Heterologous Watermelon HSP17.4 Expression Confers Improved Heat Tolerance to Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Watermelon Growth and Treatment
2.2. Bioinformatics Analysis
2.3. ClaHSP17.4 Gene Collection and ClaHSP17.4: pCAMBIA1391b-GFP Overexpression Vector Construction
2.4. Transgenic ClaHSP17.4-Overexpressing Arabidopsis Plant Selection
2.5. Heat Stress Treatment and Stress-Related Analyses
3. Results
3.1. ClaHSP17.4 Gene Characteristics
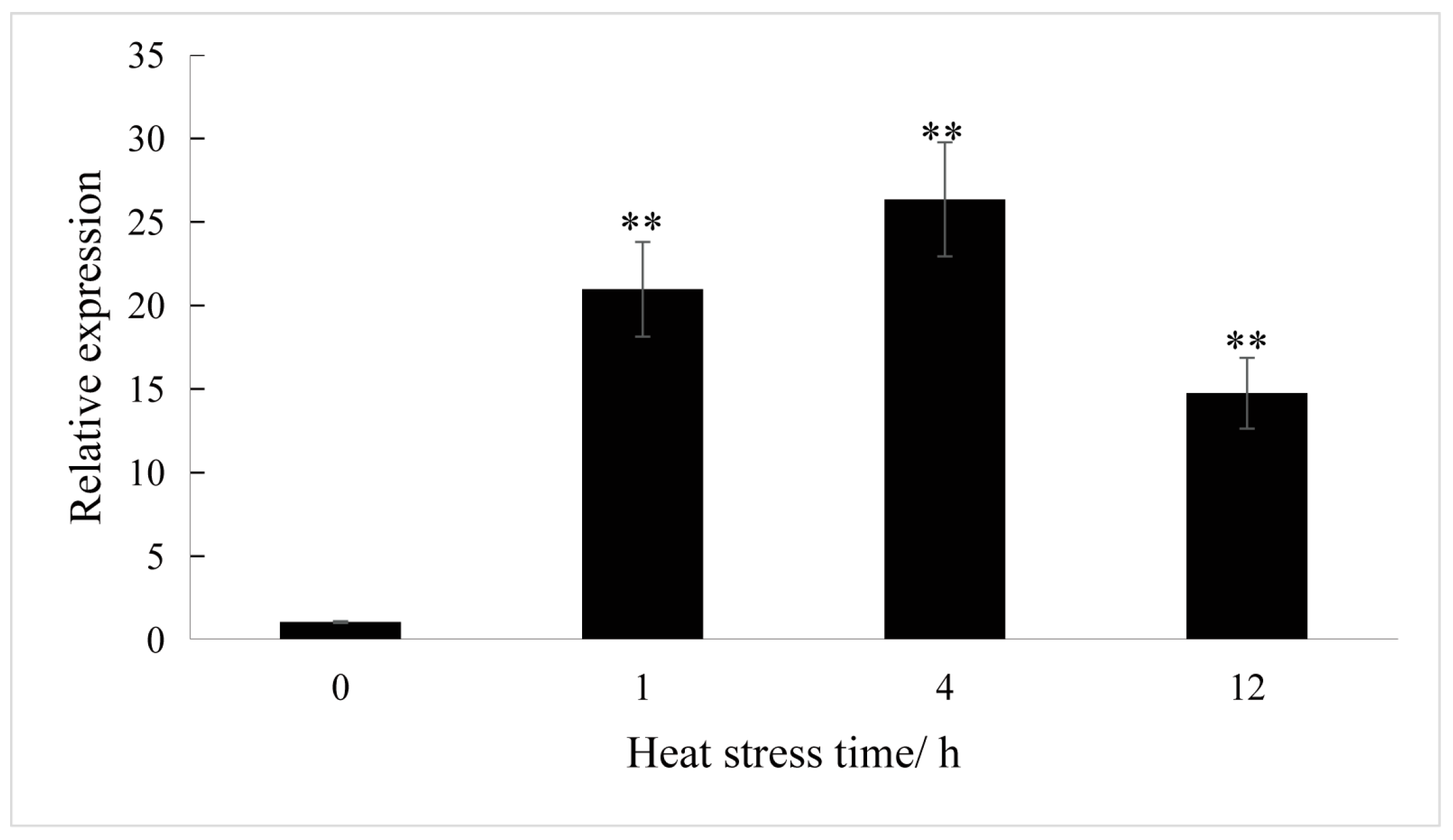
3.2. Analysis of ClaHSP17.4 Expression in Response to Heat Stress in Watermelon
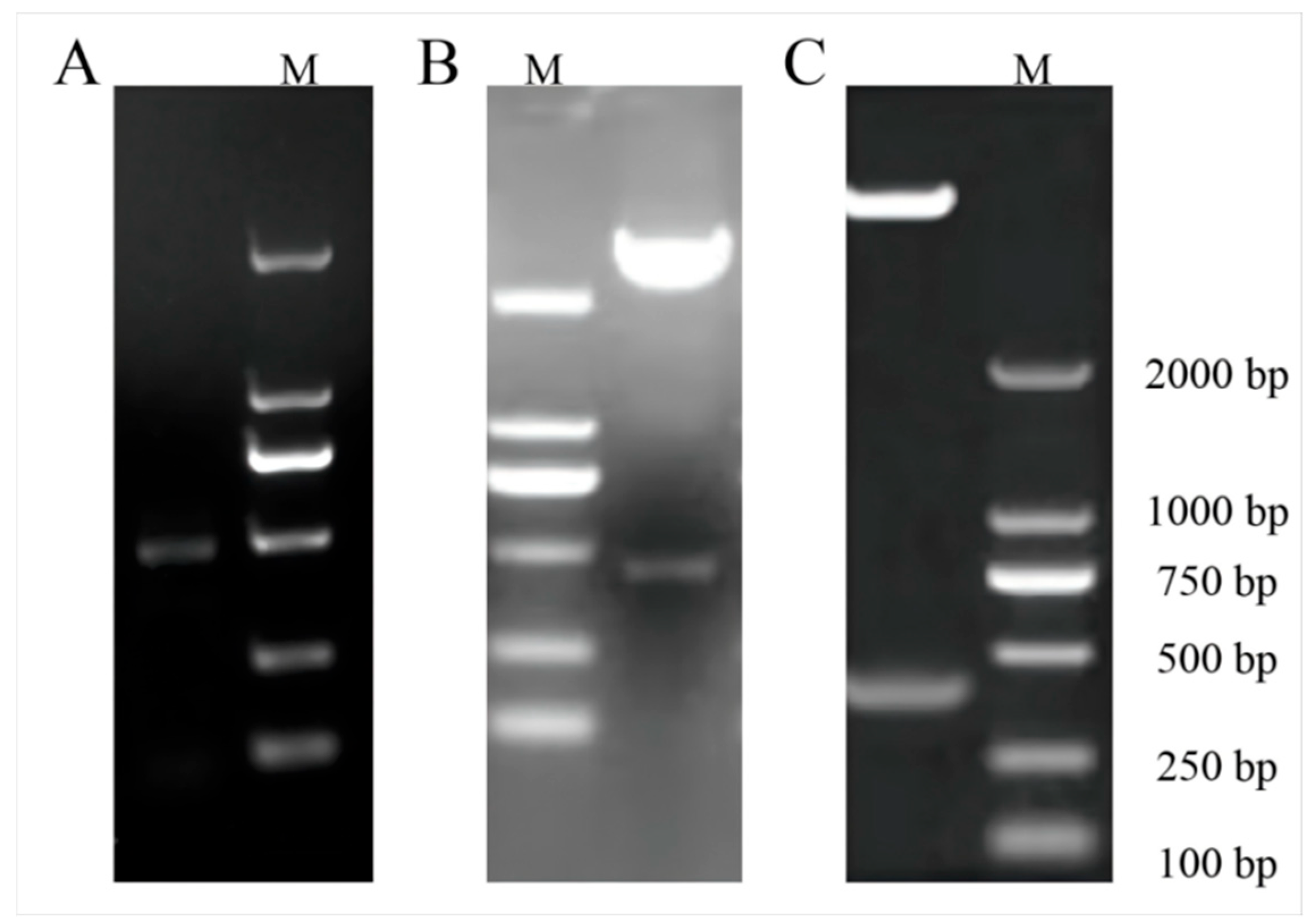
3.3. Transgenic ClaHSP17.4-Overexpressing Arabidopsis Preparation
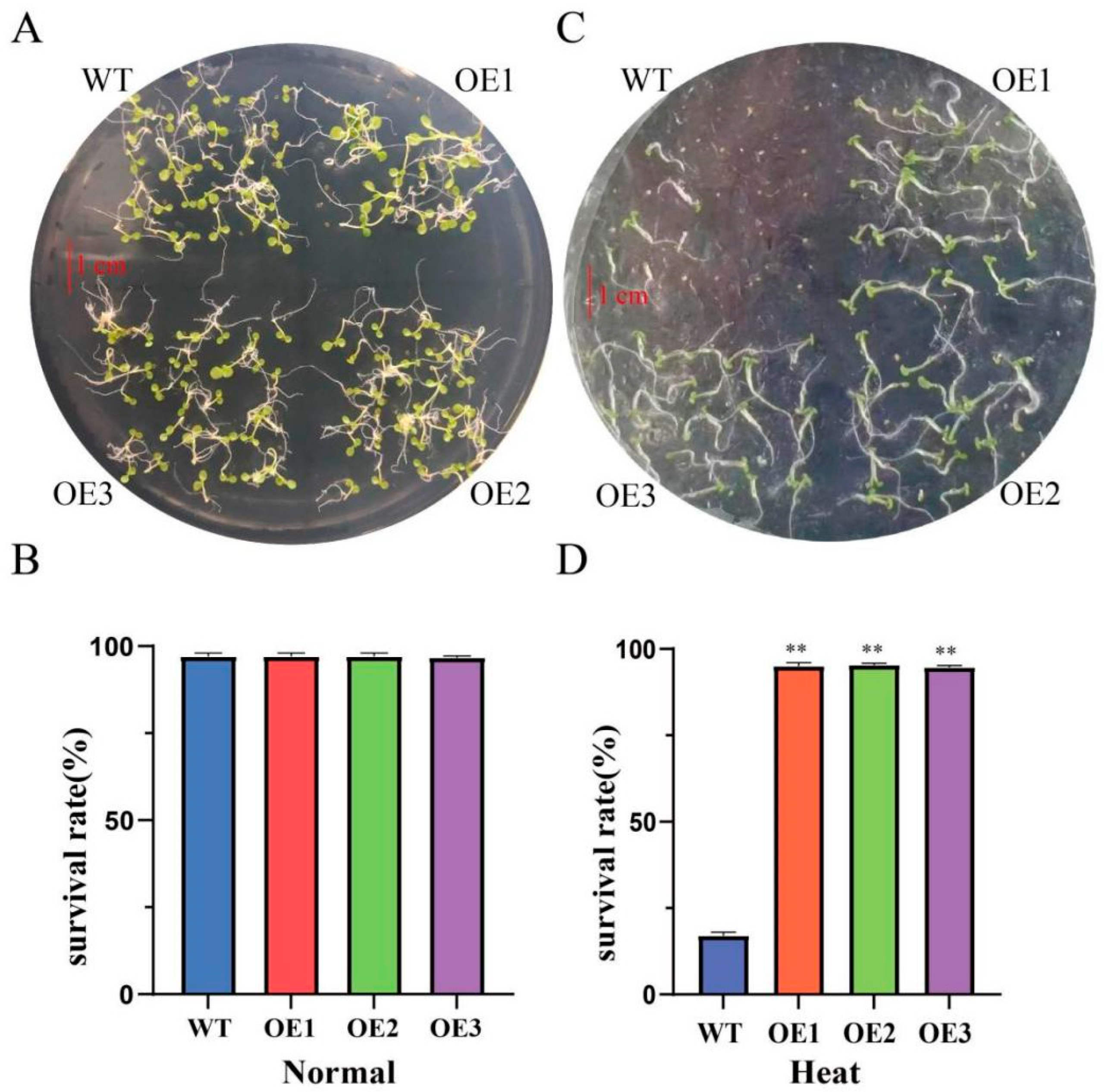
3.4. Heterologous ClaHSP17.4 Expression Enhances Arabidopsis Heat Tolerance at the Germination Stage
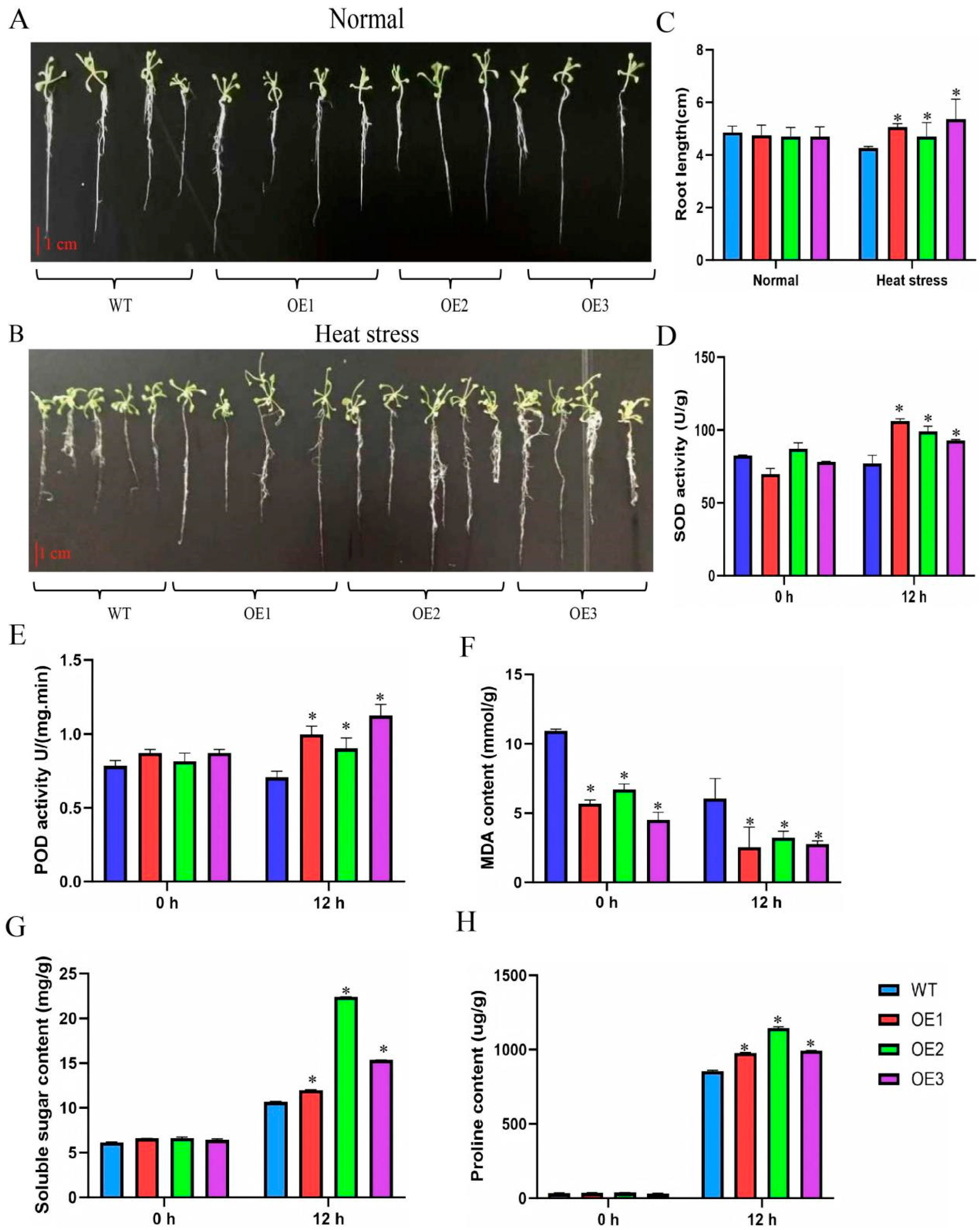
3.5. ClaHSP17.4 Improves Arabidopsis Seedling Heat Tolerance
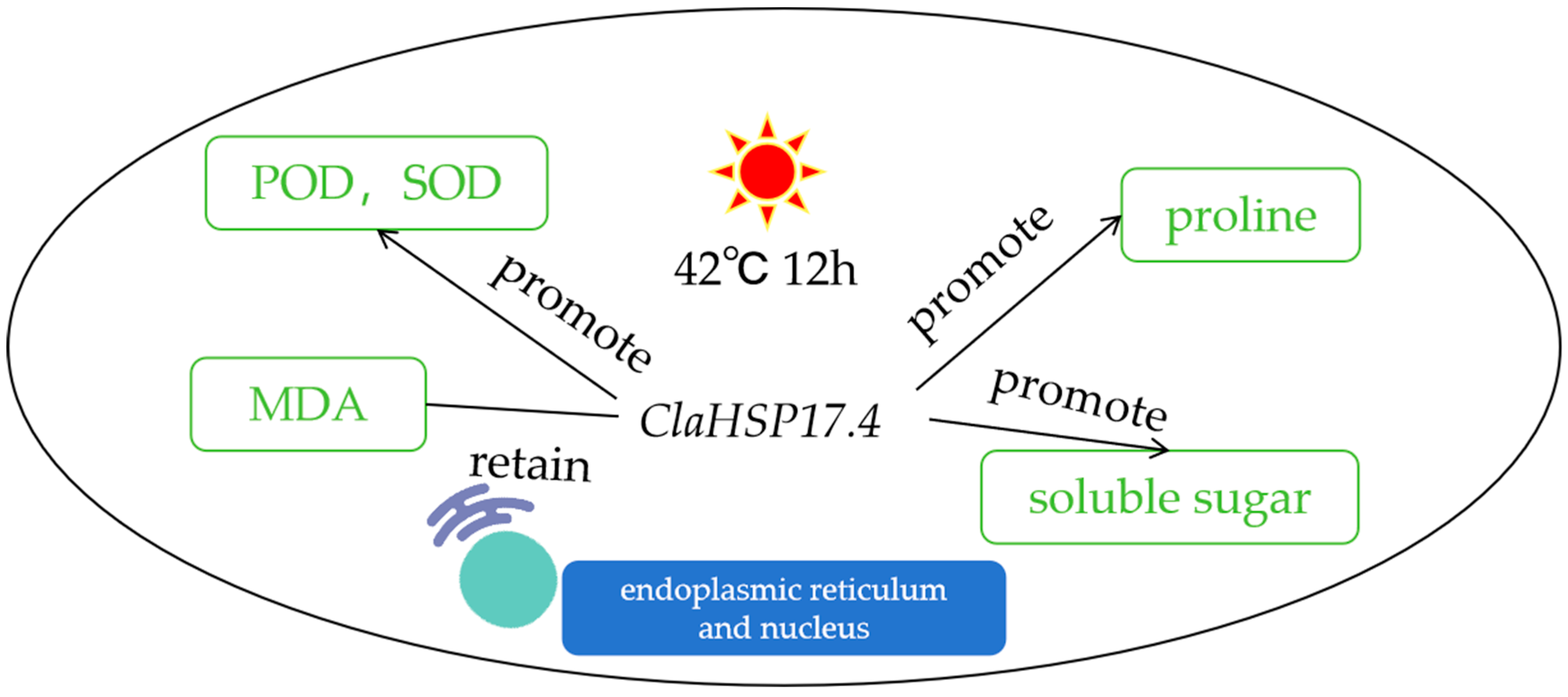
3.6. Impact of Heat Stress on MDA Content, Soluble Sugar, Proline Content, SOD Activity, and POD Activity in Arabidopsis Seedlings Overexpressing ClaHSP17.4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, X.; Mao, K.; Wang, P.; Wang, Y.; Jia, X.; Huo, L.; Sun, X.; Che, R.; Gong, X.; Ma, F. Overexpression of mdatg8i improves water use efficiency in transgenic apple by modulating photosynthesis, osmotic balance, and autophagic activity under moderate water deficit. Hortic. Res. 2021, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Garstka, M.; Venema, J.H.; Rumak, I.; Gieczewska, K.; Rosiak, M.; Koziol-Lipinska, J.; Kierdaszuk, B.; Vredenberg, W.J.; Mostowska, A. Contrasting effect of dark-chilling on chloroplast structure and arrangement of chlorophyll–protein complexes in pea and tomato: Plants with a different susceptibility to non-freezing temperature. Planta 2007, 226, 1165–1181. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.; Mishra, K.B.; Kuusisto, I.; Mishra, A.; Novotná, K.; Šebela, D.; Tyystjärvi, E. Effects of low temperature on photoinhibition and singlet oxygen production in four natural accessions of Arabidopsis. Planta 2020, 252, 19. [Google Scholar] [CrossRef]
- Garg, A.; Bordoloi, S.; Ganesan, S.P.; Sekharan, S.; Sahoo, L. A relook into plant wilting: Observational evidence based on unsaturated soil–plant-photosynthesis interaction. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Yang, K.Y.; Dong, Q.L.; Wu, J.H.; Li, H.; Luan, H.A.; Jia, P.; Zhang, X.M.; Guo, S.P.; Yang, M.S.; Qi, G.H. Genome-wide analysis of the R2R3-MYB transcription factor gene family expressed in Juglans regia under abiotic and biotic stresses. Ind. Crops Prod. 2023, 198, 279–289. [Google Scholar] [CrossRef]
- Neto, V.G.; Barbosa, R.R.; Carosio, M.G.; Ferreira, A.G.; Fernandez, L.G.; Castro, R.D.; Ligterink, W.; Hilhorst, H.; Ribeiro, P.R. Sequence analysis of Ricinus communis small heat-shock protein (sHSP) subfamily and its role in abiotic stress responses. Ind. Crops Prod. 2020, 152, 112541. [Google Scholar] [CrossRef]
- Kiang, J.G.; Tsokos, G.C. Heat shock protein 70 kDa: Molecular biology, biochemistry and physiology. Pharmacol Ther. 1998, 80, 183–201. [Google Scholar] [CrossRef]
- Panasenko, O.O.; Kim, M.V.; Gusev, N.B. Structure and properties of small heat shock protein (sHSP). Biochemistry 2002, 67, 511–519. [Google Scholar]
- Gao, T.; Mo, Z.; Tang, L.; Yu, X.Z.; Du, G.Y.; Mao, Y.X. Heat shock protein 20 gene superfamilies in red algae: Evolutionary and functional diversities. Front. Plant Sci. 2022, 13, 125–130. [Google Scholar] [CrossRef]
- Wu, J.T.; Gao, T.; Hu, J.N.; Zhao, L.; Yu, C.; Ma, F. Research advances in function and regulation mechanisms of plant small heat shock proteins (sHSPs) under environmental stresses. Sci. Total Environ. 2022, 825, 154054. [Google Scholar] [CrossRef]
- Peng, L.N.; Huang, L.B.; Gui, T.Y.; Gao, D.H.; Yan, X.H. Identification and expression profiling of HSP20 genes in Neoporphyra haitanensis. J. Appl. Phycol. 2022, 34, 1089–1097. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.Z.; Quan, X.Y.; Chen, J.F.; Wang, Z.X. Heterologously expressing a wheat CI small heat shock protein gene enhances the salinity tolerance of Arabidopsis thaliana. J. Plant Growth Regul. 2022, 41, 236–243. [Google Scholar] [CrossRef]
- Mu, C.J.; Zhang, S.J.; Yu, G.Z.; Chen, N.; Li, X.F.; Liu, H. Overexpression of small heat shock protein LimHSP16.45 in Arabidopsis enhances tolerance to abiotic stresses. PLoS ONE. 2013, 8, e82264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, H.Y.; Shi, J.W.; Wu, Y.Y.; Jiang, J. Functional characterization of class I SlHSP17.7 gene responsible for tomato cold-stress tolerance. Plant Sci. 2020, 298, 110568. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.; Takahashi, Y.; Munemasa, S.; Schroeder, J. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694, Correction in Nat. Rev. Mol. Cell Biol. 2022, 23, 516. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Dong, J.; Tian, X.Y.; Wang, J.Z.; Palta, J.A.; Xu, S.B.; Fang, Y.; Wang, Z.H. Over-expression of the heat-responsive wheat gene TaHSP23.9 in transgenic Arabidopsis conferred tolerance to heat and salt stress. Front. Plant Sci. 2020, 11, 243. [Google Scholar] [CrossRef]
- Yang, R.; Yu, G.; Li, H.; Li, X.; Mu, C. Overexpression of small heat shock protein LimHSP16.45 in Arabidopsis hsp17.6II mutant enhances tolerance to abiotic stresses. Russ. J. Plant Physiol. 2020, 67, 98–105. [Google Scholar] [CrossRef]
- He, Y.J.; Fan, M.; Sun, Y.Y.; Li, L.L. Genome-wide analysis of watermelon HSP20s and their expression profiles and subcellular locations under stresses. Int. J. Mol. Sci. 2019, 20, 12. [Google Scholar] [CrossRef]
- Shireen, F.; Nawaz, M.A.; Xiong, M.; Ahmad, A.; Sohail, H.; Chen, Z.; Abouseif, Y.; Huang, Y.; Bie, Z. Pumpkin rootstock improves the growth and development of watermelon by enhancing uptake and transport of boron and regulating the gene expression. Plant Physiol. Biochem. 2020, 154, 204–218. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Chen, C.; Shireen, F.; Zheng, Z.; Sohail, H.; Afzal, M.; Ali, M.A.; Bie, Z.; Huang, Y. Genome-wide expression profiling of leaves and roots of watermelon in response to low nitrogen. BMC Genom. 2018, 19, 456. [Google Scholar] [CrossRef]
- Liu, T.; Zheng, Y.; Yang, J.; Li, R.; Chang, H.; Li, N.; Suna, W.; Wang, L.; Wang, X. Identification of MYC genes in four Cucurbitaceae species and their roles in the response to temperature stress. BMC Genom. 2024, 25, 867. [Google Scholar] [CrossRef]
- Li, Q.P.; Yan, W.H.; Chen, H.X.; Tan, C.; Han, Z.M.; Yao, W.; Li, G.W.; Yuan, M.Q.; Xing, Y.Z. Duplication of OsHAP family genes and their association with heading date in rice. J. Exp. Bot. 2016, 67, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, N.; Ries, K. Superoxide dismutase I: Occurrence in higher plants. Plant Physiol. 1977, 59, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.J.; Lioe, H.; Robinson, C.V.; Kay, L.E.; Benesch, J.L.P. α B-crystallin polydispersity is a consequence of unbiased quaternary dynamics. J. Mol. Biol. 2011, 413, 297–309. [Google Scholar] [CrossRef]
- Waters, E.R. The evolution, function, structure, and expression of the plant sHSPs. J. Exp. Bot. 2013, 64, 391–403. [Google Scholar] [CrossRef]
- Tardieu, A.; Delaye, M. Eye lens proteins and transparency: From light transmission theory to solution X-ray structural analysis. J. Annu. Rev. Biophys. 1988, 17, 47–70. [Google Scholar] [CrossRef]
- McLoughlin, F.; Basha, E.; Fowler, M.E.; Kim, M.; Bordowitz, J.; Katiyar-Agarwal, B.; Vierling, E. Class I and II small heat shock proteins together with HSP101 protect protein translation factors during heat stress. Plant Physiol. 2016, 172, 1221–1236. [Google Scholar]
- Tang, S.; Ling, Q.; Ma, Q.; Cheng, Y.; Mei, P.; Miao, Y.; Pan, Y.; Jia, Y.; Wu, M.; Yong, X.; et al. LrHSP17.2 plays an important role in abiotic stress responses by regulating ROS scavenging and stress-related genes in Lilium regale. Plants 2024, 13, 2416. [Google Scholar] [CrossRef]
- Wehmeyer, N.; Vierling, E. The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiol. 2000, 122, 1099–1108. [Google Scholar] [CrossRef]
- Dirk, L.M.A.; Downie, A.B. An examination of Job’s rule: Protection and repair of the proteins of the translational apparatus in seeds. Seed Sci. Res. 2018, 28, 168–181. [Google Scholar] [CrossRef]
- Waters, E.R.; Vierling, E. Plant small heat shock proteins-evolutionary and functional diversity. New Phytol. 2020, 227, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.Y.; Wang, H.H.; Li, B.; Yi, Y.J.; Kong, F.J.; Liu, J.Y.; Zhang, H.X. Constitutive expression of a tomato small heat shock protein gene LeHSP21 improves tolerance to high-temperature stress by enhancing antioxidation capacity in tobacco. Plant Mol. Biol. Report. 2015, 34, 399–409. [Google Scholar] [CrossRef]
- Huang, L.J.; Cheng, G.X.; Khan, A.; Wei, A.M.; Yu, Q.H.; Yang, S.B.; Luo, D.X.; Gong, Z.H. CaHSP16.4, a small heat shock gene in pepper, is involved in heat and drought tolerance. Protoplasma. 2018, 256, 39–51. [Google Scholar] [CrossRef]
- Li, J.B.; Zhang, J.; Jia, H.X.; Li, Y.; Xu, X.D.; Wang, L.J.; Lu, M.Z. The Populus trichocarpa PtHSP17.8 involved in heat and salt stress tolerances. Plant Cell Rep. 2016, 35, 1587–1599. [Google Scholar] [CrossRef]
- Feng, X.H.; Zhang, H.X.; Ali, M.; Gai, W.X.; Cheng, G.X.; Yu, Q.H.; Yang, S.B.; Li, X.X.; Gong, Z.H. A small heat shock protein CaHsp25.9 positively regulates heat, salt, and drought stress tolerance in pepper (Capsicum annuum L.). Plant Physiol. Biochem. 2019, 142, 151–162. [Google Scholar] [CrossRef]
- Wasson, A.P.; Richards, R.; Chatrath, R.; Misra, S.; Prasad, S.S.; Rebetzke, G.; Kirkegaard, J.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenol oxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Liu, B.; Li, L.; Cheng, G.; Li, F.; Zhang, S. A pumpkin heat shock factor CmHSF30 positively regulates thermotolerance in transgenic plants. Plant Physiol. Biochem. 2025, 223, 109834. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, X.; Miao, Z.; Wang, H.; Pang, L.; Pan, Y. Hot air treatment alleviates chilling injury of sweet potato tuberous roots by regulating osmoregulatory substances and inducing antioxidant defense system. Food Chem. 2024, 459, 140393. [Google Scholar] [CrossRef]
- Zafar, S.; Yu, Y.K.; Zhu, K.M.; Wang, W.J.; Tan, X.L. Overexpression of Nicotiana tabacum Hsp17.6 enhances abiotic stress tolerance in Brassica napus. Int. J. Agric. Biol. 2020, 23, 164–170. [Google Scholar]

| Name | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| qPCR-ClaHSP17.4 | CGGGGTACCATGGATCTCAGAATCATGG | CGCGGATCCTTGACCTTGACCTCAACG |
| qRT-PCR-ClaHSP17.4 | GAGGGAGGAGGAGAAAGAG | CCTGACAAACCGCTGATAT |
| β-actin (Cla007792) | CCATGTATGTTGCCATCCAG | GGATAGCATGGGGTAGAGCA |
| Component Name | Function | Quantity |
|---|---|---|
| Box 4, TCT-motif, AE-box | part of light responsiveness | 3 |
| ARE | anaerobic induction | 4 |
| O2-site | zein metabolism regulation | 1 |
| MYB, Myb-binding site, Myb | MYB | 7 |
| TGACG-motif, CGTCA-motif | MeJA responsiveness | 2 |
| WRE3 | WRE3 | 1 |
| TGA-element | auxin-responsive element | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Li, Y.; Chen, J.; Xing, N.; Ding, W.; Chen, L.; Huang, Y.; Li, Q.; Lu, K. Heterologous Watermelon HSP17.4 Expression Confers Improved Heat Tolerance to Arabidopsis thaliana. Curr. Issues Mol. Biol. 2025, 47, 606. https://doi.org/10.3390/cimb47080606
Hong Y, Li Y, Chen J, Xing N, Ding W, Chen L, Huang Y, Li Q, Lu K. Heterologous Watermelon HSP17.4 Expression Confers Improved Heat Tolerance to Arabidopsis thaliana. Current Issues in Molecular Biology. 2025; 47(8):606. https://doi.org/10.3390/cimb47080606
Chicago/Turabian StyleHong, Yajie, Yurui Li, Jing Chen, Nailin Xing, Wona Ding, Lili Chen, Yunping Huang, Qiuping Li, and Kaixing Lu. 2025. "Heterologous Watermelon HSP17.4 Expression Confers Improved Heat Tolerance to Arabidopsis thaliana" Current Issues in Molecular Biology 47, no. 8: 606. https://doi.org/10.3390/cimb47080606
APA StyleHong, Y., Li, Y., Chen, J., Xing, N., Ding, W., Chen, L., Huang, Y., Li, Q., & Lu, K. (2025). Heterologous Watermelon HSP17.4 Expression Confers Improved Heat Tolerance to Arabidopsis thaliana. Current Issues in Molecular Biology, 47(8), 606. https://doi.org/10.3390/cimb47080606






