Abstract
Natural products play a pivotal role in human health by exerting bioactive effects, including the modulation of the gut microbiome. Cacao, a widely consumed natural product, is rich in polyphenols and dietary fiber, which may influence microbial composition and metabolic functions. However, its effects on the gut microbiota remain poorly understood, particularly regarding inter-individual differences. This study investigated the impact of cacao on gut microbiota using an in vitro fecal incubation model with samples from healthy Korean adults. Our findings classified the gut microbiota of Korean individuals into two distinct enterotypes: Bacteroides and Prevotella. In the Bacteroides enterotype, cacao treatment significantly increased the relative abundance of beneficial bacterial genera, including Roseburia, Lachnospiraceae NK4A136, Faecalibacterium, and Agathobacter. Conversely, in the Prevotella enterotype, cacao treatment was associated with an increase in the relative abundance of Prevotella; however, the small sample size and community shifts during incubation limited the robustness of this observation. Functional predictions based on KEGG pathways further revealed enterotype-specific differences. In the Bacteroides enterotype, the cacao-treated group exhibited enhanced pathways associated with starch, sucrose, galactose, and thiamine metabolism, which was not observed in the Prevotella enterotype. These findings suggest a potential role for cacao as a gut microbiome modulator, highlighting its possible utility in microbiome-targeted dietary interventions and therapeutic strategies.
1. Introduction
Cacao (Theobroma cacao) and its products are consumed globally for their nutritional and sensory properties [1]. Cacao is a rich source of proteins, lipids, minerals, starch, and pentosans [2] and is abundant in bioactive compounds, particularly polyphenols, which contribute to its antioxidant activity [2,3]. Numerous studies have highlighted the health benefits of cacao consumption, including reduced risks of cancer, cardiovascular diseases, and inflammatory conditions, as well as modulatory effects on the immune, nervous, and gastrointestinal systems [1]. In addition to these systemic effects, cacao has been shown to influence gut microbiota composition. Some studies have suggested that cacao consumption can selectively enhance or inhibit microbial populations [4,5,6]. For instance, fecal samples from cacao-fed rats demonstrated a reduction in Bacteroides, Clostridium, and Staphylococcus populations [4]. Similarly, a cacao-enriched diet in rats led to decreased Firmicutes and Proteobacteria populations, alongside increased Tenericutes and Cyanobacteria [5]. While these findings highlight the potential role of cacao in modulating gut microbiota, most studies have been limited to animal models or investigations of specific cacao components in human trials [4,5,6,7]. However, direct investigations into the effects of whole cacao consumption on the human gut microbiota remain scarce, highlighting the need for further research.
The human gut microbiota plays a crucial role in health, contributing to immune regulation, nutrient metabolism, and defense against pathogens [8]. Based on microbial composition, the gut microbiota is classified into three distinct enterotypes characterized by the predominance of Bacteroides, Prevotella, or Ruminococcus [9]. Among these, Bacteroides and Prevotella enterotypes are the most prevalent worldwide, as shown by fecal microbiota analyses of healthy individuals across five continents [10]. Diet is a key determinant of enterotypes. A protein- and animal-fat-rich diet is associated with the Bacteroides enterotype, whereas a carbohydrate- and fiber-rich diet favors the Prevotella enterotype [11,12,13]. Given the distinct metabolic capabilities and dietary responses of each enterotype [14], it is essential to evaluate the effects of food in an enterotype-specific context. However, no study has investigated the impact of cacao consumption on the gut microbiota based on enterotype classification, highlighting a critical gap in the literature.
In vitro models that simulate the human gastrointestinal environment are widely used to study the gut microbiota, providing cost-effective, rapid, and high-throughput alternatives to in vivo models [15,16]. These models have been successfully employed to compare short-chain fatty acid production in response to various dietary fibers [17]. In this study, we investigated the impact of cacao on gut microbiota using an in vitro fecal incubation model. Fecal samples from healthy Korean volunteers were analyzed to assess cacao-induced changes in the microbial composition, structure, and predicted metabolic function. Furthermore, we examined whether the microbiota response to cacao varied according to enterotype, providing insights into the potential for personalized dietary interventions tailored to gut microbiota profiles.
2. Materials and Methods
2.1. Participants Information
This study recruited 48 healthy Korean adults (25 male and 23 female participants) aged 20 to 40 years. Written informed consent was obtained from all participants prior to the sample collection. Data on participants’ age, sex, and body mass index (BMI) were recorded, along with self-reported medical histories, including recent antibiotic therapy (within the past six months) and any diagnosis of inflammatory bowel syndrome (IBS) or inflammatory bowel disease (IBD). The study protocol was approved by the Institutional Review Board (IRB) of Sejong University (IRB No. SJU-BR-E-2020-025).
2.2. Preparation of Cacao Products and In Vitro Fecal Incubation
Cacao powder (The Hershey Company, Hershey, PA, USA) was diluted to a 10% (v/v) solution in 1× phosphate-buffered saline (PBS), following a previously established method [18]. The in vitro fecal incubation model was prepared using a basal medium designed to simulate intestinal conditions, as previously described [19]. Fresh fecal samples (5–10 g per participant) were collected in sterile containers and processed within 30 min of their collection. The samples were diluted at a 1:3 ratio with basal medium, homogenized, and filtered through a sterile nylon mesh (985 µm) [18,19] under anaerobic conditions (5% CO2, 5% H2 5%, 90% N2). The homogenized fecal samples were adjusted to a final concentration of 2% (0.2 g/mL) and loaded into sterile 96-deep well plates. The diluted cacao solution was added to the fecal samples at a final concentration of 1% (0.1 g/mL). The fecal-cacao mixtures were incubated for 24 h at 37 °C with shaking at 150 rpm in an anaerobic chamber to allow fermentation. Following incubation, the samples were stored at −80 °C until further analysis.
2.3. 16S rRNA Gene Amplicon Sequencing
16S rRNA gene sequencing was performed according to the Earth Microbiome Project (EMP) protocols [20]. DNA extraction was performed using the DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Fecal samples were homogenized using a TissueLyser II (Qiagen Retsch GmbH, Hanover, Germany) at 30 Hz for 10 min to ensure efficient disruption of microbial cells. The V4 region of the bacterial 16S rRNA gene was amplified using 515F (AATGATACGGCGACCACCGAGATCTACACGCT) and 806R (CAAGCAGAAGACGGCATACGAGAT) primers with barcode sequence [21]. PCR mixtures consist of 10 μL of Gold hot-start Taq PCR master mix (Bioneer, Daejeon, Korea), 1 μL of each forward and reverse primer (5 μM), 1 μL of the template, and PCR-grade water (Sigma-Aldrich, St. Louis, MO, USA) to a final volume of 25 μL. The cycling conditions were as follows: initial denaturation step at 94 °C for 3 min, 35 cycles of denaturation at 94 °C for 45 s, annealing at 50 °C for 60 s, extension at 72 °C for 90 s, and final extension cycle for 10 min at 72 °C. The PCR products were quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA) and normalized to a uniform concentration. Sequencing was performed on an Illumina MiSeq platform using the 2 × 300 v3 Kit. Primer sequences were trimmed from raw reads, and any read pairs lacking either forward or reverse sequences were removed.
2.4. Gut Microbiota Data Analysis
The gut microbiota data were analyzed as described in previous studies [22,23]. Microbial diversity and community composition were analyzed using the QIIME 2 software pipeline (v. 2022.2) [24]. Demultiplexing and quality filtering were performed using the q2-demux plugin, and denoising was conducted using DADA2 [25]. Taxonomic classification was assigned based on SILVA (v.138). Alpha-diversity metrics (Faith’s Phylogenetic Diversity (PD), observed features, and Shannon entropy) were measured using the non-parametric Kruskal−Wallis test. Beta diversity (weighted and unweighted UniFrac) metrics were used to assess differences between groups via permutational multivariate analysis of variance (PERMANOVA) using q2-diversity [26]. Linear discriminant effect size (LEfSe) analysis was carried out to detect significant differences in bacterial composition (linear discrimination analysis (LDA) score > 3.0) [27]. For species-level prediction analysis, which enables the identification of specific amplicon sequence variants (ASVs) at the species level, the Basic Local Alignment Search Tool (BLAST; v2.12.0) was employed. The phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt2) was used based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) ortholog classification to predict the metabolic function of the metagenomes from the 16S rRNA gene dataset [28]. To identify gut microbiota enterotypes, baseline fecal samples were analyzed at the genus level. Clustering was conducted using the Partitioning Around Medoids (PAM) method based on Jensen–Shannon divergence (JSD) as the distance metric within the R environment [9]. The optimal number of clusters and cluster robustness were determined by evaluating the Calinski–Harabasz (CH) index and silhouette analysis [29].
2.5. Metabolite Profiling of Cacao Powder
Metabolites derived from cacao powder were analyzed using a Vanquish U-HPLC system (Thermo Fisher Scientific, Waltham, MA, USA) coupled to an Orbitrap Exploris 120 mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Cacao powder (10 mg) was extracted with 1 mL of 80% methanol by vortexing, followed by centrifugation at 4 °C and 13,000× g for 15 min. The resulting supernatant was analyzed by U-HPLC-MS/MS using a C18 column (ACQUITY UPLC BEH C18, 2.1 × 100 mm, 1.7 μm; Waters Corp., Milford, MA, USA) maintained at 45 °C. The mobile phase consisted of water (A) and acetonitrile (B), both containing 0.1% formic acid. Mass spectrometry was performed in the positive and negative ionization modes with a scan range of 55–800 m/z. Raw data were processed using Xcalibur (version 4.6) and Compound Discoverer (version 3.3). Blank samples were prepared using 80% methanol, and blank subtraction was performed to eliminate background signals. Metabolite identification was performed by matching m/z values and MS/MS spectra against the mzCloud (accessed in April 2025), ChemSpider (accessed in April 2025), and HMDB (version 5.0) databases.
3. Results and Discussion
3.1. Comparison of Gut Microbial Composition Between the Two Enterotypes
In vitro fermentation models are essential tools for investigating the effects of specific foods or food-derived components on the gut microbiota [30,31]. In this study, we utilized an in vitro fecal incubation model to evaluate the impact of cacao on gut microbiota.
Enterotypes, primarily dominated by the genera Bacteroides, Prevotella, and Ruminococcus, have been proposed as a way to differentiate individual gut microbiota [9], with Bacteroides and Prevotella enterotypes being the most frequently observed [32]. A previous study has also shown a correlation between dietary patterns and enterotypes [33], with food intake affecting gut microbiota composition in an enterotype-specific manner. However, to our knowledge, no study has reported how cacao consumption modulates gut microbial composition according to enterotype classification. In this study, we aimed to investigate the enterotype-dependent responses of the gut microbiota to cacao treatment. Microbiota information from fecal samples collected before incubation (0 h) was used for enterotyping, and post-incubation data (24 h) were analyzed to investigate the effect of cacao treatment on the gut microbiota composition.
Enterotype analysis of the initial fecal samples revealed two distinct groups corresponding to the Bacteroides (n = 39) and Prevotella (n = 9) enterotypes (Figure 1A and Table S1). The clustering accuracy was moderate, with a silhouette index value of 0.20 [34] (Figure 1B). This is consistent with previous findings that identified Bacteroides and Prevotella as the dominant enterotypes in the Korean population [12,33]. Two samples (Sub.17 and Sub.19) were excluded from further analysis due to inconsistent Prevotella ratios, which could hinder accurate classification (Table S1).
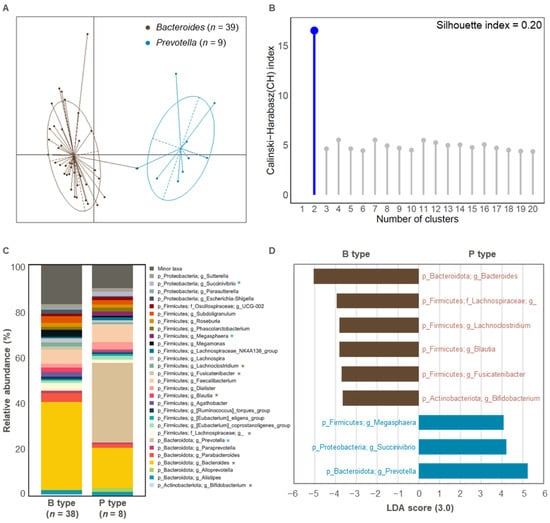
Figure 1.
The gut enterotype of initial fecal samples and microbial composition according to enterotype. (A) Enterotypes identified in 48 initial fecal samples. The clustering results were visualized using PCoA. (B) The optimal number of clusters was obtained using the CH and Silhouette indices (n = 48). (C) Bacterial taxonomy at the phylum and genus levels (top 30 bacterial taxonomies) of Bacteroides (n = 38) and Prevotella (n = 8) types. Significant differences are marked with an asterisk (*, p < 0.05). Significant increases in B type or P type are indicated in brown or blue, respectively. (D) Relative abundance of overrepresented bacterial taxa (LDA > 3.0) in Bacteroides (n = 38; brown) and Prevotella (n = 8; blue) types. B type, Bacteroides type; P type, Prevotella type.
At the genus level, we observed significant differences in microbial composition between the two enterotypes, as shown by the top 30 most abundant taxa (Figure 1C). The Bacteroides enterotype was predominantly characterized by Bacteroides, Lachnospiraceae, Lachnoclostridium, Blautia, Fusicatenibacter, and Bifidobacterium, whereas the Prevotella enterotype was dominated by Megasphaera, Succinivibrio, and Prevotella (LDA > 3.0 for bacterial features in proportions > 1%; Figure 1D).
3.2. Differences in Gut Microbiota According to Cacao Treatment
To determine the effect of cacao on the gut microbiota, we compared the non-treated (control) and cacao-treated (cacao) groups using enriched samples after 24 h of incubation. No statistically significant differences were observed in the alpha diversity metrics (Faith’s PD, observed features, and Shannon Entropy) between the control and cacao groups (Figure 2A–C). Similarly, no significant difference was observed in beta diversity based on unweighted UniFrac distances between the control and cacao groups (Figure 2D). However, beta diversity based on weighted intra-group distances was significantly higher in the cacao group than in the control group (Figure 2E).
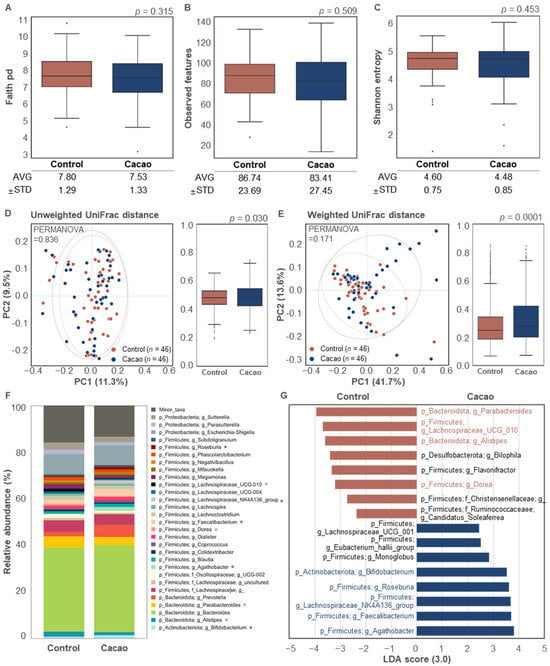
Figure 2.
Differences in bacterial structure in the gut microbiota according to cacao treatment (n = 46). (A–C) Comparison of alpha diversity indices between the control and cacao groups. Distribution of (A) Faith’s phylogenetic diversity (PD), (B) observed features, and (C) Shannon entropy was used to evaluate alpha diversity in the gut microbiota. (D,E) Comparison of beta diversity indices between the control and cacao groups. (D) Unweighted and (E) weighted UniFrac distances were used to evaluate beta diversity. (F) Bacterial taxonomy at the phylum and genus levels (top 30 bacterial taxonomies) in the two groups. Significant differences between the control and cacao groups are marked with an asterisk (*, p < 0.05). A significant increase in the control or cacao groups is indicated in brown or blue, respectively. (G) Relative abundance of overrepresented bacterial taxa (LDA > 3.0) in the control and cacao groups.
Microbial composition also differed significantly between the two groups (Figure 2F,G). In the control group, Parabacteroides, Lachnospiraceae UCG 010, Alistipes, and Dorea were overrepresented compared to the cacao group (LDA > 3.0 for bacterial features in proportions > 1%; Figure 2F,G). Previous studies have suggested that these genera may have both beneficial and pathogenic effects on human health. Parabacteroides, a Gram-negative bacterium found in the intestinal tract, has been linked to potential pathogenic effects on gut wall integrity [35]. Although Dorea is part of a healthy gut microbiota, it is also abundant in patients with inflammatory bowel diseases, such as Crohn’s disease [36]. Alistipes has been associated with both protective and harmful effects, depending on the disease context. While it has been linked to beneficial roles in some disorders, other studies suggest that it may contribute to colorectal cancer, mood disorders, and cardiovascular diseases [37,38,39].
In contrast, the cacao group showed a significant increase in the relative abundance of Bifidobacterium, Roseburia, Lachnospiraceae NK4A136 group, Faecalibacterium, and Agathobacter compared to the control group (LDA > 3.0 for bacterial features in proportions > 1%; Figure 2F,G). Bifidobacterium, a well-known probiotic, plays a crucial role in gastrointestinal health by alleviating symptoms such as constipation and diarrhea [40,41]. Previous studies have reported increased levels of Bifidobacterium in cacao- or its ingredient-treated groups compared to non-treated groups [42,43].
To better understand the components responsible for these effects, we conducted a metabolomic analysis of the cacao powder used in this study. The analysis revealed a wide range of bioactive compounds, including amino acids (e.g., valine, alanine, aspartic acid, glutamic acid, and arginine), short-chain fatty acid (SCFA) precursors (e.g., citric acid, fumaric acid, and malic acid), and various polyphenols and flavonoids, such as epicatechin, isoquercitrin, procyanidin B2, eriodictyol, and vanillin (Table S2).
Notably, the high polyphenol content of cacao has been shown to promote the growth of Bifidobacterium in the gut microbiota. For instance, polyphenols derived from pomegranates have been shown to specifically enhance the growth of Bifidobacterium spp. [44]. Similarly, wine polyphenols have been linked to increased bifidobacteria levels in rats [45]. Faecalibacterium, one of the most abundant and essential commensal bacteria in the human gut, supports host health by producing anti-inflammatory metabolites and contributing to energy metabolism [46]. Additionally, the abundance of SCFA-producing bacteria, including Roseburia, Lachnospiraceae, and Agathobacter, was higher in the cacao group than in the control group (Figure 2F,G). SCFAs play crucial roles in intestinal epithelial cell function, differentiation, and expansion of regulatory T (Treg) cells and exhibit potent anti-inflammatory properties [47,48].
Taken together, these findings suggest that cacao consumption may have beneficial effects on human gut health by promoting the growth of beneficial bacteria and reducing the abundance of potentially pathogenic bacteria in the gut microbiota.
3.3. Effect of Cacao on Gut Microbiota Diversity and Structure According to Enterotype
To determine the effect of cacao on gut microbial diversity according to enterotype, we compared the control and cacao groups for each enterotype. Bacterial alpha diversity did not differ significantly between the control and cacao groups in either of the enterotypes. Similarly, in the weighted UniFrac distance-based PCoA analysis, no distinct clustering pattern was observed between the control and cacao groups in the Bacteroides enterotype. In contrast, a clustering trend was observed for the Prevotella enterotype (p = 0.084) (Figure 3A,B). This trend was further supported by inter-group distance analysis, which showed significantly greater distances between groups in the Prevotella enterotype than in the Bacteroides enterotype (Figure 3C).
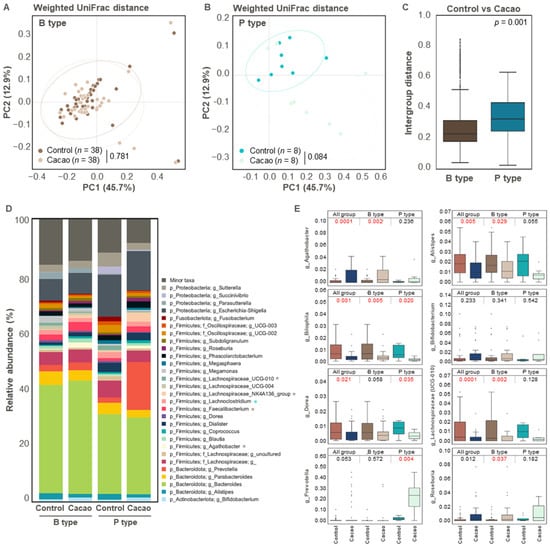
Figure 3.
Differences in the gut microbiota according to enterotype (n = 46). (A–C) Comparison of beta diversity indices between the control and cacao groups according to enterotype. Principal Coordinate Analysis (PCoA) plot based on weighted UniFrac distance of two enterotypes, (A) Bacteroides and (B) Prevotella types, and (C) intergroup distance were used to evaluate beta diversity in gut microbiota between the control and cacao groups. (D) Bacterial taxonomy at the phylum and genus levels (top 30 bacterial taxonomies) of the two groups in the Bacteroides and Prevotella types. Significant differences between the control and cacao groups are marked with an asterisk (*, p < 0.05). Significant changes in B type or P type are indicated in brown or blue, respectively. (E) Relative abundance of Agathobacter, Alistipes, Bilophila, Bifidobacterium, Dorea, Lachnospiraceae (UCG-010), Prevotella, and Roseburia in enriched fecal samples according to cacao treatment. B type, Bacteroides type; P type, Prevotella type.
We then compared the microbial composition between the control and cacao groups within each enterotype. In the Bacteroides enterotype, cacao treatment significantly increased the relative abundance of Lachnospiraceae NK4A136 group, Faecalibacterium, and Agathobacter, while it decreased that of Lachnospiraceae UCG 010 (Figure 3D,E). In the Prevotella enterotype, cacao treatment was associated with a reduction in the relative abundance of Lachnoclostridium, Bilophila, and Dorea and an increase in the relative abundance of Prevotella (Figure 3D,E and Figure S1). These results suggest that cacao contributes to the maintenance of Prevotella populations, particularly in Prevotella-dominant microbiota in our dataset. For future studies, incorporating shorter incubation periods (e.g., 6 or 12 h) or in vivo validation may provide a more accurate reflection of microbiota dynamics in response to cacao treatment while minimizing compositional shifts introduced by prolonged in vitro incubation.
Moreover, we further examined these taxa at the species level using the BLAST analysis of DADA2-derived ASVs, identifying Prevotella copri, a common Prevotella species in the human gut [49], as the most significantly increased species following cacao treatment in all fecal samples classified as the Prevotella enterotype (Figures S1 and S2). This finding suggests that P. copri is particularly responsive to cacao (Figure S2).
As mentioned earlier, a higher relative abundance of Bifidobacterium was observed in the cacao group than in the control group (Figure 2G). In particular, the relative abundance of Bifidobacterium species, including Bifidobacterium faecale and Bifidobacterium longum subsp. longum, and Bifidobacterium pseudocatenulatum, increased following cacao treatment (Figure S3); however, this increase was not statistically significant (Figure 3E). Enterotype-specific analysis revealed that B. faecalis increased in the Bacteroides enterotype, while both B. faecalis and B. longum subsp. longum were elevated in the Prevotella enterotype following cacao treatment (Figure S3). This trend suggests that cacao may promote the growth of beneficial Bifidobacterium species, which are known to support gut health. Further studies are required to confirm these effects in vivo and to clarify individual variability.
3.4. Predictive KEGG Functional Profiling According to Cacao Treatment
To analyze the functional profiles associated with cacao treatment, we used PICRUSt2 to predict the KEGG functional pathways. In the cacao group, a higher proportion of bacterial genes were associated with carbohydrate metabolism pathways, including starch, sucrose, and galactose metabolism, as well as thiamine and riboflavin metabolism. Conversely, a lower proportion of genes was observed for glycine/serine/threonine/tryptophan metabolism, valine/leucine/isoleucine degradation, tropane/piperidine/pyridine/alkaloid biosynthesis, propanoate/butanoate metabolism, methane metabolism, lipoic acid metabolism, taurine/hypotaurine metabolism, and aminobenzoate/nitrotoluene degradation (LDA > 2.0; Table 1) in the cacao group. Notably, Bifidobacterium, which was more abundant in the cacao group, can produce thiamine pyrophosphate (TPP) [50], which aligns with the observed overrepresentation of thiamine metabolism genes in the cacao group (Table 1).

Table 1.
Predictive KEGG functional profiling of the control and cacao groups.
KEGG functional profiling further revealed that in the Bacteroides enterotype, the cacao group exhibited a higher representation of genes related to starch, sucrose, and galactose metabolism, secondary and primary bile acid biosynthesis, and thiamine metabolism (LDA > 2.0, Table 1). In contrast, the control group of the Bacteroides enterotype showed a higher representation of genes associated with glycine/serine/threonine metabolism, valine/leucine/isoleucine degradation, tropane/piperidine/pyridine/alkaloid biosynthesis, propanoate/butanoate metabolism, lipoic acid metabolism, taurine/hypotaurine metabolism, and aminobenzoate degradation. Bacteroides species in the gut are known for their abundance of genes involved in carbohydrate-active enzymes and vitamin/cofactor metabolism [51], supporting these findings.
In the Prevotella enterotype, cacao treatment was associated with an increased representation of pantothenate/CoA biosynthesis and a decreased representation of methane metabolism (LDA > 2.0, Table 1). P. copri, a species that showed increased relative abundance following cacao treatment, possesses a biosynthesis pathway for vitamin B5 (pantothenate) [50], suggesting a potential link between its enrichment and increased production of vitamin B5. However, further studies, particularly those using metabolomics, are required to validate these predicted functional differences.
4. Conclusions
Our findings suggest that cacao can modulate the composition and functional profile of the human gut microbiota. Using an in vitro fecal incubation model with samples from 48 healthy Korean adults, we observed significant shifts in microbial community structure and predicted metabolic pathways following cacao treatment. These alterations included an increase in health-associated bacterial genera and enhancement of pathways related to carbohydrate and vitamin metabolism. While some variation in response appeared to be associated with enterotype, this observation should be interpreted with caution due to several limitations of this study. First, the number of individuals classified into the Prevotella enterotype was relatively small (n = 9), which may limit the generalizability of the findings related to this group. Second, the in vitro fecal incubation model, while useful for screening, does not fully recapitulate host-related factors, such as digestion, absorption, metabolism, and host–microbe immune interactions. In addition, a shift toward Bacteroides dominance was observed in most samples after 24 h of incubation, including those initially enriched with Prevotella, indicating that the in vitro system may not fully preserve the baseline microbial community structures. While this study used whole cacao powder in an in vitro fecal model, we acknowledge that digestion may alter its components in vivo. Further, in vivo studies are required to validate these results and gain deeper insights into the impact of cacao on the gut microbiome.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cimb47060414/s1.
Author Contributions
Conceptualization, H.S.; methodology, S.J. and H.S.; validation, J.K. (Jinshil Kim), G.K., B.S. and H.S.; formal analysis, S.J. and J.K. (Jinshil Kim) and J.K. (Jinwoo Kim); investigation, S.J. and J.K. (Jinshil Kim), G.K. and J.K. (Jinwoo Kim); resources, H.S.; data curation, S.J. and J.K. (Jinshil Kim), G.K. and J.K. (Jinwoo Kim); writing—original draft preparation, S.J., J.K. (Jinshil Kim) and J.K. (Jinwoo Kim); writing—review and editing, J.K. (Jinshil Kim), B.S. and H.S.; visualization, J.K. (Jinshil Kim), G.K. and B.S.; supervision, H.S.; project administration, H.S.; and funding acquisition, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the “National Institute of Health (NIH)” research project (project No. 2024ER211400) and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science and ICT (NRF-2022M3A9I5082349). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MIST) (RS-2024-00358007).
Institutional Review Board Statement
This study was approved by the Institutional Review Board (IRB) of Sejong University (IRB no. SJU-BR-E-2020-025). Participation was voluntary, and written informed consent was obtained from all the participants. The participants were Korean individuals who had not been prescribed antibiotics in the month prior to the experiment and were free of chronic conditions.
Informed Consent Statement
Informed consent was obtained from all the participants involved in the study.
Data Availability Statement
All amplicon sequence data and metadata have been made public through the EMP data portal (Qiita, https://qiita.ucsd.edu; study ID: 15919) accessed on 18 March 2025.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Andújar, I.; Recio, M.C.; Giner, R.M.; Ríos, J. Cocoa polyphenols and their potential benefits for human health. Oxidative Med. Cell. Longev. 2012, 2012, 906252. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Massot-Cladera, M.; Pérez-Cano, F.J.; Castell, M. Influence of a Cocoa-Enriched Diet on the Intestinal Immune System and Microbiota; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–225. [Google Scholar]
- Di Mattia, C.D.; Sacchetti, G.; Mastrocola, D.; Serafini, M. From cocoa to chocolate: The impact of processing on in vitro antioxidant activity and the effects of chocolate on antioxidant markers in vivo. Front. Immunol. 2017, 8, 1207. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Pérez-Berezo, T.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012, 527, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M. Gut microbiota in a rat oral sensitization model: Effect of a cocoa-enriched diet. Oxidative Med. Cell. Longev. 2017, 2017, 7417505. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Abril-Gil, M.; Torres, S.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Impact of cocoa polyphenol extracts on the immune system and microbiota in two strains of young rats. Br. J. Nutr. 2014, 112, 1944–1954. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Gorvitovskaia, A.; Holmes, S.P.; Huse, S.M. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome 2016, 4, 15. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Lim, M.Y.; Rho, M.; Song, Y.-M.; Lee, K.; Sung, J.; Ko, G. Stability of gut enterotypes in Korean monozygotic twins and their association with biomarkers and diet. Sci. Rep. 2014, 4, 7348. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, A.C.; Fernandes, G.R.; Da Silva, I.T.; Almeida-Pititto, B.; Gomes, E.P.; Pereira, A.d.C.; Ferreira, S.R. Enterotype may drive the dietary-associated cardiometabolic risk factors. Front. Cell. Infect. Microbiol. 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.; Roager, H.M.; Astrup, A.; Hjorth, M.F. Microbial enterotypes in personalized nutrition and obesity management. Am. J. Clin. Nutr. 2018, 108, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Sayar, S.; Jannink, J.-L.; White, P.J. Digestion residues of typical and high-β-glucan oat flours provide substrates for in vitro fermentation. J. Agric. Food Chem. 2007, 55, 5306–5311. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Pylkas, A.M.; Juneja, L.R.; Slavin, J.L. Comparison of different fibers for in vitro production of short chain fatty acids by intestinal microflora. J. Med. Food 2005, 8, 113–116. [Google Scholar] [CrossRef]
- Gamage, H.K.; Tetu, S.G.; Chong, R.W.; Ashton, J.; Packer, N.H.; Paulsen, I.T. Cereal products derived from wheat, sorghum, rice and oats alter the infant gut microbiota in vitro. Sci. Rep. 2017, 7, 14312. [Google Scholar] [CrossRef]
- Li, L.; Abou-Samra, E.; Ning, Z.; Zhang, X.; Mayne, J.; Wang, J.; Cheng, K.; Walker, K.; Stintzi, A.; Figeys, D. An in vitro model maintaining taxon-specific functional activities of the gut microbiome. Nat. Commun. 2019, 10, 4146. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Kim, G.; Kim, S.; Jung, H.; Kang, S.; Park, G.; Shin, H. The impact of makgeolli consumption on gut microbiota: An enterotype-based preliminary study. J. Microbiol. 2024, 62, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, S.; Lee, W.; Shin, H. The impact of coffee on gut microbial structure based on in vitro fecal incubation system. Food Sci. Biotechnol. 2024, 34, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Beiko, R.G. 16S rRNA Gene Analysis with QIIME2; Springer: Berlin/Heidelberg, Germany, 2018; pp. 113–129. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Calinski, T.; Harabasz, J. A dendrite method for cluster analysis. Commun. Stat. 1974, 3, 1–27. [Google Scholar] [CrossRef]
- Venema, K.; Van den Abbeele, P. Experimental models of the gut microbiome. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 115–126. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Ai, C.; Wen, C.; Dong, X.; Sun, X.; Cao, C.; Zhang, X.; Zhu, B.; Song, S. Gut microbiota response to sulfated sea cucumber polysaccharides in a differential manner using an in vitro fermentation model. Food Res. Int. 2021, 148, 110562. [Google Scholar] [CrossRef]
- Wu, G.D.; Bushmanc, F.D.; Lewis, J.D. Diet, the human gut microbiota, and IBD. Anaerobe 2013, 24, 117–120. [Google Scholar] [CrossRef]
- Wu, X.; Unno, T.; Kang, S.; Park, S. A Korean-style balanced diet has a potential connection with Ruminococcaceae enterotype and reduction of metabolic syndrome incidence in Korean adults. Nutrients 2021, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, L.; Rousseeuw, P.J. Finding Groups in Data: An Introduction to Cluster Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ezeji, J.C.; Sarikonda, D.K.; Hopperton, A.; Erkkila, H.L.; Cohen, D.E.; Martinez, S.P.; Cominelli, F.; Kuwahara, T.; Dichosa, A.E.; Good, C.E. Parabacteroides distasonis: Intriguing aerotolerant gut anaerobe with emerging antimicrobial resistance and pathogenic and probiotic roles in human health. Gut Microbes 2021, 13, 1922241. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.K.; Freedman, S.N.; Mangalam, A.K. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes 2017, 8, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Das, C.; Mande, S.S. In silico analysis of putrefaction pathways in bacteria and its implication in colorectal cancer. Front. Microbiol. 2017, 8, 2166. [Google Scholar] [CrossRef]
- Zuo, K.; Li, J.; Li, K.; Hu, C.; Gao, Y.; Chen, M.; Hu, R.; Liu, Y.; Chi, H.; Wang, H. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience 2019, 8, giz058. [Google Scholar] [CrossRef]
- Bangsgaard Bendtsen, K.M.; Krych, L.; Sørensen, D.B.; Pang, W.; Nielsen, D.S.; Josefsen, K.; Hansen, L.H.; Sørensen, S.J.; Hansen, A.K. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS ONE 2012, 7, e46231. [Google Scholar] [CrossRef]
- O’Callaghan, A.; Van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and their health-promoting effects. Microbiol. Spectr. 2017, 5, BAD-0010-2016. [Google Scholar] [CrossRef]
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F.; Arlorio, M.; Costabile, A.; Klinder, A.; Gibson, G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011, 55, S44–S55. [Google Scholar] [CrossRef]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Dolara, P.; Luceri, C.; De Filippo, C.; Femia, A.P.; Giovannelli, L.; Caderni, G.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2005, 591, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Halder, C.V.; de Sousa Faria, A.V.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Tett, A.; Huang, K.D.; Asnicar, F.; Fehlner-Peach, H.; Pasolli, E.; Karcher, N.; Armanini, F.; Manghi, P.; Bonham, K.; Zolfo, M. The Prevotella copri complex comprises four distinct clades underrepresented in westernized populations. Cell Host Microbe 2019, 26, 666–679.e7. [Google Scholar] [CrossRef]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Ussery, D.W.; Nielsen, J.; Nookaew, I. A closer look at bacteroides: Phylogenetic relationship and genomic implications of a life in the human gut. Microb. Ecol. 2011, 61, 473–485. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).