The Regulatory Role of Non-Coding RNAs in Autophagy-Dependent Ischemia–Reperfusion Injury of the Brain
Abstract
1. Introduction
2. General Characteristics of Long Non-Coding RNAs, MicroRNAs and Circular RNAs
3. Differentially Expressed Non-Coding RNAs and Messenger RNAs in Ischemic and Reperfused Brain
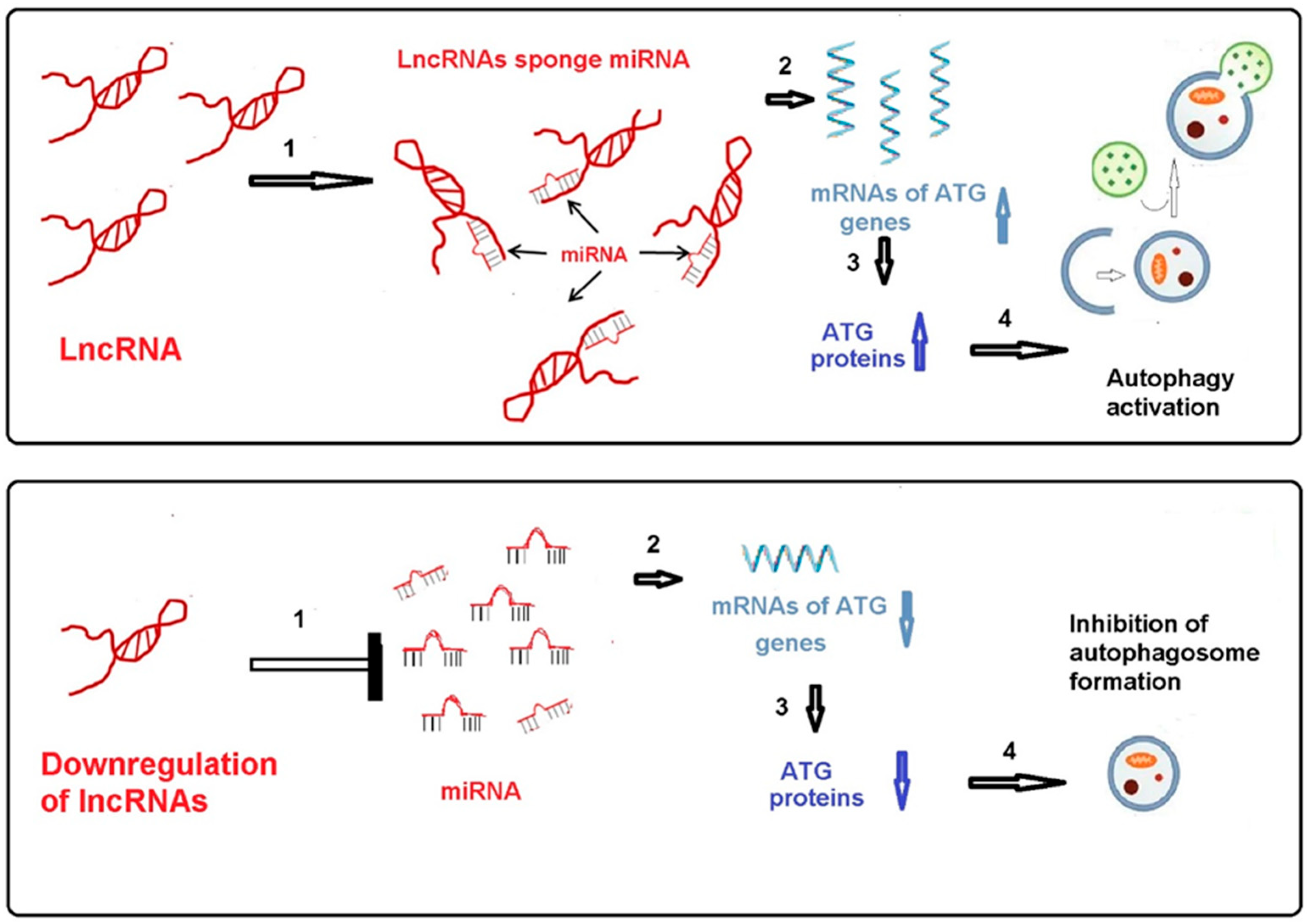
4. The Regulatory Effect of Long Non-Coding RNA, MicroRNA and Autophagy-Related Proteins on the Intensity of Autophagy and Brain Ischemia and Reperfusion Injury (LncRNA/MiRNA/ATG Protein and MiRNA/ATG Protein Axes)
5. The Regulatory Effect of Long Non-Coding RNAs, MicroRNAs and Various Target Proteins on the Intensity of Autophagy and Brain Ischemia–Reperfusion Injury
6. The Regulatory Effect of Circular RNAs, MicroRNAs and Various Target Proteins on the Intensity of Autophagy and Brain Ischemia–ReperfusionIschemia-Reperfusion Injury
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| AMPK | Adenosine monophosphate-activated protein kinase |
| ATG proteins | Autophagy-related proteins |
| BBB | Blood–brain barrier |
| BMEC | Brain microvascular endothelial cells |
| BNIP3 | Bcl2/adenovirus E1B 19 kDa interacting protein 3 |
| ceRNAs | Competing endogenous RNAs |
| circCDR1as | Circular cerebellar degeneration-related protein 1 antisense RNA |
| circRNAs | Circular RNAs |
| EA | Electroacupuncture |
| eIF4E | Eukaryotic translation initiation factor 4E |
| EP300 | E1A-associated protein p300 (E1A = adenovirus early region 1A) |
| FOXO3 | Forkhead box O3 |
| GO | Gene Ontology |
| HT22 | Mouse hippocampal neuronal cell line |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC3 | Light chain 3 |
| lncKCNQ1OT1 | Long non-coding RNA potassium voltage-gated channel subfamily Q member 1opposite strand 1 |
| lncMalat1 | Long non-coding RNA metastasis-associated lung adenocarcinoma transcript-1 |
| lncRNAs | Long non-coding RNAs |
| lncPEG11as | Long non-coding paternally expressed gene 11 antisense transcript |
| lncSNHG15 | Long non-coding RNA small nucleolar RNA host gene 15 |
| lncTUG1 | LncRNA taurine activating gene |
| MAPK | Mitogen-activated protein kinase |
| MCAO | Middle cerebral artery occlusion |
| MCAO/R | Middle cerebral artery occlusion and reperfusion |
| miRNAs | microRNAs |
| mTOR | The mammalian/mechanistic target of rapamycin |
| OGD | Oxygen and glucose deprivation |
| PC12 | Pheochromocytoma cell line of the rat adrenal medulla |
| SH-SY5Y | Stable human neuroblastoma cell line |
| SIRT1 | Sirtuin 1 |
| ULK1/2 | Unc-51-like kinase 1/2 |
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Li, X.Y.; Kong, X.M.; Yang, C.H.; Cheng, Z.F.; Lv, J.J.; Guo, H.; Liu, X.H. Global, regional, and national burden of ischemic stroke, 1990-2021: An analysis of data from the global burden of disease study 2021. EClinicalMedicine 2024, 75, 102758. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Wang, L.; Zhang, R.; Zhao, T.; Jiang, Y.; Han, L. Projected Global Trends in Ischemic Stroke Incidence, Deaths and Disability-Adjusted Life Years From 2020 to 2030. Stroke 2023, 54, 1330–1339. [Google Scholar] [CrossRef]
- Tuttolomondo, A. Ischemic Stroke Pathogenesis: Genetics, Epigenetics and Inflammation. Curr. Pharm. Des. 2020, 26, 4207–4208. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Chen, X.; Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int. J. Mol. Med. 2022, 49, 15. [Google Scholar] [CrossRef]
- Zhang, K.; Loong, S.; Yuen, L.Z.H.; Venketasubramanian, N.; Chin, H.L.; Lai, P.S.; Tan, B.Y.Q. Genetics in Ischemic Stroke: Current Perspectives and Future Directions. J. Cardiovasc. Dev. Dis. 2023, 10, 495. [Google Scholar] [CrossRef]
- Cárcel-Márquez, J.; Muiño, E.; Gallego-Fabrega, C.; Cullell, N.; Lledós, M.; Llucià-Carol, L.; Martín-Campos, J.M.; Sobrino, T.; Campos, F.; Castillo, J.; et al. Sex-Stratified Genome-Wide Association Study in the Spanish Population Identifies a Novel Locus for Lacunar Stroke. Stroke 2024, 55, 2462–2471. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Schmidt-Kastner, R. Genomic approach to selective vulnerability of the hippocampus in brain ischemia-hypoxia. Neuroscience 2015, 309, 259–279. [Google Scholar] [CrossRef]
- Einenkel, A.M.; Salameh, A. Selective vulnerability of hippocampal CA1 and CA3 pyramidal cells: What are possible pathomechanisms and should more attention be paid to the CA3 region in future studies? J. Neurosci. Res. 2024, 102, e25276. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.R.; French, B.R.; Gomez, F.E.; Qureshi, A.I. Neuroendovascular Rescue 2025: Trends in Stroke Endovascular Therapy. Neurol. Clin. 2024, 42, 717–738. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.W.; Yogendrakumar, V.; Churilov, L.; Garcia-Esperon, C.; Campbell, B.C.V.; Russell, M.L.; Sharma, G.; Chen, C.; Lin, L.; Chew, B.L.; et al. TASTE investigators. Tenecteplase versus alteplase for thrombolysis in patients selected by use of perfusion imaging within 4·5 h of onset of ischaemic stroke (TASTE): A multicentre, randomised, controlled, phase 3 non-inferiority trial. Lancet Neurol. 2024, 23, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gu, H.Q.; Feng, B.; Li, H.; Wang, X.; Dong, Q.; Fan, D.; Xu, Y.; Zhu, S.; Dai, H.; et al. PROST-2 investigators. Safety and efficacy of intravenous recombinant human prourokinase for acute ischaemic stroke within 4·5 h after stroke onset (PROST-2): A phase 3, open-label, non-inferiority, randomised controlled trial. Lancet Neurol. 2025, 24, 33–41. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakagomi, N.; Doe, N.; Nakano-Doi, A.; Sawano, T.; Takagi, T.; Matsuyama, T.; Yoshimura, S.; Nakagomi, T. Early Reperfusion Following Ischemic Stroke Provides Beneficial Effects, Even After Lethal Ischemia with Mature Neural Cell Death. Cells 2020, 9, 1374. [Google Scholar] [CrossRef]
- Jurcau, A.; Ardelean, A.I. Oxidative Stress in Ischemia/Reperfusion Injuries following Acute Ischemic Stroke. Biomedicines 2022, 10, 574. [Google Scholar] [CrossRef]
- Brait, V.H.; Jackman, K.A.; Walduck, A.K.; Selemidis, S.; Diep, H.; Mast, A.E.; Guida, E.; Broughton, B.R.; Drummond, G.R.; Sobey, C.G. Mechanisms contributing to cerebral infarct size after stroke: Gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J. Cereb. Blood Flow Metab. 2010, 30, 1306–1317. [Google Scholar] [CrossRef]
- Xie, L.; Li, W.; Hersh, J.; Liu, R.; Yang, S.H. Experimental ischemic stroke induces long-term T cell activation in the brain. J. Cereb. Blood Flow Metab. 2019, 39, 2268–2276. [Google Scholar] [CrossRef]
- Goodman, G.W.; Do, T.H.; Tan, C.; Ritzel, R.M. Drivers of Chronic Pathology Following Ischemic Stroke: A Descriptive Review. Cell. Mol. Neurobiol. 2023, 44, 7. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Gao, L.; Peng, L.; Wang, J.; Zhang, J.H.; Xia, Y. Mitochondrial stress: A key role of neuroinflammation in stroke. J. Neuroinflamm. 2024, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Maida, C.D.; Norrito, R.L.; Rizzica, S.; Mazzola, M.; Scarantino, E.R.; Tuttolomondo, A. Molecular Pathogenesis of Ischemic and Hemorrhagic Strokes: Background and Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 6297. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.; Ezazi Erdi, S.; Jorba, G.; Maestro, I.; Farrés, J.; Kirkin, V.; Martinez, A.; Pless, O. Targeting autophagy in disease: Established and new strategies. Autophagy 2022, 18, 473–495. [Google Scholar] [CrossRef]
- Chu, C.T. The Role of Autophagy in Excitotoxicity, Synaptic Mitochondrial Stress and Neurodegeneration. Autophagy Rep. 2025, 4, 2464376. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Z.; Zhao, Y.; Huang, L.; Wang, J.; Li, H.; Chen, X.; Wang, J.; Xie, J. Mechanism of ameliorating cerebral ischemia/reperfusion injury by antioxidant inhibition of autophagy based on network pharmacology and experimental verification. Aging 2024, 16, 7474–7486. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Hao, Y.; Sun, L.; Zhao, Y.; Zheng, X.; Song, L. The dual roles of autophagy and the GPCRs-mediating autophagy signaling pathway after cerebral ischemic stroke. Mol. Brain 2022, 15, 14. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, W.; Liao, Y.; Wang, W.; Deng, X.; Wang, C.; Shi, W. Autophagy: A double-edged sword in ischemia-reperfusion injury. Cell. Mol. Biol. Lett. 2025, 30, 42. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, J.; Jiang, J.; Stavrovskaya, I.G.; Li, M.; Li, W.; Wu, Q.; Zhang, X.; Luo, C.; Zhou, S.; et al. N-acetyl-serotonin offers neuroprotection through inhibiting mitochondrial death pathways and autophagic activation in experimental models of ischemic injury. J. Neurosci. 2014, 34, 2967–2978. [Google Scholar] [CrossRef]
- Li, L.; Tian, J.; Long, M.K.; Chen, Y.; Lu, J.; Zhou, C.; Wang, T. Protection against Experimental Stroke by Ganglioside GM1 Is Associated with the Inhibition of Autophagy. PLoS ONE 2016, 11, e0144219. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Yu, G.; Ye, Y.; Zhu, H.; Wang, J.; Li, Y.; Chen, L.; Gu, L. G6PD protects against cerebral ischemia-reperfusion injury by inhibiting excessive mitophagy. Life Sci. 2025, 362, 123367. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.J.; Ding, Y.; Zhang, H.N.; Sun, T.; Zhang, K.; Yang, L.; Guo, Y.Y.; Liu, S.B.; Zhao, M.G.; et al. Silibinin Prevents Autophagic Cell Death upon Oxidative Stress in Cortical Neurons and Cerebral Ischemia-Reperfusion Injury. Mol. Neurobiol. 2016, 53, 932–943. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xu, Y.; Sun, J.; Li, L.; Zhang, J.H.; Wang, Y. Autophagy and Apoptosis in Acute Brain Injuries: From Mechanism to Treatment. Antioxid. Redox Signal. 2023, 38, 234–257. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Chen, W.; Yang, Y.; Wang, Y.; Wan, H.; Zhu, Z. Danhong injection alleviates cerebral ischemia-reperfusion injury by inhibiting autophagy through miRNA-132-3p/ATG12 signal axis. J. Ethnopharmacol. 2023, 300, 115724. [Google Scholar] [CrossRef]
- Liu, K.; Yao, X.; Gao, J.; Wang, J.; Qi, J. A study on the mechanism of Beclin-1 m6A modification mediated by catalpol in protection against neuronal injury and autophagy following cerebral ischemia. Mol. Med. 2024, 30, 65. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Yuan, Q.; Lu, J.; Xi, S.; Liu, Y.; Yang, G.; Xie, Z.; Wang, B.; Ma, L.; Fu, X.; et al. Tetrahydropiperine, a natural alkaloid with neuroprotective effects in ischemic stroke. J. Chem. Neuroanat. 2024, 136, 102397. [Google Scholar] [CrossRef]
- Zakharova, I.O.; Bayunova, L.V.; Avrova, D.K.; Tretyakova, A.D.; Shpakov, A.O.; Avrova, N.F. The Autophagic and Apoptotic Death of Forebrain Neurons of Rats with Global Brain Ischemia Is Diminished by the Intranasal Administration of Insulin: Possible Mechanism of Its Action. Curr. Issues Mol. Biol. 2024, 46, 6580–6599. [Google Scholar] [CrossRef]
- Buckley, K.M.; Hess, D.L.; Sazonova, I.Y.; Periyasamy-Thandavan, S.; Barrett, J.R.; Kirks, R.; Grace, H.; Kondrikova, G.; Johnson, M.H.; Hess, D.C.; et al. Rapamycin up-regulation of autophagy reduces infarct size and improves outcomes in both permanent MCAL, and embolic MCAO, murine models of stroke. Exp. Transl. Stroke Med. 2014, 6, 8. [Google Scholar] [CrossRef]
- Liu, X.; Tian, F.; Wang, S.; Wang, F.; Xiong, L. Astrocyte autophagy flux protects neurons against oxygen-glucose deprivation and ischemic/reperfusion injury. Rejuvenation Res. 2018, 215, 405–415. [Google Scholar] [CrossRef]
- Carloni, S.; Balduini, W. Simvastatin preconditioning confers neuroprotection against hypoxia-ischemia induced brain damage in neonatal rats via autophagy and silent information regulator 1 (SIRT1) activation. Exp. Neurol. 2020, 324, 113117. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Y.; Fan, H.; Wang, Y.; Fan, S.; Hu, S.; Shen, H.; Li, H.; Xue, Q.; Ni, J.; et al. GluA1 Degradation by Autophagy Contributes to Circadian Rhythm Effects on Cerebral Ischemia Injury. J. Neurosci. 2023, 43, 2381–2397. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Hu, Y.; Yun, T.; Yu, D.; Yang, G. USP7 promotes PINK1/Parkin-dependent mitophagy to ameliorate cerebral ischemia-reperfusion injury by deubiquitinating and stabilizing SIRT1. Brain Res. 2025, 1858, 149638. [Google Scholar] [CrossRef]
- Taft, R.J.; Pheasant, M.; Mattick, J.S. The relationship between non-protein-coding DNA and eukaryotic complexity. BioEssays 2007, 29, 288–299. [Google Scholar] [CrossRef]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.; Wolvetang, E.J.; Mattick, J.S.; Rinn, J.L.; Barry, G. Mechanisms of Long Non-coding RNAs in Mammalian Nervous System Development, Plasticity, Disease, and Evolution. Neuron 2015, 88, 861–877. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Hu, Q. Non-Coding RNA in Brain Development and Disorder. Curr. Med. Chem. 2017, 24, 1983–1997. [Google Scholar] [CrossRef]
- Diedrich, S. Nichtcodierende RNA in malignen Tumoren. Eine neue Welt von Tumor-Biomarkern und Zielstrukturen in Krebszellen [Non-coding RNA in malignant tumors. A new world of tumor biomarkers and target structures in cancer cells]. Pathologe 2010, 31 (Suppl. 2), 258–262. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Nakamori, M.; Nakatani, K. Modulating RNA secondary and tertiary structures by mismatch binding ligands. Methods 2019, 167, 78–91. [Google Scholar] [CrossRef]
- Gong, C.; Zhou, X.; Lai, S.; Wang, L.; Liu, J. Long noncoding RNA/circular RNA-miRNA-mRNA axes in ischemia-reperfusion injury. Biomed. Res. Int. 2020, 2020, 8838524. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Kuo, H.C. Functional roles and networks of non-coding RNAs in the pathogenesis of neurodegenerative diseases. J. Biomed. Sci. 2020, 27, 49. [Google Scholar] [CrossRef]
- Yang, Q.; Li, F.; He, A.T.; Yang, B.B. Circular RNAs: Expression, localization, and therapeutic potentials. Mol. Ther. 2021, 29, 1683–1702. [Google Scholar] [CrossRef]
- Wolska, M.; Jarosz-Popek, J.; Junger, E.; Wicik, Z.; Porshoor, T.; Sharif, L.; Czajka, P.; Postula, M.; Mirowska-Guzel, D.; Czlonkowska, A.; et al. Long non-coding RNAs as promising therapeutic approach in ischemic stroke: A comprehensive review. Mol. Neurobiol. 2021, 58, 1664–1682. [Google Scholar] [CrossRef]
- Kyzar, E.J.; Bohnsack, J.P.; Pandey, S.C. Current and future perspectives of noncoding RNAs in brain function and neuropsychiatric disease. Biol. Psychiatry 2022, 91, 183–193. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Holsinger, R.M.D. Roles of non-coding RNA in Alzheimer’s disease pathophysiology. Int. J. Mol. Sci. 2023, 24, 12498. [Google Scholar] [CrossRef]
- Zong, Y.; Dai, Y.; Yan, J.; Yu, B.; Wang, D.; Mao, S. The roles of circular RNAs in nerve injury and repair. Front. Mol. Neurosci. 2024, 17, 1419520. [Google Scholar] [CrossRef]
- Garimella, K.V.; England, E.; Weisburd, B.; Aguet, F.; Bacino, C.; Murdock, D.R.; Dai, H.; Rosenfeld, J.A.; Emrick, L.T.; Ketkar, S.; et al. Neurodevelopmental disorder caused by deletion of CHASERR, a lncRNA gene. N. Engl. J. Med. 2024, 391, 1511–1518. [Google Scholar] [CrossRef]
- Karami, Y.; Ehtiati, S.; Ghasemi, H.; Rafiee, M.; Zamani Sani, M.; Hosseini, S.E.; Moradi Kazerouni, H.; Movahedpour, A.; Aiiashi, S.; Khatami, S.H. Non-coding RNA biosensors for early detection of brain cancer. Clin. Chim. Acta 2025, 566, 120041. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, P. Roles of non-coding RNAs and exosomal non-coding RNAs, particularly microRNAs, long non-coding RNAs, and circular RNAs, in pathogenic mechanisms behind chronic pain: A review. Int. J. Biol. Macromol. 2025, 307 Pt 3, 141945. [Google Scholar] [CrossRef]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Liu, S.; Guo, X.Y.; Shang, Q.J.; Gao, P. The biogenesis, biological functions and modifications of circular RNAs. Exp. Mol. Pathol. 2023, 131, 104861. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, Y.; Wang, X.; Shen, J.; An, W. Advances in circular RNA and its applications. Int. J. Med. Sci. 2022, 19, 975–985. [Google Scholar] [CrossRef]
- Chuang, T.J.; Chiang, T.W.; Chen, C.Y. Assessing the impacts of various factors on circular RNA reliability. Life Sci. Alliance 2023, 6, e202201793. [Google Scholar] [CrossRef]
- Neag, M.A.; Mitre, A.O.; Burla777cu, C.C.; Inceu, A.I.; Mihu, C.; Melincovici, C.S.; Bichescu, M.; Buzoianu, A.D. miRNA involvement in cerebral ischemia-reperfusion injury. Front. Neurosci. 2022, 16, 901360. [Google Scholar] [CrossRef]
- Huang, A.; Zheng, H.; Wu, Z.; Chen, M.; Huang, Y. Circular RNA-protein interactions: Functions, mechanisms, and identification. Theranostics 2020, 10, 3503–3517. [Google Scholar] [CrossRef]
- Yao, Z.T.; Yang, Y.M.; Sun, M.M.; He, Y.; Liao, L.; Chen, K.S.; Li, B. New insights into the interplay between long non-coding RNAs and RNA-binding proteins in cancer. Cancer Commun. 2022, 42, 117–140. [Google Scholar] [CrossRef]
- Ferrè, F.; Colantoni, A.; Helmer-Citterich, M. Revealing protein-lncRNA interaction. Brief. Bioinform. 2016, 17, 106–116. [Google Scholar] [CrossRef]
- Das, A.; Sinha, T.; Shyamal, S.; Panda, A.C. Emerging Role of Circular RNA-Protein Interactions. Non-Coding RNA 2020, 7, 48. [Google Scholar] [CrossRef]
- Duan, X.; Han, L.; Peng, D.; Peng, C.; Xiao, L.; Bao, Q.; Peng, H. Bioinformatics analysis of a long non-coding RNA and mRNA regulation network in rats with middle cerebral artery occlusion based on RNA sequencing. Mol. Med. Rep. 2019, 20, 417–432. [Google Scholar] [CrossRef]
- Liu, C.; Yang, J.; Zhang, C.; Liu, M.; Geng, X.; Ji, X.; Du, H.; Zhao, H. Analysis of long non-coding RNA expression profiles following focal cerebral ischemia in mice. Neurosci. Lett. 2018, 665, 123–129. [Google Scholar] [CrossRef]
- Dharap, A.; Bowen, K.; Place, R.; Li, L.C.; Vemuganti, R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J. Cereb. Blood Flow Metab. 2009, 29, 675–687. [Google Scholar] [CrossRef]
- Duan, X.; Li, L.; Gan, J.; Peng, C.; Wang, X.; Chen, W.; Peng, D. Identification and functional analysis of circular RNAs induced in rats by middle cerebral artery occlusion. Gene 2019, 701, 139–145. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, C.; Yang, J.; Geng, X.; Du, H.; Ji, X.; Zhao, H. Screening circular RNA expression patterns following focal cerebral ischemia in mice. Oncotarget 2017, 8, 86535–86547. [Google Scholar] [CrossRef]
- Duan, X.; Gan, J.; Peng, D.Y.; Bao, Q.; Xiao, L.; Wei, L.; Wu, J. Identification and functional analysis of microRNAs in rats following focal cerebral ischemia injury. Mol. Med. Rep. 2019, 19, 4175–4184. [Google Scholar] [CrossRef]
- Barrera-Vázquez, O.S.; Gomez-Verjan, J.C.; Ramírez-Aldana, R.; Torre, P.G.; Rivero-Segura, N.A. Structural and pharmacological network analysis of miRNAs involved in acute aschemic stroke: A systematic review. Int. J. Mol. Sci. 2022, 23, 4663. [Google Scholar] [CrossRef]
- Hu, X.; Ma, F.; Cheng, Z.; Zeng, S.; Shen, R.; Li, X.; Hu, J.; Jin, Z.; Cheng, J. LncRNA PEG11as silencing sponges miR-874-3p to alleviate cerebral ischemia stroke via regulating autophagy in vivo and in vitro. Aging 2022, 14, 5177–5194. [Google Scholar] [CrossRef]
- Yu, S.; Yu, M.; He, X.; Wen, L.; Bu, Z.; Feng, J. KCNQ1OT1 promotes autophagy by regulating miR-200a/FOXO3/ATG7 pathway in cerebral ischemic stroke. Aging Cell 2019, 18, e12940. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, Y.; Zhou, H. LncRNA SNHG15 regulates autophagy and prevents cerebral ischaemia-reperfusion injury through mediating miR-153-3p/ATG5 axis. J. Cell Mol. Med. 2024, 28, e17956. [Google Scholar] [CrossRef]
- Cao, Y.; Pan, L.; Zhang, X.; Guo, W.; Huang, D. LncRNA SNHG3 promotes autophagy-induced neuronal cell apoptosis by acting as a ceRNA for miR-485 to up-regulate ATG7 expression. Metab. Brain Dis. 2020, 35, 1361–1369. [Google Scholar] [CrossRef]
- Wei, L.; Peng, Y.; Yang, X.J.; Zhou, P. Knockdown of long non-coding RNA RMRP protects cerebral ischemia-reperfusion injury via the microRNA-613/ATG3 axis and the JAK2/STAT3 pathway. Kaohsiung J. Med. Sci. 2021, 37, 468–478. [Google Scholar] [CrossRef]
- Sun, B.; Ou, H.; Ren, F.; Guan, Y.; Huan, Y.; Cai, H. Propofol Protects against Cerebral Ischemia/Reperfusion Injury by Down-Regulating Long Noncoding RNA SNHG14. ACS Chem. Neurosci. 2021, 12, 3002–3014. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tan, J.; Lin, L.; Zhang, W.; Yuan, W. Sevoflurane up-regulates miR-7a to protect against ischemic brain injury in rats by down-regulating ATG7 and reducing neuronal autophagy. Brain Res. Bull. 2022, 188, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Rong, J.; Shi, W.; Liu, W.; Wang, J.; Tan, J.; Yu, B.; Tong, J. GATA6 inhibits neuronal autophagy and ferroptosis in cerebral ischemia-reperfusion injury through a miR-193b/ATG7 axis-dependent mechanism. Neurochem. Res. 2023, 48, 2552–2567. [Google Scholar] [CrossRef]
- Guo, D.; Ma, J.; Yan, L.; Li, T.; Li, Z.; Han, X.; Shui, S. Down-Regulation of Lncrna MALAT1 Attenuates Neuronal Cell Death Through Suppressing Beclin1-Dependent Autophagy by Regulating Mir-30a in Cerebral Ischemic Stroke. Cell. Physiol. Biochem. 2017, 43, 182–194. [Google Scholar] [CrossRef]
- Deng, Z.; Ou, H.; Ren, F.; Guan, Y.; Huan, Y.; Cai, H.; Sun, B. LncRNA SNHG14 promotes OGD/R-induced neuron injury by inducing excessive mitophagy via miR-182-5p/BINP3 axis in HT22 mouse hippocampal neuronal cells. Biol. Res. 2020, 53, 38. [Google Scholar] [CrossRef]
- Li, B.; Huang, Z.; Meng, J.; Yu, W.; Yang, H. MiR-202-5p attenuates neurological deficits and neuronal injury in MCAO model rats and OGD-induced injury in Neuro-2a cells by targeting eIF4E-mediated induction of autophagy and inhibition of Akt/GSK-3β pathway. Mol. Cell. Probes 2020, 51, 101497. [Google Scholar] [CrossRef]
- Liu, N.; Peng, A.; Sun, H.; Zhuang, Y.; Yu, M.; Wang, Q.; Wang, J. LncRNA AC136007.2 alleviates cerebral ischemic-reperfusion injury by suppressing autophagy. Aging 2021, 13, 19587–19597. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, Y.; Cai, J.; Zhang, D.; Liu, S.; Pang, B. LncRNA SNHG12 Improves Cerebral Ischemic-reperfusion Injury by Activating SIRT1/FOXO3a Pathway through I nhibition of Autophagy and Oxidative Stress. Curr. Neurovasc. Res. 2020, 17, 394–401. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Tang, N. Long noncoding RNA Malat1 is a potent autophagy inducer protecting brain microvascular endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury by sponging miR-26b and upregulating ULK2 expression. Neuroscience 2017, 354, 1–10. [Google Scholar] [CrossRef]
- Yao, X.; Yao, R.; Huang, F.; Yi, J. LncRNA SNHG12 as a potent autophagy inducer exerts neuroprotective effects against cerebral ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 2019, 514, 490–496. [Google Scholar] [CrossRef]
- Ren, Z.; Xie, P.; Lv, J.; Hu, Y.; Guan, Z.; Chen, L.; Yu, W. MiR-187-3p inhibitor attenuates cerebral ischemia/reperfusion injury by regulating Seipin-mediated autophagic flux. Int. J. Mol. Med. 2020, 46, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.X.; Chen, S.F.; Xue, S.X.; Liu, P.D.; Liu, H.B. LncRNA TUG1 compromised neuronal mitophagy in cerebral ischemia/reperfusion injury by targeting sirtuin 1. Cell Biol. Toxicol. 2022, 38, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Lv, Q.Y.; Li, Q.L.; Zhang, H.; Chen, C.T.; Tian, H.M. Impact of acupuncture on ischemia/reperfusion injury: Unraveling the role of miR-34c-5p and autophagy activation. Brain Res. Bull. 2024, 215, 111031. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zou, Z.; Zou, R.; Zhou, X.; Cui, S. Electroacupuncture pretreatment induces tolerance against cerebral ischemia/reperfusion injury through inhibition of the autophagy pathway. Mol. Med. Rep. 2015, 11, 4438–4446. [Google Scholar] [CrossRef]
- Mei, Z.G.; Huang, Y.G.; Feng, Z.T.; Luo, Y.N.; Yang, S.B.; Du, L.P.; Jiang, K.; Liu, X.L.; Fu, X.Y.; Deng, Y.H.; et al. Electroacupuncture ameliorates cerebral ischemia/reperfusion injury by suppressing autophagy via the SIRT1-FOXO1 signaling pathway. Aging 2020, 12, 13187–13205. [Google Scholar] [CrossRef]
- Tian, W.; Zhu, M.; Zhou, Y.; Mao, C.; Zou, R.; Cui, Y.; Li, S.; Zhu, J.; Hu, C. Electroacupuncture pretreatment alleviates cerebral ischemia-reperfusion injury by regulating mitophagy via mTOR-ULK1/FUNDC1 axis in rats. J. Stroke Cerebrovasc. Dis. 2022, 31, 106202. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, T.; Li, S.; Zhao, R.; Li, H.; Zhang, X.; Li, Y.; Xia, Y.; Ni, G. Acupuncture extended the thrombolysis window by suppressing blood-brain barrier disruption and regulating autophagy-apoptosis balance after ischemic stroke. Brain Sci. 2024, 14, 399. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, H.; Peng, J.; Hu, X.; Xia, Y. EIF4A3-induced circ_0029941 promotes astrocyte activation through enhancing autophagy via miR-224-5p/NFAT5 axis. Mol. Neurobiol. 2025, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.; Cai, Y.; Zhang, Y.; Shen, L.; Xi, W.; Zhou, Z.; Xu, L.; Liu, X.; Han, B.; et al. Circular RNA SCMH1 suppresses KMO expression to inhibit mitophagy and promote functional recovery following stroke. Theranostics 2024, 14, 7292–7308. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, C.; Wen, X.; Liu, W.; Huang, X.; Li, Y.; Zang, J.; Weng, Z.; Lu, D.; Tsang, C.K.; et al. Circular RNA circ-FoxO3 attenuates blood-brain barrier damage by inducing autophagy during ischemia/reperfusion. Mol. Ther. 2022, 30, 1275–1287. [Google Scholar] [CrossRef]
- Mehta, S.L.; Chokkalla, A.K.; Bathula, S.; Arruri, V.; Chelluboina, B.; Vemuganti, R. CDR1as regulates α-synuclein-mediated ischemic brain damage by controlling miR-7 availability. Mol. Ther. Nucleic Acids 2022, 31, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, Y.; Zhang, Y.; Bai, Y.; Chen, X.; Huang, R.; Wu, F.; Leng, S.; Chao, J.; Zhang, J.H.; et al. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: Implications for cerebral ischemic stroke. Autophagy 2018, 14, 1164–1184. [Google Scholar] [CrossRef] [PubMed]
| References and the Authors | Models | The Axis or Changes that Regulate Brain Ischemia and Reperfusion Injury | How the Protection Was Achieved | The Changes of Autophagy to Reach the Protection |
|---|---|---|---|---|
| [34]—Zhang et al., 2023 | MCAO/R, rats, PC12 cells (OGD/R) | miR-132-3p/ATG12 | MiR-132-3p overexpression | Autophagy was inhibited |
| [76]—Hu et al., 2022 | MCAO/R, mice, Neuro-2a cells (OGD/R) | LncPEG11as/miR-874-3p/ATG16L1 | Knockdown of lncPEG11as | Autophagy was inhibited |
| [77]—Yu et al., 2019 | MCAO/R, mice, Neuro2A cells (OGD/R) | LncKCNQ1OT1/miR-200a/FOXO3/ATG7 | Knockdown of lncKCNQ1OT1 | Autophagy was inhibited |
| [78]—Yu et al., 2024 | Neuro 2a cells (OGD/R) | LncSNHG15/miR-153-3p/ATG5 | Downregulation of SNHG15 | Autophagy was inhibited |
| [79]—Cao et al., 2020 | MCAO/R, mice, Neuro-2a cells (OGD/R) | LncSNHG3/miR-485/ATG7 | Downregulation of lncSNHG3 | Autophagy was inhibited |
| [80]—Wei et al., 2021 | MCAO/R, mice, Neuro-2a cells (OGD/R) | LncRMRP/miR-613/ATG3 | Knockdown of lncRMRP | Autophagy was inhibited |
| [81]—Sun et al., 2021 | MCAO/R, mice HT22 cells (OGD/R) | LncSNHG14/miR-30b-5p/ATG5 | Inhibition of SNHG14 expression by propofol | Autophagy was inhibited |
| [82]—Wu et al., 2022 | MCAO/R, rats | miR-7a/ATG7 | Upregulation of miR-7a by sevoflurane | Autophagy was inhibited |
| [83]—Fan et al., 2023 | MCAO/R, rats, cortical neurons (OGD/R) | GATA6/miR-193b/ATG7 (GATA6 is a transcription factor) | Upregulation of miR-193b by GATA6 | Autophagy was inhibited |
| References and the Authors | Models | The Axis or Changes that Regulate Brain Ischemia and Reperfusion Injury | How the Protection Was Achieved | The Changes of Autophagy to Reach the Protection |
|---|---|---|---|---|
| [84]—Guo et al., 2017 | MCAO/R, mice, cortical neurons (OGD/R) | LncMalat1/miR-30a/Beclin1 | Decrease in lncMALAT1 level | Autophagy was inhibited |
| [85]—Deng et al., 2020 | HT22 cells (OGD/R) | LncSNHG14/miR-182-5p/ BNIP3 | MiR-182-5p overexpression | Autophagy was inhibited |
| [86]—Li et al., 2020 | MCAO/R, rats, Neuro-2a cells (OGD/R) | miR-202-5p/elF4E | Upregulation of miR-202-5p | Autophagy was inhibited |
| [87]—Liu et al., 2021 | MCAO/R, rats, SH-SY5Y cells (OGD/R) | Overexpression of Ac136997.2 | Overexpression of lncAC136007.2 | Autophagy was inhibited |
| [88]—Wu et al., 2020 | HT22 cells (OGD/R) | LncSNHG12 level was increased | Increase in lncSNHG12 level | Autophagy was inhibited |
| [89]—Li et al., 2017 | Primary BMECs (OGD/R) | LncMALAT1/mi-R-26b/ULK2 | Decrease in lncMALAT1 level | Autophagy was activated |
| [90]—Yao et al., 2019 | MCAO/R, mice, SH-SY5Y cells (OGD/R) | LncSNHG12 level was increased | Increase in lncSNHG12 level | Autophagy was activated |
| [91]—Ren et al., 2020 | PC12 cells (OGD/R) | MiR-187-3p-Seipin | miR-187-3p inhibition | Autophagy was activated |
| [92]—Xue et al., 2022 | MCAO/R, rats, SH-SY5Y cells (OGD/R) | LncTUG1 is Overexpressed | Knockdown of lncTUG1 | Mitotophagy was activated |
| [93]—Lu et al., 2024 | MCAO/R, rats, | Electroacupuncture, miR-34c-5p | Increase in miR-34c-5p expression | Autophagy was activated |
| References and the Authors | Model | The Axis or Changes that Regulate Brain Ischemia and Reperfusion Injury | How the Protection Was Achieved | The Changes of Autophagy to Reach the Protection |
|---|---|---|---|---|
| [98]—Jiang et al., 2025 | MCAO/R, mice, astro- cytes, A172 cells (OGD/R) | EIF4A3/circ_0029941/miR-224-5p/NFAT5 | Knockdown of circ_0029941 | Autophagy was inhibited |
| [99]—Wang et al., 2024 | MCAO/R, mice | CircSCMH1/KMO | CircSCMH1 suppresses the expression of KMO | Mitophagy was inhibited |
| [100]—Yang et al., 2022 | MCAO/R, mice, BMECs (OGD/R) | CircFOXO3 | Upregulation of circFOXO3 | Autophagy was activated |
| [101]—Mehta et al., 2022 | MCAO/R, rats | CircCDR1as, miR-7 | Overexpression of circCDR1as | Autophagy was inhibited |
| [102]—Han et al., 2018 | MCAO/R, mice | CircHECTD/miR-142 | Knock-down of circHECTD | Autophagy was inhibited |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharova, I.O.; Bayunova, L.V.; Avrova, N.F. The Regulatory Role of Non-Coding RNAs in Autophagy-Dependent Ischemia–Reperfusion Injury of the Brain. Curr. Issues Mol. Biol. 2025, 47, 462. https://doi.org/10.3390/cimb47060462
Zakharova IO, Bayunova LV, Avrova NF. The Regulatory Role of Non-Coding RNAs in Autophagy-Dependent Ischemia–Reperfusion Injury of the Brain. Current Issues in Molecular Biology. 2025; 47(6):462. https://doi.org/10.3390/cimb47060462
Chicago/Turabian StyleZakharova, Irina O., Liubov V. Bayunova, and Natalia F. Avrova. 2025. "The Regulatory Role of Non-Coding RNAs in Autophagy-Dependent Ischemia–Reperfusion Injury of the Brain" Current Issues in Molecular Biology 47, no. 6: 462. https://doi.org/10.3390/cimb47060462
APA StyleZakharova, I. O., Bayunova, L. V., & Avrova, N. F. (2025). The Regulatory Role of Non-Coding RNAs in Autophagy-Dependent Ischemia–Reperfusion Injury of the Brain. Current Issues in Molecular Biology, 47(6), 462. https://doi.org/10.3390/cimb47060462






