Targeting Cancer Cell Fate: Apoptosis, Autophagy, and Gold Nanoparticles in Treatment Strategies
Abstract
1. Introduction
2. Mechanisms of Life and Death: Apoptosis and Autophagy in Cellular Dynamics
2.1. Apoptosis
2.1.1. Apoptotic Pathways
2.1.2. Role of Apoptosis in Maintaining Tissue Homeostasis
2.1.3. Apoptosis in Cancer
2.2. Autophagy
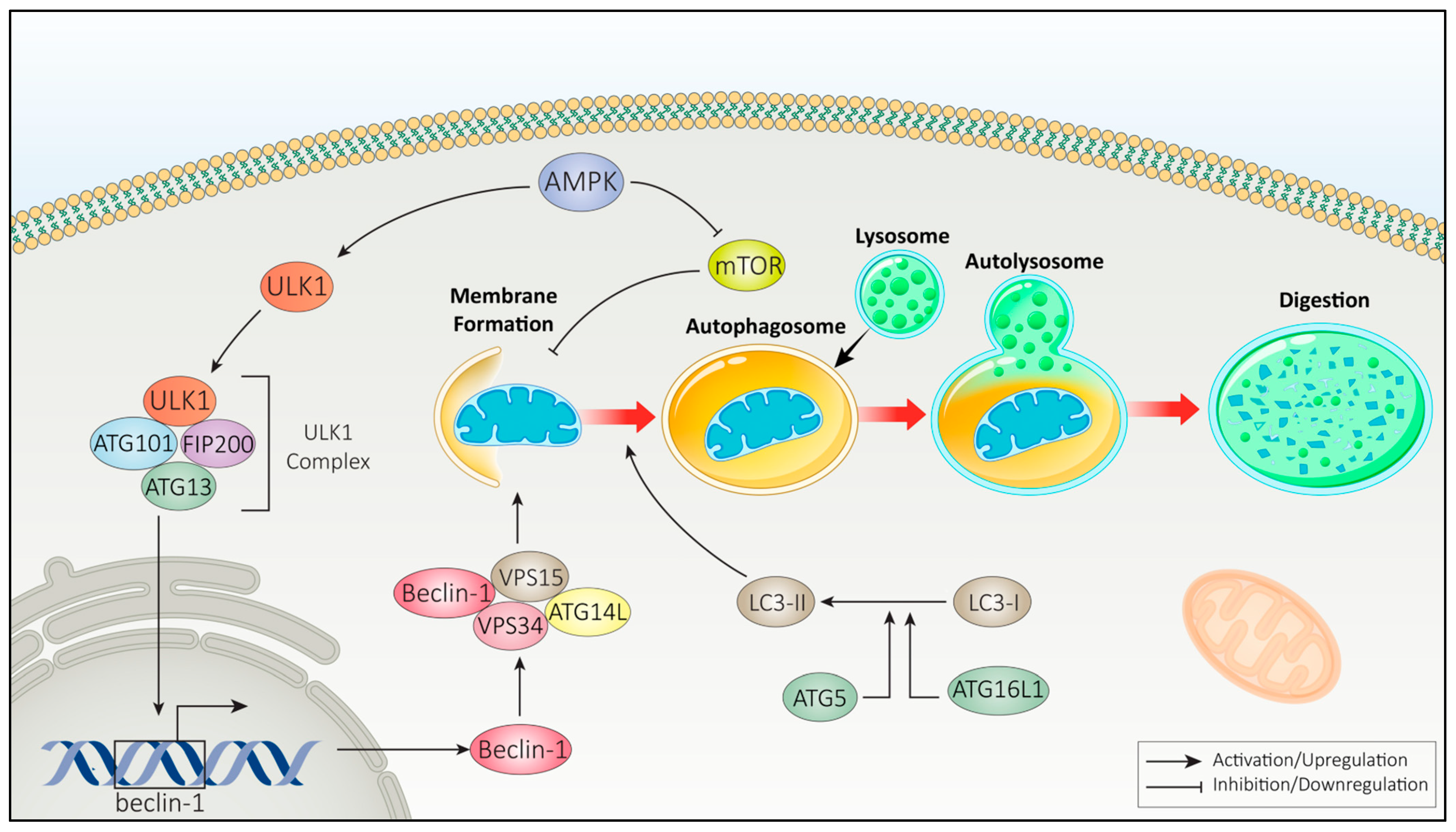
2.2.1. Autophagy Pathways
2.2.2. Autophagy in Cancer
2.3. Interplay Between Apoptosis and Autophagy
2.3.1. Regulation of Apoptosis on Autophagy
2.3.2. Regulation of Autophagy in Apoptosis
2.3.3. Interplay of Apoptosis and Autophagy in Cancer
3. Gold Nanoparticles in Cancer Treatment
3.1. Gold Nanoparticles as Radiosensitizers
Optimal Radiosensitization with Gold Nanoparticles
3.2. Gold Nanoparticles as Drug Carriers
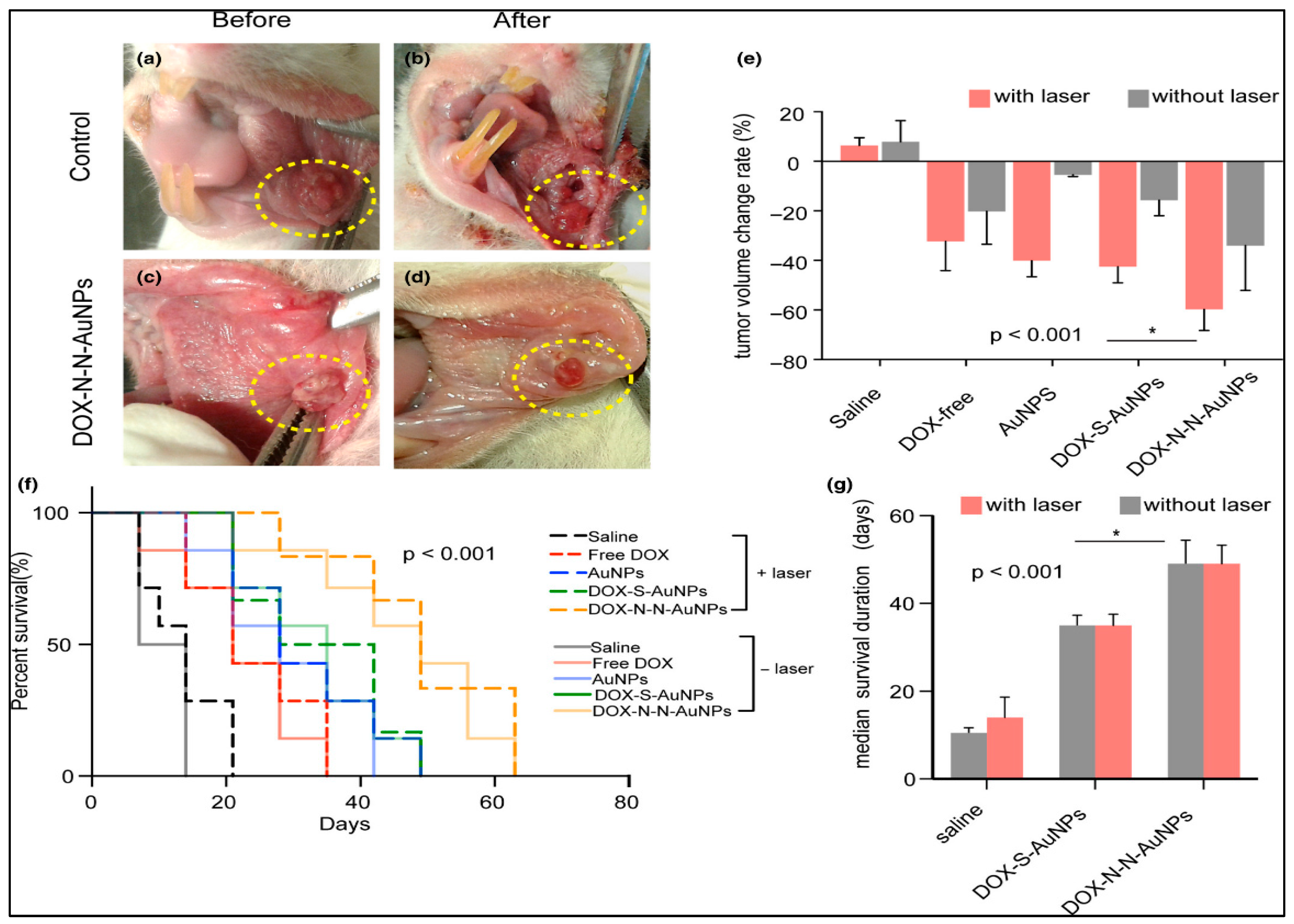
Optimal Drug Delivery with Gold Nanoparticles
4. Gold Nanoparticles in Regulating Autophagy and Apoptosis for Cancer Treatment
4.1. Gold Nanoparticles in Apoptosis
4.1.1. Gold Nanoparticles as Radiosensitizers in Apoptosis Induction
4.1.2. Gold Nanoparticles as Drug Carriers in Apoptosis Induction
4.2. Gold Nanoparticles and Autophagy
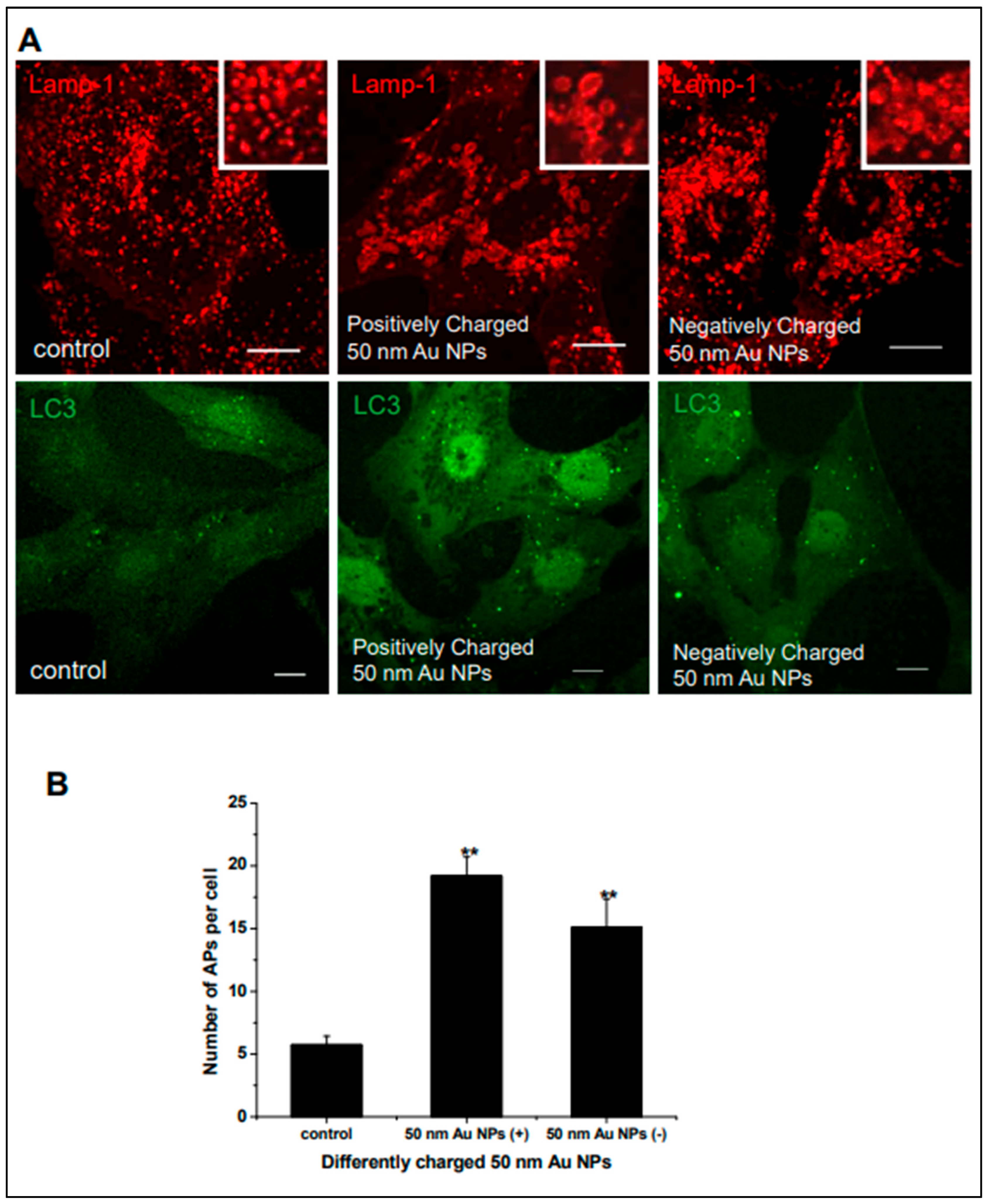
Gold Nanoparticles as Radiosensitizers in Autophagy Induction
4.3. Gold Nanoparticles as Drug Carriers in Autophagy Induction
4.3.1. Autophagy Regulation by AuNPs-Based Chemotherapeutic Agents
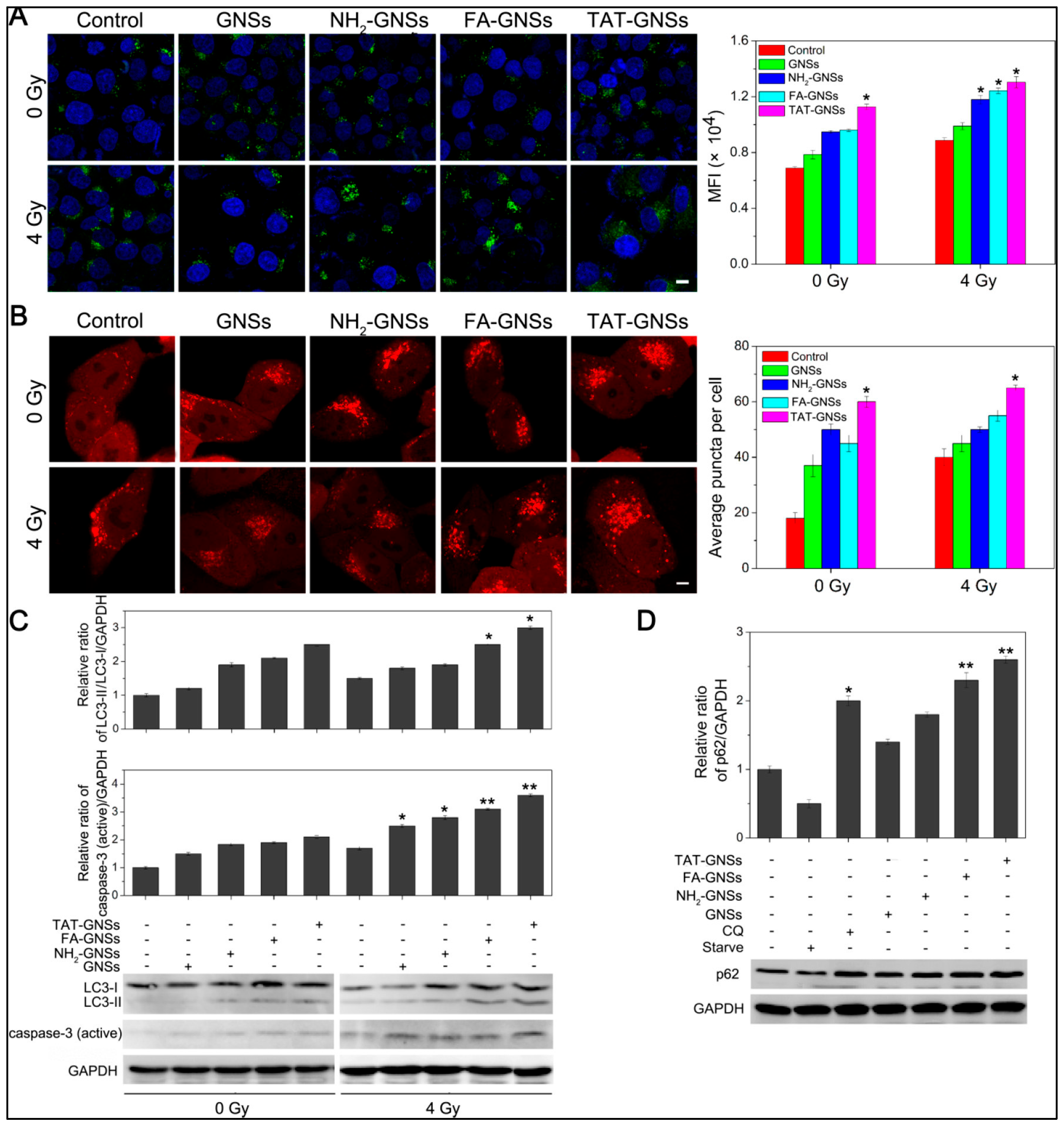
4.3.2. Autophagy Enhancement by AuNPs-Based Drug Delivery
4.4. Gold Nanoparticles in Dual Modulation of Apoptosis and Autophagy
5. Discussion
5.1. Current Research Progress and Observations
5.1.1. Gold Nanoparticles in Radiation Therapy: Apoptosis and Autophagy Through Radiosensitization
5.1.2. Gold Nanoparticles in Chemotherapy: Apoptosis and Autophagy Through Drug Delivery
5.1.3. Dual Modulation of Apoptosis and Autophagy with Gold Nanoparticles: A Synergistic Approach
5.2. Limitations
Clinical Translation and Regulatory Considerations
5.3. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andoh, V.; Ocansey, D.; Naveed, H.; Wang, N.; Chen, L.; Chen, K.; Mao, F. The Advancing Role of Nanocomposites in Cancer Diagnosis and Treatment. IJN 2024, 19, 6099–6126. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Chang, J.-E. Enhancing Cancer Treatment Through Combined Approaches: Photodynamic Therapy in Concert with Other Modalities. Pharmaceutics 2024, 16, 1420. [Google Scholar] [CrossRef] [PubMed]
- Ahire, V.; Ahmadi Bidakhvidi, N.; Boterberg, T.; Chaudhary, P.; Chevalier, F.; Daems, N.; Delbart, W.; Baatout, S.; Deroose, C.M.; Fernandez-Palomo, C.; et al. Radiobiology of Combining Radiotherapy with Other Cancer Treatment Modalities. In Radiobiology Textbook; Baatout, S., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 311–386. ISBN 978-3-031-18809-1. [Google Scholar]
- Looney, W.B.; Hopkins, H.A. Rationale for Different Chemotherapeutic and Radiation Therapy Strategies in Cancer Management. Cancer 1991, 67, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, C.; Huang, Y.; He, M.; Xu, W.W.; Li, B. Molecular Mechanisms of Chemo- and Radiotherapy Resistance and the Potential Implications for Cancer Treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef]
- Jurkovicova, D.; Neophytou, C.M.; Gašparović, A.Č.; Gonçalves, A.C. DNA Damage Response in Cancer Therapy and Resistance: Challenges and Opportunities. IJMS 2022, 23, 14672. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. IJN 2020, 15, 9407–9430. [Google Scholar] [CrossRef]
- Huang, H.; Liu, R.; Yang, J.; Dai, J.; Fan, S.; Pi, J.; Wei, Y.; Guo, X. Gold Nanoparticles: Construction for Drug Delivery and Application in Cancer Immunotherapy. Pharmaceutics 2023, 15, 1868. [Google Scholar] [CrossRef]
- Badir, A.; Refki, S.; Sekkat, Z. Utilizing Gold Nanoparticles in Plasmonic Photothermal Therapy for Cancer Treatment. Heliyon 2025, 11, e42738. [Google Scholar] [CrossRef]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-Consumption: The Interplay of Autophagy and Apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, B.; Xie, X.; Xiao, Y.-F.; Yang, S.-M.; Zhang, J.-W. The Interplay between DNA Repair and Autophagy in Cancer Therapy. Cancer Biol. Ther. 2015, 16, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ravendranathan, N.; Frisbee, J.C.; Singh, K.K. Complex Interplay between DNA Damage and Autophagy in Disease and Therapy. Biomolecules 2024, 14, 922. [Google Scholar] [CrossRef]
- Lei, Z.; Tian, Q.; Teng, Q.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z. Understanding and Targeting Resistance Mechanisms in Cancer. MedComm 2023, 4, e265. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Li, Y.; Rasheed, M.; Liu, J.; Chen, Z.; Deng, Y. Deciphering the Molecular Nexus: An In-Depth Review of Mitochondrial Pathways and Their Role in Cell Death Crosstalk. Cells 2024, 13, 863. [Google Scholar] [CrossRef]
- Cordani, M.; Butera, G.; Pacchiana, R.; Masetto, F.; Mullappilly, N.; Riganti, C.; Donadelli, M. Mutant P53-Associated Molecular Mechanisms of ROS Regulation in Cancer Cells. Biomolecules 2020, 10, 361. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting P53 Pathways: Mechanisms, Structures and Advances in Therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA Damage Response Revisited: The P53 Family and Its Regulators Provide Endless Cancer Therapy Opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The Role of BCL-2 Family Proteins in Regulating Apoptosis and Cancer Therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Hubenak, J.R.; Zhang, Q.; Branch, C.D.; Kronowitz, S.J. Mechanisms of Injury to Normal Tissue after Radiotherapy: A Review. Plast. Reconstr. Surg. 2014, 133, 49e–56e. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of Cell Death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Kim, J. Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life. Cells 2018, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Kausar, M.A.; Anwar, S.; Khan, Y.S.; Saleh, A.A.; Ahmed, M.A.A.; Kaur, S.; Iqbal, N.; Siddiqui, W.A.; Najm, M.Z. Autophagy and Cancer: Insights into Molecular Mechanisms and Therapeutic Approaches for Chronic Myeloid Leukemia. Biomolecules 2025, 15, 215. [Google Scholar] [CrossRef]
- Vitto, V.A.M.; Bianchin, S.; Zolondick, A.A.; Pellielo, G.; Rimessi, A.; Chianese, D.; Yang, H.; Carbone, M.; Pinton, P.; Giorgi, C.; et al. Molecular Mechanisms of Autophagy in Cancer Development, Progression, and Therapy. Biomedicines 2022, 10, 1596. [Google Scholar] [CrossRef]
- Kang, W.; Ishida, E.; Yamatoya, K.; Nakamura, A.; Miyado, M.; Miyamoto, Y.; Iwai, M.; Tatsumi, K.; Saito, T.; Saito, K.; et al. Autophagy-Disrupted LC3 Abundance Leads to Death of Supporting Cells of Human Oocytes. Biochem. Biophys. Rep. 2018, 15, 107–114. [Google Scholar] [CrossRef]
- White, E.; Karp, C.; Strohecker, A.M.; Guo, Y.; Mathew, R. Role of Autophagy in Suppression of Inflammation and Cancer. Curr. Opin. Cell Biol. 2010, 22, 212–217. [Google Scholar] [CrossRef]
- Rangel, M.; Kong, J.; Bhatt, V.; Khayati, K.; Guo, J.Y. Autophagy and Tumorigenesis. FEBS J. 2022, 289, 7177–7198. [Google Scholar] [CrossRef]
- Bhutia, S.K.; Mukhopadhyay, S.; Sinha, N.; Das, D.N.; Panda, P.K.; Patra, S.K.; Maiti, T.K.; Mandal, M.; Dent, P.; Wang, X.-Y.; et al. Autophagy. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2013; Volume 118, pp. 61–95. ISBN 978-0-12-407173-5. [Google Scholar]
- Pimentel, J.M.; Zhou, J.Y.; Wu, G.S. Autophagy and Cancer Therapy. Cancer Lett. 2024, 605, 217285. [Google Scholar] [CrossRef]
- Gump, J.M.; Thorburn, A. Autophagy and Apoptosis: What Is the Connection? Trends Cell Biol. 2011, 21, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Mei, Y.; Sinha, S. Role of the Crosstalk between Autophagy and Apoptosis in Cancer. J. Oncol. 2013, 2013, 102735. [Google Scholar] [CrossRef]
- Booth, L.A.; Roberts, J.L.; Dent, P. The Role of Cell Signaling in the Crosstalk between Autophagy and Apoptosis in the Regulation of Tumor Cell Survival in Response to Sorafenib and Neratinib. Semin. Cancer Biol. 2020, 66, 129–139. [Google Scholar] [CrossRef]
- Li, D.; Peng, X.; He, G.; Liu, J.; Li, X.; Lin, W.; Fang, J.; Li, X.; Yang, S.; Yang, L.; et al. Crosstalk between Autophagy and CSCs: Molecular Mechanisms and Translational Implications. Cell Death Dis. 2023, 14, 409. [Google Scholar] [CrossRef]
- Prajapati, A.; Rangra, S.; Patil, R.; Desai, N.; Jyothi, V.G.S.S.; Salave, S.; Amate, P.; Benival, D.; Kommineni, N. Receptor-Targeted Nanomedicine for Cancer Therapy. Receptors 2024, 3, 323–361. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing Nanotechnology to Improve Targeted Cancer Treatment: Overcoming Hurdles in Its Clinical Implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Zeng, Q.; Ma, X.; Song, Y.; Chen, Q.; Jiao, Q.; Zhou, L. Targeting Regulated Cell Death in Tumor Nanomedicines. Theranostics 2022, 12, 817–841. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, Pyroptosis and Apoptosis: An Intricate Game of Cell Death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Samir, P.; Malireddi, R.K.S.; Kanneganti, T.-D. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 238. [Google Scholar] [CrossRef]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, Pyroptosis, and Necroptosis—Oh My! The Many Ways a Cell Can Die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar] [CrossRef]
- Sorice, M. Crosstalk of Autophagy and Apoptosis. Cells 2022, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- Baena-Lopez, L.A.; Wang, L.; Wendler, F. Cellular Stress Management by Caspases. Curr. Opin. Cell Biol. 2024, 86, 102314. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-J.; Jiang, S.-S.; Zhang, J.; Luo, D.; Yu, B.; Yang, L.-Y.; Zhong, H.-H.; Yang, M.-W.; Liu, L.-Y.; Hong, F.-F.; et al. Effects of Apoptosis on Liver Aging. WJCC 2019, 7, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Wei, S.; Nguyen, T.H.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.-M.; Kim, H.H.; Ha, K.-T.; et al. Mitochondria-Associated Programmed Cell Death as a Therapeutic Target for Age-Related Disease. Exp. Mol. Med. 2023, 55, 1595–1619. [Google Scholar] [CrossRef]
- Nössing, C.; Ryan, K.M. 50 Years on and Still Very Much Alive: ‘Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue Kinetics. Br. J. Cancer 2023, 128, 426–431. [Google Scholar] [CrossRef]
- Yin, S.; Ji, C.; Wu, P.; Jin, C.; Qian, H. Human Umbilical Cord Mesenchymal Stem Cells and Exosomes: Bioactive Ways of Tissue Injury Repair. Am. J. Transl. Res. 2019, 11, 1230–1240. [Google Scholar]
- Lee, S.-B.; Lee, S.; Park, J.-Y.; Lee, S.-Y.; Kim, H.-S. Induction of P53-Dependent Apoptosis by Prostaglandin A2. Biomolecules 2020, 10, 492. [Google Scholar] [CrossRef]
- Verma, R.; Verma, P.; Budhwar, S.; Singh, K. S100 Proteins: An Emerging Cynosure in Pregnancy & Adverse Reproductive Outcome. Indian J. Med. Res. 2018, 148, S100–S106. [Google Scholar] [CrossRef]
- Mehrbod, P.; Ande, S.R.; Alizadeh, J.; Rahimizadeh, S.; Shariati, A.; Malek, H.; Hashemi, M.; Glover, K.K.M.; Sher, A.A.; Coombs, K.M.; et al. The Roles of Apoptosis, Autophagy and Unfolded Protein Response in Arbovirus, Influenza Virus, and HIV Infections. Virulence 2019, 10, 376–413. [Google Scholar] [CrossRef]
- Ryoo, H.D.; Bergmann, A. The Role of Apoptosis-Induced Proliferation for Regeneration and Cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008797. [Google Scholar] [CrossRef]
- Mehrotra, P.; Ravichandran, K.S. Drugging the Efferocytosis Process: Concepts and Opportunities. Nat. Rev. Drug Discov. 2022, 21, 601–620. Available online: https://www.nature.com/articles/s41573-022-00470-y (accessed on 29 May 2025). [CrossRef] [PubMed]
- Banerjee, D.S.; Banerjee, S. Design Principles and Feedback Mechanisms in Organelle Size Control. Curr. Opin. Cell Biol. 2025, 95, 102533. [Google Scholar] [CrossRef]
- Eckert, J.; Viasnoff, V.; Yap, A.S. New Directions in Epithelial Mechanoadaptation. Curr. Opin. Cell Biol. 2025, 95, 102536. [Google Scholar] [CrossRef]
- Fukagawa, T.; Torres-Padilla, M.E. Exploring the Cell Nucleus: From Chromosome Structure to Single-Cell Omics. Curr. Opin. Cell Biol. 2025, 95, 102530. [Google Scholar] [CrossRef] [PubMed]
- Hollenstein, D.M.; Kraft, C. Autophagosomes Are Formed at a Distinct Cellular Structure. Curr. Opin. Cell Biol. 2020, 65, 50–57. [Google Scholar] [CrossRef]
- Liang, C.; Jung, J.U. Autophagy Genes as Tumor Suppressors. Curr. Opin. Cell Biol. 2010, 22, 226–233. [Google Scholar] [CrossRef]
- Llères, D.; Gizzi, A.C.; Nollmann, M. Redefining Enhancer Action: Insights from Structural, Genomic, and Single-Molecule Perspectives. Curr. Opin. Cell Biol. 2025, 95, 102527. [Google Scholar] [CrossRef] [PubMed]
- New Approach Methods in Immunology. Available online: https://www.frontiersin.org/research-topics/55518/new-approach-methods-in-immunology/magazine (accessed on 29 May 2025).
- Mullen, N.J.; Singh, P.K. Nucleotide Metabolism: A Pan-Cancer Metabolic Dependency. Nat. Rev. Cancer 2023, 23, 275–294. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Fridman, J.S.; Lowe, S.W. Control of Apoptosis by P53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef]
- Chi, S.-W. Structural Insights into the Transcription-Independent Apoptotic Pathway of P53. BMB Rep. 2014, 47, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Egle, A.; Harris, A.W.; Bouillet, P.; Cory, S. Bim Is a Suppressor of Myc-Induced Mouse B Cell Leukemia. Proc. Natl. Acad. Sci. USA 2004, 101, 6164–6169. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Tumor Resistance to Apoptosis. Int. J. Cancer 2009, 124, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martinez, O.; De Leon-Oliva, D.; Boaru, D.L.; Lopez-Gonzalez, L.; García-Montero, C.; Alvarez-Mon, M.A.; Guijarro, L.G.; Torres-Carranza, D.; Saez, M.A.; et al. Autophagy in Its (Proper) Context: Molecular Basis, Biological Relevance, Pharmacological Modulation, and Lifestyle Medicine. Int. J. Biol. Sci. 2024, 20, 2532–2554. [Google Scholar] [CrossRef]
- Gómez-Virgilio, L.; Silva-Lucero, M.-C.; Flores-Morelos, D.-S.; Gallardo-Nieto, J.; Lopez-Toledo, G.; Abarca-Fernandez, A.-M.; Zacapala-Gómez, A.-E.; Luna-Muñoz, J.; Montiel-Sosa, F.; Soto-Rojas, L.O.; et al. Autophagy: A Key Regulator of Homeostasis and Disease: An Overview of Molecular Mechanisms and Modulators. Cells 2022, 11, 2262. [Google Scholar] [CrossRef]
- Khandia, R.; Dadar, M.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Iqbal, H.M.N.; Singh, K.P.; Joshi, S.K.; et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells 2019, 8, 674. [Google Scholar] [CrossRef]
- Pecoraro, A.; Pagano, M.; Russo, G.; Russo, A. Role of Autophagy in Cancer Cell Response to Nucleolar and Endoplasmic Reticulum Stress. IJMS 2020, 21, 7334. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Codogno, P.; Zhang, H. Machinery, Regulation and Pathophysiological Implications of Autophagosome Maturation. Nat. Rev. Mol. Cell Biol. 2021, 22, 733–750. [Google Scholar] [CrossRef]
- Sehrawat, A.; Mishra, J.; Mastana, S.S.; Navik, U.; Bhatti, G.K.; Reddy, P.H.; Bhatti, J.S. Dysregulated Autophagy: A Key Player in the Pathophysiology of Type 2 Diabetes and Its Complications. Biochim. et Biophys. Acta (BBA)—Mol. Basis Dis. 2023, 1869, 166666. [Google Scholar] [CrossRef]
- Wu, N.; Zheng, W.; Zhou, Y.; Tian, Y.; Tang, M.; Feng, X.; Ashrafizadeh, M.; Wang, Y.; Niu, X.; Tambuwala, M.; et al. Autophagy in Aging-Related Diseases and Cancer: Principles, Regulatory Mechanisms and Therapeutic Potential. Ageing Res. Rev. 2024, 100, 102428. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.-L. Macroautophagy in Mammalian Cells; Landes Bioscience: Austin, TX, USA, 2000; Volume Madame Curie Bioscience Database. [Google Scholar]
- Yang, Z.; Klionsky, D.J. An Overview of the Molecular Mechanism of Autophagy. In Autophagy in Infection and Immunity; Levine, B., Yoshimori, T., Deretic, V., Eds.; Current Topics in Microbiology and Immunology; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2009; Volume 335, pp. 1–32. ISBN 978-3-642-00301-1. [Google Scholar]
- Jiang, M.; Wu, W.; Xiong, Z.; Yu, X.; Ye, Z.; Wu, Z. Targeting Autophagy Drug Discovery: Targets, Indications and Development Trends. Eur. J. Med. Chem. 2024, 267, 116117. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK Signalling Pathway Coordinates Cell Growth, Autophagy and Metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-Activated Protein Kinase—An Energy Sensor That Regulates All Aspects of Cell Function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef]
- Smiles, W.J.; Ovens, A.J.; Oakhill, J.S.; Kofler, B. The Metabolic Sensor AMPK: Twelve Enzymes in One. Mol. Metab. 2024, 90, 102042. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.-Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.-L. ULK1 Induces Autophagy by Phosphorylating Beclin-1 and Activating VPS34 Lipid Kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Seo, M.; Jung, C.H.; Grunwald, D.; Stone, M.; Otto, N.M.; Toso, E.; Ahn, Y.; Kyba, M.; Griffin, T.J.; et al. ULK1 Phosphorylates Ser30 of BECN1 in Association with ATG14 to Stimulate Autophagy Induction. Autophagy 2018, 14, 584–597. [Google Scholar] [CrossRef]
- Park, J.-M.; Jung, C.H.; Seo, M.; Otto, N.M.; Grunwald, D.; Kim, K.H.; Moriarity, B.; Kim, Y.-M.; Starker, C.; Nho, R.S.; et al. The ULK1 Complex Mediates MTORC1 Signaling to the Autophagy Initiation Machinery via Binding and Phosphorylating ATG14. Autophagy 2016, 12, 547–564. [Google Scholar] [CrossRef]
- De La Ballina, L.R.; Munson, M.J.; Simonsen, A. Lipids and Lipid-Binding Proteins in Selective Autophagy. J. Mol. Biol. 2020, 432, 135–159. [Google Scholar] [CrossRef]
- Iriondo, M.N.; Etxaniz, A.; Varela, Y.R.; Ballesteros, U.; Lázaro, M.; Valle, M.; Fracchiolla, D.; Martens, S.; Montes, L.R.; Goñi, F.M.; et al. Effect of ATG12–ATG5-ATG16L1 Autophagy E3-like Complex on the Ability of LC3/GABARAP Proteins to Induce Vesicle Tethering and Fusion. Cell. Mol. Life Sci. 2023, 80, 56. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Entezari, M.; Clark, C.; Zabolian, A.; Ranjbar, E.; Farahani, M.V.; Saleki, H.; Sharifzadeh, S.O.; Far, F.B.; Ashrafizadeh, M.; et al. Targeted Regulation of Autophagy Using Nanoparticles: New Insight into Cancer Therapy. Biochim. et Biophys. Acta (BBA)—Mol. Basis Dis. 2022, 1868, 166326. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (4th Edition)1. Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the Pathogenesis of Disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Jia, R.; Dai, Z.; Zhou, J.; Ruan, J.; Chng, W.; Cai, Z.; Zhang, X. Stress Granules in Cancer: Adaptive Dynamics and Therapeutic Implications. iScience 2024, 27, 110359. [Google Scholar] [CrossRef]
- Yun, C.W.; Jeon, J.; Go, G.; Lee, J.H.; Lee, S.H. The Dual Role of Autophagy in Cancer Development and a Therapeutic Strategy for Cancer by Targeting Autophagy. IJMS 2020, 22, 179. [Google Scholar] [CrossRef]
- Mathew, R.; Karantza-Wadsworth, V.; White, E. Role of Autophagy in Cancer. Nat. Rev. Cancer 2007, 7, 961–967. [Google Scholar] [CrossRef]
- Mathur, A.; Ritu; Chandra, P.; Das, A. Autophagy: A Necessary Evil in Cancer and Inflammation. 3 Biotech 2024, 14, 87. [Google Scholar] [CrossRef]
- Tripathi, S.; Sharma, Y.; Kumar, D. Unveiling the Link between Chronic Inflammation and Cancer. Metab. Open 2025, 25, 100347. [Google Scholar] [CrossRef]
- Rakesh, R.; PriyaDharshini, L.C.; Sakthivel, K.M.; Rasmi, R.R. Role and Regulation of Autophagy in Cancer. Biochim. et Biophys. Acta (BBA)—Mol. Basis Dis. 2022, 1868, 166400. [Google Scholar] [CrossRef]
- Taucher, E.; Mykoliuk, I.; Fediuk, M.; Smolle-Juettner, F.-M. Autophagy, Oxidative Stress and Cancer Development. Cancers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.-H.; Li, N. Tumor Dormancy and Relapse: Understanding the Molecular Mechanisms of Cancer Recurrence. Mil. Med. Res. 2025, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Tian, K.; Ran, Y.; Zhou, H.; Zhou, L.; Ding, Y.; Tang, X. Beclin-1: A Therapeutic Target at the Intersection of Autophagy, Immunotherapy, and Cancer Treatment. Front. Immunol. 2024, 15, 1506426. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rubín-de-Celis, S. The Role of Beclin 1-Dependent Autophagy in Cancer. Biology 2019, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, J.; Zhu, Y.; Wang, L.; Jiang, X.; Liu, B.; He, G. Targeting Autophagy and beyond: Deconvoluting the Complexity of Beclin-1 from Biological Function to Cancer Therapy. Acta Pharm. Sin. B 2023, 13, 4688–4714. [Google Scholar] [CrossRef]
- Pajares, M.J.; Alemany-Cosme, E.; Goñi, S.; Bandres, E.; Palanca-Ballester, C.; Sandoval, J. Epigenetic Regulation of microRNAs in Cancer: Shortening the Distance from Bench to Bedside. IJMS 2021, 22, 7350. [Google Scholar] [CrossRef]
- Mandhair, H.K.; Novak, U.; Radpour, R. Epigenetic Regulation of Autophagy: A Key Modification in Cancer Cells and Cancer Stem Cells. WJSC 2021, 13, 542–567. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, H.; Li, Y.; Zhao, H. Advances in Epigenetic Modifications and Cervical Cancer Research. Biochim. et Biophys. Acta (BBA)—Rev. Cancer 2023, 1878, 188894. [Google Scholar] [CrossRef]
- Qin, Y.; Ashrafizadeh, M.; Mongiardini, V.; Grimaldi, B.; Crea, F.; Rietdorf, K.; Győrffy, B.; Klionsky, D.J.; Ren, J.; Zhang, W.; et al. Autophagy and Cancer Drug Resistance in Dialogue: Pre-Clinical and Clinical Evidence. Cancer Lett. 2023, 570, 216307. [Google Scholar] [CrossRef]
- Xi, H.; Wang, S.; Wang, B.; Hong, X.; Liu, X.; Li, M.; Shen, R.; Dong, Q. The Role of Interaction between Autophagy and Apoptosis in Tumorigenesis (Review). Oncol. Rep. 2022, 48, 208. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Panda, P.K.; Sinha, N.; Das, D.N.; Bhutia, S.K. Autophagy and Apoptosis: Where Do They Meet? Apoptosis 2014, 19, 555–566. [Google Scholar] [CrossRef]
- Wirawan, E.; Vande Walle, L.; Kersse, K.; Cornelis, S.; Claerhout, S.; Vanoverberghe, I.; Roelandt, R.; De Rycke, R.; Verspurten, J.; Declercq, W.; et al. Caspase-Mediated Cleavage of Beclin-1 Inactivates Beclin-1-Induced Autophagy and Enhances Apoptosis by Promoting the Release of Proapoptotic Factors from Mitochondria. Cell Death Dis. 2010, 1, e18. [Google Scholar] [CrossRef] [PubMed]
- Al-Odat, O.S.; Guirguis, D.A.; Schmalbach, N.K.; Yao, G.; Budak-Alpdogan, T.; Jonnalagadda, S.C.; Pandey, M.K. Autophagy and Apoptosis: Current Challenges of Treatment and Drug Resistance in Multiple Myeloma. IJMS 2022, 24, 644. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.G.; Wodetzki, D.; Thorburn, A. Autophagy and Cancer: Modulation of Cell Death Pathways and Cancer Cell Adaptations. J. Cell Biol. 2019, 219, e201909033. [Google Scholar] [CrossRef]
- Noguchi, M.; Hirata, N.; Tanaka, T.; Suizu, F.; Nakajima, H.; Chiorini, J.A. Autophagy as a Modulator of Cell Death Machinery. Cell Death Dis. 2020, 11, 517. [Google Scholar] [CrossRef]
- Zada, S.; Hwang, J.S.; Ahmed, M.; Lai, T.H.; Pham, T.M.; Elashkar, O.; Kim, D.R. Cross Talk between Autophagy and Oncogenic Signaling Pathways and Implications for Cancer Therapy. Biochim. et Biophys. Acta (BBA)—Rev. Cancer 2021, 1876, 188565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, R.; Wang, S.-S.; Jiang, X.-Y.; Cui, H.-Y.; Guo, Y.; Song, X.-Y.; Guo, Q.-Q.; Cao, L. Autophagy-Related Proteins in Genome Stability: Autophagy-Dependent and Independent Actions. Int. J. Biol. Sci. 2022, 18, 5329–5344. [Google Scholar] [CrossRef]
- Cabrera-Serrano, A.J.; Sánchez-Maldonado, J.M.; González-Olmedo, C.; Carretero-Fernández, M.; Díaz-Beltrán, L.; Gutiérrez-Bautista, J.F.; García-Verdejo, F.J.; Gálvez-Montosa, F.; López-López, J.A.; García-Martín, P.; et al. Crosstalk Between Autophagy and Oxidative Stress in Hematological Malignancies: Mechanisms, Implications, and Therapeutic Potential. Antioxidants 2025, 14, 264. [Google Scholar] [CrossRef]
- Filippone, A.; Esposito, E.; Mannino, D.; Lyssenko, N.; Praticò, D. The Contribution of Altered Neuronal Autophagy to Neurodegeneration. Pharmacol. Ther. 2022, 238, 108178. [Google Scholar] [CrossRef]
- Barmaki, H.; Nourazarian, A.; Shademan, B.; Khaki-Khatibi, F. The Autophagy Paradox: A New Hypothesis in Neurodegenerative Disorders. Neurochem. Int. 2024, 179, 105827. [Google Scholar] [CrossRef]
- Dalby, K.; Tekedereli, I.; Lopez-Berestein, G.; Ozpolat, B. Targeting the Pro-Death and pro-Survival Functions of Autophagy as Novel Therapeutic Strategies in Cancer. Autophagy 2010, 6, 322–329. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.A.; Kirby, R. Apoptosis: A Review of Pro-apoptotic and Anti-apoptotic Pathways and Dysregulation in Disease. J. Vet. Emerg. Crit. Care 2008, 18, 572–585. [Google Scholar] [CrossRef]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- Rubinstein, A.D.; Eisenstein, M.; Ber, Y.; Bialik, S.; Kimchi, A. The Autophagy Protein Atg12 Associates with Antiapoptotic Bcl-2 Family Members to Promote Mitochondrial Apoptosis. Mol. Cell 2011, 44, 698–709. [Google Scholar] [CrossRef]
- Yousefi, S.; Perozzo, R.; Schmid, I.; Ziemiecki, A.; Schaffner, T.; Scapozza, L.; Brunner, T.; Simon, H.-U. Calpain-Mediated Cleavage of Atg5 Switches Autophagy to Apoptosis. Nat. Cell Biol. 2006, 8, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Fitzwalter, B.E.; Thorburn, A. Recent Insights into Cell Death and Autophagy. FEBS J. 2015, 282, 4279–4288. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The Mechanism of HMGB1 Secretion and Release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Foglio, E.; Pellegrini, L.; Germani, A.; Russo, M.A.; Limana, F. HMGB1-Mediated Apoptosis and Autophagy in Ischemic Heart Diseases. Vasc. Biol. 2019, 1, H89–H96. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zou, J.; Zhong, X.; Li, J.; Kang, R.; Tang, D. HMGB1 in the Interplay between Autophagy and Apoptosis in Cancer. Cancer Lett. 2024, 581, 216494. [Google Scholar] [CrossRef]
- Livesey, K.M.; Kang, R.; Vernon, P.; Buchser, W.; Loughran, P.; Watkins, S.C.; Zhang, L.; Manfredi, J.J.; Zeh, H.J.; Li, L.; et al. P53/HMGB1 Complexes Regulate Autophagy and Apoptosis. Cancer Res. 2012, 72, 1996–2005. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Y.; Zhang, J.; Dong, X.; Gao, P.; Liu, K.; Ma, C.; Zhao, G. Breaking Bad: Autophagy Tweaks the Interplay Between Glioma and the Tumor Immune Microenvironment. Front. Immunol. 2021, 12, 746621. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Ramírez, A.; Castillo-Rodríguez, R.A.; Zavala-Vega, S.; Jimenez-Farfan, D.; Anaya-Rubio, I.; Briseño, E.; Palencia, G.; Guevara, P.; Cruz-Salgado, A.; Sotelo, J.; et al. Autophagy as a Potential Therapy for Malignant Glioma. Pharmaceuticals 2020, 13, 156. [Google Scholar] [CrossRef]
- Du, J.; Li, J.; Song, D.; Li, Q.; Li, L.; Li, B.; Li, L. Matrine exerts anti-breast cancer activity by mediating apoptosis and protective autophagy via the AKT/mTOR pathway in MCF-7 cells. Mol. Med. Rep. 2020, 22, 3659–3666. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.; Yang, C.; Hu, Y.; Liao, X.; Liu, Q. Autophagy Modulation in Therapeutic Strategy of Breast Cancer Drug Resistance. J. Cancer 2024, 15, 5462–5476. [Google Scholar] [CrossRef]
- Raza, S.; Siddiqui, J.A.; Srivastava, A.; Chattopadhyay, N.; Sinha, R.A.; Chakravarti, B. Autophagy as a Therapeutic Target in Breast Tumors: The Cancer Stem Cell Perspective. Autophagy Rep. 2024, 3, 2358648. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Paranandi, K.S.; Sridharan, S.; Basu, A. Autophagy in Breast Cancer and Its Implications for Therapy. Am. J. Cancer Res. 2013, 3, 251–265. [Google Scholar]
- Vera-Ramirez, L.; Vodnala, S.K.; Nini, R.; Hunter, K.W.; Green, J.E. Autophagy Promotes the Survival of Dormant Breast Cancer Cells and Metastatic Tumour Recurrence. Nat. Commun. 2018, 9, 1944. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, H.; Li, Y.; Wang, B.; Li, Q.; Zhang, L.; Liu, X.; Wang, C.; Ertas, Y.N.; Shi, H. Autophagy Flux in Bladder Cancer: Cell Death Crosstalk, Drug and Nanotherapeutics. Cancer Lett. 2024, 591, 216867. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Y.; Lian, X. Targeted Inhibition of GRP78 by HA15 Promotes Apoptosis of Lung Cancer Cells Accompanied by ER Stress and Autophagy. Biol. Open 2020, 11, bio.053298. [Google Scholar] [CrossRef]
- Lei, W.; Huo, Z. Jervine Inhibits Non-Small Cell Lung Cancer (NSCLC) Progression by Suppressing Hedgehog and AKT Signaling via Triggering Autophagy-Regulated Apoptosis. Biochem. Biophys. Res. Commun. 2020, 533, 397–403. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Paskeh, M.D.A.; Mirzaei, S.; Gholami, M.H.; Zarrabi, A.; Hashemi, F.; Hushmandi, K.; Hashemi, M.; Nabavi, N.; Crea, F.; et al. Targeting Autophagy in Prostate Cancer: Preclinical and Clinical Evidence for Therapeutic Response. J. Exp. Clin. Cancer Res. 2022, 41, 105. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, D.; Pandolfo, S.D.; Rogers, D.; Cerrato, C.; Di Meo, N.A.; Autorino, R.; Mirone, V.; Ferro, M.; Porta, C.; Stella, A.; et al. Novel Insights into Autophagy and Prostate Cancer: A Comprehensive Review. IJMS 2022, 23, 3826. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Singh, M.P.; Amaravadi, R.K. Recent Advances in Targeting Autophagy in Cancer. Trends Pharmacol. Sci. 2023, 44, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.M.; Fok, M.; Grundy, G.; Parsons, J.L.; Rocha, S. The Role of Autophagy in Hypoxia-Induced Radioresistance. Radiother. Oncol. 2023, 189, 109951. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, X.; Torabian, P.; Yang, Z. Investigating Autophagy and Intricate Cellular Mechanisms in Hepatocellular Carcinoma: Emphasis on Cell Death Mechanism Crosstalk. Cancer Lett. 2024, 588, 216744. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Xu, H.; Xu, L.; Chen, D.; Wang, J.; Huang, S.; Wen, Y.; Fang, L. Long Non-Coding RNA SNHG1 Regulates the Wnt/β-Catenin and PI3K/AKT/mTOR Signaling Pathways via EZH2 to Affect the Proliferation, Apoptosis, and Autophagy of Prostate Cancer Cell. Front. Oncol. 2020, 10, 552907. [Google Scholar] [CrossRef]
- Lin, M.; Zhu, Q.; Li, Y.; Pan, J. Peperomin E Induces Apoptosis and Cytoprotective Autophagy in Human Prostate Cancer DU145 Cells In Vitro and In Vivo. Planta Medica 2021, 87, 620–630. [Google Scholar] [CrossRef]
- Yang, G.; Li, Z.; Dong, L.; Zhou, F. lncRNA ADAMTS9-AS1 Promotes Bladder Cancer Cell Invasion, Migration, and Inhibits Apoptosis and Autophagy through PI3K/AKT/mTOR Signaling Pathway. Int. J. Biochem. Cell Biol. 2021, 140, 106069. [Google Scholar] [CrossRef]
- Georgeous, J.; AlSawaftah, N.; Abuwatfa, W.H.; Husseini, G.A. Review of Gold Nanoparticles: Synthesis, Properties, Shapes, Cellular Uptake, Targeting, Release Mechanisms and Applications in Drug Delivery and Therapy. Pharmaceutics 2024, 16, 1332. [Google Scholar] [CrossRef]
- Kouri, M.A.; Polychronidou, K.; Loukas, G.; Megapanou, A.; Vagena, I.-A.; Gerardos, A.M.; Spyratou, E.; Eftsathopoulos, E.P. Consolidation of Gold and Gadolinium Nanoparticles: An Extra Step towards Improving Cancer Imaging and Therapy. JNT 2023, 4, 127–149. [Google Scholar] [CrossRef]
- Carbone, G.G.; Mariano, S.; Gabriele, A.; Cennamo, S.; Primiceri, V.; Aziz, M.R.; Panzarini, E.; Calcagnile, L. Exploring the Potential of Gold Nanoparticles in Proton Therapy: Mechanisms, Advances, and Clinical Horizons. Pharmaceutics 2025, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xie, S.; Li, Z.; Dong, S.; Teng, L. Precise Nanoscale Fabrication Technologies, the “Last Mile” of Medicinal Development. Acta Pharm. Sin. B 2025, 15, 2372–2401. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Cong, Y.; Ovais, M.; Cai, R.; Chen, C.; Wang, L. Performance Modulation and Analysis for Catalytic Biomedical Nanomaterials in Biological Systems. Cell Rep. Phys. Sci. 2023, 4, 101453. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Santhosh, P.B.; Genova, J.; Chamati, H. Green Synthesis of Gold Nanoparticles: An Eco-Friendly Approach. Chemistry 2022, 4, 345–369. [Google Scholar] [CrossRef]
- Penninckx, S.; Heuskin, A.-C.; Michiels, C.; Lucas, S. Gold Nanoparticles as a Potent Radiosensitizer: A Transdisciplinary Approach from Physics to Patient. Cancers 2020, 12, 2021. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, X.; Xiong, Z.; Ihsan, A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Anadón, A.; Wang, X.; et al. Cancer Metabolism: The Role of ROS in DNA Damage and Induction of Apoptosis in Cancer Cells. Metabolites 2023, 13, 796. [Google Scholar] [CrossRef]
- Ricci, J.-E.; Muñoz-Pinedo, C.; Fitzgerald, P.; Bailly-Maitre, B.; Perkins, G.A.; Yadava, N.; Scheffler, I.E.; Ellisman, M.H.; Green, D.R. Disruption of Mitochondrial Function during Apoptosis Is Mediated by Caspase Cleavage of the P75 Subunit of Complex I of the Electron Transport Chain. Cell 2004, 117, 773–786. [Google Scholar] [CrossRef]
- Stern, S.T.; Adiseshaiah, P.P.; Crist, R.M. Autophagy and Lysosomal Dysfunction as Emerging Mechanisms of Nanomaterial Toxicity. Part. Fibre Toxicol. 2012, 9, 20. [Google Scholar] [CrossRef]
- Luobin, L.; Wanxin, H.; Yingxin, G.; Qinzhou, Z.; Zefeng, L.; Danyang, W.; Huaqin, L. Nanomedicine-Induced Programmed Cell Death in Cancer Therapy: Mechanisms and Perspectives. Cell Death Discov. 2024, 10, 386. [Google Scholar] [CrossRef]
- He, M.; Chen, S.; Yu, H.; Fan, X.; Wu, H.; Wang, Y.; Wang, H.; Yin, X. Advances in Nanoparticle-Based Radiotherapy for Cancer Treatment. iScience 2025, 28, 111602. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, S.; Guo, J.; Du, Q.; Wu, C.; Wu, Y.; Zhang, Y. Cell Death Pathways: Molecular Mechanisms and Therapeutic Targets for Cancer. MedComm 2024, 5, e693. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shen, H.; Mi, Z. Enhancing Proton Therapy Efficacy Through Nanoparticle-Mediated Radiosensitization. Cells 2024, 13, 1841. [Google Scholar] [CrossRef]
- Cooper, D.R.; Bekah, D.; Nadeau, J.L. Gold Nanoparticles and Their Alternatives for Radiation Therapy Enhancement. Front. Chem. 2014, 2, 86. [Google Scholar] [CrossRef]
- Mesbahi, A. A Review on Gold Nanoparticles Radiosensitization Effect in Radiation Therapy of Cancer. Rep. Pract. Oncol. Radiother. 2010, 15, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Thomas, M.; Mehtala, J.; Wang, J. Gold Nanoparticles (GNPs) as Multifunctional Materials for Cancer Treatment. In Biomaterials for Cancer Therapeutics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 349e–389e. ISBN 978-0-85709-664-7. [Google Scholar]
- Çağlar, M.; Eşitmez, D.; Cebe, M.S. The Effect of Dose Enhancement in Tumor with Silver Nanoparticles on Surrounding Healthy Tissues: A Monte Carlo Study. Technol. Cancer Res. Treat. 2024, 23, 15330338241235771. [Google Scholar] [CrossRef]
- Kouri, M.A.; Spyratou, E.; Kalkou, M.-E.; Patatoukas, G.; Angelopoulou, E.; Tremi, I.; Havaki, S.; Gorgoulis, V.G.; Kouloulias, V.; Platoni, K.; et al. Nanoparticle-Mediated Radiotherapy: Unraveling Dose Enhancement and Apoptotic Responses in Cancer and Normal Cell Lines. Biomolecules 2023, 13, 1720. [Google Scholar] [CrossRef]
- Teimouri, H.; Taheri, S.; Saidabad, F.E.; Nakazato, G.; Maghsoud, Y.; Babaei, A. New Insights into Gold Nanoparticles in Virology: A Review of Their Applications in the Prevention, Detection, and Treatment of Viral Infections. Biomed. Pharmacother. 2025, 183, 117844. [Google Scholar] [CrossRef]
- Zarska, M.; Novotný, F.; Havel, F.; Šrámek, M.; Babelova, A.; Benada, O.; Novotny, M.; Saran, H.; Kuca, K.; Musilek, K.; et al. A Two-Step Mechanism of Cellular Uptake of Cationic Gold Nanoparticles Modified by (16-Mercaptohexadecyl) Trimethylammonium Bromide (MTAB). Bioconjug. Chem. 2016, 27, 2558–2574. [Google Scholar] [CrossRef]
- Javid, H.; Oryani, M.A.; Rezagholinejad, N.; Esparham, A.; Tajaldini, M.; Karimi-Shahri, M. RGD Peptide in Cancer Targeting: Benefits, Challenges, Solutions, and Possible Integrin–RGD Interactions. Cancer Med. 2024, 13, e6800. [Google Scholar] [CrossRef]
- Wu, P.-H.; Onodera, Y.; Ichikawa, Y.; Rankin, E.; Giaccia, A.; Watanabe, Y.; Qian, W.; Hashimoto, T.; Shirato, H.; Nam, J.-M. Targeting Integrins with RGD-Conjugated Gold Nanoparticles in Radiotherapy Decreases the Invasive Activity of Breast Cancer Cells. IJN 2017, 12, 5069–5085. [Google Scholar] [CrossRef] [PubMed]
- Hlapisi, N.; Songca, S.P.; Ajibade, P.A. Capped Plasmonic Gold and Silver Nanoparticles with Porphyrins for Potential Use as Anticancer Agents—A Review. Pharmaceutics 2024, 16, 1268. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Wu, J.; Lan, F.; Liu, W.; Yang, X.; Zeng, F.; Xu, H. Nano Titanium Dioxide Induces the Generation of ROS and Potential Damage in HaCaT Cells Under UVA Irradiation. J. Nanosci. Nanotechnol. 2010, 10, 8500–8507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Y.; Li, P.; Dong, R.; Sun, C.; Song, G.; Wang, Y. Titanium Dioxide Nanoparticles Induce Apoptosis through ROS-Ca2+ -p38/AKT/mTOR Pathway in TM4 Cells. J. Appl. Toxicol. 2024, 44, 818–832. [Google Scholar] [CrossRef]
- Adzavon, K.P.; Zhao, W.; He, X.; Sheng, W. Ferroptosis Resistance in Cancer Cells: Nanoparticles for Combination Therapy as a Solution. Front. Pharmacol. 2024, 15, 1416382. [Google Scholar] [CrossRef]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.-P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M.; et al. NBTXR3, a First-in-Class Radioenhancer Hafnium Oxide Nanoparticle, plus Radiotherapy versus Radiotherapy Alone in Patients with Locally Advanced Soft-Tissue Sarcoma (Act.In.Sarc): A Multicentre, Phase 2–3, Randomised, Controlled Trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef]
- Sant’Angelo, D.; Descamps, G.; Lecomte, V.; Stanicki, D.; Penninckx, S.; Dragan, T.; Van Gestel, D.; Laurent, S.; Journe, F. Therapeutic Approaches with Iron Oxide Nanoparticles to Induce Ferroptosis and Overcome Radioresistance in Cancers. Pharmaceuticals 2025, 18, 325. [Google Scholar] [CrossRef]
- Marill, J.; Anesary, N.M.; Zhang, P.; Vivet, S.; Borghi, E.; Levy, L.; Pottier, A. Hafnium Oxide Nanoparticles: Toward an in Vitropredictive Biological Effect? Radiat. Oncol. 2014, 9, 150. [Google Scholar] [CrossRef]
- Singpanna, K.; Pornpitchanarong, C.; Patrojanasophon, P.; Rojanarata, T.; Ngawhirunpat, T.; Li, S.K.; Opanasopit, P. Gold Nanoparticles and Their Applications in Transdermal Drug Delivery: A Review. J. Drug Deliv. Sci. Technol. 2023, 90, 105174. [Google Scholar] [CrossRef]
- Ma, T.; Tran, T.B.; Lin, E.; Hunt, S.; Haveman, R.; Castro, K.; Lu, J. Size-Transformable Nanotherapeutics for Cancer Therapy. Acta Pharm. Sin. B 2025, 15, 834–851. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the Efficacy of Nanoparticle-Based Drug Delivery Systems for Cancer Treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Prajapati, B.G.; Singh, S. A Critical Review on the Dissemination of PH and Stimuli-Responsive Polymeric Nanoparticular Systems to Improve Drug Delivery in Cancer Therapy. Crit. Rev. Oncol. Hematol. 2023, 185, 103961. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, S.; Chamanara, M.; Nasiri, S.S.; Ghasri, M.; Mouraki, A.; Heidari, R.; Nourmohammadi, A. Advances in Stimuli-Responsive Gold Nanorods for Drug-Delivery and Targeted Therapy Systems. Biomed. Pharmacother. 2024, 180, 117493. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- El-Tanani, M.; Satyam, S.M.; Rabbani, S.A.; El-Tanani, Y.; Aljabali, A.A.A.; Al Faouri, I.; Rehman, A. Revolutionizing Drug Delivery: The Impact of Advanced Materials Science and Technology on Precision Medicine. Pharmaceutics 2025, 17, 375. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, K.L.; Barnes, T.J.; Prestidge, C.A. Surface Chemical Modification to Control Molecular Interactions with Porous Silicon. J. Colloid Interface Sci. 2011, 363, 327–333. [Google Scholar] [CrossRef]
- Ghazal, H.; Waqar, A.; Yaseen, F.; Shahid, M.; Sultana, M.; Tariq, M.; Bashir, M.K.; Tahseen, H.; Raza, T.; Ahmad, F. Role of Nanoparticles in Enhancing Chemotherapy Efficacy for Cancer Treatment. Next Mater. 2024, 2, 100128. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in Cancer Theragnostic and Drug Delivery: A Comprehensive Review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Ferreira, D.; Fontinha, D.; Martins, C.; Pires, D.; Fernandes, A.R.; Baptista, P.V. Gold Nanoparticles for Vectorization of Nucleic Acids for Cancer Therapeutics. Molecules 2020, 25, 3489. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, M.; Zhang, Y.; Shafiq, M.; Ullah, M.; Abbas, A.; Xianxiang, X.; Chen, G.; Diao, Y. Advancing Therapeutic Strategies with Polymeric Drug Conjugates for Nucleic Acid Delivery and Treatment. IJN 2025, 20, 25–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, M.; Zheng, J. Proximal Tubules Eliminate Endocytosed Gold Nanoparticles through an Organelle-Extrusion-Mediated Self-Renewal Mechanism. Nat. Nanotechnol. 2023, 18, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Panzarini, E.; Mariano, S.; Carata, E.; Mura, F.; Rossi, M.; Dini, L. Intracellular Transport of Silver and Gold Nanoparticles and Biological Responses: An Update. IJMS 2018, 19, 1305. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez De La Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. IJMS 2021, 22, 4642. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Kam, W.W.-Y.; Banati, R.B. Effects of Ionizing Radiation on Mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Ichim, G.; Lopez, J.; Ahmed, S.U.; Muthalagu, N.; Giampazolias, E.; Delgado, M.E.; Haller, M.; Riley, J.S.; Mason, S.M.; Athineos, D.; et al. Limited Mitochondrial Permeabilization Causes DNA Damage and Genomic Instability in the Absence of Cell Death. Mol. Cell 2015, 57, 860–872. [Google Scholar] [CrossRef]
- Cai, J.; Yang, J.; Jones, D. Mitochondrial Control of Apoptosis: The Role of Cytochrome c. Biochim. et Biophys. Acta (BBA)—Bioenerg. 1998, 1366, 139–149. [Google Scholar] [CrossRef]
- Bemidinezhad, A.; Mirzavi, F.; Gholamhosseinian, H.; Gheybi, F.; Soukhtanloo, M. Gold-Containing Liposomes and Glucose-Coated Gold Nanoparticles Enhances the Radiosensitivity of B16F0 Melanoma Cells via Increasing Apoptosis and ROS Production. Life Sci. 2023, 318, 121495. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Dong, X.; Guan, Z.; Wang, Z.; Hao, Y.; Lu, R.; Chen, L. Gold Nanoparticles Enhances Radiosensitivity in Glioma Cells by Inhibiting TRAF6/NF-KB Induced CCL2 Expression. Heliyon 2023, 9, e14362. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W.; Lo, C.-Y.; Yu, S.-Y.; Chen, F.-H.; Huang, H.-C.; Wang, L.-K.; Liaw, J.-W. Gold Nanoparticles Enhancing Generation of ROS for Cs-137 Radiotherapy. Nanoscale Res. Lett. 2022, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Kamalabadi, M.; Neshastehriz, A.; Ghaznavi, H.; Amini, S.M. Folate Functionalized Gold-Coated Magnetic Nanoparticles Effect in Combined Electroporation and Radiation Treatment of HPV-Positive Oropharyngeal Cancer. Med. Oncol. 2022, 39, 196. [Google Scholar] [CrossRef]
- Lopes, J.; Rodrigues, C.M.; Godinho-Santos, A.; Coelho, J.M.P.; Cabaço, L.C.; Barral, D.C.; Faísca, P.; Catarino, J.; Nunes, D.; Fortunato, E.; et al. Combination of Gold Nanoparticles with Near-Infrared Light as an Alternative Approach for Melanoma Management. Int. J. Pharm. 2025, 668, 124952. [Google Scholar] [CrossRef]
- Taheri, R.A.; Fathi, H.; Sharafi, A.; Mirzaei, M.; Jafari, S.; Darvishi, M.H. Niosomes Loaded with Gold Nanoparticles for Enhanced Radiation Therapy in Lung Cancer. Nanomedicine 2024, 19, 2257–2270. [Google Scholar] [CrossRef]
- Engelbrecht-Roberts, M.; Miles, X.; Vandevoorde, C.; de Kock, M. An Evaluation of the Potential Radiosensitization Effect of Spherical Gold Nanoparticles to Induce Cellular Damage Using Different Radiation Qualities. Molecules 2025, 30, 1038. [Google Scholar] [CrossRef]
- Saberi, A.; Shahbazi-Gahrouei, D.; Abbasian, M.; Fesharaki, M.; Baharlouei, A.; Arab-Bafrani, Z. Gold Nanoparticles in Combination with Megavoltage Radiation Energy Increased Radiosensitization and Apoptosis in Colon Cancer HT-29 Cells. Int. J. Radiat. Biol. 2017, 93, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, R.C.; Kutzner, B.C.; Truong, M.; Sanchez-Dardon, J.; McLean, J.R.N. Analysis of Radiation-Induced Apoptosis in Human Lymphocytes: Flow Cytometry Using Annexin V and Propidium Iodide versus the Neutral Comet Assay. Cytometry 2002, 48, 14–19. [Google Scholar] [CrossRef]
- Tabatabaie, F.; Franich, R.; Feltis, B.; Geso, M. Oxidative Damage to Mitochondria Enhanced by Ionising Radiation and Gold Nanoparticles in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 6887. [Google Scholar] [CrossRef]
- Neshastehriz, A.; Khosravi, Z.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold-Coated Iron Oxide Nanoparticles Trigger Apoptosis in the Process of Thermo-Radiotherapy of U87-MG Human Glioma Cells. Radiat. Environ. Biophys. 2018, 57, 405–418. [Google Scholar] [CrossRef]
- Khoei, S.; Hosseini, V.; Hosseini, M.; Khoee, S.; Shirvalilou, S.; Mahdavi, S.R.; Pirayesh Islamian, J. Enhancement of Radio-Thermo-Sensitivity of 5-Iodo-2-Deoxyuridine-Loaded Polymeric-Coated Magnetic Nanoparticles Triggers Apoptosis in U87MG Human Glioblastoma Cancer Cell Line. Cell. Mol. Bioeng. 2021, 14, 365–377. [Google Scholar] [CrossRef]
- Kudarha, R.; Colaco, V.; Gupta, A.; Kulkarni, S.; Soman, S.; Kulkarni, J.; Rana, K.; Navti, P.; Tiwari, R.; Osmani, R.; et al. Recent Advancements in Selenium Nanoconstructs as a Potential Carrier in Cancer Therapy. Nano-Struct. Nano-Objects 2024, 40, 101399. [Google Scholar] [CrossRef]
- Zheng, Z.; Su, J.; Bao, X.; Wang, H.; Bian, C.; Zhao, Q.; Jiang, X. Mechanisms and Applications of Radiation-Induced Oxidative Stress in Regulating Cancer Immunotherapy. Front. Immunol. 2023, 14, 1247268. [Google Scholar] [CrossRef]
- Hu, X.; Li, H.; Huang, X.; Zhu, Z.; Zhu, H.; Gao, Y.; Zhu, Z.; Chen, H. Cell Membrane-Coated Gold Nanoparticles for Apoptosis Imaging in Living Cells Based on Fluorescent Determination. Microchim. Acta 2020, 187, 175. [Google Scholar] [CrossRef]
- Ren, M.; Chen, Z.; Ge, C.; Hu, W.; Xu, J.; Yang, L.; Luan, M.; Wang, N. Visualizing MiRNA Regulation of Apoptosis for Investigating the Feasibility of MiRNA-Targeted Therapy Using a Fluorescent Nanoprobe. Pharmaceutics 2022, 14, 1349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, N.; Chen, G.; Zheng, A.; Zeng, Y.; Liu, X.; Liu, J. A Fluorescent Turn on Nanoprobe for Simultaneous Visualization of Dual-Targets Involved in Cell Apoptosis and Drug Screening in Living Cells. Nanoscale 2017, 9, 10861–10868. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Goindi, S.; Khurana, R. Formulation, Characterization and Evaluation of an Optimized Microemulsion Formulation of Griseofulvin for Topical Application. Colloids Surf. B Biointerfaces 2013, 105, 158–166. [Google Scholar] [CrossRef]
- Synthesis and Evaluation of Paclitaxel-Loaded Gold Nanoparticles for Tumor-Targeted Drug Delivery. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC5356508/ (accessed on 28 March 2025).
- Ranjan, A.P.; Mukerjee, A.; Helson, L.; Vishwanatha, J.K. Scale up, Optimization and Stability Analysis of Curcumin C3 Complex-Loaded Nanoparticles for Cancer Therapy. J. Nanobiotechnol. 2012, 10, 38. [Google Scholar] [CrossRef]
- Yang, S.-J.; Pai, J.-A.; Shieh, M.-J.; Chen, J.L.; Chen, K.-C. Cisplatin-Loaded Gold Nanoshells Mediate Chemo-Photothermal Therapy against Primary and Distal Lung Cancers Growth. Biomed. Pharmacother. 2023, 158, 114146. [Google Scholar] [CrossRef]
- Craig, G.E.; Brown, S.D.; Lamprou, D.A.; Graham, D. Cisplatin Tethered Gold Nanoparticles Which Exhibit Enhanced Reproducibility, Drug Loading and Stability—A Step Closer to Pharmaceutical Approval? Inorg. Chem. 2012, 51, 3490–3497. [Google Scholar] [CrossRef]
- Du, Y.; Xia, L.; Jo, A.; Davis, R.M.; Bissel, P.; Ehrich, M.F.; Kingston, D.G.I. Synthesis and Evaluation of Doxorubicin-Loaded Gold Nanoparticles for Tumor-Targeted Drug Delivery. Bioconjug. Chem. 2018, 29, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Zonouz, A.; Taghavi, S.; Nekooei, S.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Synthesis of Targeted Doxorubicin-Loaded Gold Nanorod −mesoporous Manganese Dioxide Core–Shell Nanostructure for Ferroptosis, Chemo-Photothermal Therapy in Vitro and in Vivo. Int. J. Pharm. 2024, 665, 124725. [Google Scholar] [CrossRef] [PubMed]
- Surapaneni, S.K.; Bashir, S.; Tikoo, K. Gold Nanoparticles-Induced Cytotoxicity in Triple Negative Breast Cancer Involves Different Epigenetic Alterations Depending upon the Surface Charge. Sci. Rep. 2018, 8, 12295. [Google Scholar] [CrossRef]
- Sargazi, S.; Laraib, U.; Er, S.; Rahdar, A.; Hassanisaadi, M.; Zafar, M.N.; Díez-Pascual, A.M.; Bilal, M. Application of Green Gold Nanoparticles in Cancer Therapy and Diagnosis. Nanomaterials 2022, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Piktel, E.; Ościłowska, I.; Suprewicz, Ł.; Depciuch, J.; Marcińczyk, N.; Chabielska, E.; Wolak, P.; Wollny, T.; Janion, M.; Parlinska-Wojtan, M.; et al. ROS-Mediated Apoptosis and Autophagy in Ovarian Cancer Cells Treated with Peanut-Shaped Gold Nanoparticles. Int. J. Nanomed. 2021, 16, 1993–2011. [Google Scholar] [CrossRef]
- Hsiang, C.H.; Tunoda, T.; Whang, Y.E.; Tyson, D.R.; Ornstein, D.K. The Impact of Altered Annexin I Protein Levels on Apoptosis and Signal Transduction Pathways in Prostate Cancer Cells. Prostate 2006, 66, 1413–1424. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK Signaling in Apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Vivas-Mejia, P.; Benito, J.M.; Fernandez, A.; Han, H.-D.; Mangala, L.; Rodriguez-Aguayo, C.; Chavez-Reyes, A.; Lin, Y.G.; Nick, A.M.; Stone, R.L.; et al. JNK-1 Inhibition Leads to Antitumor Activity in Ovarian Cancer. Clin. Cancer Res. 2010, 16, 184–194. [Google Scholar] [CrossRef]
- Gumireddy, K.; Sutton, L.N.; Phillips, P.C.; Reddy, C.D. All-Trans-Retinoic Acid-Induced Apoptosis in Human Medulloblastoma: Activation of Caspase-3/Poly(ADP-Ribose) Polymerase 1 Pathway. Clin. Cancer Res. 2003, 9, 4052–4059. [Google Scholar]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.-Y.; Chen, Y.-H.; Liu, C.; Tung, K.-L.; Wu, Y.-T.; Lin, S.-C.; Wu, C.-H.; Chang, H.-Y.; Chen, Y.-C.; Huang, B.-M. Role of JNK Activation in Paclitaxel-Induced Apoptosis in Human Head and Neck Squamous Cell Carcinoma. Oncol. Lett. 2021, 22, 705. [Google Scholar] [CrossRef]
- Farrell, L.; Puig-Barbe, A.; Haque, M.I.; Amcheslavsky, A.; Yu, M.; Bergmann, A.; Fan, Y. Actin Remodeling Mediates ROS Production and JNK Activation to Drive Apoptosis-Induced Proliferation. PLoS Genet. 2022, 18, e1010533. [Google Scholar] [CrossRef]
- Maddah, A.; Ziamajidi, N.; Khosravi, H.; Danesh, H.; Abbasalipourkabir, R. Gold Nanoparticles Induce Apoptosis in HCT-116 Colon Cancer Cell Line. Mol. Biol. Rep. 2022, 49, 7863–7871. [Google Scholar] [CrossRef]
- Iglesias-Guimarais, V.; Gil-Guiñon, E.; Sánchez-Osuna, M.; Casanelles, E.; García-Belinchón, M.; Comella, J.X.; Yuste, V.J. Chromatin Collapse during Caspase-Dependent Apoptotic Cell Death Requires DNA Fragmentation Factor, 40-kDa Subunit-/Caspase-Activated Deoxyribonuclease-Mediated 3′-OH Single-Strand DNA Breaks. J. Biol. Chem. 2013, 288, 9200–9215. [Google Scholar] [CrossRef]
- Recent Advances in Nanomedicine Preparative Methods and Their Therapeutic Potential for Colorectal Cancer: A Critical Review—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10325729/ (accessed on 28 March 2025).
- Radaic, A.; Joo, N.E.; Jeong, S.-H.; Yoo, S.-I.; Kotov, N.; Kapila, Y.L. Phosphatidylserine-Gold Nanoparticles (PS-AuNP) Induce Prostate and Breast Cancer Cell Apoptosis. Pharmaceutics 2021, 13, 1094. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Yang, Y.-C.; Yang, K.-C.; Shieh, H.-R.; Wang, T.-Y.; Hwu, Y.; Chen, Y.-J. Pegylated Gold Nanoparticles Induce Apoptosis in Human Chronic Myeloid Leukemia Cells. BioMed. Res. Int. 2014, 2014, 182353. [Google Scholar] [CrossRef] [PubMed]
- Daei, S.; Ziamajidi, N.; Abbasalipourkabir, R.; Khanaki, K.; Bahreini, F. Anticancer Effects of Gold Nanoparticles by Inducing Apoptosis in Bladder Cancer 5637 Cells. Biol. Trace Elem. Res. 2022, 200, 2673–2683. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, X.; Liu, D.; Che, X.; Wu, G. A Novel Approach for Bladder Cancer Treatment: Nanoparticles as a Drug Delivery System. Int. J. Nanomed. 2024, 19, 13461–13483. [Google Scholar] [CrossRef]
- Essawy, M.M.; El-Sheikh, S.M.; Raslan, H.S.; Ramadan, H.S.; Kang, B.; Talaat, I.M.; Afifi, M.M. Function of Gold Nanoparticles in Oral Cancer beyond Drug Delivery: Implications in Cell Apoptosis. Oral Dis. 2021, 27, 251–265. [Google Scholar] [CrossRef]
- Gold Nanomaterials for Oral Cancer Diagnosis and Therapy: Advances, Challenges, and Prospects—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9237953/ (accessed on 28 March 2025).
- P, B.S.; Periasamy, T.; Alarfaj, A.A.; Arulselvan, P.; Ravindran, R.; Suriyaprakash, J.; Thangavelu, I. Pemetrexed Loaded Gold Nanoparticles as Cytotoxic and Apoptosis Inducers in Lung Cancer Cells through ROS Generation and Mitochondrial Dysfunction Pathway. Biotechnol. Appl. Biochem. 2024, 71, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shukla, A.; Singh, S.P.; Singh, R.K.; Patel, A.K.; Verma, P.K.; Kumar, S.; Kumar, N.; Singh, V.; Wasnik, K.; et al. Synthesized Gold Nanoparticles with Moringa Oleifera Leaf Extract Induce Mitotic Arrest (G2/M Phase) and Apoptosis in Dalton’s Lymphoma Cells. Cell Biochem. Biophys. 2024, 82, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Programmed Cell Death Detection Methods: A Systematic Review and a Categorical Comparison—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9308588/ (accessed on 28 March 2025).
- Steckiewicz, K.P.; Barcinska, E.; Sobczak, K.; Tomczyk, E.; Wojcik, M.; Inkielewicz-Stepniak, I. Assessment of Anti-Tumor Potential and Safety of Application of Glutathione Stabilized Gold Nanoparticles Conjugated with Chemotherapeutics. Int. J. Med. Sci. 2020, 17, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Florance, I.; Cordani, M.; Pashootan, P.; Moosavi, M.A.; Zarrabi, A.; Chandrasekaran, N. The Impact of Nanomaterials on Autophagy across Health and Disease Conditions. Cell. Mol. Life Sci. 2024, 81, 184. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, Apoptotic and Autophagic Cell Fates Triggered by Nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Jin, S.; Tian, Y.; Zhang, X.; Zhao, Y.; Yu, L.; Liang, X.-J. Gold Nanoparticles Induce Autophagosome Accumulation through Size-Dependent Nanoparticle Uptake and Lysosome Impairment. ACS Nano 2011, 5, 8629–8639. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Zeng, J.; Acin-Perez, R.; Assali, E.A.; Martin, A.; Brownstein, A.J.; Petcherski, A.; Fernández-del-Rio, L.; Xiao, R.; Lo, C.H.; Shum, M.; et al. Restoration of Lysosomal Acidification Rescues Autophagy and Metabolic Dysfunction in Non-Alcoholic Fatty Liver Disease. Nat. Commun. 2023, 14, 2573. [Google Scholar] [CrossRef]
- Tian, X.; Shi, A.; Yin, H.; Wang, Y.; Liu, Q.; Chen, W.; Wu, J. Nanomaterials Respond to Lysosomal Function for Tumor Treatment. Cells 2022, 11, 3348. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhang, J.; Peng, Q.; Wang, X.; Xiao, X.; Shi, K. Nanotherapeutics Targeting Autophagy Regulation for Improved Cancer Therapy. Acta Pharm. Sin. B 2024, 14, 2447–2474. [Google Scholar] [CrossRef]
- Li, J.J.; Hartono, D.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y.L. Autophagy and Oxidative Stress Associated with Gold Nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef] [PubMed]
- Pantelis, P.; Theocharous, G.; Lagopati, N.; Veroutis, D.; Thanos, D.-F.; Lampoglou, G.-P.; Pippa, N.; Gatou, M.-A.; Tremi, I.; Papaspyropoulos, A.; et al. The Dual Role of Oxidative-Stress-Induced Autophagy in Cellular Senescence: Comprehension and Therapeutic Approaches. Antioxidants 2023, 12, 169. [Google Scholar] [CrossRef]
- Pérez-Hernández, M.; Arias, A.; Martínez-García, D.; Pérez-Tomás, R.; Quesada, R.; Soto-Cerrato, V. Targeting Autophagy for Cancer Treatment and Tumor Chemosensitization. Cancers 2019, 11, 1599. [Google Scholar] [CrossRef]
- Hossain, A.; Rayhan, M.T.; Mobarak, M.H.; Rimon, M.I.H.; Hossain, N.; Islam, S.; Kafi, S.M.A.A. Advances and Significances of Gold Nanoparticles in Cancer Treatment: A Comprehensive Review. Results Chem. 2024, 8, 101559. [Google Scholar] [CrossRef]
- Yasin, D.; Sami, N.; Afzal, B.; Zaki, A.; Naaz, H.; Husain, S.; Siddiqui, T.; Rizvi, M.A.; Fatma, T. Biogenic Nanoparticles: Understanding Their Potential Role in Cancer Theranostics. Next Nanotechnol. 2025, 8, 100149. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.N. Autophagy as a Second Level Protective Process in Conferring Resistance to Environmentally-Induced Oxidative Stress. Autophagy 2008, 4, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, L.; Ning, Z.; Li, C.; Yin, Y.; Chen, K.; Li, L.; Xu, F.; Gao, J. Autophagy-Modulating Biomaterials: Multifunctional Weapons to Promote Tissue Regeneration. Cell Commun. Signal 2024, 22, 124. [Google Scholar] [CrossRef]
- Sun, K.; Guo, X.; Zhao, Q.; Jing, Y.; Kou, X.; Xie, X.; Zhou, Y.; Cai, N.; Gao, L.; Zhao, X.; et al. Paradoxical Role of Autophagy in the Dysplastic and Tumor-Forming Stages of Hepatocarcinoma Development in Rats. Cell Death Dis. 2013, 4, e501. [Google Scholar] [CrossRef]
- Ma, N.; Liu, P.; He, N.; Gu, N.; Wu, F.-G.; Chen, Z. Action of Gold Nanospikes-Based Nanoradiosensitizers: Cellular Internalization, Radiotherapy, and Autophagy. ACS Appl. Mater. Interfaces 2017, 9, 31526–31542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Jiang, M.; Li, S.; Yuan, H.; Sun, H.; Yang, F.; Liang, H. Developing a Novel Gold(III) Agent to Treat Glioma Based on the Unique Properties of Apoferritin Nanoparticles: Inducing Lethal Autophagy and Apoptosis. J. Med. Chem. 2020, 63, 13695–13708. [Google Scholar] [CrossRef] [PubMed]
- Maia, P.I.d.S.; Nguyen, H.H.; Ponader, D.; Hagenbach, A.; Bergemann, S.; Gust, R.; Deflon, V.M.; Abram, U. Neutral Gold Complexes with Tridentate SNS Thiosemicarbazide Ligands. Inorg. Chem. 2012, 51, 1604–1613. [Google Scholar] [CrossRef]
- Pandey, A.; Vighetto, V.; Di Marzio, N.; Ferraro, F.; Hirsch, M.; Ferrante, N.; Mitra, S.; Grattoni, A.; Filgueira, C.S. Gold Nanoparticles Radio-Sensitize and Reduce Cell Survival in Lewis Lung Carcinoma. Nanomaterials 2020, 10, 1717. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Gharoonpour, A.; Simiyari, D.; Yousefzadeh, A.; Badragheh, F.; Rahmati, M. Autophagy Modulation in Breast Cancer Utilizing Nanomaterials and Nanoparticles. Front. Oncol. 2023, 13, 1150492. [Google Scholar] [CrossRef]
- López-Méndez, T.B.; Sánchez-Álvarez, M.; Trionfetti, F.; Pedraz, J.L.; Tripodi, M.; Cordani, M.; Strippoli, R.; González-Valdivieso, J. Nanomedicine for Autophagy Modulation in Cancer Therapy: A Clinical Perspective. Cell Biosci. 2023, 13, 44. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, F.; Li, K.; Zhang, H.; Yin, Y.; Yu, Y.; Lu, G.; Zhang, S.; Wei, Y.; Xu, K.; et al. Gold Nanoparticle-Directed Autophagy Intervention for Antitumor Immunotherapy via Inhibiting Tumor-Associated Macrophage M2 Polarization. Acta Pharm. Sin. B 2022, 12, 3124–3138. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef]
- Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef]
- Basu, B. The Radiophiles of Deinococcaceae Family: Resourceful Microbes for Innovative Biotechnological Applications. Curr. Res. Microb. Sci. 2022, 3, 100153. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chatterjee, D.K.; Lee, M.H.; Krishnan, S. Gold Nanoparticles in Breast Cancer Treatment: Promise and Potential Pitfalls. Cancer Lett. 2014, 347, 46–53. [Google Scholar] [CrossRef]
- Araki, T.; Kanda, S.; Horinouchi, H.; Ohe, Y. Current Treatment Strategies for EGFR-Mutated Non-Small Cell Lung Cancer: From First Line to beyond Osimertinib Resistance. Jpn. J. Clin. Oncol. 2023, 53, 547–561. [Google Scholar] [CrossRef]
- Huang, K.-B.; Wang, F.-Y.; Tang, X.-M.; Feng, H.-W.; Chen, Z.-F.; Liu, Y.-C.; Liu, Y.-N.; Liang, H. Organometallic Gold(III) Complexes Similar to Tetrahydroisoquinoline Induce ER-Stress-Mediated Apoptosis and Pro-Death Autophagy in A549 Cancer Cells. J. Med. Chem. 2018, 61, 3478–3490. [Google Scholar] [CrossRef] [PubMed]
- Tadic, V.; Prell, T.; Lautenschlaeger, J.; Grosskreutz, J. The ER Mitochondria Calcium Cycle and ER Stress Response as Therapeutic Targets in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2014, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-Based Drugs for Cancer Therapy and Anti-Tumor Strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Frik, M.; Fernández-Gallardo, J.; Gonzalo, O.; Mangas-Sanjuan, V.; González-Alvarez, M.; del Valle, A.S.; Hu, C.; González-Alvarez, I.; Bermejo, M.; Marzo, I.; et al. Cyclometalated Iminophosphorane Gold(III) and Platinum(II) Complexes. A Highly Permeable Cationic Platinum(II) Compound with Promising Anticancer Properties. J. Med. Chem. 2015, 58, 5825–5841. Available online: https://pubs.acs.org/doi/10.1021/acs.jmedchem.5b00427 (accessed on 30 March 2025). [CrossRef]
- De Andrade Querino, A.L.; De Sousa, A.M.; Thomas, S.R.; De Lima, G.M.; Dittz, D.; Casini, A.; Do Monte-Neto, R.L.; Silva, H. Organogold(III)-Dithiocarbamate Compounds and Their Coordination Analogues as Anti-Tumor and Anti-Leishmanial Metallodrugs. J. Inorg. Biochem. 2023, 247, 112346. [Google Scholar] [CrossRef]
- Glioblastoma Therapy: Past, Present and Future. Available online: https://www.mdpi.com/1422-0067/25/5/2529 (accessed on 30 March 2025).
- Zhang, X.; Li, W.; Wang, C.; Leng, X.; Lian, S.; Feng, J.; Li, J.; Wang, H. Inhibition of Autophagy Enhances Apoptosis Induced by Proteasome Inhibitor Bortezomib in Human Glioblastoma U87 and U251 Cells. Mol. Cell. Biochem. 2014, 385, 265–275. [Google Scholar] [CrossRef]
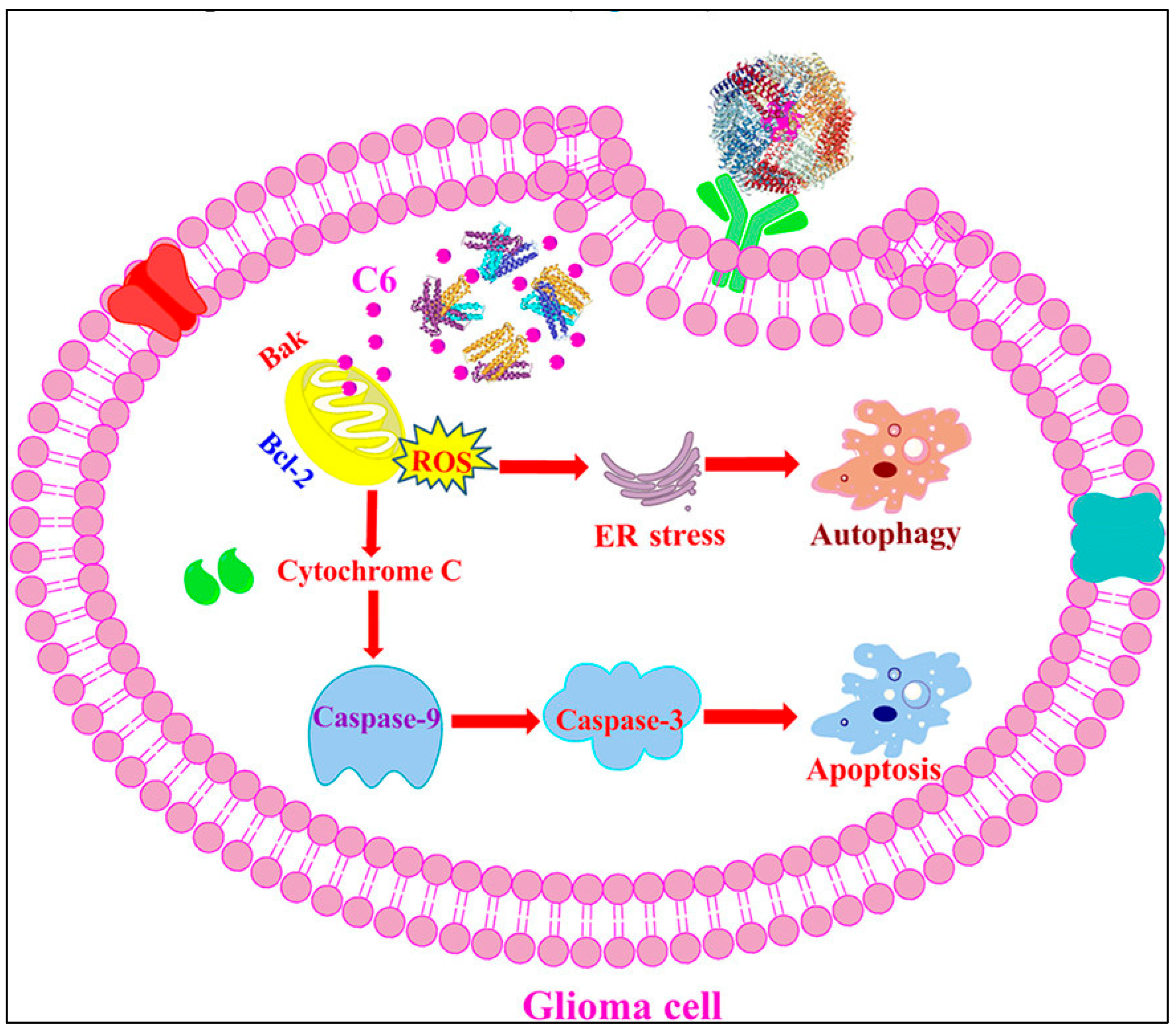
- Fernandes, R.S.; Kirszberg, C.; Rumjanek, V.M.; Monteiro, R.Q. On the Molecular Mechanisms for the Highly Procoagulant Pattern of C6 Glioma Cells. J. Thromb. Haemost. 2006, 4, 1546–1552. [Google Scholar] [CrossRef]
- Nisha; Sachan, R.S.K.; Singh, A.; Karnwal, A.; Shidiki, A.; Kumar, G. Plant-Mediated Gold Nanoparticles in Cancer Therapy: Exploring Anti-Cancer Mechanisms, Drug Delivery Applications, and Future Prospects. Front. Nanotechnol. 2024, 6, 1490980. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, Y.; Li, X. The Interaction Mechanism between Autophagy and Apoptosis in Colon Cancer. Transl. Oncol. 2020, 13, 100871. [Google Scholar] [CrossRef]
- Altman, B.J.; Rathmell, J.C. Metabolic Stress in Autophagy and Cell Death Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a008763. [Google Scholar] [CrossRef] [PubMed]
- El-Khattouti, A.; Selimovic, D.; Haikel, Y.; Hassan, M. Crosstalk Between Apoptosis and Autophagy: Molecular Mechanisms and Therapeutic Strategies in Cancer. J. Cell Death 2013, 6, 37–55. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ding, H.; Li, L.; Chen, J.; Mo, X.; Ding, Y.; Chen, W.; Tang, Q.; Wang, Y. Gold Nanoparticles Enhance the Ability of Radiotherapy to Induce Immunogenic Cell Death in Glioblastoma. IJN 2023, 18, 5701–5712. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of Cytochrome c Release from Mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Interactions between Reactive Oxygen Species and Autophagy: Special Issue: Death Mechanisms in Cellular Homeostasis. Biochim. et Biophys. Acta (BBA)—Mol. Cell Res. 2021, 1868, 119041. [Google Scholar] [CrossRef]
- Niżnik, Ł.; Noga, M.; Kobylarz, D.; Frydrych, A.; Krośniak, A.; Kapka-Skrzypczak, L.; Jurowski, K. Gold Nanoparticles (AuNPs)—Toxicity, Safety and Green Synthesis: A Critical Review. Int. J. Mol. Sci. 2024, 25, 4057. [Google Scholar] [CrossRef]
- Eliopoulos, A.G.; Havaki, S.; Gorgoulis, V.G. DNA Damage Response and Autophagy: A Meaningful Partnership. Front. Genet. 2016, 7, 204. [Google Scholar] [CrossRef]
- Autophagy in DNA Damage Response. Available online: https://www.mdpi.com/1422-0067/16/2/2641 (accessed on 30 March 2025).
- Wu, J.; Wang, X.; Wang, Y.; Xun, Z.; Li, S. Application of PLGA in Tumor Immunotherapy. Polymers 2024, 16, 1253. [Google Scholar] [CrossRef]
- Imtiaz, S.; Ferdous, U.T.; Nizela, A.; Hasan, A.; Shakoor, A.; Zia, A.W.; Uddin, S. Mechanistic Study of Cancer Drug Delivery: Current Techniques, Limitations, and Future Prospects. Eur. J. Med. Chem. 2025, 290, 117535. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive Oxygen Species (ROS) Scavenging Biomaterials for Anti-Inflammatory Diseases: From Mechanism to Therapy. J. Hematol. Oncol. 2023, 16, 116. Available online: https://jhoonline.biomedcentral.com/articles/10.1186/s13045-023-01512-7 (accessed on 30 March 2025). [CrossRef] [PubMed]
- Chuang, J.C.; Jones, P.A. Epigenetics and microRNAs. Pediatr. Res. 2007, 61, 24R–29R. [Google Scholar] [CrossRef]
- Ke, S.; Zhou, T.; Yang, P.; Wang, Y.; Zhang, P.; Chen, K.; Ren, L.; Ye, S. Gold Nanoparticles Enhance TRAIL Sensitivity through Drp1-Mediated Apoptotic and Autophagic Mitochondrial Fission in NSCLC Cells. Int. J. Nanomed. 2017, 12, 2531–2551. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Ma, R.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.-S.; Zhou, Y. Gold Nanoparticles and Gold Nanorods in the Landscape of Cancer Therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef]
- Ojha, R.; Ishaq, M.; Singh, S.K. Caspase-Mediated Crosstalk between Autophagy and Apoptosis: Mutual Adjustment or Matter of Dominance. J. Cancer Res. Ther. 2015, 11, 514–524. [Google Scholar] [CrossRef]
- Adiseshaiah, P.P.; Clogston, J.D.; McLeland, C.B.; Rodriguez, J.; Potter, T.M.; Neun, B.W.; Skoczen, S.L.; Shanmugavelandy, S.S.; Kester, M.; Stern, S.T.; et al. Synergistic Combination Therapy with Nanoliposomal C6-Ceramide and Vinblastine Is Associated with Autophagy Dysfunction in Hepatocarcinoma and Colorectal Cancer Models. Cancer Lett. 2013, 337, 254–265. [Google Scholar] [CrossRef]
- Fuchs, R.P.; Isogawa, A.; Paulo, J.A.; Onizuka, K.; Takahashi, T.; Amunugama, R.; Duxin, J.P.; Fujii, S. Crosstalk between Repair Pathways Elicits Double-Strand Breaks in Alkylated DNA and Implications for the Action of Temozolomide. eLife 2021, 10, e69544. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.-Q.; Luo, Q.; Wang, L.; Song, G.-B.; Sheng, J.-P.; Xu, B. Signaling Pathways Involved in Colorectal Cancer: Pathogenesis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Garg, P.; Malhotra, J.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Emerging Therapeutic Strategies to Overcome Drug Resistance in Cancer Cells. Cancers 2024, 16, 2478. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Kodiha, M.; Wang, Y.M.; Hutter, E.; Maysinger, D.; Stochaj, U. Off to the Organelles—Killing Cancer Cells with Targeted Gold Nanoparticles. Theranostics 2015, 5, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.M.; Kibanov Solomonov, V.V.; Sazykina, E.; Schwartz, J.A.; Gad, S.C.; Goodrich, G.P. Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. Int. J. Toxicol. 2016, 35, 38–46. [Google Scholar] [CrossRef]
- Bruckman, M.A.; Randolph, L.N.; VanMeter, A.; Hern, S.; Shoffstall, A.J.; Taurog, R.E.; Steinmetz, N.F. Biodistribution, Pharmacokinetics, and Blood Compatibility of Native and PEGylated Tobacco Mosaic Virus Nano-Rods and -Spheres in Mice. Virology 2014, 449, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Faheem, A.; El-Tanani, M. Targeted Drug Delivery to the Spleen and Its Implications for the Prevention and Treatment of Cancer. Pharmaceutics 2025, 17, 651. [Google Scholar] [CrossRef]
- Mahalmani, V.; Sinha, S.; Prakash, A.; Medhi, B. Translational Research: Bridging the Gap between Preclinical and Clinical Research. Indian J. Pharmacol. 2022, 54, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Aubrun, C.; Doussineau, T.; Carmès, L.; Meyzaud, A.; Boux, F.; Dufort, S.; Delfour, A.; De Beaumont, O.; Mirjolet, C.; Le Duc, G. Mechanisms of Action of AGuIX as a Pan-Cancer Nano-Radiosensitizer: A Comprehensive Review. Pharmaceuticals 2025, 18, 519. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.D.; Monferrer, D.; Penon, O.; Rivera-Gil, P. Regulatory Pathways and Guidelines for Nanotechnology-Enabled Health Products: A Comparative Review of EU and US Frameworks. Front. Med. 2025, 12, 1544393. [Google Scholar] [CrossRef]
- Liao, Z.; Liu, X.; Fan, D.; Sun, X.; Zhang, Z.; Wu, P. Autophagy-Mediated Nanomaterials for Tumor Therapy. Front. Oncol. 2023, 13, 1194524. [Google Scholar] [CrossRef]
- Ligero, M.; Gielen, B.; Navarro, V.; Cresta Morgado, P.; Prior, O.; Dienstmann, R.; Nuciforo, P.; Trebeschi, S.; Beets-Tan, R.; Sala, E.; et al. A Whirl of Radiomics-Based Biomarkers in Cancer Immunotherapy, Why Is Large Scale Validation Still Lacking? NPJ Precis. Oncol. 2024, 8, 42. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Zenkin, S.; Ak, M.; Mamindla, P.; Peddagangireddy, V.; Hsieh, R.; Anderson, J.L.; Delgoffe, G.M.; Menk, A.; Skinner, H.D.; et al. Evaluation of Radiomics as a Predictor of Efficacy and the Tumor Immune Microenvironment in anti-PD-1 mAb Treated Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck Patients. Head Neck 2025, 47, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Maleki, E.H.; Bahrami, A.R.; Matin, M.M. Cancer Cell Cycle Heterogeneity as a Critical Determinant of Therapeutic Resistance. Genes Dis. 2023, 11, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the Mechanisms and Challenges of Cancer Drug Resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, V.W.; Amaravadi, R.K. Emerging Strategies to Effectively Target Autophagy in Cancer. Oncogene 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Alalmaie, A.; Alshahrani, H.T.; Alqahtani, M.; Alshahrani, Z.; Alahmari, S.; Asiri, A.; Alqadi, B.; Alshahrani, A.; Alshahrani, S.; Akhter, M.H. Integrating Computational Insights in Gold Nanoparticle-Mediated Drug Delivery: Enhancing Efficacy and Precision. Front. Med. Technol. 2025, 7, 1528826. [Google Scholar] [CrossRef]





| Study | Nanoparticle Type | Key Findings | Mechanisms Observed |
|---|---|---|---|
| Bemidi-nezhad et al. [196] | Glucose-coated AuNPs (Glu-GNPs), liposome-encapsulated gold ions (Gold-Lips) | Enhanced ROS production, mitochondrial dysfunction, apoptosis; improved radiosensitivity in melanoma cells | Enhanced Bax, p53, Caspase-3/-7; downregulated Bcl-2 |
| Tsai et al. [198] | 55 nm AuNPs | Enhanced ROS accumulation, mitochondrial dysfunction, apoptosis in epidermoid carcinoma cells post γ-ray exposure | Increased ROS levels, mitochondrial damage, caspase activation |
| Saberi et al. [203] | AuNPs with 9 MV radiation | Increased apoptosis in HT-29 colorectal cancer cells | Increased DNA damage, mitochondrial dysfunction |
| Neshast-ehriz et al. [206] | Gold-coated iron oxide nanoparticles (Au@IONPs) | Enhanced apoptosis via ROS-mediated damage, mitochondrial stress; synergistic effects with hyperthermia and X-ray | Significant apoptotic markers increase, reduced cell viability |
| Hu et al. [210] | AuNP-pep@Mem | Developed probe detecting apoptosis via caspase-3; real-time tracking of apoptotic events | Caspase-3 activation, mitochondrial membrane disruption |
| Surapaneni et al. [220] | Citrate-capped and cysteamine-capped AuNPs | Citrate-AuNPs increased histone deacetylation, induced apoptosis; cysteamine-AuNPs activated p38 MAPK pathway | Differential gene regulation (caspase-3, Bax, Bcl-2) |
| Piktel et al. [222] | Gold nanopeanuts (AuP NPs) | Induced apoptosis and autophagy in ovarian cancer via ROS overproduction | Upregulated LC3-II, Bax, caspase-3; downregulated Bcl-2 |
| Maddah et al. [231] | AuNPs in HCT-116 colon cancer cells | Enhanced apoptosis via Bax, p53 upregulation, Bcl-2 downregulation | Significant chromatin condensation, apoptotic cell death |
| Radaic et al. [234] | Phosphatidylserine-capped AuNPs (PS-AuNPs) | Induced apoptosis in breast and prostate cancer cells | Increased caspase-3 activity, histone fragmentation |
| Zhang et al. [268] | AFt-C6 NPs | Dual-mode activation of apoptosis and autophagy in glioma cells; minimal cross-regulation | Caspase-3, LC3-II upregulation; downregulated p62 |
| Pandey et al. [264] | AuNPs (~37 nm) in Lewis lung carcinoma (LLC) cells | Enhanced radiosensitization, DNA damage, therapeutic efficacy | Improved apoptotic, autophagic responses via ROS elevation |
| Xiaowei Ma et al. [246] | AuNPs in lysosomes | Disrupted lysosomal degradation; autophagosome accumulation | Increased LC3-II levels, p62 accumulation |
| Li et al. [252] | AuNPs in MRC-5 lung fibroblasts | Triggered autophagy, oxidative stress, lipid peroxidation | Increased MAP-LC3, ATG7, malondialdehyde (MDA) adducts |
| Ma et al. [261] | PEG-coated gold nanospikes (GNSs) | TAT-GNSs highest radiosensitization; enhanced ROS production, impaired autophagic flux | Increased LC3-II, p62 accumulation; enhanced apoptosis with 3-MA inhibitor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouri, M.A.; Tsaroucha, A.; Axakali, T.-M.; Varelas, P.; Kouloulias, V.; Platoni, K.; Efstathopoulos, E.P. Targeting Cancer Cell Fate: Apoptosis, Autophagy, and Gold Nanoparticles in Treatment Strategies. Curr. Issues Mol. Biol. 2025, 47, 460. https://doi.org/10.3390/cimb47060460
Kouri MA, Tsaroucha A, Axakali T-M, Varelas P, Kouloulias V, Platoni K, Efstathopoulos EP. Targeting Cancer Cell Fate: Apoptosis, Autophagy, and Gold Nanoparticles in Treatment Strategies. Current Issues in Molecular Biology. 2025; 47(6):460. https://doi.org/10.3390/cimb47060460
Chicago/Turabian StyleKouri, Maria Anthi, Alexandra Tsaroucha, Theano-Marina Axakali, Panagiotis Varelas, Vassilis Kouloulias, Kalliopi Platoni, and Efstathios P. Efstathopoulos. 2025. "Targeting Cancer Cell Fate: Apoptosis, Autophagy, and Gold Nanoparticles in Treatment Strategies" Current Issues in Molecular Biology 47, no. 6: 460. https://doi.org/10.3390/cimb47060460
APA StyleKouri, M. A., Tsaroucha, A., Axakali, T.-M., Varelas, P., Kouloulias, V., Platoni, K., & Efstathopoulos, E. P. (2025). Targeting Cancer Cell Fate: Apoptosis, Autophagy, and Gold Nanoparticles in Treatment Strategies. Current Issues in Molecular Biology, 47(6), 460. https://doi.org/10.3390/cimb47060460










