Genetic Mechanisms of Antimicrobial Non-Susceptibility to Novel Fluoroquinolone Delafloxacin Among Bulgarian Clinical Isolates of Streptococcus agalactiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. GBS Strains
2.3. DNA Extraction
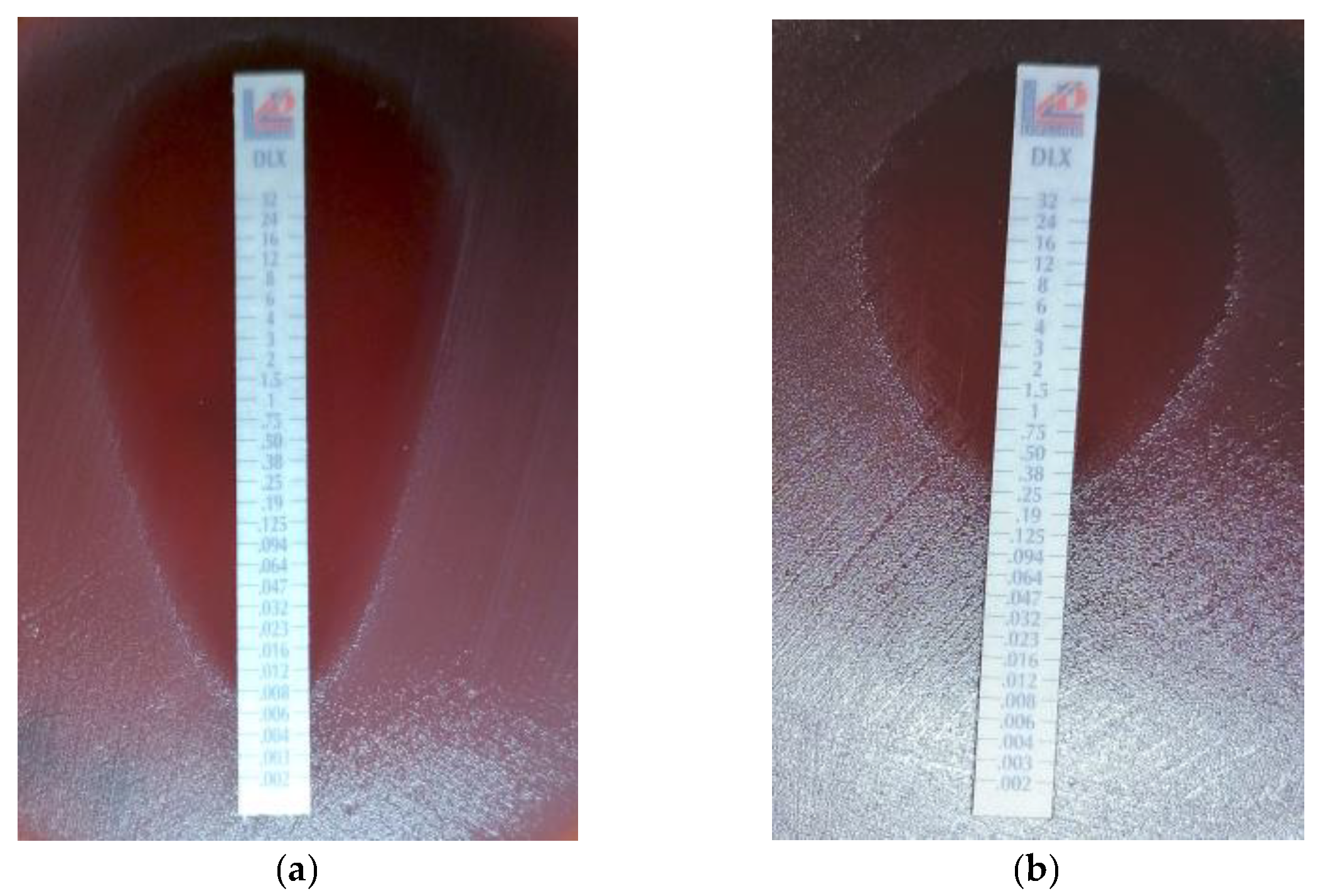
2.4. Antimicrobial Susceptibility Testing
2.5. PCR Amplification of Quinolone-Resistant Genes
2.6. Automated Sequencing
2.7. Statistical Analysis
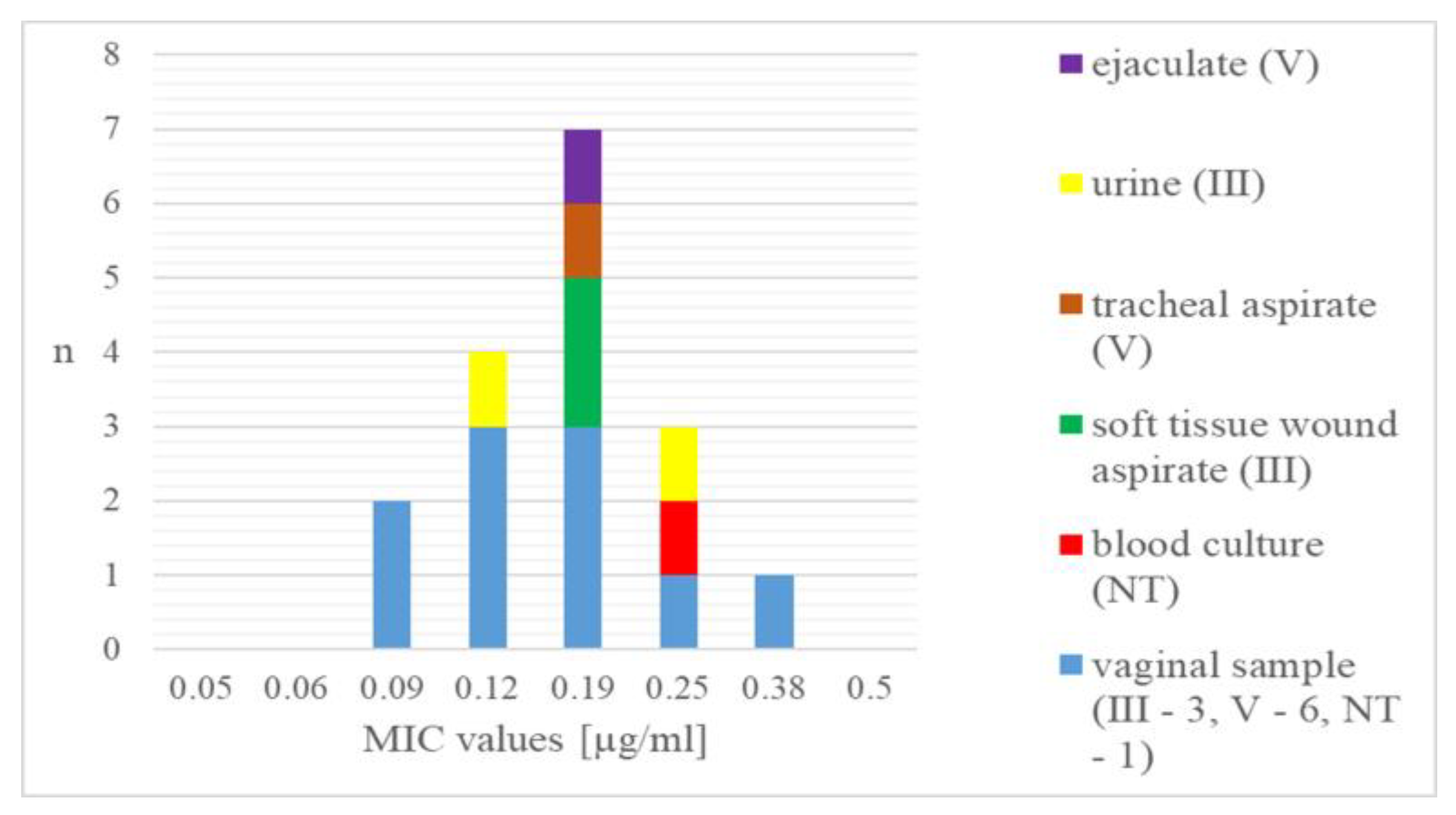
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of quinolone action and resistance: Where do we stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Lv, J.X.; Huang, Y.H.; Kafauit, F.; Wang, Y.H.; Su, C.; Ma, J.H.; Xu, Y.; Huang, C.C.; Zhang, Q.; Su, Y.W. Pharmacokinetics and pharmacodynamics of intravenous delafloxacin in healthy subjects: Model-based dose optimization. Antimicrob. Agents Chemother. 2024, 68, e0042824. [Google Scholar] [CrossRef] [PubMed]
- Tulkens, P.M.; Van Bambeke, F.; Zinner, S.H. Profile of a Novel Anionic Fluoroquinolone-Delafloxacin. Clin. Infect. Dis. 2019, 68, 213–222. [Google Scholar] [CrossRef]
- Kocsis, B.; Gulyás, D.; Szabó, D. Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline. Antibiotics 2021, 10, 1506. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Mercuro, N.J.; Davis, S.L.; Rybak, M.J. Delafloxacin: Place in Therapy and Review of Microbiologic, Clinical and Pharmacologic Properties. Infect. Dis. Ther. 2018, 7, 197–217. [Google Scholar] [CrossRef]
- Mogle, B.T.; Steele, J.M.; Thomas, S.J.; Bohan, K.H.; Kufel, W.D. Clinical review of delafloxacin: A novel anionic fluoroquinolone. J. Antimicrob. Chemother. 2018, 73, 1439–1451. [Google Scholar] [CrossRef]
- Remy, J.M.; Tow-Keogh, C.A.; McConnell, T.S.; Dalton, J.M.; DeVito, J.A. Activity of delafloxacin against methicillin-resistant Staphylococcus aureus: Resistance selection and characterization. J. Antimicrob. Chemother. 2012, 67, 2814–2820. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Markovska, R.; Medeiros, J.; Gergova, G.; Mitov, I. Delafloxacin against Helicobacter pylori, a potential option for improving eradication success? Diagn. Microbiol. Infect. Dis. 2020, 96, 114980. [Google Scholar] [CrossRef]
- Scott, L.J. Delafloxacin: A Review in Acute Bacterial Skin and Skin Structure Infections. Drugs 2020, 80, 1247–1258. [Google Scholar] [CrossRef]
- Rusu, A.; Lungu, I.A.; Moldovan, O.L.; Tanase, C.; Hancu, G. Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones-Shaping the Fifth Generation. Pharmaceutics 2021, 13, 1289. [Google Scholar] [CrossRef] [PubMed]
- Zasheva, A.; Batcheva, E.; Ivanova, K.D.; Yanakieva, A. Differences in Patient Access to Newly Approved Antibacterial Drugs in EU/EEA Countries. Antibiotics 2024, 13, 1077. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Sato, K.; Kuwahara, O.; Habadera, S.; Tsukamoto, N.; Ohuchi, H.; Akizawa, H.; Himi, T.; Fujii, N. Fluoroquinolone-resistant Streptococcus pneumoniae strains occur frequently in elderly patients in Japan. Antimicrob. Agents Chemother. 2002, 46, 3311–3315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawamura, Y.; Fujiwara, H.; Mishima, N.; Tanaka, Y.; Tanimoto, A.; Ikawa, S.; Itoh, Y.; Ezaki, T. First Streptococcus agalactiae isolates highly resistant to quinolones, with point mutations in gyrA and parC. Antimicrob. Agents Chemother. 2003, 47, 3605–3609. [Google Scholar] [CrossRef]
- Wehbeh, W.; Rojas-Diaz, R.; Li, X.; Mariano, N.; Grenner, L.; Segal-Maurer, S.; Tommasulo, B.; Drlica, K.; Urban, C.; Rahal, J.J. Fluoroquinolone-resistant Streptococcus agalactiae: Epidemiology and mechanism of resistance. Antimicrob. Agents Chemother. 2005, 49, 2495–2497. [Google Scholar] [CrossRef]
- Llaneza, E.; Seral, C.; Castillo, F.J.; Durán, E.; Rubio-Calvo, C.; Gómez-Lus, R. Characterization of clinical blood isolates of Streptococcus agalactiae with reduced susceptibility to levofloxacin. J. Chemother. 2009, 21, 463–464. [Google Scholar] [CrossRef]
- Barros, R.R.; Kegele, F.C.; Paula, G.R.; Brito, M.A.; Duarte, R.S. Molecular characterization of the first fluoroquinolone resistant strains of Streptococcus agalactiae isolated in Brazil. Braz. J. Infect. Dis. 2012, 16, 476–478. [Google Scholar] [CrossRef][Green Version]
- Piccinelli, G.; Gargiulo, F.; Corbellini, S.; Ravizzola, G.; Bonfanti, C.; Caruso, A.; De Francesco, M.A. Emergence of the first levofloxacin-resistant strains of Streptococcus agalactiae isolated in Italy. Antimicrob. Agents Chemother. 2015, 59, 2466–2469. [Google Scholar] [CrossRef]
- Neemuchwala, A.; Teatero, S.; Patel, S.N.; Fittipaldi, N. Fluoroquinolone Resistance among Clonal Complex 1 Group B Streptococcus Strains. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 6403928. [Google Scholar] [CrossRef]
- Li, C.; Sapugahawatte, D.N.; Yang, Y.; Wong, K.T.; Lo, N.W.S.; Ip, M. Multidrug-Resistant Streptococcus agalactiae Strains Found in Human and Fish with High Penicillin and Cefotaxime Non-Susceptibilities. Microorganisms 2020, 8, 1055. [Google Scholar] [CrossRef]
- Fàbrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of action of and resistance to quinolones. Microb. Biotechnol. 2009, 2, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Sader, H.S.; Rhomberg, P.R.; Flamm, R.K. In Vitro Activity of Delafloxacin against Contemporary Bacterial Pathogens from the United States and Europe, 2014. Antimicrob. Agents Chemother. 2017, 61, e02609-16. [Google Scholar] [CrossRef]
- Iregui, A.; Khan, Z.; Malik, S.; Landman, D.; Quale, J. Emergence of Delafloxacin-Resistant Staphylococcus aureus in Brooklyn, New York. Clin. Infect. Dis. 2020, 70, 1758–1760. [Google Scholar] [CrossRef]
- Bolaños, S.; Acebes, C.; Martínez-Expósito, Ó.; Boga, J.A.; Fernández, J.; Rodríguez-Lucas, C. Role of parC Mutations at Position 84 on High-Level Delafloxacin Resistance in Methicillin-Resistant Staphylococcus aureus. Antibiot. 2024, 13, 641. [Google Scholar] [CrossRef]
- Turban, A.; Guérin, F.; Dinh, A.; Cattoir, V. Updated Review on Clinically-Relevant Properties of Delafloxacin. Antibiotics 2023, 12, 1241. [Google Scholar] [CrossRef]
- Bianchi-Jassir, F.; Paul, P.; To, K.N.; Carreras-Abad, C.; Seale, A.C.; Jauneikaite, E.; Madhi, S.A.; Russell, N.J.; Hall, J.; Madrid, L.; et al. Systematic review of Group B Streptococcal capsular types, sequence types and surface proteins as potential vaccine candidates. Vaccine 2020, 38, 6682–6694. [Google Scholar] [CrossRef]
- Sadeh, M.; Salehi-Abargouei, A.; Azartoos, N.; Mirzaei, F.; Khalili, M. Distribution of Streptococcus agalactiae Among Iranian Women from 1992 to 2018: A Systematic Review and Meta-Analysis. Jundishapur J. Microbiol. 2020, 13, e102314. [Google Scholar] [CrossRef]
- Gergova, R.; Boyanov, V.; Muhtarova, A.; Alexandrova, A. A Review of the impact of streptococcal infections and antimicrobial resistance on human health. Antibiotics 2024, 13, 360. [Google Scholar] [CrossRef]
- Lohrmann, F.; Hufnagel, M.; Kunze, M.; Afshar, B.; Creti, R.; Detcheva, A.; Kozakova, J.; Rodriguez-Granger, J.; Sørensen, U.B.S.; Margarit, I.; et al. Neonatal invasive disease caused by Streptococcus agalactiae in Europe: The DEVANI multi-center study. Infection 2023, 51, 981–991. [Google Scholar] [CrossRef]
- Raabe, V.N.; Shane, A.L. Group B streptococcus (Streptococcus agalactiae). Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.G.; Burnard, D.; Sowden, D.; McMillan, D. Whole genome sequencing for antimicrobial resistance mechanisms, virulence factors and clonality in invasive Streptococcus agalactiae blood culture isolates recovered in Australia. Pathology 2020, 52, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, X.; Li, J.; Ji, W.; Zhou, H.; Gong, X.; Miao, B.; Meng, S.; Duan, L.; Shi, Q.; et al. Molecular characteristics and antibiotic resistance mechanisms of clindamycin-resistant Streptococcus agalactiae isolates in China. Front. Microbiol. 2023, 14, 1138039. [Google Scholar] [CrossRef] [PubMed]
- Watkins, F.L.K.; McGee, L.; Schrag, S.J.; Beall, B.; Jain, J.H.; Pondo, T.; Farley, M.M.; Harrison, L.H.; Zansky, S.M.; Baumbach, J. Epidemiology of invasive group B streptococcal infections among nonpregnant adults in the United States, 2008–2016. JAMA Intern. Med. 2019, 179, 479–488. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 15.0. 2025. Available online: http://www.eucast.org (accessed on 1 January 2025).
- Delannoy, C.M.; Crumlish, M.; Fontaine, M.C.; Pollock, J.; Foster, G.; Dagleish, M.P.; Turnbull, J.F.; Zadoks, R.N. Human Streptococcus agalactiae strains in aquatic mammals and fish. BMC Microbiol. 2013, 13, 41. [Google Scholar] [CrossRef]
- Poyart, C.; Tazi, A.; Réglier-Poupet, H.; Billoët, A.; Tavares, N.; Raymond, J.; Trieu-Cuot, P. Multiplex PCR assay for rapid and accurate capsular typing of group B streptococci. J. Clin. Microbiol. 2007, 45, 1985–1988. [Google Scholar] [CrossRef]
- Murayama, S.Y.; Seki, C.; Sakata, H.; Sunaoshi, K.; Nakayama, E.; Iwata, S.; Sunakawa, K.; Ubukata, K. Invasive Streptococcal Disease Working Group. Capsular type and antibiotic resistance in Streptococcus agalactiae isolates from patients, ranging from newborns to the elderly, with invasive infections. Antimicrob. Agents Chemother. 2009, 53, 2650–2653. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Boyanov, V.S.; Alexandrova, A.S.; Hristova, P.M.; Hitkova, H.Y.; Gergova, R.T. Antibiotic Resistance and Serotypes Distribution in Streptococcus agalactiae Bulgarian Clinical Isolates During the Years of 2021-2024. Pol. J. Microbiol. 2024, 73, 505–514. [Google Scholar] [CrossRef]
- Gergova, R.T.; Muhtarova, A.; Tsitou, V.M.; Mitov, I. Emergence of multidrug-resistant and -hypervirulent Streptococcus agalactiae in Bulgarian patients. Balk. Med. J. 2021, 38, 143–144. [Google Scholar] [CrossRef]
- Aygar, S. Resistance Rates of Streptococcus agalactiae Strains Isolated from Urine Samples to Various Antibiotics. Value Health Sci. 2023, 13, 373–377. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Lin, M.; Bao, J.; Wang, G.; Dong, R.; Zou, P.; Chen, Y.; Li, N.; Zhang, T.; et al. Maternal colonization with group B Streptococcus and antibiotic resistance in China: Systematic review and meta-analyses. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 5, Erratum in Ann. Clin. Microbiol. Antimicrob. 2023, 22, 17. [Google Scholar] [CrossRef]
- Cho, J.C.; Crotty, M.P.; White, B.P.; Worley, M.V. What Is Old Is New Again: Delafloxacin, a Modern Fluoroquinolone. Pharmacotherapy 2018, 38, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Giordano, P.A.; Pogue, J.M.; Cammarata, S. Analysis of Pooled Phase III Efficacy Data for Delafloxacin in Acute Bacterial Skin and Skin Structure Infections. Clin. Infect. Dis. 2019, 68, 223–232. [Google Scholar] [CrossRef]
- Cercenado, E.; Loras, C.; Cobos, A.; Sanz, J.C. In vitro activity of delafloxacin against highly levofloxacin-resistant invasive isolates of Streptococcus pneumoniae. Enferm. Infecc. Microbiol. Clin. 2022, 40, 131–133. [Google Scholar] [CrossRef]
- Ríos, E.; Pérez, M.; Sanz, J.C.; Delgado-Iribarren, A.; Rodríguez-Avial, I. Efficacy of delafloxacin alone and in combination with cefotaxime against cefotaxime non-susceptible invasive isolates of Streptococcus pneumoniae. Rev. Esp. Quimioter. 2024, 37, 158–162. [Google Scholar] [CrossRef]
| Missense Mutations | Silent Mutations | |||
|---|---|---|---|---|
| Substitution | Frequency | Substitution | Frequency | |
| parC | S79F (TCC →TTC) | 100% | A142A (GCG → GCA/GCC) | 94.1% |
| I81I (ATC → ATT) | 5.9% | |||
| gyrA | S81L (TCA → TTA) | 100% | G49G (GGT → GGG) | 5.9% |
| E52A (GAA → GCA) | 5.9% | |||
| N51D (AAT → GAT) | 5.9% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyanov, V.; Alexandrova, A.; Gergova, R. Genetic Mechanisms of Antimicrobial Non-Susceptibility to Novel Fluoroquinolone Delafloxacin Among Bulgarian Clinical Isolates of Streptococcus agalactiae. Curr. Issues Mol. Biol. 2025, 47, 446. https://doi.org/10.3390/cimb47060446
Boyanov V, Alexandrova A, Gergova R. Genetic Mechanisms of Antimicrobial Non-Susceptibility to Novel Fluoroquinolone Delafloxacin Among Bulgarian Clinical Isolates of Streptococcus agalactiae. Current Issues in Molecular Biology. 2025; 47(6):446. https://doi.org/10.3390/cimb47060446
Chicago/Turabian StyleBoyanov, Vasil, Alexandra Alexandrova, and Raina Gergova. 2025. "Genetic Mechanisms of Antimicrobial Non-Susceptibility to Novel Fluoroquinolone Delafloxacin Among Bulgarian Clinical Isolates of Streptococcus agalactiae" Current Issues in Molecular Biology 47, no. 6: 446. https://doi.org/10.3390/cimb47060446
APA StyleBoyanov, V., Alexandrova, A., & Gergova, R. (2025). Genetic Mechanisms of Antimicrobial Non-Susceptibility to Novel Fluoroquinolone Delafloxacin Among Bulgarian Clinical Isolates of Streptococcus agalactiae. Current Issues in Molecular Biology, 47(6), 446. https://doi.org/10.3390/cimb47060446







