Molecular Mechanisms of Radiation Resistance in Breast Cancer: A Systematic Review of Radiosensitization Strategies
Abstract
1. Introduction
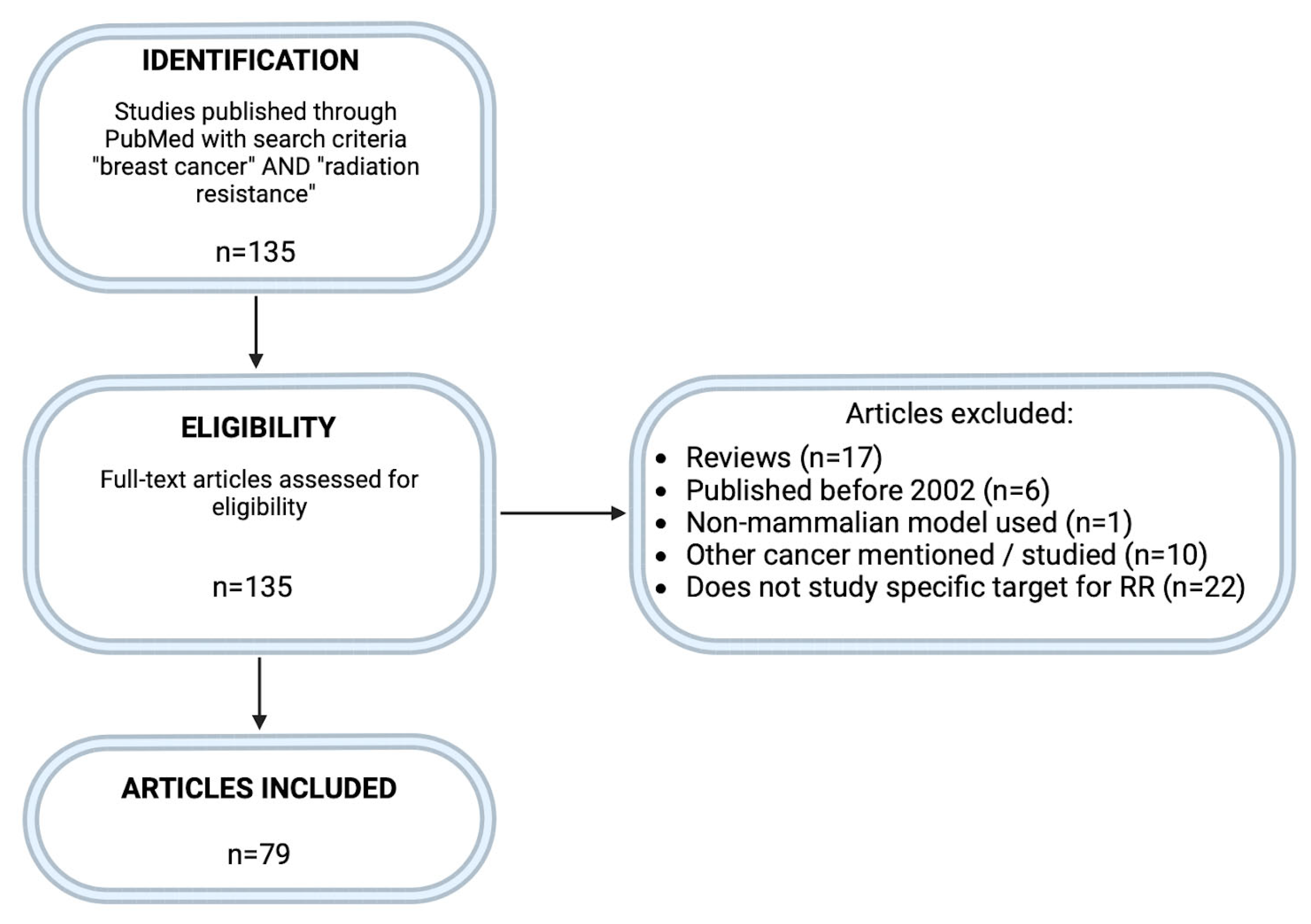
2. Methodology
3. Results and Discussion
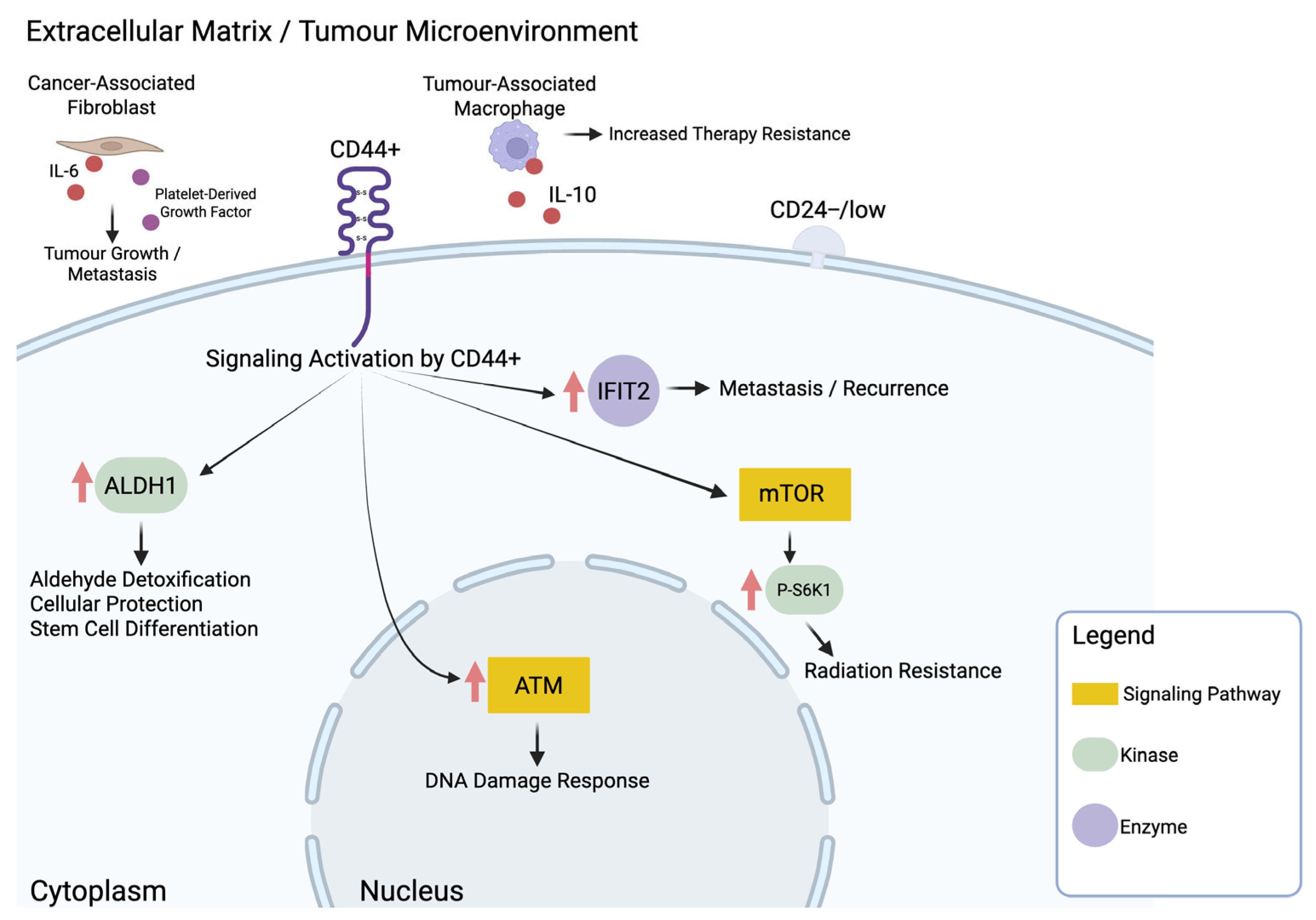
3.1. The Role of Cancer Stem Cells in Breast Cancer Radiation Resistance
| Authors, Year of Publication | PMID | Model System |
Molecular Target/
Intervention |
Effect on Radiation Resistance
[Increase (I)/Decrease (D)] |
|---|---|---|---|---|
| Anand et al., 2023 [34] | 36891450 | CD44+/CD24− breast cancer biopsies | CD44+/CD24− | I |
| Bensimon et al., 2016 [39] | 25641732 | MCF-7, MCF-7-CD24low, MCF-7-CD24neg, MDA-MB-436 and MDA-MB-436-CD24high | CD24 | D |
| Bensimon et al., 2013 [40] | 22330142 | T-47D, BT-20, MDA-MB-157, MDA-MB-231 | CD24(−/low) | I |
| Bontemps et al., 2022 [41] | 36367190 | MCF-7, MCF-7_CD24−, T47D, T47D_CD24−, HMLE | CD24(−/low) | I |
| Choi et al., 2020 [30] | 31959810 | MCF7 (CD44high/CD24low), T47D, ZR-751, BT474, SKBR3, MDA-MB-453, MDA-MB-231 | p-S6K1 | I |
| Croker & Allan, 2012 [31] | 21818590 | MDA-MB-231, MDA-MB-468 (ALDHhiCD44+/ALDHlowCD44−) | ALDH | I |
| Inalegwu et al., 2022 [42] | 35579852 | MCF-7, Fractionally irradiated cells (FIR20) | ↑ Stemness | I |
| Koh et al., 2019 [29] | 30875792 | MDA-MB-231, MDA-MB-231/IR | IFIT2 | I |
| Li et al., 2013 [38] | 24002052 | MCF-7 | GRP78 | I |
| Mal et al., 2021 [35] | 33490064 | ZR-75-1, ZR-75-1EpCAM, ZR-75-1FR and MCF-7, MCF-7FR | EpCAM | I |
| Sabol et al., 2020 [43] | 32326381 | MCF-7, ZR-75, T47D | Obesity-altered adipose stem cells | I |
| Wang et al., 2013 [36] | 23840685 | T47D, MCF-7, Bcap-37, SK-BR-3 | Lin28 | I |
| Wang et al., 2023 [37] | 36333630 | SK-BR-3, MDA-MB-468, MDA-MB-231, MCF-7, MCF10A | NRP1 | I |
| Wei et al., 2011 [44] | 22023707 | AS-B145 (ALDH+), AS-B244 (ALDH+), 4T1 (ALDH+), MDA-MB-231 (ALDH+) | Hsp27 | I |
| Woodward et al., 2007 [45] | 17202265 | MCF-7 | Wnt/β-catenin pathway | I |
| Yan et al., 2016 [46] | 27036550 | MCF-7/C6 (CD44(+)/CD24(−/low)) | ATRA | D |
| Yin & Glass, 2011 [33] | 21935375 | CD44(+)/CD24(− or low) subset of MCF-7, MDA-MB-231, MDA-MB-436, BD20, HCC38, HCC1937 | ATM signaling | I |
| Zielske et al., 2011 [47] | 21804918 | MC1, UM2, patient-derived xenografts | CD44(+) CD24(−) lin(−) | D |
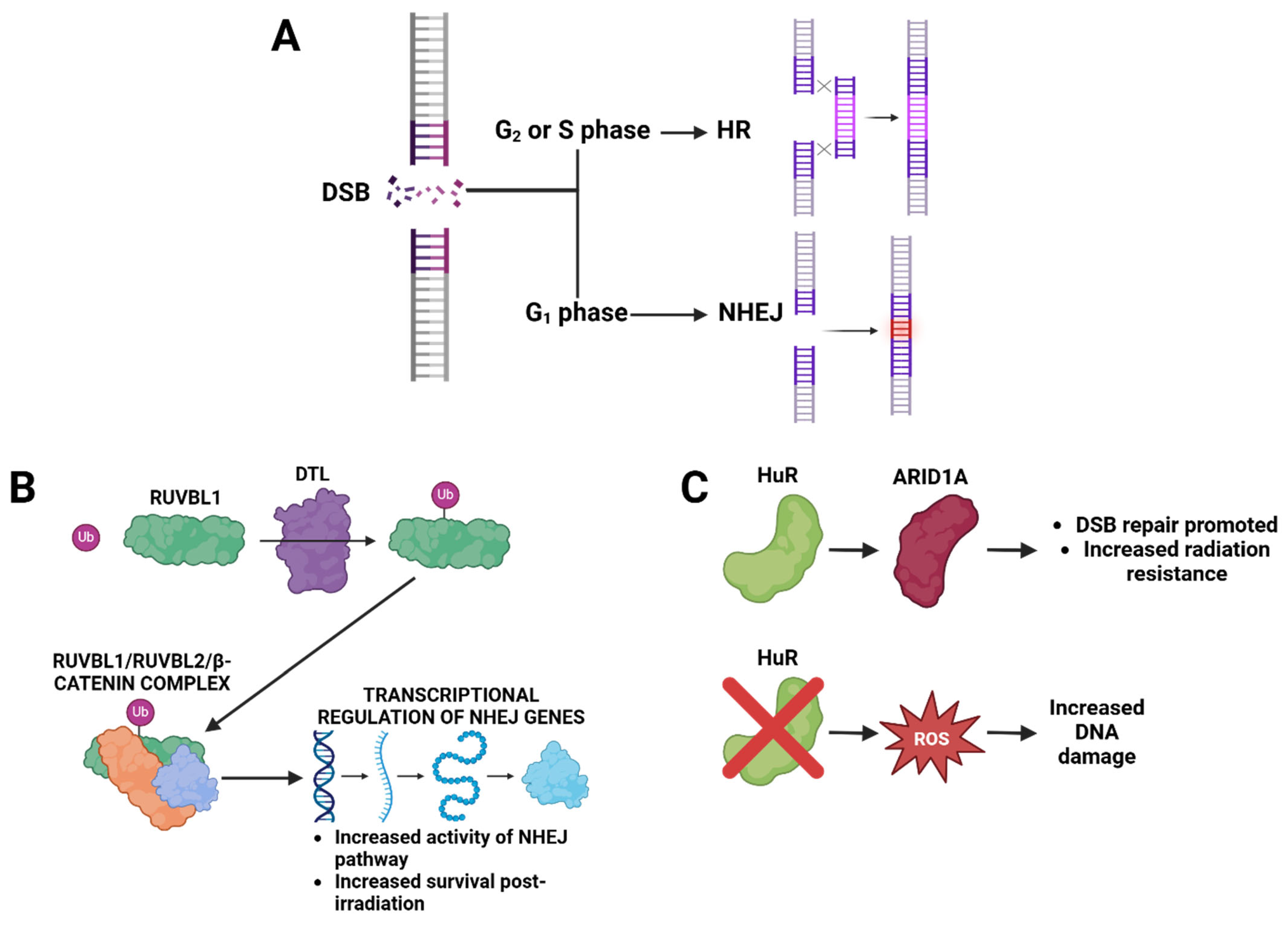
3.2. DNA Repair and Redox Pathways as Determinants of Radiation Resistance
| Authors, Year of Publication | PMID | Model System |
Molecular Target/
Intervention | Effect on Radiation Resistance [Increase (I)/Decrease (D)] |
|---|---|---|---|---|
| Abdullah et al., 2021 [61] | 33768386 | MDA-MB-231, MDA-MB-468, MDA-MB-436, MCF-7, T47D, | Thioredoxin reductase | I |
| Andrade et al., 2019 [50] | 31847141 | MDA-MB-231, Hs578t, MCF-7, MDA-MB-468 | HuR and ARID1A | I |
| Barlow et al., 2024 [64] | 38467328 | MDA-MB-468, MDA-MB-231 | FASN | I |
| Chiu et al., 2019 [58] | 31683883 | 4T1, MDA-MB-231, MCF-10A | Histone deacetylase inhibitor to induce misfolded proteins | D |
| Diaz et al., 2013 [63] | 24648762 | MCF-7 | Peroxiredoxin II | I |
| Fu et al., 2022 [65] | 35497337 | MDA-MB-231 | NSMRH (G2/M) | D |
| Kumar et al., 2020 [56] | 33385162 | TP53+/+, Fusion-Reporter (TP53+/+, PCNA-mCherry, 53BP1-mVenus) | DNA-PK- and Pol θ-dependent end joining repair | I |
| Lamb et al., 2015 [57] | 26087309 | MCF-7, T47D | DNA-PK | I |
| Luzhna et al., 2013 [66] | 23467667 | MCF-7 | ↑ pATM, KU70, RAD51, and low fidelity DNA polymerase | I |
| Mehta et al., 2016 [51] | 27588488 | MDA-MB-231, MDA-MB-468 and Hs578t | HuR | I |
| Nashir Udden et al., 2023 [54] | 36693944 | T-47D and MCF-7 | BET | I |
| Nolte et al., 2023 [67] | 36835001 | MCF-7, MDA-MB-231, BT-20 | Microtubule disruption via ESE-16 molecule | D |
| Park et al., 2017 [68] | 28554201 | MDA-MB-231 | Induction of apoptosis | D |
| Sencan et al., 2021 [55] | 33515382 | MDA-MB-231, MDA-MB-436, BT-20, MCF-7, T47D, ZR-75.1, MCF-10A | UVRAG | I |
| Tian et al., 2024 [49] | 38609375 | MMTV-PyMT | DTL-RUVBL1/2-β-catenin | I |
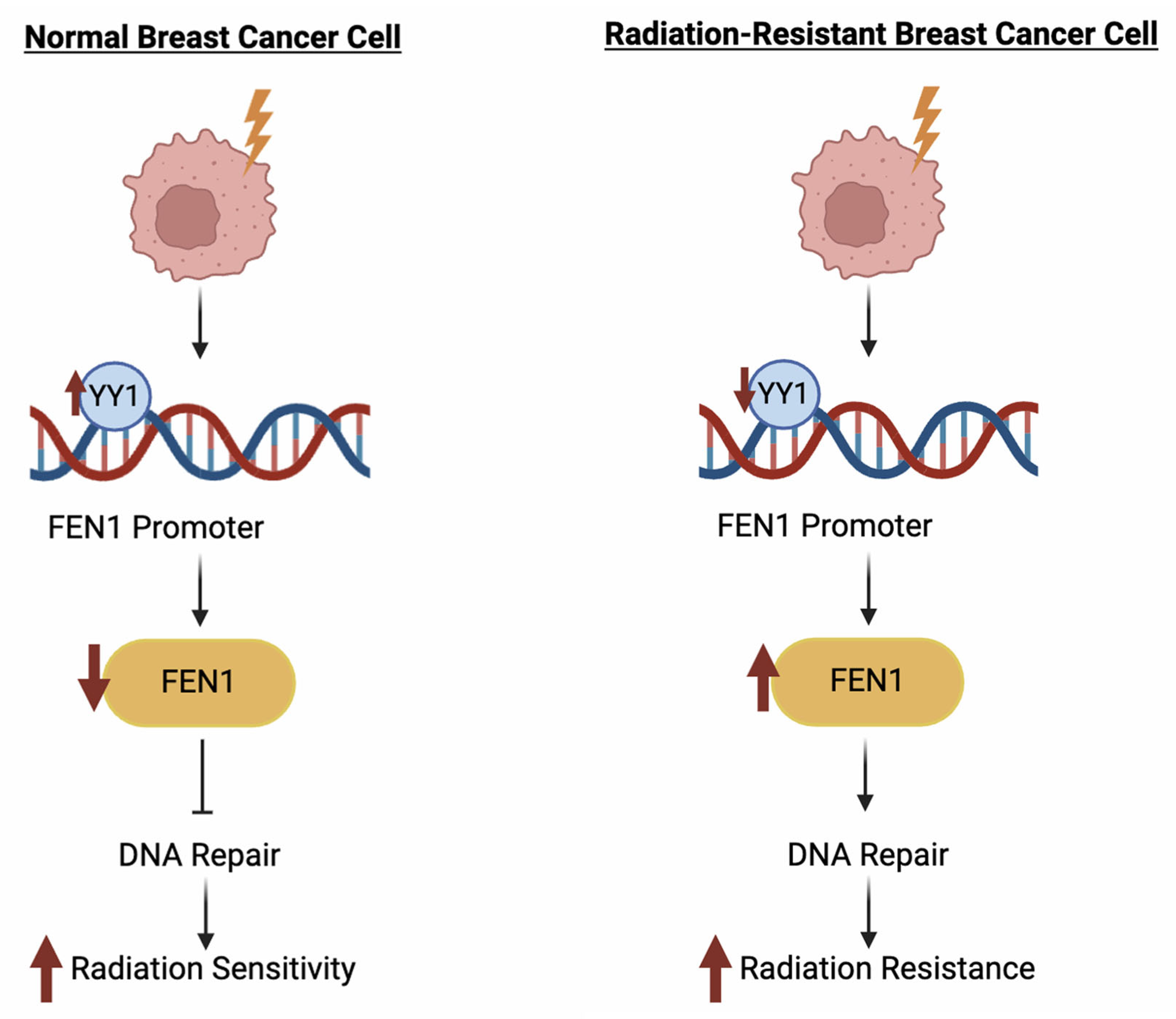
| Wang et al., 2015 [48] | 25885449 | 293 T, HeLa, MCF-7, MDA-MB-231 | FEN1 | I |
| Wang et al., 2021 [53] | 33813001 | MCF7, MDA-MB-231, MDA-MB-468, T47D | FASN | I |
| Wang et al., 2023 [59] | 37485315 | 4T1 cells in vitro and in BALB/c mice | Cu-doped polypyrrole-based hydrogel injected intratumorally to suppress antioxidant capabilities and increase reactive oxygen species production | I |
| Wu et al., 2014 [60] | 25409124 | MCF-7, ZR-75-1, MDA-MB-231 | Bcl-2 | I |
| Yang et al., 2020 [69] | 33000219 | MCF-7, T47D | MV-Edm infection mediated ↓ 53BP1 and ↓ NHEJ | D |
| Zhou et al., 2020 [70] | 32175401 | MDA-MB-231 | SOD2, CDKN1A | I |
3.3. Signaling Pathways Associated with Radiation Resistance in Breast Cancer
| Authors, Year of Publication | PMID | Model System |
Molecular Target/ Intervention | Effect on Radiation Resistance [Increase (I)/Decrease (D)] |
|---|---|---|---|---|
| Boelens et al., 2014 [71] | 25417103 | MDA-MB-231, 1833, MDA-436, MDA-157, HCC1937, MDA-468, MCF7, SKBR3, T47D, HCC70 | Antiviral/NOTCH3 pathways | I |
| Braunstein et al., 2008 [72] | 18234964 | MCF10A, UACC-893, HCC70, BT474 | NF-kappaB | I |
| Brennan et al., 2006 [73] | 17085655 | Human case study samples, MDA-MB-231, MCF-7, T47D, SKBR3, Hs578T, BT474, HeLa | CA IX | I |
| Cordes et al., 2003 [74] | 14703944 | MDA-MB-231 | Fibronectin and laminin | I |
| Heravi et al., 2012 [75] | 22357220 | 4T1 | RAF/MEK/ERK/MAP, VEGFR-2, VEGFR-3, PDGFR-β | I |
| Hu et al., 2016 [76] | 27624978 | HBL-100, MCF–7, MDA-MB-231, HCC1937, SKBR-3, and BT549 | ITGA6 | I |
| Iijima et al., 2018 [77] | 29393397 | MDA-MB-231 | HIF-1α | I |
| Ji et al., 2023 [78] | 37614420 | 4T-1 | Axl | I |
| Jung et al., 2019 [79] | 30893896 | T47D, MDA-MB-231, MCF7 | TCTP | D |
| Krautschneider et al., 2022 [80] | 36411172 | MDA-MB-231, HCC1806 | HS chains | I |
| La Verde et al., 2022 [81] | 36091449 | MCF10A, MDA-MB-231 | YAP | |
| Lei et al., 2022 [82] | 36329030 | MCF-12A, MCF-12 F, MCF-7, T47D, ZR-75-1, HCC-1806, MDA-MB-468, BT-549, MDA-MB-231, MCF-10A | lncRNA DUXAP8 | I |
| Li et al., 2021 [83] | 34221989 | MCF-7R, MDA-MB-231R | LncRNA FGD5-AS1 | I |
| Liang et al., 2022 [84] | 35944750 | MDA-MB-231, BT- 549, MCF-7, T-47D | CD146, ITGB1 | I |
| Ling et al., 2009 [85] | 19956451 | MCF-7, ZR-75 and MDA-MB-231 | Survivin | I |
| Luo et al., 2009 [86] | 19513620 | MCF-7, MDA-MB-453, SK-BR-3 | ERBB2 | I |
| Marvaso et al., 2014 [87] | 24657936 | MDA-MB-361 | FTY720 | D |
| Mast & Kuppusamy, 2018 [88] | 30524959 | MDA-MB-231 | Hypoxia | I |
| Miao et al., 2021 [89] | 33739118 | MCF-7, MDA-MB-231 | TAF9 | I |
| Onaga et al., 2022 [90] | 35813014 | The Molecular Taxonomy of Breast Cancer International Consortium dataset | SLC20A1 | I |
| Paramanantham et al., 2021 [91] | 34066541 | MDA-MB-231 | ERK | I |
| Steelman et al., 2011 [92] | 21869603 | MCF-7 | Akt/mTOR | I |
| Tao et al., 2024 [93] | 38167446 | MDA-MB-231, MDA-MB-468 | PDIA4 | I |
| Thewes et al., 2010 [94] | 20459791 | N202.1A | AP-2 transcription factors | I |
| Wolfe et al., 2015 [95] | 25832697 | SUM 149, KPL4 | VLDL; LDL | I for VLDL; D for LDL |
| Zhou et al., 2018 [62] | 29317253 | MDA-MB-231 | SDF-1 receptor CXCR4 | I |
| Zou et al., 2017 [96] | 29169152 | BT474, SKBR3, Hs578T and MDA-MB-231 | CAVEOLIN-1 | I |
3.3.1. PI3K/Akt/mTOR Signaling Pathway
3.3.2. JNK Signaling Pathway
3.3.3. MAPK, MEK/ERK, and Downstream Resistance Pathways
3.3.4. Hypoxia and Metabolic Signaling

3.4. MicroRNAs as Post-Transcriptional Regulators of Radiation Resistance in Breast Cancer
| Authors, Year of Publication | PMID | Model System | Molecular Target/ Intervention | Effect on Radiation Resistance [Increase (I)/Decrease (D)] |
|---|---|---|---|---|
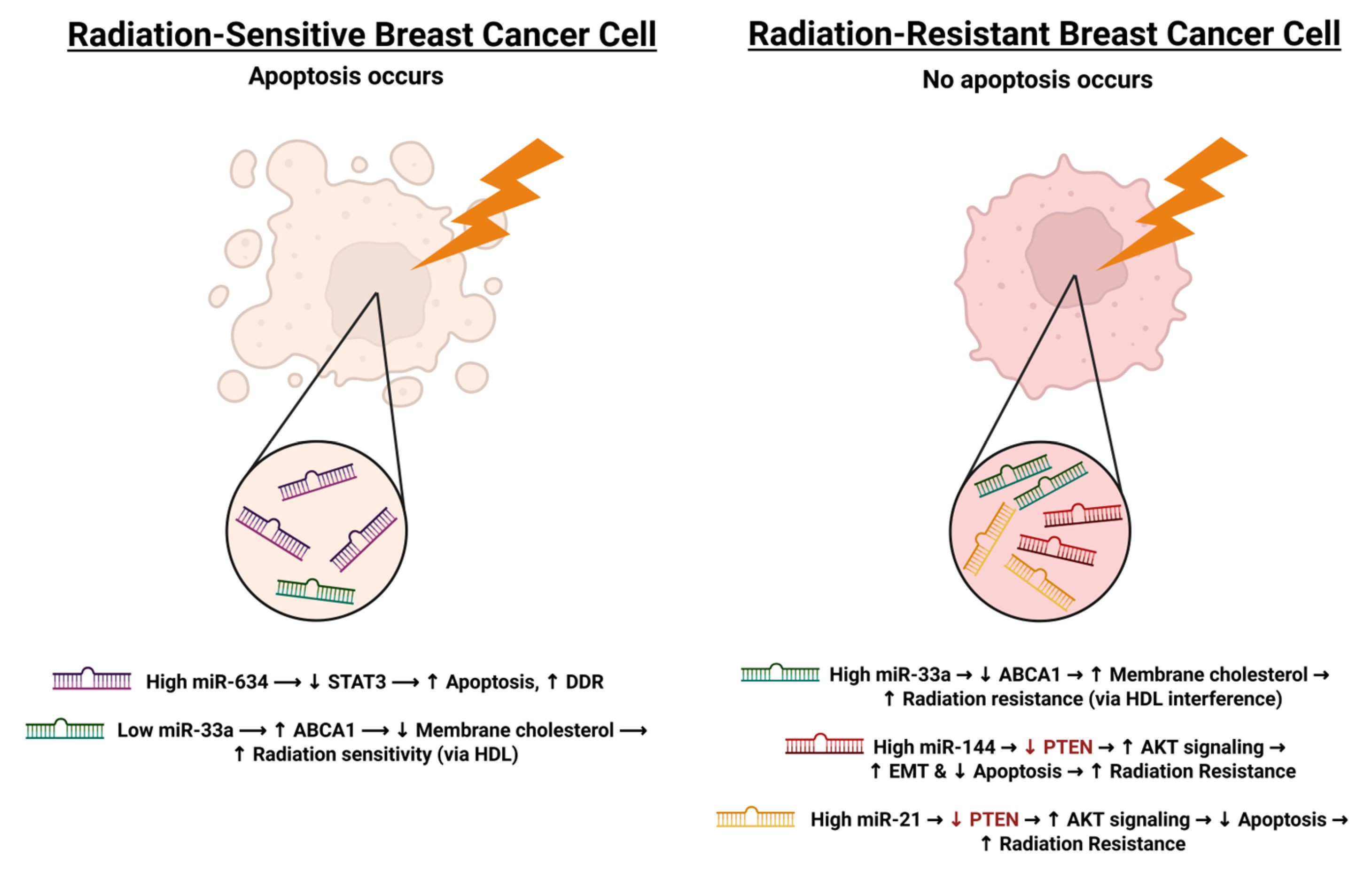
| Anastasov et al., 2012 [111] | 23216894 | T47D, MDA-MB-361 | miR-21 | I |
| Masoudi-Khoram et al., 2020 [113] | 32493932 | MDA-MB-231, T47D | miR-16-5p | D |
| Mesci et al., 2017 [114] | 28419078 | MDA-MB-231 | miR-330-3p and CCBE1 | I |
| Wang et al., 2022 [115] | 35818245 | human breast cancer cells (specific not mentioned) | miR-143-3p (through FGF1) | D |
| Wolfe et al., 2016 [110] | 27055396 | SUM149, SUM159, KPL4, MDA-MB-231 | miR-33a | I |
| Yang et al., 2020 [69] | 32077744 | MCF-7, MDA-MB-231 | miR-634, STAT3 | D |
| Yu et al., 2015 [112] | 26252024 | MDA-MB-231, SKBR3 | miR-144 | I |
| Zhang et al., 2020 [116] | 32374522 | MCF-7, T47D, LM-MCF-7,34 BT-474, SKBR-3, MDA-MB-231 | miR-449b-5p | D |
3.5. Novel and Less-Explored Molecular Targets in Radiation Resistance
3.5.1. Nanoparticle-Based Approaches

3.5.2. Immune Modulation and Stromal Targets
| Authors, Year of Publication | PMID | Model System |
Molecular Target/
Intervention | Effect on Radiation Resistance [Increase (I)/Decrease (D)] |
|---|---|---|---|---|
| Choi et al., 2022 [126] | 36555137 | MDA-MB-231 | Natural killer cell mediated cytotoxicity increased following mebendazole and radiation | D |
| Hullo et al., 2021 [119] | 33922713 | T47D, MDA-MB-231 | Platinum nanoparticles | N/A |
| Jain et al., 2014 [117] | 24444528 | MDA-MB-231 | Gold nanoparticles | D |
| Jian et al., 2024 [127] | 38214439 | 4T1 | CAFs | I |
| Zuo et al., 2023 [118] | 36686245 | MCF-7, MCF-10A | Gold nanoparticles | D |
4. Summary and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arzanova, E.; Mayrovitz, H.N. The Epidemiology of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022; ISBN 978-0-645-33203-2. [Google Scholar]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Menta, A.; Fouad, T.M.; Lucci, A.; Le-Petross, H.; Stauder, M.C.; Woodward, W.A.; Ueno, N.T.; Lim, B. Inflammatory Breast Cancer. Surg. Clin. N. Am. 2018, 98, 787–800. [Google Scholar] [CrossRef]
- Weigelt, B.; Geyer, F.C.; Reis-Filho, J.S. Histological Types of Breast Cancer: How Special Are They? Mol. Oncol. 2010, 4, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Dave, R.; Sanadya, J.; Sharma, P.; Sharma, K.K. Various Types and Management of Breast Cancer: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 109. [Google Scholar] [CrossRef]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-Mesenchymal Transition in Cancer: Complexity and Opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef]
- Marrazzo, E.; Frusone, F.; Milana, F.; Sagona, A.; Gatzemeier, W.; Barbieri, E.; Bottini, A.; Canavese, G.; Rubino, A.O.; Eboli, M.G.; et al. Mucinous Breast Cancer: A Narrative Review of the Literature and a Retrospective Tertiary Single-Centre Analysis. Breast 2020, 49, 87–92. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent Advances in Therapeutic Strategies for Triple-Negative Breast Cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Y.; Lyu, M.; Chan, C.H.; Sun, M.; Yang, X.; Qiao, S.; Chen, Z.; Yu, S.; Ren, M.; et al. Classifications of Triple-Negative Breast Cancer: Insights and Current Therapeutic Approaches. Cell Biosci. 2025, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Trayes, K.P.; Cokenakes, S.E.H. Breast Cancer Treatment. Am. Fam. Physician 2021, 104, 171–178. [Google Scholar] [PubMed]
- Maughan, K.L.; Lutterbie, M.A.; Ham, P.S. Treatment of Breast Cancer. Am. Fam. Physician 2010, 81, 1339–1346. [Google Scholar]
- Nathanson, S.D.; Detmar, M.; Padera, T.P.; Yates, L.R.; Welch, D.R.; Beadnell, T.C.; Scheid, A.D.; Wrenn, E.D.; Cheung, K. Mechanisms of Breast Cancer Metastasis. Clin. Exp. Metastasis 2022, 39, 117–137. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Kim, B.; Hong, Y.; Lee, S.; Liu, P.; Lim, J.; Lee, Y.; Lee, T.; Chang, K.; Hong, Y. Therapeutic Implications for Overcoming Radiation Resistance in Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 26880–26913. [Google Scholar] [CrossRef]
- Bi, Z.-F.; Wu, S.-G.; Chen, Z.-S. Editorial: Radioresistance in Breast Cancer. Front. Oncol. 2024, 14, 1514173. [Google Scholar] [CrossRef]
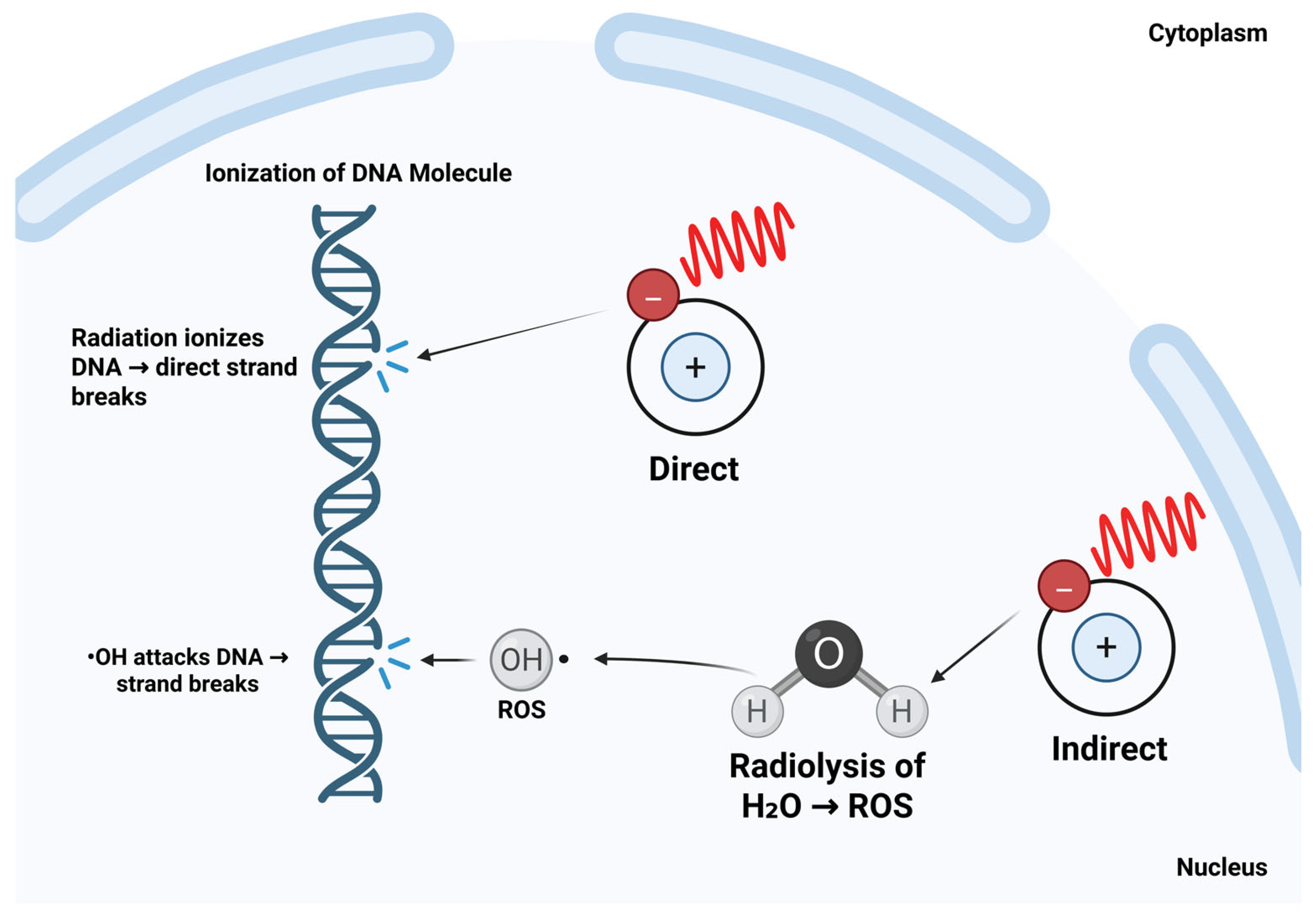
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological Response of Cancer Cells to Radiation Treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef]
- Borrego-Soto, G.; Ortiz-López, R.; Rojas-Martínez, A. Ionizing Radiation-Induced DNA Injury and Damage Detection in Patients with Breast Cancer. Genet. Mol. Biol. 2015, 38, 420–432. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Y.; Wang, R.; Wang, T. Molecular Mechanisms of Tumor Resistance to Radiotherapy. Mol. Cancer 2023, 22, 96. [Google Scholar] [CrossRef]
- Cooper, G.M. Tumor Suppressor Genes. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Goldstein, M.; Kastan, M.B. The DNA Damage Response: Implications for Tumor Responses to Radiation and Chemotherapy. Annu. Rev. Med. 2015, 66, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Borrero, L.J.H.; El-Deiry, W.S. Tumor Suppressor P53: Biology, Signaling Pathways, and Therapeutic Targeting. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef]
- Carlos-Reyes, A.; Muñiz-Lino, M.A.; Romero-Garcia, S.; López-Camarillo, C.; Hernández-de la Cruz, O.N. Biological Adaptations of Tumor Cells to Radiation Therapy. Front. Oncol. 2021, 11, 718636. [Google Scholar] [CrossRef]
- Koh, S.Y.; Moon, J.Y.; Unno, T.; Cho, S.K. Baicalein Suppresses Stem Cell-Like Characteristics in Radio- and Chemoresistant MDA-MB-231 Human Breast Cancer Cells through Up-Regulation of IFIT2. Nutrients 2019, 11, 624. [Google Scholar] [CrossRef]
- Choi, J.; Yoon, Y.N.; Kim, N.; Park, C.S.; Seol, H.; Park, I.-C.; Kim, H.-A.; Noh, W.C.; Kim, J.-S.; Seong, M.-K. Predicting Radiation Resistance in Breast Cancer with Expression Status of Phosphorylated S6K1. Sci. Rep. 2020, 10, 641. [Google Scholar] [CrossRef]
- Croker, A.K.; Allan, A.L. Inhibition of Aldehyde Dehydrogenase (ALDH) Activity Reduces Chemotherapy and Radiation Resistance of Stem-like ALDHhiCD44+ Human Breast Cancer Cells. Breast Cancer Res. Treat. 2012, 133, 75–87. [Google Scholar] [CrossRef]
- Nakshatri, H. Radiation Resistance in Breast Cancer: Are CD44+/CD24-/Proteosomelow/PKH26+cells to Blame? Breast Cancer Res. 2010, 12, 105. [Google Scholar] [CrossRef]
- Yin, H.; Glass, J. The Phenotypic Radiation Resistance of CD44+/CD24−or Low Breast Cancer Cells Is Mediated through the Enhanced Activation of ATM Signaling. PLoS ONE 2011, 6, e24080. [Google Scholar] [CrossRef]
- Anand, A.; Gaurav, K.; Miller, J.L.; Singh, K.R.; Agrawal, M.K.; Kumar, S.; Husain, N.; Agarwal, P.; Agarwal, A.; Sonkar, A.A. Clinicopathologic Correlation of CD44 +/CD24 \textminus Expression in Breast Cancer: A Report from Tertiary Care Medical University in India. Indian J. Surg. Oncol. 2023, 14, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Mal, A.; Bukhari, A.B.; Singh, R.K.; Kapoor, A.; Barai, A.; Deshpande, I.; Wadasadawala, T.; Ray, P.; Sen, S.; De, A. EpCAM-Mediated Cellular Plasticity Promotes Radiation Resistance and Metastasis in Breast Cancer. Front. Cell Dev. Biol. 2021, 8, 597673. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, C.; Lv, K.; Xie, S.; Fu, P.; Liu, X.; Chen, Y.; Qin, C.; Deng, W.; Hu, W. Lin28 Mediates Radiation Resistance of Breast Cancer Cells via Regulation of Caspase, H2A.X and Let-7 Signaling. PLoS ONE 2013, 8, e67373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Sun, X.-L.; Lu, Y.-C.; Chen, S.; Pei, D.-S.; Zhang, L.-S. NRP1 Contributes to Stemness and Potentiates Radioresistance via WTAP-Mediated m6A Methylation of Bcl-2 mRNA in Breast Cancer. Apoptosis 2023, 28, 233–246. [Google Scholar] [CrossRef]
- Li, B.; Cheng, X.L.; Yang, Y.P.; Li, Z.Q. GRP78 Mediates Radiation Resistance of a Stem Cell-like Subpopulation within the MCF-7 Breast Cancer Cell Line. Oncol. Rep. 2013, 30, 2119–2126. [Google Scholar] [CrossRef]
- Bensimon, J.; Biard, D.; Paget, V.; Goislard, M.; Morel-Altmeyer, S.; Konge, J.; Chevillard, S.; Lebeau, J. Forced Extinction of CD24 Stem-like Breast Cancer Marker Alone Promotes Radiation Resistance through the Control of Oxidative Stress. Mol. Carcinog. 2016, 55, 245–254. [Google Scholar] [CrossRef]
- Bensimon, J.; Altmeyer-Morel, S.; Benjelloun, H.; Chevillard, S.; Lebeau, J. CD24−/Low Stem-like Breast Cancer Marker Defines the Radiation-Resistant Cells Involved in Memorization and Transmission of Radiation-Induced Genomic Instability. Oncogene 2013, 32, 251–258. [Google Scholar] [CrossRef]
- Bontemps, I.; Lallemand, C.; Biard, D.; Dechamps, N.; Kortulewski, T.; Bourneuf, E.; Siberchicot, C.; Boussin, F.; Chevillard, S.; Campalans, A.; et al. Loss of CD24 Promotes Radiation- and Chemo-resistance by Inducing Stemness Properties Associated with a Hybrid E/M State in Breast Cancer Cells. Oncol. Rep. 2022, 49, 4. [Google Scholar] [CrossRef]
- Inalegwu, A.; Cuypers, B.; Claesen, J.; Janssen, A.; Coolkens, A.; Baatout, S.; Laukens, K.; De Vos, W.H.; Quintens, R. Fractionated Irradiation of MCF7 Breast Cancer Cells Rewires a Gene Regulatory Circuit towards a Treatment-resistant Stemness Phenotype. Mol. Oncol. 2022, 16, 3410–3435. [Google Scholar] [CrossRef]
- Sabol, R.A.; Villela, V.A.; Denys, A.; Freeman, B.T.; Hartono, A.B.; Wise, R.M.; Harrison, M.A.A.; Sandler, M.B.; Hossain, F.; Miele, L.; et al. Obesity-Altered Adipose Stem Cells Promote Radiation Resistance of Estrogen Receptor Positive Breast Cancer through Paracrine Signaling. Int. J. Mol. Sci. 2020, 21, 2722. [Google Scholar] [CrossRef]
- Wei, L.; Liu, T.-T.; Wang, H.-H.; Hong, H.-M.; Yu, A.L.; Feng, H.-P.; Chang, W.-W. Hsp27 Participates in the Maintenance of Breast Cancer Stem Cells through Regulation of Epithelial-Mesenchymal Transition and Nuclear Factor-κB. Breast Cancer Res. 2011, 13, R101. [Google Scholar] [CrossRef]
- Woodward, W.A.; Chen, M.S.; Behbod, F.; Alfaro, M.P.; Buchholz, T.A.; Rosen, J.M. WNT/β-Catenin Mediates Radiation Resistance of Mouse Mammary Progenitor Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 618–623. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Z.; Xu, X.; Chen, C.; Wei, W.; Fan, M.; Chen, X.; Li, J.J.; Wang, Y.; Huang, J. All-Trans Retinoic Acids Induce Differentiation and Sensitize a Radioresistant Breast Cancer Cells to Chemotherapy. BMC Complement. Altern. Med. 2016, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Zielske, S.P.; Spalding, A.C.; Wicha, M.S.; Lawrence, T.S. Ablation of Breast Cancer Stem Cells with Radiation. Transl. Oncol. 2011, 4, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, L.; Li, Z.; Zhang, T.; Liu, W.; Liu, Z.; Yuan, Y.-C.; Su, F.; Xu, L.; Wang, Y.; et al. YY1 Suppresses FEN1 Over-Expression and Drug Resistance in Breast Cancer. BMC Cancer 2015, 15, 50. [Google Scholar] [CrossRef]
- Tian, J.; Wen, M.; Gao, P.; Feng, M.; Wei, G. RUVBL1 Ubiquitination by DTL Promotes RUVBL1/2-β-Catenin-Mediated Transcriptional Regulation of NHEJ Pathway and Enhances Radiation Resistance in Breast Cancer. Cell Death Dis. 2024, 15, 259. [Google Scholar] [CrossRef]
- Andrade, D.; Mehta, M.; Griffith, J.; Oh, S.; Corbin, J.; Babu, A.; De, S.; Chen, A.; Zhao, Y.D.; Husain, S.; et al. HuR Reduces Radiation-Induced DNA Damage by Enhancing Expression of ARID1A. Cancers 2019, 11, 2014. [Google Scholar] [CrossRef]
- Mehta, M.; Basalingappa, K.; Griffith, J.N.; Andrade, D.; Babu, A.; Amreddy, N.; Muralidharan, R.; Gorospe, M.; Herman, T.; Ding, W.-Q.; et al. HuR Silencing Elicits Oxidative Stress and DNA Damage and Sensitizes Human Triple-Negative Breast Cancer Cells to Radiotherapy. Oncotarget 2016, 7, 64820–64835. [Google Scholar] [CrossRef]
- Mao, Z.; Bozzella, M.; Seluanov, A.; Gorbunova, V. DNA Repair by Nonhomologous End Joining and Homologous Recombination during Cell Cycle in Human Cells. Cell Cycle 2008, 7, 2902–2906. [Google Scholar] [CrossRef]
- Wang, C.J.; Li, D.; Danielson, J.A.; Zhang, E.H.; Dong, Z.; Miller, K.D.; Li, L.; Zhang, J.-T.; Liu, J.-Y. Proton Pump Inhibitors Suppress DNA Damage Repair and Sensitize Treatment Resistance in Breast Cancer by Targeting Fatty Acid Synthase. Cancer Lett. 2021, 509, 1–12. [Google Scholar] [CrossRef]
- Nashir Udden, S.M.; Baek, G.; Pandey, K.; Vidal, C.; Liu, Y.; Rahimi, A.S.; Kim, D.N.; Nwachukwu, C.R.; Mani, R.S.; Alluri, P.G. Towards Precision Radiation Oncology: Endocrine Therapy Response as a Biomarker for Personalization of Breast Radiotherapy. NPJ Precis. Oncol. 2023, 7, 11. [Google Scholar] [CrossRef]
- Sencan, S.; Tanriover, M.; Ulasli, M.; Karakas, D.; Ozpolat, B. UV Radiation Resistance-Associated Gene (UVRAG) Promotes Cell Proliferation, Migration, Invasion by Regulating Cyclin-Dependent Kinases (CDK) and Integrin-β/Src Signaling in Breast Cancer Cells. Mol. Cell. Biochem. 2021, 476, 2075–2084. [Google Scholar] [CrossRef]
- Kumar, R.J.; Chao, H.X.; Simpson, D.A.; Feng, W.; Cho, M.-G.; Roberts, V.R.; Sullivan, A.R.; Shah, S.J.; Wozny, A.-S.; Fagan-Solis, K.; et al. Dual Inhibition of DNA-PK and DNA Polymerase Theta Overcomes Radiation Resistance Induced by P53 Deficiency. NAR Cancer 2020, 2, zcaa038. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.; Fiorillo, M.; Chadwick, A.; Ozsvari, B.; Reeves, K.J.; Smith, D.L.; Clarke, R.B.; Howell, S.J.; Cappello, A.R.; Martinez-Outschoorn, U.E.; et al. Doxycycline Down-Regulates DNA-PK and Radiosensitizes Tumor Initiating Cells: Implications for More Effective Radiation Therapy. Oncotarget 2015, 6, 14005–14025. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-W.; Yeh, Y.-L.; Ho, S.-Y.; Wu, Y.-H.; Wang, B.-J.; Huang, W.-J.; Ho, Y.-S.; Wang, Y.-J.; Chen, L.-C.; Tu, S.-H. A New Histone Deacetylase Inhibitor Enhances Radiation Sensitivity through the Induction of Misfolded Protein Aggregation and Autophagy in Triple-Negative Breast Cancer. Cancers 2019, 11, 1703. [Google Scholar] [CrossRef]
- Wang, S.; Fei, H.; Ma, Y.; Zhu, D.; Zhang, H.; Li, X.; Huang, Q. Cu-Doped Polypyrrole Hydrogel with Tumor Catalyst Activity for NIR-II Thermo-Radiotherapy. Front. Bioeng. Biotechnol. 2023, 11, 1225937. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Schiff, D.S.; Lin, Y.; Neboori, H.J.R.; Goyal, S.; Feng, Z.; Haffty, B.G. Ionizing Radiation Sensitizes Breast Cancer Cells to Bcl-2 Inhibitor, ABT-737, through Regulating Mcl-1. Radiat. Res. 2014, 182, 618–625. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Inman, M.; Moody, C.J.; Storr, S.J.; Martin, S.G. Cytotoxic and Radiosensitising Effects of a Novel Thioredoxin Reductase Inhibitor in Breast Cancer. Investig. New Drugs 2021, 39, 1232–1241. [Google Scholar] [CrossRef]
- Zhou, K.X.; Xie, L.H.; Peng, X.; Guo, Q.M.; Wu, Q.Y.; Wang, W.H.; Zhang, G.L.; Wu, J.F.; Zhang, G.J.; Du, C.W. CXCR4 Antagonist AMD3100 Enhances the Response of MDA-MB-231 Triple-Negative Breast Cancer Cells to Ionizing Radiation. Cancer Lett. 2018, 418, 196–203. [Google Scholar] [CrossRef]
- Diaz, A.J.; Wang, T.; Li, J.-J.; Yen, Y.; Tamae, D. Enhanced Radiation Response in Radioresistant MCF-7 Cells by Targeting Peroxiredoxin II. Breast Cancer Targets Ther. 2013, 5, 87–101. [Google Scholar] [CrossRef]
- Barlow, L.; Josephraj, S.; Gu, B.; Dong, Z.; Zhang, J.-T. FASN Negatively Regulates P65 Expression by Reducing Its Stability via Thr254 Phosphorylation and Isom-erization by Pin1. J. Lipid Res. 2024, 65, 100529. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, H.; Xue, P.; Yu, H.; Yang, S.; Tao, C.; Li, W.; Wang, Y.; Zhang, J.; Wang, Y. Implantable Bioresponsive Hydrogel Prevents Local Recurrence of Breast Cancer by Enhancing Radiosensitivity. Front. Bioeng. Biotechnol. 2022, 10, 881544. [Google Scholar] [CrossRef] [PubMed]
- Luzhna, L.; Golubov, A.; Ilnytskyy, S.; Chekhun, V.F.; Kovalchuk, O. Molecular Mechanisms of Radiation Resistance in Doxorubicin-Resistant Breast Adenocarcinoma Cells. Int. J. Oncol. 2013, 42, 1692–1708. [Google Scholar] [CrossRef] [PubMed]
- Nolte, E.M.; Joubert, A.M.; Lafanechère, L.; Mercier, A.E. Radiosensitization of Breast Cancer Cells with a 2-Methoxyestradiol Analogue Affects DNA Damage and Repair Signaling In Vitro. Int. J. Mol. Sci. 2023, 24, 3592. [Google Scholar] [CrossRef]
- Park, J.E.; Piao, M.J.; Kang, K.A.; Shilnikova, K.; Hyun, Y.J.; Oh, S.K.; Jeong, Y.J.; Chae, S.; Hyun, J.W. A Benzylideneacetophenone Derivative Induces Apoptosis of Radiation-Resistant Human Breast Cancer Cells via Oxidative Stress. Biomol. Ther. 2017, 25, 404–410. [Google Scholar] [CrossRef]
- Yang, B.; Kuai, F.; Chen, Z.; Fu, D.; Liu, J.; Wu, Y.; Zhong, J. miR-634 Decreases the Radioresistance of Human Breast Cancer Cells by Targeting STAT3. Cancer Biother. Radiopharm. 2020, 35, 241–248. [Google Scholar] [CrossRef]
- Zhou, Z.-R.; Wang, X.-Y.; Yu, X.-L.; Mei, X.; Chen, X.-X.; Hu, Q.-C.; Yang, Z.-Z.; Guo, X.-M. Building Radiation-Resistant Model in Triple-Negative Breast Cancer to Screen Radioresistance-Related Molecular Markers. Ann. Transl. Med. 2020, 8, 108. [Google Scholar] [CrossRef]
- Boelens, M.C.; Wu, T.J.; Nabet, B.Y.; Xu, B.; Qiu, Y.; Yoon, T.; Azzam, D.J.; Victor, C.T.-S.; Wiemann, B.Z.; Ishwaran, H.; et al. Exosome Transfer from Stromal to Breast Cancer Cells Regulates Therapy Resistance Pathways. Cell 2014, 159, 499–513. [Google Scholar] [CrossRef]
- Braunstein, S.; Formenti, S.C.; Schneider, R.J. Acquisition of Stable Inducible Up-Regulation of Nuclear Factor-κB by Tumor Necrosis Factor Exposure Confers Increased Radiation Resistance without Increased Transformation in Breast Cancer Cells. Mol. Cancer Res. 2008, 6, 78–88. [Google Scholar] [CrossRef]
- Brennan, D.J.; Jirstrom, K.; Kronblad, A.; Millikan, R.C.; Landberg, G.; Duffy, M.J.; Rydén, L.; Gallagher, W.M.; O’Brien, S.L. CA IX is an Independent Prognostic Marker in Premenopausal Breast Cancer Patients with One to Three Positive Lymph Nodes and a Putative Marker of Radiation Resistance. Clin. Cancer Res. 2006, 12, 6421–6431. [Google Scholar] [CrossRef] [PubMed]
- Cordes, N.; Blaese, M.A.; Plasswilm, L.; Rodemann, H.P.; Van Beuningen, D. ibronectin and Laminin Increase Resistance to Ionizing Radiation and the Cytotoxic Drug Ukrain® in Human Tumour and Normal Cells in Vitro. Int. J. Radiat. Biol. 2003, 79, 709–720. [Google Scholar] [CrossRef]
- Heravi, M.; Tomic, N.; Liang, L.; Devic, S.; Holmes, J.; Deblois, F.; Radzioch, D.; Muanza, T. Sorafenib in Combination with Ionizing Radiation Has a Greater Anti-Tumour Activity in a Breast Cancer Model. Anti-Cancer Drugs 2012, 23, 525–533. [Google Scholar] [CrossRef]
- Hu, T.; Zhou, R.; Zhao, Y.; Wu, G. Integrin A6/Akt/Erk Signaling Is Essential for Human Breast Cancer Resistance to Radiotherapy. Sci. Rep. 2016, 6, 33376. [Google Scholar] [CrossRef]
- Iijima, M.; Gombodorj, N.; Tachibana, Y.; Tachibana, K.; Yokobori, T.; Honma, K.; Nakano, T.; Asao, T.; Kuwahara, R.; Aoyama, K.; et al. Development of Single Nanometer-Sized Ultrafine Oxygen Bubbles to Overcome the Hypoxia-Induced Re-sistance to Radiation Therapy via the Suppression of Hypoxia-Inducible Factor1α. Int. J. Oncol. 2018, 52, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Ding, Y.; Kong, Y.; Fang, M.; Yu, X.; Lai, X.; Gu, Q. Triple-negative Breast Cancer Cells That Survive Ionizing Radiation Exhibit an Axl-dependent Aggressive Radioresistant Phenotype. Exp. Ther. Med. 2023, 26, 448. [Google Scholar] [CrossRef]
- Jung, J.; Lee, J.-S.; Lee, Y.-S.; Lee, K. Radiosensitivity of Cancer Cells Is Regulated by Translationally Controlled Tumor Protein. Cancers 2019, 11, 386. [Google Scholar] [CrossRef]
- Krautschneider, S.L.; Troschel, F.M.; Vadillo, E.; Eich, H.T.; Götte, M.; Espinoza-Sánchez, N.A.; Greve, B. Enzymatic Digestion of Cell-surface Heparan Sulfate Alters the Radiation Response in Triple-negative Breast Cancer Cells. Arch. Med. Res. 2022, 53, 826–839. [Google Scholar] [CrossRef]
- La Verde, G.; Artiola, V.; Pugliese, M.; La Commara, M.; Arrichiello, C.; Muto, P.; Netti, P.A.; Fusco, S.; Panzetta, V. Radiation Therapy Affects YAP Expression and Intracellular Localization by Modulating Lamin A/C Levels in Breast Cancer. Front. Bioeng. Biotechnol. 2022, 10, 969004. [Google Scholar] [CrossRef]
- Lei, C.; Li, S.; Fan, Y.; Hua, L.; Pan, Q.; Li, Y.; Long, Z.; Yang, R. LncRNA DUXAP8 induces breast cancer radioresistance by modulating the PI3K/AKT/mTOR pathway and the EZH2-E-cadherin/RHOB pathway. Cancer Biol. Ther. 2022, 23, 1–13. [Google Scholar] [CrossRef]
- Li, J.; Lei, C.; Chen, B.; Zhu, Q. LncRNA FGD5-AS1 Facilitates the Radioresistance of Breast Cancer Cells by Enhancing MACC1 Expression Through Competitively Sponging miR-497-5p. Front. Oncol. 2021, 11, 671853. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, X.; Xie, Q.; Sun, H.; Huang, K.; Chen, H.; Wang, W.; Zhou, B.; Wei, X.; Zeng, D.; et al. CD146 Interaction with Integrin Β1 Activates LATS1-YAP Signaling and Induces Radiation-Resistance in Breast Cancer Cells. Cancer Lett. 2022, 546, 215856. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; He, X.; Apontes, P.; Cao, F.; Azrak, R.G.; Li, F. Enhancing Effectiveness of the MDR-Sensitive Compound T138067 Using Advanced Treatment with Negative Modulators of the Drug-Resistant Protein Survivin. Am. J. Transl. Res. 2009, 1, 393–405. [Google Scholar] [PubMed]
- Luo, B.; Yu, S.; Zhuang, L.; Xia, S.; Zhao, Z.; Rong, L. Induction of ERBB2 Nuclear Transport after Radiation in Breast Cancer Cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Marvaso, G.; Barone, A.; Amodio, N.; Raimondi, L.; Agosti, V.; Altomare, E.; Scotti, V.; Lombardi, A.; Bianco, R.; Bianco, C.; et al. Sphingosine Analog Fingolimod (FTY720) Increases Radiation Sensitivity of Human Breast Cancer Cells in Vitro. Cancer Biol. Ther. 2014, 15, 797–805. [Google Scholar] [CrossRef]
- Mast, J.M.; Kuppusamy, P. Hyperoxygenation as a Therapeutic Supplement for Treatment of Triple Negative Breast Cancer. Front. Oncol. 2018, 8, 527. [Google Scholar] [CrossRef]
- Miao, W.; Bade, D.; Wang, Y. Targeted Proteomic Analysis Revealed Kinome Reprogramming during Acquisition of Radioresistance in Breast Cancer Cells. J. Proteome Res. 2021, 20, 2830–2838. [Google Scholar] [CrossRef]
- Onaga, C.; Tamori, S.; Matsuoka, I.; Ozaki, A.; Motomura, H.; Nagashima, Y.; Sato, T.; Sato, K.; Tahata, K.; Xiong, Y.; et al. High SLC20A1 Expression Is Associated With Poor Prognosis for Radiotherapy of Estrogen Receptor-positive Breast Cancer. Cancer Diagn. Progn. 2022, 2, 429–442. [Google Scholar] [CrossRef]
- Paramanantham, A.; Jung, E.J.; Go, S.-I.; Jeong, B.K.; Jung, J.-M.; Hong, S.C.; Kim, G.S.; Lee, W.S. Activated ERK Signaling Is One of the Major Hub Signals Related to the Acquisition of Radiotherapy-Resistant MDA-MB-231 Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 4940. [Google Scholar] [CrossRef]
- Steelman, L.S.; Navolanic, P.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.T.; Martelli, A.M.; Cocco, L.; Stivala, F.; Libra, M.; Nicoletti, F.; et al. Involvement of Akt and mTOR in Chemotherapeutic- and Hormonal-Based Drug Resistance and Response to Radiation in Breast Cancer Cells. Cell Cycle 2011, 10, 3003–3015. [Google Scholar] [CrossRef]
- Tao, J.; Xue, C.; Cao, M.; Ye, J.; Sun, Y.; Chen, H.; Guan, Y.; Zhang, W.; Zhang, W.; Yao, Y. Protein Disulfide Isomerase Family Member 4 Promotes Triple-Negative Breast Cancer Tumorigenesis and Radiotherapy Resistance through JNK Pathway. Breast Cancer Res. 2024, 26, 1. [Google Scholar] [CrossRef]
- Thewes, V.; Orso, F.; Jäger, R.; Eckert, D.; Schäfer, S.; Kirfel, G.; Garbe, S.; Taverna, D.; Schorle, H. Interference with Activator Protein-2 Transcription Factors Leads to Induction of Apoptosis and an Increase in Chemo- and Radiation-Sensitivity in Breast Cancer Cells. BMC Cancer 2010, 10, 192. [Google Scholar] [CrossRef]
- Wolfe, A.R.; Atkinson, R.L.; Reddy, J.P.; Debeb, B.G.; Larson, R.; Li, L.; Masuda, H.; Brewer, T.; Atkinson, B.J.; Brewster, A.; et al. High-Density and Very-Low-Density Lipoprotein Have Opposing Roles in Regulating Tumor-Initiating Cells and Sensitivity to Radiation in Inflammatory Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Li, Y.; Xia, S.; Chu, Q.; Xiao, X.; Qiu, H.; Chen, Y.; Zheng, Z.; Liu, F.; Zhuang, L.; et al. Knockdown of CAVEOLIN-1 Sensitizes Human Basal-Like Triple-Negative Breast Cancer Cells to Radiation. Cell Physiol. Biochem. 2017, 44, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Paplomata, E.; O’Regan, R. The PI3K/AKT/mTOR Pathway in Breast Cancer: Targets, Trials and Biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR Pathways: Cross-Talk and Compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef]
- Serra, V.; Scaltriti, M.; Prudkin, L.; Eichhorn, P.J.A.; Ibrahim, Y.H.; Chandarlapaty, S.; Markman, B.; Rodriguez, O.; Guzman, M.; Rodriguez, S.; et al. PI3K Inhibition Results in Enhanced HER Signaling and Acquired ERK Dependency in HER2-Overexpressing Breast Cancer. Oncogene 2011, 30, 2547–2557. [Google Scholar] [CrossRef]
- Wang, D.; Li, C.; Zhang, Y.; Wang, M.; Jiang, N.; Xiang, L.; Li, T.; Roberts, T.M.; Zhao, J.J.; Cheng, H.; et al. Combined Inhibition of PI3K and PARP Is Effective in the Treatment of Ovarian Cancer Cells with Wild-Type PIK3CA Genes. Gynecol. Oncol. 2016, 142, 548–556. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK Signaling in Apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.-J. Pathological Roles of MAPK Signaling Pathways in Human Diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Zhong, Z.; Jiao, Z.; Yu, F.-X. The Hippo Signaling Pathway in Development and Regeneration. Cell Rep. 2024, 43, 113926. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward Understanding the Origin and Evolution of Cellular Organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological Systems Database as a Model of the Real World. Nucleic Acids Res. 2025, 53, D672–D677. [Google Scholar] [CrossRef]
- Peterson, J.; McTiernan, C.D.; Thome, C.; Khaper, N.; Lees, S.J.; Boreham, D.R.; Tai, T.C.; Tharmalingam, S. Identification of Radiation-Induced miRNA Biomarkers Using the CGL1 Cell Model System. Bioengineering 2022, 9, 214. [Google Scholar] [CrossRef]
- Tharmalingam, S.; Sreetharan, S.; Kulesza, A.V.; Boreham, D.R.; Tai, T.C. Low-Dose Ionizing Radiation Exposure, Oxidative Stress and Epigenetic Programing of Health and Disease. Radiat. Res. 2017, 188, 525–538. [Google Scholar] [CrossRef]
- Wolfe, A.R.; Bambhroliya, A.; Reddy, J.P.; Debeb, B.G.; Huo, L.; Larson, R.; Li, L.; Ueno, N.T.; Woodward, W.A. MiR-33a Decreases High-Density Lipoprotein-Induced Radiation Sensitivity in Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 791–799. [Google Scholar] [CrossRef]
- Anastasov, N.; Höfig, I.; Vasconcellos, I.G.; Rappl, K.; Braselmann, H.; Ludyga, N.; Auer, G.; Aubele, M.; Atkinson, M.J. Radiation Resistance Due to High Expression of miR-21 and G2/M Checkpoint Arrest in Breast Cancer Cells. Radiat. Oncol. 2012, 7, 206. [Google Scholar] [CrossRef]
- Yu, L.; Yang, Y.; Hou, J.; Zhai, C.; Song, Y.; Zhang, Z.; Qiu, L.; Jia, X. MicroRNA-144 Affects Radiotherapy Sensitivity by Promoting Proliferation, Migration and Invasion of Breast Cancer Cells. Oncol. Rep. 2015, 34, 1845–1852. [Google Scholar] [CrossRef]
- Masoudi-Khoram, N.; Abdolmaleki, P.; Hosseinkhan, N.; Nikoofar, A.; Mowla, S.J.; Monfared, H.; Baldassarre, G. Differential miRNAs Expression Pattern of Irradiated Breast Cancer Cell Lines Is Correlated with Radiation Sensitivity. Sci. Rep. 2020, 10, 9054. [Google Scholar] [CrossRef] [PubMed]
- Mesci, A.; Huang, X.; Taeb, S.; Jahangiri, S.; Kim, Y.; Fokas, E.; Bruce, J.; Leong, H.S.; Liu, S.K. Targeting of CCBE1 by miR-330-3p in Human Breast Cancer Promotes Metastasis. Br. J. Cancer 2017, 116, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Sun, L.; Lu, X.; Shi, J.; Chen, J.; Zhang, X. MiR-143-3p Increases the Radiosensitivity of Breast Cancer Cells Through FGF1. Cell. Mol. Biol. 2022, 67, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, B.; Xiao, H.; Dong, J.; Li, Y.; Zhu, C.; Jin, Y.; Li, H.; Cui, M.; Fan, S. LncRNA HOTAIR Enhances Breast Cancer Radioresistance through Facilitating HSPA1A Expression via Sequestering miR-449b-5p. Thorac. Cancer 2020, 11, 1801–1816. [Google Scholar] [CrossRef]
- Jain, S.; Coulter, J.A.; Butterworth, K.T.; Hounsell, A.R.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.M.; Currell, F.J.; et al. Gold Nanoparticle Cellular Uptake, Toxicity and Radiosensitisation in Hypoxic Conditions. Radiother. Oncol. 2014, 110, 342–347. [Google Scholar] [CrossRef]
- Zuo, S.; Wang, Z.; Zhao, L.; Wang, J. Gold Nanoplatform for Near-Infrared Light-Activated Radio-Photothermal Gas Therapy in Breast Cancer. Front. Bioeng. Biotechnol. 2023, 10, 1098986. [Google Scholar] [CrossRef]
- Hullo, M.; Grall, R.; Perrot, Y.; Mathé, C.; Ménard, V.; Yang, X.; Lacombe, S.; Porcel, E.; Villagrasa, C.; Chevillard, S.; et al. Radiation Enhancer Effect of Platinum Nanoparticles in Breast Cancer Cell Lines: In Vitro and In Silico Analyses. Int. J. Mol. Sci. 2021, 22, 4436. [Google Scholar] [CrossRef]
- Tharmalingam, S.; Sreetharan, S.; Brooks, A.L.; Boreham, D.R. Re-Evaluation of the Linear No-Threshold (LNT) Model Using New Paradigms and Modern Molecular Studies. Chem. Biol. Interact. 2019, 301, 54–67. [Google Scholar] [CrossRef]
- Scott, B.R.; Tharmalingam, S. The LNT Model for Cancer Induction Is Not Supported by Radiobiological Data. Chem. Biol. Interact. 2019, 301, 34–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, N.; Li, M.; Liu, M.; Wu, C. Cancer-Associated Fibroblasts: Tumor Defenders in Radiation Therapy. Cell Death Dis. 2023, 14, 541. [Google Scholar] [CrossRef]
- Vigneux, G.; Pirkkanen, J.; Laframboise, T.; Prescott, H.; Tharmalingam, S.; Thome, C. Radiation-Induced Alterations in Proliferation, Migration, and Adhesion in Lens Epithelial Cells and Implications for Cataract Development. Bioengineering 2022, 9, 29. [Google Scholar] [CrossRef]
- Al-khayyat, W.; Pirkkanen, J.; Dougherty, J.; Laframboise, T.; Dickinson, N.; Khaper, N.; Lees, S.J.; Mendonca, M.S.; Boreham, D.R.; Tai, T.C.; et al. Overexpression of FRA1 (FOSL1) Leads to Global Transcriptional Perturbations, Reduced Cellular Adhesion and Altered Cell Cycle Progression. Cells 2023, 12, 2344. [Google Scholar] [CrossRef]
- Pirkkanen, J.; Tharmalingam, S.; Morais, I.H.; Lam-Sidun, D.; Thome, C.; Zarnke, A.M.; Benjamin, L.V.; Losch, A.C.; Borgmann, A.J.; Sinex, H.C.; et al. Transcriptomic Profiling of Gamma Ray Induced Mutants from the CGL1 Human Hybrid Cell System Reveals Novel Insights into the Mechanisms of Radiation-Induced Carcinogenesis. Free Radic. Biol. Med. 2019, 145, 300–311. [Google Scholar] [CrossRef]
- Choi, H.S.; Ko, Y.S.; Jin, H.; Kang, K.M.; Ha, I.B.; Jeong, H.; Lee, J.; Jeong, B.K.; Kim, H.J. Mebendazole Increases Anticancer Activity of Radiotherapy in Radiotherapy-Resistant Triple-Negative Breast Cancer Cells by Enhancing Natural Killer Cell-Mediated Cytotoxicity. Int. J. Mol. Sci. 2022, 23, 15493. [Google Scholar] [CrossRef]
- Jian, C.; Wu, T.; Wang, L.; Gao, C.; Fu, Z.; Zhang, Q.; Shi, C. Biomimetic Nanoplatform for Dual-Targeted Clearance of Activated and Senescent Cancer-Associated Fibroblasts to Improve Radiation Resistance in Breast Cancer. Small 2024, 20, 2309279. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Morales-Vasquez, F.; Hortobagyi, G.N. Overview of Resistance to Systemic Therapy in Patients with Breast Cancer. Adv. Exp. Med. Biol. 2007, 608, 1–22. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mageau, E.; Derbowka, R.; Dickinson, N.; Lefort, N.; Kovala, A.T.; Boreham, D.R.; Tai, T.C.; Thome, C.; Tharmalingam, S. Molecular Mechanisms of Radiation Resistance in Breast Cancer: A Systematic Review of Radiosensitization Strategies. Curr. Issues Mol. Biol. 2025, 47, 589. https://doi.org/10.3390/cimb47080589
Mageau E, Derbowka R, Dickinson N, Lefort N, Kovala AT, Boreham DR, Tai TC, Thome C, Tharmalingam S. Molecular Mechanisms of Radiation Resistance in Breast Cancer: A Systematic Review of Radiosensitization Strategies. Current Issues in Molecular Biology. 2025; 47(8):589. https://doi.org/10.3390/cimb47080589
Chicago/Turabian StyleMageau, Emma, Ronan Derbowka, Noah Dickinson, Natalie Lefort, A. Thomas Kovala, Douglas R. Boreham, T. C. Tai, Christopher Thome, and Sujeenthar Tharmalingam. 2025. "Molecular Mechanisms of Radiation Resistance in Breast Cancer: A Systematic Review of Radiosensitization Strategies" Current Issues in Molecular Biology 47, no. 8: 589. https://doi.org/10.3390/cimb47080589
APA StyleMageau, E., Derbowka, R., Dickinson, N., Lefort, N., Kovala, A. T., Boreham, D. R., Tai, T. C., Thome, C., & Tharmalingam, S. (2025). Molecular Mechanisms of Radiation Resistance in Breast Cancer: A Systematic Review of Radiosensitization Strategies. Current Issues in Molecular Biology, 47(8), 589. https://doi.org/10.3390/cimb47080589







