Investigating the Effects of ONC206 Alone and in Combination with Cisplatin on Ovarian Cancer Cell Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Drugs
2.3. MTT
2.4. Trypan Blue Exclusion Method
2.5. Synergy Analysis
2.6. Wound Healing Assay
2.7. Three-Dimensional Culture and Sphere-Formation Assay
2.8. Statistical Analysis
3. Results
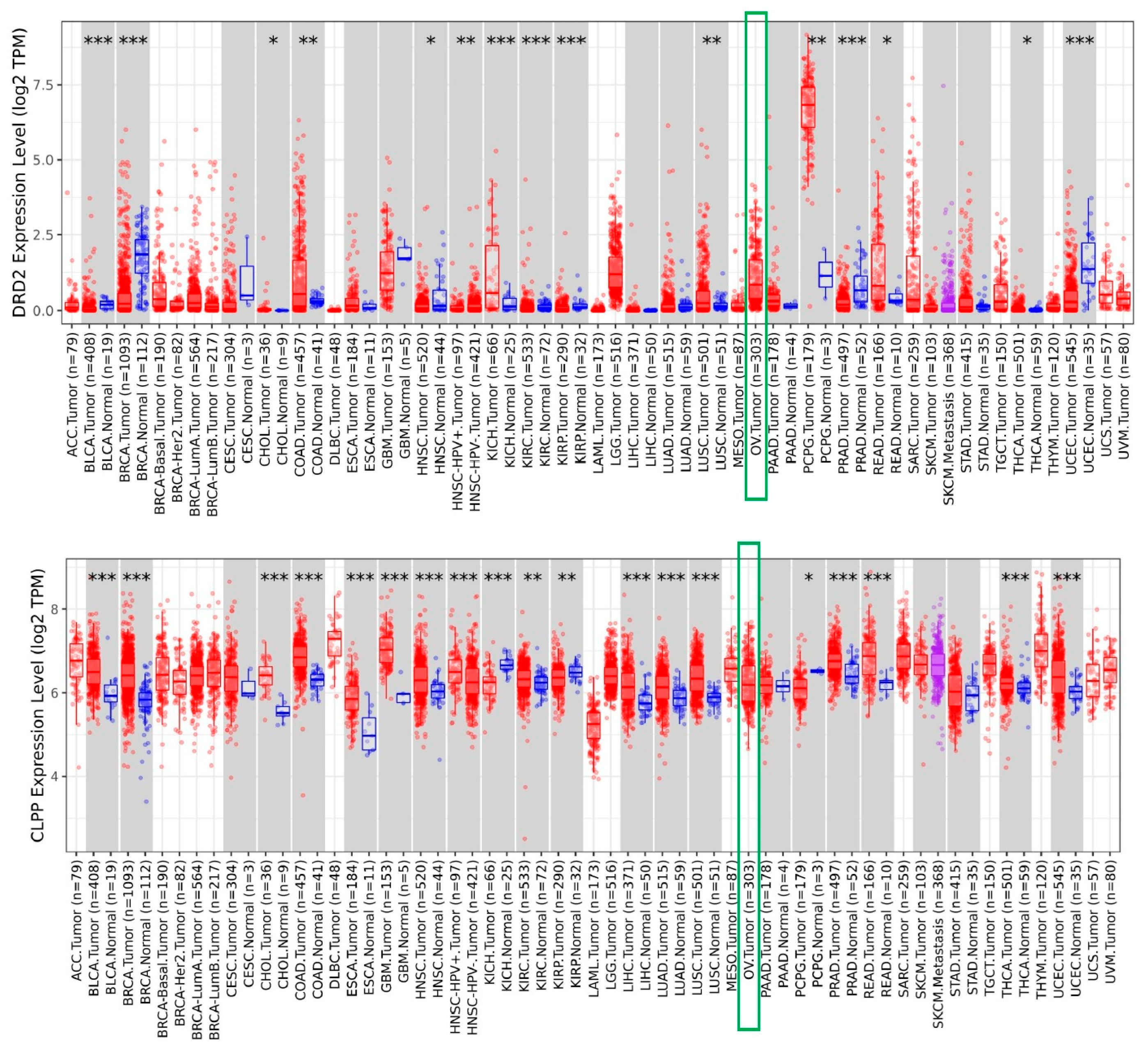
3.1. Increased Expression of DRD2 and CLPP in Ovarian Tumors
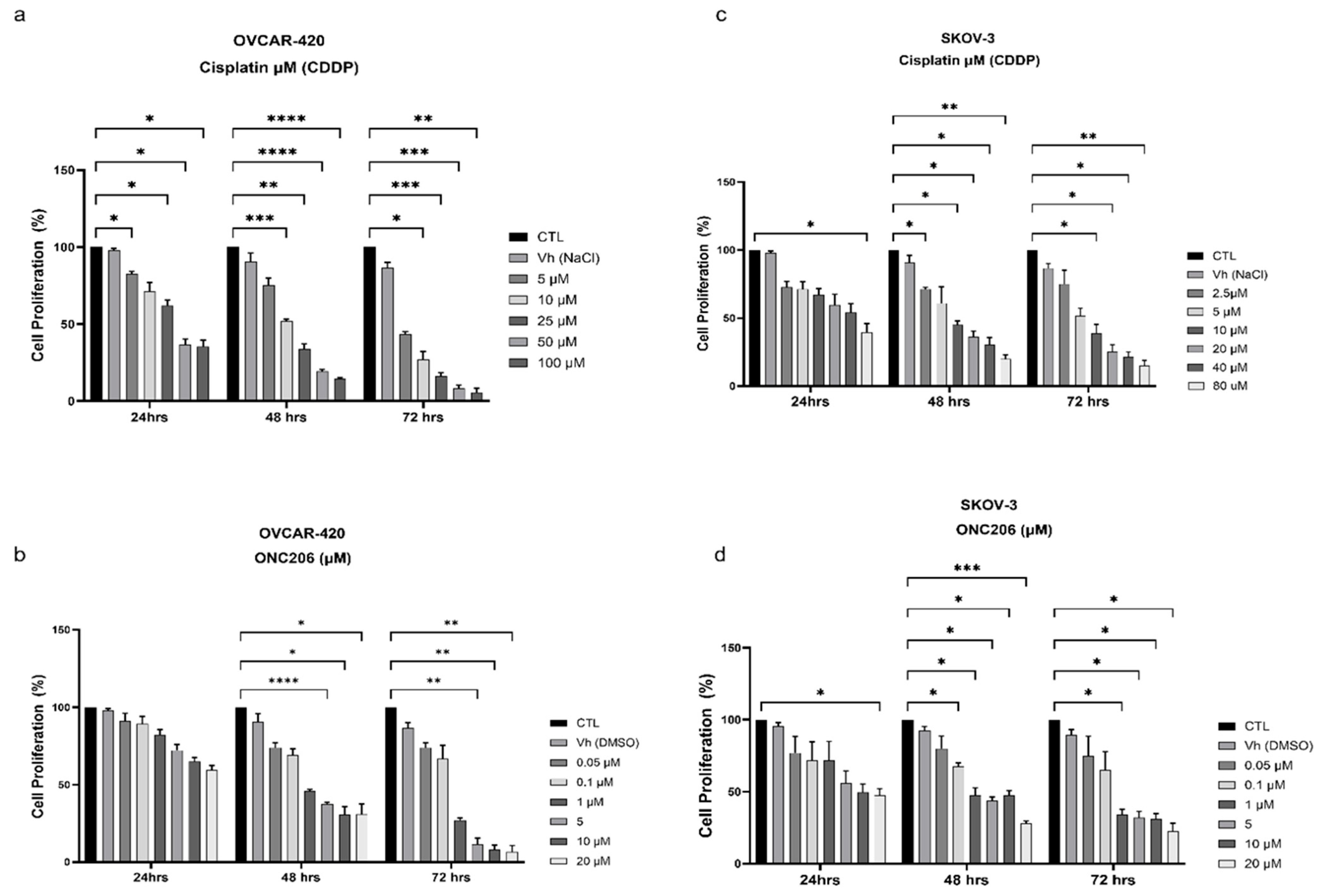
3.2. ONC206 and CDDP Decrease the Proliferation of Ovarian Cancer Cells
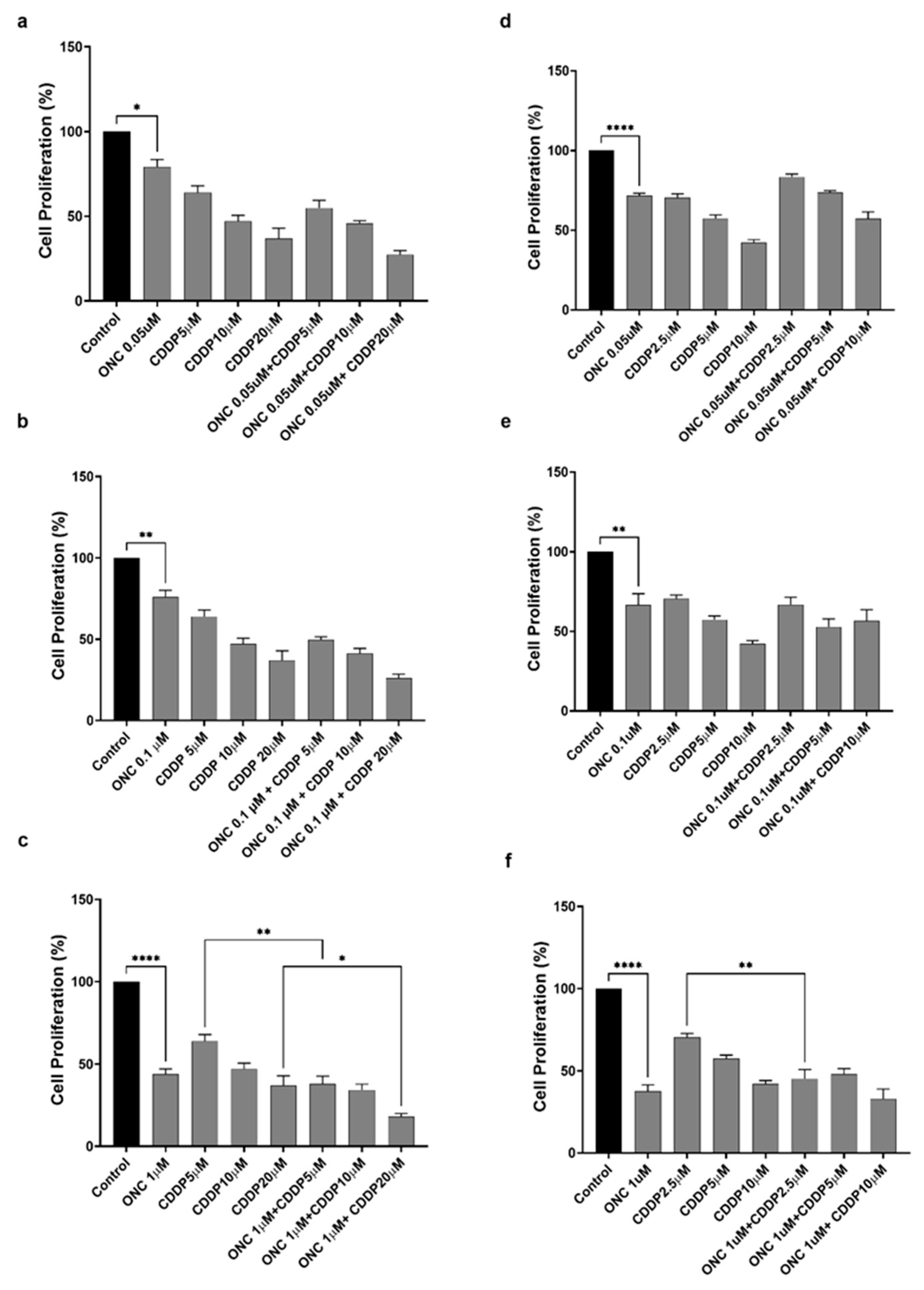
3.3. ONC206 Reduces the Proliferation of Ovarian Cancer Cells Alone and in Combination with CDDP
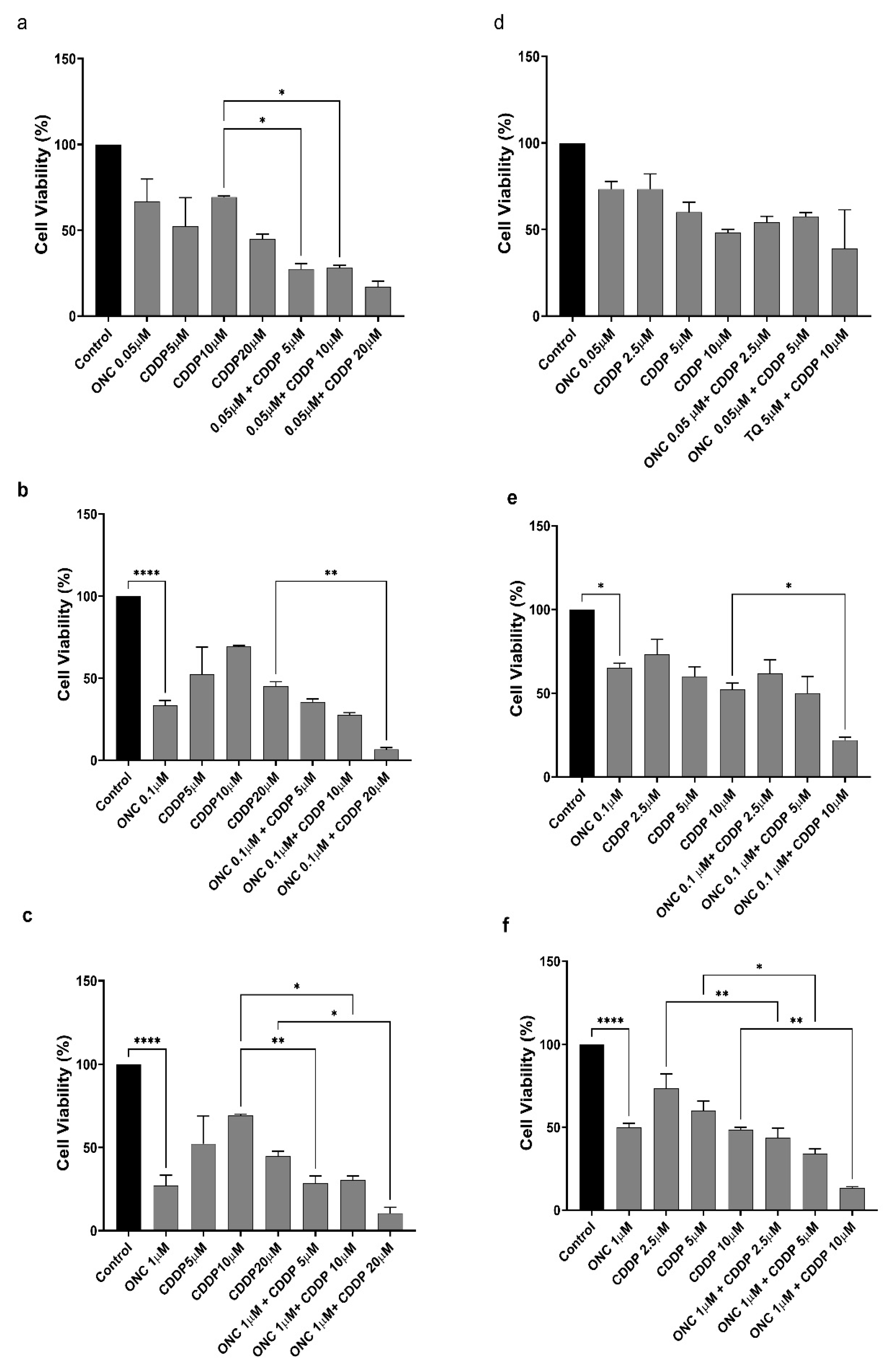
3.4. ONC206 Decreases the Cellular Viability of Ovarian Cancer Cells Alone and in Combination with CDDP
3.5. ONC206 and CDDP Exhibit Synergistic Effects on Ovarian Cancer Cells
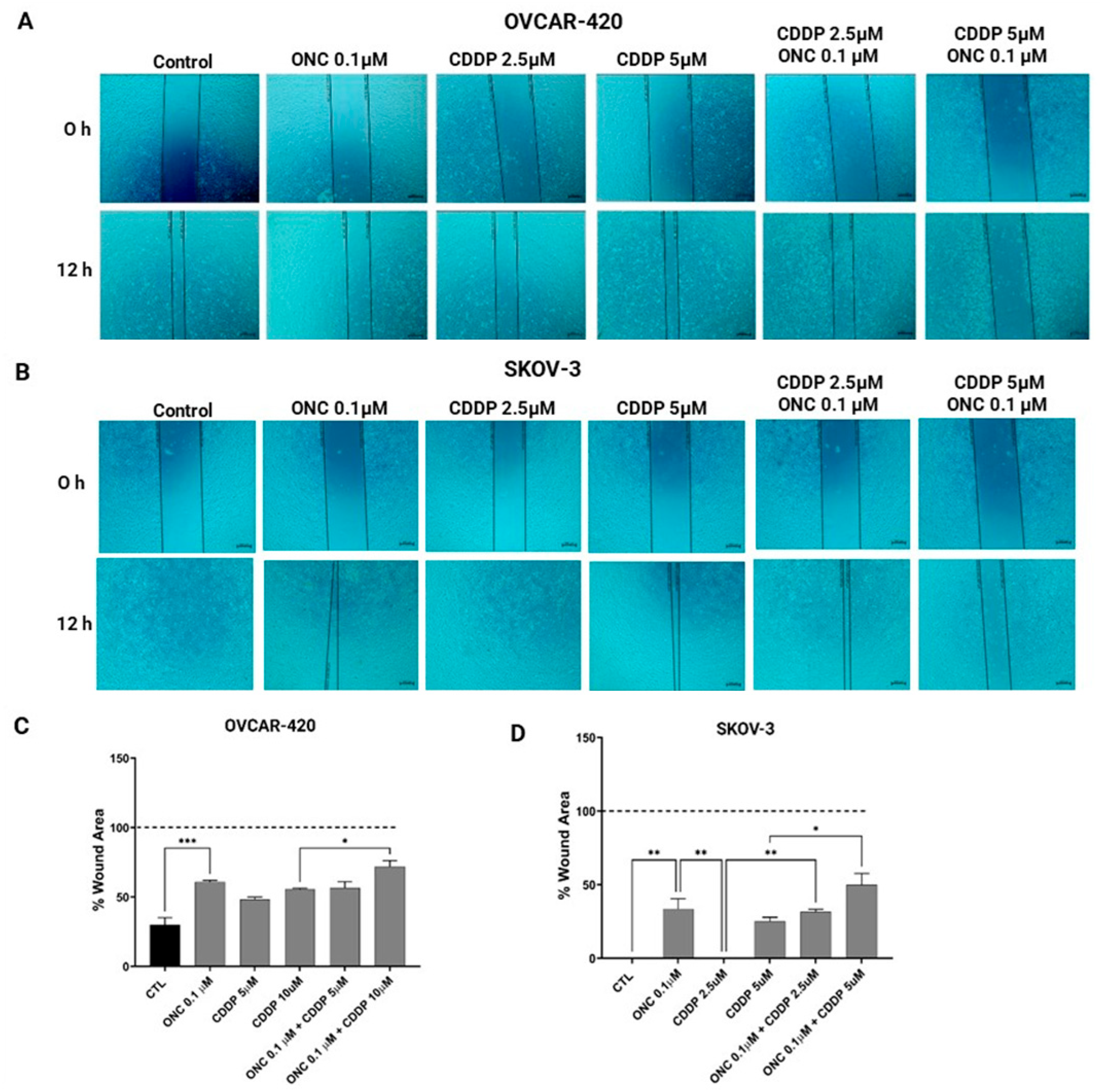
3.6. ONC206 Reduced the Migration of Ovarian Cancer Cell Lines Alone and in Combination with CDDP
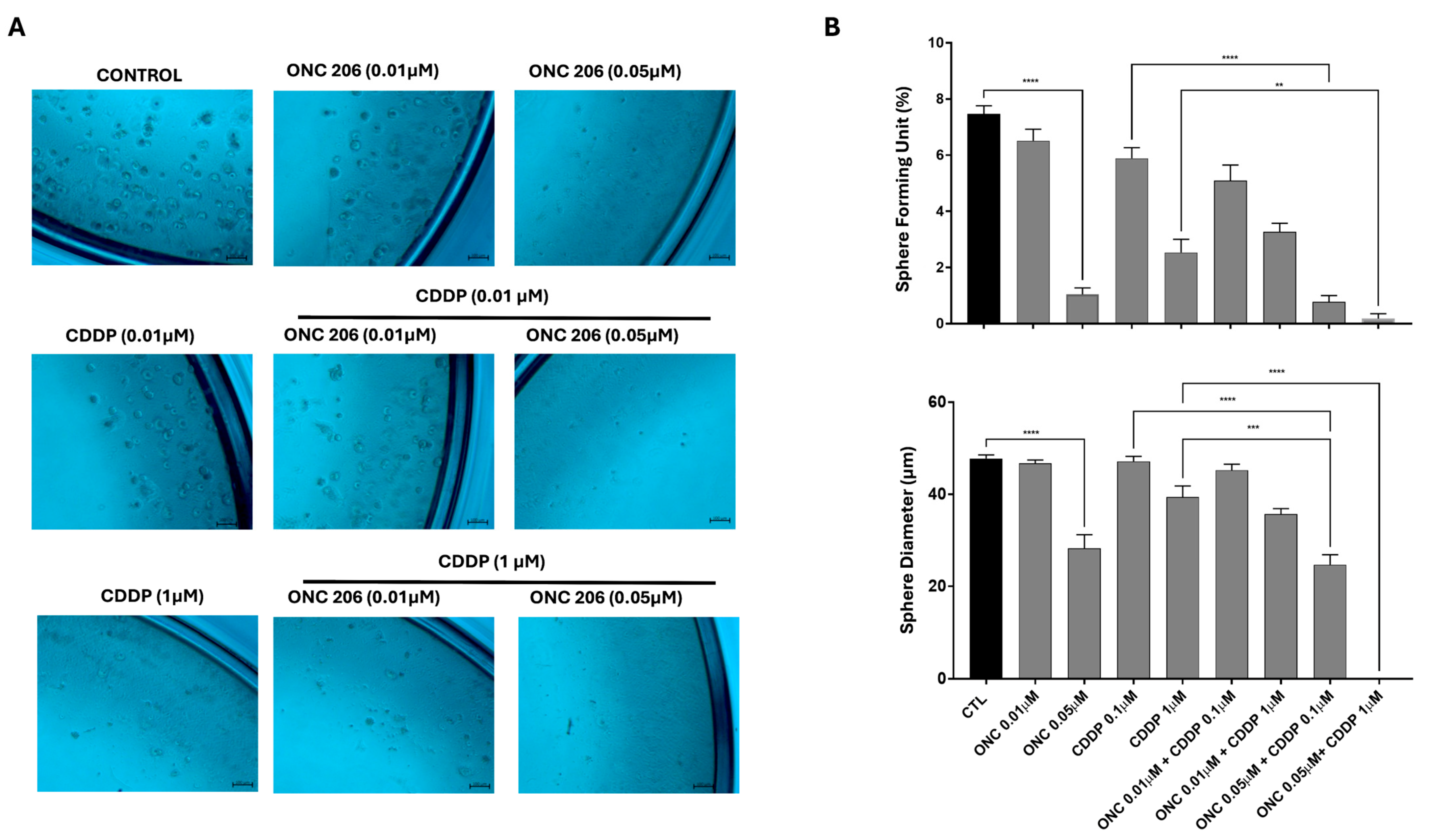
3.7. ONC206 Decreases the Growth of Cell-Derived Spheres of Ovarian Cancer Cells Alone and in Combination with CDDP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Li, C.; Lin, M.; Welch, W.; Bell, D.; Wong, Y.-F.; Berkowitz, R.; Mok, S.C.; Bandera, C.A. Ovarian cancer is a heterogeneous disease. Cancer Genet. Cytogenet. 2005, 161, 170–173. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G.; Singh, N.; Gilks, C.B. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology 2022, 80, 762–778. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; Santini, D.; Ceccarelli, C.; Santandrea, G.; Palicelli, A.; Acquaviva, G.; Chiarucci, F.; Rosini, F.; Ravegnini, G.; Pession, A.; et al. What Is New on Ovarian Carcinoma: Integrated Morphologic and Molecular Analysis Following the New 2020 World Health Organization Classification of Female Genital Tumors. Diagnostics 2021, 11, 697. [Google Scholar] [CrossRef]
- Ali, A.T.; Al-Ani, O.; Al-Ani, F. Epidemiology and risk factors for ovarian cancer. Prz. Menopauzalny 2023, 22, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. 2023, 245. Available online: https://www.ncbi.nlm.nih.gov/books/NBK65921/#:~:text=Family%20history%20and%20inherited%20susceptibility,carcinomas.%5B2%2C3%5D (accessed on 17 April 2025).
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef]
- Alatise, K.L.; Gardner, S.; Alexander-Bryant, A. Mechanisms of Drug Resistance in Ovarian Cancer and Associated Gene Targets. Cancers 2022, 14, 6246. [Google Scholar] [CrossRef]
- Celià-Terrassa, T.; Jolly, M.K. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition in Cancer Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a036905. [Google Scholar] [CrossRef]
- Li, S.S.; Ma, J.; Wong, A.S.T. Chemoresistance in ovarian cancer: Exploiting cancer stem cell metabolism. J. Gynecol. Oncol. 2018, 29, e32. [Google Scholar] [CrossRef] [PubMed]
- Bapat, S.A.; Mali, A.M.; Koppikar, C.B.; Kurrey, N.K. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005, 65, 3025–3029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tapinos, N.; Lulla, R.; El-Deiry, W.S. Dopamine pre-treatment impairs the anti-cancer effect of integrated stress response- and TRAIL pathway-inducing ONC201, ONC206 and ONC212 imipridones in pancreatic, colorectal cancer but not DMG cells. Am. J. Cancer Res. 2024, 14, 2453–2464. [Google Scholar] [CrossRef]
- Cao, J.; Cao, F.; Wang, C.; Jiao, Z.; You, Y.; Wang, X.; Zhao, W. ONC206 targeting ClpP induces mitochondrial dysfunction and protective autophagy in hepatocellular carcinoma cells. Neoplasia 2024, 55, 101015. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, L.; Ferri-Borgogno, S.; Kwan, S.-Y.; Lewis, K.E.; Cun, H.T.; Yeung, T.-L.; Soliman, P.T.; Tarapore, R.S.; Allen, J.E.; et al. Targeting Dopamine Receptor D2 by Imipridone Suppresses Uterine Serous Cancer Malignant Phenotype. Cancers 2020, 12, 2436. [Google Scholar] [CrossRef] [PubMed]
- Przystal, J.M.; Cosentino, C.C.; Yadavilli, S.; Zhang, J.; Laternser, S.; Bonner, E.R.; Prasad, R.; Dawood, A.A.; Lobeto, N.; Chong, W.C.; et al. Imipridones affect tumor bioenergetics and promote cell lineage differentiation in diffuse midline gliomas. Neuro. Oncol. 2022, 24, 1438–1451. [Google Scholar] [CrossRef]
- Staley, A.; Tucker, K.; Yin, Y.; Zhang, X.; Fan, Y.; Zhang, Y.; Fang, Z.; Sun, W.; Suo, H.; Zhao, X.; et al. Highly potent dopamine receptor D2 antagonist ONC206 demonstrates anti-tumorigenic activity in endometrial cancer. Am. J. Cancer Res. 2021, 11, 5374–5387. [Google Scholar] [CrossRef]
- Tucker, K.; Yin, Y.; Staley, S.-A.; Zhao, Z.; Fang, Z.; Fan, Y.; Zhang, X.; Suo, H.; Sun, W.; Prabhu, V.V.; et al. ONC206 has anti-tumorigenic effects in human ovarian cancer cells and in a transgenic mouse model of high-grade serous ovarian cancer. Am. J. Cancer Res. 2022, 12, 521–536. [Google Scholar]
- Marchetti, C.; De Felice, F.; Romito, A.; Iacobelli, V.; Sassu, C.M.; Corrado, G.; Ricci, C.; Scambia, G.; Fagotti, A. Chemotherapy resistance in epithelial ovarian cancer: Mechanisms and emerging treatments. Semin. Cancer Biol. 2021, 77, 144–166. [Google Scholar] [CrossRef]
- Chou, T.C. The mass-action law based algorithm for cost-effective approach for cancer drug discovery and development. Am. J. Cancer Res. 2011, 1, 925–954. [Google Scholar]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Matulonis, U.A. Clinical and translational advances in ovarian cancer therapy. Nat. Cancer 2023, 4, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Paraghamian, S.E.; Qiu, J.; Hawkins, G.M.; Zhao, Z.; Sun, W.; Fan, Y.; Zhang, X.; Suo, H.; Hao, T.; Prabhu, V.V.; et al. A novel dopamine receptor D2 antagonist (ONC206) potentiates the effects of olaparib in endometrial cancer. Cancer Biol. Ther. 2023, 24, 2202104. [Google Scholar] [CrossRef]
- Kobayashi, M.; Salomon, C.; Tapia, J.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. Ovarian cancer cell invasiveness is associated with discordant exosomal sequestration of Let-7 miRNA and miR-200. J. Transl. Med. 2014, 12, 4. [Google Scholar] [CrossRef]
- Mei, J.; Tian, H.; Huang, H.; Hsu, C.; Liou, Y.; Wu, N.; Zhang, W.; Chu, T. Cellular models of development of ovarian high-grade serous carcinoma: A review of cell of origin and mechanisms of carcinogenesis. Cell Prolif. 2021, 54, e13029. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. Methods Mol. Biol. 2017, 1601, 1–17. [Google Scholar]
- Koyanagi, M.; Kawakabe, S.; Arimura, Y. A comparative study of colorimetric cell proliferation assays in immune cells. Cytotechnology 2016, 68, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Monzer, A.; Ghamlouche, F.; Wakimian, K.; Ballout, F.; Al Bitar, S.; Yehya, A.; Kanso, M.; Saheb, N.; Tawil, A.; Doughan, S.; et al. ONC206, an imipridone derivative, demonstrates anti-colorectal cancer activity against stem/progenitor cells in 3D cell cultures and in patient-derived organoids. Pharmacol. Rep. 2024, 77, 229–246. [Google Scholar] [CrossRef]
- He, L.; Bhat, K.; Ioannidis, A.; Zhang, L.; Nguyen, N.T.; Allen, J.E.; Nghiemphu, P.L.; Cloughesy, T.F.; Liau, L.M.; Kornblum, H.I.; et al. Effects of the DRD2/3 antagonist ONC201 and radiation in glioblastoma. Radiother. Oncol. 2021, 161, 140–147. [Google Scholar] [CrossRef]
- Yeung, T.L.; Leung, C.S.; Yip, K.-P.; Yeung, C.L.A.; Wong, S.T.C.; Mok, S.C. Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am. J. Physiol. Cell Physiol. 2015, 309, C444–C4456. [Google Scholar] [CrossRef]
- Bouchalova, P.; Bouchal, P. Current methods for studying metastatic potential of tumor cells. Cancer Cell Int. 2022, 22, 394. [Google Scholar] [CrossRef] [PubMed]
- Hallas-Potts, A.; Dawson, J.C.; Herrington, C.S. Ovarian cancer cell lines derived from non-serous carcinomas migrate and invade more aggressively than those derived from high-grade serous carcinomas. Sci. Rep. 2019, 9, 5515. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Yin, Y.; Fan, Y.; Sun, W.; Zhao, X.; Tucker, K.; Staley, A.; Paraghamian, S.; Hawkins, G.; et al. ONC206, an Imipridone Derivative, Induces Cell Death Through Activation of the Integrated Stress Response in Serous Endometrial Cancer In Vitro. Front. Oncol. 2020, 10, 577141. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Moon, A. Epithelial-mesenchymal Transition and Cell Invasion. Toxicol. Res. 2010, 26, 245–252. [Google Scholar] [CrossRef]
- Markowska, A.; Sajdak, S.; Huczyński, A.; Rehlis, S.; Markowska, J. Ovarian cancer stem cells: A target for oncological therapy. Adv. Clin. Exp. Med. 2018, 27, 1017–1020. [Google Scholar] [CrossRef]
- Burgos-Ojeda, D.; Rueda, B.R.; Buckanovich, R.J. Ovarian cancer stem cell markers: Prognostic and therapeutic implications. Cancer Lett. 2012, 322, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, Z.; Amaya-Padilla, M.A.; Singomat, T.; Binju, M.; Madjid, B.D.; Yu, Y.; Kaur, P. Ovarian cancer stem cells and their role in drug resistance. Int. J. Biochem. Cell Biol. 2019, 106, 117–126. [Google Scholar] [CrossRef]
- Kryczek, I.; Liu, S.; Roh, M.; Vatan, L.; Szeliga, W.; Wei, S.; Banerjee, M.; Mao, Y.; Kotarski, J.; Wicha, M.S.; et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int. J. Cancer 2012, 130, 29–39. [Google Scholar] [CrossRef]


| Cell Lines | OVCAR-420 | SKOV-3 | ||||
|---|---|---|---|---|---|---|
| Time points | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
| IC50 of CDDP | 16.85 μM | 10.92 μM | 8.62 μM | 6.8 μM | 5.3 μM | 5.2 μM |
| IC50 of ONC206 | 0.16 μM | 0.09 μM | 0.152 μM | 0.336 μM | 0.27 μM | 0.245 μM |
| Dose CDDP | Dose ONC206 | Effect | CI | Indication |
|---|---|---|---|---|
| 5.0 | 0.05 | 0.69 | 0.55963 | Synergism |
| 10.0 | 0.05 | 0.7 | 1.04693 | Additive |
| 20.0 | 0.05 | 0.84 | 0.66549 | Synergism |
| 5.0 | 0.1 | 0.6 | 0.97897 | Synergism |
| 10.0 | 0.1 | 0.72 | 0.88727 | Synergism |
| 20.0 | 0.1 | 0.93 | 0.17915 | Synergism |
| 5.0 | 1.0 | 0.71 | 0.49044 | Synergism |
| 10.0 | 1.0 | 0.69 | 1.12129 | Additive |
| 20.0 | 1.0 | 0.9 | 0.31066 | Synergism |
| Dose CDDP | Dose ONC206 | Effect | CI | Indication |
|---|---|---|---|---|
| 2.5 | 0.05 | 0.43 | 1.35872 | Antagonism |
| 5.0 | 0.05 | 0.55 | 1.37010 | Antagonism |
| 10.0 | 0.05 | 0.775 | 0.70073 | Synergism |
| 2.5 | 0.1 | 0.5125 | 0.90821 | Synergism |
| 5.0 | 0.1 | 0.575 | 1.22318 | Antagonism |
| 10.0 | 0.1 | 0.8 | 0.57864 | Synergism |
| 2.5 | 1.0 | 0.575 | 1.09485 | Additive |
| 5.0 | 1.0 | 0.68 | 0.81825 | Synergism |
| 10.0 | 1.0 | 0.78 | 0.71879 | Synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhael, S.; Fayyad, R.; Harfouch, L.A.; Prabhu, V.V.; Bahmad, H.F.; Abou-Kheir, W.; Daoud, G. Investigating the Effects of ONC206 Alone and in Combination with Cisplatin on Ovarian Cancer Cell Models. Curr. Issues Mol. Biol. 2025, 47, 451. https://doi.org/10.3390/cimb47060451
Mikhael S, Fayyad R, Harfouch LA, Prabhu VV, Bahmad HF, Abou-Kheir W, Daoud G. Investigating the Effects of ONC206 Alone and in Combination with Cisplatin on Ovarian Cancer Cell Models. Current Issues in Molecular Biology. 2025; 47(6):451. https://doi.org/10.3390/cimb47060451
Chicago/Turabian StyleMikhael, Sara, Rona Fayyad, Leen Abi Harfouch, Varun Vijay Prabhu, Hisham F. Bahmad, Wassim Abou-Kheir, and Georges Daoud. 2025. "Investigating the Effects of ONC206 Alone and in Combination with Cisplatin on Ovarian Cancer Cell Models" Current Issues in Molecular Biology 47, no. 6: 451. https://doi.org/10.3390/cimb47060451
APA StyleMikhael, S., Fayyad, R., Harfouch, L. A., Prabhu, V. V., Bahmad, H. F., Abou-Kheir, W., & Daoud, G. (2025). Investigating the Effects of ONC206 Alone and in Combination with Cisplatin on Ovarian Cancer Cell Models. Current Issues in Molecular Biology, 47(6), 451. https://doi.org/10.3390/cimb47060451








