A Theoretical and Practical Analysis of Membrane Protein Genes Altered in Neutrophils in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Microarray Database from Gene Expression Omnibus (GEO)
2.2. Microarray Data Processing
2.3. Atlas Protein Review
2.4. Enrichment and Protein–Protein Network Analysis
2.5. Statistical Analyses
2.6. Selection Criteria for Blood Samples
- CG inclusion criteria: healthy individuals ≥60 years of age, of either sex, providing informed consent without compensation.
- CG exclusion criteria: subjects with infectious diseases, symptoms of chronic inflammation (unrelated to PD), autoimmune disorders, or anti-inflammatory treatment.
- CG elimination criteria: coagulated samples and/or processing failures.
2.7. Sample Collection
2.8. Neutrophil Isolation
2.9. RNA Extraction from Neutrophils
2.10. Transcription of mRNA to cDNA
2.11. End-Point PCR
2.12. Digital PCR
2.13. Ethical Considerations
3. Results
3.1. Selection of Main Genes in Granulocytes and/or Neutrophils Associated with PD
3.2. Enrichment Pathways Analysis
3.3. Protein-Protein Interaction (PPI) Network Analysis
3.4. Selection of Genes as Potential Biomarkers of PD
3.5. Demographic Characteristics of PD and CG
3.6. Digital PCR Analysis
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| DEG | Differentially regulated genes |
| dPCR | Digital polymerase chain reaction |
| α-syn | Alpha synuclein |
| LBs | Lewy bodies |
| GEO | Gene expression omnibus |
| SBMA | Spinal and bulbar muscular atrophy |
| BPs | Biological processes |
| CCs | Cellular components |
| MFs | Molecular functions |
| FDR | False discovery rate |
| HAP | Hospital Ángeles Puebla |
| CG | Control group |
| PPI | Protein–protein interaction |
| HBP | High blood pressure |
| SD | Standard deviation |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| SOCE | Store-operated calcium entry |
| CRAC | Calcium release-activated calcium |
| ER | Endoplasmic Reticulum |
| BSP | Bone sialoprotein |
| OMD | Osteomodulin |
| ACY1 | Aminoacylase-1 |
| GHR | Growth hormone receptor |
| MSA | Multiple system atrophy |
| ROS | Reactive oxigen species |
| ClCs | Chloride channels |
References
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The Epidemiology of Parkinson’s Disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson’s Disease in 204 Countries/Territories from 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef] [PubMed]
- Salemi, M.; Mogavero, M.P.; Lanza, G.; Mongioì, L.M.; Calogero, A.E.; Ferri, R. Examples of Inverse Comorbidity between Cancer and Neurodegenerative Diseases: A Possible Role for Noncoding RNA. Cells 2022, 11, 1930. [Google Scholar] [CrossRef]
- Gallagher, D.A.; Lees, A.J.; Schrag, A. What Are the Most Important Nonmotor Symptoms in Patients with Parkinson’s Disease and Are We Missing Them? Mov. Disord. 2010, 25, 2493–2500. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- González-González, G.; Velasco Herrera, V.M.; Ortega-Aguilar, A. Electroencephalographic Characterization by Covariance Analysis in Men with Parkinson’s Disease Reveals Sex- and Age-Related Differences. Appl. Sci. 2023, 13, 9618. [Google Scholar] [CrossRef]
- González-González, G.; Velasco-Herrera, V.M.; Ortega-Aguilar, A. Use of Covariance Analysis in Electroencephalogram Reveals Abnormalities in Parkinson’s Disease. Appl. Sci. 2021, 11, 9633. [Google Scholar] [CrossRef]
- Kulcsarova, K.; Skorvanek, M.; Postuma, R.B.; Berg, D. Defining Parkinson’s Disease: Past and Future. J. Park. Dis. 2024, 14, S257–S271. [Google Scholar] [CrossRef]
- Peña-Díaz, S.; García-Pardo, J.; Ventura, S. Development of Small Molecules Targeting α-Synuclein Aggregation: A Promising Strategy to Treat Parkinson’s Disease. Pharmaceutics 2023, 15, 839. [Google Scholar] [CrossRef]
- Feng, S.; Gui, J.; Qin, B.; Ye, J.; Zhao, Q.; Guo, A.; Sang, M.; Sun, X. Resveratrol Inhibits VDAC1-Mediated Mitochondrial Dysfunction to Mitigate Pathological Progression in Parkinson’s Disease Model. Mol. Neurobiol. 2024, 62, 6636–6654. [Google Scholar] [CrossRef]
- Bonato, G.; Antonini, A.; Pistonesi, F.; Campagnolo, M.; Guerra, A.; Biundo, R.; Pilleri, M.; Bertolin, C.; Salviati, L.; Carecchio, M. Genetic Mutations in Parkinson’s Disease: Screening of a Selected Population from North-Eastern Italy. Neurol. Sci. 2025, 46, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.W.; Tan, A.H.; Lim, J.L.; Lohmann, K.; Ibrahim, K.A.; Abdul Aziz, Z.; Chin, Y.T.; Mawardi, A.S.; Lim, T.T.; Looi, I.; et al. Genetic Study of Early-Onset Parkinson’s Disease in the Malaysian Population. Park. Relat. Disord. 2023, 111, 105399. [Google Scholar] [CrossRef] [PubMed]
- Atterling Brolin, K.; Schaeffer, E.; Kuri, A.; Rumrich, I.K.; Schumacher Schuh, A.F.; Darweesh, S.K.L.; Kaasinen, V.; Tolppanen, A.; Chahine, L.M.; Noyce, A.J. Environmental Risk Factors for Parkinson’s Disease: A Critical Review and Policy Implications. Mov. Disord. 2024, 40, 204–221. [Google Scholar] [CrossRef]
- Bruce, H.J.; Tripodis, Y.; McClean, M.; Korell, M.; Tanner, C.M.; Contreras, B.; Gottesman, J.; Kirsch, L.; Karim, Y.; Martin, B.; et al. American Football Play and Parkinson Disease Among Men. JAMA Netw. Open 2023, 6, e2328644. [Google Scholar] [CrossRef] [PubMed]
- Cocoros, N.M.; Svensson, E.; Szépligeti, S.K.; Vestergaard, S.V.; Szentkúti, P.; Thomsen, R.W.; Borghammer, P.; Sørensen, H.T.; Henderson, V.W. Long-Term Risk of Parkinson Disease Following Influenza and Other Infections. JAMA Neurol. 2021, 78, 1461. [Google Scholar] [CrossRef]
- Islam, M.S.; Azim, F.; Saju, H.; Zargaran, A.; Shirzad, M.; Kamal, M.; Fatema, K.; Rehman, S.; Azad, M.A.M.; Ebrahimi-Barough, S. Pesticides and Parkinson’s Disease: Current and Future Perspective. J. Chem. Neuroanat. 2021, 115, 101966. [Google Scholar] [CrossRef]
- Levine, K.S.; Leonard, H.L.; Blauwendraat, C.; Iwaki, H.; Johnson, N.; Bandres-Ciga, S.; Ferrucci, L.; Faghri, F.; Singleton, A.B.; Nalls, M.A. Virus Exposure and Neurodegenerative Disease Risk across National Biobanks. Neuron 2023, 111, 1086–1093.e2. [Google Scholar] [CrossRef]
- Araújo, B.; Caridade-Silva, R.; Soares-Guedes, C.; Martins-Macedo, J.; Gomes, E.D.; Monteiro, S.; Teixeira, F.G. Neuroinflammation and Parkinson’s Disease—From Neurodegeneration to Therapeutic Opportunities. Cells 2022, 11, 2908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Hui, K.Y.; Fernandez-Hernandez, H.; Hu, J.; Schaffner, A.; Pankratz, N.; Hsu, N.-Y.; Chuang, L.-S.; Carmi, S.; Villaverde, N.; Li, X.; et al. Functional Variants in the LRRK2 Gene Confer Shared Effects on Risk for Crohn’s Disease and Parkinson’s Disease. Sci. Transl. Med. 2018, 10, eaai7795. [Google Scholar] [CrossRef]
- Witoelar, A.; Jansen, I.E.; Wang, Y.; Desikan, R.S.; Gibbs, J.R.; Blauwendraat, C.; Thompson, W.K.; Hernandez, D.G.; Djurovic, S.; Schork, A.J.; et al. Genome-Wide Pleiotropy Between Parkinson Disease and Autoimmune Diseases. JAMA Neurol. 2017, 74, 780. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, J.; Qin, Q.; Wang, D.; Zhao, J.; An, K.; Mao, Z.; Min, Z.; Xiong, Y.; Li, J.; et al. A Systematic Review and Meta-Analysis of Inflammatory Biomarkers in Parkinson’s Disease. npj Park. Dis. 2023, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Luo, Q.; Chen, J.; Fan, W. Assessing the Causal Relationship between Genetically Determined Inflammatory Cytokines and Parkinson’s Disease Risk: A Bidirectional Two-Sample Mendelian Randomization Study. J. Immunol. Res. 2024, 2024, 9069870. [Google Scholar] [CrossRef]
- Hartmann, A. Postmortem Studies in Parkinson’s Disease. Dialogues Clin. Neurosci. 2004, 6, 281–293. [Google Scholar] [CrossRef]
- Smajić, S.; Prada-Medina, C.A.; Landoulsi, Z.; Ghelfi, J.; Delcambre, S.; Dietrich, C.; Jarazo, J.; Henck, J.; Balachandran, S.; Pachchek, S.; et al. Single-Cell Sequencing of Human Midbrain Reveals Glial Activation and a Parkinson-Specific Neuronal State. Brain 2022, 145, 964–978. [Google Scholar] [CrossRef]
- Yacoubian, T.A.; Fang, Y.D.; Gerstenecker, A.; Amara, A.; Stover, N.; Ruffrage, L.; Collette, C.; Kennedy, R.; Zhang, Y.; Hong, H.; et al. Brain and Systemic Inflammation in De Novo Parkinson’s Disease. Mov. Disord. 2023, 38, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cuadrado, A.; Saiz-Sanchez, D.; Mohedano-Moriano, A.; Lamas-Cenjor, E.; Leon-Olmo, V.; Martinez-Marcos, A.; Ubeda-Bañon, I. Astrogliosis and Sexually Dimorphic Neurodegeneration and Microgliosis in the Olfactory Bulb in Parkinson’s Disease. npj Park. Dis. 2021, 7, 11. [Google Scholar] [CrossRef]
- Rossi, B.; Constantin, G.; Zenaro, E. The Emerging Role of Neutrophils in Neurodegeneration. Immunobiology 2020, 225, 151865. [Google Scholar] [CrossRef]
- Van Avondt, K.; Strecker, J.; Tulotta, C.; Minnerup, J.; Schulz, C.; Soehnlein, O. Neutrophils in Aging and Aging-related Pathologies. Immunol. Rev. 2023, 314, 357–375. [Google Scholar] [CrossRef]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A Comprehensive Map of Molecular Drug Targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef]
- Shamir, R.; Klein, C.; Amar, D.; Vollstedt, E.-J.; Bonin, M.; Usenovic, M.; Wong, Y.C.; Maver, A.; Poths, S.; Safer, H.; et al. Analysis of Blood-Based Gene Expression in Idiopathic Parkinson Disease. Neurology 2017, 89, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Blanz, J.; Schweizer, M.; Auberson, M.; Maier, H.; Muenscher, A.; Hubner, C.A.; Jentsch, T.J. Leukoencephalopathy upon Disruption of the Chloride Channel ClC-2. J. Neurosci. 2007, 27, 6581–6589. [Google Scholar] [CrossRef]
- Martínez-Rojas, V.A.; Jiménez-Garduño, A.M.; Michelatti, D.; Tosatto, L.; Marchioretto, M.; Arosio, D.; Basso, M.; Pennuto, M.; Musio, C. ClC-2-like Chloride Current Alterations in a Cell Model of Spinal and Bulbar Muscular Atrophy, a Polyglutamine Disease. J. Mol. Neurosci. 2021, 71, 662–674. [Google Scholar] [CrossRef]
- Stölting, G.; Fischer, M.; Fahlke, C. CLC Channel Function and Dysfunction in Health and Disease. Front. Physiol. 2014, 5, 378. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar]
- Yang, L.; Zhang, Y.-H.; Huang, F.; Li, Z.; Huang, T.; Cai, Y.-D. Identification of Protein–Protein Interaction Associated Functions Based on Gene Ontology and KEGG Pathway. Front. Genet. 2022, 13, 1011659. [Google Scholar] [CrossRef]
- Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING V10: Protein–Protein Interaction Networks, Integrated over the Tree of Life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Singh, G.; Khatri, D.K. MicroRNA-Gene Regulatory Network of TLR Signaling in Neuroinflammation-Induced Parkinson’s Disease: A Bioinformatics Approach. Netw. Model. Anal. Health Inform. Bioinform. 2024, 13, 7. [Google Scholar] [CrossRef]
- Jiang, F.; Wu, Q.; Sun, S.; Bi, G.; Guo, L. Identification of Potential Diagnostic Biomarkers for Parkinson’s Disease. FEBS Open Bio. 2019, 9, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Posavi, M.; Diaz-Ortiz, M.; Liu, B.; Swanson, C.R.; Skrinak, R.T.; Hernandez-Con, P.; Amado, D.A.; Fullard, M.; Rick, J.; Siderowf, A.; et al. Characterization of Parkinson’s Disease Using Blood-Based Biomarkers: A Multicohort Proteomic Analysis. PLoS Med. 2019, 16, e1002931. [Google Scholar] [CrossRef] [PubMed]
- Diaz, K.; Kohut, M.L.; Russell, D.W.; Stegemöller, E.L. Peripheral Inflammatory Cytokines and Motor Symptoms in Persons with Parkinson’s Disease. Brain Behav. Immun.—Health 2022, 21, 100442. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.-L.; Du, R.-L.; Niu, R.-Z.; Xue, L.-L.; Chen, L.; Huangfu, L.-R.; Cai, X.-X.; He, X.-Y.; Huang, J.; Huang, X.-Y.; et al. Single-Cell RNA Sequencing Reveals Peripheral Immunological Features in Parkinson’s Disease. npj Park. Dis. 2024, 10, 185. [Google Scholar] [CrossRef]
- Maier-Begandt, D.; Alonso-Gonzalez, N.; Klotz, L.; Erpenbeck, L.; Jablonska, J.; Immler, R.; Hasenberg, A.; Mueller, T.T.; Herrero-Cervera, A.; Aranda-Pardos, I.; et al. Neutrophils—Biology and Diversity. Nephrol. Dial. Transplant. 2024, 39, 1551–1564. [Google Scholar] [CrossRef]
- Xie, X.; Shi, Q.; Wu, P.; Zhang, X.; Kambara, H.; Su, J.; Yu, H.; Park, S.-Y.; Guo, R.; Ren, Q.; et al. Single-Cell Transcriptome Profiling Reveals Neutrophil Heterogeneity in Homeostasis and Infection. Nat. Immunol. 2020, 21, 1119–1133. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in Chronic Inflammatory Diseases. Cell Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Kara, S.P.; Altunan, B.; Unal, A. Investigation of the Peripheral Inflammation (Neutrophil–Lymphocyte Ratio) in Two Neurodegenerative Diseases of the Central Nervous System. Neurol. Sci. 2022, 43, 1799–1807. [Google Scholar] [CrossRef]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef]
- Williams, T.D.; Rousseau, A. Actin Dynamics in Protein Homeostasis. Biosci. Rep. 2022, 42, BSR20210848. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, N.; Henty-Ridilla, J.L. Actin Filament Dynamics at Barbed Ends: New Structures, New Insights. Curr. Opin. Cell Biol. 2024, 90, 102419. [Google Scholar] [CrossRef] [PubMed]
- Sprenkeler, E.G.G.; Tool, A.T.J.; Henriet, S.S.V.; Van Bruggen, R.; Kuijpers, T.W. Formation of Neutrophil Extracellular Traps Requires Actin Cytoskeleton Rearrangements. Blood 2022, 139, 3166–3180. [Google Scholar] [CrossRef]
- Sprenkeler, E.G.G.; Webbers, S.D.S.; Kuijpers, T.W. When Actin Is Not Actin’ Like It Should: A New Category of Distinct Primary Immunodeficiency Disorders. J. Innate Immun. 2021, 13, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Alsegiani, A.; Shah, Z. The Role of Cofilin in Age-Related Neuroinflammation. Neural Regen. Res. 2020, 15, 1451. [Google Scholar] [CrossRef]
- Wurz, A.I.; Schulz, A.M.; O’Bryant, C.T.; Sharp, J.F.; Hughes, R.M. Cytoskeletal Dysregulation and Neurodegenerative Disease: Formation, Monitoring, and Inhibition of Cofilin-Actin Rods. Front. Cell. Neurosci. 2022, 16, 982074. [Google Scholar] [CrossRef]
- Ramirez-Soto, I.; Ortega-Aguilar, A. Skeletal Muscle is a Source of α-Synuclein with a Sarcolemmal Non-Lipid Raft Distribution. Cell Physiol Biochem 2022, 56, 382–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Underwood, R.; Kamath, A.; Britain, C.; McFerrin, M.B.; McLean, P.J.; Volpicelli-Daley, L.A.; Whitaker, R.H.; Placzek, W.J.; Becker, K.; et al. 14-3-3 Proteins Reduce Cell-to-Cell Transfer and Propagation of Pathogenic α-Synuclein. J. Neurosci. 2018, 38, 8211–8232. [Google Scholar] [CrossRef]
- Gao, V.; Briano, J.A.; Komer, L.E.; Burré, J. Functional and Pathological Effects of α-Synuclein on Synaptic SNARE Complexes. J. Mol. Biol. 2023, 435, 167714. [Google Scholar] [CrossRef]
- Lehrer, S.; Rheinstein, P.H. α-Synuclein Enfolds Tyrosine Hydroxylase and Dopamine ß-Hydroxylase, Potentially Reducing Dopamine and Norepinephrine Synthesis. J. Proteins Proteom. 2022, 13, 109–115. [Google Scholar] [CrossRef]
- Liu, Z.; Chan, R.B.; Cai, Z.; Liu, X.; Wu, Y.; Yu, Z.; Feng, T.; Yang, Y.; Zhang, J. α-Synuclein-Containing Erythrocytic Extracellular Vesicles: Essential Contributors to Hyperactivation of Monocytes in Parkinson’s Disease. J. Neuroinflamm. 2022, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Kuter, K.Z.; Śmiałowska, M.; Ossowska, K. The Influence of Preconditioning with Low Dose of LPS on Paraquat-Induced Neurotoxicity, Microglia Activation and Expression of α-Synuclein and Synphilin-1 in the Dopaminergic System. Pharmacol. Rep. 2022, 74, 67–83. [Google Scholar] [CrossRef]
- Shishido, T.; Nagano, Y.; Araki, M.; Kurashige, T.; Obayashi, H.; Nakamura, T.; Takahashi, T.; Matsumoto, M.; Maruyama, H. Synphilin-1 Has Neuroprotective Effects on MPP+-Induced Parkinson’s Disease Model Cells by Inhibiting ROS Production and Apoptosis. Neurosci. Lett. 2019, 690, 145–150. [Google Scholar] [CrossRef]
- Amadeo, A.; Pizzi, S.; Comincini, A.; Modena, D.; Calogero, A.M.; Madaschi, L.; Faustini, G.; Rolando, C.; Bellucci, A.; Pezzoli, G.; et al. The Association between α-Synuclein and α-Tubulin in Brain Synapses. IJMS 2021, 22, 9153. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.M.; Basellini, M.J.; Isilgan, H.B.; Longhena, F.; Bellucci, A.; Mazzetti, S.; Rolando, C.; Pezzoli, G.; Cappelletti, G. Acetylated α-Tubulin and α-Synuclein: Physiological Interplay and Contribution to α-Synuclein Oligomerization. IJMS 2023, 24, 12287. [Google Scholar] [CrossRef]
- Lin, Y.M.; Banoth, D.; Wali, M.H.; Bekova, K.; Abdulla, N.; Gurugubelli, S.; Khan, S. The Role of Alpha-Synuclein and Tubulin-Associated Unit (Tau) Proteins in the Diagnosis, Prognosis, and Treatment of Parkinson’s Disease: A Systematic Review. Cureus 2024, 16, e64766. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, C.; Meng, L.; Tian, Y.; He, M.; Yuan, X.; Zhang, G.; Zhang, Z.; Xiong, J.; Chen, G.; et al. Tau Accelerates α-Synuclein Aggregation and Spreading in Parkinson’s Disease. Brain 2022, 145, 3454–3471. [Google Scholar] [CrossRef]
- Clemens, R.A.; Lowell, C.A. CRAC Channel Regulation of Innate Immune Cells in Health and Disease. Cell Calcium 2019, 78, 56–65. [Google Scholar] [CrossRef]
- Wang, H.; Xu, M.; Kong, Q.; Sun, P.; Yan, F.; Tian, W.; Wang, X. Research and Progress on ClC-2. Mol. Med. Rep. 2017, 16, 11–22. [Google Scholar] [CrossRef]
- Almasoudi, W.; Nilsson, C.; Kjellström, U.; Sandeman, K.; Puschmann, A. Co-Occurrence of CLCN2-Related Leukoencephalopathy and SPG56. Clin. Park. Relat. Disord. 2023, 8, 100189. [Google Scholar] [CrossRef]
- Accardi, A. Structure and Gating of CLC Channels and Exchangers. J. Physiol. 2015, 593, 4129–4138. [Google Scholar] [CrossRef] [PubMed]
- Elorza-Vidal, X.; Gaitán-Peñas, H.; Estévez, R. Chloride Channels in Astrocytes: Structure, Roles in Brain Homeostasis and Implications in Disease. IJMS 2019, 20, 1034. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Stein, V.; Weinreich, F.; Zdebik, A.A. Molecular Structure and Physiological Function of Chloride Channels. Physiol. Rev. 2002, 82, 503–568. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Parishani, M.; Akbartabar Touri, M.; Ghavamzadeh, M.; Jafari Barmak, M.; Zarezade, V.; Delaviz, H.; Sadeghi, H. Pramipexole reduces inflammation in the experimental animal models of inflammation. Immunopharmacol. Immunotoxicol. 2017, 39, 80–86. [Google Scholar] [CrossRef] [PubMed]
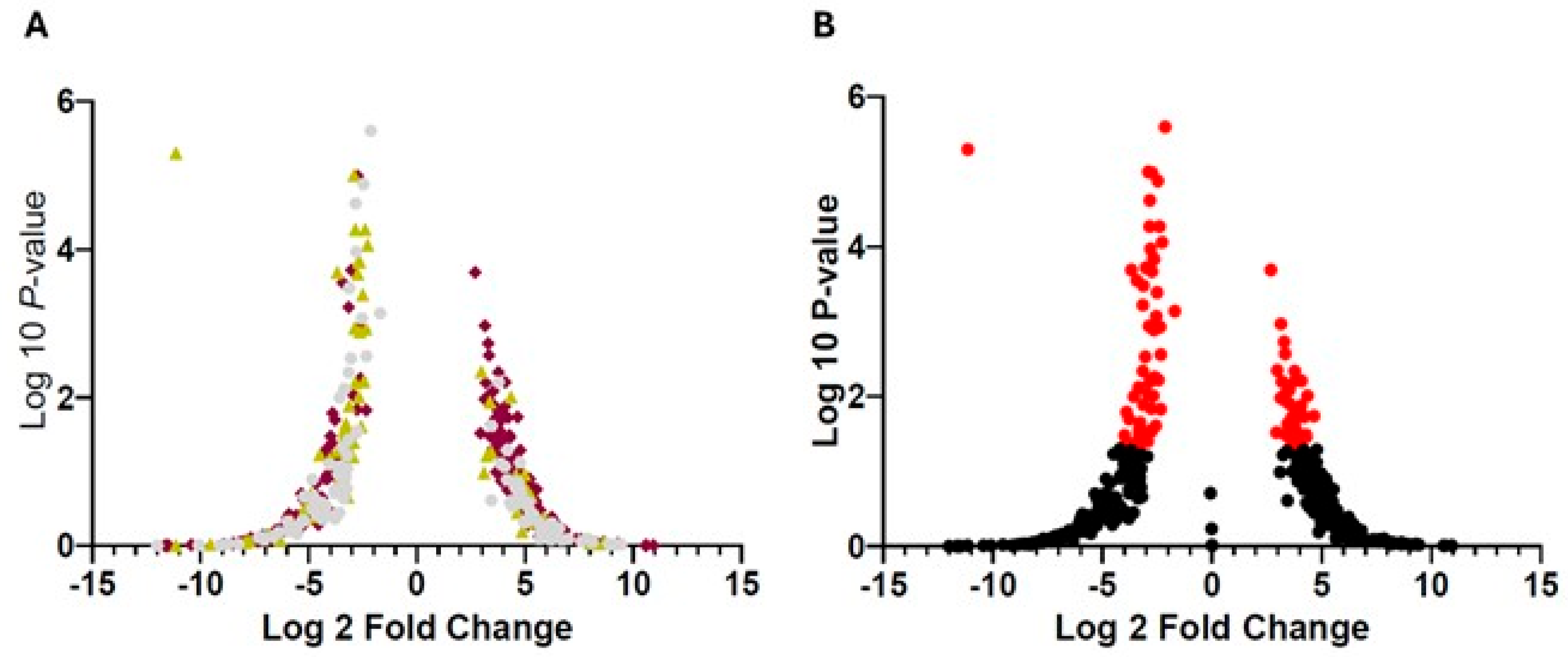

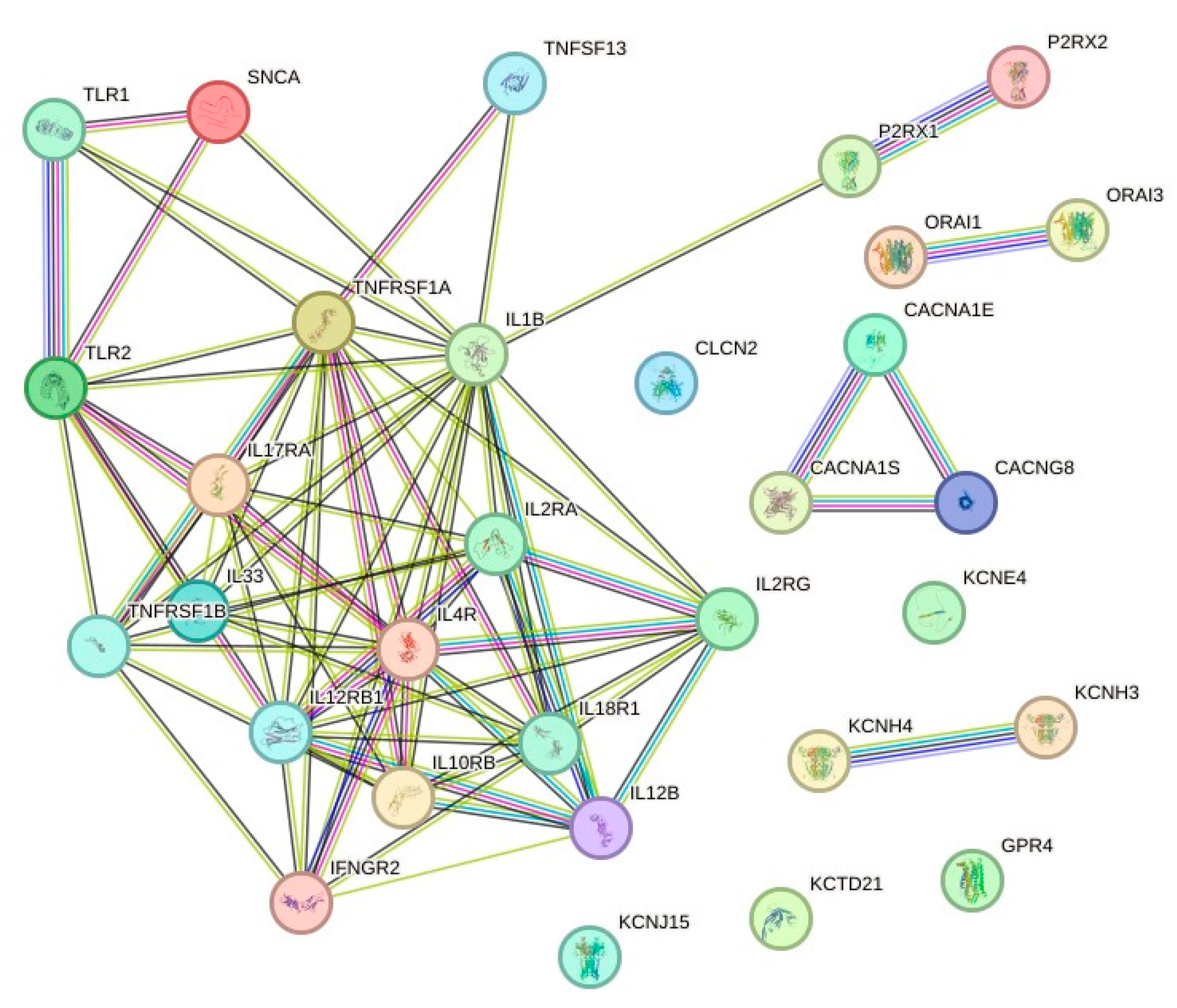
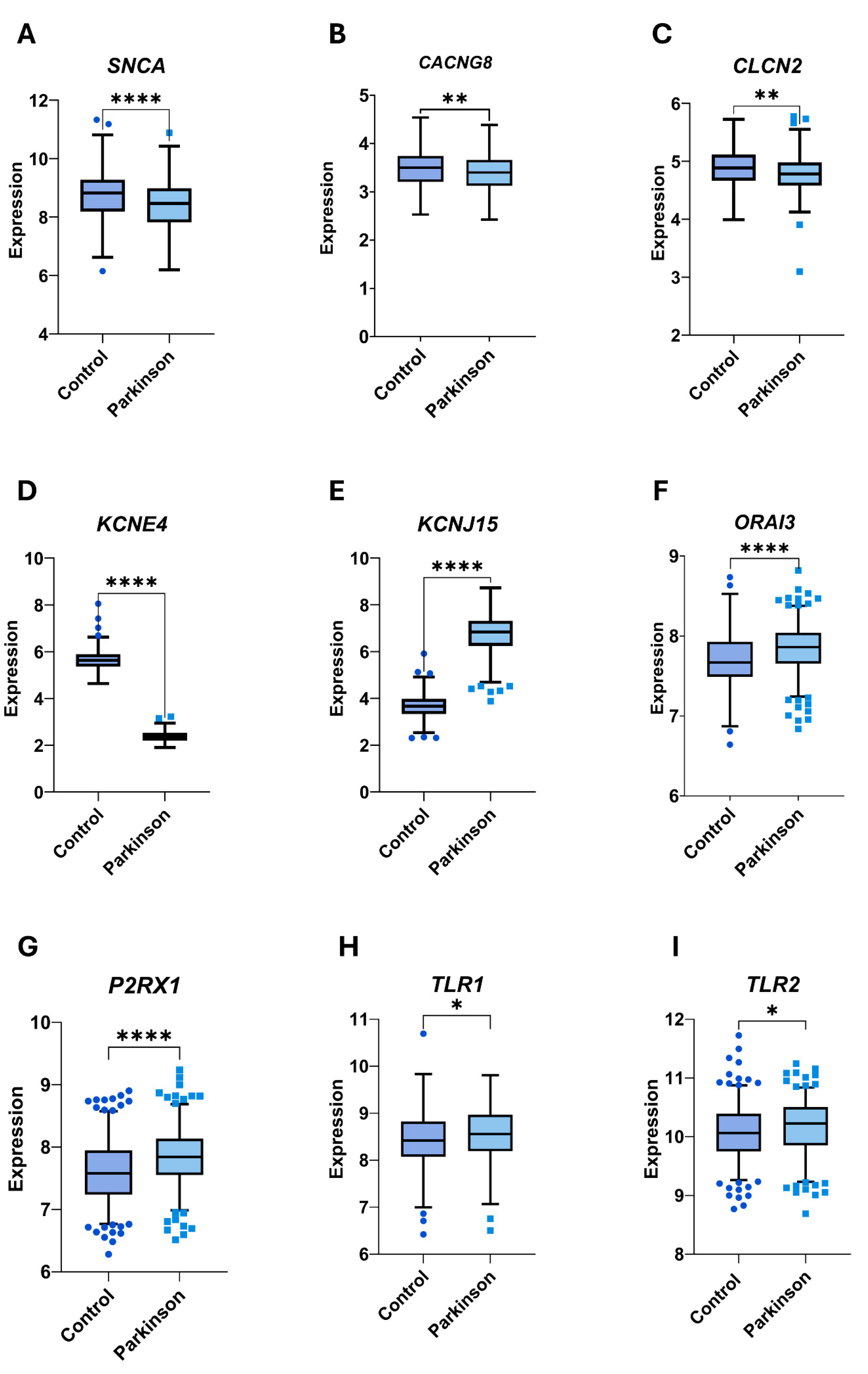
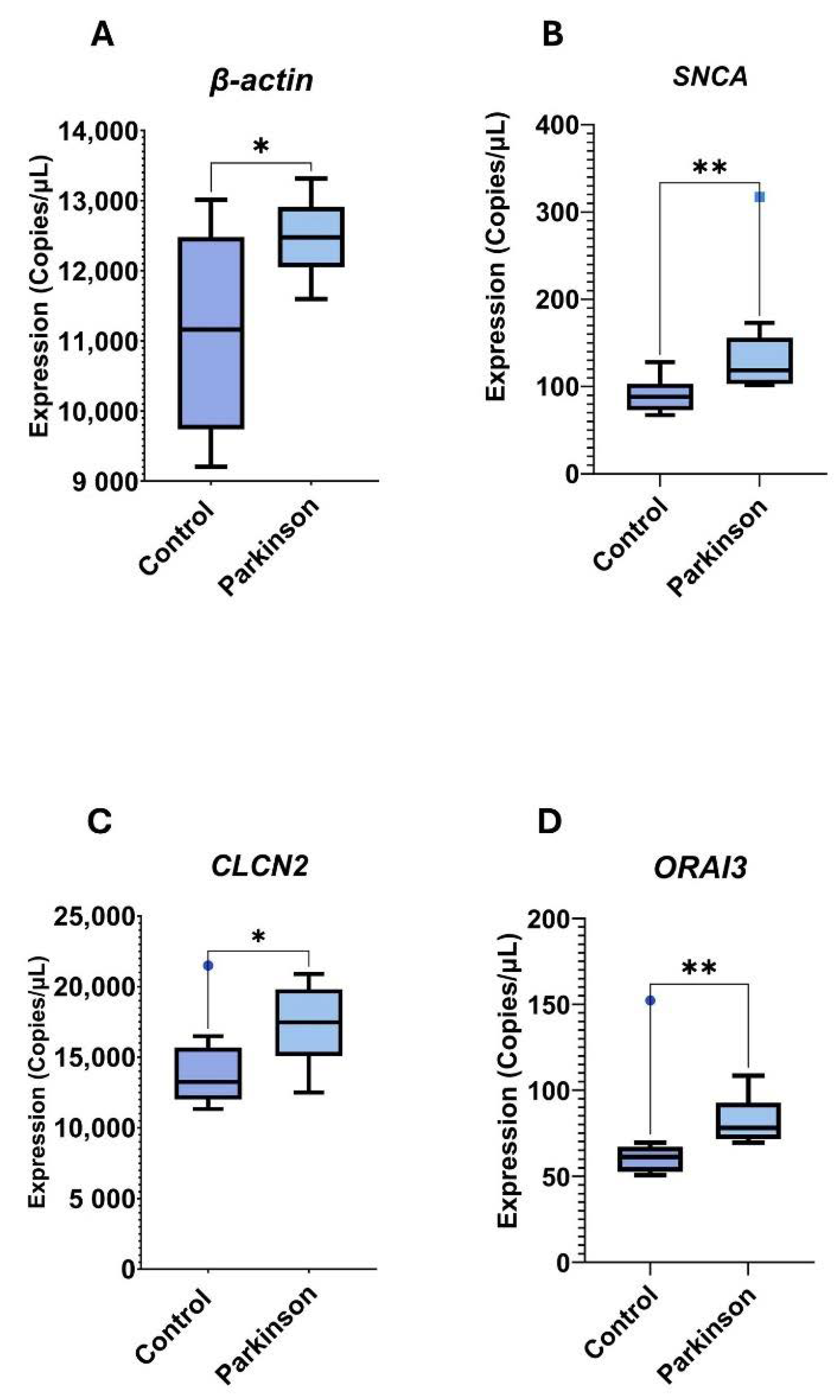
| # | ID | Symbol | SD | p-Value | FDR | Regulation |
|---|---|---|---|---|---|---|
| 1 | 5023 | P2RX1 | −4.46 × 10−1 | 1.00 × 10−4 | 0.00000303 | Up |
| 2 | 7133 | TNFRSF1B | −4.32 × 10−1 | 1.00 × 10−4 | 0.00000606 | Up |
| 3 | 93129 | ORAI3 | −4.09 × 10−1 | 1.00 × 10−4 | 0.00000909 | Up |
| 4 | 6622 | SNCA | 2.96 × 10−1 | 1.00 × 10−4 | 0.00003636 | Up |
| 5 | 3553 | IL1B | −2.03 × 10−1 | 1.00 × 10−4 | 0.00005152 | Up |
| 6 | 23704 | KCNE4 | 8.48 × 10+00 | 1.00 × 10−4 | 0.00005758 | Down |
| 7 | 3772 | KCNJ15 | −4.38 × 10+00 | 1.00 × 10−4 | 0.00007273 | Up |
| 8 | 3594 | IL12RB1 | −1.51 × 10−1 | 1.00 × 10−4 | 0.00008182 | Up |
| 9 | 3559 | IL2RA | 1.53 × 10−1 | 1.00 × 10−4 | 0.00008485 | Down |
| 10 | 59283 | CACNG8 | 1.66 × 10−1 | 1.00 × 10−4 | 0.00008788 | Down |
| 11 | 777 | CACNA1E | −6.74 × 10−2 | 1.00 × 10−4 | 0.00009091 | Down |
| 12 | 2828 | GPR4 | 6.42 × 10−1 | 1.00 × 10−4 | 0.00009394 | Down |
| 13 | 23416 | KCNH3 | 7.43 × 10+00 | 1.00 × 10−4 | 0.00009697 | Down |
| 14 | 23415 | KCNH4 | −2.90 × 10+00 | 1.00 × 10−4 | 0.00010000 | Up |
| 15 | 23765 | IL17RA | −3.91 × 10−1 | 1.01 × 10−4 | 0.00001219 | Up |
| 16 | 7132 | TNFRSF1A | −3.65 × 10−1 | 1.48 × 10−4 | 0.00002242 | Up |
| 17 | 3460 | IFNGR2 | −3.58 × 10−1 | 2.00 × 10−4 | 0.00003636 | Up |
| 18 | 3566 | IL4R | −3.41 × 10−1 | 5.00 × 10−4 | 0.00010606 | Up |
| 19 | 8741 | TNFSF13 | −3.14 × 10−1 | 8.00 × 10−4 | 0.00019394 | Up |
| 20 | 22953 | P2RX4 | −8.03 × 10−2 | 4.80 × 10−3 | 0.00378182 | Up |
| 21 | 8809 | IL18R1 | −2.62 × 10−1 | 6.40 × 10−3 | 0.00193939 | Up |
| 22 | 90865 | IL33 | 2.53 × 10−1 | 8.20 × 10−3 | 0.00273333 | Down |
| 23 | 3588 | IL10RB | −3.23 × 10−2 | 1.72 × 10−2 | 0.00781818 | Up |
| 24 | 283219 | KCTD21 | −2.27 × 10−1 | 1.89 × 10−2 | 0.00801818 | Up |
| 25 | 1181 | CLCN2 | 3.00 × 10−1 | 1.90 × 10−2 | 0.00518182 | Down |
| 26 | 7097 | TLR2 | −1.65 × 10−1 | 2.09 × 10−2 | 0.01456667 | Up |
| 27 | 779 | CACNA1S | 1.98 × 10−1 | 3.93 × 10−2 | 0.02143636 | Down |
| 28 | 7096 | TLR1 | −1.80 × 10−1 | 4.05 × 10−2 | 0.02700000 | Up |
| 29 | 3593 | IL12B | 1.56 × 10−1 | 4.13 × 10−2 | 0.03128788 | Down |
| 30 | 3561 | IL2RG | 3.32 × 10−2 | 4.13 × 10−2 | 0.02503030 | Down |
| 31 | 84876 | ORAI1 | −1.91 × 10−1 | 4.71 × 10−2 | 0.02997273 | Up |
| Control (n = 9) | PD (n = 9) | |
|---|---|---|
| Sex Men %(n) Women %(n) | 66.7% (6) 33.3% (3) | 55.5% (5) 44.5% (4) |
| Age (Mean ± SD) | 63.92 ± 7.73 | 65.42 ± 9.75 |
| High Blood Pressure Diagnosis %(n) | 8.33% (1) | 16.66% (2) |
| Years of PD diagnosis (Mean ± SD) | -- | 9.75 ± 6.38 |
| Gene | Control Copies/uL (Mean ± SD) | PD Copies/uL (Mean ± SD) | p |
|---|---|---|---|
| SNCA | 90.16 ± 20.13 | 144.5 ± 68.9 | 0.0040 |
| ORAI3 | 69.38 ± 31.71 | 81.7 ± 14.56 | 0.0044 |
| Actin | 11039 ± 1449 | 12511 ± 573 | 0.0120 |
| CLCN2 | 14233 ± 3151 | 17429 ± 2821 | 0.0400 |
| P2RX1 | 26.79 ± 4.748 | 22.77 ± 6.007 | 0.1135 |
| TLR2 | 1326 ± 440.3 | 1641 ± 361.7 | 0.1172 |
| KCNE4 | 713.4 ± 58.1 | 743.6 ± 55.53 | 0.2763 |
| TLR1 | 2503 ± 1555 | 2997 ± 1220 | 0.4649 |
| CACNG8 | 24.86 ± 4.102 | 25.04 ± 6.521 | 0.9436 |
| KCNJ15 | 57.11 ± 16.11 | 56.67 ± 7.811 | 0.9641 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Pintor, A.; Nolasco López, M.; Lozada-Ramírez, J.D.; Serrano-Meneses, M.A.; Ortega Aguilar, A.; Oropeza Canto, D.; Flores-de los Ángeles, C.; Anaya-Muñoz, V.H.; Jiménez-Garduño, A.M. A Theoretical and Practical Analysis of Membrane Protein Genes Altered in Neutrophils in Parkinson’s Disease. Curr. Issues Mol. Biol. 2025, 47, 459. https://doi.org/10.3390/cimb47060459
López Pintor A, Nolasco López M, Lozada-Ramírez JD, Serrano-Meneses MA, Ortega Aguilar A, Oropeza Canto D, Flores-de los Ángeles C, Anaya-Muñoz VH, Jiménez-Garduño AM. A Theoretical and Practical Analysis of Membrane Protein Genes Altered in Neutrophils in Parkinson’s Disease. Current Issues in Molecular Biology. 2025; 47(6):459. https://doi.org/10.3390/cimb47060459
Chicago/Turabian StyleLópez Pintor, Araliz, Miriam Nolasco López, José Daniel Lozada-Ramírez, Martín Alejandro Serrano-Meneses, Alicia Ortega Aguilar, Dante Oropeza Canto, César Flores-de los Ángeles, Victor Hugo Anaya-Muñoz, and Aura Matilde Jiménez-Garduño. 2025. "A Theoretical and Practical Analysis of Membrane Protein Genes Altered in Neutrophils in Parkinson’s Disease" Current Issues in Molecular Biology 47, no. 6: 459. https://doi.org/10.3390/cimb47060459
APA StyleLópez Pintor, A., Nolasco López, M., Lozada-Ramírez, J. D., Serrano-Meneses, M. A., Ortega Aguilar, A., Oropeza Canto, D., Flores-de los Ángeles, C., Anaya-Muñoz, V. H., & Jiménez-Garduño, A. M. (2025). A Theoretical and Practical Analysis of Membrane Protein Genes Altered in Neutrophils in Parkinson’s Disease. Current Issues in Molecular Biology, 47(6), 459. https://doi.org/10.3390/cimb47060459





