Fluorescence Reduction Neutralization Test: A Novel, Rapid, and Efficient Method for Characterizing the Neutralizing Activity of Antibodies Against Dengue Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Cultures and Cell Lines
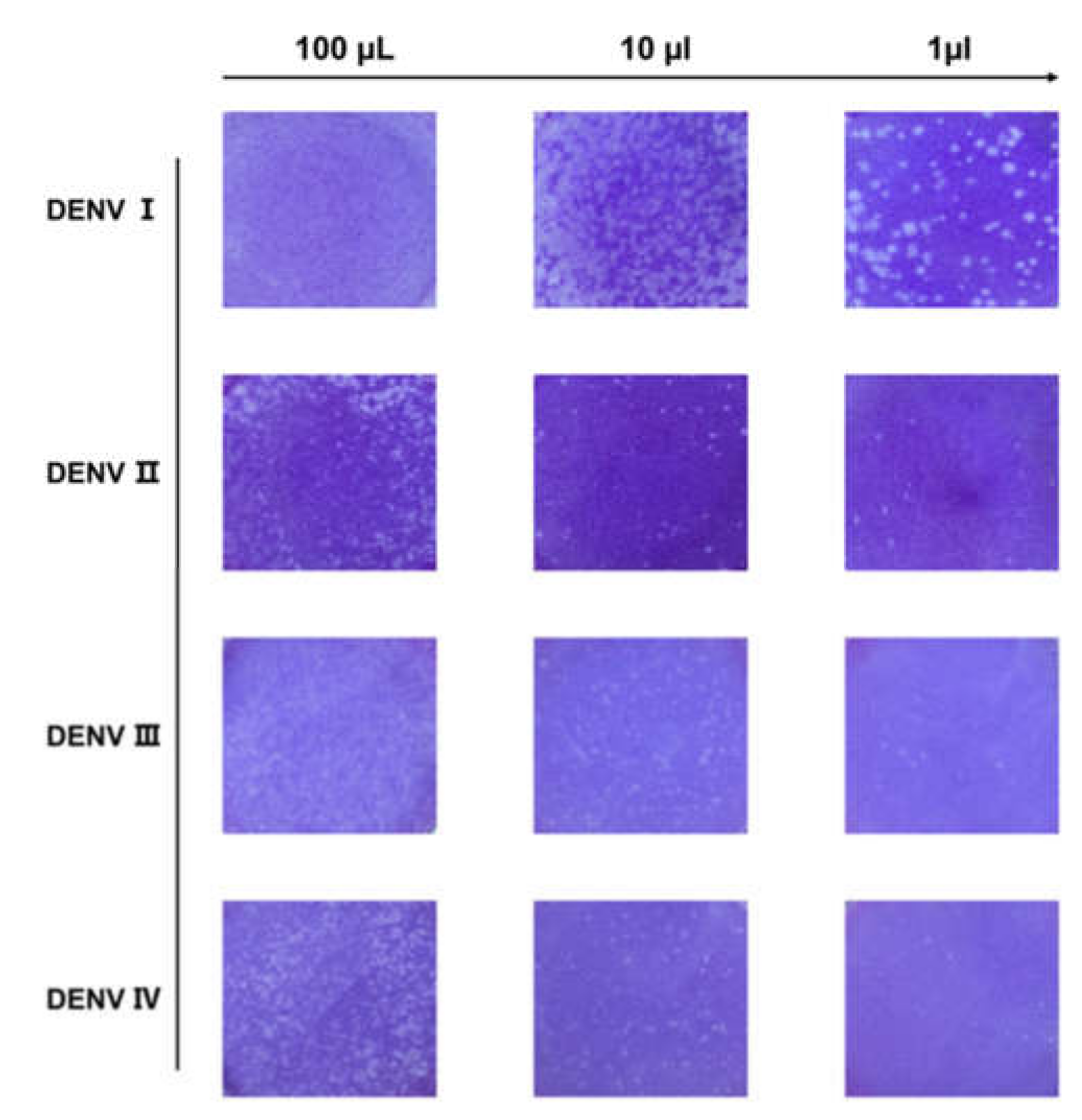
2.2. Viral Titer Assay: Plaque Test
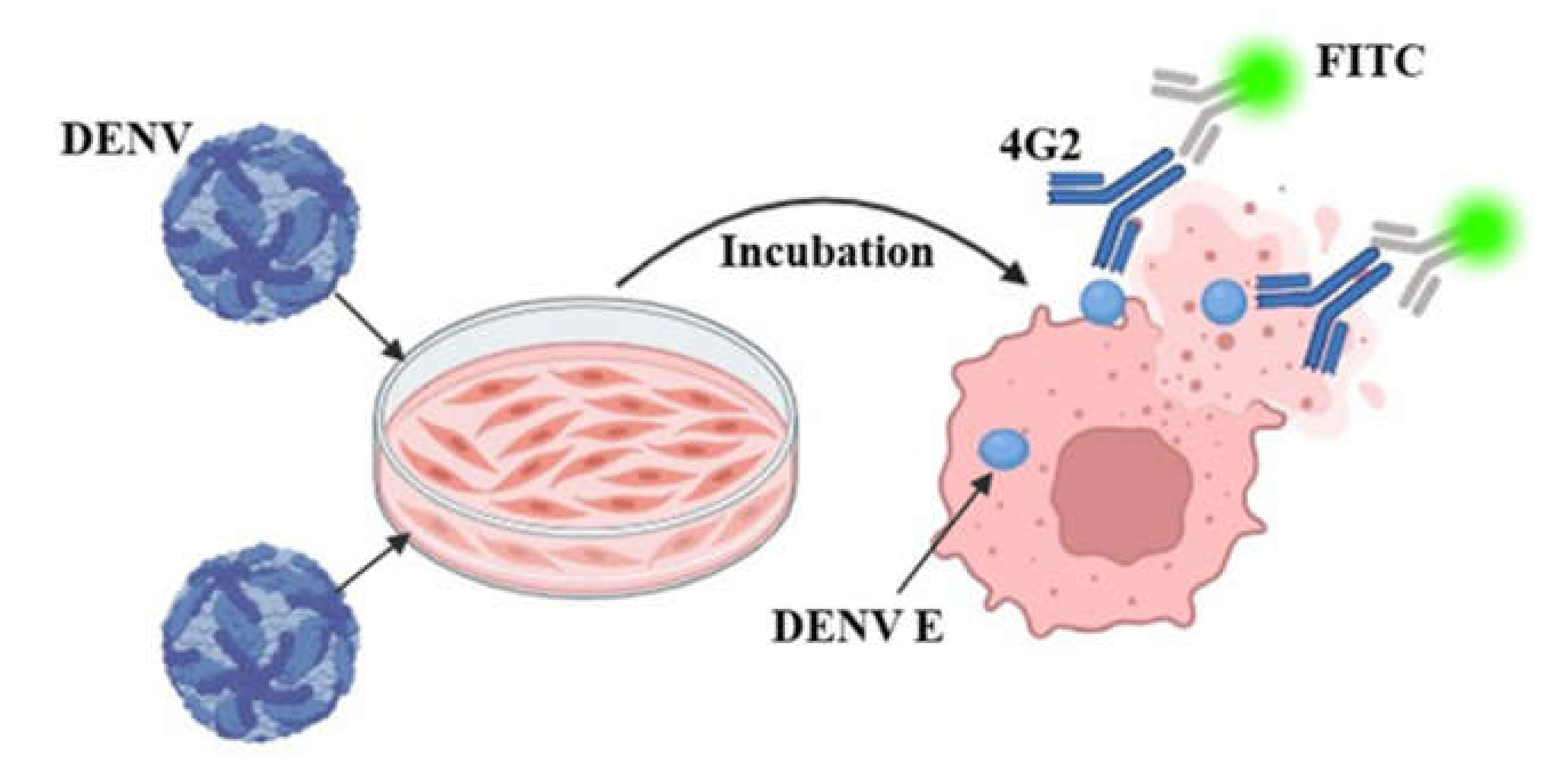
2.3. Viral Titer Assay—Fluorescence Test
2.4. Plaque Reduction Neutralization Test (PRNT)
2.5. Fluorescence Reduction Neutralization Test (FRNT)
3. Results
3.1. Production and Detection of DENV
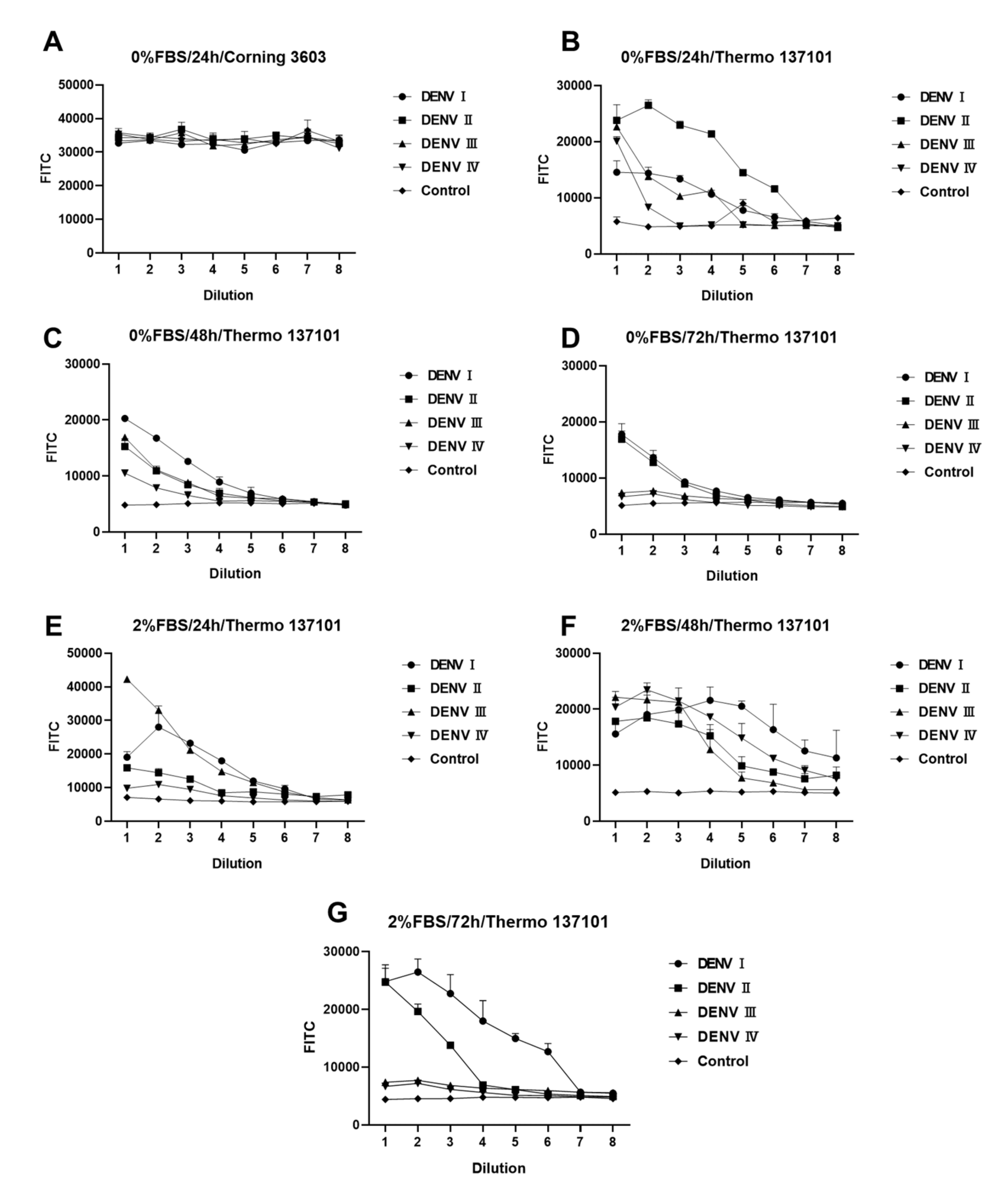
3.2. Construction and Optimization of the FRNT for DENV
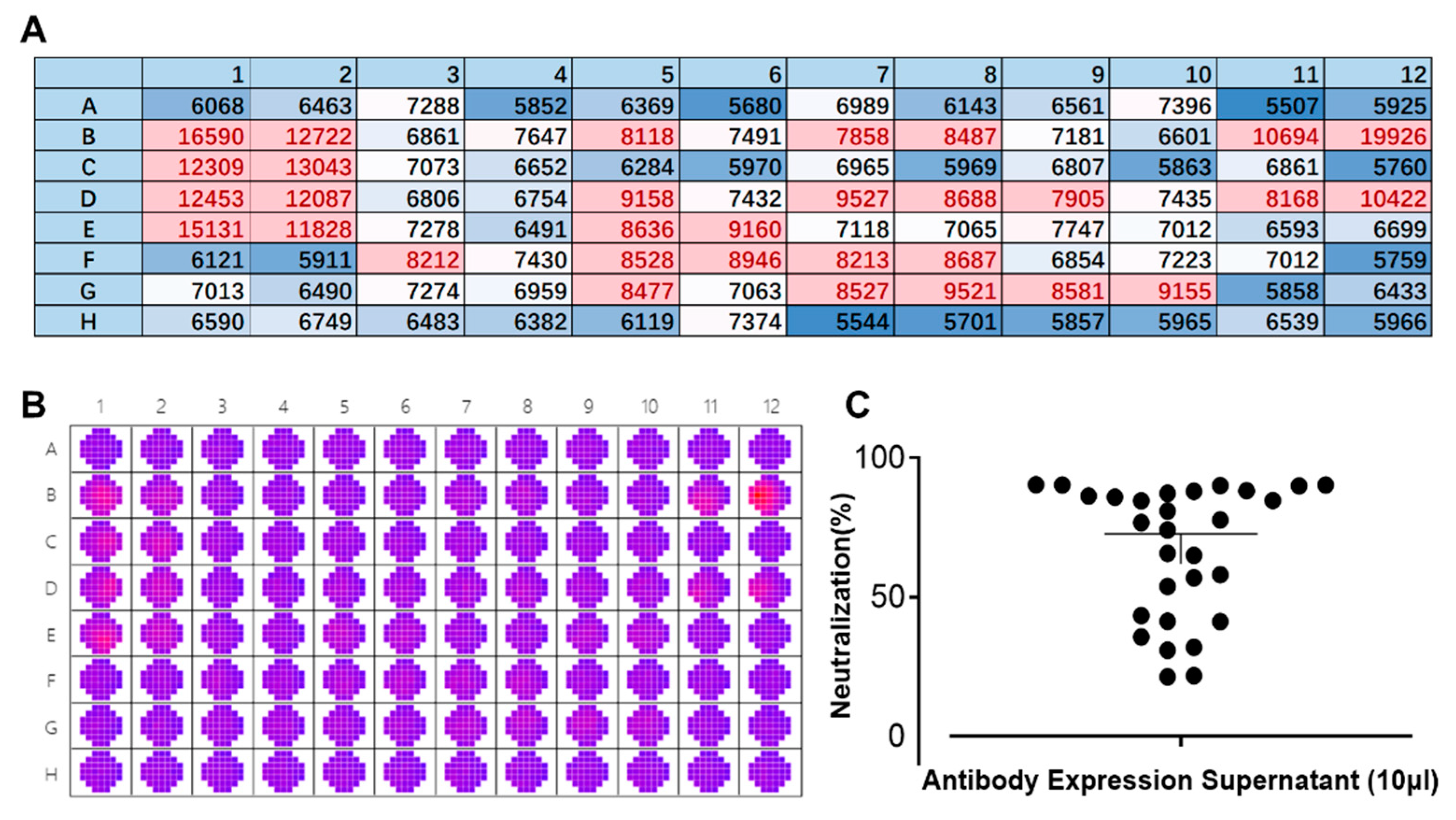
3.3. Rapid Screening of Neutralizing Antibodies Against DENV Using the FRNT
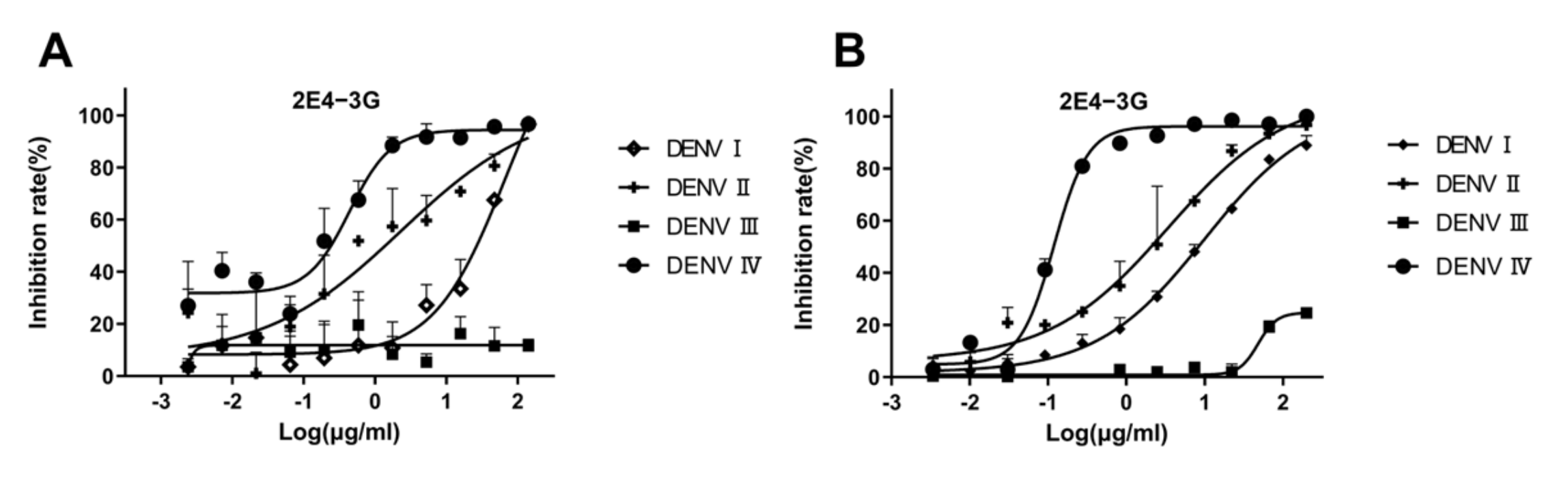
3.4. Validation of Antibody Neutralization Activity In Vitro
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, S.K.; Bhattacharjee, S. Dengue virus: Epidemiology, biology, and disease aetiology. Can. J. Microbiol. 2021, 67, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Pierson, T.C. Molecular Insight into Dengue Virus Pathogenesis and Its Implications for Disease Control. Cell 2015, 162, 488–492. [Google Scholar] [CrossRef]
- Wells, T.J.; Esposito, T.; Henderson, I.R.; Labzin, L.I. Mechanisms of antibody-dependent enhancement of infectious disease. Nat. Rev. Immunol. 2024, 25, 6–21. [Google Scholar] [CrossRef]
- Murphy, B.R.; Whitehead, S.S. Immune response to dengue virus and prospects for a vaccine. Annu. Rev. Immunol. 2011, 29, 587–619. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, J.; Du, P.; Lu, J.; Chen, L.; Huang, Y.; Yu, Y.; Xie, Q.; Wang, R.; Yang, Z. Generation and characterization of humanized synergistic neutralizing antibodies against SARS-CoV-2. J. Med. Virol. 2022, 94, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Guo, J.; Lu, J.; Du, P.; Zhang, J.; Yu, Y.; Chen, L.; Xiong, Z.; Xiang, Y.; Ni, X.; et al. A potential broad-spectrum neutralizing antibody against Betacoronavirus. J. Med. Virol. 2023, 95, e29252. [Google Scholar] [CrossRef]
- Xu, M.; Zuest, R.; Velumani, S.; Tukijan, F.; Toh, Y.X.; Appanna, R.; Tan, E.Y.; Cerny, D.; MacAry, P.; Wang, C.I.; et al. A potent neutralizing antibody with therapeutic potential against all four serotypes of dengue virus. NPJ Vaccines 2017, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, J.; Chen, L.; Yu, Y.; Yang, Z. A human bispecific neutralization antibody against four serotypes of dengue virus. Virology 2021, 558, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Fibriansah, G.; Lok, S.M. The development of therapeutic antibodies against dengue virus. Antivir. Res. 2016, 128, 7–19. [Google Scholar] [CrossRef]
- Russell, P.K.; Nisalak, A.; Sukhavachana, P.; Vivona, S. A plaque reduction test for dengue virus neutralizing antibodies. J. Immunol. 1967, 99, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Halstead, S.B.; Repik, P.M.; Putvatana, R.; Raybourne, N. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: Comparison of the BHK suspension test with standard plaque reduction neutralization. J. Clin. Microbiol. 1985, 22, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- Timiryasova, T.M.; Bonaparte, M.I.; Luo, P.; Zedar, R.; Hu, B.T.; Hildreth, S.W. Optimization and validation of a plaque reduction neutralization test for the detection of neutralizing antibodies to four serotypes of dengue virus used in support of dengue vaccine development. Am. J. Trop. Med. Hyg. 2013, 88, 962–970. [Google Scholar] [CrossRef]
- Durbin, A.P.; Karron, R.A.; Sun, W.; Vaughn, D.W.; Reynolds, M.J.; Perreault, J.R.; Thumar, B.; Men, R.; Lai, C.J.; Elkins, W.R.; et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am. J. Trop. Med. Hyg. 2001, 65, 405–413. [Google Scholar] [CrossRef]
- Vora, A.; Zhou, W.; Londono-Renteria, B.; Woodson, M.; Sherman, M.B.; Colpitts, T.M.; Neelakanta, G.; Sultana, H. Arthropod EVs mediate dengue virus transmission through interaction with a tetraspanin domain containing glycoprotein Tsp29Fb. Proc. Natl. Acad. Sci. USA 2018, 115, E6604–E6613. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhu, Z.; Li, S.; Deng, Y.; Wu, Y.; Zhang, N.; Puri, V.; Wang, C.; Zou, P.; Lei, C.; et al. A broadly neutralizing germline-like human monoclonal antibody against dengue virus envelope domain III. PLoS Pathog. 2019, 15, e1007836. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.B.; Yang, Z.S.; Lin, C.Y.; Hsu, M.C.; Urbina, A.N.; Assavalapsakul, W.; Wang, W.H.; Chen, Y.H.; Wang, S.F. Dengue overview: An updated systemic review. J. Infect. Public Health 2023, 16, 1625–1642. [Google Scholar] [CrossRef]
- Paz-Bailey, G.; Adams, L.E.; Deen, J.; Anderson, K.B.; Katzelnick, L.C. Dengue. Lancet 2024, 403, 667–682. [Google Scholar] [CrossRef]
- Thomas, S.J.; Nisalak, A.; Anderson, K.B.; Libraty, D.H.; Kalayanarooj, S.; Vaughn, D.W.; Putnak, R.; Gibbons, R.V.; Jarman, R.; Endy, T.P. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: How alterations in assay conditions impact performance. Am. J. Trop. Med. Hyg. 2009, 81, 825–833. [Google Scholar] [CrossRef]
- Morens, D.M.; Halstead, S.B.; Larsen, L.K. Comparison of dengue virus plaque reduction neutralization by macro and ‘semi-micro’ methods in LLC-MK2 cells. Microbiol. Immunol. 1985, 29, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.C.; Bogardus, L.; Giacone, D.G.; Rubinstein, L.J.; Antonello, J.M.; Sun, D.; Daijogo, S.; Gurney, K.B. Virus Reduction Neutralization Test: A Single-Cell Imaging High-Throughput Virus Neutralization Assay for Dengue. Am. J. Trop. Med. Hyg. 2018, 99, 1430–1439. [Google Scholar] [CrossRef]
- Putnak, J.R.; de la Barrera, R.; Burgess, T.; Pardo, J.; Dessy, F.; Gheysen, D.; Lobet, Y.; Green, S.; Endy, T.P.; Thomas, S.J.; et al. Comparative evaluation of three assays for measurement of dengue virus neutralizing antibodies. Am. J. Trop. Med. Hyg. 2008, 79, 115–122. [Google Scholar] [CrossRef] [PubMed]
| Virus | Titer (pfu/mL) |
|---|---|
| DENV I | 1.8 × 105 |
| DENV II | 2.6 × 104 |
| DENV III | 4.2 × 104 |
| DENV IV | 1.7 × 104 |
| Factor | Conditions |
|---|---|
| Incubation time | 24 h/48 h/72 h |
| FBS concentration | 0%/2% |
| 96-well plate | Corning 3603/Thermo 137101 |
| Cell seeding density | 4 × 104 |
| 4G2 | 1 μg/mL |
| Secondary antibody | 1:3000 |
| Virus | FRNT50 (µg/mL) | PRNT50 (µg/mL) |
|---|---|---|
| DENV I | 33.66 | 9.95 |
| DENV II | 2.33 | 3.38 |
| DENV III | none | none |
| DENV IV | 0.44 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Lu, J.; Du, P.; Cheng, K.; Lei, C.; Jiang, Y.; Peng, M.; Li, Y.; Sun, K.; Xu, C.; et al. Fluorescence Reduction Neutralization Test: A Novel, Rapid, and Efficient Method for Characterizing the Neutralizing Activity of Antibodies Against Dengue Virus. Curr. Issues Mol. Biol. 2025, 47, 140. https://doi.org/10.3390/cimb47030140
Guo J, Lu J, Du P, Cheng K, Lei C, Jiang Y, Peng M, Li Y, Sun K, Xu C, et al. Fluorescence Reduction Neutralization Test: A Novel, Rapid, and Efficient Method for Characterizing the Neutralizing Activity of Antibodies Against Dengue Virus. Current Issues in Molecular Biology. 2025; 47(3):140. https://doi.org/10.3390/cimb47030140
Chicago/Turabian StyleGuo, Jiazheng, Jiansheng Lu, Peng Du, Kexuan Cheng, Chao Lei, Yujia Jiang, Meiling Peng, Yating Li, Kaiyue Sun, Changyan Xu, and et al. 2025. "Fluorescence Reduction Neutralization Test: A Novel, Rapid, and Efficient Method for Characterizing the Neutralizing Activity of Antibodies Against Dengue Virus" Current Issues in Molecular Biology 47, no. 3: 140. https://doi.org/10.3390/cimb47030140
APA StyleGuo, J., Lu, J., Du, P., Cheng, K., Lei, C., Jiang, Y., Peng, M., Li, Y., Sun, K., Xu, C., Yu, Y., Gao, C., Kang, Q., Zhang, Y., Wang, R., & Yang, Z. (2025). Fluorescence Reduction Neutralization Test: A Novel, Rapid, and Efficient Method for Characterizing the Neutralizing Activity of Antibodies Against Dengue Virus. Current Issues in Molecular Biology, 47(3), 140. https://doi.org/10.3390/cimb47030140






