Abstract
Osteoarthritis (OA) is a debilitating joint disorder characterized by cartilage degradation, inflammation, and loss of joint function. While mesenchymal stem cells (MSCs) hold promise for OA therapy due to their regenerative and immunomodulatory properties, challenges such as poor survival, suboptimal differentiation, and an inflammatory microenvironment limit their clinical efficacy. Natural products, including curcumin, resveratrol, quercetin, and epigallocatechin gallate (EGCG), have emerged as a complementary strategy to enhance MSC-based therapies for OA. These bioactive compounds modulate key inflammatory pathways (NF-κB, MAPK, PI3K/AKT), reduce oxidative stress, and promote chondrogenic differentiation of MSCs. Preclinical studies demonstrate the synergistic effects of MSCs and natural products in attenuating inflammation, enhancing cartilage repair, and improving joint function in OA models. However, clinical translation is hindered by challenges in bioavailability, standardization of MSC protocols, and regulatory hurdles. Future research should focus on optimizing delivery systems, conducting large-scale randomized controlled trials, and establishing personalized treatment strategies based on patient biomarkers. By addressing these challenges, the integration of natural products into MSC-based therapies could revolutionize OA treatment, offering a disease-modifying approach for millions of patients worldwide.
1. Introduction
Osteoarthritis (OA) is a prevalent and debilitating joint disorder characterized by the progressive degradation of cartilage, synovial inflammation, and subsequent loss of joint function. Affecting millions worldwide, OA imposes a significant socioeconomic burden, with current treatments primarily focused on symptom management rather than halting or reversing disease progression [1]. These conventional therapies (Table 1), including nonsteroidal anti-inflammatory drugs (NSAIDs) and surgical interventions, fail to address the underlying mechanisms of cartilage degradation and inflammation, highlighting the urgent need for novel therapeutic strategies [2,3].

Table 1.
Current and potential treatment for osteoarthritis (OA).
Mesenchymal stem cells (MSCs) have emerged as a promising approach for OA treatment due to their inherent properties, including their ability to modulate immune responses, secrete trophic factors, and differentiate into chondrocytes for cartilage repair (Table 1) [4,5]. Despite these advantages, the clinical efficacy of MSC-based therapies remains inconsistent, often limited by challenges such as low cell survival, suboptimal delivery methods, and insufficient modulation of the inflammatory microenvironment in the osteoarthritic joint [6].
Natural products derived from plants, microbes, and marine organisms offer a complementary strategy to enhance the therapeutic potential of MSCs in OA [7,8,9]. Known for their diverse pharmacological properties, including anti-inflammatory, antioxidant, and cartilage-protective effects, natural products have been extensively studied in OA and regenerative medicine. Emerging evidence suggests that certain bioactive compounds, such as curcumin, resveratrol, quercetin, and epigallocatechin gallate (EGCG), can potentiate the effects of MSCs by improving cell viability, modulating differentiation, and enhancing their capacity to mitigate inflammation and promote tissue repair [10,11].
This narrative review aims to synthesize the latest findings on how natural products can significantly improve the efficacy of injected mesenchymal stem cells in treating osteoarthritis. We will discuss the mechanisms through which natural products influence MSC function, the synergistic effects observed in combination therapies, and the role of biomaterials in optimizing delivery and efficacy. Furthermore, we will address key challenges, such as standardization, bioavailability, and regulatory considerations, and highlight future research opportunities to advance this promising therapeutic paradigm.
Literature Search Methodology
This approach ensured a balanced and up-to-date synthesis of the literature, emphasizing clinically relevant findings. This narrative review employed a comprehensive literature search across PubMed, Scopus, and Web of Science, focusing on studies published between 2018 and 2024. The search strategy utilized keywords such as “Mesenchymal stem cells” AND “osteoarthritis” AND “natural products”, “MSC therapy” AND “cartilage regeneration”, and “Curcumin OR Resveratrol OR Quercetin OR EGCG” AND “stem cell differentiation”. Inclusion criteria encompassed preclinical and clinical studies investigating the effects of natural products on MSC survival, differentiation, and OA progression, as well as review articles providing mechanistic insights into OA treatment strategies. Exclusion criteria comprised studies published before 2018, non-peer-reviewed sources, articles lacking experimental evidence, and studies focusing on non-MSC-based regenerative therapies (e.g., PRP, gene therapy). This approach ensured a balanced and comprehensive review of the current literature on the subject.
2. Osteoarthritis and MSC Therapies
2.1. Overview of Osteoarthritis (OA)
Osteoarthritis (OA) is a prevalent degenerative joint disease characterized by the deterioration of joint components, including cartilage, bone, and synovial fluid, leading to pain, stiffness, and functional limitations. It is a leading cause of disability, particularly among older adults, affecting millions of people worldwide. This condition is complex, involving mechanical wear and tear, inflammation, and cellular senescence, with no cure. Risk factors include age, female sex, obesity, genetics, and joint injuries [12]. Cellular senescence, particularly in chondrocytes and synoviocytes, contributes to the disease by producing inflammatory proteins [13]. OA is the most common joint disorder, affecting approximately 10% of men and 18% of women aged 60 years and older [14]. Its prevalence has doubled over the past decade, with significant economic and social impacts [15].
Symptoms of OA include joint pain, stiffness, swelling, and reduced mobility, which often worsen with age [16,17]. Morning stiffness and functional limitations are common, with severe cases leading to joint deformities, such as bony enlargements or misalignment, and pain at night [16,17]. Diagnosis is typically based on clinical examination and imaging techniques [17]. Treatment options include lifestyle changes, pain management, physical therapy, and surgery [16]. Pharmacological treatments often involve nonsteroidal anti-inflammatory drugs (NSAIDs) and chondroprotectors such as chondroitin sulfate. Emerging therapies include nerve growth factor inhibitors and stem cell treatments [18].
Effective management of OA requires a multidisciplinary approach that combines patient education, pharmaceuticals, and complementary therapies [19]. Early diagnosis, prevention, and tailoring of treatment plans are crucial to achieving optimal outcomes. Person-centered care focusing on modifiable factors, such as exercise and weight loss, is recommended [12]. While osteoarthritis remains a significant health challenge, ongoing research into its pathophysiology and treatment options offers hope for improved management strategies.
With the global rise in aging populations and obesity rates, the prevalence of OA is expected to increase, further exacerbating the societal and economic burden associated with this disease. Current treatments for OA primarily focus on symptom management through pharmacological interventions, such as NSAIDs, corticosteroids, and hyaluronic acid injections, alongside physical therapy and, in severe cases, joint replacement surgery [20]. However, these strategies do not address the underlying pathophysiology of OA, and their long-term efficacy is often limited [3]. This gap in therapeutic options has driven interest in regenerative medicine approaches that aim to restore joint structure and function.
2.2. Pathophysiology of Osteoarthritis
Osteoarthritis (OA) is a complex, multifactorial joint disorder characterized by the degeneration of articular cartilage, synovial inflammation, and subchondral bone changes. OA pathophysiology involves biomechanical, biochemical, and cellular factors contributing to joint degradation and pain.
Recent research has provided insights into the molecular and cellular mechanisms underlying OA, highlighting the roles of inflammation, cartilage metabolism, and bone remodeling. Cartilage degradation is a hallmark of OA, primarily due to an imbalance between the anabolic and catabolic processes within the joint. Biochemical markers such as collagen type II cleavage products (C2C) and cartilage oligomeric matrix protein (COMP) are elevated in OA, indicating cartilage degradation [21]. Mechanical and immune-mediated injuries contribute to cartilage destruction, with inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α) playing significant roles [22].
Chronic low-grade synovial membrane inflammation is a central feature of OA and is driven by innate and adaptive immune responses [23]. Inflammatory cytokines, including IL-6, are elevated in the synovial fluid of OA patients, correlating with pain and disease progression [21,24]. MicroRNAs (miRNAs) regulate inflammatory processes and cartilage metabolism, influencing OA progression [25]. Subchondral bone lesions often precede cartilage degeneration, suggesting they play a pivotal role in OA pathogenesis [23]. Mechanical loading and inflammatory stimuli affect osteocyte activity, leading to bone remodeling and the osteophyte formation [24]. Understanding the interplay between bone and cartilage changes is crucial for understanding OA progression and developing therapeutic strategies [26].
Chondrocyte apoptosis and other forms of programmed cell death, such as PANoptosis, contribute to cartilage degradation in OA [27]. These cellular processes are influenced by mechanical stress and inflammatory mediators, highlighting the complexity of the OA pathophysiology (Figure 1) [27]. Current treatments focus on symptom management; however, novel therapies target underlying pathophysiological mechanisms. Native collagen type II and Aflapin have shown promise in slowing OA progression by modulating immune responses and cartilage metabolism [22]. Regenerative approaches, including cell and gene therapies, have been explored to promote cartilage repair and joint functions [26]. While the pathophysiology of osteoarthritis is increasingly understood, challenges remain in translating this knowledge into effective treatments. The heterogeneity of OA among patients complicates the development of universal therapies and necessitates personalized approaches. Additionally, neuroinflammation and central sensitization in pain chronicity present further complexities for managing OA symptoms [23,28].
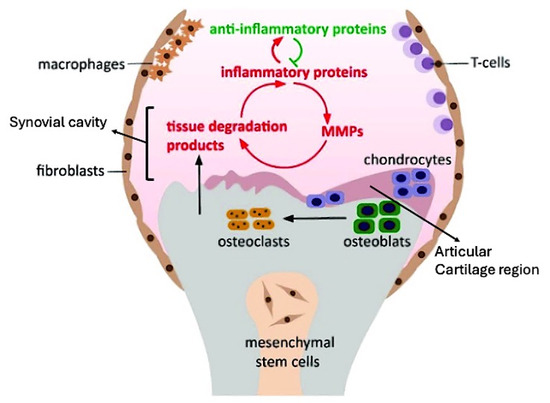
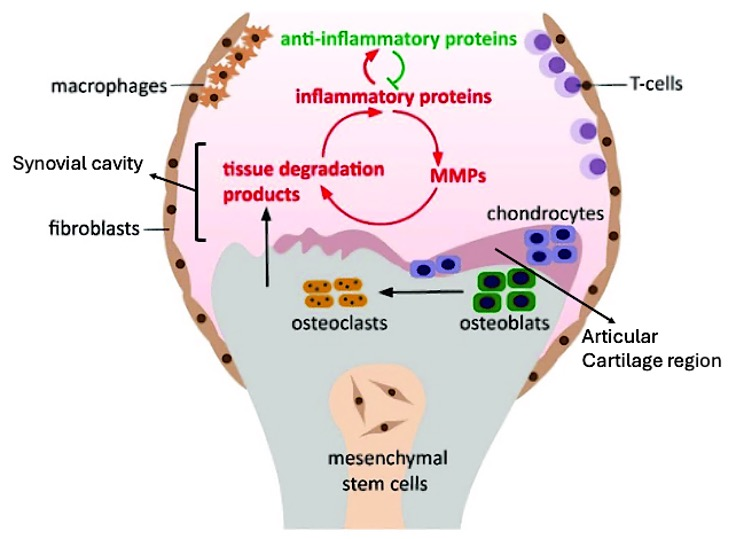
Figure 1.
Schematic representation of osteoarthritis (OA) pathology involving various cellular and molecular components. The diagram highlights the “Articular Cartilage Region” (purple section in the upper middle), which consists of chondrocytes, that are responsible for maintaining cartilage structure. Surrounding this region, the “Synovial Cavity” (pink area), the space above chondrocytes, that facilitates fluid exchange and mediates interactions between inflammatory proteins, anti-inflammatory agents, and tissue degradation processes. Mesenchymal stem cells (MSCs) are centrally located, surrounded by macrophages, fibroblasts, and T-cells, indicating an inflammatory milieu. The interplay between inflammatory and anti-inflammatory proteins, depicted by green and red arrows respectively, stimulates matrix metalloproteinases (MMPs) and generates tissue degradation products, thereby exacerbating the inflammatory cycle. Osteoclasts and osteoblasts are involved in bone remodeling, while chondrocytes contribute to cartilage repair. This dynamic balance between inflammation and tissue repair is a hallmark of OA progression.
2.3. Inflammatory Pathways in Osteoarthritis
Inflammatory pathways play a crucial role in the pathogenesis and progression of osteoarthritis (OA), a degenerative joint disease characterized by cartilage degradation, synovial inflammation, and subchondral bone changes. These pathways involve a complex interplay of cytokines, signaling molecules, and cellular responses that contribute to the chronic nature of the disease.
Cytokines such as interleukin-1 beta (IL-1β), IL-6, IL-17, and TNF-α drive inflammation and cartilage degradation (Figure 2). These cytokines activate signaling pathways such as NF-κB and MAPK, which produce MMPs that degrade the extracellular matrix [29,30,31]. Synovial macrophages and synoviocytes release inflammatory cytokines that perpetuate synovitis and joint damage. The TNF and chemokine signaling pathways are particularly significant in synovial inflammation [32,33].
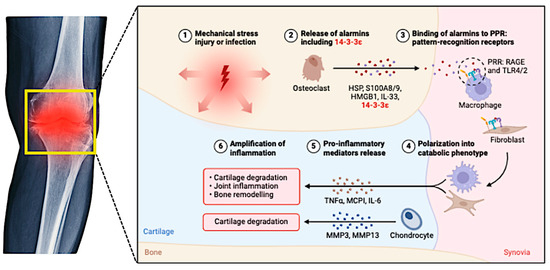
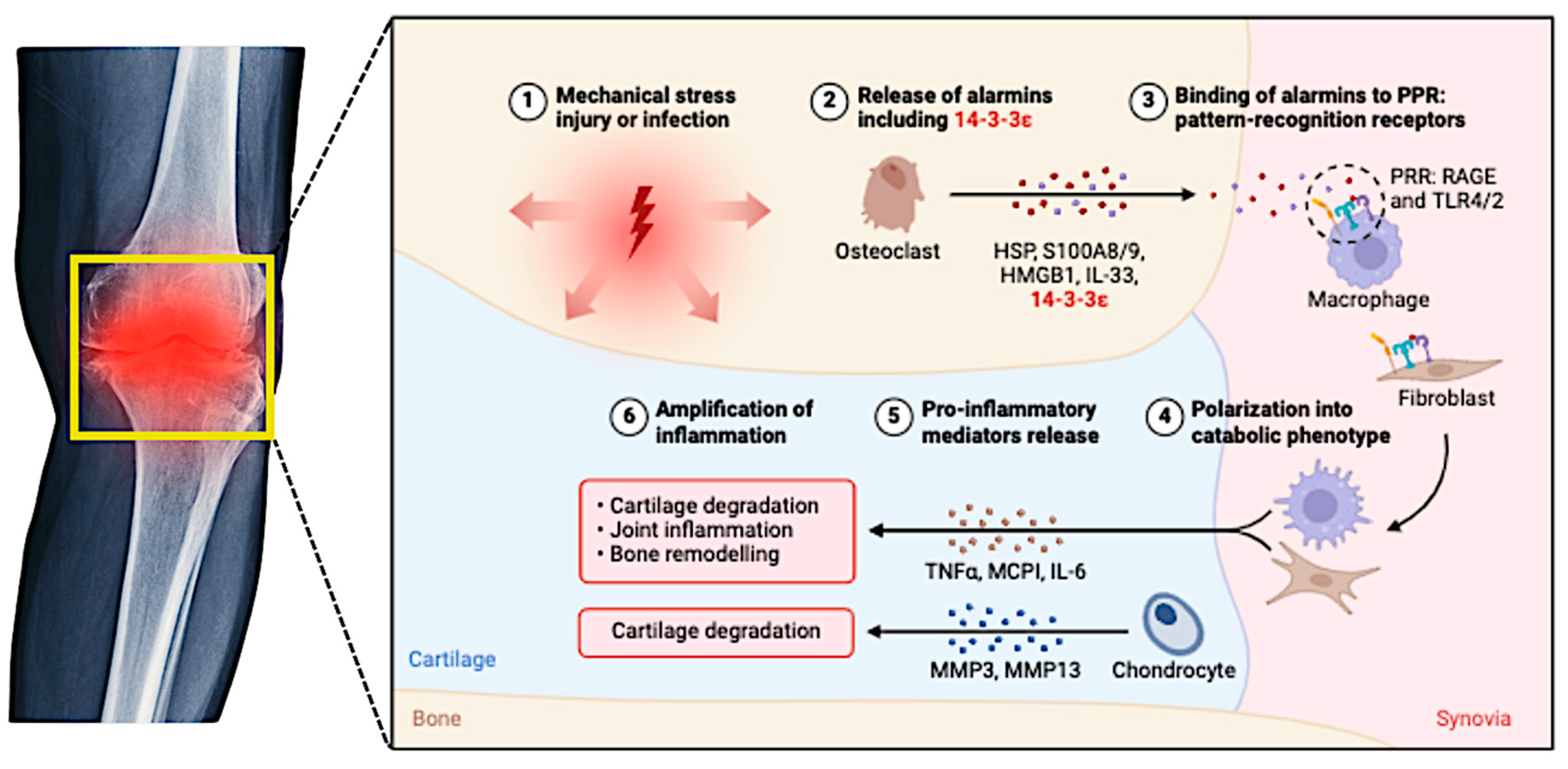
Figure 2.
This figure illustrates the complex inflammatory pathways involved in the pathogenesis and progression of osteoarthritis (OA).
Oxidative stress is closely linked to inflammation in OA, with reactive oxygen species (ROS) and nitric oxide (NO) contributing to cartilage damage and inflammation. The Nrf2 signaling pathway, which regulates oxidative stress responses, interacts with NF-κB and influences inflammation and cellular metabolism [34,35]. Several other signaling pathways have been implicated in OA, including the Wnt, Notch, and TGF-β pathways, which regulate chondrocyte apoptosis, cartilage matrix synthesis, and degradation [30,36]. MAPK and toll-like receptor (TLR) pathways are critical for mediating inflammatory responses and promoting the release of inflammatory mediators, thereby exacerbating cartilage destruction [30,36]. In post-traumatic osteoarthritis (PTOA), inflammation is triggered by joint trauma, which leads to mitochondrial dysfunction and cytokine release. Calcium/calmodulin-dependent protein kinase kinase 2 (CaMKK2) has been identified as a critical regulator of inflammatory responses and a potential therapeutic target [36,37].
Targeting the inflammatory pathways offers promising therapeutic avenues for OA treatment. Modifying the pro-inflammatory and anti-inflammatory prostaglandin balance could help cartilage regeneration and reduce inflammation [38]. The recruitment of mesenchymal stem cells and the transition from pro-inflammatory to anti-inflammatory states are potential strategies for developing new OA treatments [38]. Natural compounds targeting oxidative stress pathways have shown potential in reducing inflammation and protecting cartilage, highlighting the therapeutic potential of modulating oxidative stress in OA [34]. Although inflammation is a central component of OA pathogenesis, it is essential to consider the multifactorial nature of the disease, including mechanical stress and genetic predisposition, when developing comprehensive treatment strategies. Understanding the complex inflammatory pathways in OA highlights the need for diverse treatment strategies, including the exploration of current pharmacological interventions, as detailed in the next section.
2.4. Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells (MSCs) have garnered significant attention as a promising regenerative therapy for OA [39]. MSCs are multipotent progenitor cells that differentiate into mesenchymal lineages, including chondrocytes, osteoblasts, and adipocytes. They also possess potent immunomodulatory and anti-inflammatory properties, which are particularly relevant for the treatment of OA [40,41]. By secreting bioactive molecules such as cytokines, growth factors, and extracellular vesicles, MSCs can modulate the inflammatory microenvironment, inhibit the catabolic activity of inflammatory cytokines, and promote cartilage repair. Despite their potential, the clinical application of MSCs in OA therapy faces several challenges. These include low survival rates following intra-articular injection, poor engraftment and retention within the joint, and limited capacity to overcome the hostile inflammatory microenvironment in osteoarthritic joints [42]. Additionally, variations in MSC source, isolation techniques, and culture conditions have contributed to inconsistent therapeutic outcomes in preclinical and clinical studies [6,43]. Addressing these limitations requires innovative strategies to enhance the therapeutic efficacy of MSCs.
Recent studies have highlighted the potential of combining MSC-based therapies with natural products to overcome these barriers [44,45]. Natural products can modulate the inflammatory and oxidative stress pathways implicated in OA pathogenesis while enhancing MSC viability, functionality, and differentiation [46,47]. These synergistic effects offer a compelling rationale for integrating natural products into MSC-based therapeutic regimens for OA. This integration could potentially enhance the therapeutic efficacy of MSCs, leading to improved outcomes in patients suffering from osteoarthritis. Additionally, incorporating natural compounds may help modulate the inflammatory response and promote tissue regeneration, thereby addressing the disease’s underlying pathology.
3. Natural Products
3.1. Natural Products in Enhancing MSC Therapies
Natural products derived from diverse biological sources, such as plants, microbes, and marine organisms, have long been recognized for their pharmacological properties in treating various diseases [48,49,50]. Their bioactive compounds exhibit a wide range of effects, including anti-inflammatory, antioxidant, and chondroprotective activities, which are particularly relevant to treating osteoarthritis (OA) [34,46,51]. In recent years, there has been growing interest in leveraging natural products to enhance the therapeutic potential of mesenchymal stem cells (MSCs) in OA management [52]. This trend is driven by the increasing evidence suggesting that natural compounds possess unique properties supporting MSC proliferation, differentiation, and overall functionality. Researchers have begun investigating various natural substances, including plant extracts, vitamins, and minerals, to determine their synergistic effects when combined with MSC therapies [53].
The pathophysiology of OA marks an imbalance between pro-inflammatory and anti-inflammatory mediators, oxidative stress, and extracellular matrix degradation [54]. While MSCs exhibit significant potential in modulating these pathological processes, their therapeutic efficacy can be hindered by low viability and limited functionality within the hostile inflammatory microenvironment of osteoarthritic joints. Natural products can address these challenges by modulating key signaling pathways and creating a more favorable environment for MSC-based therapies [45]. By enhancing the viability and functionality of injected MSCs, these natural products can lead to improved outcomes in osteoarthritis management. This enhancement can be attributed to the synergistic effects of specific natural compounds that promote cellular proliferation and mitigate senescence [55]. Recent studies have highlighted various natural products, such as curcumin, resveratrol, and certain flavonoids, that have significantly improved MSC characteristics under inflammatory conditions typical of osteoarthritis [56,57,58,59].
Several natural products have demonstrated the ability to enhance MSC survival, proliferation, and differentiation [53]. For instance, curcumin, a polyphenol derived from turmeric, has been shown to exert strong anti-inflammatory and antioxidant effects, reducing the expression of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α) [54,60]. Similarly, resveratrol, a stilbene found in grapes and red wine, promotes MSC proliferation and differentiation into chondrocytes while mitigating oxidative stress and inflammatory signaling pathways [61]. Natural products can also enhance MSC functionality and immunomodulatory properties. Quercetin, a flavonoid commonly found in fruits and vegetables, has been reported to reduce the levels of matrix metalloproteinases (MMPs) that degrade cartilage and modulate the polarization of macrophages toward an anti-inflammatory phenotype [47,52,62]. Furthermore, epigallocatechin gallate (EGCG), which is recognized as a major catechin found in green tea, has been shown through various studies to protect mesenchymal stem cells (MSCs) from apoptosis [63,64,65,66]. This beneficial compound also enhances their differentiation potential significantly and plays a crucial role in reducing the production of various inflammatory mediators that can affect cell health.
The integration of natural products into MSC therapies offers several advantages. These compounds can precondition MSCs in vitro, enhancing their viability and therapeutic properties before administration. Alternatively, they can be co-administered with MSCs to create a synergistic effect within the joint microenvironment. However, the efficacy of these approaches depends on factors such as the bioavailability, stability, and dosage of the natural products, which remain critical challenges to address. The potential of natural products to enhance MSC-based therapies for OA represents an exciting frontier in regenerative medicine. Combining the regenerative capabilities of MSCs with the pharmacological benefits of natural products may allow for the development of more effective and targeted therapies for OA patients.
A growing body of evidence supports the role of natural products in improving the efficacy of MSC-based therapies for osteoarthritis. Table 2 summarizes key studies evaluating the effects of various bioactive compounds on MSC survival, differentiation, and cartilage repair in preclinical and clinical models.

Table 2.
Summary of key studies on natural products enhancing MSC therapy for osteoarthritis.
3.2. Specific Natural Products and Their Effects on MSCs
Integrating bioactive natural products can significantly enhance the therapeutic potential of mesenchymal stem cells (MSCs) in osteoarthritis (OA). These compounds, derived from plants and other natural sources, possess unique properties that address key challenges associated with MSC-based therapies, such as limited cell survival, suboptimal differentiation, and inadequate immunomodulation. Below, we discuss the effects of several well-studied natural products and their mechanisms of action in augmenting MSC function for OA treatment.
3.2.1. Curcumin
Curcumin, a polyphenolic compound derived from the turmeric root (Curcuma longa), is renowned for its potent anti-inflammatory and antioxidant properties [76,77]. It inhibits key inflammatory pathways, such as nuclear factor-kappa B (NF-κB), and reduces the production of pro-inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). Studies have demonstrated that curcumin enhances MSC survival and proliferation under oxidative stress conditions, making it a valuable agent for preconditioning MSCs before their therapeutic application [78,79,80,81]. Additionally, curcumin has been shown to promote the chondrogenic differentiation of mesenchymal stem cells (MSCs) [82,83], thereby facilitating and enhancing cartilage repair in various osteoarthritis (OA) models. This process is crucial for improving joint function and reducing pain in affected individuals.
3.2.2. Resveratrol
Resveratrol, a stilbene found in grapes, red wine, and berries, has garnered attention for its antioxidative and chondroprotective effects. It activates the sirtuin-1 (SIRT1) signaling pathway, which regulates mitochondrial function and reduces oxidative damage in MSCs [72]. Resveratrol has also been shown to enhance MSC-mediated cartilage repair by promoting the expression of cartilage-specific markers, such as collagen type II and aggrecan [84]. Furthermore, resveratrol suppresses matrix metalloproteinases (MMPs) and inflammatory mediators, contributing to the preservation of cartilage integrity in osteoarthritic joints [61,71,85]. This action not only aids in significantly reducing inflammation but also actively promotes the regeneration of chondrocytes, which are essential for maintaining the structural integrity of cartilage. Furthermore, various studies have clearly shown that combining resveratrol with mesenchymal stem cells may notably enhance the therapeutic effects [68,69,78], ultimately leading to improved outcomes in osteoarthritis treatment, making it a promising avenue for further research and application in clinical settings.
3.2.3. Quercetin
Quercetin is a flavonoid abundant in fruits, vegetables, and teas. Its anti-inflammatory and immunomodulatory properties make it an attractive candidate for MSC-based therapies. Quercetin reduces the activity of MMPs, which are involved in cartilage degradation, and modulates the polarization of macrophages in the joint microenvironment [73,86]. This shifts the inflammatory profile toward a reparative state, enhancing the therapeutic effects of MSCs. Quercetin has also been shown to promote MSC differentiation into chondrocytes, further supporting cartilage regeneration in OA [87]. This specific process significantly enhances the therapeutic potential of mesenchymal stem cells (MSCs) and may ultimately lead to improved clinical outcomes for individuals suffering from osteoarthritis.
3.2.4. Epigallocatechin Gallate (EGCG)
EGCG, the primary catechin in green tea, is recognized for protecting MSCs from apoptosis and oxidative stress. This protective effect is crucial in osteoarthritis, where oxidative stress plays a significant role in the degeneration of cartilage and the overall pathology of the disease [81]. Recent studies have demonstrated that the application of EGCG enhances the viability of MSCs and promotes their regenerative properties, leading to improved outcomes in joint health [75]. By reducing reactive oxygen species (ROS) levels and inhibiting the activation of NF-κB, EGCG enhances MSC viability and function. Additionally, EGCG has been shown to improve the chondrogenic potential of MSCs by upregulating the expression of chondrocyte-specific genes [88]. Its significant ability to effectively suppress pro-inflammatory cytokines and various chemokines further strengthens and supports its potential use in MSC-based therapies designed specifically for osteoarthritis (OA).
Natural products combined with MSCs create synergistic effects that enhance therapeutic potential. Preconditioning MSCs with curcumin or EGCG improves their survival and regenerative capacity [74,89], while resveratrol or quercetin can modulate inflammation in osteoarthritic joints [69,86]. These combinations address the limitations of standalone MSC therapies, yet clinical translation encounters challenges with bioavailability, stability, and delivery methods. Innovative strategies, such as nanoparticle encapsulation or hydrogels, should be explored to optimize efficacy in MSC applications. Advanced delivery methods could also enhance the retention and viability of MSCs in osteoarthritic environments, improving their regenerative capabilities and tissue repair. These advancements may facilitate the delivery of bioactive compounds to modulate inflammation, supporting MSC therapeutic effects in osteoarthritis. Integrating natural products improves MSC viability and differentiation, contributing to effective treatment strategies [55]. Furthermore, these compounds enhance immunomodulatory properties, promoting better MSC integration within the osteoarthritic microenvironment and boosting their regenerative capacity [88]. Thus, natural products are vital for enhancing MSC therapeutic efficacy and patient outcomes in osteoarthritis. This section outlines how natural products enhance MSC function, paving the way for integrated therapeutic approaches for OA. Future research should optimize these combinations and identify additional natural compounds with complementary effects.
4. Combination Therapies: MSCs and Natural Products
Combining mesenchymal stem cells (MSCs) with natural products represents a novel and promising approach to treating osteoarthritis (OA). This strategy leverages the regenerative and immunomodulatory properties of MSCs alongside the bioactive potential of natural compounds, creating synergistic effects that can enhance therapeutic outcomes. Here, we explore the evidence supporting these combination therapies, focusing on their synergistic effects, preclinical and clinical studies, and the mechanisms underlying their enhanced efficacy.
4.1. Synergistic Effects of MSCs and Natural Products
The synergistic effects of mesenchymal stem cells (MSCs) and natural products, such as curcumin, resveratrol, quercetin, and epigallocatechin gallate (EGCG), have been shown to enhance MSC function by addressing limitations like poor cell survival, inadequate differentiation, and insufficient modulation of the inflammatory microenvironment (Table 3). These natural compounds, known for their antioxidant and anti-inflammatory properties, interact with MSCs to improve their therapeutic efficacy in various pathological conditions. The following sections explore the mechanisms through which these natural products enhance MSC function.
Curcumin, a polyphenolic compound, has improved MSC survival by reducing oxidative stress and inflammation. It interacts with key proteins involved in inflammation and cancer progression, such as COX-2 and NF-κB, enhancing MSC viability and differentiation potential [79,81,90]. Resveratrol promotes osteogenic differentiation of MSCs by modulating the NRF2/HO-1 and NF-κB pathways, which are crucial for reducing inflammation and enhancing bone regeneration [91]. Quercetin, another polyphenol, has been found to synergize with other compounds to promote osteogenesis and reduce inflammation in MSCs, particularly in the context of age-related bone loss [69,92,93]. Curcumin and resveratrol have demonstrated a synergistic ability to inhibit TNF-α-induced inflammation by suppressing the NF-κB signaling pathway, which is vital for reducing vascular inflammation and enhancing the function of mesenchymal stem cells (MSCs) in inflammatory conditions [94,95,96]. Additionally, quercetin, with its strong antioxidant properties, modulates signaling pathways that influence inflammation, thereby strengthening the immunomodulatory capacity of MSCs [97,98].
Despite curcumin’s low bioavailability, its therapeutic efficacy can be improved through structural modifications or nano-based delivery systems. These approaches enhance solubility and absorption, maximizing its potential when used with MSCs [99,100,101,102]. Notably, the combination of resveratrol and curcumin at low concentrations has been observed to produce a synergistic effect, which is more effective than the individual compounds alone, thereby improving the therapeutic outcomes of MSC-based therapies [103,104,105,106]. Research has also shown that MSCs preconditioned with natural antioxidants, such as crocin, significantly improve cell survival and reduce oxidative stress. This highlights the promising role of natural products in enhancing MSC efficacy, particularly in lung injury models [1,107]. Furthermore, resveratrol has been found to alleviate inflammation and promote osteogenic differentiation in periodontal tissues, showcasing its potential applications in dental MSC therapies [108].

Table 3.
The synergistic effects of mesenchymal stem cells (MSCs) and natural products.
Table 3.
The synergistic effects of mesenchymal stem cells (MSCs) and natural products.
| Category | Natural Product | Model Used | Outcomes | Author |
|---|---|---|---|---|
| Enhancement of MSC Survival and Differentiation | Curcumin Dosage regimens: General wellness: 500–1000 mg daily. Arthritis and joint pain: 1000–1500 mg daily, often divided into smaller doses. Heart health: 500–700 mg daily. Cognitive health: 500–1000 mg daily. Maximum safe limit: up to 8000 mg per day for short durations. | in vitro/ clinical studies | Decreased oxidative stress and inflammation improve MSC viability and differentiation potential. | [79,81,90] |
| Resveratrol Dosage regimens: Anti-aging: 150–500 mg per day; up to 1000 mg daily for more pronounced effects. Heart health: 100–250 mg daily; higher doses of 250–500 mg per day for existing cardiovascular conditions. Anti-inflammatory: up to 1500 mg daily for up to 3 months; higher doses of 2000–3000 mg daily for 2–6 months. | in vitro | Promotes bone growth, reduces inflammation, and enhances regeneration. | [69,78,108] | |
| Quercetin Dosage regimens: General wellness: 500 mg per day, often combined with vitamin C or bromelain to enhance absorption. Allergies or inflammation: 500–1000 mg per day, divided into multiple doses. | in vitro | Supports bone formation and decreases inflammation, especially in age-related bone loss. | [69,92,93] | |
| Modulation of Inflammatory Microenvironment | Curcumin and resveratrol combination (no information) | in vitro/in vivo | Inhibits TNF-α-induced inflammation, suppresses NF-κB signaling, reduces vascular inflammation, and enhances MSC function in inflammatory conditions. | [95,96,109,110] |
| Quercetin Dosage regimens: General wellness: 500 mg per day, often combined with vitamin C or bromelain to enhance absorption. Allergies or inflammation: 500–1000 mg per day, divided into multiple doses. | in vitro/in vivo | Enhances MSCs’ immunomodulatory capacity and modulates inflammatory signaling pathways. | [97,98] | |
| Improvement of Bioavailability and Efficacy | Curcumin Dosage regimens: General wellness: 500–1000 mg daily. Arthritis and joint pain: 1000–1500 mg daily, often divided into smaller doses. Heart health: 500–700 mg daily. Cognitive health: 500–1000 mg daily. Maximum safe limit: up to 8000 mg per day for short durations | in vitro/in vivo | Improved efficacy through structural modifications or nano-delivery systems enhances solubility and absorption, maximizing the therapeutic potential of MSCs. | [99,100,101,102] |
| Resveratrol and curcumin (no information) | in vitro/in vivo | Low concentrations of compounds work synergistically, improving the therapeutic outcomes of MSC-based therapies more effectively than when used individually. | [103,104,105,106] | |
| Case Studies and Applications | Crocin (no information) | in vivo | Significant improvements in cell survival and reductions in oxidative stress highlight the potential of natural products to enhance MSC efficacy in lung injury models. | [1,107] |
| Resveratrol Dosage regimens: Anti-aging: 150–500 mg per day; up to 1000 mg daily for more pronounced effects. Heart health: 100–250 mg daily; higher doses of 250–500 mg per day for existing cardiovascular conditions. Anti-inflammatory: up to 1500 mg daily for up to 3 months; higher doses of 2000–3000 mg daily for 2–6 months | in vivo | Ameliorates inflammation, promotes osteogenic differentiation in periodontal tissues, and demonstrates potential in dental applications of MSC therapy. | [108] |
Integrating mesenchymal stem cells (MSCs) with natural products like curcumin and resveratrol offers promise in regenerative medicine, but low bioavailability remains a key challenge. While these compounds are identified for enhancing MSC functionality, their clinical application is hindered by poor solubility and absorption [69,111,112]. Nano-formulations and structural modifications may improve bioavailability, yet current research has not sufficiently explored these strategies. Curcumin is known for its anti-inflammatory and potential anti-cancer effects, but a lack of substantial research on its safety and absorption limits its clinical use. Effective dosing regimens need to be established for practical applications. Similarly, the role of resveratrol in inflammation, particularly regarding bone regeneration, requires further investigation to understand its long-term effects. The absence of rigorous clinical trials for natural compounds like the BlastiMin Complex exacerbates the situation. While some studies suggest that curcumin and resveratrol may work synergistically to reduce inflammation, their long-term effects on diverse populations warrant more research [113]. Additionally, there are challenges in achieving optimal blood levels of these phytochemicals, emphasizing the need for better absorption methods. The interaction between crocin and dexamethasone in mitigating lung cell injuries raises questions about long-term MSC survival and functionality [114], which remain unexplored. In conclusion, while the combination of MSCs and natural products holds significant potential, critical gaps in research concerning absorption, safety, and long-term effects must be addressed to enhance clinical applications and improve patient outcomes.
4.2. Evidence from Preclinical Studies
Preclinical research has demonstrated that applying mesenchymal stem cells (MSCs) alongside natural products can exhibit significant potential in treating osteoarthritis. These investigations indicate that including natural compounds, particularly those derived from various plants, enhances the proliferation and differentiation of MSCs, facilitating cartilage repair and mitigating inflammation.
For instance, the administration of curcumin-treated MSCs into the joint has shown effectiveness in osteoarthritis models by diminishing inflammation and promoting cartilage regeneration more effectively than using MSCs alone [115]. Curcumin operates by inhibiting the NF-κB/JNK signaling pathway, which is crucial in the progression of osteoarthritis [81,116]. Innovative delivery systems, such as curcumin-loaded nanoparticles and microgels, have improved the bioavailability and efficacy of these treatments, resulting in reduced joint swelling and slower cartilage degradation [117,118]. Furthermore, curcumin provides protective benefits to cartilage cells and aids cartilage repair by modulating various biological pathways while decreasing oxidative stress and cell apoptosis [88,119]. Curcumin’s efficacy in addressing cartilage damage is further enhanced when combined with other substances like chondroitin sulfate [120]. In conclusion, curcumin, particularly when integrated with advanced delivery techniques or MSCs, presents a promising method for augmenting cartilage repair and diminishing inflammation in osteoarthritis.
Nonetheless, some limitations and challenges involve the possibility of differing bioavailability and the necessity for standardized formulations. Additionally, while the initial findings are encouraging, further clinical trials are essential to validate the efficacy and safety of these combinations in a broader patient population. This is particularly critical given the complex nature of osteoarthritis and the varying responses observed among different demographic groups. Future studies should optimize the dosages and combinations of these natural products to ensure maximum therapeutic benefit and minimal adverse effects.
In addition to curcumin, resveratrol and MSCs also demonstrate the potential to reduce cartilage damage and oxidative stress in osteoarthritis animal models [71,83,85]. Resveratrol is recognized for its anti-inflammatory and antioxidant properties, playing a significant role in managing osteoarthritis, a condition characterized by joint inflammation and cartilage degradation [85]. This natural compound influences several vital biological pathways, offering protection to cartilage and enhancing the effectiveness of MSC therapies [61,121]. MSCs, especially those isolated from adipose or joint tissues, display robust regenerative properties that help reduce oxidative stress and inflammation [122]. Combining resveratrol and MSCs may enhance cellular survival and proliferation [61,72]. Research suggests that resveratrol activates SIRT1, a critical factor in regulating oxidative stress and inflammation, thereby supporting its potential synergistic effects with MSCs in decelerating osteoarthritis progression [103,123,124]. Additionally, resveratrol can inhibit detrimental inflammatory proteins, improving MSC-based interventions [125].
The combined effects of resveratrol and mesenchymal stem cells (MSCs) indicate a potential avenue for advancing more effective treatments for osteoarthritis that target cartilage damage and oxidative stress. Nevertheless, additional research is necessary to identify the most effective dosages and combinations that enhance therapeutic benefits while reducing possible adverse effects. Moreover, it is important to comprehend how these natural products improve MSC effectiveness, as this knowledge will be vital for creating targeted treatment strategies for osteoarthritis patients. This insight could facilitate the formulation of synergistic therapies that enhance the regenerative capabilities of MSCs, ultimately benefiting clinical outcomes for individuals afflicted with osteoarthritis.
Quercetin, a flavonoid with significant health-promoting properties, has been shown to enhance mesenchymal stem cells’ survival and anti-inflammatory effects, thereby improving joint health [126]. Studies have demonstrated that quercetin can ameliorate senescence and promote osteogenesis in bone marrow mesenchymal stem cells (BMSCs) by inhibiting the repetitive element-triggered RNA sensing pathway, which is crucial for maintaining the osteogenic capacity of BMSCs [127,128,129]. Additionally, quercetin-treated BMSCs produce exosomes that deliver miR-124-3p to chondrocytes, inhibiting osteoarthritis progression by reversing the apoptotic effects of inflammatory cytokines and modulating key signaling pathways such as MAPK/p38 and NF-κB [130].
Quercetin also exhibits potent anti-inflammatory and antioxidant properties, which are essential in reducing the expression of pro-inflammatory cytokines and mitigating oxidative stress, both of which are critical in the pathogenesis of osteoarthritis and other inflammatory joint diseases [86,131,132]. Furthermore, quercetin has been shown to prevent osteoarthritis progression by maintaining the balance of inflammatory cascades and promoting cartilage repair, as evidenced by its ability to inhibit matrix metalloproteinases and enhance the expression of cartilage-protective proteins [70,73,86,133,134].
These findings collectively highlight the potential of quercetin as a therapeutic agent in enhancing MSC survival and function, offering a promising approach to improving joint health and managing conditions like osteoarthritis. However, integrating natural products like quercetin into MSC therapies requires further investigation. Future research should focus on elucidating the mechanisms by which quercetin influences stem cell behavior and conducting clinical trials to assess its efficacy in osteoarthritis patients. Moreover, exploring synergistic effects with other natural compounds that may further enhance the therapeutic potential of mesenchymal stem cells in osteoarthritis treatment is crucial.
Epigallocatechin-3-gallate (EGCG) has been shown to play a significant role in protecting mesenchymal stem cells (MSCs) from inflammation and enhancing their chondrogenic differentiation both in vitro and in vivo [135,136]. EGCG, a polyphenolic antioxidant, has been reported to support MSC-mediated collagen remodeling under oxidative stress conditions; however, it is ineffective when the collagen has already undergone oxidative damage [137]. Instead, EGCG facilitates alternative routes for collagen removal, such as internalization and transcytosis, which are crucial for maintaining ECM integrity [136,137].
In the context of arthritis, EGCG combined with extracellular vesicles has demonstrated a significant upregulation of type II collagen expression, which is essential for cartilage repair, and a reduction in apoptosis-related proteins, thereby ameliorating cartilage destruction in rheumatoid arthritis models [71,138]. Furthermore, EGCG has been shown to increase type II collagen accumulation in MSC-derived cartilage-like sheets by suppressing collagen degradation, highlighting its potential to enhance chondrogenic differentiation [139]. Additionally, EGCG’s anti-inflammatory properties have been observed to reduce cartilage inflammation and degradation in osteoarthritis models, suggesting its role as a disease-modifying agent [140]. The protective effects of EGCG extend to hypoxia-induced conditions, where it mitigates apoptosis and promotes osteogenic differentiation in MSCs by upregulating miR-210, which downregulates EFNA3, a receptor tyrosine kinase ligand [94,141].
These findings highlight EGCG’s multifaceted role in supporting MSC function and differentiation, making it a promising candidate for enhancing MSC-based therapies for cartilage regeneration and protection against inflammatory insults. Nonetheless, it is important to consider the underlying mechanisms through which EGCG operates. By enhancing the proliferative capacity of MSCs and promoting their migration to damaged tissues, EGCG not only aids in cartilage repair but also serves as an anti-inflammatory agent. Future studies should focus on optimizing the delivery methods of EGCG in conjunction with MSC injections to maximize therapeutic outcomes.
4.3. Insights from Clinical Trials
The clinical evidence regarding using mesenchymal stem cell (MSC) and natural product combination therapies in patients with osteoarthritis (OA) shows promise, though it is still in its early stages. MSCs have garnered significant attention for their potential to treat OA, largely owing to their ability to differentiate into various tissues and their strong paracrine effects, which include anti-inflammatory and immunomodulatory properties [142,143]. Clinical trials have demonstrated that MSCs can alleviate pain and enhance joint functionality, with various studies indicating favorable outcomes regarding cartilage protection and repair [144,145,146].
The exploration of combining MSCs with natural products, such as Radix Achyranthis Bidentatae (AB), has emerged as a compelling area of interest. AB is recognized for its potential to improve the internal conditions of knee joints and stimulate MSC repair mechanisms, suggesting a synergistic effect when used alongside MSCs [147,148]. Natural products generally possess anti-inflammatory and antioxidant properties that may further enhance the therapeutic effects of MSCs [149,150]. Despite these encouraging findings, the available clinical evidence remains limited, with most studies concentrating on preclinical models or small-scale clinical trials. For instance, a meta-analysis of MSCs combined with platelet-rich plasma (PRP) indicated improvements in pain and joint function [151]; however, the particular role of natural products in these combinations necessitates further investigation [152,153,154].
The complexities surrounding MSC metabolism, coupled with the differences in study designs and patient characteristics, create challenges in reaching definitive conclusions regarding the effectiveness of MSC–natural product therapies [155,156,157]. As a result, despite the promising possibilities offered by combining MSC and natural products for treating osteoarthritis, it has become evident that there is an urgent requirement for carefully structured clinical trials. Such trials are essential for a comprehensive assessment of both the safety and efficacy of these innovative therapies [158,159].
Moreover, the current clinical trials examining MSC–natural product combination therapies for OA face numerous challenges stemming from the complexities inherent to both MSC and natural product treatments. A key hurdle is the variability in the microenvironment of osteoarthritic joints, which can impact MSC efficacy. Factors such as inflammation, senescence, hypoxia, high glucose levels, and elevated lipid content in the joint environment can adversely affect MSC function, complicating their therapeutic potential [160]. Additionally, the clinical translation of MSC therapies is impeded by the variety of cell sources, isolation techniques, and culture protocols, which hinders comparisons across studies and the establishment of standardized treatment protocols [45].
Although MSCs have demonstrated promise in preclinical models, their effectiveness in human trials has not been consistent, with some studies revealing only short-term benefits and others presenting contradictory findings [161,162]. The challenges related to natural products include issues with bioavailability, precise targeting, and the heterogeneity of OA as a disease, complicating the formulation of effective natural product-based therapies [163,164,165]. Furthermore, the current clinical trial evidence is insufficient to definitively ascertain the efficacy of natural anti-inflammatory products in preventing or treating OA, highlighting the need for additional high-quality research to explore their mechanisms and optimal delivery systems [166].
The integration of MSCs with natural products in clinical trials is also challenged by the necessity for robust methodologies to evaluate the combined effects and identify specific patient sub-populations that might derive the greatest benefit from these therapies [167]. In summary, while the potential for MSC–natural product combination therapies in OA is hopeful, substantial research and methodological advancements are essential to overcome these challenges and realize reliable clinical outcomes.
4.4. Mechanisms Underlying Synergistic Effects
Natural products play a crucial role in reducing oxidative stress by decreasing levels of reactive oxygen species (ROS) and safeguarding mesenchymal stem cells (MSCs) and the joint microenvironment from harm. The beneficial effects of these products stem from key antioxidant pathways and the interactions among various natural compounds. For example, botanical extracts such as turmeric, pagoda tree seed pod, and rosemary activate the Nrf2-ARE signaling pathway, enhancing the body’s defense mechanisms against free radicals and thus lowering oxidative stress [168,169,170,171]. Additionally, polyphenols and glutathione (GSH) promote cellular redox homeostasis, demonstrating synergistic effects that support disease management [172,173,174].
In the context of MSC therapy, molecular hydrogen and cold atmospheric plasma have been shown to reduce ROS levels and activate the Nrf2 pathway, thereby improving MSC survival and functionality [175,176]. Further, combinations of natural compounds such as α-tocopherol, ascorbic acid, and selenium offer protective benefits against chondrocyte cell death, presenting a promising strategy to combat oxidative stress in joint health [177]. A supplement formula containing vitamin D, Lactobacillus rhamnosus, ginger, curcumin, and Boswellia extract effectively reduces oxidative stress and promotes MSC differentiation, indicating a synergistic effect [178]. These findings highlight the significance of leveraging the synergistic effects of natural products to alleviate oxidative stress and enhance MSC therapy in regenerative medicine.
Moreover, compounds like curcumin and quercetin not only exhibit anti-inflammatory properties but also modulate pathways such as NF-κB and IL-6. Curcumin, derived from Curcuma longa L., enhances the efficacy of chemotherapeutic drugs by regulating inflammatory cancer pathways [179,180]. When paired with other compounds such as resveratrol, curcumin displays synergistic effects in inflammatory reduction by inhibiting NF-κB signaling, which is critical for addressing endothelial dysfunction and cardiovascular diseases [109,181,182]. The combination of curcumin and quercetin has shown significant inhibition of prostate cancer cell proliferation, inducing apoptosis while modulating pathways related to inflammation, including those linked to reactive oxygen species and nitric oxide [183,184,185,186]. These synergistic effects arise from the ability of these compounds to target multiple steps within inflammatory pathways, similar to other flavonoid combinations that influence NF-κB signaling [187,188]. Additionally, curcumin and resveratrol help suppress inflammation in contrast-induced nephropathy by regulating microRNA pathways, thereby underlining their roles in managing inflammatory responses [189,190].
The combined effects of curcumin and quercetin enhance their therapeutic potential across various inflammatory conditions. The ability of natural products to promote cartilage regeneration stems primarily from their capacity to enhance MSC differentiation into chondrocytes and increase the expression of cartilage-specific extracellular matrix proteins. This process benefits from the multi-component nature of natural products, which allows them to engage multiple pathways simultaneously, thereby amplifying their therapeutic impact. For instance, traditional Chinese medicine (TCM) employs combinations of herbal ingredients that regulate various targets across different pathways, fostering synergistic actions that promote MSC differentiation and cartilage regeneration [191,192]. Furthermore, the pharmacokinetic properties of these natural products, including enhanced absorption and bioavailability, significantly contribute to their efficacy in supporting cartilage health [11,193].
Integrating natural products with conventional therapies can also enhance therapeutic outcomes by overcoming resistance mechanisms and mitigating adverse effects, as evident in cancer treatment strategies [194]. The synergistic mechanism of TCM, exemplified by the targeted combination of PepT1 and PPARα, illustrates how natural products can augment the uptake and effectiveness of active compounds, promoting cartilage regeneration [181]. Overall, incorporating natural products into therapeutic strategies presents an encouraging approach to improve MSC differentiation and cartilage regeneration through their multi-targeted and synergistic actions.
The synergistic effects of delivery systems that integrate natural products and MSCs enhance cell retention and engraftment at injury sites through various mechanisms. When used in combination, natural products have demonstrated improved therapeutic effects due to their multi-targeted approach, enhancing the bioavailability and absorption of active compounds, as shown in cardiovascular treatments [112]. This multi-component strategy is also observed in TCM, where herbal combinations like Chuanxiong Rhizome and Paeoniae Radix Rubra synergistically inhibit inflammation and oxidative stress through pathways such as PI3K/AKT and MAPK, which are crucial for cell survival and retention [195,196]. Moreover, the co-delivery of bioactive compounds can preserve and enhance radical scavenging potential, thereby sustaining the biological effects of individual components.
In cancer therapy, the combination of different therapeutic agents, including salinomycin and dasatinib, has shown improved efficacy by suppressing multiple pathways, similar to enhancing MSC retention through modulating the local microenvironment [197]. Furthermore, normalizing aberrant vasculature and endothelial activation in cancer immunotherapy can facilitate the retention and infiltration of therapeutic cells, indicating a potential mechanism for improved MSC engraftment [198]. Collectively, these findings highlight the promise of utilizing combinations of natural products and MSCs to enhance therapeutic outcomes through improved cell retention and engraftment at injury sites via synergistic mechanisms.
4.5. Challenges, Considerations and Future Direction
The integration of mesenchymal stromal cells (MSCs) with natural products holds significant promise for treating osteoarthritis (OA). However, several obstacles must be addressed to exploit this potential fully. One primary challenge is the inherent heterogeneity in MSC-based therapies, which complicates the evaluation of their clinical effectiveness due to variability in tissue sources, donor characteristics, and manufacturing methods [6]. This variability can impact the immunomodulatory and regenerative functions of MSCs, which are crucial for addressing both the inflammatory and degenerative components of OA. Additionally, the bioavailability and targeted application of natural products present substantial challenges. While these compounds can effectively influence inflammatory pathways such as NFκB, MAPK, and PI3K/AKT, their clinical application is often hindered by poor bioavailability and heterogeneity in disease presentation [164].
To enhance the therapeutic efficacy and clinical applicability of combining mesenchymal stem cells (MSC) and natural product therapies for osteoarthritis (OA), future research should focus on several key areas. One crucial aspect is optimizing delivery systems for MSC-derived exosomes, as these vesicles have demonstrated significant potential in modulating the joint microenvironment and promoting cartilage repair [199,200]. Integrating exosomes with biomaterials like hydrogels could improve their stability and targeted delivery, addressing current challenges in standardization and scalability [201]. Additionally, exploring the synergistic effects of natural products, such as Radix Achyranthis Bidentatae, with MSCs could enhance immune regulation and chondrocyte formation, potentially slowing OA progression [71]. Understanding the molecular pathways influenced by natural products, including NF-κB and PI3K/AKT, is essential for developing new application strategies and improving clinical outcomes. Furthermore, the role of senolytics in conjunction with MSC therapies should be investigated, as targeting senescent cells may mitigate OA progression by modulating inflammation and cellular senescence. Personalized medicine approaches, leveraging genomic insights to identify specific OA phenotypes, could also tailor treatments to individual patient needs, enhancing effectiveness and reducing adverse effects. Overall, future research should aim to integrate these diverse therapeutic strategies, focusing on mechanistic insights, delivery optimization, and personalized approaches to fully realize the potential of MSC–natural product therapies in OA management.
5. Mechanisms of Action
The therapeutic synergy between mesenchymal stem cells (MSCs) and natural products such as curcumin, resveratrol, quercetin, and epigallocatechin gallate (EGCG) in osteoarthritis (OA) is underpinned by their ability to modulate key cellular and molecular pathways involved in joint inflammation, cartilage degeneration, and tissue regeneration. This section highlights how natural products enhance the efficacy of MSC-based therapies, focusing on cellular processes, signaling pathways, and their combined effects on the osteoarthritic microenvironment.
5.1. Modulation of Inflammatory Pathways
These natural compounds are known for their anti-inflammatory and antioxidant properties, which are crucial in addressing the persistent inflammation characteristic of OA. Curcumin, for instance, has been shown to enhance the efficacy of MSC-derived exosomes in attenuating OA by modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signaling pathways, thereby reducing inflammation and promoting chondrocyte viability [202]. Resveratrol and EGCG, along with other polyphenols, target key inflammatory pathways such as NFκB, TGFβ, and Wnt/β-catenin and execute epigenetic modifications that can alter gene expression related to OA pathophysiology [88]. These compounds can inhibit the overactivation of inflammatory cascades like NF-κB, MAPK, and PI3K/AKT, which are central to OA pathogenesis [71,140]. Moreover, the use of nano-formulated bioactive compounds, including these natural products, has been suggested to improve their bioavailability and therapeutic efficacy by modulating gut microbiota, which plays a role in systemic inflammatory responses associated with OA [203]. The integration of these natural products with MSCs not only enhances the anti-inflammatory effects but also supports cartilage homeostasis and reduces apoptosis in chondrocytes, as seen with curcumin’s ability to inhibit the PI3K-Akt-mTOR pathway [204]. Despite these promising findings, challenges such as bioavailability and precise targeting remain, necessitating further research to optimize the clinical application of these natural compounds in OA therapy. Overall, the combination of these natural products with MSCs offers a multifaceted approach to modulating inflammatory pathways in OA, potentially leading to more effective disease management strategies.
5.2. Reduction of Oxidative Stress
The incorporation of natural compounds alongside mesenchymal stem cells (MSCs) has demonstrated a noteworthy potential in mitigating oxidative stress [88], which is a significant challenge in stem cell-based therapies. These natural compounds are well known for their antioxidant properties [205], which include scavenging free radicals and modulating signaling pathways to enhance cellular resilience against oxidative damage. Curcumin, for instance, has been demonstrated to protect bone marrow mesenchymal stem cells (BMSCs) from hydrogen peroxide-induced oxidative stress by enhancing mitochondrial function and deactivating the Akt/Erk signaling pathways, thereby improving cell viability and reducing apoptosis [89,206]. Similarly, resveratrol and curcumin have been shown to exert synergistic effects in reducing oxidative stress and inflammation, which are crucial in maintaining the therapeutic efficacy of MSCs [69]. Quercetin and EGCG also possess direct antioxidant properties, as evidenced by their ability to scavenge free radicals in cellular assays, which can be beneficial in reducing oxidative stress when used in combination with MSCs [207]. Furthermore, the combination of these polyphenols can modulate gene expression and influence microRNA activity, thereby enhancing the antioxidant defense mechanisms at a molecular level [208]. The synergistic activity of these compounds, as observed in high-glucose-induced oxidative stress models, suggests that they can significantly decrease reactive oxygen species (ROS) production and inflammatory markers, which are critical in mitigating oxidative stress in MSC therapies [209]. Overall, the integration of these natural antioxidants with MSCs offers a multifaceted approach to enhancing therapeutic outcomes by reducing oxidative stress, thereby improving cell survival and function in various pathological conditions. However, the potential for these natural products to strengthen synergistically the regenerative capabilities of MSCs must be further explored through rigorous clinical trials. Future research should also focus on identifying the optimal combinations and dosages of these natural antioxidants to maximize their efficacy in osteoarthritis treatment.
5.3. Promotion of Chondrogenic Differentiation
Recent studies have investigated the integration of natural compounds such as curcumin, resveratrol, quercetin, and EGCG with mesenchymal stem cells (MSCs) as a novel strategy to enhance chondrogenic differentiation, offering significant potential for cartilage regeneration and osteoarthritis (OA) treatment. Among these, curcumin has garnered particular attention for its ability to modulate signaling pathways such as miR-124/NF-κB and miR-143/ROCK1/TLR9, which are critical in maintaining chondrocyte viability and reducing apoptosis in OA models. Remarkably, MSC-derived exosomes treated with curcumin have demonstrated the capacity to restore normal expression levels of these pathways, effectively attenuating OA progression [202]. However, this research has yet to explore additional signaling pathways that may contribute to its therapeutic outcomes. Future studies should focus on assessing curcumin’s long-term effects, its role in combination therapies, and the mechanisms underlying its epigenetic impact to expand its applications in OA management. In addition, curcumin-loaded small extracellular vesicles (sEV-CUR) derived from adipose-derived MSCs (ADMSCs) have shown superior therapeutic efficacy compared to free curcumin or ADMSC-derived sEVs. These vesicles promote chondrocyte proliferation, reduce apoptosis, and alleviate oxidative stress more effectively, making them a promising treatment option for OA. Biweekly intra-articular injections of sEV-CUR have been shown to protect cartilage and significantly reduce oxidative damage in OA models [81]. Despite these promising findings, the study does not investigate long-term effects, potential side effects, or the integration of other natural compounds such as resveratrol. Additionally, the molecular mechanisms underlying sEV-CUR’s anti-oxidative and anti-apoptotic effects remain insufficiently explored. Future research should aim to improve targeting efficiency, evaluate its pain-relief efficacy, and examine synergies with other natural compounds to harness its therapeutic potential fully.
Recent advancements in regenerative medicine continue to highlight the transformative potential of integrating natural compounds with mesenchymal stem cells (MSCs) to enhance chondrogenic differentiation, a vital process for cartilage repair and regeneration. Among these bioactive agents, resveratrol, a polyphenol, has been extensively studied for its ability to amplify the therapeutic potential of MSCs. By modulating inflammation-related cytokines, resveratrol provides a significant advantage in hyperglycemic conditions that predispose individuals to osteoarthritis (OA) [83]. Recent studies highlight the role of sustained release systems, such as resveratrol-loaded hydrogels, in maintaining localized delivery to cartilage defects, improving bioavailability and therapeutic outcomes. Resveratrol also enhances the chondrogenic differentiation of MSCs by modulating autophagy and mitochondrial dynamics, which is crucial for cells derived from metabolic syndrome conditions. Furthermore, sustained release of resveratrol has been shown to inhibit interleukin-1β-induced metalloproteinase-13 expression, thereby protecting the cartilage matrix and promoting chondrocyte differentiation [69,89]. Recent findings have highlighted the significant role resveratrol plays in modulating the behavior of mesenchymal stem cells (MSCs). These advancements focus on harnessing the bioactive compounds found in various natural sources, which have demonstrated the potential to enhance the therapeutic efficacy of injected MSCs for osteoarthritis [210]. Curcumin, another natural compound, has been reported to synergize with resveratrol to stimulate the MAPK signaling pathway, which is vital for chondrocyte differentiation and survival. It also suppresses the nuclear factor-κB pathway, thereby promoting chondrogenic differentiation in a high-density co-culture microenvironment [85]. The combination of these natural products with MSCs not only enhances the differentiation potential but also addresses the inflammatory and oxidative stress challenges that often impede effective cartilage regeneration. While the specific effects of quercetin and EGCG in combination with MSCs were not detailed in the provided contexts, the synergistic effects of curcumin and resveratrol highlight the potential of using natural compounds to enhance MSC-based therapies for cartilage repair. Overall, these findings suggest that integrating natural products with MSCs could significantly improve the outcomes of regenerative treatments for osteochondral injuries and degenerative joint diseases like OA.
5.4. Enhanced Delivery via Biomaterials
These natural polyphenols are known for their antioxidant, anti-inflammatory, and anticancer properties, which are crucial in biomedical applications, including drug delivery and tissue engineering. For instance, resveratrol has been shown to improve the survival, self-renewal, and differentiation of MSCs, thereby enhancing their therapeutic effects in regenerative medicine [53]. Similarly, resveratrol preincubation with MSCs enhances their migration and survival in neurodegenerative disease models, such as Alzheimer’s, by modulating neuroinflammation and improving cell homing [211]. The use of nanoparticles and hydrogels as delivery systems further augments the stability and bioavailability of these polyphenols. For example, curcumin and resveratrol co-delivered via a hydrogel/nanoparticle system demonstrated significant anti-inflammatory effects in a spinal cord injury model [212]. Additionally, quercetin and resveratrol encapsulated in targeted nanoparticles improved cellular uptake and stability, enhancing their therapeutic potential [213]. The formation of phytosomes with phospholipids also increases the bioavailability of these polyphenols, facilitating their penetration into cells [214]. Moreover, the use of nanotechnology, such as sericin self-assembling nanoparticles, allows for a controlled release of polyphenols, promoting MSC metabolic activity and protecting them from oxidative stress [215]. These advanced delivery systems not only improve the pharmacokinetic properties of natural products but also enhance the regenerative capabilities of MSCs, making them promising candidates for treating various diseases and injuries.
5.5. Immune Modulation
The combination of natural products such as curcumin, resveratrol, quercetin, and epigallocatechin-3-gallate (EGCG) with mesenchymal stem cells (MSCs) has shown promising effects on immune modulation. These natural compounds are known for their anti-inflammatory and immunomodulatory properties, which can enhance the therapeutic potential of MSCs in various pathological conditions. Curcumin, quercetin, and resveratrol, when used together, have been shown to modulate the tumor microenvironment by increasing T cell recruitment and reducing immunosuppressive cell populations, thereby tipping the immune balance towards activation [79]. Quercetin, in particular, has been demonstrated to enhance the immunoregulatory effects of MSCs by modulating pathways such as Akt/IκB and Toll-like receptor-3, which are crucial for immune response regulation [216]. EGCG, another potent polyphenol, has been noted for its role in modulating neuroimmune diseases, suggesting its potential to enhance MSC-based therapies for immune-related disorders and improve the therapeutic efficacy of mesenchymal stem cells (MSCs) in treating osteoarthritis. Recent studies have indicated that EGCG can improve the viability and functionality of MSCs, potentially leading to better patient outcomes [63,217,218]. The combination of these natural products with MSCs can potentially improve the modulation of inflammatory phenotypes and immune responses, offering a novel therapeutic avenue for autoimmune and inflammatory diseases. Furthermore, these compounds have been shown to influence various immune pathways, such as NF-κB and IL-6/JAK/STAT, which are critical in controlling immune responses and inflammation [219,220,221]. Overall, the integration of these natural products with MSCs could provide a synergistic effect, enhancing the immunomodulatory capacity of MSCs and offering a promising strategy for treating immune-related diseases.
5.6. Future Perspectives
Understanding the mechanisms of action underlying mesenchymal stem cell (MSC) and natural product therapies is crucial for optimizing their application in osteoarthritis (OA) treatment. MSCs have shown promise due to their regenerative and immunomodulatory properties, which include promoting chondrocyte regeneration, inhibiting extracellular matrix degradation, and attenuating inflammation induced by macrophages [222,223]. Exosomes derived from MSCs further enhance these effects by delivering bioactive molecules that modulate the joint microenvironment, reduce inflammation, and promote cartilage repair. However, challenges such as low yield, weak activity, and inefficient targeting of exosomes need to be addressed through engineering approaches and biomaterial integration to improve their therapeutic efficacy. On the other hand, natural products (NPs) offer a complementary approach by modulating key inflammatory pathways like NF-κB, MAPK, and PI3K/AKT, which are central to OA pathogenesis. Despite their potential, the clinical translation of NPs is hindered by issues of bioavailability and precise targeting [70,224]. Future research should focus on optimizing drug delivery systems for both MSC-derived exosomes and NPs, exploring personalized medicine approaches, and conducting comparative effectiveness studies to realize their full potential in OA treatment. Additionally, understanding the interaction between MSCs and the OA microenvironment, including inflammatory and senescence factors, is essential for enhancing the efficacy of MSC therapies. By addressing these research gaps, it may be possible to develop more effective and safer therapeutic strategies for managing OA, ultimately improving patient outcomes.
5.7. Perspectives on Clinical Practice and Translation
The integration of mesenchymal stem cells (MSCs) with natural products such as curcumin, resveratrol, quercetin, and epigallocatechin gallate (EGCG) presents a promising strategy for osteoarthritis (OA) treatment. These therapies could revolutionize clinical approaches by offering disease-modifying solutions that not only reduce inflammation but also promote cartilage repair and regeneration [225]. As natural products can enhance MSC viability, differentiation, and immune modulation, their clinical application could significantly alter the current landscape of OA management [226]. Currently, OA treatments primarily focus on symptom management through pharmacological therapies, such as NSAIDs and corticosteroid injections, or invasive options such as joint replacement surgery [227]. However, these strategies fail to address the underlying degenerative processes and often come with long-term side effects [228]. The introduction of MSCs combined with natural products could provide a regenerative alternative that targets the root causes of OA, offering the potential to slow disease progression, reduce inflammation, and repair cartilage.
In clinical practice, the incorporation of MSCs with natural products could complement or even replace some current therapies. For example, MSCs preconditioned with bioactive compounds like curcumin or resveratrol could improve patient outcomes by enhancing the regenerative capacity of the stem cells, reducing the need for pain management strategies like NSAIDs or corticosteroid injections [57]. This shift could lead to a more sustainable approach to OA care, with less reliance on drugs that come with significant side effects. Despite their promising potential, several challenges must be addressed to bring MSC–natural product therapies into routine clinical practice. One of the most significant barriers is bioavailability. Natural products like curcumin and resveratrol are known for their potent therapeutic effects, but their clinical use is often limited by poor bioavailability and rapid metabolism [113]. Strategies to improve the delivery of these compounds, such as nanoparticle formulations or hydrogel-based systems, are promising, but they need further optimization for clinical application.
Another critical challenge is the variability in MSC sources and protocols. MSCs can be derived from various tissues, such as bone marrow, adipose tissue, and umbilical cord blood, and there is significant variation in how these cells are isolated, cultured, and administered [229]. This variability can lead to inconsistent therapeutic outcomes, making it difficult to standardize treatment protocols. Furthermore, the regulatory landscape remains complex, as the clinical translation of MSC therapies requires rigorous safety evaluations and long-term follow-up studies to ensure their efficacy and safety. Additionally, the heterogeneity of OA across patient populations complicates the development of a universal therapeutic approach. Factors such as age, comorbidities, disease severity, and genetic predisposition can all affect how a patient responds to MSC-based therapies. This variability requires personalized treatment strategies, where patient-specific factors are considered to tailor the therapy to individual needs.
To address these challenges, future research must focus on optimizing delivery systems for natural products, such as the use of nanoparticle encapsulation or sustained-release hydrogels. These strategies would enhance the stability and bioavailability of bioactive compounds, ensuring they reach therapeutic concentrations at the target site. Moreover, standardization of MSC protocols is crucial to ensure consistency in the production and administration of stem cells. Establishing standardized methods for MSC isolation, culture, and characterization would streamline clinical application and improve reproducibility across clinical trials. To overcome the regulatory hurdles, it is essential to establish clear guidelines for the clinical use of MSC–natural product therapies. Regulatory bodies must work alongside researchers to create frameworks that address the unique challenges posed by stem cell-based therapies, ensuring that treatments are both safe and effective. Additionally, incorporating advanced biomarkers to better understand patient-specific responses could pave the way for more effective, personalized treatments. By integrating biomarkers into clinical trials, we could identify which patients are most likely to benefit from MSC therapies combined with natural products, thereby improving patient outcomes and minimizing the risk of adverse effects.
Finally, collaborative efforts between academia, industry, and regulatory agencies are needed to accelerate the development of MSC–natural product therapies. Multi-center clinical trials and robust safety data will be essential for demonstrating the efficacy and long-term safety of these therapies. As these therapies evolve, there will also be an opportunity to explore combination therapies that integrate MSCs, natural products, and other regenerative medicine strategies, creating a more comprehensive approach to OA treatment.
6. Challenges and Considerations
The combination of mesenchymal stem cells (MSCs) and natural products offers significant possibilities for osteoarthritis (OA) treatment; nonetheless, several obstacles must be addressed to enable the shift of these therapies from research environments to clinical use. This section highlights key challenges, including the necessity for standardization, concerns related to bioavailability, safety issues, regulatory hurdles, and possible approaches to tackle these challenges effectively.
6.1. Standardization and Quality Control
A primary obstacle is the standardization of MSC-derived exosome production and characterization, which is crucial for ensuring consistent therapeutic outcomes. Exosomes, which are cell-free vesicles derived from MSCs, have shown potential in modulating the joint microenvironment, reducing inflammation, and promoting cartilage repair [230,231]. However, the scalability of production and a comprehensive understanding of their mechanisms remain significant hurdles. Additionally, the integration of MSCs with natural products like Radix Achyranthis Bidentatae (AB) has shown the potential to enhance joint function and reduce cartilage apoptosis [232]. Yet, the precise mechanisms and optimal combinations require further exploration. The use of biomaterials, such as hydrogels and magnetic polysaccharide microcarriers, has been proposed to improve the delivery and efficacy of MSC-derived exosomes [233,234,235]. Still, these approaches also face challenges related to efficient enrichment and targeted delivery. Furthermore, clinical trials have demonstrated variability in patient responses to MSC therapies [6], highlighting the need for personalized treatment strategies and a deeper understanding of patient-specific factors that influence therapeutic efficacy. Addressing these challenges through rigorous standardization, mechanistic studies, and personalized approaches will be essential for the successful clinical translation of MSC and natural product-based therapies for OA.
6.2. Bioavailability and Delivery
One significant obstacle is the efficient delivery and retention of therapeutic agents within the joint space, as conventional drug delivery systems often suffer from rapid clearance and poor bioavailability, limiting their effectiveness [236]. Innovative delivery systems, such as hydrogels and lipid-based drug delivery systems, have been explored to enhance the stability and targeted delivery of MSCs and their derivatives, such as exosomes, to the affected areas. Hydrogels, for instance, provide a biocompatible scaffold that supports MSC viability and differentiation, which is crucial for effective cartilage repair [237]. Additionally, magnetic polysaccharide microcarriers and DNA supramolecular hydrogels have shown the potential to protect MSCs from shear forces and enhance their therapeutic efficacy in OA environments [238,239]. Furthermore, the combination of MSCs with natural products like Radix Achyranthis Bidentatae and stigmasterol has demonstrated synergistic effects in promoting cartilage repair and reducing inflammation [71]. However, the delivery of these combinations requires further optimization. The development of multifunctional delivery systems that can provide sustained release and targeted delivery of therapeutic agents is crucial for improving treatment outcomes and minimizing systemic exposure. Addressing these bioavailability and delivery challenges through advanced biomaterial engineering and innovative delivery strategies is essential for the successful clinical translation of MSC-based therapies for OA.
6.3. Safety and Potential Side Effects
Several challenges must be addressed to transition these therapies from research to clinical practice, particularly concerning safety and potential side effects. MSC-derived exosomes (MSC-Exos) have shown potential in modulating the joint microenvironment and promoting cartilage repair, but their clinical application is hindered by the need for standardized production methods and long-term safety assessments [201,240]. The safety of MSC therapies has been explored in various studies, with intra-articular injections of adipose-derived MSCs (ADMSCs) demonstrating no severe treatment-related adverse events in both pilot and larger-scale trials, suggesting a favorable safety profile [161,235,236]. However, the variability in patient responses and the lack of large-scale, controlled studies highlight the need for further research to confirm these findings and understand the mechanisms underlying differential responses. Additionally, the integration of MSCs with natural products like Radix Achyranthis Bidentatae (AB) offers potential benefits in immune regulation and cartilage protection [232]. Still, the safety of such combinations requires thorough investigation. The development of delivery systems, such as magnetic polysaccharide microcarriers and gelatin methacryloyl hydrogels, aims to enhance the therapeutic efficacy and safety of MSC-based treatments by ensuring targeted delivery and sustained release [201]. Yet, these innovations also necessitate rigorous safety evaluations. Overall, while MSC-based therapies for OA show promise, addressing safety concerns through comprehensive clinical trials and mechanistic studies is crucial for their successful clinical translation.
6.4. Regulatory Hurdles
One of the primary obstacles faced in the field of regenerative medicine is the regulatory hurdles associated with MSC-based therapies. These hurdles include the urgent need for standardized protocols for the isolation, characterization, and production of mesenchymal stem cells (MSCs), which are vital to ensure consistent quality, efficacy, and safety across various studies and applications [6,91,238].
The significant variability in methodologies and outcomes observed in clinical trials further complicates the regulatory landscape, primarily because there exists a noticeable lack of consensus among researchers and regulatory bodies on the most effective MSC source, optimal dosage, and appropriate delivery methods for these therapies [6]. Moreover, the long-term safety and efficacy of MSC therapies remain under scrutiny, creating a necessity for rigorous clinical trials that can sufficiently establish their therapeutic potential while meticulously addressing any possible adverse effects that may arise during treatment [42,239]. In addition, the integration of natural products, such as the well-known Radix Achyranthis Bidentatae, with mesenchymal stem cells (MSCs) warrants careful and systematic evaluation [11,232]. This thorough investigation is necessary to fully comprehend their potential synergistic effects and optimize the corresponding treatment protocols aimed at achieving better patient outcomes. Furthermore, the ongoing development of innovative delivery systems, such as cell–tissue matchmaking nanoconstructs, highlights the critical need for advanced technologies specifically designed to enhance MSC implantation and integration within the target tissues [240]. These advancements are essential for significantly improving overall therapeutic outcomes in various clinical settings, where the merging of science and medicine can lead to groundbreaking innovations that benefit patients. The collaboration between natural therapies and modern medical practices has the potential to revolutionize treatment options and contribute to enhanced healing processes, paving the way for more effective interventions. Addressing these ever-evolving regulatory and technical challenges is not just a necessity but an essential prerequisite for the successful clinical translation of mesenchymal stem cell (MSC) therapies and natural product-based treatments, specifically for osteoarthritis (OA). This concerted and dedicated effort ultimately seeks to provide a wider array of effective and regenerative treatment options for patients who suffer from painful degenerative joint diseases, intending to significantly improve their overall quality of life through innovative and groundbreaking medical advancements that could greatly enhance their everyday experiences and functional abilities.
Despite promising findings, several challenges must be addressed before MSC–natural product therapies can be fully integrated into clinical practice. This section discusses key limitations and research priorities for future studies.
7. Challenges, Limitations, and Conflicting Evidence
Despite promising preclinical and early clinical findings, significant challenges and limitations remain in integrating natural products with MSC-based therapies for osteoarthritis (OA). This section explores four key areas of concern: bioavailability issues, variability in MSC sources, conflicting clinical data, and the need for standardized treatment protocols.
7.1. Bioavailability and Stability Challenges
One of the primary obstacles to using curcumin, resveratrol, quercetin, and EGCG in MSC therapy is their poor bioavailability. These compounds face issues such as low solubility, rapid metabolism, and inefficient systemic absorption. For instance, curcumin undergoes rapid degradation and hepatic metabolism, limiting its therapeutic efficacy in vivo [67]. Similarly, EGCG, despite its potent antioxidant properties, has a short half-life and low stability in physiological conditions, reducing its bioactive potential [63]. Resveratrol, although capable of enhancing MSC survival, is poorly absorbed in the gastrointestinal tract [68].
To address these challenges, recent strategies have focused on improving the stability and absorption of these bioactive compounds. Nanoparticle encapsulation, liposomal formulations, and hydrogel-based delivery systems have shown promise in enhancing bioavailability [52,75]. Future research should prioritize optimizing nano-formulations for controlled release in the joint microenvironment and conducting comparative studies on different bioavailability enhancement techniques.
7.2. Variability in MSC Sources and Treatment Efficacy
The therapeutic potential of MSCs varies depending on factors such as tissue source, donor age, and culture conditions. This variability presents a significant challenge in standardizing treatments. Bone marrow-derived MSCs (BM-MSCs) are commonly used but have lower proliferation rates in aged individuals. Adipose-derived MSCs (AD-MSCs) are more abundant and accessible, but their immunomodulatory potential is less well studied in OA [241,242]. Umbilical cord-derived MSCs (UC-MSCs) show high proliferative potential but are less frequently tested in clinical OA settings.
Furthermore, differences in MSC preparation protocols can lead to inconsistencies in therapeutic outcomes. To address these issues, future studies should compare different MSC sources under the same experimental conditions to determine which type offers optimal regenerative potential for OA treatment. Additionally, establishing standardized MSC culture and expansion protocols is crucial to ensure reproducibility in clinical trials.
7.3. Conflicting Clinical Data on MSC + Natural Product Therapies
While preclinical studies demonstrate strong evidence of natural products enhancing MSC function, clinical translation remains inconsistent. Some clinical trials report significant pain reduction and functional improvements in OA patients treated with MSCs and bioactive compounds [69]. However, other studies show no significant cartilage regeneration, suggesting that the therapeutic window for MSC + natural product therapy may be patient-specific [85].
These conflicting results can be attributed to differences in patient age, OA severity, MSC dosage, and natural product administration. To resolve these discrepancies, larger, multi-center randomized controlled trials (RCTs) are needed to determine long-term efficacy and optimal dosing regimens. Additionally, developing biomarker-based patient selection criteria could help identify individuals who will benefit most from MSC + natural product therapy.
7.4. Lack of Regulatory Guidelines and Standardized Protocols
The absence of a standardized regulatory framework for combining natural products with MSC-based cell therapies presents another significant challenge. Many natural compounds are classified as dietary supplements rather than pharmaceuticals, leading to variability in quality control [74]. While MSC therapies require strict Good Manufacturing Practice (GMP) compliance, the co-administration of bioactive compounds is not yet fully regulated in most countries.
To address this issue, regulatory agencies should develop clear guidelines on combining stem cell-based therapies with bioactive compounds. Future studies should also focus on standardizing treatment regimens, including optimal dosing, frequency, and delivery methods for combined MSC + natural product therapy.
7.5. Future Research Priorities
In conclusion, while the integration of natural products into MSC-based therapies for OA is a promising frontier, several key research areas must be addressed before clinical translation is fully realized. These include improving bioavailability through advanced formulations, comparing different MSC sources in head-to-head trials, conducting larger-scale human trials with ≥200 patients to confirm long-term benefits, and establishing clear regulatory pathways for combined therapies. By addressing these challenges, researchers can pave the way for more effective and standardized treatments for osteoarthritis.
8. Conclusions and Future Directions
The integration of natural products with mesenchymal stem cell (MSC) therapies represents a promising strategy for enhancing cartilage regeneration, reducing inflammation, and improving clinical outcomes in osteoarthritis (OA). This review highlights the potential of curcumin, resveratrol, quercetin, and EGCG to modulate key inflammatory pathways (NF-κB, MAPK, PI3K/AKT), enhance MSC viability, and promote chondrogenic differentiation. Despite encouraging preclinical and early clinical findings, significant challenges remain before these therapies can be widely adopted in clinical settings.
8.1. Research Priorities for Advancing MSC + Natural Product Therapies
Future research should focus on several key areas to optimize MSC–natural product therapy for OA. First, improving the bioavailability and delivery systems of natural compounds is crucial, as many have poor solubility, rapid metabolism, and low systemic absorption. Studies should explore nanoparticle-based drug delivery, hydrogel formulations, and lipid-based carriers to enhance controlled release and joint retention.
Second, it is essential to compare different MSC sources and standardize therapy protocols. Bone marrow-derived MSCs (BM-MSCs), adipose-derived MSCs (AD-MSCs), and umbilical cord MSCs (UC-MSCs) exhibit varying proliferation rates, immunomodulatory capacities, and chondrogenic potential. Direct comparisons under identical conditions will help determine the most effective MSC type for OA treatment.
Third, conducting large-scale, randomized controlled trials (RCTs) is necessary to confirm the safety, efficacy, and long-term benefits of MSC–natural product therapies. Current clinical trials are often small-scale (≤50 patients) and lack long-term follow-up data. Future multi-center RCTs with larger patient cohorts (≥200 patients) will provide more robust evidence.
Fourth, establishing mechanism-based biomarkers for patient selection will improve treatment outcomes. As not all OA patients respond equally to MSC therapy, incorporating biomarkers such as inflammatory cytokine profiles and genetic markers can help identify patients who will benefit most from MSC–natural product therapy.
Lastly, addressing regulatory and manufacturing challenges is crucial for clinical translation. Standardizing protocols for MSC isolation, expansion, and combination with natural products will ensure reproducibility and facilitate regulatory approval.
8.2. Clinical Translation: Roadmap for the Next 5–10 Years
Given the advancements in stem cell therapy, biomaterial-based drug delivery, and regenerative medicine, the clinical integration of MSC and natural product therapies for OA is feasible within the next decade. A proposed roadmap for clinical translation includes three main phases:
- Preclinical refinement (0–2 years): This phase focuses on optimizing bioavailability solutions, comparing MSC sources, and conducting high-throughput screening to identify the most effective MSC–natural product combinations.
- Small-scale clinical trials (2–5 years): During this period, phase I/II clinical trials will assess safety, dosing, and preliminary efficacy. Simultaneously, standardized MSC culture and expansion protocols will be developed, and collaboration with regulatory agencies will define safety standards for combined therapies.
- Large-scale clinical adoption (5–10 years): The final phase involves conducting multi-center phase III trials, establishing personalized treatment strategies based on patient biomarkers, and achieving regulatory approval and commercialization of MSC–natural product combination therapy for OA.
8.3. The Future of MSC + Natural Product Therapy for OA
The integration of natural products into MSC-based regenerative therapies represents a paradigm shift in OA treatment. By combining MSCs’ regenerative potential with the pharmacological benefits of bioactive compounds, these therapies could offer a disease-modifying approach rather than just symptomatic relief. However, to transition from promising research to clinical reality, future studies must prioritize bioavailability optimization, MSC standardization, and large-scale clinical trials. With continued research and development, MSC + natural product therapies have the potential to revolutionize OA treatment, offering hope for millions of patients worldwide.
Author Contributions
H.H.A., R.B.H.I., A.B.S. and A.N. conceived and designed the manuscript. H.H.A. performed the literature search. All authors contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, private, or not-for-profit sectors.
Acknowledgments
The authors thank their colleagues for their valuable discussions and constructive feedback, which significantly enhanced the quality of this review. Furthermore, they acknowledge KPJ Healthcare University for providing essential resources and support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| MSCs | Mesenchymal Stem Cells |
| OA | Osteoarthritis |
| PRP | Platelet-Rich Plasma |
| EGCG | Epigallocatechin Gallate |
| COMP | Cartilage Oligomeric Matrix Protein |
| C2C | Collagen Type II Cleavage Products |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-17 | Interleukin-17 |
| TNF-α | Tumor Necrosis Factor-Alpha |
| miRNAs | MicroRNAs |
| MMPs | Matrix Metalloproteinases |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| MAPK | Mitogen-Activated Protein Kinase |
| ROS | Reactive Oxygen Species |
| NO | Nitric Oxide |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| Wnt | Wingless-Related Integration Site |
| Notch | Notch Gene |
| TGF-β | Transforming Growth Factor-Beta |
| TLR | Toll-Like Receptor |
| PTOA | Post-Traumatic Osteoarthritis |
| CaMKK2 | Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 |
| SIRT1 | Sirtuin (Silent Mating Type Information Regulation 2 Homolog) 1 |
| COX-2 | Cyclooxygenase-2 |
| PI3K/AKT | Phosphatidylinositol 3-Kinase/Protein Kinase B (Also Known as Akt) |
| ROCK1 | Rho-Associated, Coiled-Coil-Containing Protein Kinase 1 |
| TLR9 | Toll-Like Receptor 9 |
| mTOR | Mammalian Target of Rapamycin |
| ADMSCs | Adipose-Derived MSCs |
| sEV-CUR | Curcumin-Loaded Small Extracellular Vesicles |
References
- Li, Y.; Xie, W.; Xiao, W.; Dou, D. Progress in Osteoarthritis Research by the National Natural Science Foundation of China. Bone Res. 2022, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- ur Rehman, S.; Iqbal, S.; Umair Shahid, M.; Soman Jahangir, M.; Latif Malik, A. Cartilage: Structure, Function, and the Pathogenesis of Osteoarthritis. In Advancements in Synovial Joint Science—Structure, Function, and Beyond; IntechOpen: London, UK, 2024. [Google Scholar]
- Muthu, S.; Korpershoek, J.V.; Novais, E.J.; Tawy, G.F.; Hollander, A.P.; Martin, I. Failure of Cartilage Regeneration: Emerging Hypotheses and Related Therapeutic Strategies. Nat. Rev. Rheumatol. 2023, 19, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Nurul, A.A.; Azlan, M.; Ahmad Mohd Zain, M.R.; Sebastian, A.A.; Fan, Y.Z.; Fauzi, M.B. Mesenchymal Stem Cells: Current Concepts in the Management of Inflammation in Osteoarthritis. Biomedicines 2021, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Vadhan, A.; Gupta, T.; Hsu, W.L. Mesenchymal Stem Cell-Derived Exosomes as a Treatment Option for Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 9149. [Google Scholar] [CrossRef]
- Česnik, A.B.; Švajger, U. The Issue of Heterogeneity of MSC-Based Advanced Therapy Medicinal Products–a Review. Front. Cell Dev. Biol. 2024, 12, 1400347. [Google Scholar] [CrossRef]
- Marchese, P. Development of a High Throughput Screening Strategy for Drug Discovery: From Marine Fungi to Stem Cell Applications. Ph.D. Thesis, National University of Ireland Galway, Galway, Ireland, 2021. [Google Scholar]
- Cutolo, E.A.; Caferri, R.; Campitiello, R.; Cutolo, M. The Clinical Promise of Microalgae in Rheumatoid Arthritis: From Natural Compounds to Recombinant Therapeutics. Mar. Drugs 2023, 21, 630. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, L.; Jiang, K. Polysaccharide Nanosystems for Osteoarthritis Therapy: Mechanisms, Combinations, and Future Directions. Int. J. Biol. Macromol. 2024, 279, 135146. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, L.; Wei, B.; Ji, Y.; Xu, Y. Effects of Quercetin on Apoptosis and Extracellular Matrix Degradation of Chondrocytes Induced by Oxidative Stress-Mediated Pyroptosis. J. Biochem. Mol. Toxicol. 2022, 36, e22951. [Google Scholar] [CrossRef]
- Wang, Z.; Efferth, T.; Hua, X.; Zhang, X.A. Medicinal Plants and Their Secondary Metabolites in Alleviating Knee Osteoarthritis: A Systematic Review. Phytomedicine 2022, 105, 154347. [Google Scholar] [CrossRef]
- Østerås, N.; Bunzli, S. Introduction to OA, Communication, and Person-Centered Care. In Osteoarthritis Health Professional Training Manual; Academic Press: Cambridge, MA, USA, 2023; pp. 1–14. [Google Scholar] [CrossRef]
- Surmachevska, N.; Rubio, J. Senescence in Osteoarthritis: Overview of Mechanisms and Therapeutics. Eur. J. Rheumatol. 2023, 11, S3–S6. [Google Scholar] [CrossRef]
- Fiksman, F.; Krekhovska-Lepiavko, O.M.; Lokay, B.A. Osteoarthritis: Its prevalence, risk factors, clinical manifestation, diagnosis and treatment. Медсестринствo 2023, 4, 7–9. [Google Scholar] [CrossRef]
- Bueno, N.P.; Hertel, F.C.; Fernandes e Oliveira, H.F.; Arany, P.; Beloti, M.M.; Marques, M.M.; Ferraz, E.P. Enhancing Osteoblast Differentiation and Bone Repair: The Priming Effect of Photobiomodulation on Adipose Stromal Cells. J. Photochem. Photobiol. B Biol. 2024, 260, 113040. [Google Scholar] [CrossRef] [PubMed]
- Huaco, V.; Tawil, B. Osteoarthritis: Physiology, Disease, Treatments, Market Analysis. Adv. Tissue Eng. Regen. Med. Open Access 2023, 9, 24–27. [Google Scholar] [CrossRef]
- Tuncay Duruöz, M.; Öz, N.; Gürsoy, D.E.; Hande Gezer, H. Clinical Aspects and Outcomes in Osteoarthritis. Best. Pract. Res. Clin. Rheumatol. 2023, 37, 101855. [Google Scholar] [CrossRef] [PubMed]
- Rahimi Darehbagh, R.; Seyedoshohadaei, S.A.; Ramezani, R.; Rezaei, N. Stem Cell Therapies for Neurological Disorders: Current Progress, Challenges, and Future Perspectives. Eur. J. Med. Res. 2024, 29, 386. [Google Scholar] [CrossRef]
- Shaik, M.I.; Hamdi, I.H.; Sarbon, N.M. A Comprehensive Review on Traditional Herbal Drinks: Physicochemical, Phytochemicals and Pharmacology Properties. Food Chem. Adv. 2023, 3, 100460. [Google Scholar] [CrossRef]
- Cai, X.; Yuan, S.; Zeng, Y.; Wang, C.; Yu, N.; Ding, C. New Trends in Pharmacological Treatments for Osteoarthritis. Front. Pharmacol. 2021, 12, 645842. [Google Scholar] [CrossRef]
- Ashraf, I.; Khan, Q.U.; Malik, F.; Asghar, S.S.; Mansoor, A.; Mansoor, E. Unveiling the Pathophysiology of Osteoarthritis in Joint Anatomy. J. Health Rehabil. Res. 2024, 4, 1478–1483. [Google Scholar] [CrossRef]
- Verma, R.; Nath, R.; Dhadiwal, R.K.; Daftary, G.V.; Jolapara, M.A.; Shah, R.A.; Patil, N.N. Mechanisms of Action of Native Collagen Type II and Aflapin® on the Pathophysiology of Osteoarthritis and Their Evidences. Int. J. Res. Orthop. 2024, 10, 1098–1107. [Google Scholar] [CrossRef]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef]
- Gilbert, S.J.; Jones, R.; Egan, B.J.; Bonnet, C.S.; Evans, S.L.; Mason, D.J. Investigating Mechanical and Inflammatory Pathological Mechanisms in Osteoarthritis Using MSC-Derived Osteocyte-like Cells in 3D. Front. Endocrinol. 2024, 15, 1484912. [Google Scholar] [CrossRef] [PubMed]
- Szala, D.; Kopańska, M.; Trojniak, J.; Jabłoński, J.; Hanf-Osetek, D.; Snela, S.; Zawlik, I. The Role of MicroRNAs in the Pathophysiology of Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 6352. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.U.; Aslam, H.M.; Iftakhar, T.; Abdullah, M. Pathophysiology of Cartilage Damage in Knee Osteoarthritis and Regenerative Approaches toward Recovery. J. Bone Jt. Dis. 2024, 39, 32–44. [Google Scholar] [CrossRef]
- Chen, B.; Wang, L.; Xie, D.; Wang, Y. Exploration and Breakthrough in the Mode of Chondrocyte Death—A Potential New Mechanism for Osteoarthritis. Biomed. Pharmacother. 2024, 170, 115990. [Google Scholar] [CrossRef]
- Singh, A.; Rai, V.; Pandey, S.; Chavan, M.; Ketha, D. Osteoarthritis: Insights into Pathogenesis and Futuristic Treatment Strategies. Int. J. Res. Orthop. 2022, 8, 765–774. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, B. The Role of Inflammatory Mediators and Matrix Metalloproteinases (MMPs) in the Progression of Osteoarthritis. Biomater. Biosyst. 2024, 13, 100090. [Google Scholar] [CrossRef]
- Wang, M.N.; Liu, L.; Zhao, L.P.; Yuan, F.; Fu, Y.B.; Xu, X.B.; Li, B. Research of Inflammatory Factors and Signaling Pathways in Knee Osteoarthritis. China J. Orthop. Traumatol. 2020, 33, 388–392. [Google Scholar]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef]
- Wu, P.; Li, M.; Shi, X.; Jie, L.; Mao, J.; Yin, S. The Identification of Key Genes and Pathways in Osteoarthritis-Related Inflammatory Synoviocytes by Bioinformatics Analysis. Preprint 2022. [Google Scholar] [CrossRef]
- Okikiade, A.; Osharode, A.; Oyewole, A.; Ogunesan, D.; Oladejo, D.; Oshobu, I.; Browne, K. Understanding the Role of Inflammation in Secondary Osteoarthritis. Asian J. Med. Health 2022, 20, 60–74. [Google Scholar] [CrossRef]
- Lee, Y.T.; Yunus, M.H.M.; Ugusman, A.; Yazid, M.D. Natural Compounds Affecting Inflammatory Pathways of Osteoarthritis. Antioxidants 2022, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Rebouh, N.Y. Anti-Osteoarthritis Mechanism of the Nrf2 Signaling Pathway. Biomedicines 2023, 11, 3176. [Google Scholar] [CrossRef]
- Han, P.F.; Zhang, Z.L.; Chen, T.Y.; Zhao, R.P.; Zhang, R.; Li, X.D.; Li, P.C.; Wei, L.; Lv, Z.; Wei, X.C. Initial Effects of Inflammation-Related Cytokines and Signaling Pathways on the Pathogenesis of Post-Traumatic Osteoarthritis. Front. Nurs. 2018, 5, 91–96. [Google Scholar] [CrossRef]
- Riggs, K.C.; Sankar, U. Inflammatory Mechanisms in Post-Traumatic Osteoarthritis: A Role for CaMKK2. Immunometabolism 2023, 5, e00031. [Google Scholar] [CrossRef]
- Bar-Or, D.; Rael, L.T.; Thomas, G.W.; Brody, E.N. Inflammatory Pathways in Knee Osteoarthritis: Potential Targets for Treatment. Curr. Rheumatol. Rev. 2015, 11, 50–58. [Google Scholar] [CrossRef]
- Tian, R.; Su, S.; Yu, Y.; Liang, S.; Ma, C.; Jiao, Y.; Xing, W.; Tian, Z.; Jiang, T.; Wang, J. Revolutionizing Osteoarthritis Treatment: How Mesenchymal Stem Cells Hold the Key. Biomed. Pharmacother. 2024, 173, 116458. [Google Scholar] [CrossRef]
- Kwon, D.G.; Kim, M.K.; Jeon, Y.S.; Nam, Y.C.; Park, J.S.; Ryu, D.J. State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 1618. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Q.; Tam, P.K.H. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int. J. Mol. Sci. 2022, 23, 10023. [Google Scholar] [CrossRef]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.M.; Kameishi, S.; Grainger, D.W.; Okano, T. Strategies to Address Mesenchymal Stem/Stromal Cell Heterogeneity in Immunomodulatory Profiles to Improve Cell-Based Therapies. Acta Biomater. 2021, 133, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Ceruso, A.; Gonzalez-Pujana, A.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Latest Advances to Enhance the Therapeutic Potential of Mesenchymal Stromal Cells for the Treatment of Immune-Mediated Diseases. Drug Deliv. Transl. Res. 2021, 11, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Zhidu, S.; Ying, T.; Rui, J.; Chao, Z. Translational Potential of Mesenchymal Stem Cells in Regenerative Therapies for Human Diseases: Challenges and Opportunities. Stem Cell Res. Ther. 2024, 15, 266. [Google Scholar] [CrossRef]
- Pérez-Lozano, M.L.; Cesaro, A.; Mazor, M.; Esteve, E.; Berteina-Raboin, S.; Best, T.M.; Lespessailles, E.; Toumi, H. Emerging Natural-Product-Based Treatments for the Management of Osteoarthritis. Antioxidants 2021, 10, 265. [Google Scholar] [CrossRef]
- Wang, L.; He, C. Nrf2-Mediated Anti-Inflammatory Polarization of Macrophages as Therapeutic Targets for Osteoarthritis. Front. Immunol. 2022, 13, 967193. [Google Scholar] [CrossRef]
- Chopra, B.; Dhingra, A.K. Natural Products: A Lead for Drug Discovery and Development. Phytother. Res. 2021, 35, 4660–4702. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising Bioactive Compounds from the Marine Environment and Their Potential Effects on Various Diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Huynh, T.T.T.; Quang, M.T.; Vu, H.Y.T. Modulation of Inflammatory Signaling Pathways by Natural Products in Osteoarthritis: Mechanisms, Challenges, and Future Directions. Trop. J. Pharm. Res. 2024, 23, 1387–1396. [Google Scholar] [CrossRef]
- Wang, X.; He, W.; Huang, H.; Han, J.; Wang, R.; Li, H.; Long, Y.; Wang, G.; Han, X. Recent Advances in Hydrogel Technology in Delivering Mesenchymal Stem Cell for Osteoarthritis Therapy. Biomolecules 2024, 14, 858. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, Z.; Dong, L.; Zhang, D.; Shang, F.; Li, A.; Gao, Y.; Bai, Q.; Liu, D.; Xie, X.; et al. Natural Small Molecules Synergize Mesenchymal Stem Cells for Injury Repair in Vital Organs: A Comprehensive Review. Stem Cell Res. Ther. 2024, 15, 243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Long, Y.; Wang, Y.; Chin, K.-Y. Osteoarthritis: An Integrative Overview from Pathogenesis to Management. Malays. J. Pathol. 2024, 46, 369. [Google Scholar] [PubMed]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional Heterogeneity of Mesenchymal Stem Cells from Natural Niches to Culture Conditions: Implications for Further Clinical Uses. Cell. Mol. Life Sci. 2021, 78, 447–467. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, X.; Zhang, X. Targeting Different Phenotypes of Macrophages: A Potential Strategy for Natural Products to Treat Inflammatory Bone and Joint Diseases. Phytomedicine 2023, 118, 154952. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, P.; Rodríguez-Nogales, C.; Jordan, O.; Allémann, E. Combination of Mesenchymal Stem Cells and Bioactive Molecules in Hydrogels for Osteoarthritis Treatment. Eur. J. Pharm. Biopharm. 2022, 172, 41–52. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G. Experimental Therapeutics for the Treatment of Osteoarthritis. J. Exp. Pharmacol. 2021, 13, 101–125. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Duong, C.M.; Nguyen, X.H.; Than, U.T.T. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Osteoarthritis Treatment: Extracellular Matrix Protection, Chondrocyte and Osteocyte Physiology, Pain and Inflammation Management. Cells 2021, 10, 2887. [Google Scholar] [CrossRef]
- Koroljević, Z.D.; Jordan, K.; Ivković, J.; Bender, D.V.; Perić, P. Curcuma as an Anti-Inflammatory Component in Treating Osteoarthritis. Rheumatol. Int. 2023, 43, 589–616. [Google Scholar] [CrossRef]
- Yang, S.; Sun, M.; Zhang, X. Protective Effect of Resveratrol on Knee Osteoarthritis and Its Molecular Mechanisms: A Recent Review in Preclinical and Clinical Trials. Front. Pharmacol. 2022, 13, 921003. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Xu, W.; Li, Y.; Lai, R.; Qiu, X.; Chen, X.; Chen, Z.; Mi, B.; Wu, M.; et al. Translational Challenges and Prospective Solutions in the Implementation of Biomimetic Delivery Systems. Pharmaceutics 2023, 15, 2623. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.; Liao, W.Y.; Huang, C.W.; Chang, C.H. Adipose-Derived Stem Cells Preincubated with Green Tea EGCG Enhance Pancreatic Tissue Regeneration in Rats with Type 1 Diabetes through ROS/Sirt1 Signaling Regulation. Int. J. Mol. Sci. 2022, 23, 3165. [Google Scholar] [CrossRef] [PubMed]
- Burczak, A.; Kosiedowska, M.; Borkowska, P.; Kowalski, J. Cytoprotective Roles of Epigallocatechin Gallate and Resveratrol on Staurosporine-Treated Mesenchymal Stem Cells in In Vitro Culture. Herba Pol. 2021, 67, 45–52. [Google Scholar] [CrossRef]
- El-Desoky Mohamady, R.E.; Elwia, S.K.; Abo El Wafa, S.M.; Mohamed, M.A. Effect of Mesenchymal Stem Cells Derived Exosomes and Green Tea Polyphenols on Acetic Acid Induced Ulcerative Colitis in Adult Male Albino Rats. Ultrastruct. Pathol. 2022, 46, 147–163. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Y.; Bin, X.; Chan, K.P.; Chen, S.; Qian, Z.; Yao, Y.; Yuan, X.L.; Qiu, K.; Huang, Y.; et al. Combined Treatment of Human Mesenchymal Stem Cells and Green Tea Extract on Retinal Ganglion Cell Regeneration in Rats after Optic Nerve Injury. Exp. Eye Res. 2024, 239, 109787. [Google Scholar] [CrossRef]
- Xu, S.; Chang, L.; Zhao, X.; Hu, Y.; Lin, Y.; Chen, Z.; Ren, X.; Mei, X. Preparation of Epigallocatechin Gallate Decorated Au-Ag Nano-Heterostructures as NIR-Sensitive Nano-Enzymes for the Treatment of Osteoarthritis through Mitochondrial Repair and Cartilage Protection. Acta Biomater. 2022, 144, 168–182. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Sun, Y.; Li, M.; Chang, C.; Liu, W.; Zhu, X.; Wei, L.; Wen, F.; Liu, Y. Effects of Adipose Derived Stem Cells Pretreated with Resveratrol on Sciatic Nerve Regeneration in Rats. Sci. Rep. 2023, 13, 5812. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism—A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Y.; Pathak, J.L.; Hong, W.; Zeng, J.; Qian, D.; Hao, B.; Li, H.; Gu, J.; Jaspers, R.T.; et al. Quercetin Prevents Osteoarthritis Progression Possibly via Regulation of Local and Systemic Inflammatory Cascades. J. Cell Mol. Med. 2023, 27, 515–528. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, B.; Xiang, W.; Zheng, L.; Wang, X.; Li, S.; Zhang, T.; Feng, D.; Gong, Y.; Wu, J.; et al. Natural Products in Osteoarthritis Treatment: Bridging Basic Research to Clinical Applications. Chin. Med. 2024, 19, 25. [Google Scholar] [CrossRef]
- Han, X.; Jia, G.F.; Zhu, F. Resveratrol Alleviates Osteoporosis by Promoting Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells via SITR1/PI3K/AKT Pathway. Int. J. Morphol. 2024, 42, 216–224. [Google Scholar] [CrossRef]
- Ruan, H.; Zhu, T.; Wang, T.; Guo, Y.; Liu, Y.; Zheng, J. Quercetin Modulates Ferroptosis via the SIRT1/Nrf−2/HO−1 Pathway and Attenuates Cartilage Destruction in an Osteoarthritis Rat Model. Int. J. Mol. Sci. 2024, 25, 7461. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis and Adult Adipose-Derived Stem Cells. Pharmacol. Res. 2021, 172, 105803. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Song, Z.; Yu, J.; Ren, B.; Dong, Y.; You, Y.; Zhang, Z.; Jia, C.; Zhao, Y.; Zhou, X.; et al. Supramolecular Self-Assembly of EGCG-Selenomethionine Nanodrug for Treating Osteoarthritis. Bioact. Mater. 2024, 32, 164–176. [Google Scholar] [CrossRef]
- Razavi, B.M.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. A Review of Therapeutic Potentials of Turmeric (Curcuma longa) and Its Active Constituent, Curcumin, on Inflammatory Disorders, Pain, and Their Related Patents. Phytother. Res. 2021, 35, 6489–6513. [Google Scholar] [CrossRef]
- Memarzia, A.; Khazdair, M.R.; Behrouz, S.; Gholamnezhad, Z.; Jafarnezhad, M.; Saadat, S.; Boskabady, M.H. Experimental and Clinical Reports on Anti-Inflammatory, Antioxidant, and Immunomodulatory Effects of Curcuma Longa and Curcumin, an Updated and Comprehensive Review. BioFactors 2021, 47, 311–350. [Google Scholar] [CrossRef]
- Li, W.; Xiang, Z.; Yu, W.; Huang, X.; Jiang, Q.; Abumansour, A.; Yang, Y.; Chen, C. Natural Compounds and Mesenchymal Stem Cells: Implications for Inflammatory-Impaired Tissue Regeneration. Stem Cell Res. Ther. 2024, 15, 34. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Zhang, J.; Zhang, Y.; He, W.; Ju, J.; Wu, Y.; Wang, Y. The Effect of Resveratrol, Curcumin and Quercetin Combination on Immuno-Suppression of Tumor Microenvironment for Breast Tumor-Bearing Mice. Sci. Rep. 2023, 13, 13278. [Google Scholar] [CrossRef]
- Deng, J.; Ouyang, P.; Li, W.; Zhong, L.; Gu, C.; Shen, L.; Cao, S.; Yin, L.; Ren, Z.; Zuo, Z.; et al. Curcumin Alleviates the Senescence of Canine Bone Marrow Mesenchymal Stem Cells during in Vitro Expansion by Activating the Autophagy Pathway. Int. J. Mol. Sci. 2021, 22, 11356. [Google Scholar] [CrossRef]
- Xu, C.; Zhai, Z.; Ying, H.; Lu, L.; Zhang, J.; Zeng, Y. Curcumin Primed ADMSCs Derived Small Extracellular Vesicle Exert Enhanced Protective Effects on Osteoarthritis by Inhibiting Oxidative Stress and Chondrocyte Apoptosis. J. Nanobiotechnology 2022, 20, 123. [Google Scholar] [CrossRef]
- Ghorbaninejad, M.; Khademi-Shirvan, M.; Hosseini, S.; Meyfour, A.; Shahhoseini, M.; Baghaban Eslaminejad, M. Effective Role of Curcumin on Expression Regulation of EZH2 Histone Methyltransferase as a Dynamic Epigenetic Factor in Osteogenic Differentiation of Human Mesenchymal Stem Cells. Biochim. Biophys. Acta Gene Regul. Mech. 2023, 1866, 194903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Q.; Zou, Z.; Li, Z.; Jin, M.; An, J.; Li, H.; Ma, J. Curcumin Supplementation Enhances Bone Marrow Mesenchymal Stem Cells to Promote the Anabolism of Articular Chondrocytes and Cartilage Repair. Cell Transplant. 2021, 30, 0963689721993776. [Google Scholar] [CrossRef] [PubMed]
- Strecanska, M.; Danisovic, L.; Ziaran, S.; Cehakova, M. The Role of Extracellular Matrix and Hydrogels in Mesenchymal Stem Cell Chondrogenesis and Cartilage Regeneration. Life 2022, 12, 2066. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xu, H.; Qian, W. The Role and Current Research Status of Resveratrol in the Treatment of Osteoarthritis and Its Mechanisms: A Narrative Review. Drug Metab. Rev. 2024, 56, 399–412. [Google Scholar] [CrossRef]
- Aleebrahim-Dehkordi, E.; Soveyzi, F.; Arian, A.S.; Hamedanchi, N.F.; Hasanpour-Dehkordi, A.; Rafieian-Kopaei, M. Quercetin and Its Role in Reducing the Expression of Pro-Inflammatory Cytokines in Osteoarthritis. Antiinflamm Antiallergy Agents Med. Chem. 2022, 21, 153–165. [Google Scholar] [CrossRef]
- Yamaura, K.; Nelson, A.L.; Nishimura, H.; Rutledge, J.C.; Ravuri, S.K.; Bahney, C.; Philippon, M.J.; Huard, J. Therapeutic Potential of Senolytic Agent Quercetin in Osteoarthritis: A Systematic Review and Meta-Analysis of Preclinical Studies. Ageing Res. Rev. 2023, 90, 101989. [Google Scholar] [CrossRef]
- Hridayanka, K.S.N.; Duttaroy, A.K.; Basak, S. Bioactive Compounds and Their Chondroprotective Effects for Osteoarthritis Amelioration: A Focus on Nanotherapeutic Strategies, Epigenetic Modifications, and Gut Microbiota. Nutrients 2024, 16, 3587. [Google Scholar] [CrossRef]
- Li, W.; Huang, Y.; Fan, L.; Yangzom, D.; Zhang, K.; Shen, L.; Cao, S.; Gu, C.; Yu, S. Curcumin Liposomes Alleviate Senescence of Bone Marrow Mesenchymal Stem Cells by Activating Mitophagy. Sci. Rep. 2024, 14, 31291. [Google Scholar] [CrossRef]
- Xia, S.; Weng, T.; Jin, R.; Yang, M.; Yu, M.; Zhang, W.; Wang, X.; Han, C. Curcumin-Incorporated 3D Bioprinting Gelatin Methacryloyl Hydrogel Reduces Reactive Oxygen Species-Induced Adipose-Derived Stem Cell Apoptosis and Improves Implanting Survival in Diabetic Wounds. Burns Trauma. 2022, 10, tkac001. [Google Scholar] [CrossRef]
- Ma, C.Y.; Zhai, Y.; Li, C.T.; Liu, J.; Xu, X.; Chen, H.; Tse, H.F.; Lian, Q. Translating Mesenchymal Stem Cell and Their Exosome Research into GMP Compliant Advanced Therapy Products: Promises, Problems and Prospects. Med. Res. Rev. 2024, 44, 919–938. [Google Scholar] [CrossRef]
- Ilyas, S.; Lee, J.; Lee, D. Emerging Roles of Natural Compounds in Osteoporosis: Regulation, Molecular Mechanisms and Bone Regeneration. Pharmaceuticals 2024, 17, 984. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, A.; Giordani, C.; Bonacci, S.; Giuliani, A.; Ramini, D.; Matacchione, G.; Sabbatinelli, J.; Di Valerio, S.; Pacetti, D.; Procopio, A.D.; et al. Anti-Inflammatory Effects of Olive Leaf Extract and Its Bioactive Compounds Oleacin and Oleuropein-Aglycone on Senescent Endothelial and Small Airway Epithelial Cells. Antioxidants 2023, 12, 1509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Luo, Y.; Li, X.; Huang, G.; Chen, H.; Li, A.; Qin, S. The Role of Flavonoids in the Osteogenic Differentiation of Mesenchymal Stem Cells. Front. Pharmacol. 2022, 13, 849513. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Kanika; Jori, C.; Chaudhary, A.A.; Rudayni, H.A.; Rashid, S.; Khan, R. Resveratrol and Resveratrol Nano-Delivery Systems in the Treatment of Inflammatory Bowel Disease. J. Nutr. Biochem. 2022, 109, 109101. [Google Scholar] [CrossRef]
- Ghahremani, H.; Bahramzadeh, A.; Bolandnazar, K.; Emamgholipor, S.; Hosseini, H.; Meshkani, R. Resveratrol as a Potential Protective Compound against Metabolic Inflammation. Acta Biochim. Iran. 2023, 1, 50–64. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Moradi, S.Z.; Cao, H.; Khan, H.; Xiao, J. Effects of Polyphenols on Oxidative Stress, Inflammation, and Interconnected Pathways during Spinal Cord Injury. Oxid. Med. Cell Longev. 2022, 2022, 8100195. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.A. Dietary Polyphenols Suppress Chronic Inflammation by Modulation of Multiple Inflammation-Associated Cell Signaling Pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef]
- Khan, M.I.; Hossain, M.I.; Hossain, M.K.; Rubel, M.H.K.; Hossain, K.M.; Mahfuz, A.M.U.B.; Anik, M.I. Recent Progress in Nanostructured Smart Drug Delivery Systems for Cancer Therapy: A Review. ACS Appl. Bio Mater. 2022, 5, 971–1012. [Google Scholar] [CrossRef]
- Rahiman, N.; Markina, Y.V.; Kesharwani, P.; Johnston, T.P.; Sahebkar, A. Curcumin-Based Nanotechnology Approaches and Therapeutics in Restoration of Autoimmune Diseases. J. Control. Release 2022, 348, 264–286. [Google Scholar] [CrossRef]
- Gayathri, K.; Bhaskaran, M.; Selvam, C.; Thilagavathi, R. Nano Formulation Approaches for Curcumin Delivery—A Review. J. Drug Deliv. Sci. Technol. 2023, 82, 104326. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Afzal, S.; Zheng, Y.F.; Münch, G.; Li, C.G. Synergistic Protective Effect of Curcumin and Resveratrol against Oxidative Stress in Endothelial EAhy926 Cells. Evid.-Based Complement. Altern. Med. 2021, 2021, 2661025. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic Efficacy of Curcumin and Resveratrol against Cancer: Chemoprevention, Chemoprotection, Drug Synergism and Clinical Pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Ghaeini Hesarooeyeh, Z.; Basham, A.; Sheybani-Arani, M.H.; Abbaszadeh, M.; Salimi Asl, A.; Moghbeli, M.; Saburi, E. Effect of Resveratrol and Curcumin and the Potential Synergism on Hypertension: A Mini-Review of Human and Animal Model Studies. Phytother. Res. 2024, 38, 42–58. [Google Scholar] [CrossRef]
- Laszló, I.P.; Laszló, M.R.; Popescu, T.; Toma, V.; Ion, R.M.; Moldovan, R.; Filip, G.A.; Cainap, C.; Clichici, S.; Muresan, A. The Comparative Effects of Resveratrol and Curcumin in Combination with Photodynamic Therapy. Med. Pharm. Rep. 2022, 95, 165–178. [Google Scholar] [CrossRef]
- Ra, K.; Park, S.C.; Lee, B.C. Female Reproductive Aging and Oxidative Stress: Mesenchymal Stem Cell Conditioned Medium as a Promising Antioxidant. Int. J. Mol. Sci. 2023, 24, 5053. [Google Scholar] [CrossRef]
- Ma, Y.; Qian, Y.; Chen, Y.; Ruan, X.; Peng, X.; Sun, Y.; Zhang, J.; Luo, J.; Zhou, S.; Deng, C. Resveratrol Modulates the Inflammatory Response in HPDLSCs via the NRF2/HO-1 and NF-ΚB Pathways and Promotes Osteogenic Differentiation. J. Periodontal Res. 2024, 59, 162–173. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Si, H. Synergistic Anti-Inflammatory Effects and Mechanisms of the Combination of Resveratrol and Curcumin in Human Vascular Endothelial Cells and Rodent Aorta. J. Nutr. Biochem. 2022, 108, 109083. [Google Scholar] [CrossRef]
- Salehi, B.; Cruz-Martins, N.; Butnariu, M.; Sarac, I.; Bagiu, I.C.; Ezzat, S.M.; Wang, J.; Koay, A.; Sheridan, H.; Adetunji, C.O.; et al. Hesperetin’s Health Potential: Moving from Preclinical to Clinical Evidence and Bioavailability Issues, to Upcoming Strategies to Overcome Current Limitations. Crit. Rev. Food Sci. Nutr. 2022, 62, 4449–4464. [Google Scholar] [CrossRef]
- Harney, T.L.; Harney, T.L. Resveratrol and Curcumin: Extending the Frontier of Phytomedicine. In Medicinal Plants-Harnessing the Healing Power of Plants; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Deng, M.; Ma, Y.; Jin, Z.; Shen, C. Potential Role of Herbal Remedies on Mesenchymal Stem Cells: An Overview of New Therapeutic Strategies for Osteoporosis. J. Organoid Biosci. 2024, 2, 60–88. [Google Scholar] [CrossRef]
- Vikal, A.; Maurya, R.; Bhowmik, S.; Khare, S.; Raikwar, S.; Patel, P.; Das Kurmi, B. Resveratrol: A Comprehensive Review of Its Multifaceted Health Benefits, Mechanisms of Action, and Potential Therapeutic Applications in Chronic Disease. Pharmacol. Res. Nat. Prod. 2024, 3, 100047. [Google Scholar] [CrossRef]
- Jamshidi, V.; Halabian, R.; Saeedi, P.; Bagheri, H.; Ghoochani, B.F.N.M. Accelerating Synergistic Effects of Preconditioned Mesenchymal Stem Cells with Crocin and Dexamethasone in Pulmonary Epithelial Cells Injury. Toxicol. Res. 2023, 12, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Zhang, Y.; Chang, X.; Liu, C.; Wen, X.; Tian, F.; Li, Y. Curcumin Alleviates Osteoarthritis Through the P38MAPK Pathway: Network Pharmacological Prediction and Experimental Confirmation. J. Inflamm. Res. 2024, 17, 5039–5056. [Google Scholar] [CrossRef] [PubMed]
- Makuch, S.; Więcek, K.; Woźniak, M. The Immunomodulatory and Anti-Inflammatory Effect of Curcumin on Immune Cell Populations, Cytokines, and in Vivo Models of Rheumatoid Arthritis. Pharmaceuticals 2021, 14, 309. [Google Scholar] [CrossRef]
- Wei, Y.; Guo, A.; Liu, Z.; Zhang, L.; Liao, W.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Development of Curcumin Loaded Core-Shell Zein Microparticles Stabilized by Cellulose Nanocrystals and Whey Protein Microgels through Interparticle Interactions. Food Funct. 2021, 12, 6936–6949. [Google Scholar] [CrossRef]
- Sable, A.A.; Kunwar, A.; Barik, A. Alginate and Chitosan-Based Delivery Systems for Improving the Bioavailability and Therapeutic Efficacy of Curcumin. Pharmaceutics 2024, 16, 423. [Google Scholar] [CrossRef]
- Yao, J.; Liu, X.; Sun, Y.; Dong, X.; Liu, L.; Gu, H. Curcumin-Alleviated Osteoarthritic Progression in Rats Fed a High-Fat Diet by Inhibiting Apoptosis and Activating Autophagy via Modulation of MicroRNA-34a. J. Inflamm. Res. 2021, 14, 2317–2331. [Google Scholar] [CrossRef]
- Vassallo, V.; Di Meo, C.; Toro, G.; Alfano, A.; Iolascon, G.; Schiraldi, C. Hyaluronic Acid-Based Injective Medical Devices: In Vitro Characterization of Novel Formulations Containing Biofermentative Unsulfated Chondroitin or Extractive Sulfated One with Cyclodextrins. Pharmaceuticals 2023, 16, 1429. [Google Scholar] [CrossRef]
- Sheng, S.; Wang, X.; Liu, X.; Hu, X.; Shao, Y.; Wang, G.; Mao, D.; Li, C.; Chen, B.; Chen, X. The Role of Resveratrol on Rheumatoid Arthritis: From Bench to Bedside. Front. Pharmacol. 2022, 13, 829677. [Google Scholar] [CrossRef]
- Markov, A.; Thangavelu, L.; Aravindhan, S.; Zekiy, A.O.; Jarahian, M.; Chartrand, M.S.; Pathak, Y.; Marofi, F.; Shamlou, S.; Hassanzadeh, A. Mesenchymal Stem/Stromal Cells as a Valuable Source for the Treatment of Immune-Mediated Disorders. Stem Cell Res. Ther. 2021, 12, 192. [Google Scholar] [CrossRef]
- Liang, C.C.; Xing, H.; Wang, C.Y.; Xu, X.F.; Hao, Y.; Qiu, B. Resveratrol Protection against IL-1β-Induced Chondrocyte Damage via the SIRT1/FOXO1 Signaling Pathway. J. Orthop. Surg. Res. 2022, 17, 406. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Lei, M.Y.; Liu, Z.Q.; Liu, Z.F.; Ma, Z.; Liu, K.; Li, J.; Deng, Y.; Liu, W.; Xu, B. Resveratrol Attenuates Manganese-Induced Oxidative Stress and Neuroinflammation through SIRT1 Signaling in Mice. Food Chem. Toxicol. 2021, 153, 112283. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.G.; Best, T.M.; Huard, J.; Philippon, M.; Hornicek, F.; Duan, Z.; Griswold, A.J.; Kaplan, L.D.; Hare, J.M.; Kouroupis, D. Therapeutic Perspectives for Inflammation and Senescence in Osteoarthritis Using Mesenchymal Stem Cells, Mesenchymal Stem Cell-Derived Extracellular Vesicles and Senolytic Agents. Cells 2023, 12, 1421. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Lee, Y.H.; Bat-Ulzii, A.; Chatterjee, S.; Bhattacharya, M.; Chakraborty, C.; Lee, S.S. Bioactivity, Molecular Mechanism, and Targeted Delivery of Flavonoids for Bone Loss. Nutrients 2023, 15, 919. [Google Scholar] [CrossRef]
- Wang, N.; Wang, L.; Yang, J.; Wang, Z.; Cheng, L. Quercetin Promotes Osteogenic Differentiation and Antioxidant Responses of Mouse Bone Mesenchymal Stem Cells through Activation of the AMPK/SIRT1 Signaling Pathway. Phytother. Res. 2021, 35, 2639–2650. [Google Scholar] [CrossRef]
- Xing, X.; Tang, Q.; Zou, J.; Huang, H.; Yang, J.; Gao, X.; Xu, X.; Ma, S.; Li, M.; Liang, C.; et al. Bone-Targeted Delivery of Senolytics to Eliminate Senescent Cells Increases Bone Formation in Senile Osteoporosis. Acta Biomater. 2023, 157, 352–366. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Wen, L.; Ling, Z.; Xia, J.; Cheng, B.; Peng, J. Quercetin Ameliorates Senescence and Promotes Osteogenesis of BMSCs by Suppressing the Repetitive Element-triggered RNA Sensing Pathway. Int. J. Mol. Med. 2025, 55, 4. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Zhen, Y.; Guo, T.; Wang, C.; Shen, L.; Li, W. Quercetin Inhibits Cytotoxicity of PC12 Cells Induced by Amyloid-Beta 25-35 via Stimulating Estrogen Receptor α, Activating ERK1/2, and Inhibiting Apoptosis. Open Life Sci. 2022, 17, 230–242. [Google Scholar] [CrossRef]
- Goyal, A.; Agrawal, N. Quercetin: A Potential Candidate for the Treatment of Arthritis. Curr. Mol. Med. 2021, 22, 325–335. [Google Scholar] [CrossRef]
- Kim, J.G.; Sharma, A.R.; Lee, Y.-H.; Chatterjee, S.; Choi, Y.J.; Rajvansh, R.; Chakraborty, C.; Lee, S.-S. Therapeutic Potential of Quercetin as an Antioxidant for Bone-Muscle-Tendon Regeneration and Aging. Aging Dis. 2024, 16, 1–25. [Google Scholar] [CrossRef]
- Samadi, F.; Kahrizi, M.S.; Heydari, F.; Arefnezhad, R.; Roghani-Shahraki, H.; Mokhtari Ardekani, A.; Rezaei-Tazangi, F. Quercetin and Osteoarthritis: A Mechanistic Review on the Present Documents. Pharmacology 2022, 107, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Lin, W.; Ba, X.; Huang, Y.; Chen, Z.; Han, L.; Qin, K.; Huang, Y.; Tu, S. Quercetin-Mediated SIRT1 Activation Attenuates Collagen-Induced Mice Arthritis. J. Ethnopharmacol. 2021, 279, 114213. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, Y.; Fang, X.; Yang, J.; Chen, Z. Epigallocatechin-3-Gallate Promotes Osteo-/Odontogenic Differentiation of Stem Cells from the Apical Papilla through Activating the Bmp–Smad Signaling Pathway. Molecules 2021, 26, 1580. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, B.; Esmaeilizade, Z.; Omrani, M.D.; Ghaderian, S.M.H.; Rajabibazl, M.; Fazeli, Z. The Effect of Co-Treating Human Mesenchymal Stem Cells with Epigallocatechin Gallate and Hypoxia-Inducible Factor-1 on the Expression of RANKL/RANK/OPG Signaling Pathway, Osteogenesis, and Angiogenesis Genes. Regen. Eng. Transl. Med. 2022, 8, 117–124. [Google Scholar] [CrossRef]
- Komsa-Penkova, R.; Stoycheva, S.; Tonchev, P.; Stavreva, G.; Todinova, S.; Georgieva, G.; Yordanova, A.; Kyurkchiev, S.; Altankov, G. Morphological and Quantitative Evidence for Altered Mesenchymal Stem Cell Remodeling of Collagen in an Oxidative Environment—Peculiar Effect of Epigallocatechin-3-Gallate. Polymers 2022, 14, 3957. [Google Scholar] [CrossRef]
- Song, C.; Xu, S.; Chang, L.; Zhao, X.; Mei, X.; Ren, X.; Chen, Z. Preparation of EGCG Decorated, Injectable Extracellular Vesicles for Cartilage Repair in Rat Arthritis. Regen. Biomater. 2021, 8, rbab067. [Google Scholar] [CrossRef]
- Guo, X.; Ma, Y.; Min, Y.; Sun, J.; Shi, X.; Gao, G.; Sun, L.; Wang, J. Progress and Prospect of Technical and Regulatory Challenges on Tissue-Engineered Cartilage as Therapeutic Combination Product. Bioact. Mater. 2023, 20, 501–518. [Google Scholar] [CrossRef]
- Gambari, L.; Cellamare, A.; Grassi, F.; Grigolo, B.; Panciera, A.; Ruffilli, A.; Faldini, C.; Desando, G. Overview of Anti-Inflammatory and Anti-Nociceptive Effects of Polyphenols to Halt Osteoarthritis: From Preclinical Studies to New Clinical Insights. Int. J. Mol. Sci. 2022, 23, 15861. [Google Scholar] [CrossRef]
- Wei, F.; Lin, K.; Ruan, B.; Wang, C.; Yang, L.; Wang, H.; Wang, Y. Epigallocatechin Gallate Protects MC3T3-E1 Cells from Cadmium-Induced Apoptosis and Dysfunction via Modulating PI3K/AKT/MTOR and Nrf2/HO-1 Pathways. PeerJ 2024, 12, e17488. [Google Scholar] [CrossRef]
- Molnar, V.; Pavelić, E.; Vrdoljak, K.; Čemerin, M.; Klarić, E.; Matišić, V.; Bjelica, R.; Brlek, P.; Kovačić, I.; Tremolada, C.; et al. Mesenchymal Stem Cell Mechanisms of Action and Clinical Effects in Osteoarthritis: A Narrative Review. Genes 2022, 13, 949. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Ran, C.; Wang, W.; Piao, F.; Yang, J.; Tian, S.; Li, L.; Zhao, D. Immunoregulatory Paracrine Effect of Mesenchymal Stem Cells and Mechanism in the Treatment of Osteoarthritis. Front. Cell Dev. Biol. 2024, 12, 1411507. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Rim, Y.A.; Nam, Y.; Ju, J.H. Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front. Immunol. 2021, 12, 631291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wu, W.; Qu, X. Mesenchymal Stem Cells in Osteoarthritis Therapy: A Review. Am. J. Transl. Res. 2021, 13, 448. [Google Scholar] [PubMed]
- Carneiro, D.d.C.; Araújo, L.T.d.; Santos, G.C.; Damasceno, P.K.F.; Vieira, J.L.; Santos, R.R.d.; Barbosa, J.D.V.; Soares, M.B.P. Clinical Trials with Mesenchymal Stem Cell Therapies for Osteoarthritis: Challenges in the Regeneration of Articular Cartilage. Int. J. Mol. Sci. 2023, 24, 9939. [Google Scholar] [CrossRef]
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef]
- Zou, Z.; Li, H.; Yu, K.; Ma, K.; Wang, Q.; Tang, J.; Liu, G.; Lim, K.; Hooper, G.; Woodfield, T.; et al. The Potential Role of Synovial Cells in the Progression and Treatment of Osteoarthritis. Exploration 2023, 3, 20220132. [Google Scholar] [CrossRef]
- Xia, C.; Dai, Z.; Jin, Y.; Chen, P. Emerging Antioxidant Paradigm of Mesenchymal Stem Cell-Derived Exosome Therapy. Front. Endocrinol 2021, 12, 727272. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Aixirefu, A.; Chen, R.; Wang, H. Clinical Efficacy of Mesenchymal Stem Cells and Platelet-Rich Plasma in the Therapy of Osteoarthritis: A Meta-Analysis. Am. J. Transl. Res. 2024, 16, 4256. [Google Scholar] [CrossRef]
- Chalidis, B.; Givissis, P.; Papadopoulos, P.; Pitsilos, C. Molecular and Biologic Effects of Platelet-Rich Plasma (PRP) in Ligament and Tendon Healing and Regeneration: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 2744. [Google Scholar] [CrossRef]
- Popescu, M.N.; Iliescu, M.G.; Beiu, C.; Popa, L.G.; Mihai, M.M.; Berteanu, M.; Ionescu, A.M. Autologous Platelet-Rich Plasma Efficacy in the Field of Regenerative Medicine: Product and Quality Control. Biomed. Res. Int. 2021, 2021, 4672959. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Tierno, R.; Zalduendo, M.; Alkhraisat, M.H. The Effectiveness of Platelet-Rich Plasma as a Carrier of Stem Cells in Tissue Regeneration: A Systematic Review of Pre-Clinical Research. Cells Tissues Organs 2021, 210, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Copp, G.; Robb, K.P.; Viswanathan, S. Culture-Expanded Mesenchymal Stromal Cell Therapy: Does It Work in Knee Osteoarthritis? A Pathway to Clinical Success. Cell Mol. Immunol. 2023, 20, 626–650. [Google Scholar] [CrossRef] [PubMed]
- Calcat-i-Cervera, S.; Sanz-Nogués, C.; O’Brien, T. When Origin Matters: Properties of Mesenchymal Stromal Cells From Different Sources for Clinical Translation in Kidney Disease. Front. Med. 2021, 8, 728496. [Google Scholar] [CrossRef]
- Wilson, A.J.; Brown, N.; Rand, E.; Genever, P.G. Attitudes Towards Standardization of Mesenchymal Stromal Cells—A Qualitative Exploration of Expert Views. Stem Cells Transl. Med. 2023, 12, 745–757. [Google Scholar] [CrossRef]
- Fountzilas, E.; Tsimberidou, A.M.; Vo, H.H.; Kurzrock, R. Clinical Trial Design in the Era of Precision Medicine. Genome Med. 2022, 14, 101. [Google Scholar] [CrossRef]
- Mahmoudian, A.; Lohmander, L.S.; Mobasheri, A.; Englund, M.; Luyten, F.P. Early-Stage Symptomatic Osteoarthritis of the Knee—Time for Action. Nat. Rev. Rheumatol. 2021, 17, 621–632. [Google Scholar] [CrossRef]
- Gerami, M.H.; Khorram, R.; Rasoolzadegan, S.; Mardpour, S.; Nakhaei, P.; Hashemi, S.; Al-Naqeeb, B.Z.T.; Aminian, A.; Samimi, S. Emerging Role of Mesenchymal Stem/Stromal Cells (MSCs) and MSCs-Derived Exosomes in Bone- and Joint-Associated Musculoskeletal Disorders: A New Frontier. Eur. J. Med. Res. 2023, 28, 86. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Cao, X.; Qin, A.; Zhao, J. Current Applications of Adipose-Derived Mesenchymal Stem Cells in Bone Repair and Regeneration: A Review of Cell Experiments, Animal Models, and Clinical Trials. Front. Bioeng. Biotechnol. 2022, 10, 942128. [Google Scholar] [CrossRef]
- Ivanovski, S.; Han, P.; Peters, O.A.; Sanz, M.; Bartold, P.M. The Therapeutic Use of Dental Mesenchymal Stem Cells in Human Clinical Trials. J. Dent. Res. 2024, 103, 1173–1184. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-Derived Natural Products for Drug Discovery: Current Approaches and Prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Guo, Y.; Pu, F.; Yang, C.; Xiao, X.; Du, H.; He, J.; Lu, S. Opportunities and Challenges in Enhancing the Bioavailability and Bioactivity of Dietary Flavonoids: A Novel Delivery System Perspective. Food Chem. 2024, 430, 137115. [Google Scholar] [CrossRef] [PubMed]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted Drug Delivery Strategies for Precision Medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Sarker, M.M.R.; Afrin, S.; Richi, F.T.; Zhao, C.; Zhou, J.R.; Mohamed, I.N. Traditional Herbal Medicines, Bioactive Metabolites, and Plant Products Against COVID-19: Update on Clinical Trials and Mechanism of Actions. Front. Pharmacol. 2021, 12, 671498. [Google Scholar] [CrossRef]
- Maleitzke, T.; Hildebrandt, A.; Dietrich, T.; Appelt, J.; Jahn, D.; Otto, E.; Zocholl, D.; Baranowsky, A.; Duda, G.N.; Tsitsilonis, S.; et al. The Calcitonin Receptor Protects against Bone Loss and Excessive Inflammation in Collagen Antibody-Induced Arthritis. iScience 2022, 25, 103689. [Google Scholar] [CrossRef]
- Li, W.; Sun, K.; Hu, F.; Chen, L.; Zhang, X.; Wang, F.; Yan, B. Protective Effects of Natural Compounds against Oxidative Stress in Ischemic Diseases and Cancers via Activating the Nrf2 Signaling Pathway: A Mini Review. J. Biochem. Mol. Toxicol. 2021, 35, e22658. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 Signaling: An Important Molecular Mechanism of Herbal Medicine in the Treatment of Atherosclerosis via the Protection of Vascular Endothelial Cells from Oxidative Stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the Nrf2/Are Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef]
- Zhang, M.J.; Sun, W.W.; Yang, J.; Shi, D.D.; Dai, X.F.; Li, X.M. The Effect of Preventing Oxidative Stress and Its Mechanisms in the Extract from Sonchus Brachyotus DC. Based on the Nrf2-Keap1-ARE Signaling Pathway. Antioxidants 2023, 12, 1677. [Google Scholar] [CrossRef]
- Scuto, M.; Trovato Salinaro, A.; Caligiuri, I.; Ontario, M.L.; Greco, V.; Sciuto, N.; Crea, R.; Calabrese, E.J.; Rizzolio, F.; Canzonieri, V.; et al. Redox Modulation of Vitagenes via Plant Polyphenols and Vitamin D: Novel Insights for Chemoprevention and Therapeutic Interventions Based on Organoid Technology. Mech. Ageing Dev. 2021, 199, 111551. [Google Scholar] [CrossRef]
- Machado, I.F.; Miranda, R.G.; Dorta, D.J.; Rolo, A.P.; Palmeira, C.M. Targeting Oxidative Stress with Polyphenols to Fight Liver Diseases. Antioxidants 2023, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, C.; Malfa, G.A.; Tomasello, B.; Bianchi, S.; Acquaviva, R. Natural Compounds and Glutathione: Beyond Mere Antioxidants. Antioxidants 2023, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Tudino, V.; Ibba, R.; Carullo, G.; Artamonov, M.Y.; Pyatakovich, F.A.; Minenko, I.A. Synergistic Antioxidant Effects of Molecular Hydrogen and Cold Atmospheric Plasma in Enhancing Mesenchymal Stem Cell Therapy. Antioxidants 2024, 13, 1584. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Sanz, M.; Tornín, J.; Ginebra, M.P.; Canal, C. Cold Atmospheric Plasma: A New Strategy Based Primarily on Oxidative Stress for Osteosarcoma Therapy. J. Clin. Med. 2021, 10, 893. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Z.; Liu, L.; Xiao, Y. Natural Compounds Protect against the Pathogenesis of Osteoarthritis by Mediating the NRF2/ARE Signaling. Front. Pharmacol. 2023, 14, 1188215. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Ouyang, L.; Chen, W.; Zhang, L.; Cao, Y. Antioxidants Improve the Proliferation and Efficacy of HUC-MSCs against H2O2-Induced Senescence. Antioxidants 2023, 12, 1334. [Google Scholar] [CrossRef]
- Hasan, A.A.; Tatarskiy, V.; Kalinina, E. Synthetic Pathways and the Therapeutic Potential of Quercetin and Curcumin. Int. J. Mol. Sci. 2022, 23, 14413. [Google Scholar] [CrossRef]
- Sul, O.J.; Ra, S.W. Quercetin Prevents Lps-induced Oxidative Stress and Inflammation by Modulating Nox2/Ros/Nf-kb in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef]
- Zhang, C.; Weng, Y.; Wang, H.; Zhan, S.; Li, C.; Zheng, D.; Lin, Q. A Synergistic Effect of Triptolide and Curcumin on Rheumatoid Arthritis by Improving Cell Proliferation and Inducing Cell Apoptosis via Inhibition of the IL-17/NF-ΚB Signaling Pathway. Int. Immunopharmacol. 2024, 142, 112953. [Google Scholar] [CrossRef]
- Hong, S.; Dia, V.P.; Zhong, Q. Synergistic Anti-Inflammatory Activity of Apigenin and Curcumin Co-Encapsulated in Caseins Assessed with Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Int. J. Biol. Macromol. 2021, 193, 702–712. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential Mechanisms of Quercetin in Cancer Prevention: Focus on Cellular and Molecular Targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Rhman, M.A.; Devnarain, N.; Khan, R.; Owira, P.M.O. Synergism Potentiates Oxidative Antiproliferative Effects of Naringenin and Quercetin in MCF-7 Breast Cancer Cells. Nutrients 2022, 14, 3437. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.; Umar, S.M.; Dev J.R., A.; Mendiratta, M.; Prasad, C.P. In Vitro Anticancer Efficacy of a Polyphenolic Combination of Quercetin, Curcumin, and Berberine in Triple Negative Breast Cancer (TNBC) Cells. Phytomedicine Plus 2022, 2, 100265. [Google Scholar] [CrossRef]
- Chittasupho, C.; Manthaisong, A.; Okonogi, S.; Tadtong, S.; Samee, W. Effects of Quercetin and Curcumin Combination on Antibacterial, Antioxidant, In Vitro Wound Healing and Migration of Human Dermal Fibroblast Cells. Int. J. Mol. Sci. 2022, 23, 142. [Google Scholar] [CrossRef]
- Asnaashari, S.; Amjad, E.; Sokouti, B. Synergistic Effects of Flavonoids and Paclitaxel in Cancer Treatment: A Systematic Review. Cancer Cell Int. 2023, 23, 211. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Baer-Dubowska, W. Modulation of Nrf2 and NF-ΚB Signaling Pathways by Naturally Occurring Compounds in Relation to Cancer Prevention and Therapy. Are Combinations Better than Single Compounds? Int. J. Mol. Sci. 2021, 22, 8223. [Google Scholar] [CrossRef]
- Jena, A.B.; Dash, U.C.; Duttaroy, A.K. An in Silico Investigation on the Interactions of Curcumin and Epigallocatechin-3-Gallate with NLRP3 Inflammasome Complex. Biomed. Pharmacother. 2022, 156, 113890. [Google Scholar] [CrossRef]
- Supriyadi, R.; Koswara, M.I.A.; Soelaeman, M.A.; Huang, I. The Effect of Antioxidants Supplementation on Oxidative Stress and Proinflammatory Biomarkers in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1413–1426. [Google Scholar] [CrossRef]
- Li, J.; Zhao, P.; Tian, Y.; Li, K.; Zhang, L.; Guan, Q.; Mei, X.; Qin, Y. The Anti-Inflammatory Effect of a Combination of Five Compounds From Five Chinese Herbal Medicines Used in the Treatment of COPD. Front. Pharmacol. 2021, 12, 709702. [Google Scholar] [CrossRef]
- Tang, H.; Hosein, A.; Mattioli-Belmonte, M. Traditional Chinese Medicine and Orthopedic Biomaterials: Host of Opportunities from Herbal Extracts. Mater. Sci. Eng. C 2021, 120, 111760. [Google Scholar] [CrossRef]
- Kciuk, M.; Garg, A.; Rohilla, M.; Chaudhary, R.; Dhankhar, S.; Dhiman, S.; Bansal, S.; Saini, M.; Singh, T.G.; Chauhan, S.; et al. Therapeutic Potential of Plant-Derived Compounds and Plant Extracts in Rheumatoid Arthritis—Comprehensive Review. Antioxidants 2024, 13, 775. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wan, H.; Tong, X.; He, Y.; Yang, J.; Zhang, L.; Shao, C.; Ding, Z.; Wan, H.; Li, C. An Integrative Strategy for Discovery of Functional Compound Combination from Traditional Chinese Medicine: Danhong Injection as a Model. Biomed. Pharmacother. 2021, 138, 111451. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, R.; Yu, H.; Zheng, Q.; Chen, Y. Bioactive Herbal Extracts of Traditional Chinese Medicine Applied with the Biomaterials: For the Current Applications and Advances in the Musculoskeletal System. Front. Pharmacol. 2021, 12, 778041. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, Y.; Wang, J.; Yu, C.; Shen, W. Research Status and Molecular Mechanism of the Traditional Chinese Medicine and Antitumor Therapy Combined Strategy Based on Tumor Microenvironment. Front. Immunol. 2021, 11, 609705. [Google Scholar] [CrossRef]
- Matuszewska, K.; Pereira, M.; Petrik, D.; Lawler, J.; Petrik, J. Normalizing Tumor Vasculature to Reduce Hypoxia, Enhance Perfusion, and Optimize Therapy Uptake. Cancers 2021, 13, 4444. [Google Scholar] [CrossRef]
- Deng, S.; Cao, H.; Cui, X.; Fan, Y.; Wang, Q.; Zhang, X. Optimization of Exosome-Based Cell-Free Strategies to Enhance Endogenous Cell Functions in Tissue Regeneration. Acta Biomater. 2023, 171, 68–84. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, J.; Lu, T.; Han, W.; Jiao, K.; Li, H. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Bone-Related Diseases: Intercellular Communication Messengers and Therapeutic Engineering Protagonists. Int. J. Nanomedicine 2024, 19, 3233–3257. [Google Scholar] [CrossRef]
- Chu, C.H.; Lee, R.P.; Wu, W.T.; Chen, I.H.; Yeh, K.T.; Wang, C.C. Advancing Osteoarthritis Treatment: The Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes and Biomaterial Integration. Biomedicines 2024, 12, 2478. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, Y.; Ma, Y.; Ao, Y.; Hu, X.; Meng, Q. Engineering of MSC-Derived Exosomes: A Promising Cell-Free Therapy for Osteoarthritis. Membranes 2022, 12, 739. [Google Scholar] [CrossRef]
- Kumari, S.; Goyal, A.; Gürer, E.S.; Yapar, E.A.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive Loaded Novel Nano-Formulations for Targeted Drug Delivery and Their Therapeutic Potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.J.P.; Rath, S.N.; Kotikalapudi, N.; Venkatesan, V. Beneficial Effects of Secretome Derived from Mesenchymal Stem Cells with Stigmasterol to Negate IL-1β-Induced Inflammation in-Vitro Using Rat Chondrocytes—OA Management. Inflammopharmacology 2021, 29, 1701–1717. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Khalifa, A.M.; Mohamed, A.A.; Galhom, R.A.; Korayem, H.E.; Abd El-Fadeal, N.M.; Abd-Eltawab Tammam, A.; Khalifa, M.M.; Elserafy, O.S.; Abdel-Karim, R.I. Bone-Marrow-Derived Mesenchymal Stem Cells, Their Conditioned Media, and Olive Leaf Extract Protect against Cisplatin-Induced Toxicity by Alleviating Oxidative Stress, Inflammation, and Apoptosis in Rats. Toxics 2022, 10, 526. [Google Scholar] [CrossRef] [PubMed]
- Jiang, E.; Chen, X.; Bi, Y.; Pan, C.; Li, X.; Lan, X. Curcumin Inhibits Oxidative Stress and Apoptosis Induced by H2O2 in Bovine Adipose-Derived Stem Cells (BADSCs). Animals 2024, 14, 3421. [Google Scholar] [CrossRef]
- Gopalakrishna, R.; Oh, A.; Hou, L.; Lee, E.; Aguilar, J.; Li, A.; Mack, W.J. Flavonoid Quercetin and Its Glucuronide and Sulfate Conjugates Bind to 67-KDa Laminin Receptor and Prevent Neuronal Cell Death Induced by Serum Starvation. Biochem. Biophys. Res. Commun. 2023, 671, 116–123. [Google Scholar] [CrossRef]
- Liu, H.; Guan, H.; He, F.; Song, Y.; Li, F.; Sun-Waterhouse, D.; Li, D. Therapeutic Actions of Tea Phenolic Compounds against Oxidative Stress and Inflammation as Central Mediators in the Development and Progression of Health Problems: A Review Focusing on MicroRNA Regulation. Crit. Rev. Food Sci. Nutr. 2024, 64, 8414–8444. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499. [Google Scholar]
- Al-Azab, M.; Idiiatullina, E.; Safi, M.; Hezam, K. Enhancers of Mesenchymal Stem Cell Stemness and Therapeutic Potency. Biomed. Pharmacother. 2023, 162, 114356. [Google Scholar] [CrossRef]
- Zhang, K.; Du, X.; Gao, Y.; Liu, S.; Xu, Y. Mesenchymal Stem Cells for Treating Alzheimer’s Disease: Cell Therapy and Chemical Reagent Pretreatment. J. Alzheimers Dis. 2023, 93, 863–878. [Google Scholar] [CrossRef]
- Zhou, F.; Peterson, T.; Fan, Z.; Wang, S. The Commonly Used Stabilizers for Phytochemical-Based Nanoparticles: Stabilization Effects, Mechanisms, and Applications. Nutrients 2023, 15, 3881. [Google Scholar] [CrossRef]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Abou Chahla, M.N.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, H.; Hanafi-Bojd, M.Y.; Iranshahy, M.; Zarban, A.; Raissi, H. The Combination of Polyphenols and Phospholipids as an Efficient Platform for Delivery of Natural Products. Sci. Rep. 2023, 13, 2501. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Lv, Y.; Li, X.; Bao, H.; Cao, X.; Huang, J.; Zhang, Z. SOD-Functionalized Gold Nanoparticles as ROS Scavenger and CT Contrast Agent for Protection and Imaging Tracking of Mesenchymal Stem Cells in Idiopathic Pulmonary Fibrosis Treatment. Chem. Eng. J. 2023, 459, 141603. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, Z.; Li, Y.; Wu, R.; Jiang, J. Pretreatment of Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes with Quercetin Enhances the Healing of Diabetic Skin Wounds by Modulating Host-Microbiota Interactions. Int. J. Nanomedicine 2024, 19, 12557–12581. [Google Scholar] [CrossRef] [PubMed]
- Zawani, M.; Fauzi, M.B. Epigallocatechin Gallate: The Emerging Wound Healing Potential of Multifunctional Biomaterials for Future Precision Medicine Treatment Strategies. Polymers 2021, 13, 3656. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Samanta, A.; Saha, P.; Ghosh, S.; Sinha, D. The Potential of Epigallocatechin Gallate in Targeting Cancer Stem Cells: A Comprehensive Review. Curr. Med. Chem. 2024, 31, 5255–5280. [Google Scholar] [CrossRef]
- Moudgil, K.D.; Venkatesha, S.H. The Anti-Inflammatory and Immunomodulatory Activities of Natural Products to Control Autoimmune Inflammation. Int. J. Mol. Sci. 2023, 24, 95. [Google Scholar] [CrossRef]
- Las Heras, K.; Garcia-Orue, I.; Rancan, F.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Modulating the Immune System towards a Functional Chronic Wound Healing: A Biomaterials and Nanomedicine Perspective. Adv. Drug Deliv. Rev. 2024, 210, 115342. [Google Scholar] [CrossRef]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A Systematic Review of Natural Products for Skin Applications: Targeting Inflammation, Wound Healing, and Photo-Aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory Properties of Mesenchymal Stem Cells/Dental Stem Cells and Their Therapeutic Applications. Cell Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Immunomodulation and Regeneration: A next Generation Therapeutic Tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Hristova-Panusheva, K.; Xenodochidis, C.; Georgieva, M.; Krasteva, N. Nanoparticle-Mediated Drug Delivery Systems for Precision Targeting in Oncology. Pharmaceuticals 2024, 17, 677. [Google Scholar] [CrossRef] [PubMed]
- Swami, P.N.; Andriamifidy, H.F.; Haque, S.; Reed, T.; Khan, A.; Grande, D.A. Exosomes from the Synovial Microenvironment in Joint Homeostasis and Osteoarthritis. J. Cartil. Jt. Preserv. 2024, 4, 100220. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Zeng, Y.; Pu, W.; Mu, X.; Sun, K.; Peng, Y.; Shen, B. Exosomes Rewire the Cartilage Microenvironment in Osteoarthritis: From Intercellular Communication to Therapeutic Strategies. Int. J. Oral. Sci. 2022, 14, 40. [Google Scholar] [CrossRef]
- An, X.; Wang, J.; Xu, K.; Zhao, R.C.; Su, J. Perspectives on Osteoarthritis Treatment with Mesenchymal Stem Cells and Radix Achyranthis Bidentatae. Aging Dis. 2024, 15, 1029–1045. [Google Scholar]
- Arifka, M.; Wilar, G.; Elamin, K.M.; Wathoni, N. Polymeric Hydrogels as Mesenchymal Stem Cell Secretome Delivery System in Biomedical Applications. Polymers 2022, 14, 1218. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Song, J.; Yin, Z.; Zhou, F.; Shen, H.; Wang, G.; Su, J. Multifunctional Hydrogel-Based Engineered Extracellular Vesicles Delivery for Complicated Wound Healing. Theranostics 2024, 14, 4198–4217. [Google Scholar] [CrossRef]
- Heo, S.; Noh, M.; Kim, Y.; Park, S. Stem Cell-Laden Engineered Patch: Advances and Applications in Tissue Regeneration. ACS Appl. Bio Mater. 2025, 8, 62–87. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Liang, J.; Liu, P.; Yang, X.; Liu, L.; Zhang, Y.; Wang, Q.; Zhao, H. Biomaterial-Based Scaffolds in Promotion of Cartilage Regeneration: Recent Advances and Emerging Applications. J. Orthop. Transl. 2023, 41, 54–62. [Google Scholar] [CrossRef]
- Ma, M.; Zou, F.; Abudureheman, B.; Han, F.; Xu, G.; Xie, Y.J.; Qiao, K.; Peng, J.; Guan, Y.; Meng, H.; et al. Magnetic Microcarriers with Accurate Localization and Proliferation of Mesenchymal Stem Cell for Cartilage Defects Repairing. ACS Nano 2023, 17, 6373–6386. [Google Scholar] [CrossRef] [PubMed]
- Rohila, A.; Shukla, R. Recent Advancements in Microspheres Mediated Targeted Delivery for Therapeutic Interventions in Osteoarthritis. J. Microencapsul. 2024, 41, 434–455. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; He, C. The Role and Therapeutic Potential of MSC-Derived Exosomes in Osteoarthritis. Arch. Biochem. Biophys. 2021, 710, 109002. [Google Scholar] [CrossRef]
- Kim, K.I.I.; Lee, M.C.; Lee, J.H.; Moon, Y.W.; Lee, W.S.; Lee, H.J.; Hwang, S.C.; In, Y.; Shon, O.J.; Bae, K.C.; et al. Clinical Efficacy and Safety of the Intra-Articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells for Knee Osteoarthritis: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Sports Med. 2023, 51, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Chamberlain, M.; Wickham, J.; Shah, K.; Cicuttini, F.; Wang, Y.; Solterbeck, A.; Kenihan, L.; Kelly, L.A.; Castelluccio, R.; et al. Safety and Efficacy of an Allogeneic Adipose-Derived Mesenchymal Stem Cell Preparation in the Treatment of Knee Osteoarthritis: A Phase I/IIa Randomised Controlled Trial. Osteoarthr. Cartil. Open 2024, 6, 100500. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Costa, L.A.; Eiro, N. New Era of Mesenchymal Stem Cell-Based Medicine: Basis, Challenges and Prospects. Rev. Clínica Española 2023, 223, 619–628. [Google Scholar] [CrossRef]
- Galderisi, U.; Peluso, G.; Di Bernardo, G. Clinical Trials Based on Mesenchymal Stromal Cells Are Exponentially Increasing: Where Are We in Recent Years? Stem Cell Rev. Rep. 2022, 18, 23–36. [Google Scholar] [CrossRef]
- Joshi, S.; Allabun, S.; Ojo, S.; Alqahtani, M.S.; Shukla, P.K.; Abbas, M.; Wechtaisong, C.; Almohiy, H.M. Enhanced Drug Delivery System Using Mesenchymal Stem Cells and Membrane-Coated Nanoparticles. Molecules 2023, 28, 2130. [Google Scholar] [CrossRef]
- Ghazal, M.M.; Zakiyyah, Z.M.M.; Ahmad Mohd Zain, M.R.; Ismail, S. A Narrative Review on Advances in Knee Osteoarthritis Treatment: Current Status and Emerging Trends. Niger. Health J. 2019, 25, 16–30. [Google Scholar] [CrossRef]
- Freitag, J.; Bates, D.; Wickham , J.; Shah, K.; Huguenin , L.; Tenen , A. Adipose-Derived Mesenchymal Stem Cell Therapy in the Treatment of Knee Osteoarthritis: A Randomized Controlled Trial. Regenerative Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).