Abstract
Sepsis acts as the leading cause of mortality in intensive care units, characterized by life-threatening organ dysfunction due to a dysregulated host response to infection. Vitamin D (VD) pleiotropic functions were demonstrated in different biological processes, including inflammation and immunity. VD receptor (VDR) is a member of the nuclear receptor superfamily, involved in immunoregulation and resistance to infections. Previous studies have demonstrated that VD deficiency is a potential risk factor for sepsis development, which may be regulated by VDR-related physiological processes. In this review, we present a comprehensive overview of the roles of VD and VDR in sepsis, focusing on immune modulation, anti-inflammatory and anti-infective responses, oxidative stress regulation, gut microbiome enhancement, vascular endothelial cell modulation, and antiplatelet activity. We also discuss recent advances in clinical research on VD/VDR in sepsis, considering the clinical implications and potential interventions of VD analogs and VDR ligands in treatment. Despite its challenges, VD holds potential for personalized sepsis interventions. Additionally, VD/VDR may serve as a promising bidirectional immunomodulator, capable of addressing both hyperinflammatory and immunosuppressive phases of sepsis, yet require systematic investigations into its dynamic states and functions across different sepsis phases. Ongoing study and evidence-based guidelines are crucial to maximize its therapeutic benefits and improve clinical outcomes.
1. Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. Further development of sepsis leads to septic shock and multiple organ dysfunction syndrome (MODS), which is a common complication in critically ill patients suffering from trauma/burns, infections, and shock [1]. Sepsis remains a leading cause of mortality in patients in intensive care units (ICUs) worldwide [2]. A recent analysis from the Global Burden of Disease Study reported 48.9 million annual cases of sepsis worldwide, with 11 million related deaths in 2017 [3]. Sepsis still contributes to almost 20% of all deaths every year in the world, more than 20 deaths every minute [4,5]. Furthermore, a recent meta-analysis indicated a mortality rate of 26.7% in sepsis patients, highlighting a 46% rise in incidence since 2008 [6]. However, the mechanisms underlying sepsis remain incompletely understood, potentially involving dysregulation of inflammatory responses, immune dysfunction, and coagulation disorder. Currently, the clinical management of sepsis typically includes standard approaches such as infection control, fluid resuscitation, inflammation suppression, and immune modulation, yet there is a notable lack of specific therapeutic agents targeting sepsis.
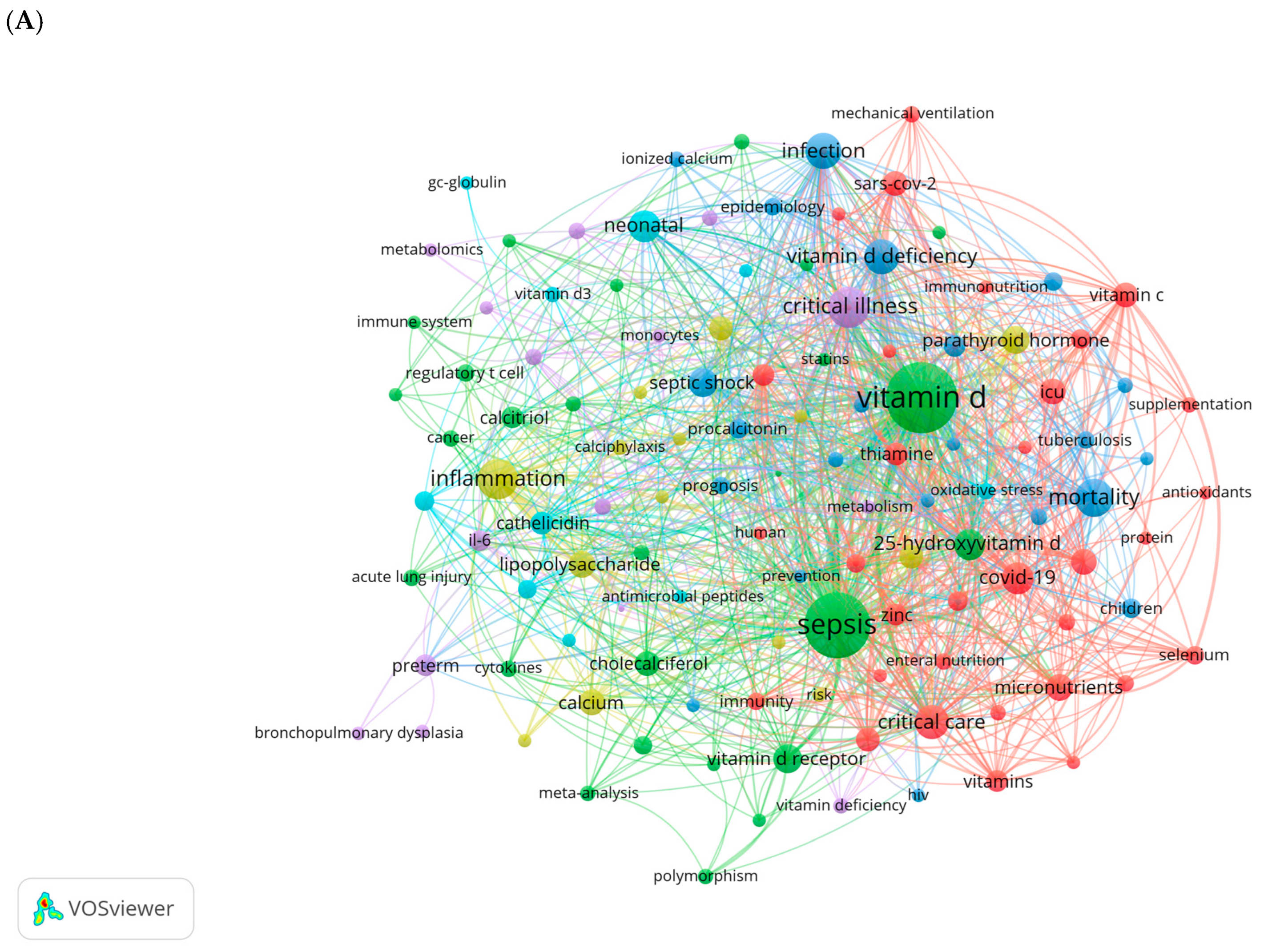
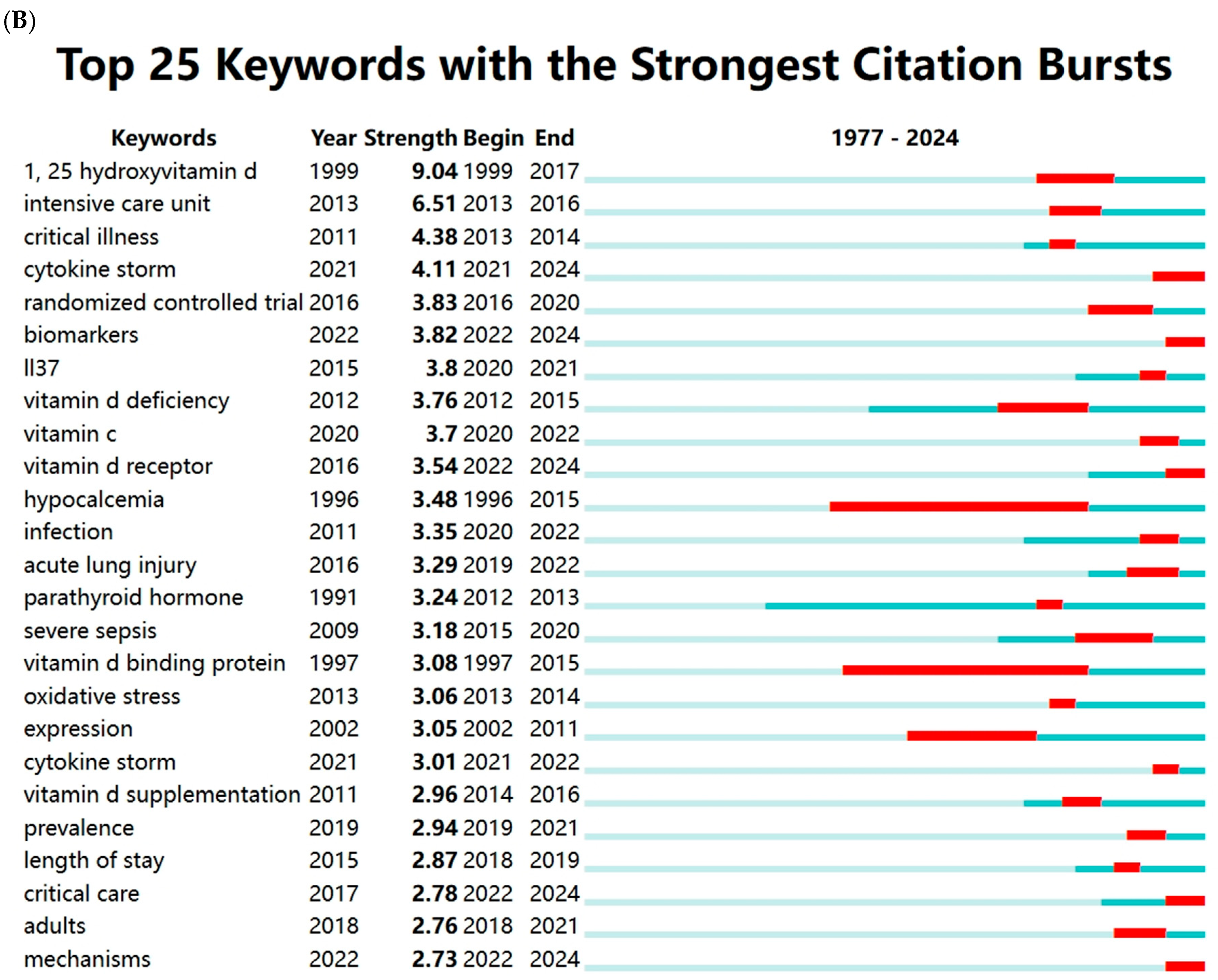
Vitamin D (VD) is a fat-soluble vitamin with a wide range of biological effects. Its active form, 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), exerts its functions by binding to the VD receptor (VDR) [7]. The roles of VD extend beyond its well-known regulation of calcium and phosphorus metabolism and bone mineralization; it also plays a critical role in modulating immune responses and inflammation, regulating cell proliferation and differentiation, and inhibiting tumor growth [8,9,10,11]. Thus, VD is essential for maintaining homeostasis within the body. Recent studies have increasingly highlighted the close relationship between VD and the onset and progression of sepsis. We conducted a comprehensive keyword analysis through a bibliometric study, retrieving publications related to sepsis and VD/VDR from the Web of Science Core Collection (WoSCC) database from its inception to 2024 (Figure 1). High-frequency keywords such as critical illness, inflammation, infection, VD deficiency, and COVID-19 indicate that there is a growing interest in understanding the role of VD in immune modulation and the inflammatory response during critical illness. This also highlights the potential significance of VD in clinical outcomes for patients with sepsis.
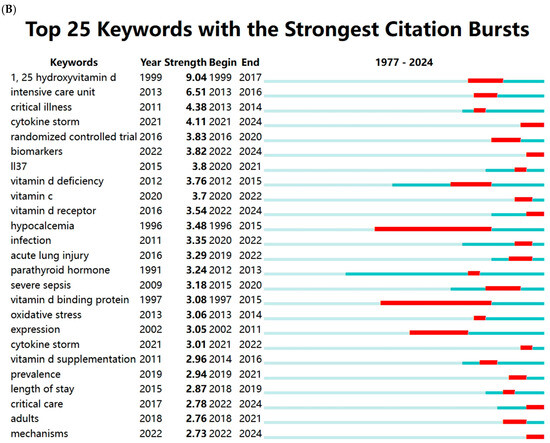
Figure 1.
Keywords cluster analysis. (A) Co-occurrence visualization network of keywords using VOSviewer (1.6.20). (B) Visualization map of top 25 keywords with the strongest citation bursts using CiteSpace (6.4.R1). The light blue lines represent the timeline, the dark blue lines indicate the time periods when the keyword appears, and the red lines represent the time periods when the keyword is burst (with high citation frequency).
VD has long been recognized as a key factor in regulating immune responses and influencing the outcomes of critical illnesses [12,13,14,15,16,17,18,19,20,21]. This review further updates the understanding of VDR, presents the roles of VD and VDR in sepsis, and describes mechanisms that contribute to their action. We also discuss the clinical implications and potential interventions of VD and its analogs in sepsis, providing valuable insights for clinical treatment of sepsis and the rational application of VD.
2. Clinical Evidence of VD Impact on Sepsis
2.1. VD Deficiency
VD deficiency (VDD) has emerged as a significant concern in critically ill patients, particularly those suffering from sepsis. A significant prevalence of low VD levels has been observed in critically ill patients, both pediatric and adult. In most pediatric ICUs (PICUs) globally, the rate of VDD varies from 30% to 70% [22]. Among adults, approximately 25% are at risk of VDD, while 8% face VD insufficiency [23]. A significant proportion of critically ill patients (ranging from 50% to 90%) admitted to the ICU exhibit low levels of VD [24,25,26,27]. Insufficient levels of VD impair immune function, disrupt hormone metabolism, and elevate the risk of various viral and bacterial infections and severe illnesses, ultimately leading to increased mortality [28]. Numerous studies have explored the prevalence and implications of VDD in sepsis patients, revealing a complex relationship between VD levels and clinical outcomes. Studies investigating VDD in patients with sepsis are summarized in Table 1. Researchers indicate that VDD or VD insufficiency is linked to an increased susceptibility to sepsis and poorer clinical outcomes, including greater sepsis severity, higher mortality rates, prolonged hospital and ICU stays, and negative correlations with lactate, CRP, acute physiology and chronic health evaluation II (APACHE II), and sequential organ failure assessment (SOFA) scores, as well as longer durations of mechanical ventilation [25,29,30,31,32,33,34,35,36,37,38,39,40,41]. However, some studies have found no significant connections between VD levels and the susceptibility to or severity of sepsis [42,43]. In pediatric populations, findings are also inconsistent. Some research shows that critically ill children and infants with sepsis may have lower levels of 25(OH)D and more severe VDD compared to those without sepsis [28,44,45,46], suggesting that lower 25(OH)D levels are associated with sepsis susceptibility and poor outcomes [46,47,48,49,50,51,52,53]. Conversely, other studies indicate that neither VD levels nor VDR polymorphisms are linked to sepsis [54,55].

Table 1.
Summary of studies on vitamin D deficiency in sepsis patients.
2.2. VD Supplementation
While the evidence regarding the role of VD in sepsis treatment remains inconclusive, the potential benefits of supplementation in improving overall health outcomes cannot be overlooked. An overview of studies investigating VD supplementation in sepsis patients is presented in Table 2. Multiple randomized controlled trials (RCTs) indicate that high-dose VD safely elevates plasma 25(OH)D levels into the sufficient range without causing adverse effects such as hypercalcemia or hypercalciuria [59,60]. However, the benefits of VD supplementation for patients with sepsis remain debated. Some studies suggest that VD supplementation positively impacts the sepsis prognosis and infectious biomarkers, leading to better clinical outcomes, reduced mortality in mechanically ventilated patients, and shorter hospital stays [59,61,62,63,64,65]. A recent pharmacological evaluation investigating the clinical efficacy of VD in COVID-19 and long COVID has shown that extensive observational studies indicate a definitive relationship between low VD levels and the severity and mortality of COVID-19. It also reports beneficial effects of VD supplementation on COVID-19. Additionally, it has been highlighted that metformin may enhance VD receptor (VDR) sensitivity and improve treatment outcomes for both COVID-19 and long COVID [66]. Conversely, other research indicates that VD supplementation does not offer advantages over a placebo in terms of mortality or other nonfatal outcomes among critically ill patients with VDD [67,68,69,70]. In pediatric care, a case–control study found that maternal VD supplementation may enhance neonatal VD levels and reduce the risk of early-onset neonatal sepsis (EONS) [48]. Additionally, research involving children (<14 years) demonstrated that a single dose of 150,000 IU of cholecalciferol improved 25(OH)D levels and reduced the incidence of septic shock in those with VD deficiency and sepsis [71]. Another RCT in neonates with sepsis revealed that VD supplementation improved sepsis scores and lowered high levels of hs-CRP [72]. Furthermore, an RCT indicated that a dose of 400 IU of VD was sufficient to treat VD deficiency in most premature infants with late-onset sepsis [73].

Table 2.
Summary of studies on vitamin D supplementation in patients with sepsis.
2.3. Association of VDR Gene Polymorphism with the Risk of Sepsis
The relationship between VDR gene polymorphisms and susceptibility to sepsis has garnered increasing attention. The VDR gene is located on chromosome 12q13.1 and contains multiple single-nucleotide polymorphisms (SNPs), such as rs2228570, rs1544410, rs7975232, and rs731236 [74]. VDR gene polymorphisms have been shown to affect the expression and function of the VDR protein. For instance, the rs2228570 polymorphism is associated with changes in VDR protein levels in immune cells, and the rs1544410 and rs731236 polymorphisms are linked to variations in VDR plasma concentration [75]. Given that the VDR protein plays a crucial role in immune regulation, polymorphisms in the VDR gene may influence immune responses and susceptibility to diseases.
A recent case–control study indicated that the VDR gene may be a sepsis susceptibility gene in Chinese Han children [76]. The rs2228570 polymorphism in the VDR gene has been consistently associated with an increased risk of sepsis. A case–control study indicated that rs2228570 variants were more prevalent in sepsis patients compared to healthy controls, indicating a significant role in predisposing individuals to sepsis and septic shock [77]. A meta-analysis found that the rs2228570 locus, particularly the AA genotype, was linked to a higher susceptibility to sepsis (OR = 0.53, 95% CI = 0.30–0.91) [29]. A prospective observational study confirmed that the VDR rs2228570 polymorphism, particularly the CC genotype, appears to be associated with lower mortality in ICU patients [78]. The rs731236 polymorphism has shown mixed results. One study found an association between rs731236 SNP and neonatal sepsis, with specific genotypes (CT vs. CC + TT) being more prevalent in sepsis cases [79]. However, another meta-analysis did not find a significant correlation between rs731236 and sepsis risk [29]. The rs1544410 and rs7975232 polymorphisms have not shown a consistent association with sepsis across studies. Some analyses did not find significant correlations, suggesting that these polymorphisms may not play a major role in sepsis susceptibility [29,77].
The frequency of VDR polymorphisms may vary significantly among different ethnic groups, potentially influencing patients’ susceptibility to diseases. Researchers have investigated specific alleles and genotypes of VDR single-nucleotide polymorphisms, including rs731236, rs7975232, and rs1544410, within the Kazakh population, discovering that these exhibit unique prevalence patterns [80]. Further prospective studies and large-scale clinical trials are needed to explore the gene–environment interactions and the potential of using VDR polymorphisms as biomarkers for disease susceptibility and a therapeutic response.
3. Possible Mechanisms of the Association Between VD/VDR and Sepsis
3.1. Pathophysiological Process of Sepsis
3.1.1. Innate Immunity and Inflammatory Mediators
Sepsis is a life-threatening syndrome characterized by organ dysfunction resulting from a dysregulated host response to infection and/or infectious agents [1]. Infection refers to the pathophysiological process of colonization and proliferation of pathogens, such as bacteria, fungi, viruses, protozoa, etc. Infectious agents are the fundamental structural molecules of these pathogens, including pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). PAMPs include bacterial lipopolysaccharides/endotoxins (LPSs), flagellin, genomic DNA, yeast polysaccharides, and single- or double-stranded RNA (ssRNA or dsRNA). DAMPs refer to intracellular material or molecules released from dead or damaged host cells, such as adenosine triphosphate (ATP) and mitochondrial DNA [81]. The first step in the host’s response to a pathogen is the activation of innate immune cells, primarily comprising macrophages, monocytes, neutrophils, and natural killer cells. During the infection process, invading pathogens, PAMPs, and DAMPs are recognized by pattern recognition receptors (PRRs) [82]. These PRRs, distributed in the immune cells, include toll-like receptors (TLRs), C-type lectin receptors, NOD-like receptors (nucleotide-binding oligomerization domain), RIG-I-like receptors (retinoic acid-inducible gene 1), etc. This recognition activates intracellular signal transduction pathways, resulting in the transcription and release of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6. Additionally, some PRRs, particularly the NOD-like receptor family, can form larger protein complexes known as inflammasomes, which play a critical role in producing vital cytokines such as IL-1β and IL-18, as well as caspases involved in programmed cell death. Pro-inflammatory cytokines promote the activation and proliferation of leukocytes, the activation of the complement system, the upregulation of endothelial adhesion molecules and chemokine expression, the production of tissue factors (TFs), and the induction of hepatic acute phase reactants. In sepsis, this immune response is exaggerated, leading to collateral damage and the death of host cells and tissues. The essence of sepsis lies in the fact that the PAMPs and DAMPs recognized by PRRs trigger immune cell activation, ultimately resulting in immune dysfunction.
3.1.2. Immunosuppression
Although the precise timeline of immunological changes in sepsis remains inadequately characterized, accumulating evidence indicates that the progression of sepsis encompasses two distinct phases: an initial cytokine storm characterized by hyperinflammation, followed by a phase of immunosuppression marked by hypoinflammation [83]. These phases may coexist but predominate at different stages of the condition. The unregulated cytokine storm is primarily responsible for fatalities occurring within the initial days of sepsis, while immunosuppression is the leading cause of mortality in the later stages [84]. Notably, over 70% of patients die after the first three days of sepsis, likely due to heightened vulnerability to weakly virulent pathogens or opportunistic bacteria, reflecting the host’s inability to eliminate invading pathogens. This phase renders patients vulnerable to secondary infections and reactivation of latent infections, ultimately leading to increased mortality rates [2].
In immunosuppression patients, T-cell numbers decrease due to apoptosis and a reduced response to inflammatory cytokines. Autopsy studies of ICU patients who died from sepsis indicated a significant loss of CD4+ and CD8+ T cells, especially in lymphoid tissues like the spleen. Research also found reduced production of essential cytokines such as IL-6 and TNF-α in response to LPS [85]. Moreover, neutrophils in septic patients showed fewer chemokine receptors, leading to decreased chemotaxis to IL-8 [86]. Myeloid-derived suppressor cells (MDSCs) expand during sepsis and significantly inhibit both innate and adaptive immune responses, ultimately suppressing T-cell activation and proliferation, worsening the immunosuppressive environment [87]. A key feature of immunosuppression is the reprogramming of monocytes/macrophages. In septic patients, reprogrammed leukocytes may exhibit excessive release of anti-inflammatory factors and reduced MHC-II expression. Epigenetic regulation related to histone and DNA methylation plays a crucial role in this reprogramming of immune cells in sepsis. Sepsis-induced immunosuppression involves epigenetic changes in immune cells, leading to lasting immune function alterations in sepsis survivors. Identified epigenetic biomarkers may predict post-septic immunosuppression risk, providing potential strategies for improved management [88]. Additionally, studies indicate that autophagy in clearing dead and dysfunctional cells contributes to immunosuppression [89,90]. Furthermore, several studies highlight the close association between sepsis-induced immune suppression and the checkpoint regulator PD-1. Increased PD-1 expression can lead to T-cell apoptosis and higher mortality in septic patients [91]. Immunosuppression in sepsis thus provides a novel understanding of the disorder as well as a new therapeutic approach.
3.1.3. Coagulation Disorder
In sepsis, inflammatory and hemostatic pathways intersect, activating both inflammatory and coagulation cascades. This disruption of the anticoagulant–coagulant balance leads to extensive thrombosis and can trigger severe disseminated intravascular coagulation (DIC). Coagulation abnormalities are closely linked to TF, expressed by endothelial cells, which activates FX and FIX when binding with FVIIa, amplifying coagulation through interactions with platelets and neutrophils [92]. Under pathological conditions, major anticoagulation pathways—antithrombin, TF pathway inhibitor, and the protein C system—are inhibited, increasing thrombosis risk [93]. Additionally, thrombin activates platelets, promoting neutrophil aggregation and releasing coagulation factors, enhancing coagulation further [94]. Research shows that coagulation factors like TF and fibrin can promote inflammation and activate a complement, which in turn can activate thrombin [95]. High-mobility group box 1 also plays a role in coagulation activation during sepsis. Recent studies indicate that Gasdermin D (GSDMD) induces pyroptosis, releasing TF and activating coagulation [96]. Targeting GSDMD may offer a new approach to inhibit abnormal coagulation in sepsis.
3.1.4. Organ Dysfunction
The underlying mechanism of tissue and organ dysfunction in sepsis is decreased perfusion, resulting in reduced oxygen delivery and utilization by cells. This hypoperfusion is caused by cardiovascular dysfunction associated with sepsis [97]. The incidence of septic cardiomyopathy ranges from 18% to 60%, believed to be linked to circulating cytokines such as TNF-α and IL-1β, which can depress cardiac myocytes and disrupt mitochondrial function. The combination of hemodynamic changes and microvascular thrombosis can lead to inadequate perfusion of tissues and organs. Consequently, anaerobic glycolysis increases in cells, producing lactic acid. Additionally, reactive oxygen species (ROS) generated by the inflammatory response lead to mitochondrial dysfunction and decreased ATP levels. These factors cause cellular damage, resulting in widespread changes in tissues and organs that significantly contribute to the morbidity and mortality associated with sepsis [82].
3.2. VD/VDR
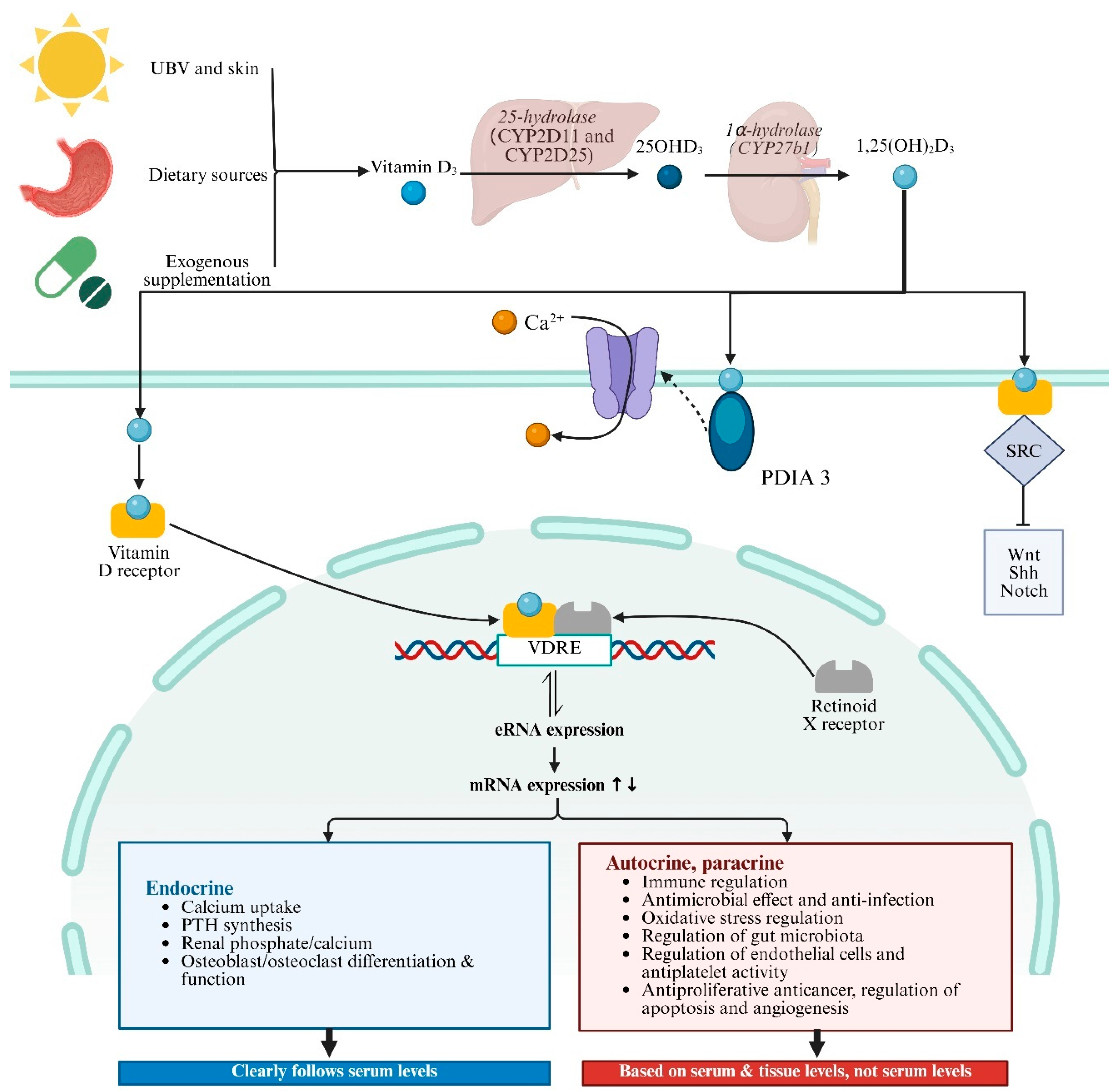
VD is obtained in three main ways: skin synthesis from UV radiation acting on 7-dehydrocholesterol, dietary sources, and exogenous supplementation, and skin synthesis is the predominant method. VD has two main forms, Vitamin D2 (ergocalciferol) and Vitamin D3 (cholecalciferol), with Vitamin D3 being more effective in raising serum 25-hydroxyvitamin D levels [98]. Vitamin D3 enters the bloodstream and is transported to the liver by vitamin D binding protein (DBP), where enzymes like CYP2D11 and CYP2D25 hydroxylate it into 25-hydroxyvitamin D3 (25(OH)D3) [99]. 25(OH)D3 is the primary form of Vitamin D3 in plasma and serves as the main storage form in the liver. It is then further hydroxylated in the kidneys by 1α-hydroxylase to form 1,25(OH)2D3, which is critical for calcium homeostasis [98]. 1,25(OH)2D3 is the most biologically active form of VD, exerting its effects by binding to VDR in target cells (Figure 2). Global consensus recommendations on prevention and management of nutritional rickets define VD sufficiency as serum 25(OH)D3 levels above 50 nmol/L, deficiency as below 30 nmol/L, and insufficiency as between 30 and 50 nmol/L [100]. VD levels in populations are influenced by various factors including geographical location, seasonal variation, sunlight exposure, skin pigmentation, dietary sources, and supplement intake. Deficiency is particularly prevalent in high-latitude regions, during winter, and in overcast weather [101]. Currently, VD measurement methods include immunoassays and liquid chromatography–tandem mass spectrometry (LC-MS/MS). Immunoassays are popular due to their speed and convenience, but they cannot differentiate between 1,25(OH)2D3 and 25(OH)D3 and may have cross-reactivity issues [102]. In contrast, LC-MS/MS is highly sensitive and specific, regarded as the gold standard for measuring VD metabolites, though it is more time-consuming and costly [103,104].
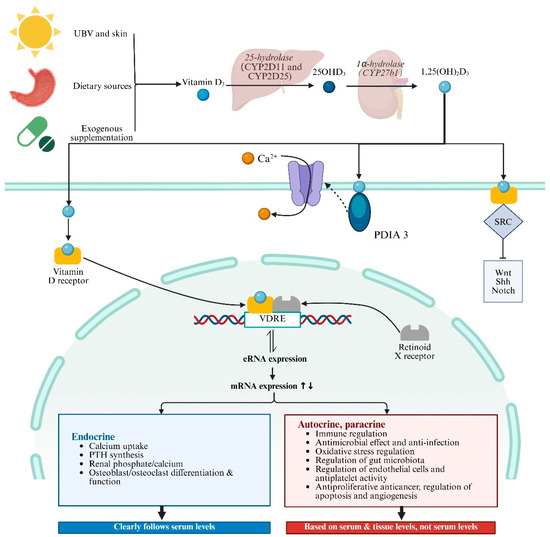
Figure 2.
Vitamin D synthesis, signaling, and pathophysiological process. Abbreviations: UBV, ultraviolet radiation b; VDRE, vitamin D response elements; SRC, SRC proto-oncogene, non-receptor tyrosine kinase; PDIA3, protein disulfide isomerase family A member 3; PTH, parathyroid hormone; SRC, SRC proto-oncogene, non-receptor tyrosine kinase. ↑↓ indicates the impact of the upregulation or downregulation of mRNA expression levels on the following physiological functions.
The biological roles of VD in health and disease are linked to changes in the transcriptome mediated by 1,25(OH)2D3 in VDR-expressing cells (Figure 2) [105]. VDR, a member of the nuclear receptor superfamily, influences many genes across various cell types and tissues [106,107]. When 1,25(OH)2D3 binds to VDR, it undergoes a conformational change, allowing it to heterodimerize with the retinoid X receptor (RXR) [108,109]. This complex then travels to the nucleus, where it binds to VD response elements (VDREs) in gene promoters and recruits co-regulatory complexes to modulate transcription. The VDR–ligand complex regulates the expression of over 1000 genes through both activation and repression mechanisms [99,110,111,112]. VDR-mediated transcriptional regulation involves epigenetic modifications and chromatin reorganization. Enhancer RNAs (eRNAs), a class of non-coding RNAs, have emerged as potential VDR target genes. They facilitate chromatin looping and enhancer–promoter interactions, crucial for the activation of mRNA-encoding genes [113]. Genome-wide analyses have identified numerous VDR binding sites, with a significant number being unique to specific cellular contexts. These sites often contain DR3-type sequences, which are preferentially found at highly ligand-responsive VDR loci [114]. Besides the genomic pathway, there is increasing evidence that a subset of plasma-membrane-bound VDRs elicit rapid biological effects through nongenomic pathways [115,116]. Recent studies have identified rapid, nongenomic effects mediated by membrane-bound VDRs and protein disulfide isomerase family A member 3 (PDIA3) [117,118].
3.3. Immune Regulation
VDR is expressed in a variety of immune cells, including monocytes/macrophages, dendritic cells (DCs), T cells, B cells, etc., indicating its essential role in modulating immune responses. 1,25(OH)2D3 modulates the activation, proliferation, and differentiation of immune cells through the VDR expressed in these cells [119,120,121,122,123,124]. Additionally, immune and inflammatory cells can convert 25(OH)D3 into 1,25(OH)2D3 [125,126,127,128]. The expression of CYP27B1 is increased by immune stimuli (such as IFN-g and LPS) that activate the C/EBPb transcription factor, which binds to the CYP27B1 genes in both mice and humans [129].
3.3.1. Monocytes/Macrophages
Monocytes are a type of white blood cell derived from the bone marrow, circulating in the blood. Macrophages are differentiated forms of monocytes found in tissues, possessing enhanced phagocytic and immune functions. 1,25(OH)2D3 stimulates the differentiation of monocytes to macrophages [130]. Activated macrophages, which function as antigen-presenting cells (APCs), release inflammatory mediators to attract various cell types to the site of inflammation for pathogen elimination. Macrophages are typically categorized into two types: M1 and M2. M1 macrophages generate pro-inflammatory mediators such as nitric oxide (NO), TNF-α, IL-23, IL-12, and IL-1β, facilitating pathogen clearance and promoting the polarization of T helper 1 (Th1) and Th17 cells. M2 macrophages produce IL-10, which possesses anti-inflammatory properties [131]. 1,25(OH)2D3 can influence macrophage polarization, promoting a shift towards an anti-inflammatory phenotype [132]. VDR activation promotes M2 macrophage polarization and then reduces liver ischemia and reperfusion injury. Autophagy is involved in this process by mediating the suppressor of cytokine signaling [133].
In monocytes and macrophages, the expression of the 1α-hydroxylase isoenzyme (CYP27B1) induces the production of active 1,25(OH)2D3, which activates the VDR and promotes cell adhesion and differentiation. Additionally, activated VDR induces the production of cathelicidin, thereby contributing to antimicrobial activity [134]. 1,25(OH)2D3 inhibits LPS-induced p38 activation and cytokine production in monocytes/macrophages by upregulating MKP-1 [135]. 1,25(OH)2D3 triggers IL-15-differentiated macrophages, inducing antimicrobial activity against intracellular Mycobacterium tuberculosis (Mtb) [136]. 1,25(OH)2D3 restores macrophage lysosome acidification to protect against Clostridioides difficile infection in mice [137]. 1,25(OH)2D3 suppresses LPS-induced inflammation by promoting negative feedback regulation of TLR signaling that targets microRNA-155-SOCS1 in macrophages [138]. VDR activation exhibits strong anti-inflammatory effects in mouse hepatic macrophages, including those isolated from diet-induced obesity livers, and mice with genetic loss of VDR developed spontaneous hepatic inflammation at 6 months of age [139]. Consistently with the data above, VDR KO mice are less responsive to LPS stimulation [140].
3.3.2. DCs
DCs are pivotal in orchestrating immune responses, acting as APCs that bridge innate and adaptive immunity. DCs patrol tissues for pathogens, process foreign antigens, and present peptides to T cells, which then differentiate into effector cells. 1,25(OH)2D3 decreases the production of pro-inflammatory cytokines, such as IL-12 and TNF-α, while simultaneously enhancing the production of the anti-inflammatory cytokine IL-10 in DCs [141,142,143]. Furthermore, 1,25(OH)2D3 diminishes the expression of class II MHC and costimulatory molecules (CD40, CD80, CD86) in DCs. These DCs exhibit a limited capacity to induce T-cell proliferation and autoreactive T-cell activation, instead promoting the differentiation of regulatory T (Treg) cells [143,144,145,146]. 1,25(OH)2D3 suppresses IL-12 expression through the binding of the VDR/RXR complex to the NF-kB site within the IL-12p40 promoter [147]. Moreover, VD has been extensively proposed as a modulator of DCs towards tolerance, reducing their ability to stimulate effector T-cell generation while enhancing their potential to induce anti-inflammatory Treg cells [148,149,150]. DCs metabolize VD for the programming of T cells, and directly interact with DCs to influence their migration and their ability to instruct T cells, thereby initiating, fine-tuning, or attenuating immune responses [151].
3.3.3. B Cells
B cells are essential components of the immune system, primarily responsible for producing antibodies that neutralize pathogens. They also present antigens to T cells, form memory B cells for long-term immunity, and secrete cytokines to regulate immune responses. In antibody-mediated autoimmune conditions, B cells are crucial for producing auto-reactive antibodies.
1,25(OH)2D3 has distinct effects at different stages of B-cell differentiation. It inhibits B-cell proliferation, immunoglobulin class switching, and antibody production while also inducing apoptosis [152,153]. 1,25(OH)2D3 directly regulates the homoeostasis of B cells, by promoting the apoptosis of immunoglobulin-secreting B cells and inhibiting the formation of plasma and memory cells [15,148,152]. The VDR directly and indirectly regulates IgE production in B cells. Through the VDR, VD is an environmental factor that helps to maintain low serum IgE responses [154].
Human B cells activated by CD40 and IL-4 signals can generate and secrete significant amounts of 1,25(OH)2D3. Moreover, 1,25(OH)2D3 enhances IL-10 expression in activated B cells by recruiting the VD receptor to the promoter of IL-10, and it can also increase IgE expression [127]. 1,25(OH)2D3 efficiently induces CCR10 expression in terminally differentiating human B cells in vitro. The human CCR10 promoter is cooperatively activated by Ets-1 and VDR in the presence of 1,25(OH)2D3 [155].
Furthermore, 1,25(OH)2D3 can influence the effect of B cells on regulating the activation and function of T cells. Naive T cells co-cultured with these B cells exhibited reduced expansion, decreasing cytokine production upon restimulation. The reduced CD86 expression on B cells was identified as a key mechanism, as T-cell activation and expansion were restored by anti-CD28 antibodies. 1,25(OH)2D3-primed B cells have a diminished capacity to activate T cells [156]. 1,25(OH)2D3 reduces the expression of CD74, which plays a role in the assembly and presentation of MHC-II molecules on the cell surface [157]. The impact of 1,25(OH)2D3 on the NF-kB pathway mediated CD40 activation to suppress the differentiation of B cells into plasma cells [155,158].
3.3.4. T Cells
The expression level of VDR in naive T cells is low, rendering them insensitive to 1,25(OH)2D3. However, studies have shown that 1,25(OH)2D3 may directly regulate T-cell receptor (TCR) signaling and induce the expression of nuclear VDR and phospholipase C gamma 1 (PLC-γ1) through the MAPK p38 pathway [128,159]. Both VDR and PLC-γ1 play crucial roles in TCR signaling and T-cell activation [160]. T cells can differentiate into various subtypes, with 1,25(OH)2D3 exerting different effects on each subtype, influencing their differentiation, proliferation, and cytokine production [143,161,162]. TH lymphocytes are the specific cellular targets for the immunoinhibitory effects of 1,25(OH)2D3, which shifts T cells from a pro-inflammatory immune state to a more tolerant phenotype [163]. VDR is essential for the normal development and function of invariant natural killer T (iNKT) cells. The development of iNKT cells relies on the expression of functional nuclear VDR in the thymus. VDR KO mice exhibit impaired responses of iNKT cells to TCR stimulation [164].
1,25(OH)2D3 has been found to modulate the balance between pro-inflammatory and anti-inflammatory cytokines produced by T cells. This modulation is particularly important in the context of chronic inflammatory conditions, where an imbalance in cytokine production can lead to tissue damage and disease progression. 1,25(OH)2D3 inhibits the production of interferon gamma (IFN-γ), IL-2, IL-4, IL-9 (Th9), and IL-22 (Th22) in Th1 cells [120,162,165,166,167,168,169,170]. CD4+ T cells from VDR KO mice produce higher levels of IFN-γ and IL-17 compared to their wild-type counterparts [171]. Mechanistically, VDR/RXR can suppress IFN-γ expression by binding to a silencing region in the hIFN-γ promoter [172]. Additionally, 1,25(OH)2D3 inhibits IL-2 production in Th1 cells by directly interfering with the NFATp/AP-1 complex and sequestering nVDR from Runx1 through the VDR/RXR pathway [166].
1,25(OH)2D3 not only inhibits Th2 cell differentiation but also promotes the secretion of anti-inflammatory Th2 cytokines (such as IL-3, IL-4, IL-5, and IL-10) and GATA3 in Th2 cells without polarization [173,174]. During the in vitro polarization of naive CD62 ligand+ CD4+ T cells, calcitriol inhibits the production of the Th2 cytokine IL-4. Notably, VD exhibits its strongest inhibitory effect when administered from the beginning of the polarization process; however, adding VD after polarization significantly diminishes this effect. In activated CD62 ligand-CD4+ T cells, the inhibitory effect of 1,25(OH)2D3 on IL-4 production is absent. A potential mechanism underlying this is that, during Th2 differentiation, the VDR reduces IL-4 transcription [162,165].
Th17 cells are crucial for defending against certain pathogens, including Helicobacter pylori, Mtb, Candida albicans, Klebsiella pneumoniae, and Staphylococcus aureus, all of which are associated with inflammation and tissue damage [175]. In vitro, 1,25(OH)2D3 inhibits the production of Th17-related cytokines and transcription factors, such as IL-17A, IL-17F, RORC, and CCR6, thereby suppressing Th17 cell differentiation and activation [169,176]. 1,25(OH)2D3 also reduces the production of IFN-γ and TNF-α in CD8+ T cells [177]. 1,25(OH)2D3 significantly decreases the expansion of TCRγδ T cells, IFN-γ production, and CD25 expression in a dose-dependent manner. Additionally, it downregulates Akt and ERK signaling pathways and enhances antigen-induced cell death at higher concentrations [178].
Tregs, characterized as CD4+ CD25+ T cells, not only produce anti-inflammatory cytokines such as IL-10 and TGF-β but also downregulate the activity of macrophages, dendritic cells, and both CD4+ and CD8+ T cells. Tregs are essential for maintaining immune tolerance and preventing autoimmune diseases by helping to suppress excessive immune responses [179]. VD has been shown to influence the development, differentiation, and apoptosis of Tregs [180,181]. Forkhead box P3 (Foxp3) is a vital regulator of Treg development and function. 1,25(OH)2D3 modulates FOXP3 expression in Tregs through direct binding of the VDR to the FOXP3 gene [182]. In vivo administration of vitamin D3, along with the adoptive transfer of vitamin D3-induced IDO (+) immature/tolerogenic DCs, leads to a significant increase in Treg proportions in the lymph nodes of a rat model of multiple sclerosis [183]. Moreover, 1,25(OH)2D3 inhibits the production of pro-inflammatory cytokines, including IFN-γ, IL-17, and IL-21 in Tregs. Additionally, 1,25(OH)2D3 stimulates high levels of cytotoxic T-lymphocyte-associated protein 4 and FoxP3 expression, the latter of which requires the presence of IL-2 [180]. Furthermore, VD facilitates the polarization of CD4+ T cells towards Tregs or regulatory Th2 phenotypes by regulating the gene transcription of cytokines [184,185,186]. These two phenotypes are crucial for VD’s ability to suppress Th1-mediated autoimmune responses [161,186].
3.4. Antimicrobial Effect and Anti-Inflammation
3.4.1. VD/VDR Induced Cathelicidin Pathway in Antimicrobial Effect
The 1,25(OH)2D3-VDR-RXR complex activates the transcription of genes encoding antimicrobial peptides (AMPs), which have direct antimicrobial activities against various pathogens via the formation of ion channels and increasing membrane permeability [187,188]. In addition to their antimicrobial roles, AMPs have significant regulatory effects on innate immunity and adaptive immunity, as well as on the aggregation and activation of immune cells and the expression of genes related to inflammatory factors [189]. AMPs directly regulated by VD signaling include human cationic AMP 18 (hCAP-18) and Defensin β2 (DEFB) [188]. The hCAP-18 is located in the phagolysosome with azurophil granule proteins, including serine protease. It converts hCAP-18 into the active AMP LL-37, which is a specific form of human cathelicidin [190]. The expression of LL-37 is significantly enhanced by 1,25(OH)2D3 in all assessed cell types, including epithelial cells, macrophages, and neutrophils, while the regulation of DEFB expression was less pronounced [191]. There has been a growing interest in designing new peptides based on AMPs [192]. Wang et al. identified the core antimicrobial region of LL-37 using 3D structural analysis, which can be function-dependent. They modified LL-37 into 17BIPHE2, which emerged as a stable, selective, and potent antimicrobial, antibiofilm, and anticancer peptide. Additionally, they proposed the application strategies of VD as a peptide-inducing factor [193].
AMPs acts as a vital regulator and effector of antimicrobial responses mediated by 1,25(OH)2D3. Gottlieb et al. developed a high-throughput cathelicidin assay using CRISPR/Cas9 edited human monocyte-macrophage cell lines, which serves as a new tool for evaluating VD-dependent antimicrobial responses [194]. A meta-analysis revealed a negative correlation between serum 25(OH)D3 levels and LL-37 in asthma patients [195]. Similarly, an RCT conducted in patients with acute respiratory infections also found a negative correlation between serum 25(OH)D3 levels and LL-37, suggesting that sufficient serum 25(OH)D levels may help prevent acute respiratory infections, potentially mediated by the induction of AMP production [196]. A pilot clinical trial involving 76 hospitalized patients with SARS-CoV-2 infection demonstrated the potential efficacy of VD supplementation in reducing the severity of COVID-19, with oral calcifediol reducing the need for intensive care unit (ICU) treatment [197]. Research by Mok et al. indicated that 1,25(OH)2D3 exhibits significant efficacy against SARS-CoV-2 in cell models, functioning by enhancing the expression of the AMP cathelicidin through the regulation of the VDR pathway [198].
LL-37 exhibits broad antimicrobial activity, which has been reported against Gram-positive and Gram-negative pathogenic bacteria, viruses, and fungi [187,199,200]. Moreover, LL-37 is considered an immunomodulator that exerts pro-inflammatory or anti-inflammatory effects depending on the specific cells, tissues, or microenvironments [201]. A substantial body of research indicates that VD exerts antibacterial effects against Mtb through the induction of antimicrobial peptides, highlighting its protective role against tuberculosis [202,203,204,205]. Studies have shown that the TLR family plays a crucial role in activating the expression of VDR and CYP27B1 in human macrophages, which in turn induces the activation of cathelicidin [134,206]. Cathelicidin-dependent autophagy is involved in inhibiting the replication of Mtb and HIV in co-infected macrophages [207]. Additionally, another study found that VD mediates antibacterial effects by regulating paracrine signaling in epithelial cells, including IL1R1 signaling and IL-1β-driven production of the antimicrobial peptide defensin beta 4 (DEFB4)/human beta-defensin 2, independent of 1,25(OH)2D3-stimulated autophagy in macrophages [208]. Fernandez et al. found that 1,25(OH)2D3 can limit the infection of monocyte-derived macrophages (MDMs) by the Zika virus (ZIKV). Transcriptional profiling revealed that VD can reduce the levels of pro-inflammatory cytokines and chemokines in MDMs, while enhancing the expression of degranulation-related genes like cathelicidin. Additionally, treatment with LL-37 was shown to decrease the proportion of ZIKV-infected MDMs [209]. Furthermore, Tang et al. discovered that the P2X7 receptor (P2X7R) plays an important role in the internalization of LL-37 by human macrophages, contributing to the intracellular clearance of bacteria [210].
3.4.2. VD/VDR Regulating Autophagy Pathway
VD and VDR regulate autophagy through a different signaling pathway [211]. The role of the VD/VDR-antimicrobial peptide axis in regulating autophagy during infection has been extensively studied [191]. 1,25(OH)2D3/VDR-induced antimicrobial peptides activate autophagy in Mtb-infected monocytes, promoting the maturation of intracellular Mtb phagosomes and thereby clearing Mtb [202]. Antimicrobial peptides can be internalized into lysosomes, enhancing the bactericidal activity of target cells against Staphylococcus aureus, Helicobacter pylori, and Salmonella [210,212,213]. Mechanistically, VD-induced cathelicidin expression upregulates Beclin-1 and ATG5, thereby increasing autophagy levels. Moreover, the antimicrobial effects of 1,25(OH)2D3 and cathelicidin can be abrogated by autophagolysosome inhibitors [204,214]. Another study indicated that the combination of IL-12 and IL-18 triggered an antimicrobial response against Mtb through the activation of VDR, cathelicidin, and autophagy in p-38/MAPK- and STAT4-dependent pathways in macrophages and lung epithelial cells [215].
As a target gene of VDR, autophagy-related 16 like 1 (ATG16L1) serves as a crucial mediator in the VDR-regulated autophagy pathway. Studies in intestinal epithelial cells demonstrate that diminished VDR expression is associated with decreased ATG16L1 levels and compromised autophagy, consequently elevating the risk of inflammatory bowel disease [216]. In Paneth cell-specific VDR knockout mice, lysozyme production was markedly reduced in the Paneth cell. It demonstrated impaired antimicrobial capacity and diminished autophagic responses, highlighting VDR’s essential role in maintaining Paneth cell function and inflammatory regulation [217]. Interestingly, in LPS-tolerant macrophages, elevated VDR expression negatively regulates ATG16L1 transcription, resulting in suppressed autophagy and a reduced bacterial clearance capacity alongside diminished pro-inflammatory cytokine production [218]. These findings reveal that VDR’s transcriptional control of ATG16L1 exhibits cell-type specificity and varies according to immunological and inflammatory conditions.
In HK-2 cells, microtubule-associated protein 1 light chain 3 (LC3) interacts with VDR to facilitate its nuclear translocation and form activated VDR-RXR dimers, with the binding site distinct from the LC3-interacting region. LC3-RXR dimerization inhibits high glucose-induced activation and nuclear translocation [219]. Recently investigations demonstrate that P62 directly binds VDR through its nuclear receptor box motif, promoting VDR nuclear translocation and transcriptional activation of autophagy to suppress ferroptosis in the airway epithelium [220]. Lai et al. reported that VD reverses endothelial damage caused by ROS accumulation, mitophagy impairment, and inflammation [221]. Ma’s research identified E3 ubiquitin ligase Parkin as an interaction partner of VDR, promoting VDR degradation through P62-associated autophagolysosomal pathways [222]. In wrap-restraint stress-exposed rats, 1,25(OH)2D3 administration alleviates stress-induced colitis by enhancing AMPK activity and suppressing mTOR-STATS signaling to augment colonic autophagy [223]. In monosodium iodoacetate-induced osteoarthritis rat models, VD supplementation significantly ameliorated pain, synovial inflammation, and cartilage degradation by downregulating matrix metalloproteinase-13, IL-1β, and monocyte chemoattractant protein-1 expression while inducing autophagy activation [224].
3.4.3. Modulation of VD/VDR on Inflammatory Cytokines
The inhibitory role of 1,25(OH)2D3 in inflammation has been widely studied in innate immune responses. VD has been found to reduce levels of pro-inflammatory cytokines (TNF-α and IL-6), while promoting the secretion of anti-inflammatory cytokines like IL-10 [225]. This shift in cytokine balance is crucial for mitigating the excessive inflammation that often accompanies sepsis, which can lead to organ dysfunction and increased mortality [226]. Cui et al. demonstrated that VDR-deficient microglia/macrophages exhibited a hyperinflammatory phenotype, marked by excessive secretion of TNF-α and IFN-γ, which further amplified endothelial CXCL10 production and T-cell infiltration, exacerbating brain injury. Conversely, VDR activation restrained these cytokines, preserving blood–brain barrier integrity and mitigating inflammatory damage [227].
1,25(OH)2D3 directly activates transcription of the IL-1β gene in human macrophages by promoting VDR binding to its promoter VDRE, thereby enhancing IL-1β production—a pivotal early cytokine in pathogen defense. This effect is amplified by NLRP3 inflammasome activation during Mtb infection. In a macrophage–epithelial co-culture model, 1,25D-driven IL-1β secretion reduced bacterial burden and improved macrophage survival via paracrine induction of epithelial AMPs. Additionally, 1,25D broadly amplifies cytokine/chemokine responses, including CCL3, CCL4, CCL8, and IL-8/CXCL8, highlighting its role in orchestrating innate immunity against infections [208]. In human peripheral blood mononuclear cells infected by Mtb, treatment with 1,25(OH)2D3 results in a dose-dependent inhibition of the pro-inflammatory cytokines IL-6, TNF-α, and IFN-γ [228]. Zhang et al. found that 1,25(OH)2D3 and 25(OH)2D3 dose-dependently inhibit LPS-induced p38 phosphorylation in human monocytes. This reduces IL-6 and TNF-α production via MKP-1 upregulation of pro-inflammatory cytokine production by targeting mitogen [135].
Through binding to the VDR, 1,25(OH)2D3 regulates innate immune responses of PRR signaling by activating transcription of the genes [229]. Interestingly, VD differentially regulates human innate cytokine responses to bacterial versus viral PRR stimuli. 1,25(OH)2D3 was found to inhibit inflammatory innate cytokine responses stimulated by bacterial PRR ligands but not by viral PRR ligands or infectious respiratory syncytial virus. This indicates a complex role of VD in immune modulation, particularly in bacterial infections [230].
The TLRs are the most prominent family of receptors triggering anti-inflammatory activity. TLR4 is a critical PRR that recognizes LPS from Gram-negative bacteria, triggering an inflammatory response. 1,25(OH)2D3 can negatively regulate the TLR4/myeloid differentiation primary response gene 88 (MyD88)/Toll-IL-1 resistance-domain-containing adapter-inducing interferon-β (TRIF) signaling pathway, thereby reducing the production of pro-inflammatory cytokines such as IL-6, IL-10, and TNF-α in monocytes/macrophages [231]. VD has been shown to reduce the expression of various TLRs, specifically TLR2, TLR4, and TLR9. Additionally, it inhibits the production of several pro-inflammatory cytokines in monocytes and macrophages, including IL-6, IL-23, TNF-α, inducible nitric oxide synthase (iNOS), IL-1, and various chemokines that recruit T cells [232]. Research has shown that VD can modulate cytokine production by enhancing the synergistic effect provided by NOD2 and TLR co-activation. This modulation results in increased levels of IL-10 and IL-23, while inhibiting IL-12p70 production [233]. VD/VDR signaling enhances innate immunity by directly upregulating the PRR NOD2/CARD15 in monocytes and epithelial cells. This induction facilitates synergistic activation of NF-κB upon stimulation by muramyl dipeptide (MDP), amplifying the expression of AMPs (cathelicidin antimicrobial peptide [CAMP] and DEFB4). Notably, this synergy is absent in macrophages from Crohn’s disease patients harboring non-functional NOD2 variants, underscoring the critical role of VD in bridging NOD2-mediated immune responses and inflammation regulation. These findings highlight VD’s dual role in modulating inflammatory pathways and antimicrobial defenses via VDR-dependent mechanisms [234].
VDR suppresses NF-ĸB-driven inflammatory responses in the gut through direct interactions with NF-ĸB and its upstream kinase-inhibitory kappa B kinase beta (IKKβ)IKKβ, thereby mitigating intestinal inflammation [235]. In macrophages, VD-activated VDR binds to the NF-κB p50 subunit, inhibiting its colocalization with Kruppel-like factor 5 and thereby suppressing LPS-induced cellular proliferation [236]. Studies have shown that the VDR negatively regulates bacteria-stimulated NF-κB activity by interacting with NF-κB p65, such as reducing its phosphorylation and nuclear translocation [237,238]. Interaction of VDR with NF-ĸB p65 impedes its migration into the nucleus, thereby dampening inflammatory signaling pathways. VD treatment can prevent LPS-induced placental inflammation by promoting dimerization of VDR with NF-ĸB p65 [239]. Artesunate interacts with VDR and disrupts its association with NF-κB p65 in LPS-tolerant macrophages, facilitating NF-κB p65 nuclear translocation. This activated the transcription of NF-κB p65 target genes such as pro-inflammatory cytokine-related genes [218].
3.4.4. Modulation of VD/VDR on microRNA
The VD/VDR also participates in the regulation of the immune system through the modulation of microRNAs, which is closely associated with systemic or local inflammation. Karkein et al. reported that 1,25(OH)2D3 regulates the NF-κB signaling pathway by influencing miR-146a, miR-150, and miR-155 in adipocytes, thereby limiting inflammation [240]. Another study indicated that 1,25(OH)2D3 modulates the expression of inflammation-related miR-146a-5p and miR-155-5p during dengue virus type 2 infection in MDMs, thus affecting the inflammatory response [241]. The in vivo and in vitro studies also suggest that VD downregulates miR-155, thereby influencing the NF-κB signaling pathway and reducing the p65 subunit [240,242]. Interestingly, research has found that inflammation conversely downregulates VDR expression in epithelial cells by inducing miR-346 targeting VDR, thereby impairing mucosal barrier function [243]. Other studies suggest that VD/VDR regulates the immune system through microRNAs to assist in the treatment of sepsis. Shang et al. discovered that inhibiting miR-874-5p expression increases VDR expression, reduces NLRP3 expression, decreases caspase-1 activation and IL-1β secretion, and alleviates pyroptosis and inflammatory responses, thereby protecting from intestinal barrier damage in sepsis—all of which can be reversed by downregulating VDR [244]. Sepsis-induced acute lung injury (ALI) endangers patients’ lives, and controlling ALI helps improve the survival rate of sepsis patients. Ahmad et al. found that vitamin D-related microRNAs regulate the endoplasmic reticulum (ER) stress pathway in ALI to improve clinical outcomes. In a mouse model of sepsis combined with ALI, researchers found that VD improves the lung tissue structure, neutrophil infiltration, endothelial barrier, and reduces the upregulation of ER stress markers activating transcription factor 6 (ATF6) and C/EBP homologous protein induced by CLP and LPS. VD enhances the expression of miR-149-5p, which inhibits the ER-specific ATF6 inflammatory pathway in LPS-stimulated macrophages and improves pulmonary edema in mice [245]. These pieces of evidence indicate that the mechanism by which VD/VDR regulates the inflammatory level in sepsis involves its influence on microRNAs.
3.5. Oxidative Stress Regulation
Oxidative stress, characterized by excessive reactive oxygen species (ROS) and lipid peroxidation, plays a pivotal role in sepsis pathogenesis, driving tissue damage, mitochondrial dysfunction, and dysregulated immune responses. VD and VDR modulate oxidative stress through multiple mechanisms, particularly by targeting ferroptosis, and key redox signaling pathways. In a study on cardiomyocytes, Chen et al. demonstrated that organic oxidants, such as tert-butyl hydroperoxide, induce ferroptosis via glutathione depletion, glutathione peroxidase 4 (GPX4) degradation, and mitochondrial iron overload through the Bach1/heme oxygenase 1 (HO-1) pathway. While hydrogen peroxide (H2O2) induced milder oxidative stress, organic oxidants promoted robust lipid peroxidation and iron accumulation, illustrating the differential impacts of oxidative species on cell death mechanisms. This highlights the critical role of VD in mitigating ferroptosis: VD activates antioxidant pathways that scavenge ROS and stabilize mitochondrial function, countering excessive lipid peroxidation and iron-dependent cytotoxicity [246].
Cai et al. investigated neonatal hypoxic–ischemic encephalopathy and found that VD suppresses ferroptosis by activating the Nrf2/HO-1 pathway. VD treatment increased glutathione (GSH) levels, reduced the levels of malondialdehyde (MDA) and ROS, and upregulated GPX4, a key enzyme in lipid peroxide reduction [247]. Similarly, Li et al. showed that in aging mice, VD alleviates ferroptosis in hippocampal neurons by enhancing nuclear factor erythroid 2–related factor 2 (Nrf2) signaling, reducing the levels of intracellular iron and ROS, and improving mitochondrial morphology [248]. These findings suggest VD-mediated Nrf2 activation as a universal mechanism to counteract oxidative stress-induced lipid peroxidation and iron overload, which are critical in sepsis-associated organ injury. Hu et al. explored VDR’s role in cisplatin-induced acute kidney injury (AKI), revealing that VDR activation upregulates GPX4 transcription, inhibits lipid peroxidation (measured by 4HNE and MDA), and attenuates ferroptosis. This mechanism is particularly relevant to sepsis-induced AKI, where oxidative stress and renal tubular cell death contribute to morbidity. The study identified GPX4 as a direct target of VDR, linking VDR signaling to the regulation of ferroptotic pathways in renal protection [249]. In neuroinflammatory contexts, Ribeiro et al. showed that VD supplementation rescues aberrant NF-κB pathway activation in Mecp2 mutant mice. NF-κB, a key regulator of inflammation and oxidative stress, is hyperactivated in sepsis, driving pro-inflammatory cytokine production. VD-mediated inhibition of NF-κB reduces pro-inflammatory gene expression, potentially dampening the excessive immune response and oxidative tissue damage seen in severe sepsis [250].
3.6. Regulation of Gut Microbiota
Emerging evidence highlights a bidirectional regulatory interplay between VD/VDR signaling and gut microbiome dynamics, particularly in inflammatory bowel disease (IBD). Clinical and preclinical studies demonstrate that the VD status modulates the microbial composition, while supplementation enhances diversity via VDR-mediated antimicrobial and immune pathways. Ismailova et al. observed a higher prevalence of VD deficiency in patients with Crohn’s disease. Interestingly, Crohn’s disease patients with higher VD levels exhibited lower risks of disease relapse [251]. In addition, both British and American guidelines suggest that for IBD patients, VD levels should be checked in a timely manner. If VD deficiency occurs, VD supplementation should be carried out promptly [252,253]. A clear association exists between VD and IBD, particularly Crohn’s disease, which demonstrate significant connections with gut microbiome alterations.
Wang et al. found that human VDR gene variations significantly affect the composition of the gut microbiota. The study enrolled 1812 individuals from Germany and, after comprehensively controlling for dietary and non-genetic parameters, identified significant genome-wide associations between overall microbial variation/individual taxa and multiple genetic loci (including the VDR gene). Integrated with preclinical studies, they found that both non-genetic and genetic factors each accounted for approximately 10% of gut microbiota variation [254]. Luthold et al. reported higher Prevotella but lower Haemophilus and Veillonella levels in fecal samples from individuals with a higher VD intake [255]. A systematic meta-analysis encompassing 25 clinical trials demonstrated that an elevated VD intake enhances gut microbiota diversity, inducing compositional shifts marked by altered alpha/beta diversity and increased microbial species richness [256]. Fabisiak et al. demonstrated that colonic mucosal expression of VDR and the antimicrobial peptide CAMP (LL-37) was significantly elevated in ulcerative colitis patients compared to healthy controls. VD maintains intestinal barrier integrity, regulates autophagy and apoptosis, and modulates gut microbiota composition through VDR signaling. VD deficiency is associated with exacerbated inflammation and microbial dysbiosis in IBD patients, while VD supplementation may improve gut microbial homeostasis by regulating VDR and antimicrobial peptide expression [257]. Kanhere et al. point out that weekly high-dose VD3 supplementation affects the intestinal and respiratory microbiota of patients with cystic fibrosis [258]. Interestingly, one study has also found that the gut microbiota is related to the absorption or metabolism of VD [259]. Aggeletopoulou et al. demonstrated in animal studies that VD modifies the gut microbial composition. VD deficiency or VDR knockout alters microbial flora. VDR knockout mice showed increased Bacteroides levels, while dietary restriction and CYP27B1 deficiency mice showed increased Firmicutes and Proteobacteria levels [260]. These pieces of evidence suggest that there may be a complex relationship between VD and gut microbiota in the human body.
3.7. Regulation of Endothelial Cells and Antithrombotic Activity
Sepsis involves endothelial dysfunction, where VD and VDR play crucial roles in modulating endothelial cell (EC) functions critical for immune balance and vascular integrity. Amersfoot et al. point out that some ECs serve as “immunomodulatory ECs (IMECs)”, which act as an early defender in sepsis. Those IMECs express VDR and regulate immune cell recruitment via adhesion molecules (e.g., vascular cell adhesion protein 1, intercellular adhesion molecule 1) and cytokines [261]. 1,25(OH)2D3 activates VDR in ECs to suppress pro-inflammatory pathways like NF-κB, reducing cytokines (IL-6, TNF-α) and improving endothelial nitric oxide synthase (eNOS) activity, which mitigates sepsis-induced vascular leakage and oxidative stress [262]. Liu et al. demonstrated that VD reduces apoptosis, promotes migration, and enhances the viability of ECs, while decreasing TNF-α-induced protein 8-like 1 (TIPE1) expression under high-glucose conditions. Their findings suggest that VD counteracts the detrimental effects of high glucose on ECs by suppressing TIPE1 expression, further indicating that VD supplementation may mitigate microvascular damage [263]. Song et al.’s knockout of the VDR of mice’s retina ECs exhibited impaired retinal vascular regeneration, increased inflammatory responses, and compromised EC regeneration compared to wild-type controls (VDR+/+). It showed that maintaining the physiological functionality of VDR in ECs is essential for preserving normal endothelial cell function [264]. Some evidence suggests that VD/VDR could benefit endothelial progenitor cells (EPCs). In vitro studies and animal models indicated that activation of VDR signaling enhances EPC functionality, and it may improve EC activity and stimulate angiogenic processes [265]. VD/VDR regulates EC adhesion, inflammatory responses, and repair mechanisms, potentially mitigating sepsis-induced endothelial dysfunction and immune dysregulation.
VD/VDR primarily exerts antithrombotic effects through antiplatelet and anticoagulant mechanisms [266]. Research on antiplatelet activity, concerning VD/VDR in sepsis, is limited but highlights key interactions. Cox proposed that platelets play a dual role in infectious diseases, exhibiting both protective and pathogenic effects. In sepsis, platelets release antimicrobial peptides (e.g., platelet factor 4 [PF4]), enhance monocyte bactericidal activity, and form microthrombi to limit pathogen dissemination. However, excessive platelet activation may contribute to DIC and organ failure. Some studies suggest that antiplatelet therapy may improve clinical outcomes [267]. A clinical study involving 503 patients requiring dual antiplatelet therapy (DAPT) demonstrated an association between VD and high residual platelet reactivity (HRPR). Specifically, the study revealed that lower VD levels were significantly correlated with increased adenosine diphosphate (ADP)-mediated platelet reactivity (p < 0.05). Researchers suggest that VD may potentiate the therapeutic efficacy of antiplatelet medications [268]. The United Kingdom’s clinical guidelines indicate that VD exerts antithrombotic effects through three primary mechanisms. Notably, 50% of thrombotic antiphospholipid syndrome (APS) patients were found to have VD deficiency. Importantly, APS patients with confirmed VD deficiency demonstrated a significantly higher incidence of thrombosis compared to those without deficiency (77% vs. 53%, respectively) [269]. VD/VDR is also associated with anticoagulation. Çakır et al. found in a study involving 201 patients with atrial fibrillation that low 25(OH)D3 levels were correlated with the occurrence of left atrial thrombus in patients taking non-vitamin K antagonist oral anticoagulants. They suggested that VD supplementation or deficiency might influence the efficacy of anticoagulant drugs, warranting further investigation [270]. Khansari et al. reported similar findings, indicating that VD levels affect the therapeutic efficacy of warfarin [271]. An animal study demonstrated that VD supplementation was associated with downregulation of TNF-α-induced TF expression and NF-κB signaling, significant attenuation of PAR-2 expression, restoration of VDR levels, and enhanced TF pathway inhibitor (TFPI) expression. TFPI is an anticoagulant protein that functions as a dual coagulation inhibitor by binding to TF/factor VIIa and factor Xa [272,273].
4. Critical Knowledge Gaps and Future Perspectives
4.1. Limitations and Gaps in Current Research
Current evidence on the association between VD deficiency and sepsis susceptibility remains inconclusive, plagued by heterogeneous study designs, divergent diagnostic thresholds for VD insufficiency, and inconsistent patient populations. Observational studies report correlations between hypovitaminosis D and an increased sepsis incidence or mortality, yet RCTs evaluating VD supplementation in critically ill patients have yielded conflicting results, failing to establish a consensus on its therapeutic efficacy. For example, while preclinical models demonstrate that 1,25(OH)2D3 enhances AMP production and modulates cytokine profiles in immune cells, these findings are not consistently replicated in human sepsis trials. This translational gap likely stems from multifactorial challenges, including the complexity of sepsis pathophysiology, interindividual variability in VD metabolism, and suboptimal timing or dosing of supplementation in clinical settings.
Moreover, methodological inconsistencies further confound interpretation. Serum 25(OH)2D3—the widely accepted biomarker of VD status—exhibits limitations in critically ill populations. Hemodilution from fluid resuscitation can artificially lower 25(OH)D levels by up to 35%, while immune cell-specific activation of VD may render serum measurements inadequate for assessing tissue-level bioactivity [274]. Numerous studies have demonstrated significant variations in VD content across different tissues, as well as substantial differences among various VD forms. Lipkie et al. reported marked differences in 25(OH)D3 concentrations across rat soft tissues, adipose tissue, and liver [275]. Similarly, Fu et al. found distinct concentrations of 25(OH)D3 and 1,25(OH)2D3 in human brain tissue compared to other body tissues [276]. Máčová et al. highlighted several challenges in current VD testing methodologies, including sample type selection and the choice of immunoassays versus chromatography or mass spectrometry. Notably, there is no globally standardized VD measurement protocol. The researchers emphasized the need for improved antibody specificity and standardized mass spectrometry procedures [277]. Nevertheless, the global management of VD shows significant variations. Across different regions, local guidelines demonstrate substantial disparities in the defined concentration thresholds for VD deficiency, insufficiency, sufficiency, and toxicity (Table 3) [278,279]. Additionally, our understanding of VD toxicity and side effects remains limited. For patients with hepatic or renal impairment (the two major organs involved in VD metabolism), sepsis, and other critically ill patients, the definitions of VD standard are poorly established. The potential side effects of VD supplementation in these populations are also unclear [277,278]. There are no widely accepted standards regarding the timing and dosage of VD supplementation for these patient groups.

Table 3.
Vitamin D guidelines in different regions.
Animal models, though instrumental in elucidating mechanistic pathways (e.g., VDR-mediated transcriptional regulation of IL-1β or NLRP3 inflammasome modulation), poorly replicate the immunometabolic dysregulation of human sepsis. These discrepancies underscore the need for standardized protocols to define VD sufficiency in sepsis, stratified RCTs targeting deficient subpopulations, and advanced models that bridge the gap between preclinical promise and clinical reality.
4.2. Future Directions
Future studies can prioritize elucidating the physiological role of VD/VDR in sepsis, particularly in resolving controversies surrounding its immunomodulatory effects. This requires systematic investigations into its dynamic states and functions across different sepsis phases, especially sepsis-induced immunosuppression. Our research team has made some strides in understanding VDR’s role during sepsis-associated immunosuppression. Our findings highlight VDR as a bidirectional immunoregulatory checkpoint in the sepsis pathogenesis and suggest that pharmacological modulation of the VDR–autophagy–NF-κB axis could represent a promising therapeutic strategy [218]. Our recent work uncovered a previously unrecognized form of VDR-membrane-bound VDR (mVDR) on macrophages, which plays a pivotal role in LPS tolerance formation [116]. Building on these findings, future studies may benefit from exploring the temporal dynamics of VDR activation in human sepsis, clarifying its stage-dependent functions, and investigating potential therapeutic synergies through integration of VD/VDR modulation with established immunoadjuvant strategies.
Emerging evidence underscores the need for population-specific VD thresholds. Genetic polymorphisms in VDR (e.g., FokI and BsmI variants) and ethnic disparities in VD metabolism necessitate tailored approaches [77,80]. For instance, populations in high-latitude regions with limited sunlight exposure, such as Finland, exhibit higher incidences of autoimmune diseases like type 1 diabetes—conditions potentially modifiable by VD optimization [280,281,282]. Similarly, infants, elderly patients, and those with renal/hepatic impairment require distinct supplementation protocols to mitigate toxicity risks while maximizing immune benefits [28,44,45,46]. Future guidelines can integrate genetic, geographic, and comorbidity factors to define precision thresholds for deficiency and sufficiency.
To bridge this gap, stratified clinical trials targeting VD-deficient subpopulations, such as critically ill adults and children, are essential. The ongoing VITdALIZE and VITdALIZE-KIDS trials exemplify this approach. The VITdALIZE study (NCT03188796) is an international (Austria, Germany, Belgium, Switzerland, and UK), multicenter (more than 30 sites), placebo-controlled, double-blind, phase 3 randomized trial, which aims to evaluate the outcomes (such as 28-day morality) of VD supplementation on the prognosis of critically ill adult patients with VD deficiency [283]. Subsequently, in September 2024, VITdALIZE-KIDS (NCT03742505) was designed to assess the effect of VD supplementation on outcomes in children (>37 weeks, <18 years), using a study method similar to the VITDALIZE study [284]. These two studies may provide insights into the clinical response to VD supplementation in different critically ill patients, and both have infection-related end points that warrant continued attention.
VD analogs have been identified in over 3000 species. The majority of these analogs have not yet progressed to clinical trials or received regulatory approval for use as drugs or dietary supplements [285]. Beyond classical roles in bone health, VD analogs show promise in modulating immune dysregulation. Tacalcitol, a synthetic VD analog, has demonstrated efficacy in autoimmune diseases (psoriasis and vitiligo) without disrupting calcium and phosphorus homeostasis [286,287]. The pharmacological actions of Tacalcitol primarily involve the inhibition of DNA synthesis and the suppression of proliferation in human epidermal cells derived from psoriasis lesions [286,288]. Tacalcitol also influences the immune system, particularly in acute lymphoblastic leukemia (ALL) B cells, such as those expressing CD27, CD24, CD38, and CD23 [289]. Similarly, Oxcalcitriol, approved for secondary hyperparathyroidism, has shown potential in autoimmune diseases like rheumatoid arthritis (RA), reducing joint inflammation with fewer hypercalcemic side effects compared to calcitriol [290,291]. Oxcalcitriol also exhibits renoprotective effects in both diabetic nephropathy and acute kidney injury by attenuating inflammatory mediators (IL-16, TLR-4, IFN-γ), reducing apoptosis/fibrosis, and enhancing autophagy to promote cell survival [292,293]. Preclinical studies of Maxacalcitol reveal its ability to attenuate psoriasiform inflammation by upregulating regulatory T cells and downregulating IL-23/IL-17 pathways, outperforming corticosteroids in reducing MHC Class II-driven immune infiltration [294]. These findings suggest VD analogs could be repurposed for sepsis-associated hyperinflammation, particularly given their pleiotropic effects on autophagy, apoptosis, and TLR4 signaling—key pathways dysregulated in septic organ failure.
Research investigating VD/VDR–microRNA interactions in sepsis warrants significant expansion. Current investigations predominantly focus on localized complications such as intestinal barrier dysfunction and acute lung injury [244,245], and a definitive empirical delineation of their holistic impact on sepsis pathophysiology concurrently lacks clinical validation. Future trials can also incorporate tissue-specific biomarkers (e.g., LL-37 levels, VDR activity) and advanced technologies (single-cell sequencing, organoid models) to refine VD’s therapeutic window. Combining dynamic serum profiling with tissue biopsies or immune cell assays may better predict clinical responses. Additionally, leveraging AI-driven pharmacokinetic models could optimize dosing schedules for high-risk populations, ensuring efficacy while minimizing adverse events.
5. Conclusions
VD/VDR signaling represents a promising yet underexplored avenue for sepsis management. VD/VDR may serve as a promising bidirectional immunomodulator, capable of addressing sepsis’s hyperinflammatory and immunosuppressive phases. Achieving clinical translation requires bridging mechanistic insights with precision medicine approaches, ensuring that VD-based therapies are timed, dosed, and targeted to align with sepsis’s dynamic pathophysiology. By addressing current knowledge gaps and leveraging novel analogs and technologies, VD/VDR modulation could evolve into a cornerstone of personalized sepsis care, offering dual benefits as an immune enhancer and anti-inflammatory agent across all disease stages.
Author Contributions
Conceptualization, S.S. and H.Z.; data curation, S.S., D.C., Y.W., S.Z., and Q.C.; writing—original draft, S.S. and D.C.; writing—review and editing, H.Z. and A.Y.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (82304576); China Postdoctoral Science Foundation (2022M713859); Nature Science Foundation of Hubei Province (2022CFB879); Knowledge Innovation Project of Wuhan City (2022020801020521); and Open Foundation of Hubei Key Laboratory of Central Nervous System Tumor and Intervention (ZZYKF202304).
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
| VD | vitamin D |
| VDR | vitamin D receptor |
| MODS | multiple organ dysfunction syndrome |
| ICUs | intensive care units |
| 1,25(OH)2D3 | 1α,25-dihydroxyvitamin D3 |
| VDD | vitamin D deficiency |
| PICUs | pediatric intensive care units |
| CRP | c-reactive protein |
| APACHE II | acute physiology and chronic health evaluation II |
| SOFA | sequential organ failure assessment |
| EONS | early-onset neonatal sepsis |
| RCT | randomized controlled trial |
| SNPs | single nucleotide polymorphisms |
| LPS | lipopolysaccharides (endotoxins) |
| PAMPs | pathogen-associated molecular patterns |
| DAMPs | damage-associated molecular patterns |
| PRRs | pattern recognition receptors |
| TLRs | toll-like receptors |
| NOD | nucleotide-binding oligomerization domain |
| RIG-I | retinoic acid-inducible gene 1 |
| TNF-α | tumor necrosis factor-α |
| IL-1 | interleukin-1 |
| MDSCs | myeloid-derived suppressor cells |
| MHC-II | major histocompatibility complex class II |
| PD-1 | programmed cell death protein 1 |
| DIC | disseminated intravascular coagulation |
| TF | tissue factor |
| FX | coagulation factors |
| GSDMD | gasdermin D |
| ROS | reactive oxygen species |
| ATP | adenosine triphosphate |
| DBP | vitamin D binding protein |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| RXR | retinoid X receptor |
| VDREs | vitamin D response elements |
| eRNAs | enhancer RNAs |
| PDIA3 | protein disulfide isomerase family A member 3 |
| DC(s) | dendritic cell(s) |
| IFN-g | interferon-gamma |
| APC | antigen-presenting cell |
| NO | nitric oxide |
| Th1: | T helper 1 cell |
| CYP27B1 | 25-hydroxyvitamin D3 1-alpha-hydroxylase (enzyme) |
| TCR | T cell receptor |
| PLC-γ1 | phospholipase C gamma 1 |
| iNKT | invariant natural killer T cell |
| IFN-γ | interferon gamma |
| Treg | regulatory T cell |
| Foxp3 | forkhead box P3 |
| AMP | antimicrobial peptide |
| hCAP-18 | human cationic antimicrobial peptide 18 |
| DEFB | defensin beta |
| LL-37 | cathelicidin antimicrobial peptide (Active form of hCAP-18) |
| Mtb | mycobacterium tuberculosis |
| ZIKV | zika virus |
| ATG16L1 | autophagy related 16 like 1 |
| LC3 | microtubule associated protein 1 light chain 3 (Autophagy marker) |
| MyD88 | myeloid differentiation primary response 88 |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| IKKβ | inhibitor of nuclear factor kappa B kinase subunit beta |
| GSH | glutathione |
| MDA | malondialdehyde |
| GPX4 | glutathione peroxidase 4 |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| HO-1 | heme oxygenase 1 |
| IBD | inflammatory bowel disease |
| CAMP | cathelicidin antimicrobial peptide |
| DEFB4 | defensin beta 4 |
| IMECs | immunomodulatory endothelial cells |
| eNOS | endothelial nitric oxide synthase |
| TIPE1 | TNF-α-induced protein 8-like 1 |
| EPCs | endothelial progenitor cells |
| PF4 | platelet factor 4 |
| DAPT | dual antiplatelet therapy |
| ADP | adenosine diphosphate |
| APS | antiphospholipid syndrome |
| HRPR | high residual platelet reactivity |
| WoSCC | Web of Science Core Collection |
| TF | tissue factor |
| TFPI | tissue factor pathway inhibitor |
| MDMs | monocyte-derived macrophages |
| ER | endoplasmic reticulum |
| ALI | acute lung injury |
| ATF6 | activating transcription factor 6 |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; International Forum of Acute Care Trialists Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Cribbs, S.K.; Martin, G.S. Going Global with Sepsis: The Need for National Registries. Crit. Care Med. 2009, 37, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and Mortality of Hospital- and ICU-Treated Sepsis: Results from an Updated and Expanded Systematic Review and Meta-Analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef]
- Renkema, K.Y.; Alexander, R.T.; Bindels, R.J.; Hoenderop, J.G. Calcium and Phosphate Homeostasis: Concerted Interplay of New Regulators. Ann. Med. 2008, 40, 82–91. [Google Scholar] [CrossRef]
- Veldurthy, V.; Wei, R.; Oz, L.; Dhawan, P.; Jeon, Y.H.; Christakos, S. Vitamin D, Calcium Homeostasis and Aging. Bone Res. 2016, 4, 16041. [Google Scholar] [CrossRef]
- Müller, V.; de Boer, R.J.; Bonhoeffer, S.; Szathmáry, E. An Evolutionary Perspective on the Systems of Adaptive Immunity. Biol. Rev. Camb. Philos. Soc. 2018, 93, 505–528. [Google Scholar] [CrossRef]
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Vitamin D Endocrinology on the Cross-Road between Immunity and Metabolism. Mol. Cell. Endocrinol. 2017, 453, 52–67. [Google Scholar] [CrossRef]
- Cortes, M.; Chen, M.J.; Stachura, D.L.; Liu, S.Y.; Kwan, W.; Wright, F.; Vo, L.T.; Theodore, L.N.; Esain, V.; Frost, I.M.; et al. Developmental Vitamin D Availability Impacts Hematopoietic Stem Cell Production. Cell Rep. 2016, 17, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Al-Tarrah, K.; Hewison, M.; Moiemen, N.; Lord, J.M. Vitamin D Status and Its Influence on Outcomes Following Major Burn Injury and Critical Illness. Burns Trauma 2018, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A. A Potential Role of Vitamin D on Platelet Leukocyte Aggregation and Pathological Events in Sepsis: An Updated Review. J. Inflamm. Res. 2021, 14, 3651–3664. [Google Scholar] [CrossRef]
- Alhassan Mohammed, H.; Mirshafiey, A.; Vahedi, H.; Hemmasi, G.; Moussavi Nasl Khameneh, A.; Parastouei, K.; Saboor-Yaraghi, A.A. Immunoregulation of Inflammatory and Inhibitory Cytokines by Vitamin D 3 in Patients with Inflammatory Bowel Diseases. Scand. J. Immunol. 2017, 85, 386–394. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the Immune System. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Belsky, J.B.; Wira, C.R.; Jacob, V.; Sather, J.E.; Lee, P.J. A Review of Micronutrients in Sepsis: The Role of Thiamine, l-Carnitine, Vitamin C, Selenium and Vitamin D. Nutr. Res. Rev. 2018, 31, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D Regulation of Immune Function. Curr. Osteoporos. Rep. 2022, 20, 186–193. [Google Scholar] [CrossRef]
- Briassoulis, G.; Ilia, S. Vitamin D Deficiency in Sepsis: “Body Humors” Imbalance or Sepsis “Epiphenomenon”? Crit. Care Med. 2017, 45, 376–377. [Google Scholar] [CrossRef]
- Cutuli, S.L.; Ferrando, E.S.; Cammarota, F.; Franchini, E.; Caroli, A.; Lombardi, G.; Tanzarella, E.S.; Grieco, D.L.; Antonelli, M.; De Pascale, G. Update on Vitamin D Role in Severe Infections and Sepsis. J. Anesth. Analg. Crit. Care 2024, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Gois, P.; Ferreira, D.; Olenski, S.; Seguro, A. Vitamin D and Infectious Diseases: Simple Bystander or Contributing Factor? Nutrients 2017, 9, 651. [Google Scholar] [CrossRef]
- Kempker, J.; Martin, G. Vitamin D and Sepsis: From Associations to Causal Connections. Inflamm. Allergy-Drug Targets 2013, 12, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Rippel, C.; South, M.; Butt, W.W.; Shekerdemian, L.S. Vitamin D Status in Critically Ill Children. Intensive Care Med. 2012, 38, 2055–2062. [Google Scholar] [CrossRef]
- Looker, A.C.; Johnson, C.L.; Lacher, D.A.; Pfeiffer, C.M.; Schleicher, R.L.; Sempos, C.T. Vitamin D Status: United States, 2001–2006; NCHS Data Brief No. 59; National Center for Health Statistics: Hyattsville, MD, USA, 2011; pp. 1–8.
- Braun, A.B.; Gibbons, F.K.; Litonjua, A.A.; Giovannucci, E.; Christopher, K.B. Low Serum 25-Hydroxyvitamin D at Critical Care Initiation Is Associated with Increased Mortality. Crit. Care Med. 2012, 40, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Chang, D.; Mahadevappa, K.; Gibbons, F.K.; Liu, Y.; Giovannucci, E.; Christopher, K.B. Association of Low Serum 25-Hydroxyvitamin D Levels and Mortality in the Critically Ill. Crit. Care Med. 2011, 39, 671–677. [Google Scholar] [CrossRef]
- Lee, P.; Eisman, J.A.; Center, J.R. Vitamin D Deficiency in Critically Ill Patients. N. Engl. J. Med. 2009, 360, 1912–1914. [Google Scholar] [CrossRef] [PubMed]
- Venkatram, S.; Chilimuri, S.; Adrish, M.; Salako, A.; Patel, M.; Diaz-Fuentes, G. Vitamin D Deficiency Is Associated with Mortality in the Medical Intensive Care Unit. Crit. Care 2011, 15, R292. [Google Scholar] [CrossRef]
- Yu, W.; Ying, Q.; Zhu, W.; Huang, L.; Hou, Q. Vitamin D Status Was Associated with Sepsis in Critically Ill Children: A PRISMA Compliant Systematic Review and Meta-Analysis. Medicine 2021, 100, e23827. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.; Chen, M.; Chai, Y.; Guan, L.; Chen, Y. Association of Vitamin D Receptor Gene Polymorphism with the Risk of Sepsis: A Systematic Review and Meta-Analysis. Medicine 2023, 102, e35130. [Google Scholar] [CrossRef]
- Seok, H.; Kim, J.; Choi, W.S.; Park, D.W. Effects of Vitamin D Deficiency on Sepsis. Nutrients 2023, 15, 4309. [Google Scholar] [CrossRef]
- Ghosh, A.; Prabhu M, M.; Prabhu, K.; Changani, M.; Patel, N.; Belle, V.S. Vitamin D as a Biomarker in Predicting Sepsis Outcome at a Tertiary Care Hospital. Asian J. Med. Sci. 2023, 14, 89–94. [Google Scholar] [CrossRef]
- Kahar, L.A.; Yusrawati, Y.; Jamsari, J.; Maskoen, T.; Aribowo, K.; Sari, W.M. Vitamin D-Binding Protein and the Role of Its Gene Polymorphisms in the Mortality of Sepsis Patients. Acta Medica Acad. 2024, 52, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.; Fındıklı, H.A. Novel Biomarker for Predicting Sepsis Mortality: Vitamin D Receptor. J. Int. Med. Res. 2021, 49, 030006052110347. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, S. Serum 25-Hydroxyvitamin D and the Risk of Mortality in Adult Patients with Sepsis: A Meta-Analysis. BMC Infect. Dis. 2020, 20, 189. [Google Scholar] [CrossRef]
- Olejarova, M.; Dobisova, A.; Suchankova, M.; Tibenska, E.; Szaboova, K.; Koutun, J.; Vlnieskova, K.; Bucova, M. Vitamin D Deficiency—A Potential Risk Factor for Sepsis Development, Correlation with Inflammatory Markers, SOFA Score and Higher Early Mortality Risk in Sepsis. Bratisl. Med. J. 2019, 120, 284–290. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Vallecoccia, M.S.; Schiattarella, A.; Di Gravio, V.; Cutuli, S.L.; Bello, G.; Montini, L.; Pennisi, M.A.; Spanu, T.; Zuppi, C.; et al. Clinical and Microbiological Outcome in Septic Patients with Extremely Low 25-Hydroxyvitamin D Levels at Initiation of Critical Care. Clin. Microbiol. Infect. 2016, 22, 456.e7–456.e13. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Sanguankeo, A.; Permpalung, N. Significant Association between Vitamin D Deficiency and Sepsis: A Systematic Review and Meta-Analysis. BMC Anesthesiol. 2015, 15, 84. [Google Scholar] [CrossRef]
- Amrein, K.; Zajic, P.; Schnedl, C.; Waltensdorfer, A.; Fruhwald, S.; Holl, A.; Purkart, T.; Wünsch, G.; Valentin, T.; Grisold, A.; et al. Vitamin D Status and Its Association with Season, Hospital and Sepsis Mortality in Critical Illness. Crit. Care 2014, 18, R47. [Google Scholar] [CrossRef]
- Rech, M.A.; Hunsaker, T.; Rodriguez, J. Deficiency in 25-Hydroxyvitamin D and 30-Day Mortality in Patients with Severe Sepsis and Septic Shock. Am. J. Crit. Care 2014, 23, e72–e79. [Google Scholar] [CrossRef]
- Higgins, D.M.; Wischmeyer, P.E.; Queensland, K.M.; Sillau, S.H.; Sufit, A.J.; Heyland, D.K. Relationship of Vitamin D Deficiency to Clinical Outcomes in Critically Ill Patients. J. Parenter. Enter. Nutr. 2012, 36, 713–720. [Google Scholar] [CrossRef]
- Ginde, A.A.; Camargo, C.A.; Shapiro, N.I. Vitamin D Insufficiency and Sepsis Severity in Emergency Department Patients with Suspected Infection. Acad. Emerg. Med. 2011, 18, 551–554. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Y.; Mei, X.; Zhong, J.; Huang, F. Circulating Micronutrient Levels and Their Association with Sepsis Susceptibility and Severity: A Mendelian Randomization Study. Front. Genet. 2024, 15, 1353118. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Meng, Z.; Shi, Y.; Zheng, R.; Pan, J.; Qian, S. Causal Effects of Genetically Vitamins and Sepsis Risk: A Two-Sample Mendelian Randomization Study. BMC Infect. Dis. 2023, 23, 766. [Google Scholar] [CrossRef]
- Ozdemir, A.A.; Cag, Y. Neonatal Vitamin D Status and the Risk of Neonatal Sepsis: Vitamin D Status and Neonatal Sepsis. Pak. J. Med. Sci. 2019, 35, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Cizmeci, M.N.; Kanburoglu, M.K.; Akelma, A.Z.; Ayyildiz, A.; Kutukoglu, I.; Malli, D.D.; Tatli, M.M. Cord-Blood 25-Hydroxyvitamin D Levels and Risk of Early-Onset Neonatal Sepsis: A Case–Control Study from a Tertiary Care Center in Turkey. Eur. J. Pediatr. 2015, 174, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, M.; Cekmez, F.; Buyukkale, G.; Erener-Ercan, T.; Demir, F.; Tunc, T.; Aydın, F.N.; Aydemir, G. Lower Vitamin D Levels Are Associated with Increased Risk of Early-Onset Neonatal Sepsis in Term Infants. J. Perinatol. 2015, 35, 39–45. [Google Scholar] [CrossRef]
- Workneh Bitew, Z.; Worku, T.; Alemu, A. Effects of Vitamin D on Neonatal Sepsis: A Systematic Review and Meta-analysis. Food Sci. Nutr. 2021, 9, 375–388. [Google Scholar] [CrossRef]
- Jeengar, B.; Gothwal, S.; Meena, K.K.; Garg, V.K.; Athwani, V.; Gupta, M.L.; Meena, K.; Bairwa, G.S. Vitamin D Levels and Early Onset Sepsis in Newborns. J. Neonatol. 2021, 35, 64–69. [Google Scholar] [CrossRef]
- He, M.; Cao, T.; Wang, J.; Wang, C.; Wang, Z.; Abdelrahim, M.E.A. Vitamin D Deficiency Relation to Sepsis, Paediatric Risk of Mortality III Score, Need for Ventilation Support, Length of Hospital Stay, and Duration of Mechanical Ventilation in Critically Ill Children: A Meta-analysis. Int. J. Clin. Pract. 2021, 75, e13908. [Google Scholar] [CrossRef]
- Su, G.; Jia, D. Vitamin D in Acute and Critically Sick Children with a Subgroup of Sepsis and Mortality: A Meta-Analysis. Nutr. Cancer 2021, 73, 1118–1125. [Google Scholar] [CrossRef]
- Onwuneme, C.; Carroll, A.; Doherty, D.; Bruell, H.; Segurado, R.; Kilbane, M.; Murphy, N.; McKenna, M.J.; Molloy, E.J. Inadequate Vitamin D Levels Are Associated with Culture Positive Sepsis and Poor Outcomes in Paediatric Intensive Care. Acta Paediatr. 2015, 104, e433–e438. [Google Scholar] [CrossRef]
- Razavi Khorasani, N.; Moazzami, B.; Zahedi Tajrishi, F.; Mohammadpour, Z.; Rouhi, F.; Alizadeh-Navaei, R.; Ghadimi, R. The Association Between Low Levels of Vitamin D and Clinical Outcomes in Critically-Ill Children: A Systematic Review and Meta-Analysis. Fetal Pediatr. Pathol. 2020, 39, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Cariolou, M.; Cupp, M.A.; Evangelou, E.; Tzoulaki, I.; Berlanga-Taylor, A.J. Importance of Vitamin D in Acute and Critically Ill Children with Subgroup Analyses of Sepsis and Respiratory Tract Infections: A Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e027666. [Google Scholar] [CrossRef] [PubMed]
- Donayeva, A.; Amanzholkyzy, A.; Nurgaliyeva, R.; Gubasheva, G.; Saparbayev, S.; Ayaganov, D.; Kaldybayeva, A.; Abdelazim, I.A.; Farghali, M.M. Vitamin D and Vitamin D Receptor Polymorphism in Asian Adolescents with Primary Dysmenorrhea. BMC Womens Health 2023, 23, 414. [Google Scholar] [CrossRef] [PubMed]
- Say, B.; Uras, N.; Sahin, S.; Degirmencioglu, H.; Oguz, S.S.; Canpolat, F.E. Effects of Cord Blood Vitamin D Levels on the Risk of Neonatal Sepsis in Premature Infants. Korean J. Pediatr. 2017, 60, 248. [Google Scholar] [CrossRef][Green Version]
- Xiao, D.; Zhang, X.; Ying, J.; Zhou, Y.; Li, X.; Mu, D.; Qu, Y. Association between Vitamin D Status and Sepsis in Children: A Meta-Analysis of Observational Studies. Clin. Nutr. 2020, 39, 1735–1741. [Google Scholar] [CrossRef]
- Kim, I.; Kim, S.S.; Song, J.I.; Yoon, S.H.; Park, G.Y.; Lee, Y.-W. Association between Vitamin D Level at Birth and Respiratory Morbidities in Very-Low-Birth-Weight Infants. Korean J. Pediatr. 2019, 62, 166–172. [Google Scholar] [CrossRef]
- Arnson, Y.; Gringauz, I.; Itzhaky, D.; Amital, H. Vitamin D Deficiency Is Associated with Poor Outcomes and Increased Mortality in Severely Ill Patients. QJM 2012, 105, 633–639. [Google Scholar] [CrossRef]
- Han, J.E.; Jones, J.L.; Tangpricha, V.; Brown, M.A.; Hao, L.; Hebbar, G.; Lee, M.J.; Liu, S.; Brown, L.A.S.; Ziegler, T.R.; et al. High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. J. Clin. Transl. Endocrinol. 2016, 4, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Sourij, H.; Wagner, G.; Holl, A.; Pieber, T.R.; Smolle, K.H.; Stojakovic, T.; Schnedl, C.; Dobnig, H. Short-Term Effects of High-Dose Oral Vitamin D3 in Critically Ill Vitamin D Deficient Patients: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Crit. Care 2011, 15, R104. [Google Scholar] [CrossRef]
- Sistanizad, M.; Salarian, S.; Kouchek, M.; Shojaei, S.; Miri, M.; Masbough, F. Effect of Calcitriol Supplementation on Infectious Biomarkers in Patients with Positive Systemic Inflammatory Response: A Randomized Controlled Trial. Ann. Med. Surg. 2024, 86, 875–880. [Google Scholar] [CrossRef]
- Sarhan, N.; Abou Warda, A.E.; Sarhan, R.M.; Boshra, M.S.; Mostafa-Hedeab, G.; ALruwaili, B.F.; Ibrahim, H.S.G.; Schaalan, M.F.; Fathy, S. Evidence for the Efficacy of a High Dose of Vitamin D on the Hyperinflammation State in Moderate-to-Severe COVID-19 Patients: A Randomized Clinical Trial. Med. Kaunas Lith. 2022, 58, 1358. [Google Scholar] [CrossRef] [PubMed]
- Miri, M.; Kouchek, M.; Rahat Dahmardeh, A.; Sistanizad, M. Effect of High-Dose Vitamin D on Duration of Mechanical Ventilation in ICU Patients. Iran. J. Pharm. Res. 2019, 18, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Shichen, M.; Liang, Z.; Yu, S.; Zhao, M.; Zhang, L.; Lv, R.; Liu, Y.; Chang, P.; Liu, Z. Potential Benefits of Vitamin D for Sepsis Prophylaxis in Critical Ill Patients. Front. Nutr. 2023, 10, 1073894. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhu, Y.; Zheng, X.; Li, T.; Niu, K.; Wang, Z.; Lu, X.; Zhang, Y.; Shen, C. Vitamin D Supplementation during Intensive Care Unit Stay Is Associated with Improved Outcomes in Critically Ill Patients with Sepsis: A Cohort Study. Nutrients 2023, 15, 2924. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Abdel-Wadood, Y.A.; Thabet, R.H.; Gomaa, G.A. Pharmacological Evaluation of Vitamin D in COVID-19 and Long COVID-19: Recent Studies Confirm Clinical Validation and Highlight Metformin to Improve VDR Sensitivity and Efficacy. Inflammopharmacology 2024, 32, 249–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, F.; Tang, J.; Jia, L.; Feng, Y.; Xu, P.; Faramand, A. Association between Vitamin D Supplementation and Mortality: Systematic Review and Meta-Analysis. BMJ 2019, 366, l4673. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Ginde, A.A.; Brower, R.G.; Caterino, J.M.; Finck, L.; Banner-Goodspeed, V.M.; Grissom, C.K.; Hayden, D.; Hough, C.L.; Hyzy, R.C.; et al. Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients. N. Engl. J. Med. 2019, 381, 2529–2540. [Google Scholar] [CrossRef]
- Leaf, D.E.; Raed, A.; Donnino, M.W.; Ginde, A.A.; Waikar, S.S. Randomized Controlled Trial of Calcitriol in Severe Sepsis. Am. J. Respir. Crit. Care Med. 2014, 190, 533–541. [Google Scholar] [CrossRef]
- Amrein, K.; Schnedl, C.; Holl, A.; Riedl, R.; Christopher, K.B.; Pachler, C.; Urbanic Purkart, T.; Waltensdorfer, A.; Münch, A.; Warnkross, H.; et al. Effect of High-Dose Vitamin D3 on Hospital Length of Stay in Critically Ill Patients with Vitamin D Deficiency: The VITdAL-ICU Randomized Clinical Trial. JAMA 2014, 312, 1520–1530. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Gao, L.; Cao, Z.; Wang, Q. Effects of a Single Dose of Vitamin D in Septic Children: A Randomized, Double-Blinded, Controlled Trial. J. Int. Med. Res. 2020, 48, 300060520926890. [Google Scholar] [CrossRef]
- Hagag, A.A.; El Frargy, M.S.; Houdeeb, H.A. Therapeutic Value of Vitamin D as an Adjuvant Therapy in Neonates with Sepsis. Infect. Disord.—Drug Targets 2020, 20, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hady, H.; Yahia, S.; Megahed, A.; Mosbah, A.; Seif, B.; Nageh, E.; Bhattacharjee, I.; Aly, H. Mediators in Preterm Infants With Late-Onset Sepsis: A Randomized Controlled Trial. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 578–584. [Google Scholar] [CrossRef]
- Smolders, J.; Peelen, E.; Thewissen, M.; Menheere, P.; Tervaert, J.W.C.; Hupperts, R.; Damoiseaux, J. The Relevance of Vitamin D Receptor Gene Polymorphisms for Vitamin D Research in Multiple Sclerosis. Autoimmun. Rev. 2009, 8, 621–626. [Google Scholar] [CrossRef]
- Tourkochristou, E.; Tsounis, E.P.; Tzoupis, H.; Aggeletopoulou, I.; Tsintoni, A.; Lourida, T.; Diamantopoulou, G.; Zisimopoulos, K.; Kafentzi, T.; de Lastic, A.-L.; et al. The Influence of Single Nucleotide Polymorphisms on Vitamin D Receptor Protein Levels and Function in Chronic Liver Disease. Int. J. Mol. Sci. 2023, 24, 11404. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Lu, X.; Li, W.; Wang, Y.; Li, N.; Chen, Y.; Zhang, L.; Niu, W.; Zhang, Q. Vitamin D Receptor Is a Sepsis-Susceptibility Gene in Chinese Children. Med. Sci. Monit. 2021, 27, e932518. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ru, J.; Li, Z.; Jiang, X.; Fan, C. Lower Vitamin D Levels and VDR FokI Variants Are Associated with Susceptibility to Sepsis: A Hospital-Based Case-Control Study. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2022, 27, 188–195. [Google Scholar] [CrossRef]
- Bozgul, S.M.K.; Emecen, D.A.; Akarca, F.K.; Bozkurt, D.; Aydin, O.; Koca, D.; Can, O.; Unalp, O.V.; Atik, T. Association between Vitamin D Receptor Gene FokI Polymorphism and Mortality in Patients with Sepsis. Mol. Biol. Rep. 2023, 51, 44. [Google Scholar] [CrossRef]
- Darnifayanti, D.; Rizki, D.R.; Amirah, S.; Abdurrahman, M.F.; Akmal, M.; Abdulmadjid, S.N.; Yusuf, S.; Iqhrammullah, M. Association between Vitamin D Receptor Gene Variants and Neonatal Sepsis: A Systematic Review and Meta-Analysis. J. Infect. Public Health 2024, 17, 518–526. [Google Scholar] [CrossRef]
- Protas, V.V.; Pogossyan, G.P.; Li, K.G. Frequency of Rs731236, Rs7975232 and Rs1544410 Single Nucleotide Polymorphisms of Vitamin-D Receptor (VDR) Gene among the Kazakh Ethnic Group. Bull. Karaganda Univ. “Biol. Med. Geogr. Ser.” 2024, 11429, 57–63. [Google Scholar] [CrossRef]
- van der Poll, T.; Opal, S.M. Host-Pathogen Interactions in Sepsis. Lancet Infect. Dis. 2008, 8, 32–43. [Google Scholar] [CrossRef]
- Gyawali, B.; Ramakrishna, K.; Dhamoon, A.S. Sepsis: The Evolution in Definition, Pathophysiology, and Management. SAGE Open Med. 2019, 7, 205031211983504. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in Sepsis: A Novel Understanding of the Disorder and a New Therapeutic Approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-Induced Immunosuppression: From Cellular Dysfunctions to Immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Schmieg, R.E.; Hui, J.J.; Chang, K.C.; Osborne, D.F.; Freeman, B.D.; Cobb, J.P.; Buchman, T.G.; et al. Sepsis-Induced Apoptosis Causes Progressive Profound Depletion of B and CD4+ T Lymphocytes in Humans. J. Immunol. 2001, 166, 6952–6963. [Google Scholar] [CrossRef] [PubMed]
- Heagy, W.; Hansen, C.; Nieman, K.; Cohen, M.; Richardson, C.; Rodriguez, J.L.; West, M.A. Impaired Ex Vivo Lipopolysaccharide-Stimulated Whole Blood Tumor Necrosis Factor Production May Identify “Septic” Intensive Care Unit Patients. Shock 2000, 14, 271–276; discussion 276–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fang, X.; Gao, C.; Song, C.; He, Y.; Zhou, T.; Yang, X.; Shang, Y.; Xu, J. MDSCs in Sepsis-Induced Immunosuppression and Its Potential Therapeutic Targets. Cytokine Growth Factor Rev. 2023, 69, 90–103. [Google Scholar] [CrossRef]
- Beltrán-García, J.; Osca-Verdegal, R.; Romá-Mateo, C.; Carbonell, N.; Ferreres, J.; Rodríguez, M.; Mulet, S.; García-López, E.; Pallardó, F.V.; García-Giménez, J.L. Epigenetic Biomarkers for Human Sepsis and Septic Shock: Insights from Immunosuppression. Epigenomics 2020, 12, 617–646. [Google Scholar] [CrossRef]
- Oami, T.; Watanabe, E.; Hatano, M.; Sunahara, S.; Fujimura, L.; Sakamoto, A.; Ito, C.; Toshimori, K.; Oda, S. Suppression of T Cell Autophagy Results in Decreased Viability and Function of T Cells Through Accelerated Apoptosis in a Murine Sepsis Model. Crit. Care Med. 2017, 45, e77–e85. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, B.; Zheng, X.; Chen, L.; Chen, W.; Fang, Z. The Protective Role of Autophagy in Sepsis. Microb. Pathog. 2019, 131, 106–111. [Google Scholar] [CrossRef]
- Watanabe, E.; Nishida, O.; Kakihana, Y.; Odani, M.; Okamura, T.; Harada, T.; Oda, S. Pharmacokinetics, Pharmacodynamics, and Safety of Nivolumab in Patients With Sepsis-Induced Immunosuppression: A Multicenter, Open-Label Phase 1/2 Study. Shock 2020, 53, 686–694. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; van der Poll, T. Coagulation and Sepsis. Thromb. Res. 2017, 149, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kerris, E.W.J.; Hoptay, C.; Calderon, T.; Freishtat, R.J. Platelets and Platelet Extracellular Vesicles in Hemostasis and Sepsis. J. Investig. Med. 2020, 68, 813–820. [Google Scholar] [CrossRef]
- Alberelli, M.A.; De Candia, E. Functional Role of Protease Activated Receptors in Vascular Biology. Vascul. Pharmacol. 2014, 62, 72–81. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, X.; Tang, Y.; Qiu, X.; Wang, Y.; Kang, H.; Wu, J.; Wang, Z.; Liu, Y.; Chen, F.; et al. Bacterial Endotoxin Activates the Coagulation Cascade through Gasdermin D-Dependent Phosphatidylserine Exposure. Immunity 2019, 51, 983–996.e6. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.E.; Puskarich, M.A. Sepsis-Induced Tissue Hypoperfusion. Crit. Care Nurs. Clin. N. Am. 2011, 23, 115–125. [Google Scholar] [CrossRef]
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; Silva, D.D.d.; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; et al. Vitamin D: Sources, Physiological Role, Biokinetics, Deficiency, Therapeutic Use, Toxicity, and Overview of Analytical Methods for Detection of Vitamin D and Its Metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 59, 517–554. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- Wang, S. Epidemiology of Vitamin D in Health and Disease. Nutr. Res. Rev. 2009, 22, 188–203. [Google Scholar] [CrossRef]
- Bolodeoku, J. An Assessment of Automated Vitamin D Measurement Methods Including a Point-of-Care Testing Method, I-CHROMATM Using the Randox International Quality Assurance Scheme (Riqas). Biomed. J. Sci. Tech. Res. 2018, 3, 3457–3463. [Google Scholar] [CrossRef]
- Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Nikolac Gabaj, N.; Unic, A.; Miler, M.; Pavicic, T.; Culej, J.; Bolanca, I.; Herman Mahecic, D.; Milevoj Kopcinovic, L.; Vrtaric, A. In Sickness and in Health: Pivotal Role of Vitamin D. Biochem. Medica 2020, 30, 020501. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J. Vitamin D and the RNA Transcriptome: More than mRNA Regulation. Front. Physiol. 2014, 5, 181. [Google Scholar] [CrossRef]
- Carlberg, C.; Campbell, M.J. Vitamin D Receptor Signaling Mechanisms: Integrated Actions of a Well-Defined Transcription Factor. Steroids 2013, 78, 127–136. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D Genomics: From In Vitro to In Vivo. Front. Endocrinol. 2018, 9, 250. [Google Scholar] [CrossRef]
- Haussler, M.R.; Haussler, C.A.; Bartik, L.; Whitfield, G.K.; Hsieh, J.-C.; Slater, S.; Jurutka, P.W. Vitamin D Receptor: Molecular Signaling and Actions of Nutritional Ligands in Disease Prevention. Nutr. Rev. 2008, 66, S98–S112. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Genome-Wide (over)View on the Actions of Vitamin D. Front. Physiol. 2014, 5, 167. [Google Scholar] [CrossRef]
- Ramagopalan, S.V.; Heger, A.; Berlanga, A.J.; Maugeri, N.J.; Lincoln, M.R.; Burrell, A.; Handunnetthi, L.; Handel, A.E.; Disanto, G.; Orton, S.-M.; et al. A ChIP-Seq Defined Genome-Wide Map of Vitamin D Receptor Binding: Associations with Disease and Evolution. Genome Res. 2010, 20, 1352–1360. [Google Scholar] [CrossRef]
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. Genome-Wide Analysis of the VDR/RXR Cistrome in Osteoblast Cells Provides New Mechanistic Insight into the Actions of the Vitamin D Hormone. J. Steroid Biochem. Mol. Biol. 2010, 121, 136–141. [Google Scholar] [CrossRef]
- Carlberg, C.; Seuter, S. A Genomic Perspective on Vitamin D Signaling. Anticancer Res. 2009, 29, 3485–3493. [Google Scholar] [PubMed]
- The Function of the Vitamin D Receptor and a Possible Role of Enhancer RNA in Epigenomic Regulation of Target Genes: Implications for Bone Metabolism. PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30899718/ (accessed on 20 October 2024).
- Tuoresmäki, P.; Väisänen, S.; Neme, A.; Heikkinen, S.; Carlberg, C. Patterns of Genome-Wide VDR Locations. PLoS ONE 2014, 9, e96105. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D Receptor (VDR)-Mediated Actions of 1α,25(OH)2vitamin D3: Genomic and Non-Genomic Mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Hua, L.; Li, P.; Wu, J.; Shang, S.; Deng, F.; Luo, J.; Liao, M.; Wang, N.; et al. Vitamin D Receptor (VDR) on the Cell Membrane of Mouse Macrophages Participates in the Formation of Lipopolysaccharide Tolerance: mVDR Is Related to the Effect of Artesunate to Reverse LPS Tolerance. Cell Commun. Signal. 2023, 21, 124. [Google Scholar] [CrossRef]
- Donati, S.; Palmini, G.; Aurilia, C.; Falsetti, I.; Miglietta, F.; Iantomasi, T.; Brandi, M.L. Rapid Nontranscriptional Effects of Calcifediol and Calcitriol. Nutrients 2022, 14, 1291. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A.; Carlberg, C. Vitamin D Receptor(s): In the Nucleus but Also at Membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of Vitamin D on Immune Function: Lessons Learned from Genome-Wide Analysis. Front. Physiol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Cantorna, M.T. Mechanisms Underlying the Effect of Vitamin D on the Immune System. Proc. Nutr. Soc. 2010, 69, 286–289. [Google Scholar] [CrossRef]
- Bhalla, A.K.; Amento, E.P.; Clemens, T.L.; Holick, M.F.; Krane, S.M. Specific High-Affinity Receptors for 1,25-Dihydroxyvitamin D3 in Human Peripheral Blood Mononuclear Cells: Presence in Monocytes and Induction in T Lymphocytes Following Activation. J. Clin. Endocrinol. Metab. 1983, 57, 1308–1310. [Google Scholar] [CrossRef]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-Dihydroxyvitamin D3 Receptors in Human Leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef]
- Brennan, A.; Katz, D.R.; Nunn, J.D.; Barker, S.; Hewison, M.; Fraher, L.J.; O’Riordan, J.L. Dendritic Cells from Human Tissues Express Receptors for the Immunoregulatory Vitamin D3 Metabolite, Dihydroxycholecalciferol. Immunology 1987, 61, 457–461. [Google Scholar]
- Morgan, J.W.; Kouttab, N.; Ford, D.; Maizel, A.L. Vitamin D-Mediated Gene Regulation in Phenotypically Defined Human B Cell Subpopulations. Endocrinology 2000, 141, 3225–3234. [Google Scholar] [CrossRef]
- Kongsbak, M.; von Essen, M.R.; Levring, T.B.; Schjerling, P.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Geisler, C. Vitamin D-Binding Protein Controls T Cell Responses to Vitamin D. BMC Immunol. 2014, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.E.; Wood, A.M.; Qureshi, O.S.; Hou, T.Z.; Gardner, D.; Briggs, Z.; Kaur, S.; Raza, K.; Sansom, D.M. Availability of 25-Hydroxyvitamin D(3) to APCs Controls the Balance between Regulatory and Inflammatory T Cell Responses. J. Immunol. 2012, 189, 5155–5164. [Google Scholar] [CrossRef]
- Heine, G.; Niesner, U.; Chang, H.-D.; Steinmeyer, A.; Zügel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-Dihydroxyvitamin D(3) Promotes IL-10 Production in Human B Cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Odum, N.; Geisler, C. Vitamin D Controls T Cell Antigen Receptor Signaling and Activation of Human T Cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Esteban, L.; Vidal, M.; Dusso, A. 1alpha-Hydroxylase Transactivation by Gamma-Interferon in Murine Macrophages Requires Enhanced C/EBPbeta Expression and Activation. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 131–137. [Google Scholar] [CrossRef]
- Xu, H.; Soruri, A.; Gieseler, R.K.; Peters, J.H. 1,25-Dihydroxyvitamin D3 Exerts Opposing Effects to IL-4 on MHC Class-II Antigen Expression, Accessory Activity, and Phagocytosis of Human Monocytes. Scand. J. Immunol. 1993, 38, 535–540. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and Pathogenic Functions of Macrophage Subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Qiu, P.; Liu, Y.; Zhang, J. Review: The Role and Mechanisms of Macrophage Autophagy in Sepsis. Inflammation 2019, 42, 6–19. [Google Scholar] [CrossRef]
- Fang, M.; Zhong, C. Vitamin D Receptor Mediates Liver Ischemia and Reperfusion Injury by Autophagy-Regulated M2 Macrophage Polarization. Turk. J. Biol. Turk Biyol. Derg. 2023, 47, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.M.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D Inhibits Monocyte/Macrophage Proinflammatory Cytokine Production by Targeting MAPK Phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Krutzik, S.R.; Hewison, M.; Liu, P.T.; Robles, J.A.; Stenger, S.; Adams, J.S.; Modlin, R.L. IL-15 Links TLR2/1-Induced Macrophage Differentiation to the Vitamin D-Dependent Antimicrobial Pathway. J. Immunol. 2008, 181, 7115–7120. [Google Scholar] [CrossRef]
- Chan, H.; Li, Q.; Wang, X.; Liu, W.Y.; Hu, W.; Zeng, J.; Xie, C.; Kwong, T.N.Y.; Ho, I.H.T.; Liu, X.; et al. Vitamin D3 and Carbamazepine Protect against Clostridioides Difficile Infection in Mice by Restoring Macrophage Lysosome Acidification. Autophagy 2022, 18, 2050–2067. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D Promotes Negative Feedback Regulation of TLR Signaling via Targeting microRNA-155-SOCS1 in Macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef]
- Dong, B.; Zhou, Y.; Wang, W.; Scott, J.; Kim, K.; Sun, Z.; Guo, Q.; Lu, Y.; Gonzales, N.M.; Wu, H.; et al. Vitamin D Receptor Activation in Liver Macrophages Ameliorates Hepatic Inflammation, Steatosis, and Insulin Resistance in Mice. Hepatology 2020, 71, 1559–1574. [Google Scholar] [CrossRef]
- Wittke, A.; Chang, A.; Froicu, M.; Harandi, O.F.; Weaver, V.; August, A.; Paulson, R.F.; Cantorna, M.T. Vitamin D Receptor Expression by the Lung Micro-Environment Is Required for Maximal Induction of Lung Inflammation. Arch. Biochem. Biophys. 2007, 460, 306–313. [Google Scholar] [CrossRef]
- Griffin, M.D.; Lutz, W.; Phan, V.A.; Bachman, L.A.; McKean, D.J.; Kumar, R. Dendritic Cell Modulation by 1alpha,25 Dihydroxyvitamin D3 and Its Analogs: A Vitamin D Receptor-Dependent Pathway That Promotes a Persistent State of Immaturity in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 6800–6805. [Google Scholar] [CrossRef]
- Ferreira, G.B.; van Etten, E.; Verstuyf, A.; Waer, M.; Overbergh, L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 Alters Murine Dendritic Cell Behaviour in Vitro and in Vivo. Diabetes Metab. Res. Rev. 2011, 27, 933–941. [Google Scholar] [CrossRef]
- Penna, G.; Adorini, L. 1 Alpha,25-Dihydroxyvitamin D3 Inhibits Differentiation, Maturation, Activation, and Survival of Dendritic Cells Leading to Impaired Alloreactive T Cell Activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef]
- Piemonti, L.; Monti, P.; Sironi, M.; Fraticelli, P.; Leone, B.E.; Dal Cin, E.; Allavena, P.; Di Carlo, V. Vitamin D3 Affects Differentiation, Maturation, and Function of Human Monocyte-Derived Dendritic Cells. J. Immunol. 2000, 164, 4443–4451. [Google Scholar] [CrossRef]
- Unger, W.W.J.; Laban, S.; Kleijwegt, F.S.; van der Slik, A.R.; Roep, B.O. Induction of Treg by Monocyte-Derived DC Modulated by Vitamin D3 or Dexamethasone: Differential Role for PD-L1. Eur. J. Immunol. 2009, 39, 3147–3159. [Google Scholar] [CrossRef] [PubMed]
- van Halteren, A.G.S.; Tysma, O.M.; van Etten, E.; Mathieu, C.; Roep, B.O. 1alpha,25-Dihydroxyvitamin D3 or Analogue Treated Dendritic Cells Modulate Human Autoreactive T Cells via the Selective Induction of Apoptosis. J. Autoimmun. 2004, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, D.; Cippitelli, M.; Cocciolo, M.G.; Mazzeo, D.; Di Lucia, P.; Lang, R.; Sinigaglia, F.; Panina-Bordignon, P. Inhibition of IL-12 Production by 1,25-Dihydroxyvitamin D3. Involvement of NF-kappaB Downregulation in Transcriptional Repression of the P40 Gene. J. Clin. Investig. 1998, 101, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin Effects on the Immune System: Vitamins A and D Take Centre Stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Barragan, M.; Good, M.; Kolls, J.K. Regulation of Dendritic Cell Function by Vitamin D. Nutrients 2015, 7, 8127–8151. [Google Scholar] [CrossRef]
- Adorini, L.; Penna, G. Induction of Tolerogenic Dendritic Cells by Vitamin D Receptor Agonists. In Dendritic Cells. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 251–273. [Google Scholar] [CrossRef]
- Bscheider, M.; Butcher, E.C. Vitamin D Immunoregulation through Dendritic Cells. Immunology 2016, 148, 227–236. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory Effects of 1,25-Dihydroxyvitamin D3 on Human B Cell Differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef]
- Lemire, J.M.; Adams, J.S.; Sakai, R.; Jordan, S.C. 1 Alpha,25-Dihydroxyvitamin D3 Suppresses Proliferation and Immunoglobulin Production by Normal Human Peripheral Blood Mononuclear Cells. J. Clin. Investig. 1984, 74, 657–661. [Google Scholar] [CrossRef]
- James, J.; Weaver, V.; Cantorna, M.T. Control of Circulating IgE by the Vitamin D Receptor In Vivo Involves B Cell Intrinsic and Extrinsic Mechanisms. J. Immunol. 2017, 198, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, A.-K.; Nagakubo, D.; Hieshima, K.; Nakayama, T.; Jin, Z.; Yoshie, O. 1,25-Dihydroxyvitamin D3 Induces CCR10 Expression in Terminally Differentiating Human B Cells. J. Immunol. 2008, 180, 2786–2795. [Google Scholar] [CrossRef]
- Drozdenko, G.; Scheel, T.; Heine, G.; Baumgrass, R.; Worm, M. Impaired T Cell Activation and Cytokine Production by Calcitriol-Primed Human B Cells. Clin. Exp. Immunol. 2014, 178, 364–372. [Google Scholar] [CrossRef]
- Danner, O.K.; Matthews, L.R.; Francis, S.; Rao, V.N.; Harvey, C.P.; Tobin, R.P.; Wilson, K.L.; Alema-Mensah, E.; Newell Rogers, M.K.; Childs, E.W. Vitamin D3 Suppresses Class II Invariant Chain Peptide Expression on Activated B-Lymphocytes: A Plausible Mechanism for Downregulation of Acute Inflammatory Conditions. J. Nutr. Metab. 2016, 2016, 4280876. [Google Scholar] [CrossRef]
- Geldmeyer-Hilt, K.; Heine, G.; Hartmann, B.; Baumgrass, R.; Radbruch, A.; Worm, M. 1,25-Dihydroxyvitamin D3 Impairs NF-κB Activation in Human Naïve B Cells. Biochem. Biophys. Res. Commun. 2011, 407, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Kamen, D.L.; Tangpricha, V. Vitamin D and Molecular Actions on the Immune System: Modulation of Innate and Autoimmunity. J. Mol. Med. Berl. Ger. 2010, 88, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.O.; Samaras, N.; Samaras, D.; Aspinall, R. How Important Is Vitamin D in Preventing Infections? Osteoporos. Int. 2013, 24, 1537–1553. [Google Scholar] [CrossRef]
- van Etten, E.; Mathieu, C. Immunoregulation by 1,25-Dihydroxyvitamin D3: Basic Concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef]
- Pichler, J.; Gerstmayr, M.; Szépfalusi, Z.; Urbanek, R.; Peterlik, M.; Willheim, M. 1 Alpha,25(OH)2D3 Inhibits Not Only Th1 but Also Th2 Differentiation in Human Cord Blood T Cells. Pediatr. Res. 2002, 52, 12–18. [Google Scholar] [CrossRef][Green Version]
- Lemire, J.M.; Adams, J.S.; Kermani-Arab, V.; Bakke, A.C.; Sakai, R.; Jordan, S.C. 1,25-Dihydroxyvitamin D3 Suppresses Human T Helper/Inducer Lymphocyte Activity in Vitro. J. Immunol. 1985, 134, 3032–3035. [Google Scholar] [CrossRef]
- Yu, S.; Cantorna, M.T. The Vitamin D Receptor Is Required for iNKT Cell Development. Proc. Natl. Acad. Sci. USA 2008, 105, 5207–5212. [Google Scholar] [CrossRef] [PubMed]
- Staeva-Vieira, T.P.; Freedman, L.P. 1,25-Dihydroxyvitamin D3 Inhibits IFN-Gamma and IL-4 Levels during in Vitro Polarization of Primary Murine CD4+ T Cells. J. Immunol. 2002, 168, 1181–1189. [Google Scholar] [CrossRef]
- Alroy, I.; Towers, T.L.; Freedman, L.P. Transcriptional Repression of the Interleukin-2 Gene by Vitamin D3: Direct Inhibition of NFATp/AP-1 Complex Formation by a Nuclear Hormone Receptor. Mol. Cell. Biol. 1995, 15, 5789–5799. [Google Scholar] [CrossRef]
- Van Belle, T.L.; Gysemans, C.; Mathieu, C. Vitamin D in Autoimmune, Infectious and Allergic Diseases: A Vital Player? Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 617–632. [Google Scholar] [CrossRef]
- Lemire, J.M.; Archer, D.C.; Beck, L.; Spiegelberg, H.L. Immunosuppressive Actions of 1,25-Dihydroxyvitamin D3: Preferential Inhibition of Th1 Functions. J. Nutr. 1995, 125, 1704S–1708S. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.T.; Lee, Y.K.; Maynard, C.L.; Oliver, J.R.; Bikle, D.D.; Jetten, A.M.; Weaver, C.T. Lineage-Specific Effects of 1,25-Dihydroxyvitamin D(3) on the Development of Effector CD4 T Cells. J. Biol. Chem. 2011, 286, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Korf, H.; Overbergh, L.; Verstuyf, A.; Thorrez, L.; Van Lommel, L.; Waer, M.; Schuit, F.; Gysemans, C.; Mathieu, C. The Vitamin D Analog, TX527, Promotes a Human CD4+CD25highCD127low Regulatory T Cell Profile and Induces a Migratory Signature Specific for Homing to Sites of Inflammation. J. Immunol. 2011, 186, 132–142. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T Cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef]
- Cippitelli, M.; Santoni, A. Vitamin D3: A Transcriptional Modulator of the Interferon-Gamma Gene. Eur. J. Immunol. 1998, 28, 3017–3030. [Google Scholar] [CrossRef]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1alpha,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4(+) T Cells to Enhance the Development of Th2 Cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef]
- Khoo, A.-L.; Joosten, I.; Michels, M.; Woestenenk, R.; Preijers, F.; He, X.-H.; Netea, M.G.; van der Ven, A.J.A.M.; Koenen, H.J.P.M. 1,25-Dihydroxyvitamin D3 Inhibits Proliferation but Not the Suppressive Function of Regulatory T Cells in the Absence of Antigen-Presenting Cells. Immunology 2011, 134, 459–468. [Google Scholar] [CrossRef]
- Peck, A.; Mellins, E.D. Precarious Balance: Th17 Cells in Host Defense. Infect. Immun. 2010, 78, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Chung, Y.; Dong, C. Vitamin D Suppresses Th17 Cytokine Production by Inducing C/EBP Homologous Protein (CHOP) Expression. J. Biol. Chem. 2010, 285, 38751–38755. [Google Scholar] [CrossRef] [PubMed]
- Lysandropoulos, A.P.; Jaquiéry, E.; Jilek, S.; Pantaleo, G.; Schluep, M.; Du Pasquier, R.A. Vitamin D Has a Direct Immunomodulatory Effect on CD8+ T Cells of Patients with Early Multiple Sclerosis and Healthy Control Subjects. J. Neuroimmunol. 2011, 233, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cencioni, M.T.; Angelini, D.F.; Borsellino, G.; Battistini, L.; Brosnan, C.F. Transcriptional Profiling of Gamma Delta T Cells Identifies a Role for Vitamin D in the Immunoregulation of the V Gamma 9V Delta 2 Response to Phosphate-Containing Ligands. J. Immunol. 2005, 174, 6144–6152. [Google Scholar] [CrossRef]
- Okeke, E.B.; Uzonna, J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.K.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 Combine to Inhibit T Cell Production of Inflammatory Cytokines and Promote Development of Regulatory T Cells Expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef]
- Joshi, S.; Pantalena, L.-C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-Dihydroxyvitamin D(3) Ameliorates Th17 Autoimmunity via Transcriptional Modulation of Interleukin-17A. Mol. Cell. Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, S.H.; Lee, N.; Lee, W.-W.; Hwang, K.-A.; Shin, M.S.; Lee, S.-H.; Kim, W.-U.; Kang, I. 1,25-Dihyroxyvitamin D3 Promotes FOXP3 Expression via Binding to Vitamin D Response Elements in Its Conserved Noncoding Sequence Region. J. Immunol. 2012, 188, 5276–5282. [Google Scholar] [CrossRef]
- Farias, A.S.; Spagnol, G.S.; Bordeaux-Rego, P.; Oliveira, C.O.F.; Fontana, A.G.M.; de Paula, R.F.O.; Santos, M.P.A.; Pradella, F.; Moraes, A.S.; Oliveira, E.C.; et al. Vitamin D3 Induces IDO+ Tolerogenic DCs and Enhances Treg, Reducing the Severity of EAE. CNS Neurosci. Ther. 2013, 19, 269–277. [Google Scholar] [CrossRef]
- Bansal, A.S.; Henriquez, F.; Sumar, N.; Patel, S. T Helper Cell Subsets in Arthritis and the Benefits of Immunomodulation by 1,25(OH)2 Vitamin D. Rheumatol. Int. 2012, 32, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A Helpful Immuno-Modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Ooi, J.H.; Chen, J.; Cantorna, M.T. Vitamin D Regulation of Immune Function in the Gut: Why Do T Cells Have Vitamin D Receptors? Mol. Aspects Med. 2012, 33, 77–82. [Google Scholar] [CrossRef]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human Cathelicidin Antimicrobial Peptide (CAMP) Gene Is a Direct Target of the Vitamin D Receptor and Is Strongly up-Regulated in Myeloid Cells by 1,25-Dihydroxyvitamin D3. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 1067–1077. [Google Scholar] [CrossRef]
- Wang, T.-T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardão, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound Healing Activity of the Human Antimicrobial Peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Tjabringa, G.S.; Hiemstra, P.S.; Borregaard, N. Human Cathelicidin, hCAP-18, Is Processed to the Antimicrobial Peptide LL-37 by Extracellular Cleavage with Proteinase 3. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Silwal, P.; Kim, I.; Modlin, R.L.; Jo, E.-K. Vitamin D-Cathelicidin Axis: At the Crossroads between Protective Immunity and Pathological Inflammation during Infection. Immune Netw. 2020, 20, e12. [Google Scholar] [CrossRef]
- Guo, Y.; Farhan, M.H.R.; Gan, F.; Yang, X.; Li, Y.; Huang, L.; Wang, X.; Cheng, G. Advances in Artificially Designed Antibacterial Active Antimicrobial Peptides. Biotechnol. Bioeng. 2025, 122, 247–264. [Google Scholar] [CrossRef]
- Wang, G.; Narayana, J.L.; Mishra, B.; Zhang, Y.; Wang, F.; Wang, C.; Zarena, D.; Lushnikova, T.; Wang, X. Design of Antimicrobial Peptides: Progress Made with Human Cathelicidin LL-37. Adv. Exp. Med. Biol. 2019, 1117, 215–240. [Google Scholar] [CrossRef]
- Gottlieb, C.; Henrich, M.; Liu, P.T.; Yacoubian, V.; Wang, J.; Chun, R.; Adams, J.S. High-Throughput CAMP Assay (HiTCA): A Novel Tool for Evaluating the Vitamin D-Dependent Antimicrobial Response. Nutrients 2023, 15, 1380. [Google Scholar] [CrossRef] [PubMed]
- El Abd, A.; Dasari, H.; Dodin, P.; Trottier, H.; Ducharme, F.M. Associations between Vitamin D Status and Biomarkers Linked with Inflammation in Patients with Asthma: A Systematic Review and Meta-Analysis of Interventional and Observational Studies. Respir. Res. 2024, 25, 344. [Google Scholar] [CrossRef] [PubMed]
- Laaksi, A.; Kyröläinen, H.; Pihlajamäki, H.; Vaara, J.P.; Luukkaala, T.; Laaksi, I. Effects of Vitamin D Supplementation and Baseline Vitamin D Status on Acute Respiratory Infections and Cathelicidin: A Randomized Controlled Trial. Open Forum Infect. Dis. 2024, 11, ofae482. [Google Scholar] [CrossRef]
- Lauretani, F.; Salvi, M.; Zucchini, I.; Testa, C.; Cattabiani, C.; Arisi, A.; Maggio, M. Relationship between Vitamin D and Immunity in Older People with COVID-19. Int. J. Environ. Res. Public Health 2023, 20, 5432. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.-K.; Ng, Y.L.; Ahidjo, B.A.; Aw, Z.Q.; Chen, H.; Wong, Y.H.; Lee, R.C.H.; Loe, M.W.C.; Liu, J.; Tan, K.S.; et al. Evaluation of In Vitro and In Vivo Antiviral Activities of Vitamin D for SARS-CoV-2 and Variants. Pharmaceutics 2023, 15, 925. [Google Scholar] [CrossRef]
- van der Does, A.M.; Bergman, P.; Agerberth, B.; Lindbom, L. Induction of the Human Cathelicidin LL-37 as a Novel Treatment against Bacterial Infections. J. Leukoc. Biol. 2012, 92, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.; Heilborn, J.D.; Chamorro Jimenez, C.I.; Hammarsjo, A.; Törmä, H.; Stahle, M. Vitamin D Induces the Antimicrobial Protein hCAP18 in Human Skin. J. Investig. Dermatol. 2005, 124, 1080–1082. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Kaplan, M.J. Little Peptide, Big Effects: The Role of LL-37 in Inflammation and Autoimmune Disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [CrossRef]
- Martineau, A.R.; Wilkinson, K.A.; Newton, S.M.; Floto, R.A.; Norman, A.W.; Skolimowska, K.; Davidson, R.N.; Sørensen, O.E.; Kampmann, B.; Griffiths, C.J.; et al. IFN-Gamma- and TNF-Independent Vitamin D-Inducible Human Suppression of Mycobacteria: The Role of Cathelicidin LL-37. J. Immunol. 2007, 178, 7190–7198. [Google Scholar] [CrossRef]
- Dimitrov, V.; White, J.H. Species-Specific Regulation of Innate Immunity by Vitamin D Signaling. J. Steroid Biochem. Mol. Biol. 2016, 164, 246–253. [Google Scholar] [CrossRef]
- Yuk, J.-M.; Shin, D.-M.; Lee, H.-M.; Yang, C.-S.; Jin, H.S.; Kim, K.-K.; Lee, Z.-W.; Lee, S.-H.; Kim, J.-M.; Jo, E.-K. Vitamin D3 Induces Autophagy in Human Monocytes/Macrophages via Cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Schauber, J.; Stange, E.F. Defensins and Cathelicidins in Gastrointestinal Infections. Curr. Opin. Gastroenterol. 2007, 23, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Talafha, M.M.; Qasem, A.; Naser, S.A. Mycobacterium Avium Paratuberculosis Infection Suppresses Vitamin D Activation and Cathelicidin Production in Macrophages through Modulation of the TLR2-Dependent P38/MAPK-CYP27B1-VDR-CAMP Axis. Nutrients 2024, 16, 1358. [Google Scholar] [CrossRef]
- Campbell, G.R.; Spector, S.A. Autophagy Induction by Vitamin D Inhibits Both Mycobacterium Tuberculosis and Human Immunodeficiency Virus Type 1. Autophagy 2012, 8, 1523–1525. [Google Scholar] [CrossRef]
- Verway, M.; Bouttier, M.; Wang, T.-T.; Carrier, M.; Calderon, M.; An, B.-S.; Devemy, E.; McIntosh, F.; Divangahi, M.; Behr, M.A.; et al. Vitamin D Induces Interleukin-1β Expression: Paracrine Macrophage Epithelial Signaling Controls M. tuberculosis Infection. PLoS Pathog. 2013, 9, e1003407. [Google Scholar] [CrossRef]
- Fernandez, G.J.; Ramírez-Mejía, J.M.; Castillo, J.A.; Urcuqui-Inchima, S. Vitamin D Modulates Expression of Antimicrobial Peptides and Proinflammatory Cytokines to Restrict Zika Virus Infection in Macrophages. Int. Immunopharmacol. 2023, 119, 110232. [Google Scholar] [CrossRef]
- Tang, X.; Basavarajappa, D.; Haeggström, J.Z.; Wan, M. P2X7 Receptor Regulates Internalization of Antimicrobial Peptide LL-37 by Human Macrophages That Promotes Intracellular Pathogen Clearance. J. Immunol. 2015, 195, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, S.K. Vitamin D in Autophagy Signaling for Health and Diseases: Insights on Potential Mechanisms and Future Perspectives. J. Nutr. Biochem. 2022, 99, 108841. [Google Scholar] [CrossRef]
- Zhou, A.; Li, L.; Zhao, G.; Min, L.; Liu, S.; Zhu, S.; Guo, Q.; Liu, C.; Zhang, S.; Li, P. Vitamin D3 Inhibits Helicobacter Pylori Infection by Activating the VitD3/VDR-CAMP Pathway in Mice. Front. Cell. Infect. Microbiol. 2020, 10, 566730. [Google Scholar] [CrossRef]
- Huang, F.-C. Vitamin D Differentially Regulates Salmonella-Induced Intestine Epithelial Autophagy and Interleukin-1β Expression. World J. Gastroenterol. 2016, 22, 10353–10363. [Google Scholar] [CrossRef]
- Sato, E.; Imafuku, S.; Ishii, K.; Itoh, R.; Chou, B.; Soejima, T.; Nakayama, J.; Hiromatsu, K. Vitamin D-Dependent Cathelicidin Inhibits Mycobacterium Marinum Infection in Human Monocytic Cells. J. Dermatol. Sci. 2013, 70, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yang, E.; Shen, L.; Modlin, R.L.; Shen, H.; Chen, Z.W. IL-12+IL-18 Cosignaling in Human Macrophages and Lung Epithelial Cells Activates Cathelicidin and Autophagy, Inhibiting Intracellular Mycobacterial Growth. J. Immunol. 2018, 200, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Y.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal Epithelial Vitamin D Receptor Deletion Leads to Defective Autophagy in Colitis. Gut 2015, 64, 1082–1094. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, Y.-G.; Xia, Y.; Zhang, J.; Kaser, A.; Blumberg, R.; Sun, J. Paneth Cell Alertness to Pathogens Maintained by Vitamin D Receptors. Gastroenterology 2021, 160, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Wu, J.; Li, X.; Liu, X.; Li, P.; Zheng, C.; Wang, Y.; Liu, S.; Zheng, J.; Zhou, H. Artesunate Interacts with the Vitamin D Receptor to Reverse Sepsis-Induced Immunosuppression in a Mouse Model via Enhancing Autophagy. Br. J. Pharmacol. 2020, 177, 4147–4165. [Google Scholar] [CrossRef]
- Li, A.; Zhang, H.; Han, H.; Zhang, W.; Yang, S.; Huang, Z.; Tan, J.; Yi, B. LC3 Promotes the Nuclear Translocation of the Vitamin D Receptor and Decreases Fibrogenic Gene Expression in Proximal Renal Tubules. Metabolism. 2019, 98, 95–103. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T.; Li, Q.; Ling, Y.; Ma, Y.; Tao, S. SQSTM1 Improves Acute Lung Injury via Inhibiting Airway Epithelium Ferroptosis in a Vitamin D Receptor/Autophagy-Mediated Manner. Free Radic. Biol. Med. 2024, 222, 588–600. [Google Scholar] [CrossRef]
- Lai, T.-C.; Chen, Y.-C.; Cheng, H.-H.; Lee, T.-L.; Tsai, J.-S.; Lee, I.-T.; Peng, K.-T.; Lee, C.-W.; Hsu, L.-F.; Chen, Y.-L. Combined Exposure to Fine Particulate Matter and High Glucose Aggravates Endothelial Damage by Increasing Inflammation and Mitophagy: The Involvement of Vitamin D. Part. Fibre Toxicol. 2022, 19, 25. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, J.; Wu, Y.; Sun, X.; Rao, Z.; Sun, N.; Fu, Y.; Zhang, Z.; Li, J.; Xiao, M.; et al. Parkin Increases the Risk of Colitis by Downregulation of VDR via Autophagy-Lysosome Degradation. Int. J. Biol. Sci. 2023, 19, 1633–1644. [Google Scholar] [CrossRef]
- Abdelmalak, M.F.L.; Abdelrahim, D.S.; George Michael, T.M.A.; Abdel-Maksoud, O.M.; Labib, J.M.W. Vitamin D and Lactoferrin Attenuate Stress-Induced Colitis in Wistar Rats via Enhancing AMPK Expression with Inhibiting mTOR-STAT3 Signaling and Modulating Autophagy. Cell Biochem. Funct. 2023, 41, 211–222. [Google Scholar] [CrossRef]
- Jhun, J.; Woo, J.S.; Kwon, J.Y.; Na, H.S.; Cho, K.-H.; Kim, S.A.; Kim, S.J.; Moon, S.-J.; Park, S.-H.; Cho, M.-L. Vitamin D Attenuates Pain and Cartilage Destruction in OA Animals via Enhancing Autophagic Flux and Attenuating Inflammatory Cell Death. Immune Netw. 2022, 22, e34. [Google Scholar] [CrossRef] [PubMed]
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1061–1094. [Google Scholar] [CrossRef]
- Watkins, R.R.; Yamshchikov, A.V.; Lemonovich, T.L.; Salata, R.A. The Role of Vitamin D Deficiency in Sepsis and Potential Therapeutic Implications. J. Infect. 2011, 63, 321–326. [Google Scholar] [CrossRef]
- Cui, P.; Lu, W.; Wang, J.; Wang, F.; Zhang, X.; Hou, X.; Xu, F.; Liang, Y.; Chai, G.; Hao, J. Microglia/Macrophages Require Vitamin D Signaling to Restrain Neuroinflammation and Brain Injury in a Murine Ischemic Stroke Model. J. Neuroinflammation 2023, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Khoo, A.-L.; Chai, L.Y.A.; Koenen, H.J.P.M.; Oosting, M.; Steinmeyer, A.; Zuegel, U.; Joosten, I.; Netea, M.G.; van der Ven, A.J.A.M. Vitamin D(3) down-Regulates Proinflammatory Cytokine Response to Mycobacterium Tuberculosis through Pattern Recognition Receptors While Inducing Protective Cathelicidin Production. Cytokine 2011, 55, 294–300. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef]
- Fitch, N.; Becker, A.B.; HayGlass, K.T. Vitamin D [1,25(OH)2D3] Differentially Regulates Human Innate Cytokine Responses to Bacterial versus Viral Pattern Recognition Receptor Stimuli. J. Immunol. 2016, 196, 2965–2972. [Google Scholar] [CrossRef]
- Zheng, G.; Wen, N.; Pan, M.; Huang, Y.; Li, Z. Biologically Active 1,25-Dihydroxyvitamin D3 Protects against Experimental Sepsis by Negatively Regulating the Toll-like Receptor 4/Myeloid Differentiation Primary Response Gene 88/Toll-IL-1 Resistance-Domain-Containing Adapter-Inducing Interferon-β Signaling Pathway. Int. J. Mol. Med. 2019, 44, 1151–1160. [Google Scholar] [CrossRef]
- Korf, H.; Wenes, M.; Stijlemans, B.; Takiishi, T.; Robert, S.; Miani, M.; Eizirik, D.L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 Curtails the Inflammatory and T Cell Stimulatory Capacity of Macrophages through an IL-10-Dependent Mechanism. Immunobiology 2012, 217, 1292–1300. [Google Scholar] [CrossRef]
- Dionne, S.; Calderon, M.R.; White, J.H.; Memari, B.; Elimrani, I.; Adelson, B.; Piccirillo, C.; Seidman, E.G. Differential Effect of Vitamin D on NOD2- and TLR-Induced Cytokines in Crohn’s Disease. Mucosal Immunol. 2014, 7, 1405–1415. [Google Scholar] [CrossRef]
- Wang, T.-T.; Dabbas, B.; Laperriere, D.; Bitton, A.J.; Soualhine, H.; Tavera-Mendoza, L.E.; Dionne, S.; Servant, M.J.; Bitton, A.; Seidman, E.G.; et al. Direct and Indirect Induction by 1,25-Dihydroxyvitamin D3 of the NOD2/CARD15-Defensin Β2 Innate Immune Pathway Defective in Crohn Disease. J. Biol. Chem. 2010, 285, 2227–2231. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D Receptor Inhibits Nuclear Factor κB Activation by Interacting with IκB Kinase β Protein. J. Biol. Chem. 2013, 288, 19450–19458. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, R.-N.; Wen, Y.; Yin, W.-N.; Bai, D.; Zheng, G.-Y.; Li, J.-S.; Zheng, B.; Wen, J.-K. 1, 25(OH)2D3-Induced Interaction of Vitamin D Receptor with P50 Subunit of NF-κB Suppresses the Interaction between KLF5 and P50, Contributing to Inhibition of LPS-Induced Macrophage Proliferation. Biochem. Biophys. Res. Commun. 2017, 482, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Farmer, P.; Rubin, J.; Nanes, M.S. Integration of the NfκB P65 Subunit into the Vitamin D Receptor Transcriptional Complex: Identification of P65 Domains That Inhibit 1,25-dihydroxyvitamin D3-stimulated Transcription. J. Cell. Biochem. 2004, 92, 833–848. [Google Scholar] [CrossRef]
- Wu, S.; Liao, A.P.; Xia, Y.; Chun Li, Y.; Li, J.-D.; Sartor, R.B.; Sun, J. Vitamin D Receptor Negatively Regulates Bacterial-Stimulated NF-κB Activity in Intestine. Am. J. Pathol. 2010, 177, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Yu, Z.; Fu, L.; Wang, H.; Chen, X.; Zhang, C.; Lv, Z.-M.; Xu, D.-X. Vitamin D3 Inhibits Lipopolysaccharide-Induced Placental Inflammation through Reinforcing Interaction between Vitamin D Receptor and Nuclear Factor Kappa B P65 Subunit. Sci. Rep. 2015, 5, 10871. [Google Scholar] [CrossRef]
- Karkeni, E.; Bonnet, L.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Ye, J.; Landrier, J.-F. Vitamin D Limits Inflammation-Linked microRNA Expression in Adipocytes in Vitro and in Vivo: A New Mechanism for the Regulation of Inflammation by Vitamin D. Epigenetics 2018, 13, 156–162. [Google Scholar] [CrossRef]
- Castillo, J.A.; Urcuqui-Inchima, S. Vitamin D Modulates Inflammatory Response of DENV-2-Infected Macrophages by Inhibiting the Expression of Inflammatory-Liked miRNAs. Pathog. Glob. Health 2023, 117, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Kempinska-Podhorodecka, A.; Milkiewicz, M.; Wasik, U.; Ligocka, J.; Zawadzki, M.; Krawczyk, M.; Milkiewicz, P. Decreased Expression of Vitamin D Receptor Affects an Immune Response in Primary Biliary Cholangitis via the VDR-miRNA155-SOCS1 Pathway. Int. J. Mol. Sci. 2017, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, J.; Zhang, Z.; Liu, T.; Shi, Y.; Ge, X.; Li, Y.C. MicroRNA-346 Mediates Tumor Necrosis Factor α-Induced Downregulation of Gut Epithelial Vitamin D Receptor in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2014, 20, 1910–1918. [Google Scholar] [CrossRef]
- Shang, L.; Li, J.; Zhou, F.; Zhang, M.; Wang, S.; Yang, S. MiR-874-5p Targets VDR/NLRP3 to Reduce Intestinal Pyroptosis and Improve Intestinal Barrier Damage in Sepsis. Int. Immunopharmacol. 2023, 121, 110424. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Zaki, A.; Manda, K.; Mohan, A.; Syed, M.A. Vitamin-D Ameliorates Sepsis-Induced Acute Lung Injury via Augmenting miR-149-5p and Downregulating ER Stress. J. Nutr. Biochem. 2022, 110, 109130. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Zeng, Y.; Mo, X.; Hong, S.; He, H.; Li, J.; Fatima, S.; Liu, Q. Oxidative Stress Induces Mitochondrial Iron Overload and Ferroptotic Cell Death. Sci. Rep. 2023, 13, 15515. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, X.; Tan, X.; Wang, P.; Zhao, X.; Zhang, H.; Song, Y. Vitamin D Suppresses Ferroptosis and Protects against Neonatal Hypoxic-Ischemic Encephalopathy by Activating the Nrf2/HO-1 Pathway. Transl. Pediatr. 2022, 11, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.-J.; Zheng, X.-R.; Liu, Q.-L.; Du, Q.; Lai, Y.-J.; Liu, S.-Q. Eriodictyol Ameliorates Cognitive Dysfunction in APP/PS1 Mice by Inhibiting Ferroptosis via Vitamin D Receptor-Mediated Nrf2 Activation. Mol. Med. Camb. Mass 2022, 28, 11. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Yi, B.; Yang, S.; Liu, J.; Hu, J.; Wang, J.; Cao, K.; Zhang, W. VDR Activation Attenuate Cisplatin Induced AKI by Inhibiting Ferroptosis. Cell Death Dis. 2020, 11, 73. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Moore, S.M.; Kishi, N.; Macklis, J.D.; MacDonald, J.L. Vitamin D Supplementation Rescues Aberrant NF-κB Pathway Activation and Partially Ameliorates Rett Syndrome Phenotypes in Mecp2 Mutant Mice. eNeuro 2020, 7, ENEURO.0167-20.2020. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, Infections and Immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients With Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef]
- Wang, J.; Thingholm, L.B.; Skiecevičienė, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.-A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-Wide Association Analysis Identifies Variation in Vitamin D Receptor and Other Host Factors Influencing the Gut Microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef]
- Luthold, R.V.; Fernandes, G.R.; Franco-de-Moraes, A.C.; Folchetti, L.G.D.; Ferreira, S.R.G. Gut Microbiota Interactions with the Immunomodulatory Role of Vitamin D in Normal Individuals. Metabolism. 2017, 69, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef] [PubMed]
- Fabisiak, N.; Tarasiuk-Zawadzka, A.; Fabisiak, A.; Wlodarczyk, M.; Fichna, J. Determining the Expression of Vitamin D Receptor, Regulator of Iron Metabolism Hepcidin and Cathelicidin Antimicrobial Peptide Genes for Development of Future Diagnostic and Therapeutic Options in Patients with Inflammatory Bowel Disease. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2024, 75, 409–413. [Google Scholar] [CrossRef]
- Kanhere, M.; He, J.; Chassaing, B.; Ziegler, T.R.; Alvarez, J.A.; Ivie, E.A.; Hao, L.; Hanfelt, J.; Gewirtz, A.T.; Tangpricha, V. Bolus Weekly Vitamin D3 Supplementation Impacts Gut and Airway Microbiota in Adults With Cystic Fibrosis: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2018, 103, 564–574. [Google Scholar] [CrossRef]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Oral Supplementation with Probiotic L. Reuteri NCIMB 30242 Increases Mean Circulating 25-Hydroxyvitamin D: A Post Hoc Analysis of a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2013, 98, 2944–2951. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Tsounis, E.P.; Mouzaki, A.; Triantos, C. Exploring the Role of Vitamin D and the Vitamin D Receptor in the Composition of the Gut Microbiota. Front. Biosci. Landmark Ed. 2023, 28, 116. [Google Scholar] [CrossRef]
- Amersfoort, J.; Eelen, G.; Carmeliet, P. Immunomodulation by Endothelial Cells—Partnering up with the Immune System? Nat. Rev. Immunol. 2022, 22, 576–588. [Google Scholar] [CrossRef]
- Sha, T.; Wang, Y.; Zhang, Y.; Lane, N.E.; Li, C.; Wei, J.; Zeng, C.; Lei, G. Genetic Variants, Serum 25-Hydroxyvitamin D Levels, and Sarcopenia: A Mendelian Randomization Analysis. JAMA Netw. Open 2023, 6, e2331558. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, H.; Chen, Y.; He, J.; Zhu, L.; Yang, B.; Zhao, W. High Glucose-Induced Injury in Human Umbilical Vein Endothelial Cells Is Alleviated by Vitamin D Supplementation through Downregulation of TIPE1. Diabetol. Metab. Syndr. 2024, 16, 18. [Google Scholar] [CrossRef]
- Song, Y.-S.; Jamali, N.; Sorenson, C.M.; Sheibani, N. Vitamin D Receptor Expression Limits the Angiogenic and Inflammatory Properties of Retinal Endothelial Cells. Cells 2023, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Vincent, V.; Thakkar, H.; Abraham, R.; Ramakrishnan, L. Beneficial Role of Vitamin D on Endothelial Progenitor Cells (EPCs) in Cardiovascular Diseases. J. Lipid Atheroscler. 2022, 11, 229–249. [Google Scholar] [CrossRef]
- Mohammad, S.; Mishra, A.; Ashraf, M.Z. Emerging Role of Vitamin D and Its Associated Molecules in Pathways Related to Pathogenesis of Thrombosis. Biomolecules 2019, 9, 649. [Google Scholar] [CrossRef]
- Cox, D. Sepsis—It Is All about the Platelets. Front. Immunol. 2023, 14, 1210219. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Pergolini, P.; Rolla, R.; Sartori, C.; Nardin, M.; Schaffer, A.; Barbieri, L.; Daffara, V.; Marino, P.; Bellomo, G.; et al. Vitamin D Levels and High-Residual Platelet Reactivity in Patients Receiving Dual Antiplatelet Therapy with Clopidogrel or Ticagrelor. Platelets 2016, 27, 576–582. [Google Scholar] [CrossRef]
- Sayar, Z.; Moll, R.; Isenberg, D.; Cohen, H. Thrombotic Antiphospholipid Syndrome: A Practical Guide to Diagnosis and Management. Thromb. Res. 2021, 198, 213–221. [Google Scholar] [CrossRef]
- Çakır, O.M. Low Vitamin D Levels Predict Left Atrial Thrombus in Nonvalvular Atrial Fibrillation. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1152–1160. [Google Scholar] [CrossRef]
- Khansari, N.; Bagheri, M.; Homayounfar, S.; Poorolajal, J.; Mehrpooya, M. Influence of Vitamin D Status on the Maintenance Dose of Warfarin in Patients Receiving Chronic Warfarin Therapy. Cardiol. Ther. 2022, 11, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moreno, J.M.; Herencia, C.; Montes de Oca, A.; Muñoz-Castañeda, J.R.; Rodríguez-Ortiz, M.E.; Díaz-Tocados, J.M.; Peralbo-Santaella, E.; Camargo, A.; Canalejo, A.; Rodriguez, M.; et al. Vitamin D Modulates Tissue Factor and Protease-Activated Receptor 2 Expression in Vascular Smooth Muscle Cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 1367–1376. [Google Scholar] [CrossRef]
- Wood, J.P.; Ellery, P.E.R.; Maroney, S.A.; Mast, A.E. Biology of Tissue Factor Pathway Inhibitor. Blood 2014, 123, 2934–2943. [Google Scholar] [CrossRef]
- Kempker, J.A.; Tangpricha, V.; Ziegler, T.R.; Martin, G.S. Vitamin D in Sepsis: From Basic Science to Clinical Impact. Crit. Care 2012, 16, 316. [Google Scholar] [CrossRef] [PubMed]
- Lipkie, T.E.; Janasch, A.; Cooper, B.R.; Hohman, E.E.; Weaver, C.M.; Ferruzzi, M.G. Quantification of Vitamin D and 25-Hydroxyvitamin D in Soft Tissues by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2013, 932, 6–11. [Google Scholar] [CrossRef]
- Fu, X.; Dolnikowski, G.G.; Patterson, W.B.; Dawson-Hughes, B.; Zheng, T.; Morris, M.C.; Holland, T.M.; Booth, S.L. Determination of Vitamin D and Its Metabolites in Human Brain Using an Ultra-Pressure LC-Tandem Mass Spectra Method. Curr. Dev. Nutr. 2019, 3, nzz074. [Google Scholar] [CrossRef]
- Máčová, L.; Bičíková, M. Vitamin D: Current Challenges between the Laboratory and Clinical Practice. Nutrients 2021, 13, 1758. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.; Zelzer, S.; Eibinger, G.; Herrmann, M. Vitamin D Metabolites: Analytical Challenges and Clinical Relevance. Calcif. Tissue Int. 2023, 112, 158–177. [Google Scholar] [CrossRef]
- Health Management Branch of Chinese Nutrition Society. Expert consensus on evaluation and improvement of vitamin D nutritional status. Chin. Health Manag. J. 2023, 17, 245–252. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: A Millenium Perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of Season and Latitude on the Cutaneous Synthesis of Vitamin D3: Exposure to Winter Sunlight in Boston and Edmonton Will Not Promote Vitamin D3 Synthesis in Human Skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Huang, Y.-C.; Qiao, Y.-C.; Ling, W.; Pan, Y.-H.; Geng, L.-J.; Xiao, J.-L.; Zhang, X.-X.; Zhao, H.-L. Climates on Incidence of Childhood Type 1 Diabetes Mellitus in 72 Countries. Sci. Rep. 2017, 7, 12810. [Google Scholar] [CrossRef]
- Amrein, K.; Parekh, D.; Westphal, S.; Preiser, J.-C.; Berghold, A.; Riedl, R.; Eller, P.; Schellongowski, P.; Thickett, D.; Meybohm, P.; et al. Effect of High-Dose Vitamin D3 on 28-Day Mortality in Adult Critically Ill Patients with Severe Vitamin D Deficiency: A Study Protocol of a Multicentre, Placebo-Controlled Double-Blind Phase III RCT (the VITDALIZE Study). BMJ Open 2019, 9, e031083. [Google Scholar] [CrossRef]
- O’Hearn, K.; Menon, K.; Albrecht, L.; Amrein, K.; Britz-McKibbin, P.; Cayouette, F.; Choong, K.; Foster, J.R.; Fergusson, D.A.; Floh, A.; et al. Rapid Normalization of Vitamin D Deficiency in PICU (VITdALIZE-KIDS): Study Protocol for a Phase III, Multicenter Randomized Controlled Trial. Trials 2024, 25, 619. [Google Scholar] [CrossRef]
- Maestro, M.A.; Molnár, F.; Carlberg, C. Vitamin D and Its Synthetic Analogs. J. Med. Chem. 2019, 62, 6854–6875. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.; Trompke, C. Tacalcitol Ointment for Long-Term Control of Chronic Plaque Psoriasis in Dermatological Practice. Dermatology 2002, 204, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, Z.; Wang, Y.; Chai, L.; Zhou, X. Vitamin D Analogs Combined with Different Types of Phototherapy in the Treatment of Vitiligo: A Systematic Review of Randomized Trials and within-Patient Studies. Int. Immunopharmacol. 2022, 109, 108789. [Google Scholar] [CrossRef]
- Aggarwal, P.; Aggarwal, K.; Jain, V.K. Tacalcitol: A Useful Adjunct to Narrow Band Ultraviolet B Phototherapy in Psoriasis. J. Dermatol. Treat. 2016, 27, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Turlej, E.; Goszczyński, T.M.; Drab, M.; Orzechowska, B.; Maciejewska, M.; Banach, J.; Wietrzyk, J. The Impact of Exosomes/Microvesicles Derived from Myeloid Dendritic Cells Cultured in the Presence of Calcitriol and Tacalcitol on Acute B-Cell Precursor Cell Lines with MLL Fusion Gene. J. Clin. Med. 2022, 11, 2224. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Ogata, H. Clinical Uses of 22-Oxacalcitriol. Curr. Vasc. Pharmacol. 2014, 12, 324–328. [Google Scholar] [CrossRef]
- Li, C.; Yin, S.; Yin, H.; Cao, L.; Zhang, T.; Wang, Y. Efficacy and Safety of 22-Oxa-Calcitriol in Patients with Rheumatoid Arthritis: A Phase II Trial. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 9127–9135. [Google Scholar] [CrossRef]
- Hamzawy, M.; Gouda, S.A.A.; Rashid, L.; Attia Morcos, M.; Shoukry, H.; Sharawy, N. The Cellular Selection between Apoptosis and Autophagy: Roles of Vitamin D, Glucose and Immune Response in Diabetic Nephropathy. Endocrine 2017, 58, 66–80. [Google Scholar] [CrossRef]
- Hamzawy, M.; Gouda, S.A.A.; Rashed, L.; Morcos, M.A.; Shoukry, H.; Sharawy, N. 22-Oxacalcitriol Prevents Acute Kidney Injury via Inhibition of Apoptosis and Enhancement of Autophagy. Clin. Exp. Nephrol. 2019, 23, 43–55. [Google Scholar] [CrossRef]
- Hau, C.S.; Shimizu, T.; Tada, Y.; Kamata, M.; Takeoka, S.; Shibata, S.; Mitsui, A.; Asano, Y.; Sugaya, M.; Kadono, T.; et al. The Vitamin D3 Analog, Maxacalcitol, Reduces Psoriasiform Skin Inflammation by Inducing Regulatory T Cells and Downregulating IL-23 and IL-17 Production. J. Dermatol. Sci. 2018, 92, 117–126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).