Head and Neck: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2570–2596. [Google Scholar] [CrossRef]
- Eberly, H.W.; Sciscent, B.Y.; Lorenz, F.J.; Rettig, E.M.; Goyal, N. Current and Emerging Diagnostic, Prognostic, and Predictive Biomarkers in Head and Neck Cancer. Biomedicines 2024, 12, 415. [Google Scholar] [CrossRef]
- Kanno, Y.; Chen, C.Y.; Lee, H.L.; Chiou, J.F.; Chen, Y.J. Molecular Mechanisms of Chemotherapy Resistance in Head and Neck Cancers. Front. Oncol. 2021, 11, 640392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michelon, I.; Nachtigal, G.C.; Dacoregio, M.I.; Moraes, A.C.B.K.; Moraes, M.; Piva, L.S.; da Costa, C.T.; Lund, R.G.; Michelon, D. Treatment Options for Cisplatin-Ineligible Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma: A Systematic Review. J. Cancer Res. Clin. Oncol. 2024, 150, 379. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Xu, C.; Xu, H.; Liu, B. Head and Neck Squamous Cell Carcinoma-Specific Prognostic Signature and Drug Sensitive Subtypes Based on Programmed Cell Death-Related Genes. PeerJ 2023, 11, e16364. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) Resistance and the New Strategy to Enhance the Sensitivity against Cancer: Implication of DNA Repair Inhibition. Biomed Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef]
- Tentoni, N.; Combs, R.; Hwang, M.; Ward, S.; McCracken, A.; Lowe, J.; Howard, S.C. Long-Term Outcomes of 5-Fluorouracil-Related Early-Onset Toxicities: A Retrospective Cohort Study. Cancers 2024, 16, 4050. [Google Scholar] [CrossRef]
- Head and Neck Cancer: Pathogenesis and Targeted Therapy—Liu—2024—MedComm—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/mco2.702 (accessed on 26 June 2025).
- Effective Radiosensitization of HNSCC Cell Lines by DNA-PKcs Inhibitor AZD7648 and PARP Inhibitors Talazoparib and Niraparib—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11172136/ (accessed on 26 June 2025).
- von Witzleben, A.; Wang, C.; Laban, S.; Savelyeva, N.; Ottensmeier, C.H. HNSCC: Tumour Antigens and Their Targeting by Immunotherapy. Cells 2020, 9, 2103. [Google Scholar] [CrossRef]
- Obradovic, A.; Graves, D.; Korrer, M.; Wang, Y.; Roy, S.; Naveed, A.; Xu, Y.; Luginbuhl, A.; Curry, J.; Gibson, M.; et al. Immunostimulatory Cancer-Associated Fibroblast Subpopulations Can Predict Immunotherapy Response in Head and Neck Cancer. Clin. Cancer Res. 2022, 28, 2094–2109. [Google Scholar] [CrossRef]
- Mei, Z.; Huang, J.; Qiao, B.; Lam, A.K. Immune Checkpoint Pathways in Immunotherapy for Head and Neck Squamous Cell Carcinoma. Int. J. Oral. Sci. 2020, 12, 16. [Google Scholar] [CrossRef]
- Tilsed, C.M.; Fisher, S.A.; Nowak, A.K.; Lake, R.A.; Lesterhuis, W.J. Cancer Chemotherapy: Insights into Cellular and Tumor Microenvironmental Mechanisms of Action. Front. Oncol. 2022, 12, 960317. [Google Scholar] [CrossRef]
- Villa, A.; Sonis, S. Toxicities Associated with Head and Neck Cancer Treatment and Oncology-Related Clinical Trials. Curr. Probl. Cancer 2016, 40, 244–257. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Lee, A.M.; Weaver, A.N.; Acosta, P.; Harris, L.; Bowles, D.W. Review of Current and Future Medical Treatments in Head and Neck Squamous Cell Carcinoma. Cancers 2024, 16, 3488. [Google Scholar] [CrossRef]
- Rades, D.; Zwaan, I.; Soror, T.; Idel, C.; Pries, R.; Bruchhage, K.L.; Hakim, S.G.; Yu, N.Y. Chemoradiation with Cisplatin vs. Carboplatin for Squamous Cell Carcinoma of the Head and Neck (SCCHN). Cancers 2023, 15, 3278. [Google Scholar] [CrossRef] [PubMed]
- Carinato, H.; Burgy, M.; Ferry, R.; Fischbach, C.; Kalish, M.; Guihard, S.; Brahimi, Y.; Flesch, H.; Bronner, G.; Schultz, P.; et al. Weekly Paclitaxel, Carboplatin, and Cetuximab as First-Line Treatment of Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma for Patients Ineligible to Cisplatin-Based Chemotherapy: A Retrospective Monocentric Study in 60 Patients. Front. Oncol. 2021, 11, 714551. [Google Scholar] [CrossRef]
- Chauffert, B.; Zhou, Y.; Medjkoune, L.; Ouikene, A.; Galez, A.; Belkhir, F.; Saint-Germain, P.; Youssef, A.; Chehimi, M. High Response Rate to Carboplatin-Paclitaxel-Cetuximab and Pembrolizumab in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Case Rep. Oncol. 2023, 16, 13–20. [Google Scholar] [CrossRef]
- Hanemaaijer, S.H.; Kok, I.C.; Fehrmann, R.S.N.; van der Vegt, B.; Gietema, J.A.; Plaat, B.E.C.; van Vugt, M.A.T.M.; Vergeer, M.R.; Leemans, C.R.; Langendijk, J.A.; et al. Comparison of Carboplatin With 5-Fluorouracil vs. Cisplatin as Concomitant Chemoradiotherapy for Locally Advanced Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 761. [Google Scholar] [CrossRef]
- Nassif, S.; Wichmann, J.; Strube, D.; Vassis, S.; Christiansen, H.; Steinmann, D. Cisplatin Versus Carboplatin and Paclitaxel in Radiochemotherapy for Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma. Vivo 2022, 36, 821–832. [Google Scholar] [CrossRef]
- Rühle, A.; Weymann, M.; Behrens, M.; Marschner, S.; Haderlein, M.; Fabian, A.; Senger, C.; Dickstein, D.R.; Kraft, J.; von der Grün, J.; et al. A Multicenter Evaluation of Different Chemotherapy Regimens in Older Adults With Head and Neck Squamous Cell Carcinoma Undergoing Definitive Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 1282–1293. [Google Scholar] [CrossRef]
- Dzienis, M.; Cundom, J.; Fuentes, C.S.; Spreafico, A.; Nordlinger, M.; Pastor, A.V.; Alesi, E.; Neki, A.; Fung, A.S.; Figueiredo Lima, I.P.; et al. Pembrolizumab Plus Carboplatin and Paclitaxel as First-Line Therapy for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (KEYNOTE-B10): A Single-Arm Phase IV Trial. J. Clin. Oncology. 2024, 42, 2989–2999. [Google Scholar] [CrossRef]
- Schneider, J.J.; Galettis, P.; Martin, J.H. Overcoming Barriers to Implementing Precision Dosing with 5-Fluorouracil and Capecitabine. Br. J. Clin. Pharmacol. 2021, 87, 317–325. [Google Scholar] [CrossRef]
- Sakatani, A.; Sonohara, F.; Goel, A. Melatonin-Mediated Downregulation of Thymidylate Synthase as a Novel Mechanism for Overcoming 5-Fluorouracil Associated Chemoresistance in Colorectal Cancer Cells. Carcinogenesis 2019, 40, 422–431. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Bacanu, S.; Sreekumar, L.; Ramos, A.D.; Dai, L.; Michaelis, M.; Cinatl, J.; Seki, T.; Cao, Y.; Coffill, C.R.; et al. CETSA Interaction Proteomics Define Specific RNA-Modification Pathways as Key Components of Fluorouracil-Based Cancer Drug Cytotoxicity. Cell Chem. Biol. 2022, 29, 572–585.e8. [Google Scholar] [CrossRef]
- Kogashiwa, Y.; Sakurai, H.; Kimura, T.; Kohno, N. Docetaxel Suppresses Invasiveness of Head and Neck Cancer Cells in Vitro. Cancer Sci. 2010, 101, 1382–1386. [Google Scholar] [CrossRef]
- Su, C.-C.; Lin, J.-W.; Chang, K.-Y.; Wu, C.-T.; Liu, S.-H.; Chang, K.-C.; Liu, J.-M.; Lee, K.-I.; Fang, K.-M.; Chen, Y.-W. Involvement of AMPKα and MAPK-ERK/-JNK Signals in Docetaxel-Induced Human Tongue Squamous Cell Carcinoma Cell Apoptosis. Int. J. Mol. Sci. 2022, 23, 13857. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, X. Research progress on pyroptosis in otorhinolaryngology. J. Otolaryngol. Ophthalmol. Shandong Univ. 2024, 38, 140–148. [Google Scholar]
- Morse, R.T.; Ganju, R.G.; TenNapel, M.J.; Neupane, P.; Kakarala, K.; Shnayder, Y.; Chen, A.M.; Lominska, C.E. Weekly cisplatin chemotherapy dosing versus triweekly chemotherapy with concurrent radiation for head and neck squamous cell carcinoma. Head Neck 2019, 41, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zakeri, K.; Raab, G.; Hesse, J.; Shamseddine, A.; Chen, L.; Yu, Y.; Kang, J.J.; McBride, S.M.; Riaz, N.; et al. Concurrent Carboplatin and Paclitaxel Definitive Radiation Therapy for Locally Advanced Head and Neck Cancer. Head Neck 2023, 45, 2207–2216. [Google Scholar] [CrossRef]
- Di Nardo, P.; Lisanti, C.; Garutti, M.; Buriolla, S.; Alberti, M.; Mazzeo, R.; Puglisi, F. Chemotherapy in patients with early breast cancer: Clinical overview and management of long-term side effects. Expert Opin. Drug. Saf. 2022, 21, 1341–1355. [Google Scholar] [CrossRef] [PubMed]
- Weppelmann, B.; Wheeler, R.H.; Peters, G.E.; Stephens, S.; Spencer, S.A.; Meredith, R.F.; Kim, R.Y.; Salter, M.M. A phase I study of prolonged infusion 5-fluorouracil and concomitant radiation therapy in patients with squamous cell cancer of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, T.; Shiga, K.; Katagiri, K.; Saito, D.; Oikawa, S.I.; Ikeda, A.; Tsuchida, K.; Miyaguchi, J.; Ohashi, Y.U.; Ariga, H.; et al. Treatment Outcomes and Prognostic Factors of Concurrent Chemoradiotherapy With Docetaxel, Cisplatin, and Fluorouracil in Advanced Head and Neck Cancer. Anticancer Res. 2022, 42, 6047–6056. [Google Scholar] [CrossRef] [PubMed]
- Risk Factors for Dysgeusia During Chemotherapy for Solid Tumors: A Retrospective Cross-Sectional Study | Supportive Care In Cancer. Available online: https://link.springer.com/article/10.1007/s00520-021-06219-4 (accessed on 26 June 2025).
- Goel, B.; Tiwari, A.K.; Pandey, R.K.; Singh, A.P.; Kumar, S.; Sinha, A.; Jain, S.K.; Khattri, A. Therapeutic Approaches for the Treatment of Head and Neck Squamous Cell Carcinoma—An Update on Clinical Trials. Transl. Oncol. 2022, 21, 101426. [Google Scholar] [CrossRef]
- Khamaikawin, W.; Locharoenrat, K. Evaluation of a Docetaxel-Cisplatin-Fluorouracil-Au Complex in Human Oral Carcinoma Cell Line. Artif. Cells Nanomed. Biotechnol. 2023, 51, 148–157. [Google Scholar] [CrossRef]
- Libert, L.; Abdeddaim, C.; Saleh, K.; Even, C.; Duplomb, S.; Dubreuil, J.; Rambeau, A.; Guiard, E.; Pointreau, Y.; Olympios-Gerotzortzos, N.; et al. Retrospective Multicentric Survival Analysis of Patients Receiving TPEx Regimen as First-Line Treatment of Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. ESMO Open 2025, 10, 104544. [Google Scholar] [CrossRef]
- Imamura, Y.; Tanaka, K.; Kiyota, N.; Hayashi, H.; Ota, I.; Arai, A.; Iwae, S.; Minami, S.; Yane, K.; Yamazaki, T.; et al. Docetaxel plus Cisplatin in Recurrent and/or Metastatic Non-Squamous-Cell Head and Neck Cancer: A Multicenter Phase II Trial. Med. Oncol. 2021, 38, 128. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Lein, M.-Y.; Yang, S.-N.; Wang, Y.-C.; Lin, Y.-J.; Lin, C.-Y.; Hua, C.-H.; Tsai, M.-H.; Lin, C.-C. Dose-Dense TPF Induction Chemotherapy for Locally Advanced Head and Neck Cancer: A Phase II Study. BMC Cancer 2020, 20, 832. [Google Scholar] [CrossRef]
- Modyfikowany TPEx Jako Leczenie Pierwszego Rzutu W Nawrotowym I/Lub Przerzutowym Raku Głowy I Szyi | Badania Przeciwnowotworowe. Available online: https://ar.iiarjournals.org/content/41/4/2045 (accessed on 26 June 2025).
- De Azevedo, J.; Mourtada, J.; Bour, C.; Devignot, V.; Schultz, P.; Borel, C.; Pencreach, E.; Mellitzer, G.; Gaiddon, C.; Jung, A.C. The EXTREME Regimen Associating Cetuximab and Cisplatin Favors Head and Neck Cancer Cell Death and Immunogenicity with the Induction of an Anti-Cancer Immune Response. Cells 2022, 11, 2866. [Google Scholar] [CrossRef]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burns, C.V.; Edwin, S.B.; Szpunar, S.; Forman, J. Cisplatin-induced nephrotoxicity in an outpatient setting. Pharmacotherapy 2021, 41, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Borkowska, A.; Bagues, A.; Tu, L.; Liu, J.Y.H.; Lu, Z.; Rudd, J.A.; Nurgali, K.; Abalo, R. Mechanisms of Chemotherapy-Induced Neurotoxicity. Front. Pharmacol. 2022, 13, 750507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brianna Lee, S.H. Chemotherapy: How to reduce its adverse effects while maintaining the potency? Med. Oncol. 2023, 40, 88. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Outlaw, D.; Harvey, R.D.; Lichtman, S.M.; Zamboni, W.C.; Giri, S. Chemotherapy dosing in older adults with cancer: One size does NOT fit all. J. Geriatr. Oncol. 2023, 14, 101363. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, A.; Joosten, E.A.J.; van den Beuken-van Everdingen, M.H.J. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers 2021, 13, 242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogawa, T.; Ono, K.; Ryumon, S.; Kawai, H.; Nakamura, T.; Umemori, K.; Yoshida, K.; Kanemoto, H.; Obata, K.; Yoshioka, N.; et al. Novel mechanism of cisplatin resistance in head and neck squamous cell carcinoma involving extracellular vesicles and a copper transporter system. Head Neck 2024, 46, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Sadeghi, S.; Tabatabaeian, H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int. J. Mol. Sci. 2021, 22, 9451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mori, R.; Ukai, J.; Tokumaru, Y.; Niwa, Y.; Futamura, M. The mechanism underlying resistance to 5-fluorouracil and its reversal by the inhibition of thymidine phosphorylase in breast cancer cells. Oncol. Lett. 2022, 24, 311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Dong, P.; Yang, L. The Role of Nanotherapy in Head and Neck Squamous Cell Carcinoma by Targeting Tumor Microenvironment. Front. Immunol. 2023, 14, 1189323. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, S.; Zhang, Q.; Zhang, H.; Fan, Y.; Qiu, F.; Kang, N. Biological and Pharmacological Effects of Hexahydrocurcumin, a Metabolite of Curcumin. Arch. Biochem. Biophys. 2018, 646, 31–37. [Google Scholar] [CrossRef]
- Chithra, A.; Sekar, R.; Senthil Kumar, P.; Padmalaya, G. A Review on Removal Strategies of Microorganisms from Water Environment Using Nanomaterials and Their Behavioural Characteristics. Chemosphere 2022, 295, 133915. [Google Scholar] [CrossRef]
- Assessment of a Nano-Docetaxel Combined Treatment for Head and Neck Cancer. Available online: https://www.researchgate.net/publication/355278665_Assessment_of_a_NanoDocetaxel_Combined_Treatment_for_Head_and_Neck_Cancer (accessed on 26 June 2025).
- Bhardwaj, P.; Gota, V.; Vishwakarma, K.; Pai, V.; Chaudhari, P.; Mohanty, B.; Thorat, R.; Yadav, S.; Gurjar, M.; Goda, J.S.; et al. Loco-Regional Radiosensitizing Nanoparticles-in-Gel Augments Head and Neck Cancer Chemoradiotherapy. J. Control. Release 2022, 343, 288–302. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Kounatidis, D.; Panagopoulos, F.; Geladari, E.; Karampela, I.; Stratigou, T.; Dalamaga, M. Immunotherapy in Head and Neck Cancer: Where Do We Stand? Curr. Oncol. Rep. 2023, 25, 897–912. [Google Scholar] [CrossRef]
- Spyrou, N.; Vallianou, N.; Kadillari, J.; Dalamaga, M. The Interplay of Obesity, Gut Microbiome and Diet in the Immune Check Point Inhibitors Therapy Era. Semin. Cancer Biol. 2021, 73, 356–376. [Google Scholar] [CrossRef]
- Ipilimumab (Anti-Ctla-4 Mab) in the Treatment of Metastatic Melanoma: Effectiveness and Toxicity Management—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4963052/ (accessed on 26 June 2025).
- Bhatia, A.; Burtness, B. Treating Head and Neck Cancer in the Age of Immunotherapy: A 2023 Update. Drugs 2023, 83, 217–248. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Tourneau, C.L.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus Methotrexate, Docetaxel, or Cetuximab for Recurrent or Metastatic Head-and-Neck Squamous Cell Carcinoma (KEYNOTE-040): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Uppaluri, R.; Campbell, K.M.; Egloff, A.M.; Zolkind, P.; Skidmore, Z.L.; Nussenbaum, B.; Paniello, R.C.; Rich, J.T.; Jackson, R.; Pipkorn, P.; et al. Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase II Trial. Clin. Cancer Res. 2020, 26, 5140–5152. [Google Scholar] [CrossRef]
- Wise-Draper, T.M.; Gulati, S.; Palackdharry, S.; Hinrichs, B.H.; Worden, F.P.; Old, M.O.; Dunlap, N.E.; Kaczmar, J.M.; Patil, Y.; Riaz, M.K.; et al. Phase II Clinical Trial of Neoadjuvant and Adjuvant Pembrolizumab in Resectable Local-Regionally Advanced Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2022, 28, 1345–1352. [Google Scholar] [CrossRef]
- Pembrolizumab Alone or with Chemotherapy Versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (Keynote-048): A Randomised, Open-Label, Phase 3 Study—Pubmed. Available online: https://pubmed.ncbi.nlm.nih.gov/31679945/ (accessed on 26 June 2025).
- Cirillo, A.; Marinelli, D.; Romeo, U.; Messineo, D.; De Felice, F.; De Vincentiis, M.; Valentini, V.; Mezi, S.; Valentini, F.; Vivona, L.; et al. Pembrolizumab-Based First-Line Treatment for PD-L1-Positive, Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Retrospective Analysis. BMC Cancer 2024, 24, 430. [Google Scholar] [CrossRef]
- Emancipator, K.; Huang, L.; Aurora-Garg, D.; Bal, T.; Cohen, E.E.W.; Harrington, K.; Soulières, D.; Le Tourneau, C.; Licitra, L.; Burtness, B.; et al. Comparing Programmed Death Ligand 1 Scores for Predicting Pembrolizumab Efficacy in Head and Neck Cancer. Mod. Pathol. 2021, 34, 532–541. [Google Scholar] [CrossRef]
- Meci, A.; Goyal, N.; Slonimsky, G. Mechanisms of Resistance and Therapeutic Perspectives in Immunotherapy for Advanced Head and Neck Cancers. Cancers 2024, 16, 703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daste, A.; Larroquette, M.; Gibson, N.; Lasserre, M.; Domblides, C. Immunotherapy for head and neck squamous cell carcinoma: Current status and perspectives. Immunotherapy 2024, 16, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, P.; Benamar, M.; Han, S.; Haigis, M.C.; Sharpe, A.H.; Chatila, T.A. Regulatory T cells in dominant immunologic tolerance. J. Allergy. Clin. Immunol. 2024, 153, 28–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Le, D.T.; Huynh, T.R.; Burt, B.; Van Buren, G.; Abeynaike, S.A.; Zalfa, C.; Nikzad, R.; Kheradmand, F.; Tyner, J.J.; Paust, S. Natural killer cells and cytotoxic T lymphocytes are required to clear solid tumor in a patient-derived xenograft. JCI Insight 2021, 6, e140116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, A.; Fan, T.; Liu, Y.; Yu, G.; Li, C.; Jiang, Z. Regulatory T cells in immune checkpoint blockade antitumor therapy. Mol. Cancer 2024, 23, 251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oweida, A.; Darragh, L.; Bhatia, S.; Raben, D.; Heasley, L.; Nemenoff, R.; Clambey, E.; Karam, S. Regulatory T cells mediate resistance to radiotherapy in head and neck squamous cell carcinoma. J. Clin. Oncol. 2019, 37, 8. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Zhang, G.; Shang, D. Reprogramming of regulatory T cells in inflammatory tumor microenvironment: Can it become immunotherapy turning point? Front. Immunol. 2024, 15, 1345838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ge, J.; Yin, X.; Chen, L. Regulatory T cells: Masterminds of immune equilibrium and future therapeutic innovations. Front. Immunol. 2024, 15, 1457189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.C.; Chan, L.P.; Cho, S.F. Targeting the Immune Microenvironment in the Treatment of Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 1084. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.; Zhu, Y.; He, Y.; Gu, W.; Zhou, Q.; Jin, B.; Chen, S.; Lin, H. Unraveling the immune evasion mechanisms in the tumor microenvironment of head and neck squamous cell carcinoma. Front. Immunol. 2025, 16, 1597202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliva, M.; Spreafico, A.; Taberna, M.; Alemany, L.; Coburn, B.; Mesia, R.; Siu, L.L. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann. Oncol. 2019, 30, 57–67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panagiotou, E.; Gomatou, G.; Trontzas, I.P.; Syrigos, N.; Kotteas, E. Cyclin-Dependent Kinase (CDK) Inhibitors in Solid Tumors: A Review of Clinical Trials. Clin. Transl. Oncol. 2022, 24, 161–192. [Google Scholar] [CrossRef]
- Ettl, T.; Schulz, D.; Bauer, R.J. The Renaissance of Cyclin Dependent Kinase Inhibitors. Cancers 2022, 14, 293. [Google Scholar] [CrossRef]
- Swiecicki, P.L.; Durm, G.; Bellile, E.; Bhangale, A.; Brenner, J.C.; Worden, F.P. A Multi-Center Phase II Trial Evaluating the Efficacy of Palbociclib in Combination with Carboplatin for the Treatment of Unresectable Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Invest. New Drugs 2020, 38, 1550–1558. [Google Scholar] [CrossRef]
- Huang, C.; Chen, L.; Savage, S.R.; Eguez, R.V.; Dou, Y.; Li, Y.; da Veiga Leprevost, F.; Jaehnig, E.J.; Lei, J.T.; Wen, B.; et al. Proteogenomic Insights into the Biology and Treatment of HPV-Negative Head and Neck Squamous Cell Carcinoma. Cancer Cell 2021, 39, 361–379.e16. [Google Scholar] [CrossRef]
- Adkins, D.; Ley, J.; Neupane, P.; Worden, F.; Sacco, A.G.; Palka, K.; Grilley-Olson, J.E.; Maggiore, R.; Salama, N.N.; Trinkaus, K.; et al. Palbociclib and Cetuximab in Platinum-Resistant and in Cetuximab-Resistant Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicentre, Multigroup, Phase 2 Trial. Lancet Oncol. 2019, 20, 1295–1305. [Google Scholar] [CrossRef]
- Michel, L.; Ley, J.; Wildes, T.M.; Schaffer, A.; Robinson, A.; Chun, S.-E.; Lee, W.; Lewis, J.; Trinkaus, K.; Adkins, D. Phase I Trial of Palbociclib, a Selective Cyclin Dependent Kinase 4/6 Inhibitor, in Combination with Cetuximab in Patients with Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma. Oral. Oncol. 2016, 58, 41–48. [Google Scholar] [CrossRef]
- Nadrodzina PARP—Amé—2004—BioEssays—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/bies.20085 (accessed on 28 June 2025).
- Kraus, W.L. PARPs and ADP-Ribosylation: 50 Years … and Counting. Mol. Cell 2015, 58, 902–910. [Google Scholar] [CrossRef]
- Rouleau-Turcotte, É.; Krastev, D.B.; Pettitt, S.J.; Lord, C.J.; Pascal, J.M. Captured Snapshots of PARP1 in the Active State Reveal the Mechanics of PARP1 Allostery. Mol. Cell 2022, 82, 2939–2951.e5. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Wang, Y. PARP-1 and Its Associated Nucleases in DNA Damage Response. DNA Repair 2019, 81, 102651. [Google Scholar] [CrossRef]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef]
- Chudy, P.; Kochan, J.; Wawro, M.; Nguyen, P.; Gorczyca, M.; Varanko, A.; Retka, A.; Ghadei, S.S.; Napieralska, E.; Grochot-Przęczek, A.; et al. Heme Oxygenase-1 Protects Cells from Replication Stress. Redox Biol. 2024, 75, 103247. [Google Scholar] [CrossRef]
- Harrision, D.; Gravells, P.; Thompson, R.; Bryant, H.E. Poly(ADP-Ribose) Glycohydrolase (PARG) vs. Poly(ADP-Ribose) Polymerase (PARP)—Function in Genome Maintenance and Relevance of Inhibitors for Anti-Cancer Therapy. Front. Mol. Biosci. 2020, 7, 191. [Google Scholar] [CrossRef]
- Lindström, M.S.; Bartek, J.; Maya-Mendoza, A. P53 at the Crossroad of DNA Replication and Ribosome Biogenesis Stress Pathways. Cell Death Differ. 2022, 29, 972–982. [Google Scholar] [CrossRef]
- Fischbach, A.; Krüger, A.; Assmann, G.M.; Rank, L.; Stöckl, M.T.; Fischer, J.M.F.; Veith, S.; Rossatti, P.; Ganz, M.; Ferrando-May, E.; et al. The C-Terminal Domain of P53 Orchestrates the Interplay between Non-Covalent and Covalent Poly(ADP-Ribosyl)Ation of P53 by PARP1. Nucleic Acids Res. 2018, 406, 804–822. [Google Scholar] [CrossRef]
- Ossovskaya, V.; Koo, I.C.; Kaldjian, E.P.; Alvares, C.; Sherman, B.M. Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer 2010, 1, 812–821. [Google Scholar] [CrossRef]
- Rojo, F.; García-Parra, J.; Zazo, S.; Tusquets, I.; Ferrer-Lozano, J.; Menendez, S.; Eroles, P.; Chamizo, C.; Servitja, S.; Ramírez-Merino, N.; et al. Nuclear PARP-1 Protein Overexpression Is Associated with Poor Overall Survival in Early Breast Cancer. Ann. Oncol. 2012, 23, 1156–1164. [Google Scholar] [CrossRef]
- Dziaman, T.; Ludwiczak, H.; Ciesla, J.M.; Banaszkiewicz, Z.; Winczura, A.; Chmielarczyk, M.; Wisniewska, E.; Marszalek, A.; Tudek, B.; Olinski, R. PARP-1 Expression Is Increased in Colon Adenoma and Carcinoma and Correlates with OGG1. PLoS ONE 2014, 9, e115558. [Google Scholar] [CrossRef]
- Salemi, M.; Galia, A.; Fraggetta, F.; La Corte, C.; Pepe, P.; La Vignera, S.; Improta, G.; Bosco, P.; Calogero, A.E. Poly (ADP-Ribose) Polymerase 1 Protein Expression in Normal and Neoplastic Prostatic Tissue. Eur. J. Histochem. 2013, 57, e13. [Google Scholar] [CrossRef]
- Galia, A.; Calogero, A.E.; Condorelli, R.; Fraggetta, F.; La Corte, A.; Ridolfo, F.; Bosco, P.; Castiglione, R.; Salemi, M. PARP-1 Protein Expression in Glioblastoma Multiforme. Eur. J. Histochem. 2012, 56, e9. [Google Scholar] [CrossRef]
- Kossatz, S.; Brand, C.; Gutiontov, S.; Liu, J.T.C.; Lee, N.Y.; Gönen, M.; Weber, W.A.; Reiner, T. Detection and Delineation of Oral Cancer with a PARP1 Targeted Optical Imaging Agent. Sci. Rep. 2016, 6, 21371. [Google Scholar] [CrossRef]
- Wang, F.; Gouttia, O.G.; Wang, L.; Peng, A. PARP1 Upregulation in Recurrent Oral Cancer and Treatment Resistance. Front. Cell Dev. Biol. 2022, 9, 804962. [Google Scholar] [CrossRef]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific Killing of BRCA2-Deficient Tumours with Inhibitors of Poly(ADP-Ribose) Polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA Repair Defect in BRCA Mutant Cells as a Therapeutic Strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Petermann, E.; Orta, M.L.; Issaeva, N.; Schultz, N.; Helleday, T. Hydroxyurea-Stalled Replication Forks Become Progressively Inactivated and Require Two Different RAD51-Mediated Pathways for Restart and Repair. Mol. Cell 2010, 37, 492–502. [Google Scholar] [CrossRef]
- Verhagen, C.V.M.; de Haan, R.; Hageman, F.; Oostendorp, T.P.D.; Carli, A.L.E.; O’Connor, M.J.; Jonkers, J.; Verheij, M.; van den Brekel, M.W.; Vens, C. Extent of Radiosensitization by the PARP Inhibitor Olaparib Depends on Its Dose, the Radiation Dose and the Integrity of the Homologous Recombination Pathway of Tumor Cells. Radiother. Oncol. 2015, 116, 358–365. [Google Scholar] [CrossRef]
- Khan, K.; Araki, K.; Wang, D.; Li, G.; Li, X.; Zhang, J.; Xu, W.; Hoover, R.K.; Lauter, S.; O’Malley, B., Jr.; et al. Head and Neck Cancer Radiosensitization by the Novel Poly(ADP-Ribose) Polymerase Inhibitor GPI-15427. Head Neck 2010, 32, 381–391. [Google Scholar] [CrossRef]
- Noël, G.; Godon, C.; Fernet, M.; Giocanti, N.; Mégnin-Chanet, F.; Favaudon, V. Radiosensitization by the Poly(ADP-Ribose) Polymerase Inhibitor 4-Amino-1,8-Naphthalimide Is Specific of the S Phase of the Cell Cycle and Involves Arrest of DNA Synthesis. Mol. Cancer Ther. 2006, 5, 564–574. [Google Scholar] [CrossRef][Green Version]
- Henneman, L.; van Miltenburg, M.H.; Michalak, E.M.; Braumuller, T.M.; Jaspers, J.E.; Drenth, A.P.; de Korte-Grimmerink, R.; Gogola, E.; Szuhai, K.; Schlicker, A.; et al. Selective Resistance to the PARP Inhibitor Olaparib in a Mouse Model for BRCA1-Deficient Metaplastic Breast Cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 8409–8414. [Google Scholar] [CrossRef]
- Nowsheen, S.; Bonner, J.A.; LoBuglio, A.F.; Trummell, H.; Whitley, A.C.; Dobelbower, M.C.; Yang, E.S. Cetuximab Augments Cytotoxicity with Poly (ADP-Ribose) Polymerase Inhibition in Head and Neck Cancer. PLoS ONE 2011, 6, e24148. [Google Scholar] [CrossRef]
- Dittmann, K.; Mayer, C.; Rodemann, H.-P. Inhibition of Radiation-Induced EGFR Nuclear Import by C225 (Cetuximab) Suppresses DNA-PK Activity. Radiother. Oncol. 2005, 76, 157–161. [Google Scholar] [CrossRef]
- Dorna, D.; Kleszcz, R.; Paluszczak, J. Triple Combinations of Histone Lysine Demethylase Inhibitors with PARP1 Inhibitor–Olaparib and Cisplatin Lead to Enhanced Cytotoxic Effects in Head and Neck Cancer Cells. Biomedicines 2024, 12, 1359. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Citro, S.; Ghiani, L.; Doni, M.; Miccolo, C.; Tagliabue, M.; Ansarin, M.; Chiocca, S. HPV-Mediated PARP1 Regulation and Drug Sensitization in Head and Neck Cancer. Oral. Oncol. 2025, 165, 107307. [Google Scholar] [CrossRef]
- Moutafi, M.; Koliou, G.-A.; Papaxoinis, G.; Economopoulou, P.; Kotsantis, I.; Gkotzamanidou, M.; Anastasiou, M.; Pectasides, D.; Kyrodimos, E.; Delides, A.; et al. Phase II Window Study of Olaparib Alone or with Cisplatin or Durvalumab in Operable Head and Neck Cancer. Cancer Res. Commun. 2023, 3, 1514–1523. [Google Scholar] [CrossRef]
- Wilson, T.C.; Xavier, M.-A.; Knight, J.; Verhoog, S.; Torres, J.B.; Mosley, M.; Hopkins, S.L.; Wallington, S.; Allen, P.D.; Kersemans, V.; et al. PET Imaging of PARP Expression Using 18F-Olaparib. J. Nucl. Med. 2019, 60, 504–510. [Google Scholar] [CrossRef]
- Carney, B.; Kossatz, S.; Lok, B.H.; Schneeberger, V.; Gangangari, K.K.; Pillarsetty, N.V.K.; Weber, W.A.; Rudin, C.M.; Poirier, J.T.; Reiner, T. Target Engagement Imaging of PARP Inhibitors in Small-Cell Lung Cancer. Nat. Commun. 2018, 9, 176. [Google Scholar] [CrossRef]
- Obrazowanie PARP1/2 Z 18F-PARPi U Pacjentów Z Rakiem Głowy I Szyi | Medrxiv. Available online: https://www.medrxiv.org/content/10.1101/19009381v1 (accessed on 28 June 2025).
- Carney, B.; Carlucci, G.; Salinas, B.; Di Gialleonardo, V.; Kossatz, S.; Vansteene, A.; Longo, V.A.; Bolaender, A.; Chiosis, G.; Keshari, K.R.; et al. Non-Invasive PET Imaging of PARP1 Expression in Glioblastoma Models. Mol. Imaging Biol. 2016, 18, 386–392. [Google Scholar] [CrossRef]
- Saini, K.S.; Somara, S.; Ko, H.C.; Thatai, P.; Quintana, A.; Wallen, Z.D.; Green, M.F.; Mehrotra, R.; McGuigan, S.; Pang, L.; et al. Biomarkers in head and neck squamous cell carcinoma: Unraveling the path to precision immunotherapy. Front. Oncol. 2024, 14, 1473706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsieh, J.C.; Wang, H.M.; Wu, M.H.; Chang, K.P.; Chang, P.H.; Liao, C.T.; Liau, C.T. Review of emerging biomarkers in head and neck squamous cell carcinoma in the era of immunotherapy and targeted therapy. Head Neck 2019, 41 Suppl. S1, 19–45. [Google Scholar] [CrossRef] [PubMed]
- Pekarek, L.; Garrido-Gil, M.J.; Sánchez-Cendra, A.; Cassinello, J.; Pekarek, T.; Fraile-Martinez, O.; García-Montero, C.; Lopez-Gonzalez, L.; Rios-Parra, A.; Álvarez-Mon, M.; et al. Emerging histological and serological biomarkers in oral squamous cell carcinoma: Applications in diagnosis, prognosis evaluation and personalized therapeutics (Review). Oncol. Rep. 2023, 50, 213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boguszewicz, Ł. Predictive Biomarkers for Response and Toxicity of Induction Chemotherapy in Head and Neck Cancers. Front. Oncol. 2022, 12, 900903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, H.; Kiess, A.; Chung, C.H. Emerging biomarkers in head and neck cancer in the era of genomics. Nat. Rev. Clin. Oncol. 2015, 12, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, E.; Madu, C.; Lu, Y. Epigenetic Modulators as Therapeutic Agents in Cancer. Int. J. Mol. Sci. 2023, 24, 14964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Haren, M.J.; Gao, Y.; Buijs, N.; Campagna, R.; Sartini, D.; Emanuelli, M.; Mateuszuk, L.; Kij, A.; Chlopicki, S.; Escudé Martinez de Castilla, P.; et al. Esterase-Sensitive Prodrugs of a Potent Bisubstrate Inhibitor of Nicotinamide N-Methyltransferase (NNMT) Display Cellular Activity. Biomolecules 2021, 11, 1357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, P.; Wang, G.; Li, H.; Yuan, Y.; Chen, H.; Wang, S.; Sun, Z.; Meng, F.; Li, Y.; Yang, F.; et al. Nicotinamide N-methyltransferase negatively regulates metastasis-promoting property of cancer-associated fibroblasts in lung adenocarcinoma. Cancer Commun. 2025, 45, 110–137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sartini, D.; Santarelli, A.; Rossi, V.; Goteri, G.; Rubini, C.; Ciavarella, D.; Lo Muzio, L.; Emanuelli, M. Nicotinamide N-methyltransferase upregulation inversely correlates with lymph node metastasis in oral squamous cell carcinoma. Mol. Med. 2007, 13, 415–421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Y.; van Haren, M.J.; Buijs, N.; Innocenti, P.; Zhang, Y.; Sartini, D.; Campagna, R.; Emanuelli, M.; Parsons, R.B.; Jespers, W.; et al. Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics. J. Med. Chem. 2021, 64, 12938–12963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pozzi, V.; Molinelli, E.; Campagna, R.; Serritelli, E.N.; Cecati, M.; De Simoni, E.; Sartini, D.; Goteri, G.; Martin, N.I.; van Haren, M.J.; et al. Knockdown of nicotinamide N-methyltransferase suppresses proliferation, migration, and chemoresistance of Merkel cell carcinoma cells in vitro. Hum. Cell 2024, 37, 729–738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sartini, D.; Campagna, R.; Lucarini, G.; Pompei, V.; Salvolini, E.; Mattioli-Belmonte, M.; Molinelli, E.; Brisigotti, V.; Campanati, A.; Bacchetti, T.; et al. Differential immunohistochemical expression of paraoxonase-2 in actinic keratosis and squamous cell carcinoma. Hum. Cell 2021, 34, 1929–1931. [Google Scholar] [CrossRef] [PubMed]
- Belloni, A.; Campagna, R.; Notarstefano, V.; Pozzi, V.; Orilisi, G.; Pompei, V.; Togni, L.; Mascitti, M.; Sartini, D.; Giorgini, E.; et al. Deepening Cisplatin sensitivity on Oral Squamous cell Carcinoma cell lines after PON2 knockdown: A FTIRM investigation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 330, 125726. [Google Scholar] [CrossRef] [PubMed]
- Rola Osi PI3K/Akt/mTOR W Płaskonabłonkowym Raku Głowy I Szyi. Available online: https://www.mdpi.com/2227-9059/12/7/1610 (accessed on 29 June 2025).
- Celowanie W Szlak Sygnałowy PI3K/AKT/mTOR Jako Radiosensybilizacja W Płaskonabłonkowych Rakach Głowy I Szyi. Available online: https://www.mdpi.com/1422-0067/23/24/15749 (accessed on 29 June 2025).
- Kurupi, R.; Floros, K.V.; Jacob, S.; Chawla, A.T.; Cai, J.; Hu, B.; Puchalapalli, M.; Coon, C.M.; Khatri, R.; Crowther, G.S.; et al. Pharmacologic Inhibition of SHP2 Blocks Both PI3K and MEK Signaling in Low-Epiregulin HNSCC via GAB1. Cancer Res. Commun. 2022, 2, 1061–1074. [Google Scholar] [CrossRef]
- A Phase 1b Study of Cetuximab and BYL719 (Alpelisib) Concurrent with Intensity Modulated Radiation Therapy in Stage III-IVB Head and Neck Squamous Cell Carcinoma—International Journal of Radiation Oncology, Biology, Physics. Available online: https://www.redjournal.org/article/S0360-3016(19)33963-X/abstract (accessed on 29 June 2025).
- de Wit, J.G.; Vonk, J.; Voskuil, F.J.; de Visscher, S.A.H.J.; Schepman, K.P.; Hooghiemstra, W.T.R.; Linssen, M.D.; Elias, S.G.; Halmos, G.B.; Plaat, B.E.C.; et al. EGFR-targeted fluorescence molecular imaging for intraoperative margin assessment in oral cancer patients: A phase II trial. Nat. Commun. 2023, 14, 4952. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.F.; Steuer, C.E.; Ekpenyong, A.; McCook-Veal, A.; Magliocca, K.; Patel, M.; Schmitt, N.C.; Stokes, W.; Bates, J.E.; Rudra, S.; et al. Pembrolizumab and Cabozantinib in Recurrent Metastatic Head and Neck Squamous Cell Carcinoma: A Phase 2 Trial. Nat. Med. 2023, 29, 880–887. [Google Scholar] [CrossRef]

| Phase | Aim of Study | Molecular Target | Status | Results | No. of Research |
|---|---|---|---|---|---|
| I/II | To assess the safety, determine the recommended combination dosing, and evaluate the early antitumor activity of tipifarnib and alpelisib in patients with HRAS overexpression and/or PIK3CA mutation and/or amplified recurrent/metastatic head and neck squamous cell carcinoma. | PI3K inhibition | Active, not recruiting | No results published yet | NCT04997902 |
| I | To evaluate the safety and tolerability of a conventional PI3K-alpha inhibitor (TOS-358) in adult patients with solid tumors, breast cancer, HNSCC, urothelial cancer, and endometrial cancer; monotherapy. | PI3K inhibition | Recruiting | No results published yet | NCT04997902 |
| Ib/II | To assess the safety, tolerability, and preliminary efficacy of duvelisib in combination with pembrolizumab in participants with R/M HNSCC. | PI3K inhibition | Terminated | Study stopped by a sponsor. Due to small number of participants (2 patients receiving treatment), no data published | NCT04193293 |
| II | Evaluation of the efficacy and safety of preoperative administration of MRG003 in combination with pucotenlimab ± cisplatin injection. | Anti-EGFR | Active, not recruiting | Completed | NCT06530914 |
| - | Evaluation of EGFR expression in OSCC patients. | EGFR level detection | Completed | p16, p53, and EGFR were positive in 60%, 44%, and 58% of cases, respectively. Significant associations were observed between p16 and age, tumor location, abnormal sexual habits, and lymph node involvement. p53 expression correlated with age and sexual habits, and p16 expression significantly co-occurred with p53 and EGFR | NCT06606301 |
| I/II | To assess the safety, tolerability, pharmacokinetics, and antitumor activity of YH32364 in patients with locally advanced or metastatic solid tumors. | Bispecific antibody targeting EGFR and 4-1BB | Recruiting | No results published yet | NCT06975410 |
| II | To assess the safety and feasibility of neoadjuvant PD-1 blockade, alone or in combination with TPF chemotherapy, in patients with locally advanced, resectable oral squamous cell carcinoma. | Inhibition of the immune checkpoint PD-1 | Completed | No results published yet | NCT04649476 |
| Ib/IIa | Evaluation of the efficacy, safety, and pharmacodynamics of CyPep-1 administered intralesional in combination with pembrolizumab (anti-PD-1). Analysis of the antitumor activity and local and systemic immunological effects of CyPep-1 on lesions after and without injection. | Inhibition of the immune checkpoint PD-1 and the use of a tumor membrane-targeting protein | Completed | No results published yet | NCT05383170 |
| I | The aim of the study is to assess safety, tolerability, and dose-limiting toxicity, and to determine the maximum tolerated and recommended Phase 2 dose for the further development of PRT3645. | CDK 4/6 inhibition | Completed | No results published yet | NCT05538572 |
| II | The aim of the study is to evaluate new combination therapies targeting the EGFR pathway and CDK4/6 inhibitors in patients with PD-1-resistant head and neck squamous cell carcinoma. | CDK4/6 inhibition and EGFR inhibition | Recruiting | No results published yet | NCT05721443 |
| II | The aim of the study is to evaluate new combination therapies targeting the EGFR pathway and CDK4/6 inhibitors in patients with PD-1-resistant head and neck squamous cell carcinoma. | CDK4/6 inhibition and PD-1 inhibition | Active, not recruiting | No results published yet | NCT06199271 |
| I/II | The aim of the study is to evaluate [18F]-olaparib as a radioactive tracer for imaging PARP expression in tumors using PET. | PARP inhibition | Active, not recruiting | No results published yet | NCT06482307 |
| II | Evaluation of the effectiveness of maintenance therapy with Dostarlimab and Niraparib in patients with surgically removed HNSCC. | PD-1 and PARP inhibition | Recruiting | No results published yet | NCT04681469 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarna, R.; Kubina, R.; Paździor-Heiske, M.; Halama, A.; Chudy, P.; Wala, P.; Krzykawski, K.; Nowak, I. Molecular Targets for Pharmacotherapy of Head and Neck Squamous Cell Carcinomas. Curr. Issues Mol. Biol. 2025, 47, 609. https://doi.org/10.3390/cimb47080609
Sarna R, Kubina R, Paździor-Heiske M, Halama A, Chudy P, Wala P, Krzykawski K, Nowak I. Molecular Targets for Pharmacotherapy of Head and Neck Squamous Cell Carcinomas. Current Issues in Molecular Biology. 2025; 47(8):609. https://doi.org/10.3390/cimb47080609
Chicago/Turabian StyleSarna, Robert, Robert Kubina, Marlena Paździor-Heiske, Adrianna Halama, Patryk Chudy, Paulina Wala, Kamil Krzykawski, and Ilona Nowak. 2025. "Molecular Targets for Pharmacotherapy of Head and Neck Squamous Cell Carcinomas" Current Issues in Molecular Biology 47, no. 8: 609. https://doi.org/10.3390/cimb47080609
APA StyleSarna, R., Kubina, R., Paździor-Heiske, M., Halama, A., Chudy, P., Wala, P., Krzykawski, K., & Nowak, I. (2025). Molecular Targets for Pharmacotherapy of Head and Neck Squamous Cell Carcinomas. Current Issues in Molecular Biology, 47(8), 609. https://doi.org/10.3390/cimb47080609







