Characterization of an mRNA-Encoded Antibody Against Henipavirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells and Viruses
2.3. Gene Construction
2.4. mRNA Preparation and LNP Formulation
2.5. In Vitro Expression of mRNA-1E5
2.6. Western Blot
2.7. In Vivo Expression Dynamics of mRNA-1E5-LNPs
2.8. ELISA
2.9. Pseudovirus Packaging and Neutralization Test
2.10. Challenge of BALB/C Mice with rHIV-HeV g2
2.11. Prophylactic Efficacy of mRNA-1E5-LNP in Mice
2.12. Detection of Biochemical Criteria in Mice
2.13. Histopathological Analysis in Mice
2.14. Statistical Analysis
3. Results
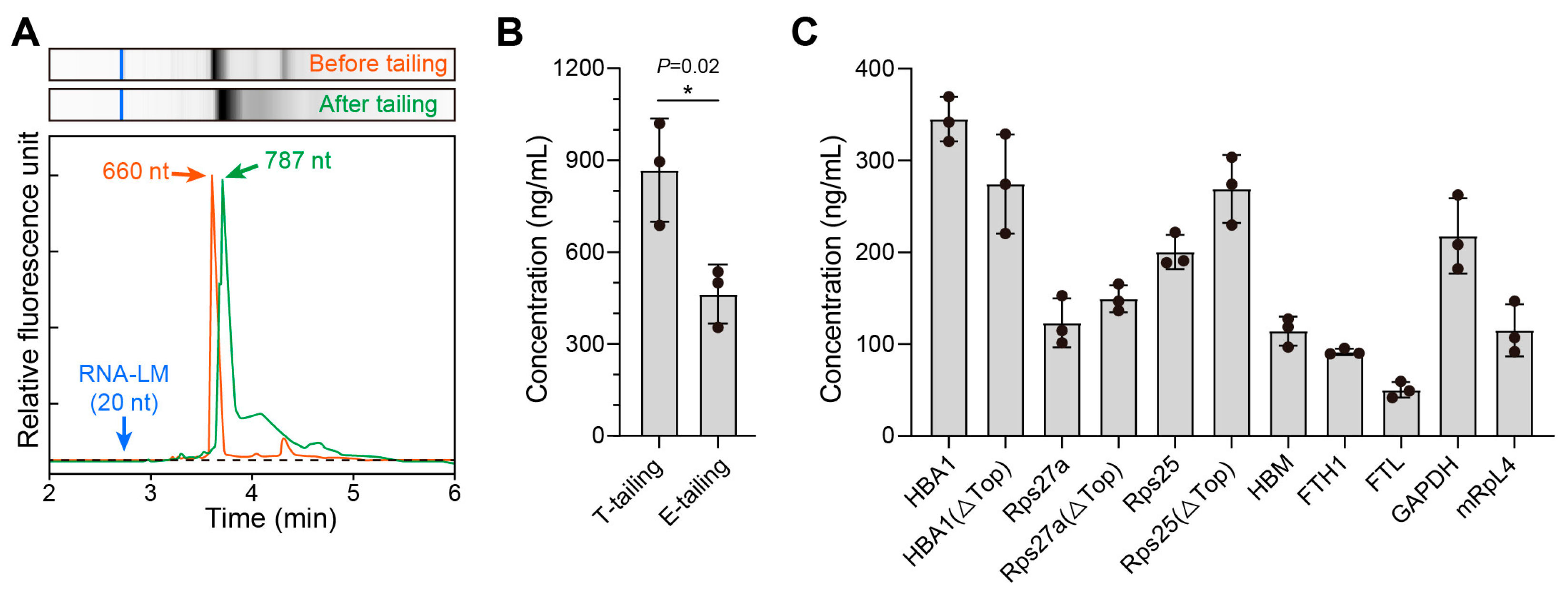
3.1. Screening of Natural Endogenous UTRs
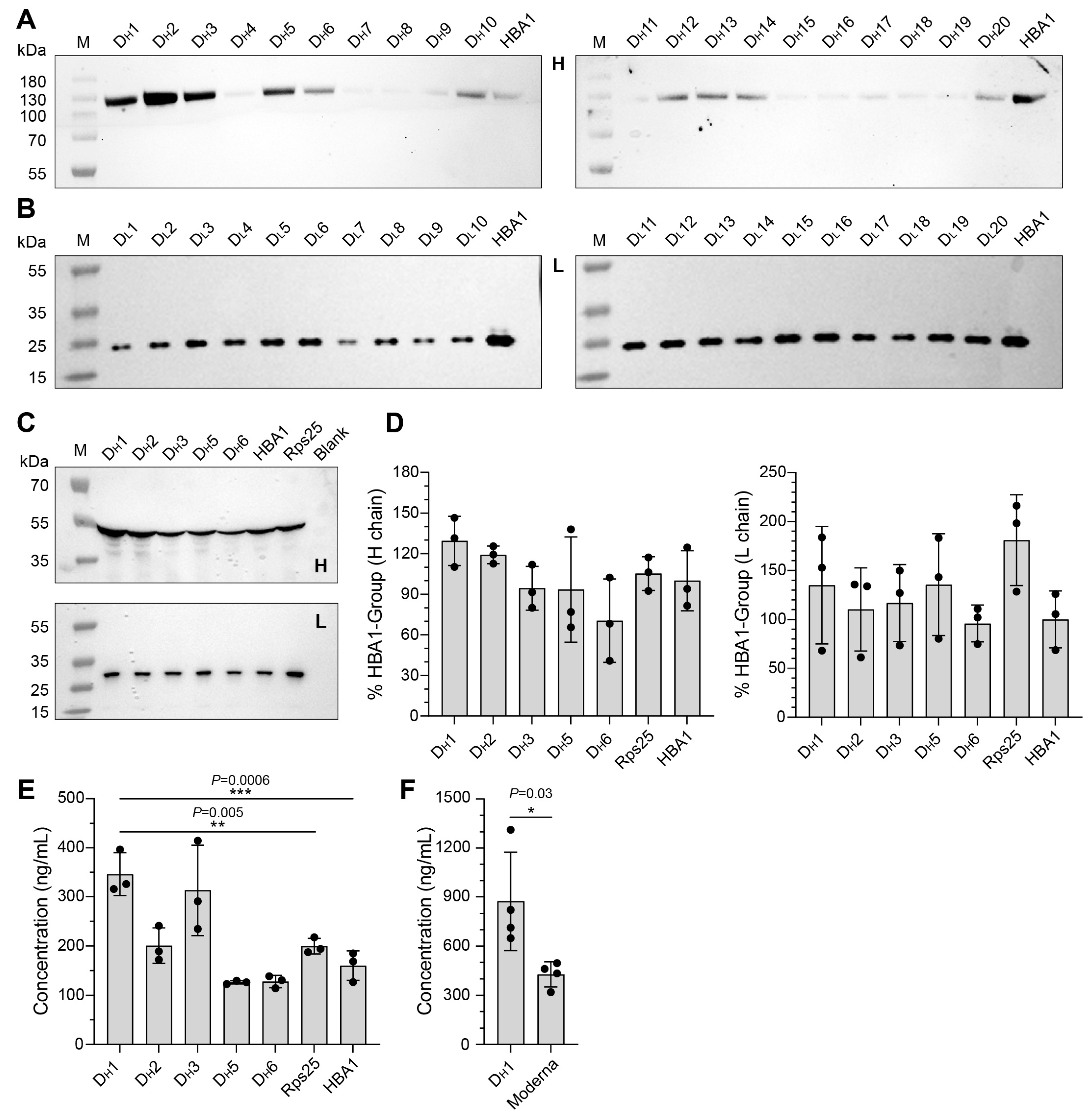
3.2. Screening of Artificially Designed UTRs and Construction of mRNA-1E5
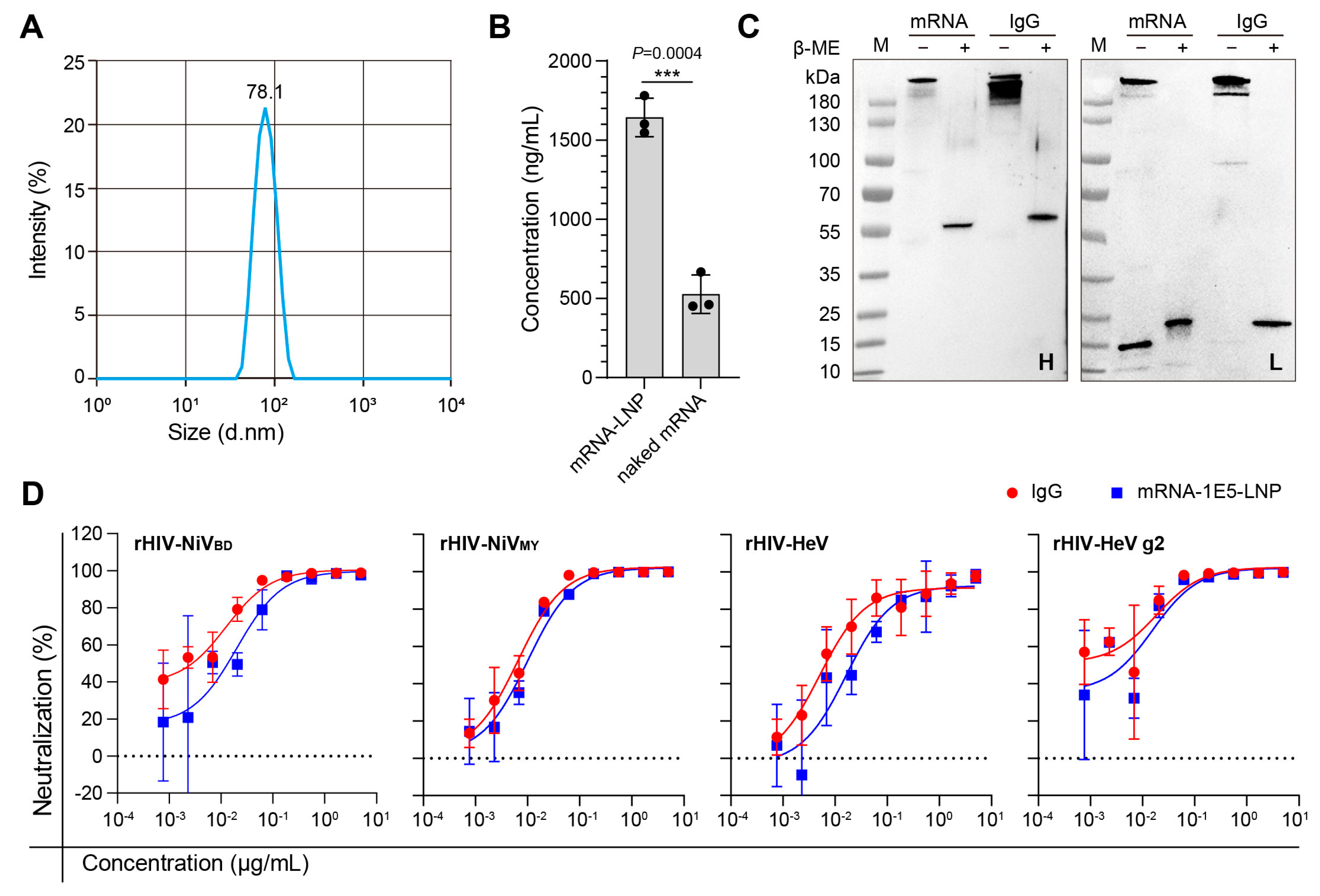
3.3. In Vitro Efficiency Characterization of mRNA-1E5-LNP
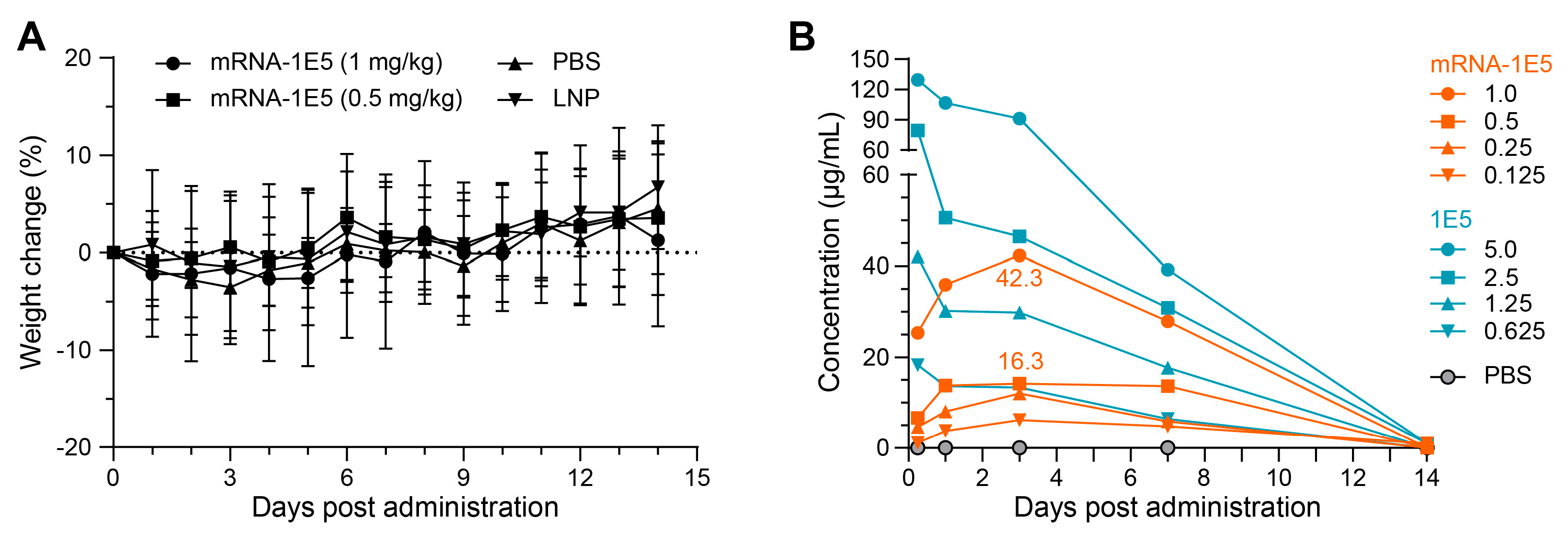
3.4. Preliminary Safety Evaluation and Expression Kinetics of mRNA-1E5-LNPs
3.5. Establishment of a Mouse Evaluation Model Based on the HeV Pseudovirus
3.6. In Vivo Prophylactic Protective Efficacy of mRNA-1E5-LNPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine albumin |
| BSL-2 | Biosafety level 2 laboratory |
| BSL-4 | Biosafety level 4 laboratory |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| ELISA | Enzyme-linked immunosorbent assay |
| HBA1 | Hemoglobin subunit alpha 1 |
| HEK 293T | Human embryonic kidney 293T |
| HeV | Hendra virus |
| HNVs | Hendra and Nipah viruses |
| HIV | Human Immunodeficiency Virus |
| IgG | Immunoglobulin G |
| IC50 | Half-maximal inhibitory concentration |
| LNP | Lipid nanoparticle |
| mRNA | Messenger RNA |
| MEM | Minimum essential medium |
| NiV | Nipah virus |
| ORF | Open reading frame |
| PBS | Phosphate-buffered saline |
| UTR | Untranslated region |
References
- Eaton, B.T.; Broder, C.C.; Middleton, D.; Wang, L.F. Hendra and Nipah viruses: Different and dangerous. Nat. Rev. Microbiol. 2006, 4, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.K.; Lowe, L.; Hummel, K.B.; Sazzad, H.M.; Gurley, E.S.; Hossain, M.J.; Luby, S.P.; Miller, D.M.; Comer, J.A.; Rollin, P.E.; et al. Characterization of Nipah virus from outbreaks in Bangladesh, 2008–2010. Emerg. Infect. Dis. 2012, 18, 248–255. [Google Scholar] [CrossRef]
- Wang, Z.; Dang, H.V.; Amaya, M.; Xu, Y.; Yin, R.; Yan, L.; Hickey, A.C.; Annand, E.J.; Horsburgh, B.A.; Reid, P.A.; et al. Potent monoclonal antibody-mediated neutralization of a divergent Hendra virus variant. Proc. Natl. Acad. Sci. USA 2022, 119, e2122769119. [Google Scholar] [CrossRef] [PubMed]
- Pallister, J.A.; Klein, R.; Arkinstall, R.; Haining, J.; Long, F.; White, J.R.; Payne, J.; Feng, Y.R.; Wang, L.F.; Broder, C.C.; et al. Vaccination of ferrets with a recombinant G glycoprotein subunit vaccine provides protection against Nipah virus disease for over 12 months. Virol. J. 2013, 10, 237. [Google Scholar] [CrossRef]
- Weatherman, S.; Feldmann, H.; de Wit, E. Transmission of henipaviruses. Curr. Opin. Virol. 2018, 28, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Soman Pillai, V.; Krishna, G.; Valiya Veettil, M. Nipah virus: Past outbreaks and future containment. Viruses 2020, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Conroy, G. Nipah virus outbreak: What scientists know so far. Nature 2023. [Google Scholar] [CrossRef]
- Stone, J.A.; Vemulapati, B.M.; Bradel-Tretheway, B.; Aguilar, H.C. Multiple Strategies Reveal a Bidentate Interaction between the Nipah Virus Attachment and Fusion Glycoproteins. J. Virol. 2016, 90, 10762–10773. [Google Scholar] [CrossRef]
- Dang, H.V.; Cross, R.W.; Borisevich, V.; Bornholdt, Z.A.; West, B.R.; Chan, Y.P.; Mire, C.E.; Da Silva, S.C.; Dimitrov, A.S.; Yan, L.; et al. Broadly neutralizing antibody cocktails targeting Nipah virus and Hendra virus fusion glycoproteins. Nat. Struct. Mol. Biol. 2021, 28, 426–434. [Google Scholar] [CrossRef]
- Dong, J.; Cross, R.W.; Doyle, M.P.; Kose, N.; Mousa, J.J.; Annand, E.J.; Borisevich, V.; Agans, K.N.; Sutton, R.; Nargi, R.; et al. Potent Henipavirus Neutralization by Antibodies Recognizing Diverse Sites on Hendra and Nipah Virus Receptor Binding Protein. Cell 2020, 183, 1536–1550.e1517. [Google Scholar] [CrossRef]
- Dang, H.V.; Chan, Y.P.; Park, Y.J.; Snijder, J.; Da Silva, S.C.; Vu, B.; Yan, L.; Feng, Y.R.; Rockx, B.; Geisbert, T.W.; et al. An antibody against the F glycoprotein inhibits Nipah and Hendra virus infections. Nat. Struct. Mol. Biol. 2019, 26, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Playford, E.G.; Munro, T.; Mahler, S.M.; Elliott, S.; Gerometta, M.; Hoger, K.L.; Jones, M.L.; Griffin, P.; Lynch, K.D.; Carroll, H.; et al. Safety, tolerability, pharmacokinetics, and immunogenicity of a human monoclonal antibody targeting the G glycoprotein of henipaviruses in healthy adults: A first-in-human, randomised, controlled, phase 1 study. Lancet Infect. Dis. 2020, 20, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Anderson, D.E.; Halpin, K.; Hong, X.; Chen, H.; Walker, S.; Valdeter, S.; van der Heide, B.; Neave, M.J.; Bingham, J.; et al. A new Hendra virus genotype found in Australian flying foxes. Virol. J. 2021, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Annand, E.J.; Horsburgh, B.A.; Xu, K.; Reid, P.A.; Poole, B.; de Kantzow, M.C.; Brown, N.; Tweedie, A.; Michie, M.; Grewar, J.D.; et al. Novel Hendra Virus Variant Detected by Sentinel Surveillance of Horses in Australia. Emerg. Infect. Dis. 2022, 28, 693–704. [Google Scholar] [CrossRef]
- Zhang, X.A.; Li, H.; Jiang, F.C.; Zhu, F.; Zhang, Y.F.; Chen, J.J.; Tan, C.W.; Anderson, D.E.; Fan, H.; Dong, L.Y.; et al. A Zoonotic Henipavirus in Febrile Patients in China. N. Engl. J. Med. 2022, 387, 470–472. [Google Scholar] [CrossRef]
- Chen, R.E.; Zhang, X.; Case, J.B.; Winkler, E.S.; Liu, Y.; VanBlargan, L.A.; Liu, J.; Errico, J.M.; Xie, X.; Suryadevara, N.; et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat. Med. 2021, 27, 717–726. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- August, A.; Attarwala, H.Z.; Himansu, S.; Kalidindi, S.; Lu, S.; Pajon, R.; Han, S.; Lecerf, J.M.; Tomassini, J.E.; Hard, M.; et al. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against Chikungunya virus. Nat. Med. 2021, 27, 2224–2233. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, M.; Seo, Y.; Moon, Y.S.; Lee, H.J.; Lee, K.; Lee, H. Emergence of synthetic mRNA: In vitro synthesis of mRNA and its applications in regenerative medicine. Biomaterials 2018, 156, 172–193. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Sun, M.; Zhang, X.; Zhang, H.; Liu, Y.; Yao, Y.; Li, M.; Fang, T.; Sun, B.; Chen, Z.; et al. A potent Henipavirus cross-neutralizing antibody reveals a dynamic fusion-triggering pattern of the G-tetramer. Nat. Commun. 2024, 15, 4330. [Google Scholar] [CrossRef]
- Stadler, C.R.; Bähr-Mahmud, H.; Celik, L.; Hebich, B.; Roth, A.S.; Roth, R.P.; Karikó, K.; Türeci, Ö.; Sahin, U. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat. Med. 2017, 23, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Türeci, O.; Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef]
- Weissman, D. mRNA transcript therapy. Expert. Rev. Vaccines 2015, 14, 265–281. [Google Scholar] [CrossRef]
- Hinnebusch, A.G.; Ivanov, I.P.; Sonenberg, N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 2016, 352, 1413–1416. [Google Scholar] [CrossRef]
- Zeng, C.; Hou, X.; Yan, J.; Zhang, C.; Li, W.; Zhao, W.; Du, S.; Dong, Y. Leveraging mRNA Sequences and Nanoparticles to Deliver SARS-CoV-2 Antigens In Vivo. Adv. Mater. 2020, 32, e2004452. [Google Scholar] [CrossRef]
- Yamashita, R.; Suzuki, Y.; Takeuchi, N.; Wakaguri, H.; Ueda, T.; Sugano, S.; Nakai, K. Comprehensive detection of human terminal oligo-pyrimidine (TOP) genes and analysis of their characteristics. Nucleic Acids Res. 2008, 36, 3707–3715. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Lin, A.; Xu, C.; Li, Z.; Liu, K.; Liu, B.; Ma, X.; Zhao, F.; Jiang, H.; et al. Algorithm for optimized mRNA design improves stability and immunogenicity. Nature 2023, 621, 396–403. [Google Scholar] [CrossRef]
- Nie, J.; Liu, L.; Wang, Q.; Chen, R.; Ning, T.; Liu, Q.; Huang, W.; Wang, Y. Nipah pseudovirus system enables evaluation of vaccines in vitro and in vivo using non-BSL-4 facilities. Emerg. Microbes Infect. 2019, 8, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Deal, C.E.; Carfi, A.; Plante, O.J. Advancements in mRNA Encoded Antibodies for Passive Immunotherapy. Vaccines 2021, 9, 108. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Sun, B.; Fang, T.; Zhao, X.; Ren, Y.; Song, Z.; He, S.; Li, J.; Fan, P.; Yu, C. Characterization of an mRNA-Encoded Antibody Against Henipavirus. Curr. Issues Mol. Biol. 2025, 47, 519. https://doi.org/10.3390/cimb47070519
Liu Z, Sun B, Fang T, Zhao X, Ren Y, Song Z, He S, Li J, Fan P, Yu C. Characterization of an mRNA-Encoded Antibody Against Henipavirus. Current Issues in Molecular Biology. 2025; 47(7):519. https://doi.org/10.3390/cimb47070519
Chicago/Turabian StyleLiu, Zixuan, Bingjie Sun, Ting Fang, Xiaofan Zhao, Yi Ren, Zhenwei Song, Sijun He, Jianmin Li, Pengfei Fan, and Changming Yu. 2025. "Characterization of an mRNA-Encoded Antibody Against Henipavirus" Current Issues in Molecular Biology 47, no. 7: 519. https://doi.org/10.3390/cimb47070519
APA StyleLiu, Z., Sun, B., Fang, T., Zhao, X., Ren, Y., Song, Z., He, S., Li, J., Fan, P., & Yu, C. (2025). Characterization of an mRNA-Encoded Antibody Against Henipavirus. Current Issues in Molecular Biology, 47(7), 519. https://doi.org/10.3390/cimb47070519





