Molecular Mechanisms of Lobelia nummularia Extract in Breast Cancer: Targeting EGFR/TP53 and PI3K-AKT-mTOR Signaling via ROS-Mediated Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ethanolic Extracts of Lobelia nummularia
2.2. Phytochemical Analysis
2.2.1. Total Flavonoid Content (TFC)
2.2.2. Total Phenolic Content (TPC)
2.2.3. Quantitative Analysis of Key Flavonoids by HPLC
2.3. Antioxidant Assays In Vitro
2.4. Anticancer Activity In Vitro
2.4.1. Cell Culture and LNE Administration
2.4.2. Cell Proliferation Assay
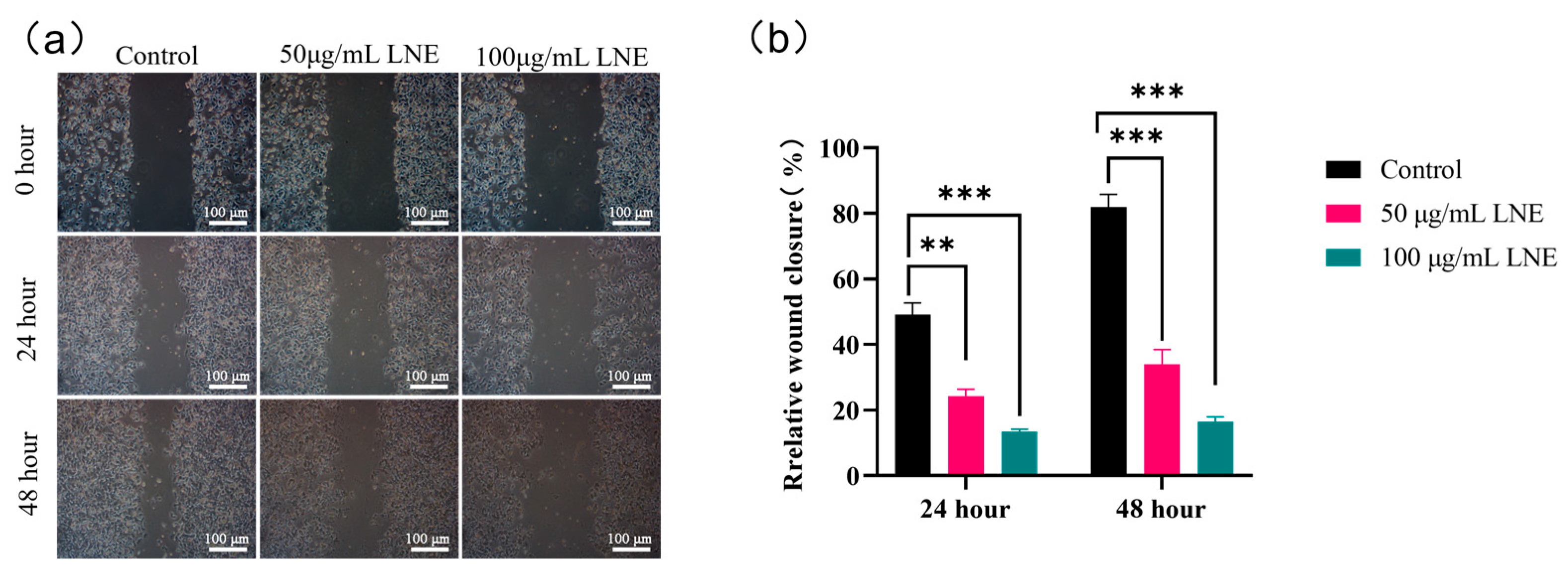
2.4.3. Wound Healing Assay to Determine the Migration Ability of MDA-MB-231
2.4.4. Hoechst Staining to Determine Cell Apoptosis
2.4.5. Study on Intracellular ROS Generation
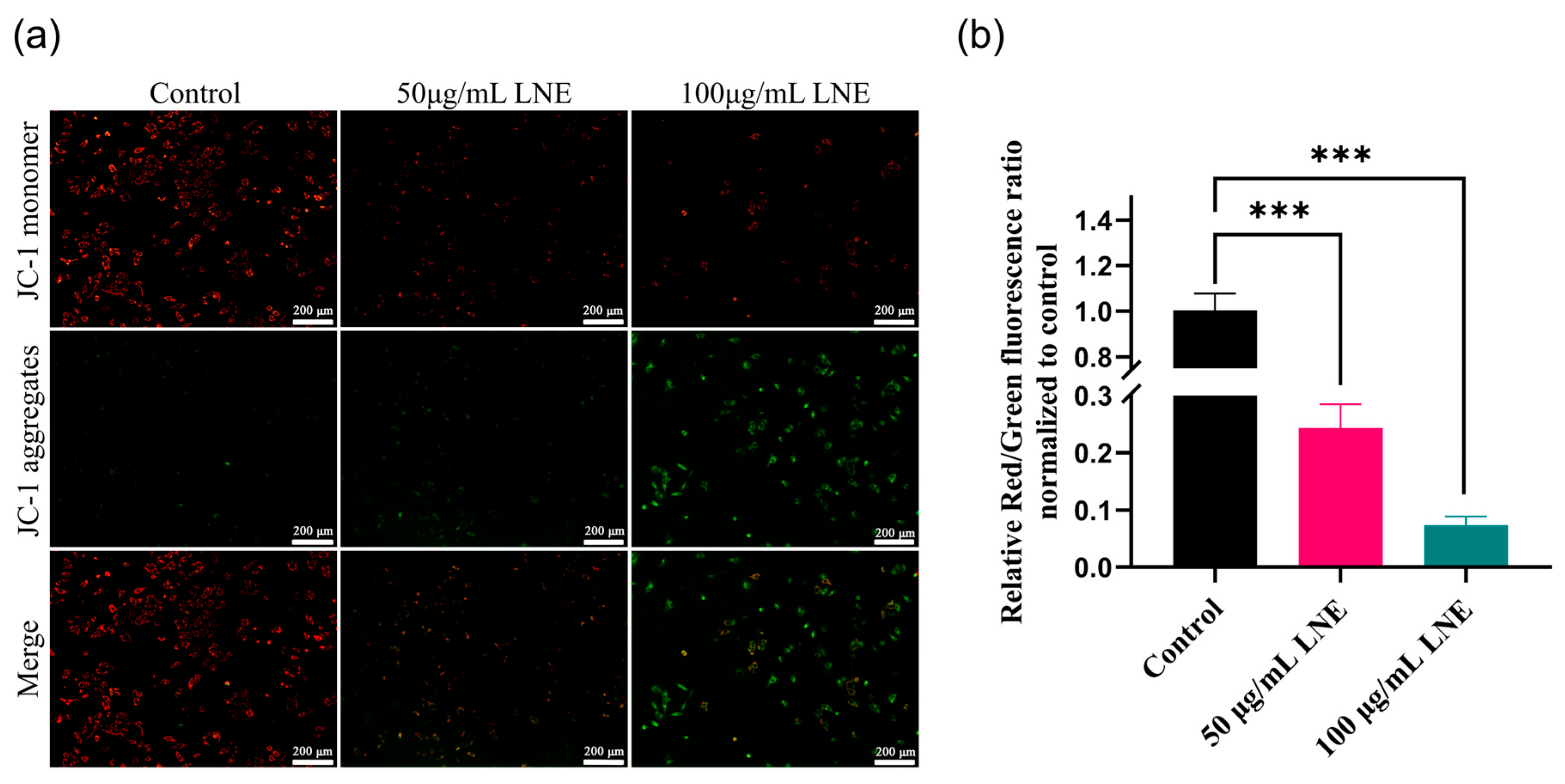
2.4.6. JC-1 Staining to Detect Mitochondrial Membrane Potential
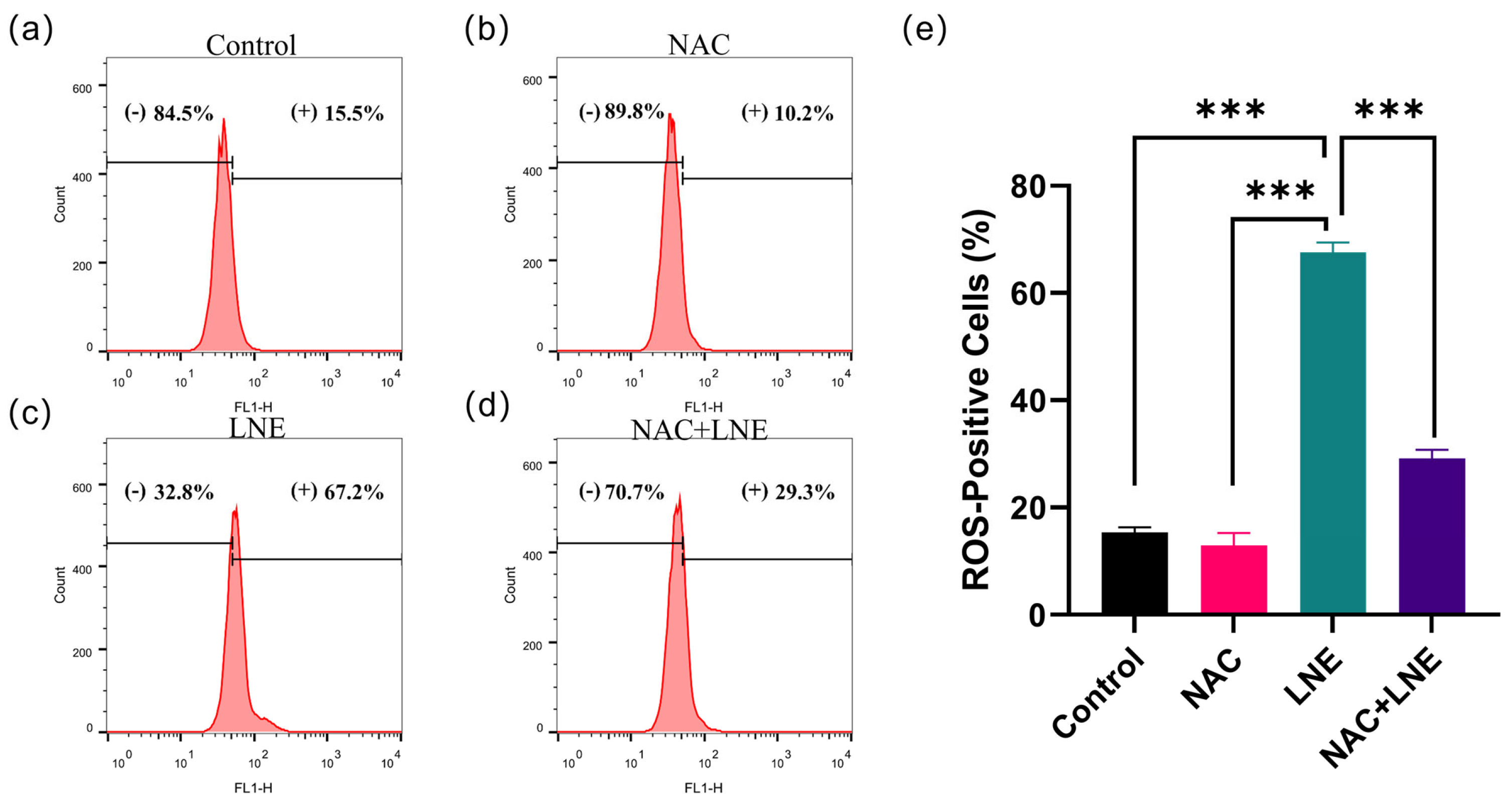
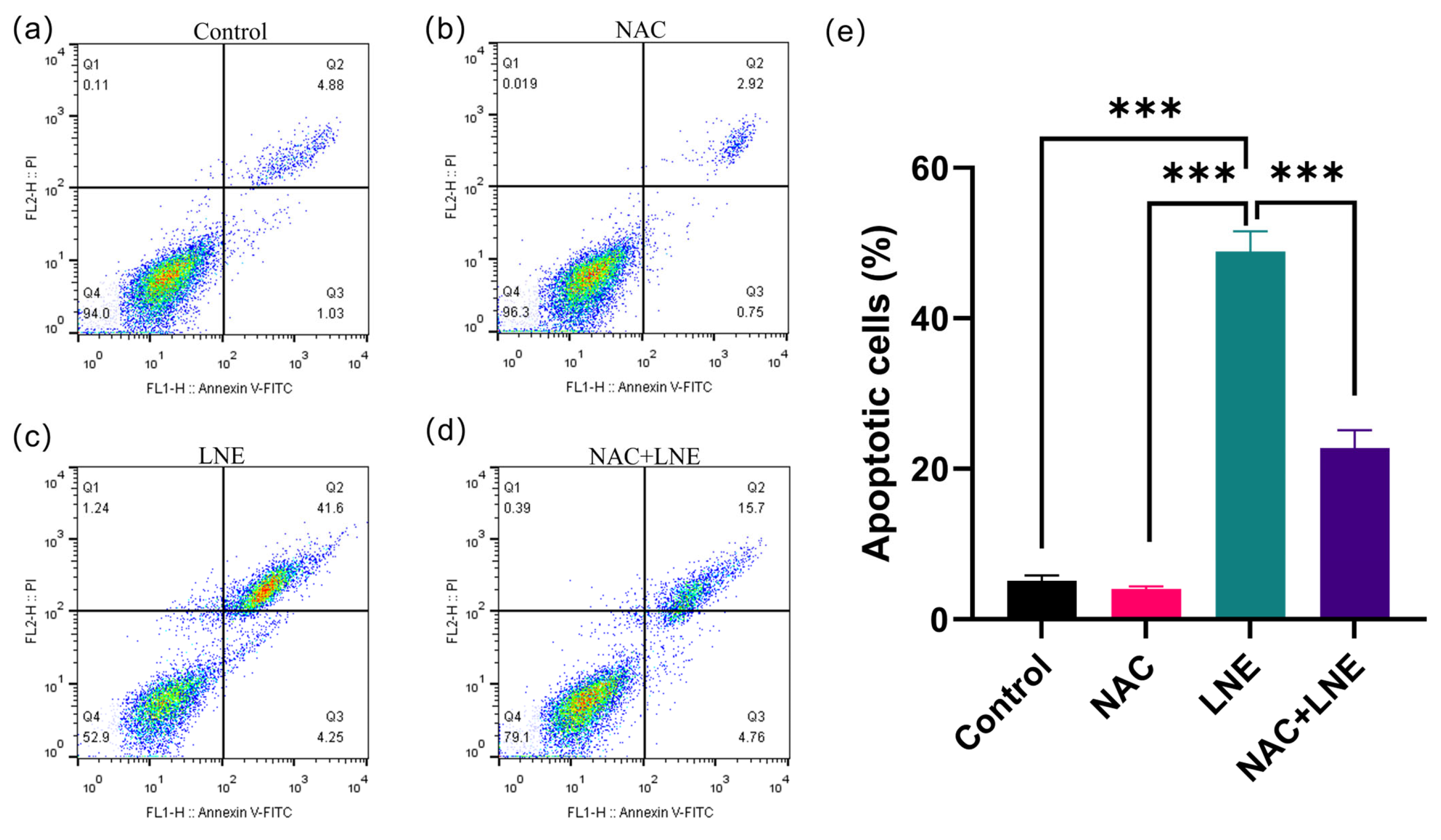
2.4.7. NAC Rescue Experiment and Flow Cytometry Analysis
2.5. Preparation of Serum Samples to Identify Chemically Absorbed Substances In Vivo
2.6. HPLC-QTOF/MS Analysis
2.7. Network Pharmacology Analysis
2.7.1. Database Construction
2.7.2. Construction of the Protein–Protein Interaction Network
2.7.3. GO and KEGG Pathway Enrichment Analysis
2.7.4. Molecular Docking
2.8. Verification of Animal Studies
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. Total Phenolic and Total Flavonoid Contents of Ethanolic Extract of Lobelia nummularia
3.2. Compounds in the Ethanolic Extract of Lobelia nummularia Identified by HPLC-QTOF-MS
3.3. Quantitative Analysis of Key Bioactive Flavonoids in LNE
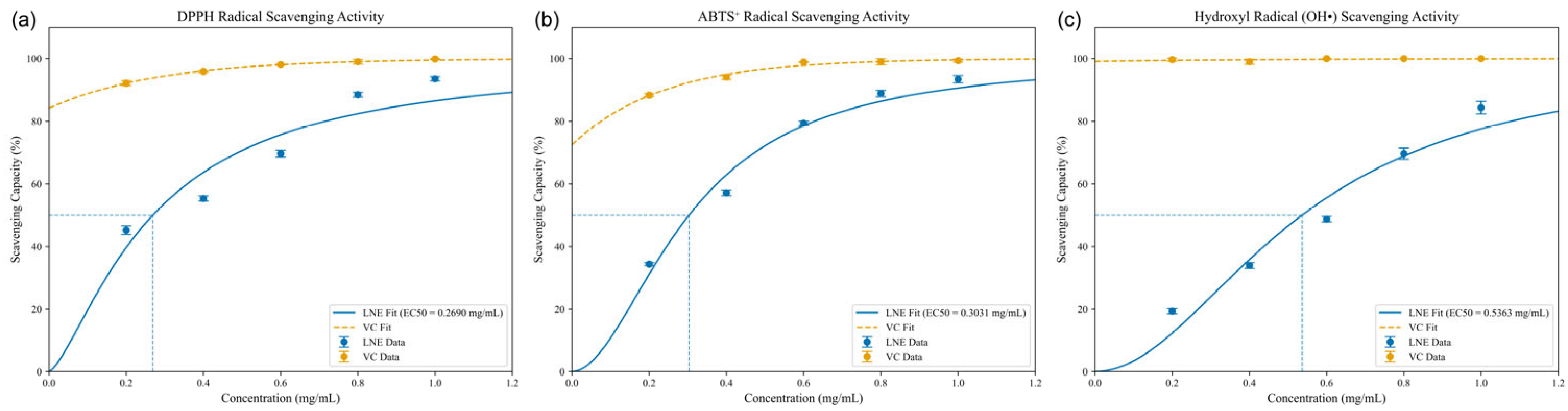
3.4. Antioxidant Activity of Ethanolic Extract of Lobelia nummularia
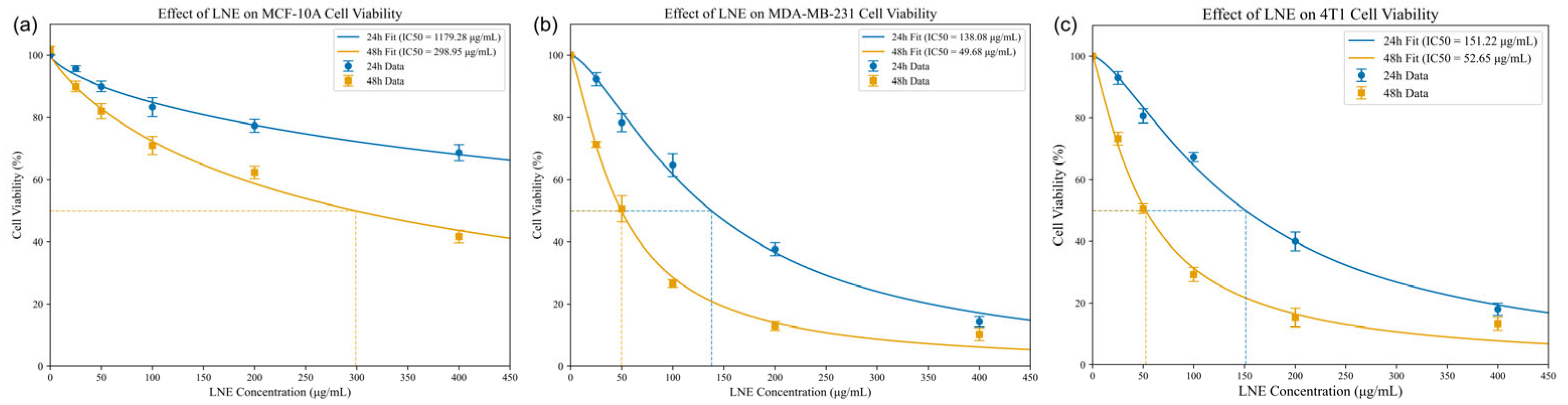
3.5. LNE Induces Cytotoxicity in MDA-MB-231 Cells
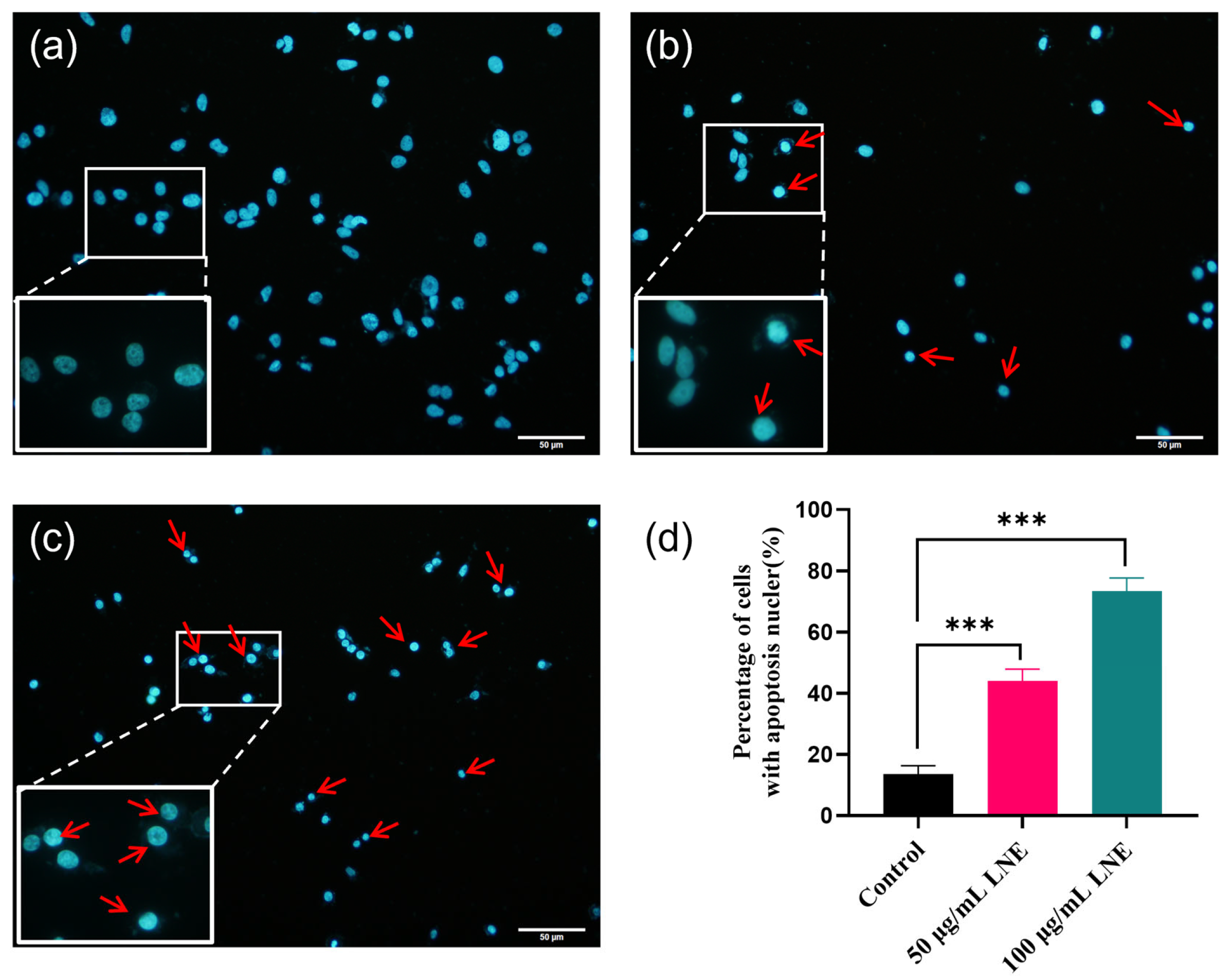
3.6. LNE Induces Apoptotic Morphology in MDA-MB-231 Cells
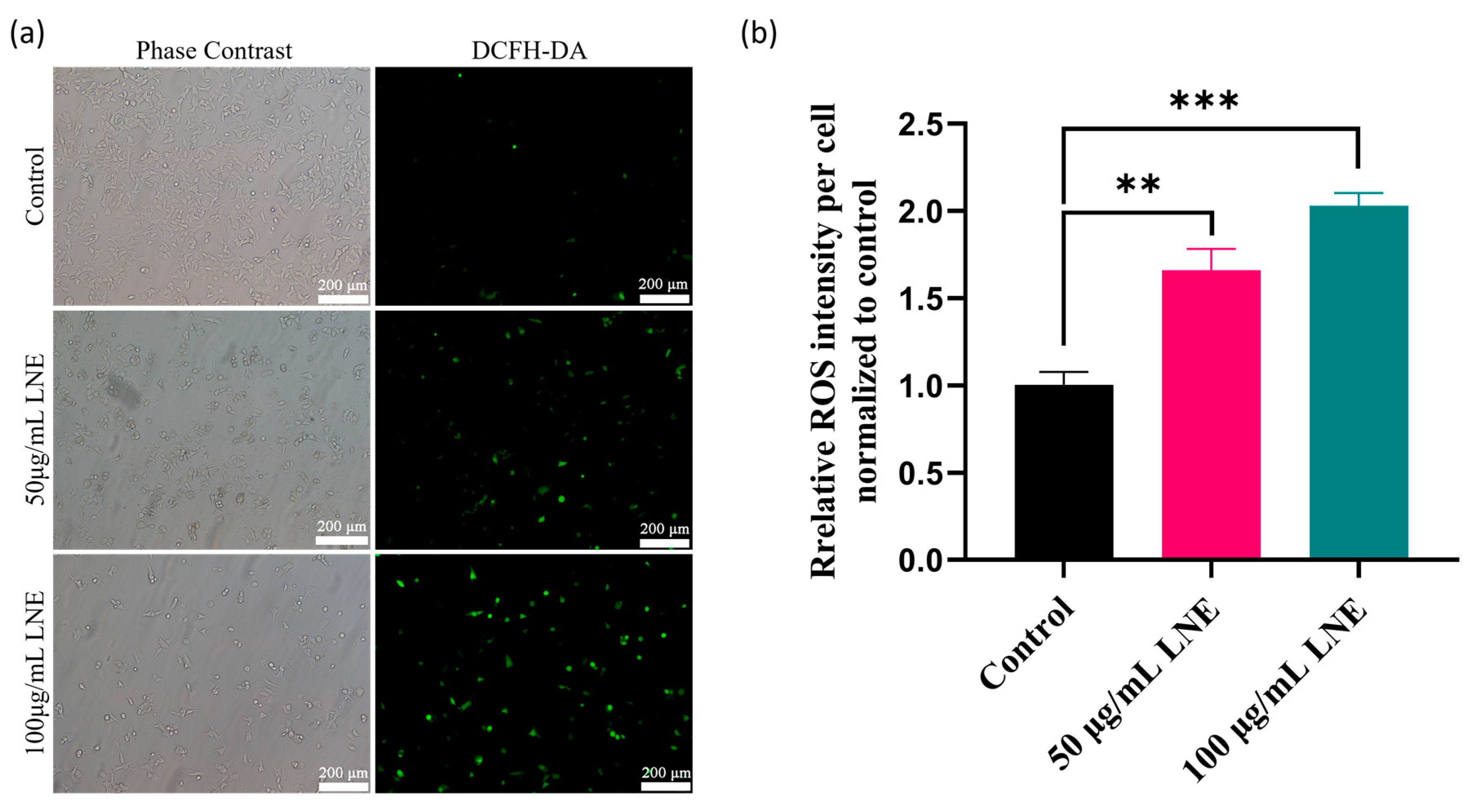
3.7. LNE Elevates Intracellular ROS and Disrupts Mitochondrial Membrane Potential
3.8. LNE-Induced Apoptosis Is Mediated by ROS Production
3.9. Effect of LNE on the Migration of MDA-MB-231 Cells Determined by Scratch Assay
3.10. In Vivo Studies
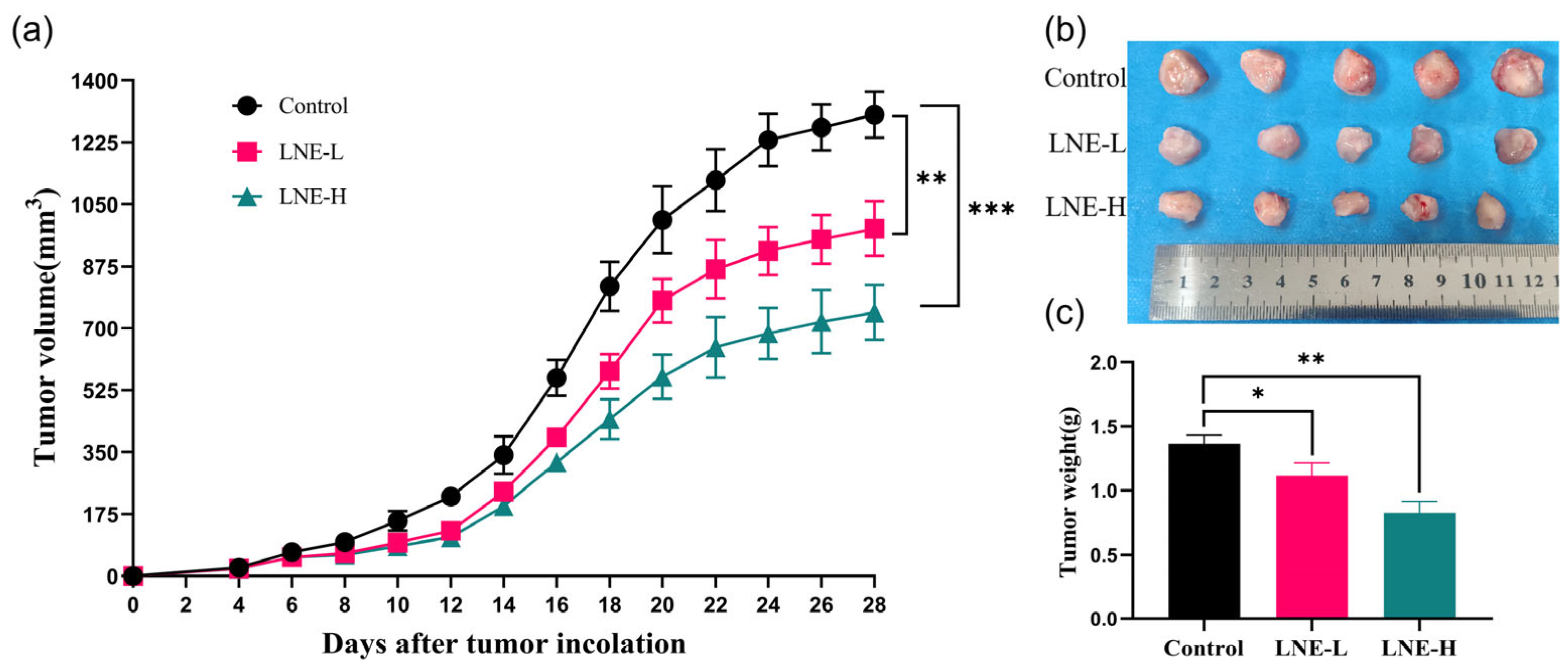
3.10.1. Effect of LNE on the Original 4T1 Breast Tumor Burden in Mice
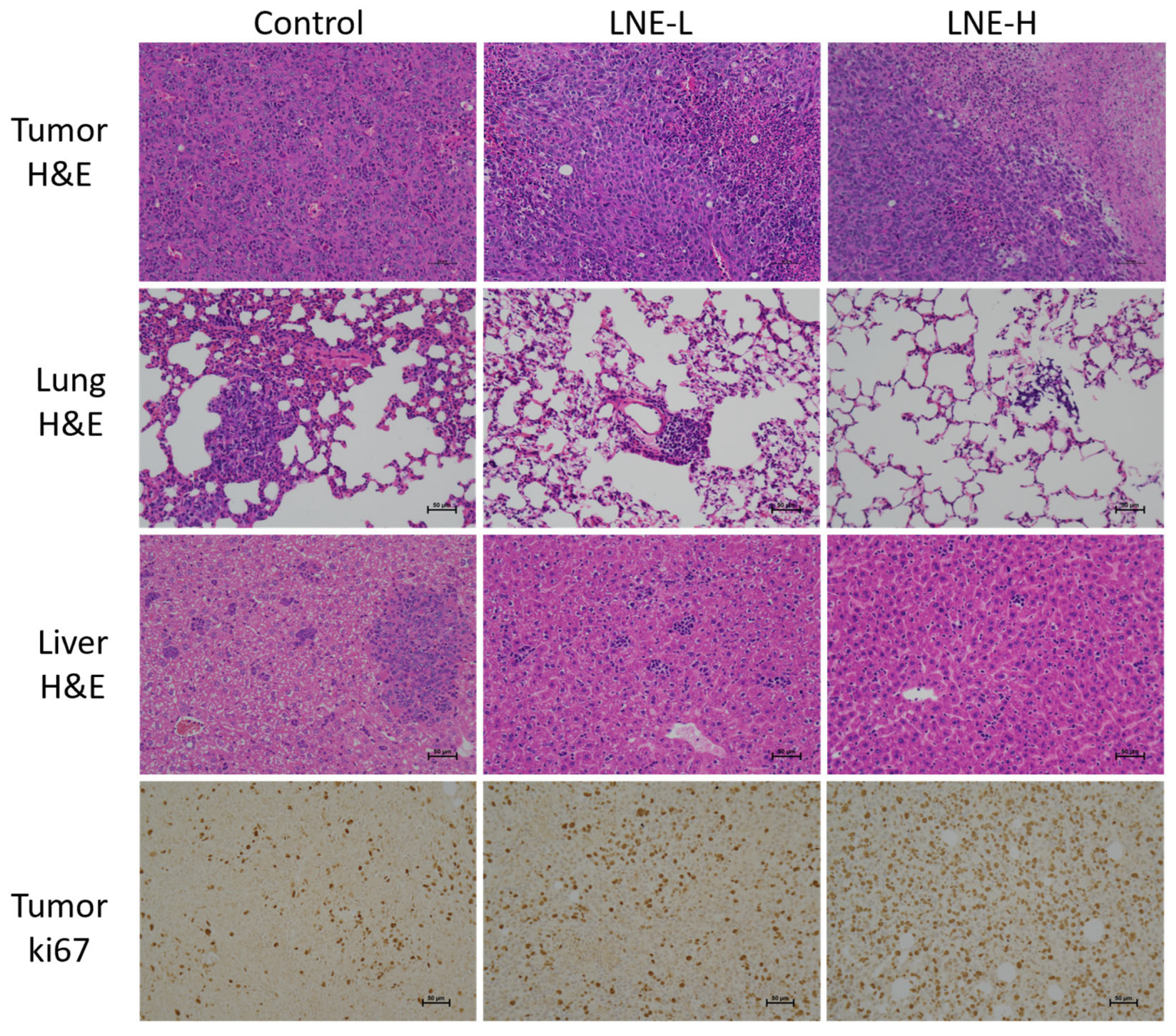
3.10.2. Effect of LNE on the Morphology of Tumor Tissues and Metastatic Tissues
3.10.3. Identification of the Blood-Absorbed Constituents of LNE via HPLC-Q-TOF-MS
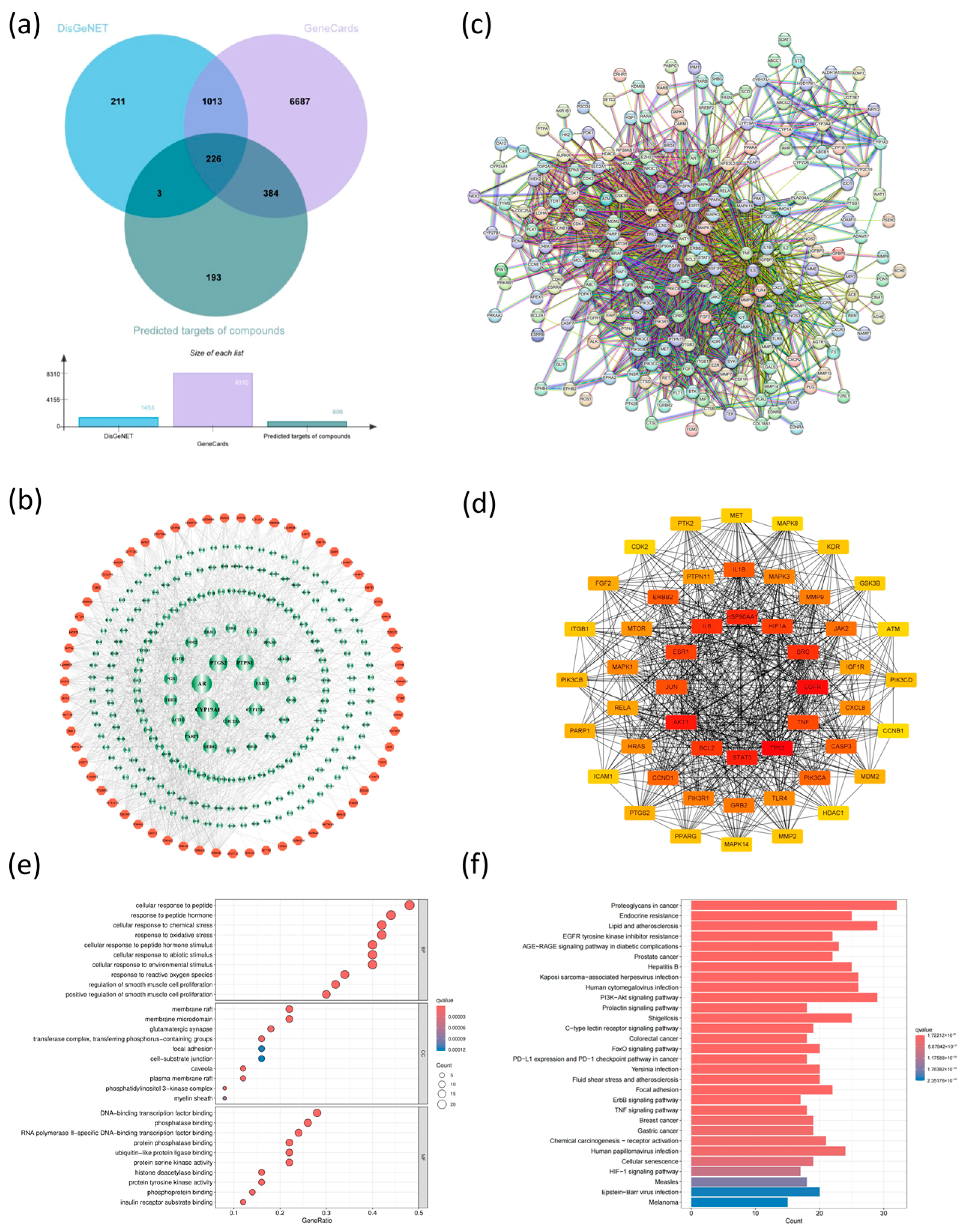
3.10.4. Screening of Anti-Breast Cancer Targets of Lobelia nummularia Ethanol Extract
3.10.5. Network Construction and Topological Analysis
3.10.6. GO and KEGG Enrichment Analysis
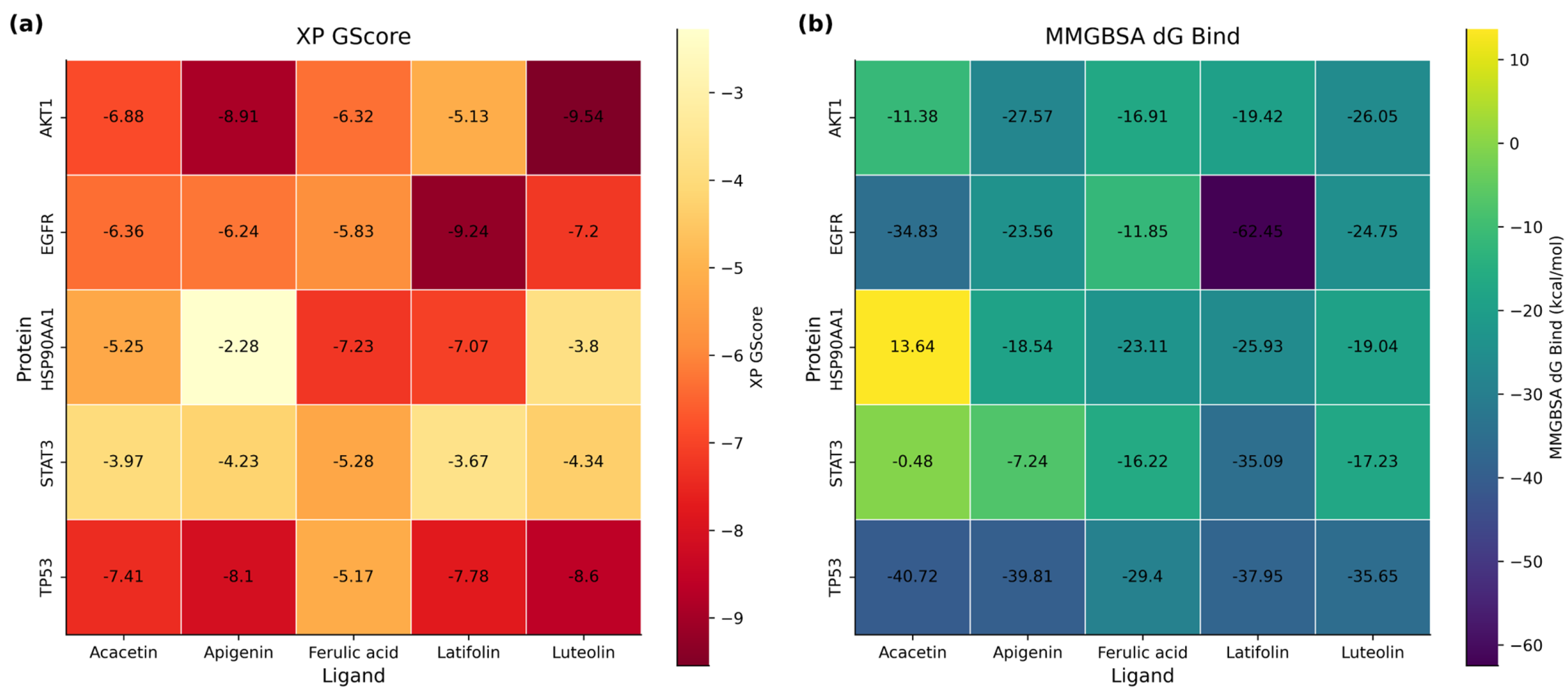
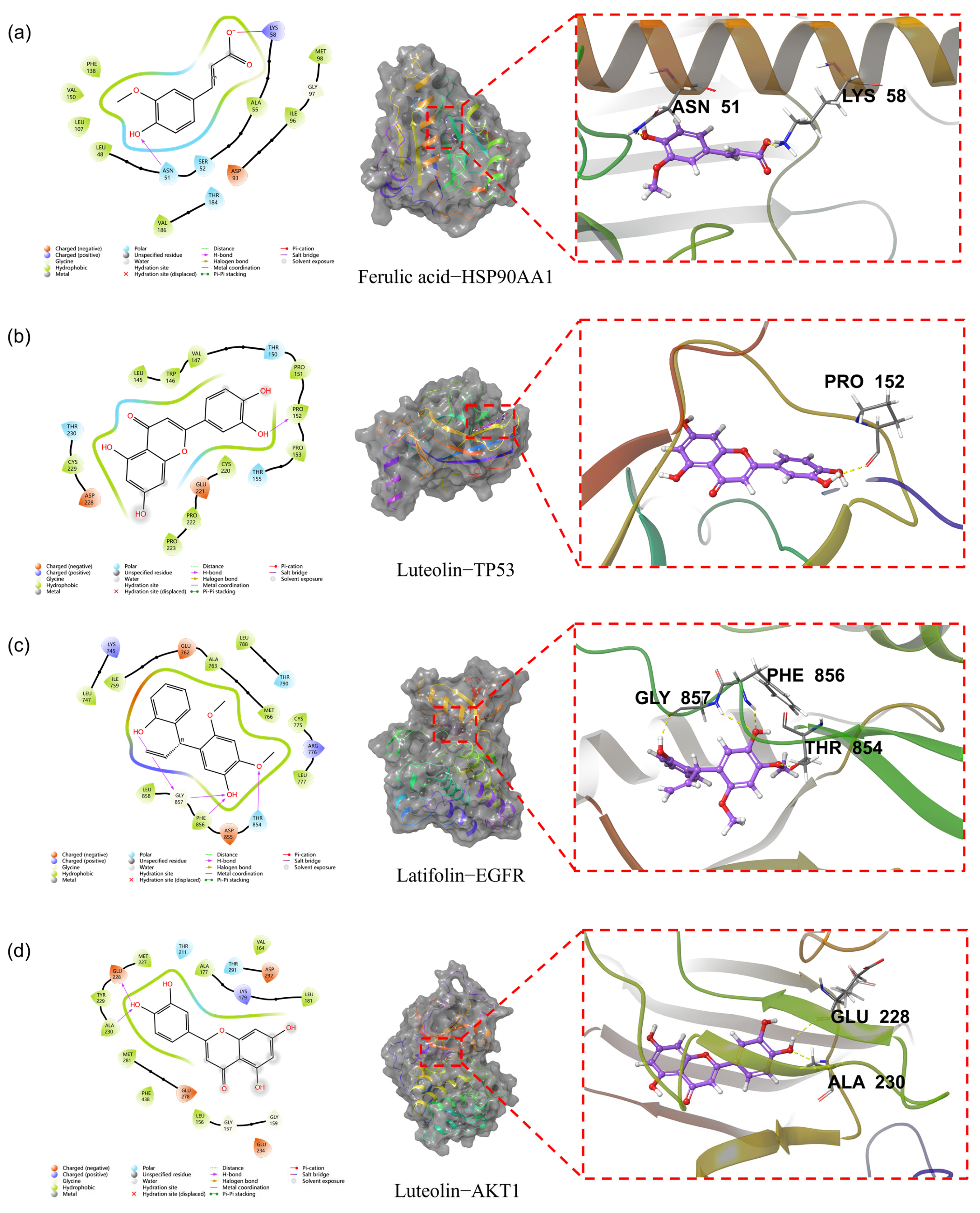
3.11. Molecular Docking Analysis of Key Compound-Target Interactions
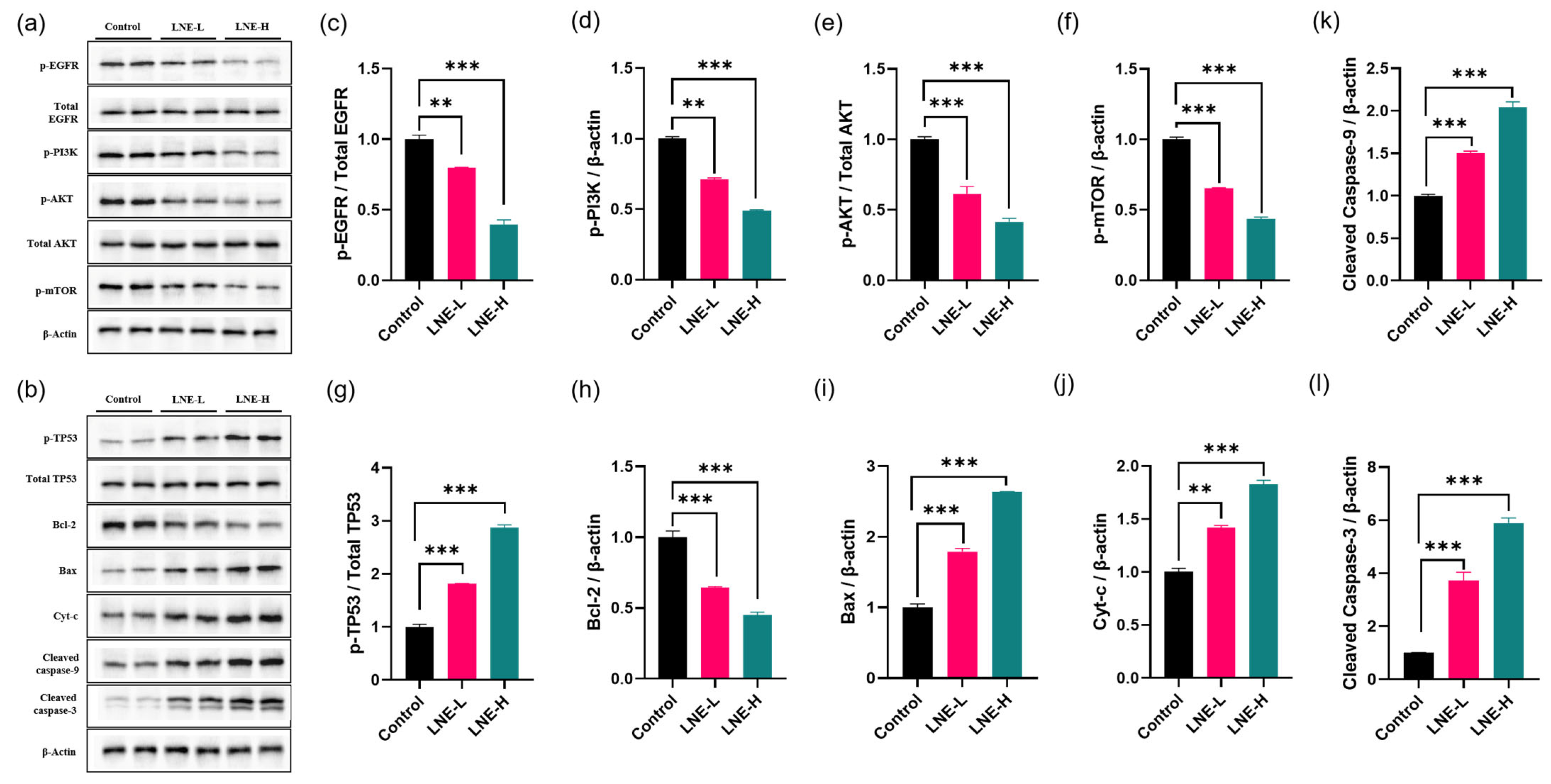
3.12. LNE Suppresses the EGFR/PI3K/AKT/mTOR Pathway and Activates the TP53-Mediated Apoptotic Cascade In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LNE | Ethanolic extract of Lobelia nummularia |
| HPLC-QTOF-MS | High-performance liquid chromatography coupled with Quadrupole Time-of-flight mass spectrometry |
| TCM | Traditional Chinese Medicine |
| TNBC | Triple-negative breast cancer |
| ROS | Reactive oxygen species |
| MMP | Mitochondrial membrane potential |
| TFC | Total flavonoid content |
| RE | Rutin equivalent |
| TPC | Total phenolic content |
| GAE | Gallic acid equivalent |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DMEM | Dulbecco’s modified eagle medium |
| DMSO | Dimethyl sulfoxide |
| CCK-8 | Cell Counting Kit-8 |
| PBS | Phosphate-buffered saline |
| JC-1 | 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide |
| H2DCF-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| NAC | N-acetylcysteine |
| PPI | Protein–protein interaction |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
References
- Ishimaru, K.; Osabe, M.; Yan, L.; Fujioka, T.; Mihashi, K.; Tanaka, N. Polyacetylene Glycosides from Pratia Nummularia Cultures. Phytochemistry 2003, 62, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.-K.; Ou, J.-C.; Sun, M.-L.; Sun, C.-M. Two Rare Alkaloids from Pratia Nummularia. Planta Medica 1995, 61, 567–568. [Google Scholar] [CrossRef]
- Matsuura, E.; Shimomura, K.; Ishimaru, K. Flavonoid and Polyacetylene from Pratia Nummularia. Nat. Med. 2000, 54, 44. [Google Scholar]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global Patterns of Breast Cancer Incidence and Mortality: A Population-based Cancer Registry Data Analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, S.-Y.; Chan, K.I.; Zhong, Z.-F.; Wang, Y.-T. Novel Anti-Cancer Products Targeting AMPK: Natural Herbal Medicine against Breast Cancer. Molecules 2023, 28, 740. [Google Scholar] [CrossRef]
- Yuan, Y.; Long, H.; Zhou, Z.; Fu, Y.; Jiang, B. PI3K-AKT-Targeting Breast Cancer Treatments: Natural Products and Synthetic Compounds. Biomolecules 2023, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Li, Y.; Li, S.; Liu, J.; Yang, X.; Xia, G.; Wang, G. Natural Products and Derivatives for Breast Cancer Treatment: From Drug Discovery to Molecular Mechanism. Phytomedicine 2024, 129, 155600. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Javed Shaikh, M.A.; Afzal, O.; Alfawaz Altamimi, A.S.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Al-Abbasi, F.A.; Singh, S.K.; Dua, K.; et al. An Overview of Epithelial Growth Factor Receptor (EGFR) Inhibitors in Cancer Therapy. Chem.-Biol. Interact. 2022, 366, 110108. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting P53 Pathways: Mechanisms, Structures and Advances in Therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, C.Y.; Duan, F.G.; Fan, X.-X.; Yao, X.-J.; Parks, R.J.; Tang, Y.-J.; Wang, M.-F.; Liu, L.; Tsang, B.K.; et al. P53 Sensitizes Chemoresistant Non-Small Cell Lung Cancer via Elevation of Reactive Oxygen Species and Suppression of EGFR/PI3K/AKT Signaling. Cancer Cell Int. 2019, 19, 188. [Google Scholar] [CrossRef]
- Hanikoglu, A.; Ozben, H.; Hanikoglu, F.; Ozben, T. Hybrid Compounds & Oxidative Stress Induced Apoptosis in Cancer Therapy. Curr. Med. Chem. 2020, 27, 2118–2132. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Li, K.; Ni, M.; Jiang, H.; Wu, H.; Yu, Q.; Li, J.; Li, X.; Wei, J.; Wu, W.; et al. Dendritic Polylysine with Paclitaxel and Triptolide Codelivery for Enhanced Cancer Ferroptosis through the Accumulation of ROS. ACS Appl. Mater. Interfaces 2024, 16, 20143–20156. [Google Scholar] [CrossRef]
- Wei, F.; Nian, Q.; Zhao, M.; Wen, Y.; Yang, Y.; Wang, J.; He, Z.; Chen, X.; Yin, X.; Wang, J.; et al. Natural Products and Mitochondrial Allies in Colorectal Cancer Therapy. Biomed. Pharmacother. 2023, 167, 115473. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Y.; Sun, Y.; Xu, H.; Wei, R.; Zhou, Y.; Li, F.; Li, J.; Wang, J.; Chen, P.; et al. Gambogic Acid Induces GSDME Dependent Pyroptotic Signaling Pathway via ROS/P53/Mitochondria/Caspase-3 in Ovarian Cancer Cells. Biochem. Pharmacol. 2025, 232, 116695. [Google Scholar] [CrossRef]
- Cheng, B.; Chu, X.; Liu, R.; Ma, X.; Wang, M.; Zhang, J.; Jiao, P.; Gao, Q.; Ma, W.; Zhang, Y.; et al. Synthesis of Novel Pentacyclic Triterpenoid Derivatives That Induce Apoptosis in Cancer Cells through a ROS-Dependent, Mitochondrial-Mediated Pathway. Mol. Pharm. 2023, 20, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, J.; Wei, Z.; Shahidi, F. Effect of in Vitro Digestion on Phenolics and Antioxidant Activity of Red and Yellow Colored Pea Hulls. Food Chem. 2021, 337, 127606. [Google Scholar] [CrossRef]
- Rinaldo, D.; Sotin, H.; Pétro, D.; Le-Bail, G.; Guyot, S. Browning Susceptibility of New Hybrids of Yam (Dioscorea Alata) as Related to Their Total Phenolic Content and Their Phenolic Profile Determined Using LC-UV-MS. LWT 2022, 162, 113410. [Google Scholar] [CrossRef]
- Meng, X.; Zheng, S.; Yin, Z.; Wang, X.; Yang, D.; Zou, T.; Li, H.; Chen, Y.; Liao, C.; Xie, Z.; et al. Apigenin Ameliorates Imiquimod-Induced Psoriasis in C57BL/6J Mice by Inactivating STAT3 and NF-κB. Food Sci. Hum. Wellness 2024, 13, 211–224. [Google Scholar] [CrossRef]
- Chen, L.-H.; Sun, Y.; Cai, H.; Guo, S.; Wang, X.-C.; Li, W.-D.; Mao, C.-Q.; Liu, X.-H.; Yan, L.-Y.; Jiang, H.-L.; et al. Simultaneous Determination of Eleven Bioactive Constituents in Honey-Processed Licorice by High-Performance Liquid Chromatography-Diode Array Detector and Its Application from the Perspective of Processing Influence under Orthogonal Design. World J. Tradit. Chin. Med. 2022, 8, 395–401. [Google Scholar] [CrossRef]
- Wu, L.; Lin, Y.; Gao, S.; Wang, Y.; Pan, H.; Wang, Z.; Pozzolini, M.; Yang, F.; Zhang, H.; Yang, Y.; et al. Luteolin Inhibits Triple-Negative Breast Cancer by Inducing Apoptosis and Autophagy through SGK1-FOXO3a-BNIP3 Signaling. Front. Pharmacol. 2023, 14, 1200843. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-T.; Liu, Y.-E.; Hsu, K.-W.; Wang, Y.-F.; Chan, Y.-C.; Chen, Y.; Chen, D.-R. MLL3 Induced by Luteolin Causes Apoptosis in Tamoxifen-Resistant Breast Cancer Cells through H3K4 Monomethylation and Suppression of the PI3K/AKT/mTOR Pathway. Am. J. Chin. Med. 2020, 48, 1221–1241. [Google Scholar] [CrossRef] [PubMed]
- Golonko, A.; Olichwier, A.J.; Szklaruk, A.; Paszko, A.; Świsłocka, R.; Szczerbiński, Ł.; Lewandowski, W. Apigenin’s Modulation of Doxorubicin Efficacy in Breast Cancer. Molecules 2024, 29, 2603. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, Z.; Cheng, Y.; Ye, J.; Li, S.; Xu, Y.; Ye, Z.; Shi, Y.; Ding, J.; Zhao, Y.; et al. Multifunctional Iron-Apigenin Nanocomplex Conducting Photothermal Therapy and Triggering Augmented Immune Response for Triple Negative Breast Cancer. Int. J. Pharm. 2024, 655, 124016. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. The Anticancer Mechanism of Action of Selected Polyphenols in Triple-Negative Breast Cancer (TNBC). Biomed. Pharmacother. 2023, 165, 115170. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Guo, Z.; Yao, L.; Sun, Y.; Dian, Y.; Zhao, D.; Ke, Y.; Zeng, F.; Zhang, C.; Deng, G.; et al. Targeting Oxidative Stress-Mediated Regulated Cell Death as a Vulnerability in Cancer. Redox Biol. 2025, 84, 103686. [Google Scholar] [CrossRef]
- Penjweini, R.; Link, K.A.; Qazi, S.; Mattu, N.; Zuchowski, A.; Vasta, A.; Sackett, D.L.; Knutson, J.R. Low Dose Taxol Causes Mitochondrial Dysfunction in Actively Respiring Cancer Cells. J. Biol. Chem. 2025, 301, 108450. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Garg, P.; Ramisetty, S.; Nair, M.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Strategic Advancements in Targeting the PI3K/AKT/mTOR Pathway for Breast Cancer Therapy. Biochem. Pharmacol. 2025, 236, 116850. [Google Scholar] [CrossRef]
- Que, Z.; Wang, P.; Hu, Y.; Xue, Y.; Liu, X.; Qu, C.; Ma, J.; Liu, Y. Dihydroartemisin Inhibits Glioma Invasiveness via a ROS to P53 to β-Catenin Signaling. Pharmacol. Res. 2017, 119, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Min, W.L.; Wang, B.F.; Liang, B.B.; Zhang, L.; Pan, J.Y.; Huang, Y.; Zhao, Y.; Lin, S.; Zhao, Y.H.; Zhang, S.Q.; et al. A ROS/Akt/NF-κB Signaling Cascade Mediates Epidermal Growth Factor-Induced Epithelial-Mesenchymal Transition and Invasion in Human Breast Cancer Cells. World J. Oncol. 2022, 13, 289–298. [Google Scholar] [CrossRef] [PubMed]

| No. | Name | Formula | PubChem CID | Experimental Mass | Retention Time (s) | Error (ppm) | ms2 Adduct | Fragment Ions |
|---|---|---|---|---|---|---|---|---|
| 1 | Stachydrine | C7H13NO2 | 115244 | 144.1020015 | 31.5758 | 0.010537654 | [M+H]+ | 144.101146; 84.080841; 58.065159; 126.128348; 119.419592 |
| 2 | Luteolin 6-C-glucoside 8-C-arabinoside | C27H30O16 | 3549960 | 609.1474678 | 44.0334 | 2.409582862 | [M−H]− | 609.148219; 369.060713; 399.068383; 489.108606; 339.051556 |
| 3 | Mecamylamine | C11H21N | 4032 | 168.1747615 | 50.00785 | 1.418221913 | M+H | 168.174269; 96.08015; 169.036027; 98.09618; 81.070174 |
| 4 | KOBUSONE | C14H22O2 | 6710676 | 240.1957444 | 55.354 | 1.063924211 | [M+NH4]+ | 240.195169; 116.107206; 58.065149; 168.138912; 96.080923 |
| 5 | Luteolin 7-diglucuronide | C27H26O18 | 5282153 | 637.1034196 | 56.5514 | 2.480547191 | M−H | 285.041504; 637.107566; 351.056026; 113.024071; 85.029135 |
| 6 | Isoviolanthin | C27H30O14 | 101422758 | 577.15554 | 92.2036 | 0.797083227 | M−H | 577.159142; 353.066993; 383.07698; 457.111106; 96.969484 |
| 7 | Complanatuside | C28H32O16 | 5492406 | 669.1661464 | 98.08 | 2.770017312 | M+HCOO | 299.055642; 461.104242; 284.030824; 285.041273; 92.680996 |
| 8 | Kaempferol-7-O-neohesperidoside | C27H30O15 | 22178437 | 593.1517476 | 104.523 | 0.425453913 | [M−H]− | 285.041179; 593.145822; 284.031119; 65.908963; 113.025004 |
| 9 | coniferin | C16H22O8 | 5280372 | 341.1240745 | 110.0625 | 0.218302657 | [M−H]− | 59.013955; 89.02417; 161.061407; 71.013681; 119.035053 |
| 10 | Ferulic acid | C10H10O4 | 445858 | 195.0652329 | 110.538 | 1.193821788 | [M+H]+ | 177.053943; 145.029184; 117.033485; 149.060482; 137.059385 |
| 11 | Fraxidin | C11H10O5 | 3083616 | 223.0600451 | 119.282 | 0.202181336 | M+H | 223.061278; 190.02662; 208.035693; 107.048879; 163.038906 |
| 12 | Riboprine | C15H21N5O4 | 24405 | 336.1666443 | 124.828 | 1.058079384 | M+H | 204.124472; 136.06205; 336.170314; 148.061031; 161.095865 |
| 13 | 5,7-Dihydroxychromone | C9H6O4 | 5281343 | 179.0340958 | 130.096 | 0.535338091 | M+H | 179.034489; 133.10047; 105.070142; 119.085012; 161.096 |
| 14 | Scolymoside | C27H30O14 | 5282152 | 579.1694132 | 136.458 | 2.739837438 | [M+H]+ | 271.05789; 433.108463; 85.02838; 71.048962; 579.161749 |
| 15 | Pinoresinol 4-O-β-D-glucopyranoside | C26H32O11 | 486614 | 519.1879002 | 144.175 | 1.733783223 | M−H | 151.040621; 357.134574; 136.01692; 342.111936; 71.013654 |
| 16 | Pinoresinol | C20H22O6 | 73399 | 341.1386165 | 144.197 | 1.807099734 | M+H-H2O | 137.059485; 291.100616; 341.194003; 323.127251; 271.094876 |
| 17 | Genistein | C15H10O5 | 5280961 | 271.0593836 | 145.892 | 2.273908332 | [M+H]+ | 271.06211; 153.017829; 119.049142; 92.655186; 272.063805 |
| 18 | Cynaroside | C21H20O11 | 5280637 | 447.092682 | 151.141 | 0.711158319 | M−H | 285.041211; 447.093001; 286.043464; 284.031065; 92.680147 |
| 19 | Rupestonic acid | C15H20O3 | 24094149 | 249.1475434 | 161.996 | 6.194885616 | M+H | 249.147868; 92.657727; 203.143353; 81.069482; 145.100533 |
| 20 | Beta-Ionone | C13H20O | 638014 | 193.1588105 | 170.629 | 0.981050279 | M+H | 193.157839; 135.11705; 109.100915; 175.147548; 133.100598 |
| 21 | DiosMetin 7-O-β-D-Glucuronide | C22H20O12 | 163006812 | 477.1027193 | 170.724 | 0.588367273 | M+H | 301.072533; 286.047122; 477.105174; 258.05208; 53.010567 |
| 21 | Diosmetin | C16H12O6 | 5281612 | 301.070716 | 269.096 | 0.943328686 | [M+H]+ | 301.072595; 286.047098; 258.052202; 81.069478; 95.08489 |
| 22 | Lobetyolin | C20H28O8 | 53486204 | 441.1766452 | 181.792 | 1.462452492 | [M+FA]- | 185.097802; 89.024214; 143.070626; 59.014012; 159.081211 |
| 23 | Spinosin | C28H32O15 | 155692 | 607.1672125 | 194.127 | 0.349939428 | M−H | 283.061329; 607.171074; 268.038726; 67.466445; 78.95929 |
| 24 | Tuberostemonine | C22H33NO4 | 100781 | 376.2479048 | 201.319 | 0.25294059 | [M+H]+ | 376.248801; 377.247515; 358.233612; 147.103664; 261.174318 |
| 25 | Fraxinellone | C14H16O3 | 124039 | 215.1067854 | 203.194 | 0.997819514 | M+H-H2O | 215.105705; 145.065711; 155.085119; 187.111516; 169.100661 |
| 26 | Usaramine | C18H25NO6 | 5281756 | 352.1726306 | 205.963 | 1.048932421 | M+H | 352.170472; 295.150637; 92.657724; 98.096297; 168.174312 |
| 27 | Luteolin | C15H10O6 | 5280445 | 287.0551804 | 209.087 | 0.628452748 | M+H | 287.05467; 153.018018; 135.044122; 288.058286; 117.032437 |
| 28 | Atractylenolide II | C15H20O2 | 14448070 | 233.1536143 | 209.134 | 1.654448966 | [M+H]+ | 233.154667; 71.048887; 105.070182; 131.084822; 91.054277 |
| 29 | Latifolin | C17H18O4 | 340211 | 287.1249013 | 213.75 | 0.343592741 | [M+H]+ | 287.054684; 153.018255; 135.044133; 288.058309; 270.150185 |
| 30 | Linarin | C28H32O14 | 5317025 | 615.1685984 | 223.922 | 0.972718738 | M+Na | 615.169949; 307.055942; 331.100059; 68.351735; 493.124227 |
| 31 | (2S,3R,4S)-4-Hydroxyisoleucine | C6H13NO3 | 6918732 | 130.0863195 | 226.623 | 2.456282791 | M+H-H2O | 84.044273; 130.064604; 107.950323; 71.929408; 125.960848 |
| 32 | Methyl 4-hydroxycinnamate | C10H10O3 | 5319562 | 177.0555744 | 230.9275 | 2.403598183 | [M−H]− | 177.054427; 145.029807; 117.034873; 121.029215; 118.043024 |
| 33 | Acacetin | C16H12O5 | 5280442 | 285.0747007 | 243.296 | 1.049777021 | M+H | 285.076868; 270.053533; 242.056158; 92.656033; 286.079336 |
| 34 | Asiaticoside | C48H78O19 | 108062 | 1003.510537 | 246.35 | 0.535444462 | M+HCOO | 487.347446; 957.508084; 957.479987; 469.151768; 101.024141 |
| 35 | Ergolide | C17H22O5 | 185786 | 324.1781173 | 248.736 | 0.361924876 | M+NH4 | 324.177938; 211.094179; 98.096178; 92.656032; 154.082545 |
| 36 | Syringaresinol | C22H26O8 | 332426 | 419.1676828 | 252.173 | 0.756695406 | [M+H]+ | 419.168493; 132.065422; 72.044052; 257.114948; 254.958445 |
| 37 | Kavain | C14H14O3 | 5281565 | 213.0910271 | 259.4415 | 0.126984272 | M+H-H2O | 213.091787; 185.096444; 142.078457; 154.078911; 157.101219 |
| 38 | Inulicin | C17H24O5 | 75528891 | 326.1958085 | 261.257 | 0.587206957 | M+NH4 | 85.028377; 326.196542; 183.101269; 267.122701; 165.091525 |
| 39 | Bisabolangelone | C15H20O3 | 10399769 | 247.1340224 | 268.899 | 0.09080725 | M−H | 247.132609; 203.143286; 248.137682; 162.837881; 92.676764 |
| 41 | Demethylnobiletin | C20H20O8 | 358832 | 389.1225257 | 284.678 | 1.21882624 | M+H | 389.119241; 359.077366; 374.098167; 341.063326; 356.089036 |
| 42 | Alloimperatorin | C16H14O4 | 69502 | 271.0938387 | 292.379 | 0.594815307 | M+H | 271.094804; 105.382367; 92.132945; 192.297717; 259.592281 |
| 43 | Polygodial | C15H22O2 | 5080908 | 235.169451 | 298.258 | 1.917771994 | M+H | 235.169945; 93.069512; 107.085554; 121.101703; 133.100577 |
| 44 | Zedoarondiol | C15H24O3 | 14632997 | 253.1797 | 305.905 | 1.184805966 | [M+H]+ | 93.069488; 81.069513; 121.10071; 79.054399; 107.085486 |
| 45 | Gymnemagenin | C30H50O6 | 10051937 | 505.3525394 | 309.753 | 0.911444093 | M−H | 505.350093; 487.347686; 56.152842; 71.013656; 506.363847 |
| 46 | Atractylenolide III | C15H20O3 | 155948 | 249.1486649 | 314.085 | 1.34514875 | [M+H]+ | 249.14809; 119.08487; 92.661102; 147.079977; 252.132632 |
| 47 | Bruceine A | C26H34O11 | 160006 | 539.2131534 | 321.513 | 0.284396213 | M−H+H2O | 539.205976; 113.024072; 85.029152; 75.008734; 71.013838 |
| 48 | Marrubiin | C20H28O4 | 73401 | 331.1916448 | 321.6995 | 1.072398059 | M−H | 331.193862; 332.196479; 313.181246; 92.67761; 133.520065 |
| 49 | Dihydroactinidiolide | C11H16O2 | 27209 | 181.1225766 | 326.329 | 3.183261855 | M+H | 181.122452; 135.11727; 163.112022; 107.085367; 121.100795 |
| 50 | Butyl isobutyl phthalate | C16H22O4 | 28813 | 301.1413047 | 341.735 | 1.011821714 | M+Na | 301.141862; 286.047221; 81.069483; 184.650306; 92.656035 |
| 51 | Saikosaponin D | C42H68O13 | 107793 | 803.4522042 | 372.685 | 2.235120875 | [M+Na]+ | 803.463922; 803.507124; 92.654344; 641.40604; 625.930823 |
| 52 | Terminolic acid | C30H48O6 | 12314613 | 503.3382395 | 385.895 | 0.475782683 | M−H | 503.341111; 55.928854; 504.338726; 311.375763; 110.400566 |
| 53 | Crocetin | C20H24O4 | 5281232 | 329.1722963 | 404.626 | 0.900207345 | [M+H]+ | 329.172948; 149.692598; 256.954708; 336.743959; 171.70608 |
| 54 | AKBA | C32H48O5 | 11168203 | 513.35481 | 418.236 | 0.370144244 | M+H | 513.35574; 57.039088; 92.658572; 495.343888; 514.360992 |
| 55 | Quillaic acid | C30H46O5 | 101810 | 485.3277089 | 430.64 | 1.460587582 | M−H | 485.32381; 53.927556; 486.328987; 92.675918; 379.981476 |
| 56 | (4aS,6aS,6bR,9R,10R,11R,12aR)-10,11-dihydroxy-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | C30H48O5 | 23757202 | 489.3567674 | 436.014 | 0.475344562 | [M+H]+ | 205.160139; 191.144531; 453.341398; 203.17906; 489.324348 |
| 57 | Pygenic acid B | C30H48O5 | 12308659 | 487.3421468 | 437.467 | 1.750717748 | [M−H]− | 487.347592; 488.34909; 54.15171; 111.009208; 92.68015 |
| 58 | Apigenin | C15H10O5 | 5280443 | 269.0452442 | 455.382 | 2.809282436 | [M−H]− | 269.044494; 225.055691; 62.963937; 270.047442; 241.050221 |
| 59 | Cimigenol | C30H48O5 | 16020000 | 471.347276 | 472.593 | 0.585572418 | M+H-H2O | 471.350356; 453.333917; 185.131764; 435.325276; 293.227612 |
| 60 | Pomolic acid | C30H48O4 | 382831 | 471.3484584 | 482.522 | 0.972499137 | M−H | 471.346489; 52.374449; 472.34771; 133.289188; 134.079265 |
| 61 | Corosolic acid | C30H48O4 | 15917996 | 473.3630846 | 515.5 | 0.178787465 | [M+H]+ | 455.35577; 437.344813; 295.242818; 121.10064; 173.131926 |
| 62 | Oleanolic acid 28-O-β-D-glucopyranoside | C36H58O8 | 14189384 | 663.4124935 | 536.728 | 0.743809988 | M+HCOO | 455.35524; 663.411329; 617.40069; 50.596811; 97.568097 |
| 63 | 20-Deoxyingenol | C20H28O4 | 11290503 | 315.1948027 | 551.025 | 2.546744331 | M+H-H2O | 315.191006; 133.100671; 125.676823; 189.090937; 51.139422 |
| 64 | Methyl[8]-Shogaol | C20H30O3 | 91721121 | 319.2248125 | 569.836 | 0.587449289 | [M+H]+ | 319.225535; 301.211427; 109.854958; 227.756837; 127.093095 |
| 65 | 11-Keto-beta-boswellic acid | C30H46O4 | 9847548 | 469.3315601 | 601.329 | 0.93730445 | M−H | 469.333839; 281.241547; 154.541827; 357.801014; 92.680998 |
| 66 | 9-hydroxy-1,4a-dimethyl-7-propan-2-yl-2,3,4,9,10,10a-hexahydrophenanthrene-1-carboxylic acid | C20H28O3 | 10448477 | 339.1902539 | 607.388 | 0.74860204 | [M+Na]+ | 339.289571; 69.069705; 55.054574; 95.085925; 83.085622 |
| 67 | Glaucocalyxin A | C20H28O4 | 10471963 | 350.2298112 | 663.461 | 0.539028221 | M+NH4 | 210.111133; 350.229669; 192.100537; 82.760478; 113.752814 |
| 68 | Enoxolone | C30H46O4 | 10114 | 469.3317111 | 665.256 | 0.615556275 | [M−H]− | 469.334369; 52.150157; 470.338904; 412.977183; 285.937884 |
| 69 | Ursolic acid | C30H48O3 | 64945 | 439.3566644 | 718.354 | 0.763858235 | [M+H]+ | 191.179464; 203.179014; 439.35968; 95.085887; 189.164795 |
| 70 | Betulinic acid | C30H48O3 | 64971 | 455.3524115 | 720.026 | 1.292391888 | [M−H]− | 455.355122; 456.352381; 50.597151; 444.575521; 203.241857 |
| 71 | Alpha-Boswellic acid | C30H48O3 | 637234 | 457.369799 | 754.031 | 3.933259197 | [M+H]+ | 457.352924; 439.35553; 421.35036; 193.157987; 121.10064 |
| 72 | 20(S)-Protopanaxadiol | C30H52O3 | 11213350 | 483.3809242 | 760.816 | 0.156811831 | [M+Na]+ | 483.387525; 53.708146; 240.276588; 217.502137; 480.633583 |
| 73 | Stigmasterol | C29H48O | 5280794 | 395.3678549 | 764.692 | 2.162277811 | [M+H]+ | 395.368602; 147.117219; 81.069484; 145.102194; 159.116863 |
| 74 | Eclalbasaponin I | C42H68O14 | 10079039 | 819.4495614 | 764.982 | 0.685051695 | M+Na | 819.456032; 819.50053; 91.050532; 365.109116; 203.052701 |
| 75 | Micheliolide | C15H20O3 | 442279 | 266.1725499 | 769.589 | 1.691129387 | M+NH4 | 266.172107; 92.65772; 129.988652; 147.429102; 141.661701 |
| Compound | Calibration Curves | R2 | Linear Ranges (mg/mL) |
|---|---|---|---|
| Ferulic acid | y = 1980x + 3.7665 | 0.999 | 0.005–0.2 |
| Luteolin | y = 2542.8x + 3.0522 | 0.999 | 0.005–0.2 |
| Latifolin | y = 1360.9x + 2.867 | 0.999 | 0.005–0.2 |
| Apigenin | y = 1157.4x + 2.8478 | 0.999 | 0.001–0.4 |
| Acacetin | y = 3451.6x + 3.184 | 0.999 | 0.001–0.2 |
| No. | Compound Name | Retention Time (min) | Content (mg/g Extract) |
|---|---|---|---|
| 1 | Ferulic acid | 16.221 | 6.25 ± 0.34 |
| 2 | Luteolin | 31.349 | 4.81 ± 0.46 |
| 3 | Latifolin | 32.571 | 1.01 ± 0.16 |
| 4 | Apigenin | 36.491 | 5.66 ± 0.35 |
| 5 | Acacetin | 46.549 | 3.12 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, F.; Qiao, Y.; Chen, Z.; He, H.; Wang, F.; Chen, J. Molecular Mechanisms of Lobelia nummularia Extract in Breast Cancer: Targeting EGFR/TP53 and PI3K-AKT-mTOR Signaling via ROS-Mediated Apoptosis. Curr. Issues Mol. Biol. 2025, 47, 546. https://doi.org/10.3390/cimb47070546
Yuan F, Qiao Y, Chen Z, He H, Wang F, Chen J. Molecular Mechanisms of Lobelia nummularia Extract in Breast Cancer: Targeting EGFR/TP53 and PI3K-AKT-mTOR Signaling via ROS-Mediated Apoptosis. Current Issues in Molecular Biology. 2025; 47(7):546. https://doi.org/10.3390/cimb47070546
Chicago/Turabian StyleYuan, Fahu, Yu Qiao, Zhongqiang Chen, Huihuang He, Fuyan Wang, and Jiangyuan Chen. 2025. "Molecular Mechanisms of Lobelia nummularia Extract in Breast Cancer: Targeting EGFR/TP53 and PI3K-AKT-mTOR Signaling via ROS-Mediated Apoptosis" Current Issues in Molecular Biology 47, no. 7: 546. https://doi.org/10.3390/cimb47070546
APA StyleYuan, F., Qiao, Y., Chen, Z., He, H., Wang, F., & Chen, J. (2025). Molecular Mechanisms of Lobelia nummularia Extract in Breast Cancer: Targeting EGFR/TP53 and PI3K-AKT-mTOR Signaling via ROS-Mediated Apoptosis. Current Issues in Molecular Biology, 47(7), 546. https://doi.org/10.3390/cimb47070546






