The Role of Tumor Microenvironment and Targeted Therapy in Chronic Lymphocytic Leukemia
Abstract
1. Introduction
2. The Tumor Microenvironment of CLL
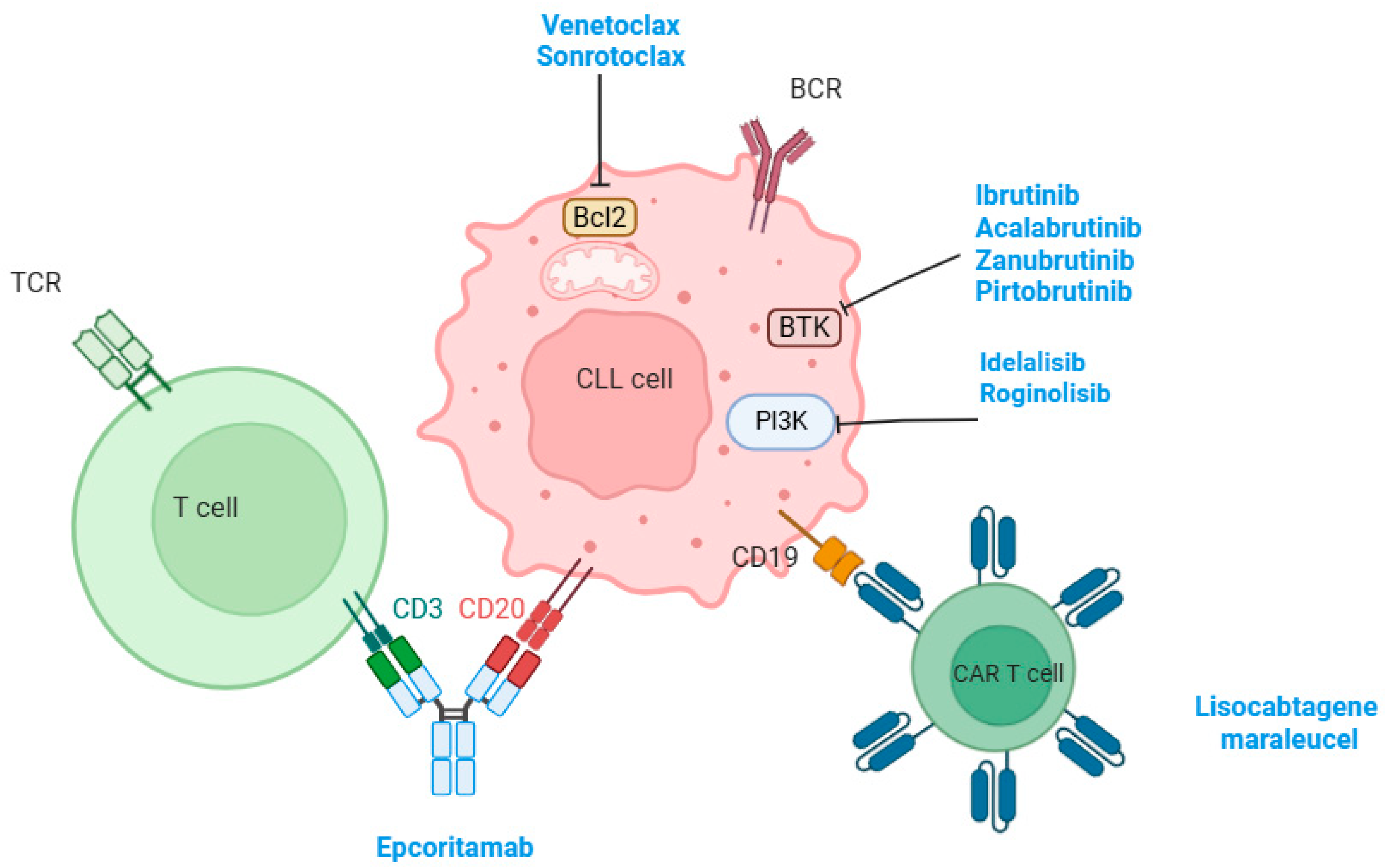
3. Targeted Therapy for the Management of CLL
3.1. BTK Inhibitors
3.2. BCL2 Inhibitors
3.3. Combination of BTK and BCL2 Inhibitors
3.4. PI3K Inhibitors
3.5. Chimeric Antigen Receptor T-Cell Therapy
3.6. Bispecific Antibodies
4. Challenges of CLL-Directed Treatments
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, X.; Li, F.; Jin, J.; Wang, H. The Global Burden and Attributable Risk Factors of Chronic Lymphocytic Leukemia in 204 Countries and Territories from 1990 to 2019: Analysis Based on the Global Burden of Disease Study 2019. Biomed. Eng. Online 2022, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Ruchlemer, R.; Polliack, A. Geography, Ethnicity and “Roots” in Chronic Lymphocytic Leukemia. Leuk. Lymphoma 2013, 54, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Huang, S.-Y.; Lin, C.-T.; Lin, Y.-J.; Chang, C.-J.; Tien, H.-F. The Incidence of Chronic Lymphocytic Leukemia in Taiwan, 1986-2005: A Distinct Increasing Trend with Birth-Cohort Effect. Blood 2010, 116, 4430–4435. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, A.; Piñeros, M.; Ferlay, J.; Soerjomataram, I.; Monnereau, A.; Bray, F. Epidemiological Patterns of Leukaemia in 184 Countries: A Population-Based Study. Lancet Haematol. 2018, 5, e14–e24. [Google Scholar] [CrossRef]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.A.; Grant, B.; Sharman, J.P.; Coleman, M.; Wierda, W.G.; et al. Targeting BTK with Ibrutinib in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2013, 369, 32–42. [Google Scholar] [CrossRef]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Byrd, J.C.; Harrington, B.; O’Brien, S.; Jones, J.A.; Schuh, A.; Devereux, S.; Chaves, J.; Wierda, W.G.; Awan, F.T.; Brown, J.R.; et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 323–332. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without Obinutuzumab versus Chlorambucil and Obinutuzmab for Treatment-Naive Chronic Lymphocytic Leukaemia (ELEVATE TN): A Randomised, Controlled, Phase 3 Trial. Lancet Lond. Engl. 2020, 395, 1278–1291, Erratum in Lancet Lond. Engl. 2020, 395, 1694. [Google Scholar] [CrossRef]
- Tam, C.S.; Brown, J.R.; Kahl, B.S.; Ghia, P.; Giannopoulos, K.; Jurczak, W.; Šimkovič, M.; Shadman, M.; Österborg, A.; Laurenti, L.; et al. Zanubrutinib versus Bendamustine and Rituximab in Untreated Chronic Lymphocytic Leukaemia and Small Lymphocytic Lymphoma (SEQUOIA): A Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2022, 23, 1031–1043, Erratum in Lancet Lond. Engl. 2023, 24, e106. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.-M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef]
- Jain, N.; Keating, M.; Thompson, P.; Ferrajoli, A.; Burger, J.; Borthakur, G.; Takahashi, K.; Estrov, Z.; Fowler, N.; Kadia, T.; et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL. N. Engl. J. Med. 2019, 380, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Munir, T.; Cairns, D.A.; Bloor, A.; Allsup, D.; Cwynarski, K.; Pettitt, A.; Paneesha, S.; Fox, C.P.; Eyre, T.A.; Forconi, F.; et al. Chronic Lymphocytic Leukemia Therapy Guided by Measurable Residual Disease. N. Engl. J. Med. 2024, 390, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Nwabo Kamdje, A.H.; Bassi, G.; Pacelli, L.; Malpeli, G.; Amati, E.; Nichele, I.; Pizzolo, G.; Krampera, M. Role of Stromal Cell-Mediated Notch Signaling in CLL Resistance to Chemotherapy. Blood Cancer J. 2012, 2, e73. [Google Scholar] [CrossRef] [PubMed]
- Montresor, A.; Toffali, L.; Rigo, A.; Ferrarini, I.; Vinante, F.; Laudanna, C. CXCR4- and BCR-Triggered Integrin Activation in B-Cell Chronic Lymphocytic Leukemia Cells Depends on JAK2-Activated Bruton’s Tyrosine Kinase. Oncotarget 2018, 9, 35123–35140. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of Bone Marrow. Analysis of Precursor Cells for Osteogenic and Hematopoietic Tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Pontikoglou, C.; Kastrinaki, M.-C.; Klaus, M.; Kalpadakis, C.; Katonis, P.; Alpantaki, K.; Pangalis, G.A.; Papadaki, H.A. Study of the Quantitative, Functional, Cytogenetic, and Immunoregulatory Properties of Bone Marrow Mesenchymal Stem Cells in Patients with B-Cell Chronic Lymphocytic Leukemia. Stem Cells Dev. 2013, 22, 1329–1341. [Google Scholar] [CrossRef]
- Pham, H.; Tonai, R.; Wu, M.; Birtolo, C.; Chen, M. CD73, CD90, CD105 and Cadherin-11 RT-PCR Screening for Mesenchymal Stem Cells from Cryopreserved Human Cord Tissue. Int. J. Stem Cells 2018, 11, 26–38. [Google Scholar] [CrossRef]
- Mangolini, M.; Götte, F.; Moore, A.; Ammon, T.; Oelsner, M.; Lutzny-Geier, G.; Klein-Hitpass, L.; Williamson, J.C.; Lehner, P.J.; Dürig, J.; et al. Notch2 Controls Non-Autonomous Wnt-Signalling in Chronic Lymphocytic Leukaemia. Nat. Commun. 2018, 9, 3839. [Google Scholar] [CrossRef]
- Patel, V.; Chen, L.S.; Wierda, W.G.; Balakrishnan, K.; Gandhi, V. Impact of Bone Marrow Stromal Cells on Bcl-2 Family Members in Chronic Lymphocytic Leukemia. Leuk. Lymphoma 2014, 55, 899–910. [Google Scholar] [CrossRef][Green Version]
- Amigo-Jiménez, I.; Bailón, E.; Aguilera-Montilla, N.; Terol, M.J.; García-Marco, J.A.; García-Pardo, A. Bone Marrow Stroma-Induced Resistance of Chronic Lymphocytic Leukemia Cells to Arsenic Trioxide Involves Mcl-1 Upregulation and Is Overcome by Inhibiting the PI3Kδ or PKCβ Signaling Pathways. Oncotarget 2015, 6, 44832–44848. [Google Scholar] [CrossRef]
- Burger, J.A.; Tsukada, N.; Burger, M.; Zvaifler, N.J.; Dell’Aquila, M.; Kipps, T.J. Blood-Derived Nurse-like Cells Protect Chronic Lymphocytic Leukemia B Cells from Spontaneous Apoptosis through Stromal Cell-Derived Factor-1. Blood 2000, 96, 2655–2663. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Endo, T.; Tsukada, N.; Ohata, J.; Kitada, S.; Reed, J.C.; Zvaifler, N.J.; Kipps, T.J. Nurselike Cells Express BAFF and APRIL, Which Can Promote Survival of Chronic Lymphocytic Leukemia Cells via a Paracrine Pathway Distinct from That of SDF-1α. Blood 2005, 106, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Smit, L.A.; Hallaert, D.Y.H.; Spijker, R.; de Goeij, B.; Jaspers, A.; Kater, A.P.; van Oers, M.H.J.; van Noesel, C.J.M.; Eldering, E. Differential Noxa/Mcl-1 Balance in Peripheral versus Lymph Node Chronic Lymphocytic Leukemia Cells Correlates with Survival Capacity. Blood 2006, 109, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Hallaert, D.Y.H.; Spijker, R.; Jak, M.; Derks, I.A.; Alves, N.L.; Wensveen, F.M.; de Boer, J.P.; de Jong, D.; Green, S.R.; van Oers, M.H.J.; et al. Crosstalk among Bcl-2 Family Members in B-CLL: Seliciclib Acts via the Mcl-1/Noxa Axis and Gradual Exhaustion of Bcl-2 Protection. Cell Death Differ. 2007, 14, 1958–1967. [Google Scholar] [CrossRef]
- Stamatopoulos, B.; Van Damme, M.; Crompot, E.; Dessars, B.; Housni, H.E.; Mineur, P.; Meuleman, N.; Bron, D.; Lagneaux, L. Opposite Prognostic Significance of Cellular and Serum Circulating MicroRNA-150 in Patients with Chronic Lymphocytic Leukemia. Mol. Med. 2015, 21, 123–133. [Google Scholar] [CrossRef]
- Schulz, A.; Toedt, G.; Zenz, T.; Stilgenbauer, S.; Lichter, P.; Seiffert, M. Inflammatory Cytokines and Signaling Pathways Are Associated with Survival of Primary Chronic Lymphocytic Leukemia Cells in Vitro: A Dominant Role of CCL2. Haematologica 2011, 96, 408–416. [Google Scholar] [CrossRef]
- Cheng, S.; Ma, J.; Guo, A.; Lu, P.; Leonard, J.P.; Coleman, M.; Liu, M.; Buggy, J.J.; Furman, R.R.; Wang, Y.L. BTK Inhibition Targets in Vivo CLL Proliferation through Its Effects on B-Cell Receptor Signaling Activity. Leukemia 2014, 28, 649–657. [Google Scholar] [CrossRef]
- Haselager, M.V.; Kater, A.P.; Eldering, E. Proliferative Signals in Chronic Lymphocytic Leukemia; What Are We Missing? Front. Oncol. 2020, 10, 592205. [Google Scholar] [CrossRef]
- Parente-Ribes, A.; Skånland, S.S.; Bürgler, S.; Os, A.; Wang, D.; Bogen, B.; Tjønnfjord, G.E.; Taskén, K.; Munthe, L.A. Spleen Tyrosine Kinase Inhibitors Reduce CD40L-Induced Proliferation of Chronic Lymphocytic Leukemia Cells but Not Normal B Cells. Haematologica 2016, 101, e59–e62. [Google Scholar] [CrossRef][Green Version]
- Vial, G.; Gensous, N.; Duffau, P. L’axe CD40-CD40L: Implications Actuelles et Futures En Immunologie Clinique. Rev. Med. Interne 2021, 42, 722–728. [Google Scholar] [CrossRef]
- Binder, M.; Léchenne, B.; Ummanni, R.; Scharf, C.; Balabanov, S.; Trusch, M.; Schlüter, H.; Braren, I.; Spillner, E.; Trepel, M. Stereotypical Chronic Lymphocytic Leukemia B-Cell Receptors Recognize Survival Promoting Antigens on Stromal Cells. PLoS ONE 2010, 5, e15992. [Google Scholar] [CrossRef]
- Burger, J.A.; Zvaifler, N.J.; Tsukada, N.; Firestein, G.S.; Kipps, T.J. Fibroblast-like Synoviocytes Support B-Cell Pseudoemperipolesis via a Stromal Cell-Derived Factor-1- and CD106 (VCAM-1)-Dependent Mechanism. J. Clin. Investig. 2001, 107, 305–315. [Google Scholar] [CrossRef]
- Möhle, R.; Failenschmid, C.; Bautz, F.; Kanz, L. Overexpression of the Chemokine Receptor CXCR4 in B Cell Chronic Lymphocytic Leukemia Is Associated with Increased Functional Response to Stromal Cell-Derived Factor-1 (SDF-1). Leukemia 1999, 13, 1954–1959. [Google Scholar] [CrossRef]
- Burger, J.A.; Burger, M.; Kipps, T.J. Chronic Lymphocytic Leukemia B Cells Express Functional CXCR4 Chemokine Receptors That Mediate Spontaneous Migration beneath Bone Marrow Stromal Cells. Blood 1999, 94, 3658–3667. [Google Scholar] [CrossRef]
- Borge, M.; Nannini, P.R.; Morande, P.E.; Jancic, C.; Bistmans, A.; Bezares, R.F.; Giordano, M.; Gamberale, R. CXCL12 Is a Costimulator for CD4+ T Cell Activation and Proliferation in Chronic Lymphocytic Leukemia Patients. Cancer Immunol. Immunother. CII 2012, 62, 113–124. [Google Scholar] [CrossRef]
- Berke Menteşe, İ.; Yegin, Z.A.; Gökçen, S.; Özkurt, Z.N.; Yağcı, M. Prognostic Significance of Serum BAFF, APRIL, TACI and BCMA Levels in Chronic Lymphocytic Leukemia. Indian J. Hematol. Blood Transfus. 2019, 35, 265–271. [Google Scholar] [CrossRef]
- Fiorcari, S.; Maffei, R.; Atene, C.G.; Potenza, L.; Luppi, M.; Marasca, R. Nurse-Like Cells and Chronic Lymphocytic Leukemia B Cells: A Mutualistic Crosstalk inside Tissue Microenvironments. Cells 2021, 10, 217. [Google Scholar] [CrossRef]
- Burger, J.A.; Chiorazzi, N. B Cell Receptor Signaling in Chronic Lymphocytic Leukemia. Trends Immunol. 2013, 34, 592–601. [Google Scholar] [CrossRef]
- Kipps, T.J.; Choi, M.Y. Targeted Therapy in Chronic Lymphocytic Leukemia. Cancer J. Sudbury Mass 2019, 25, 378–385. [Google Scholar] [CrossRef]
- Cui, B.; Chen, L.; Zhang, S.; Mraz, M.; Fecteau, J.-F.; Yu, J.; Ghia, E.M.; Zhang, L.; Bao, L.; Rassenti, L.Z.; et al. MicroRNA-155 Influences B-Cell Receptor Signaling and Associates with Aggressive Disease in Chronic Lymphocytic Leukemia. Blood 2014, 124, 546–554. [Google Scholar] [CrossRef]
- Drennan, S.; D’Avola, A.; Gao, Y.; Weigel, C.; Chrysostomou, E.; Steele, A.J.; Zenz, T.; Plass, C.; Johnson, P.W.; Williams, A.P.; et al. IL-10 Production by CLL Cells Is Enhanced in the Anergic IGHV Mutated Subset and Associates with Reduced DNA Methylation of the IL10 Locus. Leukemia 2017, 31, 1686–1694. [Google Scholar] [CrossRef]
- Niemann, C.U.; Herman, S.E.M.; Maric, I.; Gomez-Rodriguez, J.; Biancotto, A.; Chang, B.Y.; Martyr, S.; Stetler-Stevenson, M.; Yuan, C.M.; Calvo, K.R.; et al. Disruption of in Vivo Chronic Lymphocytic Leukemia Tumor-Microenvironment Interactions by Ibrutinib--Findings from an Investigator-Initiated Phase II Study. Clin. Cancer Res. 2016, 22, 1572–1582. [Google Scholar] [CrossRef]
- Zou, Y.-X.; Zhu, H.-Y.; Li, X.-T.; Xia, Y.; Miao, K.-R.; Zhao, S.-S.; Wu, Y.-J.; Wang, L.; Xu, W.; Li, J.-Y. The Impacts of Zanubrutinib on Immune Cells in Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Hematol. Oncol. 2019, 37, 392–400. [Google Scholar] [CrossRef]
- Svanberg, R.; Janum, S.; Patten, P.E.M.; Ramsay, A.G.; Niemann, C.U. Targeting the Tumor Microenvironment in Chronic Lymphocytic Leukemia. Haematologica 2021, 106, 2312–2324. [Google Scholar] [CrossRef]
- Ping, L.; Ding, N.; Shi, Y.; Feng, L.; Li, J.; Liu, Y.; Lin, Y.; Shi, C.; Wang, X.; Pan, Z.; et al. The Bruton’s Tyrosine Kinase Inhibitor Ibrutinib Exerts Immunomodulatory Effects through Regulation of Tumor-Infiltrating Macrophages. Oncotarget 2017, 8, 39218–39229. [Google Scholar] [CrossRef]
- Solman, I.G.; Blum, L.K.; Hoh, H.Y.; Kipps, T.J.; Burger, J.A.; Barrientos, J.C.; O’Brien, S.; Mulligan, S.P.; Kay, N.E.; Hillmen, P.; et al. Ibrutinib Restores Immune Cell Numbers and Function in First-Line and Relapsed/Refractory Chronic Lymphocytic Leukemia. Leuk. Res. 2020, 97, 106432. [Google Scholar] [CrossRef]
- Herman, S.E.M.; Mustafa, R.Z.; Gyamfi, J.A.; Pittaluga, S.; Chang, S.; Chang, B.; Farooqui, M.; Wiestner, A. Ibrutinib Inhibits BCR and NF-κB Signaling and Reduces Tumor Proliferation in Tissue-Resident Cells of Patients with CLL. Blood 2014, 123, 3286–3295. [Google Scholar] [CrossRef]
- Valentin, R.; Grabow, S.; Davids, M.S. The Rise of Apoptosis: Targeting Apoptosis in Hematologic Malignancies. Blood 2018, 132, 1248–1264. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a Potent and Selective BCL-2 Inhibitor, Achieves Antitumor Activity While Sparing Platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Kohlhapp, F.J.; Haribhai, D.; Mathew, R.; Duggan, R.; Ellis, P.A.; Wang, R.; Lasater, E.A.; Shi, Y.; Dave, N.; Riehm, J.J.; et al. Venetoclax Increases Intratumoral Effector T Cells and Antitumor Efficacy in Combination with Immune Checkpoint Blockade. Cancer Discov. 2021, 11, 68–79. [Google Scholar] [CrossRef]
- de Weerdt, I.; Hofland, T.; de Boer, R.; Dobber, J.A.; Dubois, J.; van Nieuwenhuize, D.; Mobasher, M.; de Boer, F.; Hoogendoorn, M.; Velders, G.A.; et al. Distinct Immune Composition in Lymph Node and Peripheral Blood of CLL Patients Is Reshaped during Venetoclax Treatment. Blood Adv. 2019, 3, 2642–2652. [Google Scholar] [CrossRef]
- Thijssen, R.; Slinger, E.; Weller, K.; Geest, C.R.; Beaumont, T.; van Oers, M.H.J.; Kater, A.P.; Eldering, E. Resistance to ABT-199 Induced by Microenvironmental Signals in Chronic Lymphocytic Leukemia Can Be Counteracted by CD20 Antibodies or Kinase Inhibitors. Haematologica 2015, 100, e302–e306. [Google Scholar] [CrossRef]
- Elias, E.E.; Sarapura Martinez, V.J.; Amondarain, M.; Colado, A.; Cordini, G.; Bezares, R.F.; Fernandez Grecco, H.; Custidiano, M.D.R.; Sánchez Ávalos, J.C.; Garate, G.; et al. Venetoclax-Resistant CLL Cells Show a Highly Activated and Proliferative Phenotype. Cancer Immunol. Immunother. 2022, 71, 979–987. [Google Scholar] [CrossRef]
- Barr, P.M.; Owen, C.; Robak, T.; Tedeschi, A.; Bairey, O.; Burger, J.A.; Hillmen, P.; Coutre, S.E.; Dearden, C.; Grosicki, S.; et al. Up to 8-Year Follow-up from RESONATE-2: First-Line Ibrutinib Treatment for Patients with Chronic Lymphocytic Leukemia. Blood Adv. 2022, 6, 3440–3450. [Google Scholar] [CrossRef]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Samoilova, O.; Novak, J.; Ben-Yehuda, D.; et al. Ibrutinib plus Obinutuzumab versus Chlorambucil plus Obinutuzumab in First-Line Treatment of Chronic Lymphocytic Leukaemia (iLLUMINATE): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2019, 20, 43–56, Erratum in Lancet Oncol. 2019, 20, e10. [Google Scholar] [CrossRef]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Novak, J.; Strugov, V.; Gill, D.; et al. First-Line Treatment of Chronic Lymphocytic Leukemia with Ibrutinib plus Obinutuzumab versus Chlorambucil plus Obinutuzumab: Final Analysis of the Randomized, Phase III iLLUMINATE Trial. Haematologica 2022, 107, 2108–2120. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Woyach, J.A.; Perez Burbano, G.; Ruppert, A.S.; Miller, C.; Heerema, N.A.; Zhao, W.; Wall, A.; Ding, W.; Bartlett, N.L.; Brander, D.M.; et al. Follow-up from the A041202 Study Shows Continued Efficacy of Ibrutinib Regimens for Older Adults with CLL. Blood 2024, 143, 1616–1627, Erratum in Blood 2025, 145, 548–549. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Hanson, C.A.; Paietta, E.M.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Long-Term Outcomes for Ibrutinib-Rituximab and Chemoimmunotherapy in CLL: Updated Results of the E1912 Trial. Blood 2022, 140, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Pitchford, A.; Bloor, A.; Broom, A.; Young, M.; Kennedy, B.; Walewska, R.; Furtado, M.; Preston, G.; Neilson, J.R.; et al. Ibrutinib and Rituximab versus Fludarabine, Cyclophosphamide, and Rituximab for Patients with Previously Untreated Chronic Lymphocytic Leukaemia (FLAIR): Interim Analysis of a Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2023, 24, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Kozak, T.; Simkovic, M.; Kaplan, P.; Kraychok, I.; Illes, A.; de la Serna, J.; et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2020, 38, 2849–2861. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Šimkovič, M.; Kriachok, I.; Illés, Á.; de la Serna, J.; Dolan, S.; Campbell, P.; et al. Acalabrutinib Versus Investigator’s Choice in Relapsed/Refractory Chronic Lymphocytic Leukemia: Final ASCEND Trial Results. HemaSphere 2022, 6, e801. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Patel, K.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Hughes, M.; et al. Acalabrutinib ± Obinutuzumab vs Obinutuzumab + Chlorambucil in Treatment-Naive Chronic Lymphocytic Leukemia: 6-Year Follow-up of Elevate-TN. Blood 2023, 142, 636. [Google Scholar] [CrossRef]
- Shadman, M.; Munir, T.; Robak, T.; Brown, J.R.; Kahl, B.S.; Ghia, P.; Giannopoulos, K.; Šimkovič, M.; Österborg, A.; Laurenti, L.; et al. Zanubrutinib Versus Bendamustine and Rituximab in Patients with Treatment-Naïve Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: Median 5-Year Follow-Up of SEQUOIA. J. Clin. Oncol. 2025, 43, 780–787. [Google Scholar] [CrossRef]
- Tam, C.S.; Robak, T.; Ghia, P.; Kahl, B.S.; Walker, P.; Janowski, W.; Simpson, D.; Shadman, M.; Ganly, P.S.; Laurenti, L.; et al. Zanubrutinib Monotherapy for Patients with Treatment Naïve Chronic Lymphocytic Leukemia and 17p Deletion. Haematologica 2021, 106, 2354–2363. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kaźmierczak, M.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 319–332. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef]
- Gomez, E.B.; Ebata, K.; Randeria, H.S.; Rosendahl, M.S.; Cedervall, E.P.; Morales, T.H.; Hanson, L.M.; Brown, N.E.; Gong, X.; Stephens, J.; et al. Preclinical Characterization of Pirtobrutinib, a Highly Selective, Noncovalent (Reversible) BTK Inhibitor. Blood 2023, 142, 62–72. [Google Scholar] [CrossRef]
- Mato, A.R.; Woyach, J.A.; Brown, J.R.; Ghia, P.; Patel, K.; Eyre, T.A.; Munir, T.; Lech-Maranda, E.; Lamanna, N.; Tam, C.S.; et al. Pirtobrutinib after a Covalent BTK Inhibitor in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 389, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Munir, T.; Grosicki, S.; Roeker, L.; Burke, J.M.; Chen, C.I.; Grzasko, N.; Follows, G.; Mátrai, Z.; Sanna, A.; et al. BRUIN CLL-321: Randomized Phase III Trial of Pirtobrutinib Versus Idelalisib Plus Rituximab (IdelaR) or Bendamustine Plus Rituximab (BR) in BTK Inhibitor Pretreated Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Blood 2024, 144, 886. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Jurczak, W.; Zhou, K.; Šimkovič, M.; Mayer, J.; et al. Sustained Benefit of Zanubrutinib vs Ibrutinib in Patients with R/R CLL/SLL: Final Comparative Analysis of ALPINE. Blood 2024, 144, 2706–2717. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawaf, O.; Robrecht, S.; Zhang, C.; Olivieri, S.; Chang, Y.M.; Fink, A.M.; Tausch, E.; Schneider, C.; Ritgen, M.; Kreuzer, K.-A.; et al. Venetoclax-Obinutuzumab for Previously Untreated Chronic Lymphocytic Leukemia: 6-Year Results of the Randomized Phase 3 CLL14 Study. Blood 2024, 144, 1924–1935. [Google Scholar] [CrossRef]
- Eichhorst, B.; Niemann, C.U.; Kater, A.P.; Fürstenau, M.; von Tresckow, J.; Zhang, C.; Robrecht, S.; Gregor, M.; Juliusson, G.; Thornton, P.; et al. First-Line Venetoclax Combinations in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 1739–1754. [Google Scholar] [CrossRef]
- Fürstenau, M.; Ritgen, M.; Robrecht, S.; Von Tresckow, J.; Zhang, C.; Schilhabel, A.; Gregor, M.; Thornton, P.; Staber, P.B.; Tadmor, T.; et al. First-Line Venetoclax Combinations in Fit Patients with CLL: 4-Year Follow-up and NGS-Based MRD Analysis from the Phase 3 GAIA/CLL13 Trial. Blood 2023, 142, 635. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- Blombery, P.; Anderson, M.A.; Gong, J.-N.; Thijssen, R.; Birkinshaw, R.W.; Thompson, E.R.; Teh, C.E.; Nguyen, T.; Xu, Z.; Flensburg, C.; et al. Acquisition of the Recurrent Gly101Val Mutation in BCL2 Confers Resistance to Venetoclax in Patients with Progressive Chronic Lymphocytic Leukemia. Cancer Discov. 2019, 9, 342–353. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Wang, Q.; Feng, Y.; Xing, H.; Yang, X.; Guo, Y.; Guo, Y.; Sun, H.; Liu, X.; et al. Sonrotoclax Overcomes BCL2 G101V Mutation-Induced Venetoclax Resistance in Preclinical Models of Hematologic Malignancy. Blood 2024, 143, 1825–1836. [Google Scholar] [CrossRef]
- Jain, N.; Keating, M.; Thompson, P.; Ferrajoli, A.; Burger, J.A.; Borthakur, G.; Takahashi, K.; Estrov, Z.; Sasaki, K.; Fowler, N.; et al. Ibrutinib Plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: A Nonrandomized Phase 2 Trial. JAMA Oncol. 2021, 7, 1213–1219. [Google Scholar] [CrossRef]
- Ghia, P.; Wierda, W.G.; Barr, P.M.; Kipps, T.J.; Siddiqi, T.; Allan, J.N.; Hunter, Z.; Zhou, C.; Szoke, A.; Dean, J.P.; et al. Relapse after First-Line Fixed Duration Ibrutinib + Venetoclax: High Response Rates to Ibrutinib Retreatment and Absence of BTK Mutations in Patients with Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL) with up to 5 Years of Follow-up in the Phase 2 Captivate Study. Blood 2023, 142, 633. [Google Scholar] [CrossRef]
- Kater, A.P.; Owen, C.; Moreno, C.; Follows, G.; Munir, T.; Levin, M.-D.; Benjamini, O.; Janssens, A.; Osterborg, A.; Robak, T.; et al. Fixed-Duration Ibrutinib-Venetoclax in Patients with Chronic Lymphocytic Leukemia and Comorbidities. NEJM Evid. 2022, 1, EVIDoa2200006. [Google Scholar] [CrossRef]
- Niemann, C.U.; Munir, T.; Moreno, C.; Owen, C.; Follows, G.A.; Benjamini, O.; Janssens, A.; Levin, M.-D.; Robak, T.; Simkovic, M.; et al. Fixed-Duration Ibrutinib-Venetoclax versus Chlorambucil-Obinutuzumab in Previously Untreated Chronic Lymphocytic Leukaemia (GLOW): 4-Year Follow-up from a Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2023, 24, 1423–1433. [Google Scholar] [CrossRef]
- Davids, M.S.; Lampson, B.L.; Tyekucheva, S.; Wang, Z.; Lowney, J.C.; Pazienza, S.; Montegaard, J.; Patterson, V.; Weinstock, M.; Crombie, J.L.; et al. Acalabrutinib, Venetoclax, and Obinutuzumab as Frontline Treatment for Chronic Lymphocytic Leukaemia: A Single-Arm, Open-Label, Phase 2 Study. Lancet Oncol. 2021, 22, 1391–1402. [Google Scholar] [CrossRef]
- Davids, M.S.; Ryan, C.E.; Lampson, B.L.; Ren, Y.; Tyekucheva, S.; Fernandes, S.M.; Crombie, J.L.; Kim, A.I.; Weinstock, M.; Montegaard, J.; et al. Phase II Study of Acalabrutinib, Venetoclax, and Obinutuzumab in a Treatment-Naïve Chronic Lymphocytic Leukemia Population Enriched for High-Risk Disease. J. Clin. Oncol. 2025, 43, 788–799, Erratum in J. Clin. Oncol. 2025, 43, 1521–1522. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Seymour, J.F.; Jurczak, W.; Aw, A.; Wach, M.; Illes, A.; Tedeschi, A.; Owen, C.; Skarbnik, A.; Lysak, D.; et al. Fixed-Duration Acalabrutinib Combinations in Untreated Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2025, 392, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Combination of Zanubrutinib + Venetoclax for Treatment-Naive (TN) CLL/SLL with del(17p) and/or TP53: Preliminary Results from SEQUOIA Arm D. Available online: https://www.beigenemedical.com/CongressDocuments/Ma_BGB-3111-304_ArmD_EHA_Abstract_2024.pdf (accessed on 21 July 2025).
- Soumerai, J.D.; Mato, A.R.; Dogan, A.; Seshan, V.E.; Joffe, E.; Flaherty, K.; Carter, J.; Hochberg, E.; Barnes, J.A.; Hamilton, A.M.; et al. Zanubrutinib, Obinutuzumab, and Venetoclax with Minimal Residual Disease-Driven Discontinuation in Previously Untreated Patients with Chronic Lymphocytic Leukaemia or Small Lymphocytic Lymphoma: A Multicentre, Single-Arm, Phase 2 Trial. Lancet Haematol. 2021, 8, e879–e890. [Google Scholar] [CrossRef] [PubMed]
- Soumerai, J.D.; Cheah, C.Y.; Anderson, M.A.; Lasica, M.; Verner, E.; Opat, S.S.; Ma, S.; Weinkove, R.; Cordoba, R.; Ghia, P.; et al. Sonrotoclax and Zanubrutinib as Frontline Treatment for CLL Demonstrates High MRD Clearance Rates with Good Tolerability: Data from an Ongoing Phase 1/1b Study BGB-11417-101. Blood 2024, 144, 1012. [Google Scholar] [CrossRef]
- Cheah, C.Y. Updated Results from the Phase 1 Study of Sonrotoclax (BGB-11417), A Novel BCL2 Inhibitor, in Combination with Zanubrutinib for Relapsed/Refractory CLL/SLL Demonstrate Deep and Durable Responses. Available online: https://library.ehaweb.org/eha/2025/eha2025-congress/4159236/chan.y.cheah.updated.results.from.the.phase.1.study.of.sonrotoclax.28bgb-1141729.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D2%2Atopic%3D1574%2Asearch%3Dcll (accessed on 21 July 2025).
- Roeker, L.E.; Woyach, J.A.; Cheah, C.Y.; Coombs, C.C.; Shah, N.N.; Wierda, W.G.; Patel, M.R.; Lamanna, N.; Tsai, D.E.; Nair, B.; et al. Fixed-Duration Pirtobrutinib plus Venetoclax with or without Rituximab in Relapsed/Refractory CLL: The Phase 1b BRUIN Trial. Blood 2024, 144, 1374–1386. [Google Scholar] [CrossRef]
- Brown, J.R.; Furman, R.R.; Flinn, I.; Coutre, S.E.; Wagner-Johnston, N.D.; Kahl, B.S.; Spurgeon, S.E.F.; Benson, D.M.; Peterman, S.; Johnson, D.M.; et al. Final Results of a Phase I Study of Idelalisib (GSE1101) a Selective Inhibitor of PI3Kδ, in Patients with Relapsed or Refractory CLL. J. Clin. Oncol. 2013, 31, 7003. [Google Scholar] [CrossRef]
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef]
- Ajina, A.; Maher, J. Strategies to Address Chimeric Antigen Receptor Tonic Signaling. Mol. Cancer Ther. 2018, 17, 1795–1815. [Google Scholar] [CrossRef]
- Jensen, M.C.; Riddell, S.R. Designing Chimeric Antigen Receptors to Effectively and Safely Target Tumors. Curr. Opin. Immunol. 2015, 33, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia. N. Engl. J. Med. 2011, 365, 725–733, Erratum in N. Engl. J. Med. 2016, 374, 998. [Google Scholar] [CrossRef] [PubMed]
- Guedan, S.; Chen, X.; Madar, A.; Carpenito, C.; McGettigan, S.E.; Frigault, M.J.; Lee, J.; Posey, A.D.; Scholler, J.; Scholler, N.; et al. ICOS-Based Chimeric Antigen Receptors Program Bipolar TH17/TH1 Cells. Blood 2014, 124, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.; Khalife, N.; Arbab, A.; Khoury, R.; Chahine, C.; Ibrahim, R.; Tikriti, Z.; Masri, N.; Hachem, M.; Le Cesne, A. Updates on Chimeric Antigen Receptor T-Cells in Large B-Cell Lymphoma. Biomedicines 2024, 12, 2810. [Google Scholar] [CrossRef]
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. KTE-X19 for Relapsed or Refractory Adult B-Cell Acute Lymphoblastic Leukaemia: Phase 2 Results of the Single-Arm, Open-Label, Multicentre ZUMA-3 Study. Lancet Lond. Engl. 2021, 398, 491–502. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Morschhauser, F.; Dahiya, S.; Palomba, M.L.; Martin Garcia-Sancho, A.; Reguera Ortega, J.L.; Kuruvilla, J.; Jäger, U.; Cartron, G.; Izutsu, K.; Dreyling, M.; et al. Lisocabtagene Maraleucel in Follicular Lymphoma: The Phase 2 TRANSCEND FL Study. Nat. Med. 2024, 30, 2199–2207, Erratum in Nat. Med. 2024, 30, 2374. [Google Scholar] [CrossRef]
- Wang, M.; Siddiqi, T.; Gordon, L.I.; Kamdar, M.; Lunning, M.; Hirayama, A.V.; Abramson, J.S.; Arnason, J.; Ghosh, N.; Mehta, A.; et al. Lisocabtagene Maraleucel in Relapsed/Refractory Mantle Cell Lymphoma: Primary Analysis of the Mantle Cell Lymphoma Cohort From TRANSCEND NHL 001, a Phase I Multicenter Seamless Design Study. J. Clin. Oncol. 2024, 42, 1146–1157. [Google Scholar] [CrossRef]
- Porter, D.L.; Hwang, W.-T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric Antigen Receptor T Cells Persist and Induce Sustained Remissions in Relapsed Refractory Chronic Lymphocytic Leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef]
- Frey, N.V.; Gill, S.; Hexner, E.O.; Schuster, S.; Nasta, S.; Loren, A.; Svoboda, J.; Stadtmauer, E.; Landsburg, D.J.; Mato, A.; et al. Long-Term Outcomes From a Randomized Dose Optimization Study of Chimeric Antigen Receptor Modified T Cells in Relapsed Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2020, 38, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, T.; Maloney, D.G.; Kenderian, S.S.; Brander, D.M.; Dorritie, K.; Soumerai, J.; Riedell, P.A.; Shah, N.N.; Nath, R.; Fakhri, B.; et al. Lisocabtagene Maraleucel in Chronic Lymphocytic Leukaemia and Small Lymphocytic Lymphoma (TRANSCEND CLL 004): A Multicentre, Open-Label, Single-Arm, Phase 1-2 Study. Lancet Lond. Engl. 2023, 402, 641–654. [Google Scholar] [CrossRef]
- Wierda, W.G.; Dorritie, K.; Gauthier, J.; Nath, R.; Kipps, T.J.; Riedell, P.A.; Eradat, H.A.; Kenderian, S.S.; Kharfan-Dabaja, M.A.; Shah, N.N.; et al. Lisocabtagene Maraleucel (Liso-Cel) Combined with Ibrutinib (Ibr) for Patients (Pts) with Relapsed or Refractory (R/R) Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL): Primary Results from the Open-Label, Phase 1/2 Transcend CLL 004 Study. Blood 2024, 144, 887. [Google Scholar] [CrossRef]
- Gill, S.; Vides, V.; Frey, N.V.; Hexner, E.O.; Metzger, S.; O’Brien, M.; Hwang, W.-T.; Brogdon, J.L.; Davis, M.M.; Fraietta, J.A.; et al. Anti-CD19 CAR T Cells in Combination with Ibrutinib for the Treatment of Chronic Lymphocytic Leukemia. Blood Adv. 2022, 6, 5774–5785. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.J.; Barba, P.; Jäger, U.; Shah, N.N.; Blaise, D.; Briones, J.; Shune, L.; Boissel, N.; Bondanza, A.; Mariconti, L.; et al. A Novel Autologous CAR-T Therapy, YTB323, with Preserved T-Cell Stemness Shows Enhanced CAR T-Cell Efficacy in Preclinical and Early Clinical Development. Cancer Discov. 2023, 13, 1982–1997. [Google Scholar] [CrossRef]
- Liang, E.C.; Albittar, A.; Huang, J.J.; Hirayama, A.V.; Kimble, E.L.; Portuguese, A.J.; Chapuis, A.; Shadman, M.; Till, B.G.; Cassaday, R.D.; et al. Factors Associated with Long-Term Outcomes of CD19 CAR T-Cell Therapy for Relapsed/Refractory CLL. Blood Adv. 2023, 7, 6990–7005. [Google Scholar] [CrossRef]
- Roddie, C.; Sandhu, K.S.; Tholouli, E.; Logan, A.C.; Shaughnessy, P.; Barba, P.; Ghobadi, A.; Guerreiro, M.; Yallop, D.; Abedi, M.; et al. Obecabtagene Autoleucel in Adults with B-Cell Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2024, 391, 2219–2230. [Google Scholar] [CrossRef]
- Roddie, C.; Tholouli, E.; Shaughnessy, P.; Jabbour, E.; Logan, A.C.; Hodby, K.; Mountjoy, L.; Bloor, A.J.C.; Irvine, D.; Linch, D.C.; et al. Long-Term Efficacy and Safety of Obecabtagene Autoleucel (Obe-Cel) in Adult Patients (Pts) with Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia ([R/R B-ALL]; Pooled Analysis from ALLCAR19 and FELIX Phase Ib Studies) or Other B-Cell Malignancies (ALLCAR19 Extension Study). Blood 2023, 142, 2114. [Google Scholar] [CrossRef]
- Luo, Y.; Qie, Y.; Gadd, M.E.; Manna, A.; Rivera-Valentin, R.; To, T.; Li, S.; Yassine, F.; Murthy, H.S.; Dronca, R.; et al. Translational Development of a Novel BAFF-R CAR-T Therapy Targeting B-Cell Lymphoid Malignancies. Cancer Immunol. Immunother. 2023, 72, 4031–4047. [Google Scholar] [CrossRef]
- Dong, Z.; Cheng, W.A.; Smith, D.L.; Huang, B.; Zhang, T.; Chang, W.-C.; Wang, X.; Forman, S.J.; Kwak, L.W.; Qin, H. Antitumor Efficacy of BAFF-R Targeting CAR T Cells Manufactured under Clinic-Ready Conditions. Cancer Immunol. Immunother. 2020, 69, 2139–2145. [Google Scholar] [CrossRef]
- Robinson, H.R.; Qi, J.; Cook, E.M.; Nichols, C.; Dadashian, E.L.; Underbayev, C.; Herman, S.E.M.; Saba, N.S.; Keyvanfar, K.; Sun, C.; et al. A CD19/CD3 Bispecific Antibody for Effective Immunotherapy of Chronic Lymphocytic Leukemia in the Ibrutinib Era. Blood 2018, 132, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Mhibik, M.; Gaglione, E.M.; Eik, D.; Herrick, J.; Le, J.; Ahn, I.E.; Chiu, C.; Wielgos-Bonvallet, M.; Hiemstra, I.H.; Breij, E.C.W.; et al. Cytotoxicity of the CD3×CD20 Bispecific Antibody Epcoritamab in CLL Is Increased by Concurrent BTK or BCL-2 Targeting. Blood Adv. 2023, 7, 4089–4101. [Google Scholar] [CrossRef] [PubMed]
- Danilov, A.; Fakhri, B.; Awan, F.T.; Bentzen, H.H.; Eradat, H.A.; Niemann, C.U.; Offner, F.; Poulsen, C.B.; Hoeyer, T.; Bellido, M.; et al. Epcoritamab Monotherapy in Patients (Pts) with Relapsed or Refractory (R/R) Chronic Lymphocytic Leukemia (CLL): Results from CLL Expansion and Optimization Cohorts of Epcore CLL-1. Blood 2024, 144, 883. [Google Scholar] [CrossRef]
- Townsend, W.; Leong, S.; Shah, M.; Batten, T.; Tucker, D.; Pottinger, B.; Paneesha, S.; El-Sharkawi, D.; Eyre, T.A.; Lam, H.P.J.; et al. Time Limited Exposure to a ROR1 Targeting Bispecific T Cell Engager (NVG-111) Leads to Durable Responses in Subjects with Relapsed Refractory Chronic Lymphocytic Leukemia (CLL) and Mantle Cell Lymphoma (MCL). Blood 2023, 142, 329. [Google Scholar] [CrossRef]
- Woyach, J.A.; Furman, R.R.; Liu, T.-M.; Ozer, H.G.; Zapatka, M.; Ruppert, A.S.; Xue, L.; Li, D.H.-H.; Steggerda, S.M.; Versele, M.; et al. Resistance Mechanisms for the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib. N. Engl. J. Med. 2014, 370, 2286–2294. [Google Scholar] [CrossRef]
- Jain, N.; Keating, M.J. Richter Transformation of CLL. Expert Rev. Hematol. 2016, 9, 793–801. [Google Scholar] [CrossRef]
- Davids, M.S.; Rogers, K.A.; Tyekucheva, S.; Wang, Z.; Pazienza, S.; Renner, S.K.; Montegaard, J.; Ihuoma, U.; Lehmberg, T.Z.; Parry, E.M.; et al. Venetoclax plus Dose-Adjusted R-EPOCH for Richter Syndrome. Blood 2022, 139, 686–689. [Google Scholar] [CrossRef]
- Wierda, W.G.; Shah, N.N.; Cheah, C.Y.; Lewis, D.; Hoffmann, M.S.; Coombs, C.C.; Lamanna, N.; Ma, S.; Jagadeesh, D.; Munir, T.; et al. Pirtobrutinib, a Highly Selective, Non-Covalent (Reversible) BTK Inhibitor in Patients with B-Cell Malignancies: Analysis of the Richter Transformation Subgroup from the Multicentre, Open-Label, Phase 1/2 BRUIN Study. Lancet Haematol. 2024, 11, e682–e692. [Google Scholar] [CrossRef]
| Trial | Treatment Arms | Line of Treatment | Population | Nb of Pts | Median Age (y) (Range) | Disease Characteristics | Primary Endpoint | U-MRD Rate (PB or BM) |
|---|---|---|---|---|---|---|---|---|
| REASONATE-2 [7,55] | Ibru vs. Clb | 1 | 65 years or older | 269 | 73 (65–89) vs. 72 (65–90) | U-IGHV: 43% vs. 45% Del(11q): 21% vs. 19% Del(17p) excluded | mPFS: 8.9 y vs. 1.3 y | NA |
| ILLUMINATE [56,57] | Ibru + Obn vs. Clb + Obn | 1 | 65 y or older younger than 65 y with comorbidities | 229 | 70 (66–75) vs. 72 (66–77) | U-IGHV: 62% vs. 53% Del(17p): 12% vs. 16% TP53 mutation: 12% vs. 15% Del(11q): 12% vs. 19% | mPFS: NR vs. 22 months | 38% vs. 25% (p = 0.033) |
| Alliance A041202 [58,59] | Benda + R vs. Ibru vs. Ibru + R | 1 | 65 y or older | 547 | 71 (65–89) | U-IGHV: 58% vs. 63% vs. 61% Del(17p): 8% vs. 5% vs. 6% TP53 mutation: 9% vs. 9% vs. 12% Del(11q): 18% vs. 19% vs. 21% | mPFS: 44 m vs. NR vs. NR 48-month PFS rate: 47% vs. 76% vs. 76% | 4% vs. 0.3% vs. 2% |
| ECOG E1912 [60,61] | Ibru + R vs. FCR | 1 | 70 years or younger | 529 | 57 ± 7.4 (mean) | U-IGHV: 75% vs. 62% Del(11q): 22% vs. 22% Del(17p) excluded | 36-month PFS rate: 89.4% vs. 72.9% (p < 0.001) | At cycle 12: 8.3% vs. 59.2% |
| FLAIR [62] | Ibru + R vs. FCR | 1 | Between 18 and 70 y | 771 | 62 y | U-IGHV: 50% vs. 50% Del(17p) excluded | mPFS: NR vs. 67 m (p < 0.0001) 48-month PFS rate: 85.6% vs. 73.0% | NA |
| ASCEND [63,64] | Acala vs. R + idela or R + benda | R/R | Pts aged 18 years and higher | 310 | 68 y (32–89) | U-IGHV: 77% vs. 82% Del(17p) : 18% vs. 14% Del(11q): 25% vs. 29% TP53 mutation: 26% vs. 22% | MPs: NR vs. 16.5 m 42-month PFS rate: 62% vs. 19% | NA |
| ELEVATE TN [9,65] | Acala + Obn vs. Acala vs. Clb + Obn | 1 | 65 y or older younger than 65 y with comorbidities | 535 | 70 y | U-IGHV: 58% vs. 67% vs. 66% Del(17p): 9% vs. 9% vs. 9% Del(11q): 17% vs. 17% vs. 19% TP53 mutation: 12% vs. 11% vs. 12% | mPFS: NR vs. NR vs. 22.6 m 72-month PFS rate: 78% vs. 62% vs. 17% | 13% (acala + Obn) vs. 8% (Clb + Obn) with CR or CRi |
| SEQUOIA [10,66] | Zanu vs. R + benda | 1 | 65 y or older younger than 65 y with comorbidities | 590 | 70 (66–75) | U-IGHV: 53% vs. 52% Del(11q): 18% vs. 19% TP53 mutation: 6% vs. 6% Del(17p) excluded | mPFS: NR vs. 44.1 m | NA |
| ALPINE [68,73] | Zanu vs. Ibru | R/R CLL | Pts aged 18 years and higher | 652 | 67 y (35–90) vs. 68 (35–89) | U-IGHV: 73% vs. 73% Del(11q): 28% vs. 27% Del(17p): 14% vs. 15% TP53 mutation: 9% vs. 8% | mPFS: NR vs. 34.2 m p = 0.002 36-month PFS rate: 65% vs. 54% | NA |
| ELEVATE RR [69] | Acala vs. Ibru | R/R CLL | Pts aged 18 years and higher | 533 | 66 (41–89) vs. 65 (28–88) | U-IGHV: 82% vs. 89% Del(17p): 45% vs. 45% Del(11q): 62% vs. 66% TP53 mutation: 37% vs. 42% | mPFS: 38.4 vs. 38.4 m | NA |
| BRUIN CLL-321 [72] | Pirto vs. R + idela or R + benda | R/R CLL | Pts aged 18 years and higher | 238 | 67 (42–90) | U-IGHV: 92.8% vs. 79.6% Del(17p): 46.2% vs. 44.5% | Follow-up of 11.6 m, HR of PFS: 0.55 | NA |
| Trial | Treatment Arms | Line of Treatment | Population | Nb of Pts | Median Age (y) (Range) | Disease Characteristics | Primary Endpoint | U-MRD Rate (PB or BM) |
|---|---|---|---|---|---|---|---|---|
| CLL14 [11,74] | Ven + Obn vs. Clb + Obn | 1 | Frail patients | 432 | 72 y (41–89) | U-IGHV: 61% vs. 59% Del(17p): 9% vs. 7% Del(11q): 18% vs. 20% TP53 mutation: 11% vs. 8% | mPFS: 76.2 vs. 36.4 m (p < 0.0001) | EOT: 76% vs. 35% At month 60: 27.4% vs. 12.9% |
| CLL13 [75] | Ven + Obn vs. Ven + Obn + Ibru vs. Ven + R vs. FCR or R + benda | 1 | Pts aged 18 years and older | 926 | 62 y (31–83) vs. 60 y (30–84) vs. 62 y (27–84) vs. 61 y (29–84) | U-IGHV: 57% vs. 53% vs. 57% vs. 57% Del(11q): 19% vs. 14% vs. 19% vs. 18% Del(17p) and TP53 mutation excluded | mPFS: NR vs. NR vs. 63.4 m vs. 59.2 m U-MRD at 15 months: 86.5% vs. 92.2% vs. 57% vs. 52% | U-MRD at 15 months: 86.5% vs. 92.2% vs. 57% vs. 52% |
| UK Flair [13] | Ibru + Ven vs. FCR | 1 | Fit for FCR | 523 | 62 y (56–67) | U-IGHV: 47% vs. 53% Del(17p): excluded Del(13q): 34% vs. 38% | 36-month PFS rate: 97.2% vs. 76.8% (p < 0.001) | PB: 1-year U-MRD: 47.5% vs. 66%; 5-year U-MRD: 92.7% vs. 67.9% |
| GLOW [82,83] | Ibru + ven vs. Clb + Obn | 1 | 65 y or older Younger than 65 y with comorbidities | 211 | 71 y (47–93) | U-IGHV: 52% vs. 51% Del(17p): excluded Del(11q): 19% vs. 17% TP53 mutation: 7% vs. 2% | mPFS: NR vs. 21 m (p < 0.001) 42-month PFS rate: 74.6% vs. 24.8% | PB 3 months after EOT: 54.7% vs. 39% |
| AMPLIFY [86] | Acala + Ven vs. Acala + Ven + Obn vs. FCR or benda + R | 1 | Pts aged 18 years and higher | 867 | 61 (26–86) | U-IGHV: 58.6% Del(17p): excluded | mPFS: NR vs. NR vs. 47.8 m 36-month PFS rate: 76.5% vs. 83.1% vs. 66.5% |
| Trial | Nb of Pts | Population | Arms | Primary Endpoint |
|---|---|---|---|---|
| ECOG-ACRIN EA9161 (NCT03701282) | 720 | Previously untreated CLL | Investigational: ibrutinib + venetoclax + obinutuzumab (IOV) Control: ibrutinib + obinutuzumab (IO) | PFS |
| MAJIC (NCT05057494) | 607 | Previously untreated CLL/SLL | Arm A: acalabrutinib + venetoclax Arm B: acalabrutinib + obinutuzumab | PFS |
| CLL16 (NCT05197192) | 650 | Previously untreated high-risk CLL defined as at least del(17p), TP53 mutation or complex karyotype | Arm A: acalabrutinib + venetoclax + obinutuzumab (GAVe) Arm B: venetoclax + obinutuzumab (GVe) | PFS |
| CLL17 (NCT04608318) | 897 | Previously untreated CLL | Arm A: Ibrutinib Arm B: venetoclax + obinutuzumab Arm C: venetoclax + ibrutinib | PFS |
| BRUIN CLL-313 (NCT05023980) | 250 | Previously untreated CLL/SLL | Arm A: pirtobrutinib Arm B: bendamustine + rituximab | PFS |
| BRUIN CLL-314 (NCT05254743) | 650 | Previously untreated CLL/SLL | Experimental: pirtobrutinib Control: Ibrutinib | ORR |
| GLORA-2 (NCT06319456) | 344 | Previously untreated CLL/SLL | Experimental: lisaftoclax + acalabrutinib Control: FCR | PFS |
| BELLWAVE-011 (NCT06136559) | 1200 | Previously untreated CLL/SLL | Experimental: nemtabrutinib Control: ibrutinib or acalabrutinib | ORR PFS |
| BGB-11417-301 (NCT06073821) | 640 | Previously untreated CLL | Experimental: zanubrutinib + sonrotoclax Control: venetoclax + obinutuzumab | PFS |
| BELLWAVE-010 (NCT05947851) | 720 | R/R CLL/SLL following at least one prior therapy | Experimental: nemtabrutinib (MK-1026) + venetoclax Control: venetoclax + rituximab | PFS |
| BRUIN-CLL-322 (NCT05254743) | 600 | R/R CLL/SLL | Experimental: venetoclax + rituximab + pirtobrutinib Control: venetoclax + rituximab | PFS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, K.; Arbab, A.; Khalife, N.; Khoury, R.; Ibrahim, R.; Hachem, M.A.; Khalil, C.; Bou Orm, C.; Sawan, J.; Lafarge, G.; et al. The Role of Tumor Microenvironment and Targeted Therapy in Chronic Lymphocytic Leukemia. Curr. Issues Mol. Biol. 2025, 47, 604. https://doi.org/10.3390/cimb47080604
Saleh K, Arbab A, Khalife N, Khoury R, Ibrahim R, Hachem MA, Khalil C, Bou Orm C, Sawan J, Lafarge G, et al. The Role of Tumor Microenvironment and Targeted Therapy in Chronic Lymphocytic Leukemia. Current Issues in Molecular Biology. 2025; 47(8):604. https://doi.org/10.3390/cimb47080604
Chicago/Turabian StyleSaleh, Khalil, Ahmadreza Arbab, Nadine Khalife, Rita Khoury, Rebecca Ibrahim, Mohamad Ali Hachem, Cynthia Khalil, Cendrella Bou Orm, Joud Sawan, Geoffroy Lafarge, and et al. 2025. "The Role of Tumor Microenvironment and Targeted Therapy in Chronic Lymphocytic Leukemia" Current Issues in Molecular Biology 47, no. 8: 604. https://doi.org/10.3390/cimb47080604
APA StyleSaleh, K., Arbab, A., Khalife, N., Khoury, R., Ibrahim, R., Hachem, M. A., Khalil, C., Bou Orm, C., Sawan, J., Lafarge, G., Masri, N., Tikriti, Z., Chahine, C., & Le Cesne, A. (2025). The Role of Tumor Microenvironment and Targeted Therapy in Chronic Lymphocytic Leukemia. Current Issues in Molecular Biology, 47(8), 604. https://doi.org/10.3390/cimb47080604






