Nitric Oxide Regulates Multiple Signal Pathways in Plants via Protein S-Nitrosylation
Abstract
1. Nitric Oxide and S-Nitrosylation
2. Identification of S-Nitrosylated Proteins in Plants: Methodological Aspects
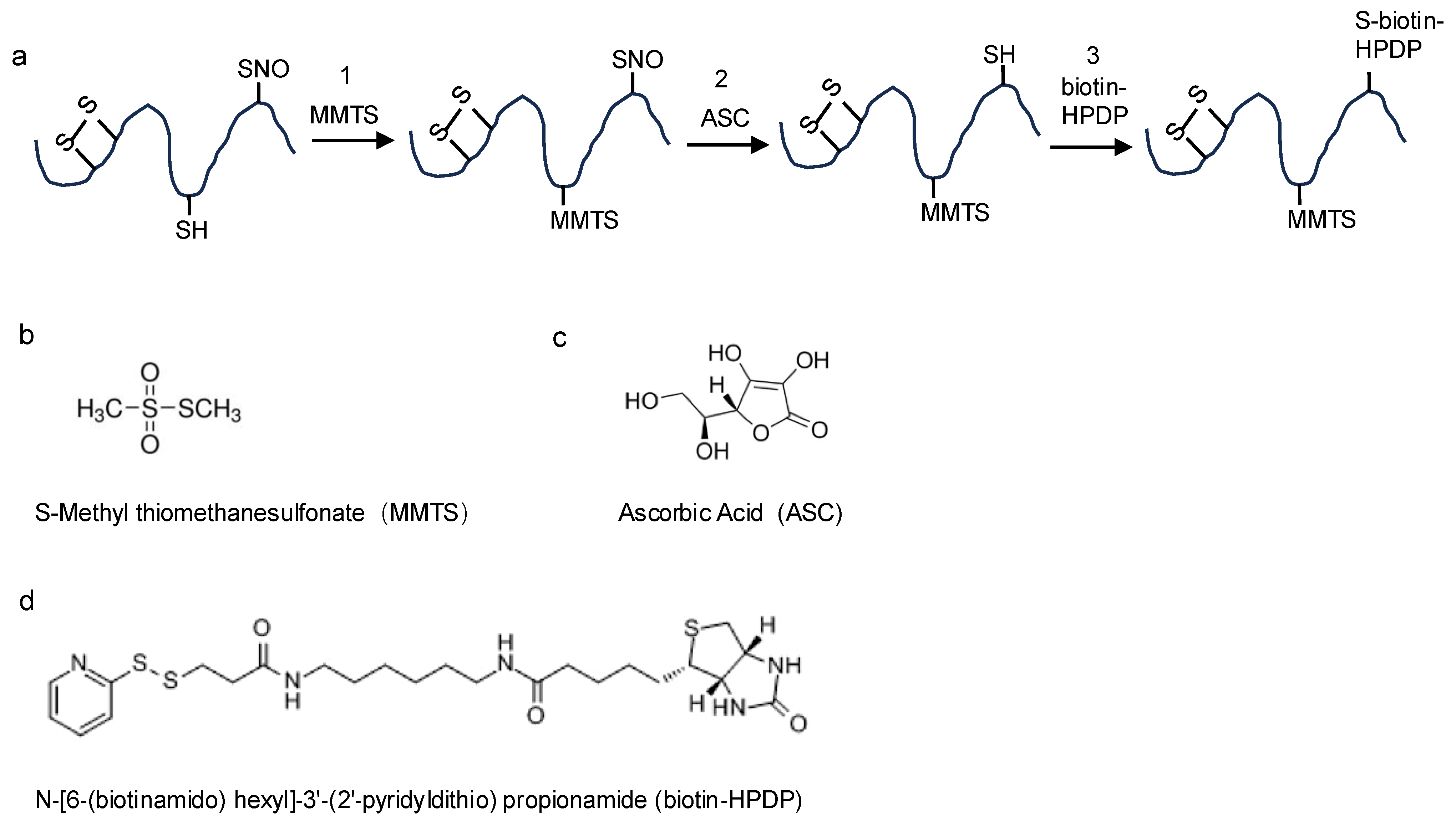
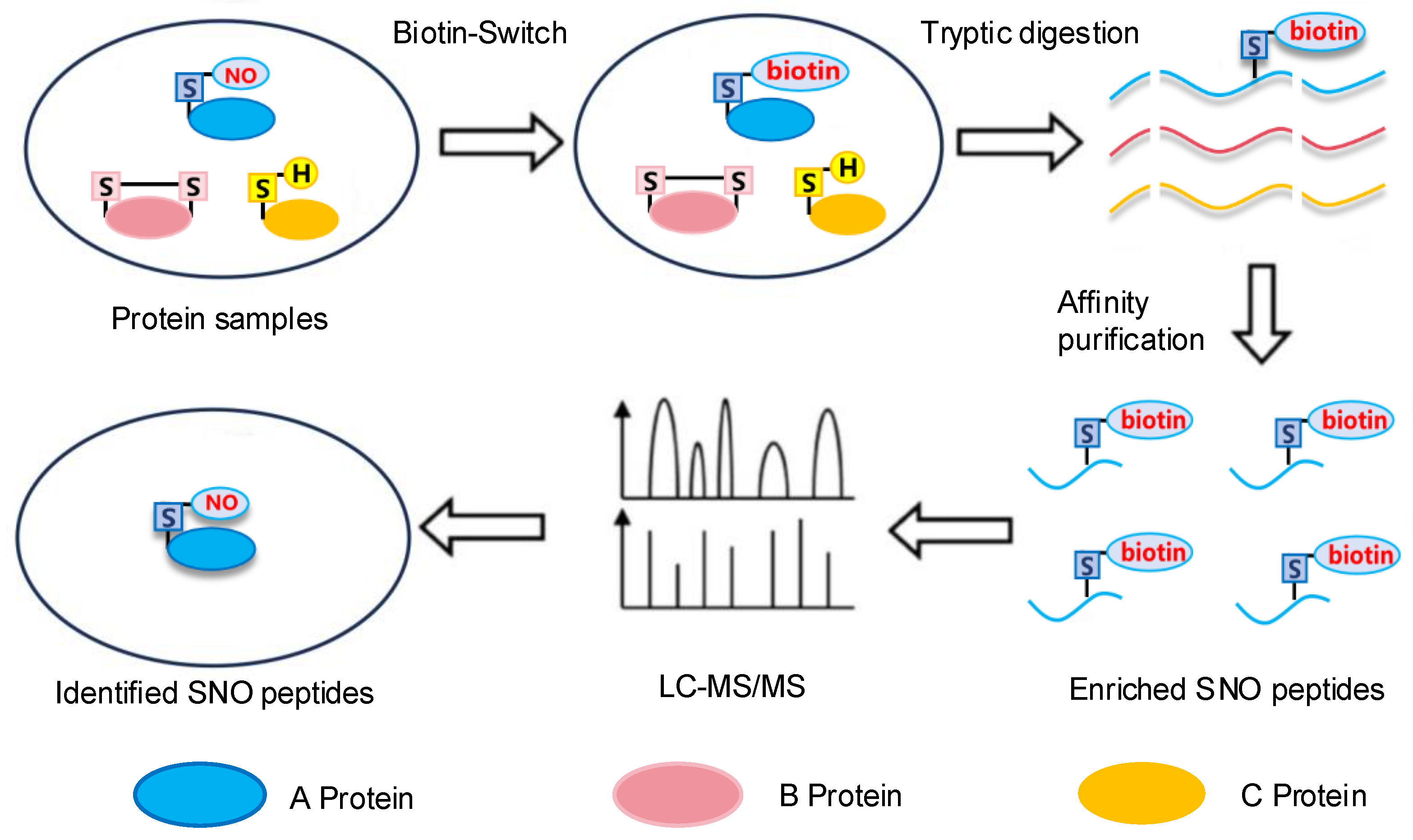
2.1. The Biotin-Switch Technique
2.2. Other Methods Used to Detect S-Nitrosopeptides
2.3. LC-MS/MS
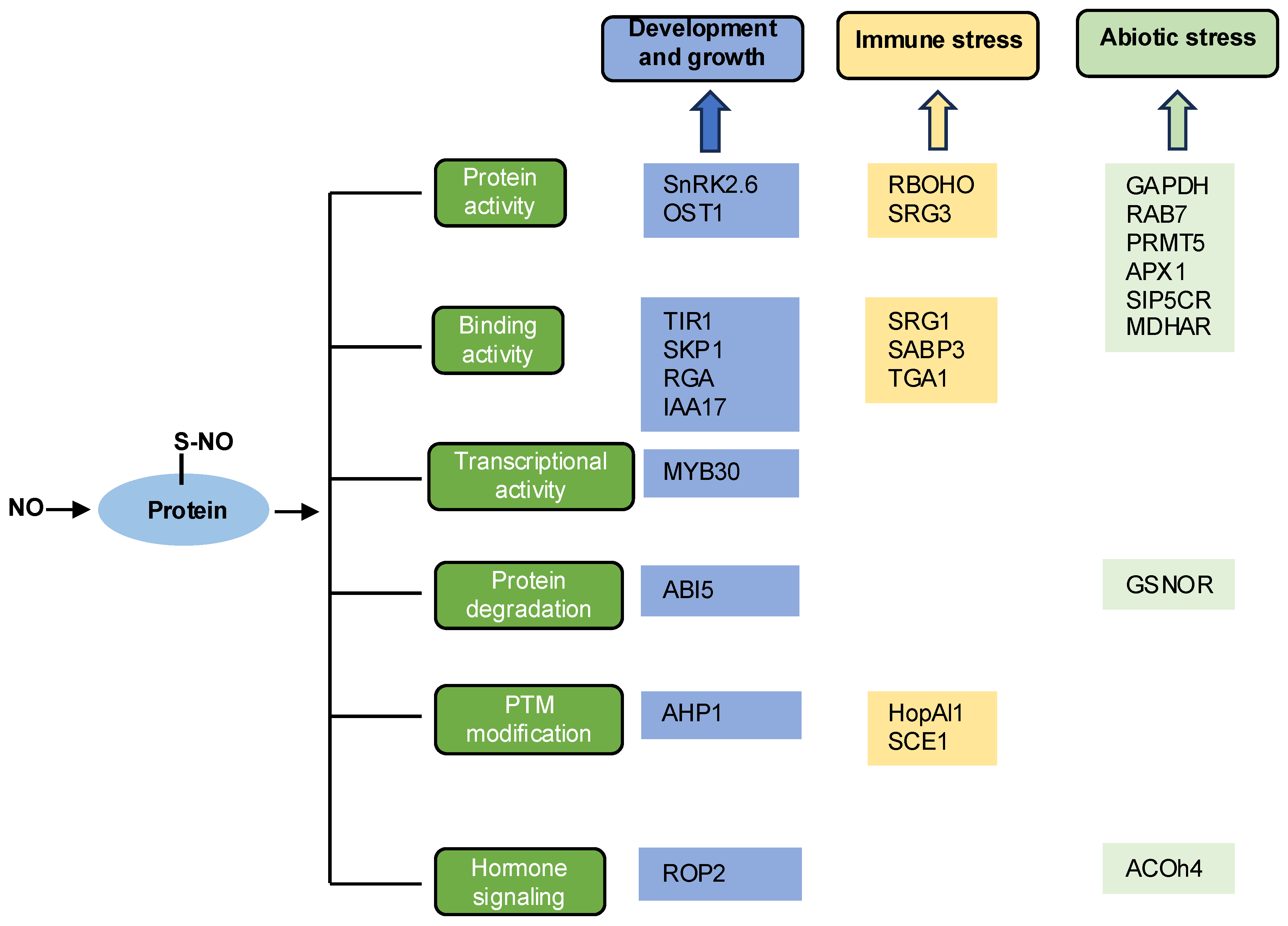
3. Functions of S-Nitrosylation in Plants
3.1. Growth and Development
3.1.1. ABA Signaling
3.1.2. Auxin Signaling
3.1.3. Other Hormone Signaling Pathways
| Species | Protein | The Detecting Techniques | Functions | Result | Reference |
|---|---|---|---|---|---|
| Arabidopsis thaliana | SnRK2.6 | BST | Inhibits enzyme activity | Promotes seed germination | [41] |
| Arabidopsis thaliana | ABI5 | BST; LC-MS/MS | Promotes ABI5 degradation | Promotes seed germination | [42] |
| Arabidopsis thaliana | MYB30 | BST; LC-MS/MS | Enhances MYB30 transcriptional activity | Promotes seed germination | [45] |
| Arabidopsis thaliana | ROP2 | BST | Reduces the auxin transport rate | Inhibits root growth | [48] |
| Arabidopsis thaliana | TIR1 | BST | Enhances TIR1-Aux/IAA interaction | Activates auxin signaling pathway | [47] |
| Arabidopsis thaliana | SKP1 | BST; LC-MS/MS | Enhances SKP1 binding to CUL1-TIR1 | Enhances auxin signal transduction | [49] |
| Arabidopsis thaliana | IAA17 | BST; LC-MS/MS; DAN Assay | Inhibits its interaction with TIR1 | Negatively regulates auxin signaling | [51] |
| Arabidopsis thaliana | RGA | BST; LC-MS/MS; DAN Assay | Inhibits its interaction with the F-box protein | Coordinates growth and stress responses | [52] |
| Arabidopsis thaliana | AHP1 | BST; LC-MS/MS; DAN Assay | Inhibits AHP1 phosphorylation | Inhibits cytokinin reactions | [15] |
| Arabidopsis thaliana | OST1 | BST | Inhibits OST1 activity | Negatively regulates ABA signaling | [53] |
3.2. Immune Response
3.2.1. Immune-Related Gene Expression
3.2.2. Regulating Protein Activity
3.2.3. Regulating Programmed Cell Death
| Species | Protein | The Detecting Techniques | Functions | Results | Reference |
|---|---|---|---|---|---|
| Arabidopsis thaliana | NPR1/ TGA1 | BST; LC-MS/MS | Enhances the DNA binding activity | Regulates plant defense response | [56] |
| Arabidopsis thaliana | SRG1 | BST; LC-MS/MS | Decreases DNA binding and transcriptional inhibition activity | Attenuates the plant immune response | [57] |
| Arabidopsis thaliana | SRG3 | BST | Abolishes the activity for SRG3 | Positively regulates plant immunity | [58] |
| Arabidopsis thaliana | SABP3 | BST; LC-MS/MS | Suppresses its binding activity | Modulates the plant defense response | [61] |
| Arabidopsis thaliana | SCE1 | BST | Increases SUMO1/2 conjugate levels | Impairs the immune response | [62] |
| Arabidopsis thaliana | HopAl1 | BST; DAN Assay | Inhibits phosphothreonine lyase activity | Activates cell death | [63] |
| Arabidopsis thaliana | RBOHO | BST; LC-MS/MS | Decreases enzyme activity | Disrupts cell death | [64] |
3.3. Abiotic Stress
3.3.1. Improving Salt Tolerance of Plants
3.3.2. Improving Temperature Tolerance of Plants
3.3.3. Improving Oxidative Tolerance of Plants
| Species | Protein | The Detecting Techniques | Functions | Results | Reference |
|---|---|---|---|---|---|
| Nicotiana tabacum | GAPDH | BST | Enhances protein kinases activity | Responses to salt stress | [67] |
| Arabidopsis thaliana | ACOh4 | BST; LC-MS/MS | Promotes the synthesis of ethylene | Enhances the salt resistance | [68] |
| Arabidopsis thaliana | RAB7 | BST; LC-MS/MS | Enhances protein activity | Maintains the ionic balance under salt stress | [69] |
| Arabidopsis thaliana | PRMT5 | BST; LC-MS/MS; DAN Assay | Enhances methyltransferase activity | Enhances stress tolerance | [34] |
| Solanum lycopersicum L. | SIP5CR | BST | Boosts both SlP5CR activity and proline synthesis | Enhances stress tolerance | [70] |
| Ganoderma lucidum | Acon | BST; LC-MS/MS | Regulates ganoderic acid biosynthesis | Enhances heat stress | [72] |
| Arabidopsis thaliana | AHb1 | BST | Reduces NO emission | Enhances hypoxic stress | [75] |
| Arabidopsis thaliana | APX1 | BST; LC-MS/MS; DAN Assay | Enhances APX1 activity | Enhances the antioxidative capacity | [77] |
| Arabidopsis thaliana | GSNOR | BST; LC-MS/MS; DAN Assay; | Induces the autophagic degradation of GSNOR | Enhances hypoxic stress | [76] |
| Helianthus annuus | GAPDH | BST; LC-MS/MS | Regulates protein activity | Responses to salt stress | [78] |
| Helianthus annuus | MDHAR | BST; LC-MS/MS | Regulates protein activity | Responses to salt stress | [78] |
3.4. Role of S-Nitrosylation in Legumes
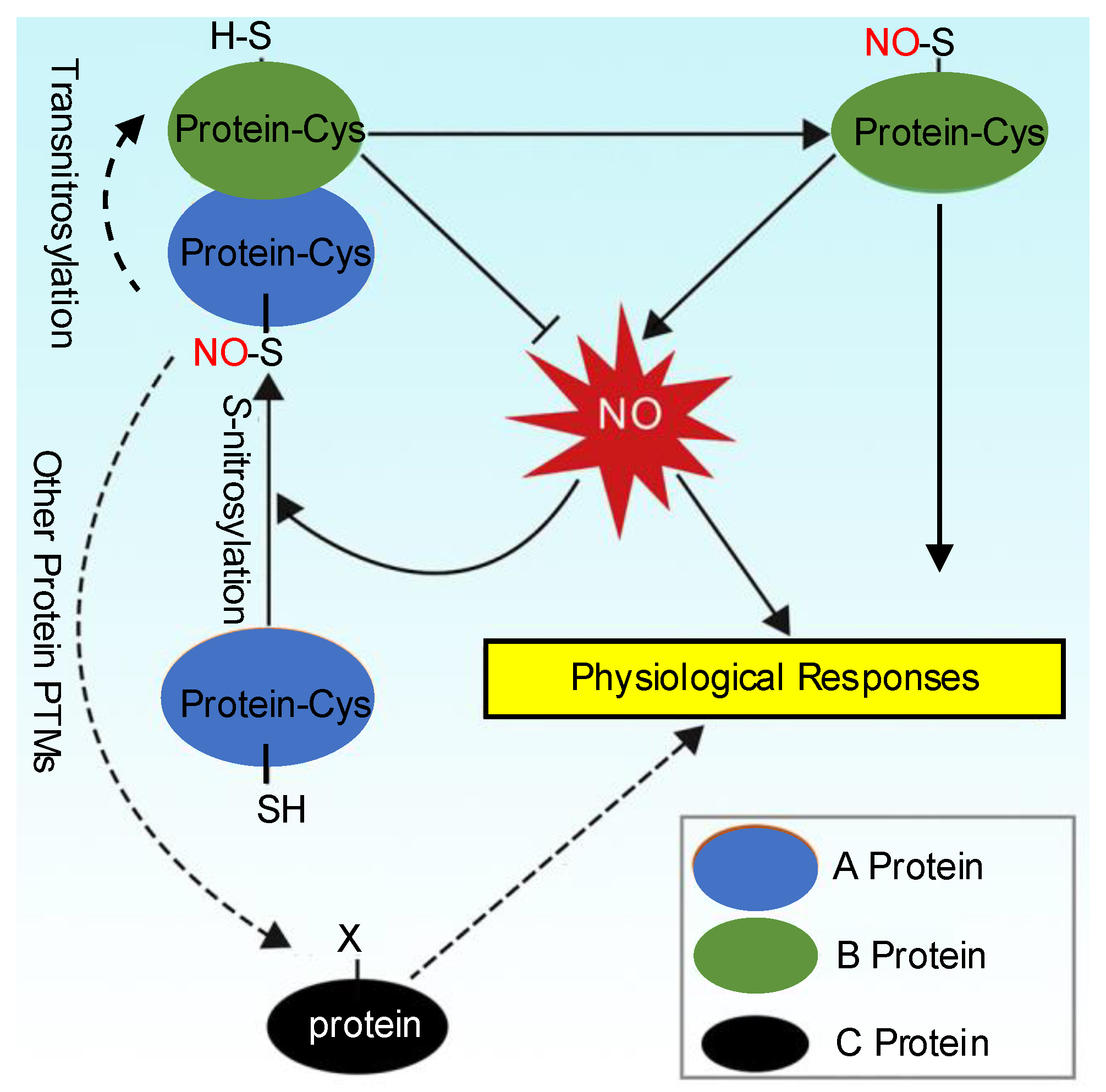
4. Denitrosylation and Transnitrosylation
5. The Crosstalk Between S-Nitrosylation and Other Protein PTMs
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Shang, J.X.; Li, X.; Li, C.; Zhao, L. The Role of nitric oxide in plant responses to salt stress. Int. J. Mol. Sci. 2022, 23, 6167. [Google Scholar] [CrossRef] [PubMed]
- Khator, K.; Parihar, S.; Jasik, J.; Shekhawat, G.S. Nitric oxide in plants: An insight on redox activity and responses toward abiotic stress signaling. Plant Signal. Behav. 2024, 19, 2298053. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 2012, 27, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; He, M.; Wang, Z.; Zhang, J.; Song, Y.; He, Z.; Dong, Y. Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regul. 2015, 77, 343–356. [Google Scholar] [CrossRef]
- Santos, M.P.; Zandonadi, D.B.; de Sa, A.F.L.; Costa, E.P.; de Oliveira, C.J.L.; Perez, L.E.P.; Façanha, A.R.; Bressan-Smith, R. Abscisic acid-nitric oxide and auxin interaction modulates salt stress response in tomato roots. Theor. Exp. Plant Physiol. 2020, 32, 301–313. [Google Scholar] [CrossRef]
- Feng, J.; Chen, L.; Zuo, J. Protein S-nitrosylation in plants: Current progresses and challenges. J. Integr. Plant Biol. 2019, 61, 1206–1223. [Google Scholar] [CrossRef]
- Hess, D.T.; Matsumoto, A.; Kim, S.O.; Marshall, H.E.; Stamler, J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166. [Google Scholar] [CrossRef]
- Hess, D.T.; Stamler, J.S. Regulation by S-nitrosylation of protein post-translational modification. J. Biol. Chem. 2012, 287, 4411–4418. [Google Scholar] [CrossRef]
- López-Sánchez, L.M.; López-Pedrera, C.; Rodríguez-Ariza, A. Proteomics insights into deregulated protein S-nitrosylation and disease. Expert. Rev. Proteom. 2012, 9, 59–69. [Google Scholar] [CrossRef]
- Zavadskiy, S.; Sologova, S.; Moldogazieva, N. Oxidative distress in aging and age-related diseases: Spatiotemporal dysregulation of protein oxidation and degradation. Biochimie 2022, 195, 114–134. [Google Scholar] [CrossRef]
- Marino, S.M.; Gladyshev, V.N. Structural analysis of cysteine S-nitrosylation: A modified acid-based motif and the emerging role of trans-nitrosylation. Nat. Commun. 2010, 395, 844–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, Y.; Wang, G.; Fang, C.; Bao, H.; Zhang, Y.; Lu, H. FluoroTRAQ: Quantitative analysis of protein S-nitrosylation through Fuorous solid-phase extraction combining with iTRAQ by mass spectrometry. Anal. Chem. 2020, 92, 15317–15322. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Qu, M.; Jia, B.; Wang, W.; Luo, Z.; Song, C.P.; Tao, W.A.; Wang, P. FAT-switch-based quantitative S-nitrosoproteomics reveals a key role of GSNOR1 in regulating ER functions. Nat. Commun. 2023, 14, 3268. [Google Scholar] [CrossRef] [PubMed]
- Borrowman, S.; Kapuganti, J.G.; Loake, G.J. Expanding roles for nitrosylation in the regulation of plant immunity. Free Radic. Biol. Med. 2023, 194, 357–368. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Snyder, S.H. The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE 2001, 2001, pl1. [Google Scholar] [CrossRef]
- Feng, J.; Wang, C.; Chen, Q.; Chen, H.; Ren, B.; Li, X.; Zuo, J. S-nitrosylation of phosphotransfer proteins represses cytokinin signaling. Nat. Commun. 2013, 4, 1529. [Google Scholar] [CrossRef]
- Zhang, Y.; Keszler, A.; Broniowska, K.A.; Hogg, N. Characterization and application of the biotin-switch assay for the identification of S-nitrosated proteins. Free Radic. Biol. Med. 2005, 38, 874–881. [Google Scholar] [CrossRef]
- Wu, C.; Parrott, A.M.; Liu, T.; Jain, M.R.; Yang, Y.; Sadoshima, J.; Li, H. Distinction of thioredoxin transnitrosylation and denitrosylation target proteins by the ICAT quantitative approach. J. Proteom 2011, 74, 2498–2509. [Google Scholar] [CrossRef]
- Qu, Z.; Meng, F.; Bomgarden, R.D.; Viner, R.I.; Li, J.; Rogers, J.C.; Cheng, J.; Greenlief, C.M.; Cui, J.; Lubahn, D.B.; et al. Proteomic quantification and site-mapping of S-nitrosylated proteins using isobaric iodoTMT reagents. J. Pro-Teome Res. 2014, 13, 3200–3211. [Google Scholar] [CrossRef]
- Gong, B.; Shi, Q. Identifying S-nitrosylated proteins and unraveling S-nitrosoglutathione reductase-modulated sodic alkaline stress tolerance in Solanum lycopersicum L. Plant Physiol Biochem. 2019, 142, 84–93. [Google Scholar] [CrossRef]
- Ibáñez-Vea, M.; Huang, H.; Martínez, X.; Pérez, E.; Gato, M.; Zuazo, M.; Arasanz, H.; Fernández-Irigoyen, J.; Santamaría, E.; Fernandez-Hinojal, G.; et al. Characterization of macrophage endogenous S-nitrosoproteome using a cysteine-specific phosphonate adap-table tag in combination with TiO2 Chromatography. J. Proteome Res. 2018, 17, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Mnatsakanyan, R.; Markoutsa, S.; Walbrunn, K.; Roos, A.; Verhelst, S.H.L.; Zahedi, R.P. Proteome-wide detection of S-nitrosylation targets and motifs using bioorthogonal cleavable-linker-based enrichment and switch technique. Nat. Commun. 2019, 10, 2195. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.T.; Thompson, J.W.; Foster, M.W.; Nogueira, L.; Moseley, M.A.; Stamler, J.S. Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat. Biotechnol 2009, 27, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, J.R.; Kang, J.W.; Lee, S.J.; Shin, G.C.; Yeo, W.S.; Kim, K.H.; Park, H.S.; Kim, K.P. Selective enrichment and mass spectrometric identification of nitrated peptides using fluorinated carbon tags. Anal. Chem. 2011, 83, 157–163. [Google Scholar] [CrossRef]
- Zhao, M.; Deng, C. Designed synthesis of fluorous-functionalized magnetic. mesoporous microspheres for specific enrichment of phosphopeptides with fluorous derivatization. Proteomics 2016, 16, 1051–1058. [Google Scholar] [CrossRef]
- Foster, M.W.; Forrester, M.T.; Stamler, J.S. A protein microarray-based analysis of S-nitrosylation. Proc. Natl. Acad. Sci. USA 2009, 106, 18948–18953. [Google Scholar] [CrossRef]
- Cho, D.H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-nitrosylation of Drp1 mediates beta-amyloid- related mitochondrial fission and neuronal injury. Science 2009, 324, 102–105. [Google Scholar] [CrossRef]
- Hu, J.; Huang, X.; Chen, L.; Sun, X.; Lu, C.; Zhang, L.; Wang, Y.; Zuo, J. Site-specific nitrosoproteomic identification of endogenously S-nitrosylated proteins in Arabidopsis. Plant Physiol. 2015, 167, 1731–1746. [Google Scholar] [CrossRef]
- Silva, A.M.; Vitorino, R.; Domingues, M.R.; Spickett, C.M.; Domingues, P. Post-translational modifications and mass spectrometry detection. Free Radic. Biol. Med. 2013, 65, 925–941. [Google Scholar] [CrossRef]
- Chicooree, N.; Unwin, R.D.; Griffiths, J.R. The application of targeted mass spectrometry-based strategies to the detection and localization of post- translational modifications. Mass Spectrom. Rev. 2014, 34, 595–626. [Google Scholar] [CrossRef]
- Astier, J.; Besson-Bard, A.; Lamotte, O.; Bertoldo, J.; Bourque, S.; Terenzi, H.; Wendehenne, D. Nitric oxide inhibits the ATPase activity of the chaperone-like AAA+ ATPase CDC48, a target for S-nitrosylation in cryptogein signalling in tobacco cells. Biochem. J. 2012, 447, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Kovacs, I.; Spannagl, M.; Lindermayr, C. Computational prediction of candidate proteins for S-nitrosylation in Arabidopsis thaliana. PLoS ONE 2014, 9, e110232. [Google Scholar] [CrossRef] [PubMed]
- Puyaubert, J.; Fares, A.; Rézé, N.; Peltier, J.B.; Baudouin, E. Identification of endogenously S-nitrosylated proteins in Arabidopsis plantlets: Effect of cold stress on cysteine nitrosylation level. Plant Sci. 2014, 215–216, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yang, H.; Mu, J.; Lu, T.; Peng, J.; Deng, X.; Kong, Z.; Bao, S.; Cao, X.; Zuo, J.; et al. Nitric oxide regulates protein methylation during stress responses in Plants. Mol. Cell 2017, 67, 702–710. [Google Scholar] [CrossRef]
- Sánchez-Vicente, I.; Fernández-Espinosa, M.G.; Lorenzo, O. Nitric oxide molecular targets: Reprogramming plant development upon stress. J. Exp. Bot. 2019, 70, 4441–4460. [Google Scholar] [CrossRef]
- Sanchez-Corrionero, A.; Sánchez-Vicente, I.; Arteaga, N.; Manrique-Gil, I.; Gómez-Jiménez, S.; Torres-Quezada, I.; Albertos, P.; Lorenzo, O. Fine-tuned nitric oxide and hormone interface in plant root development and regeneration. J. Exp. Bot. 2023, 74, 6104–6118. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Bhat, J.A.; Ahmad, P.; Allakhverdiev, S.I. Polyamines and nitric oxide crosstalk in plant development and abiotic stress tolerance. Funct. Plant Biol. FPB 2023, 50, i–iv. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2021, 35, 199–214. [Google Scholar] [CrossRef]
- Beligni, M.V.; Lamattina, L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 2000, 210, 215–221. [Google Scholar] [CrossRef]
- Bethke, P.C.; Gubler, F.; Jacobsen, J.V.; Jones, R.L. Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta 2004, 219, 847–855. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, J.K.; Lang, Z. Nitric oxide suppresses the inhibitory effect of abscisic acid on seed germination by S-nitrosylation of SnRK2 proteins. Plant Signal. Behav. 2015, 10, e1031939. [Google Scholar] [CrossRef] [PubMed]
- Albertos, P.; Romero-Puertas, M.C.; Tatematsu, K.; Mateos, I.; Sánchez-Vicente, I.; Nambara, E.; Lorenzo, O. S-nitrosylation triggers ABI5 degradation to promote seed germination and seedling growth. Nat. Commun. 2015, 6, 8669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Y.; Zheng, Y. Integration of ABA, GA, and light signaling in seed germination through the regulation of ABI5. Front. Plant Sci. 2022, 13, 1000803. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Zhao, H.; Wang, X.; Niu, Y.; Zhou, H.; Zheng, Y. The MIEL1-ABI5/MYB30 regulatory module fine tunes abscisic acid signaling during seed germination. J. Integr. Plant Biol. 2022, 64, 930–941. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, L.; Shen, J.; Zhou, H.; Zhen, Y. S-nitrosylation of the transcription factor MYB30 facilitates nitric oxide-promoted seed germination in Arabidopsis. Plant Cell 2024, 36, 794. [Google Scholar] [CrossRef]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef]
- Terrile, M.C.; París, R.; Calderón-Villalobos, L.I.; Iglesias, M.J.; Lamattina, L.; Estelle, M.; Casalongué, C.A. Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. Plant J. 2012, 70, 492–500. [Google Scholar] [CrossRef]
- Kenesi, E.; Kolbert, Z.; Kaszler, N.; Klement, E.; Menesi, D.; Molnar, A.; Valkai, I.; Feigl, G.; Rigó, G.; Cséplő, A.; et al. The ROP2 GTPase participates in nitric oxide (NO)-induced root shortening in Arabidopsis. Plants 2023, 12, 750. [Google Scholar] [CrossRef]
- Iglesias, M.J.; Terrile, M.C.; Correa-Aragunde, N.; Colman, S.L.; Izquierdo-Álvarez, A.; Fiol, D.F.; París, R.; Sánchez-López, N.; Marina, A.; Villalobos, L.I.A.C.; et al. Regulation of SCFTIR1/AFBs E3 ligase assembly by S-nitrosylation of Arabidopsis SKP1-like1 impacts on auxin signaling. Redox Biol. 2018, 18, 200–210. [Google Scholar] [CrossRef]
- Terrile, M.C.; Tebez, N.M.; Colman, S.L.; Mateos, J.L.; Morato-Lopez, E.; Sanchez-Lopez, N.; Izquierdo-Álvarez, A.; Marina, A.; Villalobos, L.I.A.C.; Estelle, M.; et al. S-nitrosation of E3 ubiquitin ligase complex components regulates hormonal signalings in Arabidopsis. Front. Plant Sci. 2021, 12, 794582. [Google Scholar] [CrossRef]
- Jing, H.; Yang, X.; Emenecker, R.J.; Feng, J.; Zhang, J.; Figueiredo, M.R.A.; Chaisupa, P.; Wright, R.C.; Holehouse, A.S.; Strader, L.C.; et al. Nitric oxide-mediated S-nitrosylation of IAA17 protein in intrinsically disordered region represses auxin signaling. J. Genet. Genom. 2023, 50, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, S.; Song, C.P.; Zhou, J.M.; Li, J.; Zuo, J. Nitric oxide negatively regulates gibberellin signaling to coordinate growth and salt tolerance in Arabidopsis. J. Genet. Genom. 2022, 49, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Du, Y.; Hou, Y.J.; Zhao, Y.; Hsu, C.C.; Yuan, F.; Zhu, X.; Tao, W.A.; Song, C.P.; Zhu, J.K.; et al. Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc. Natl. Acad. Sci. USA 2015, 112, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.W.; Skelly, M.J.; Yin, M.; Yu, M.; Mun, B.G.; Lee, S.U.; Hussain, A.; Spoel, S.H.; Loake, G.J. Nitric oxide and S-nitrosoglutathione function additively during plant immunity. New Phytol. 2016, 211, 516–526. [Google Scholar] [CrossRef]
- Imran, Q.M.; Hussain, A.; Lee, S.U.; Mun, B.G.; Falak, N.; Loake, G.J.; Yun, B.W. Transcriptome profile of NO-induced Arabidopsis transcription factor genes suggests their putative regulatory role in multiple biological processes. Sci. Rep. 2018, 8, 771. [Google Scholar] [CrossRef]
- Lindermayr, C.; Sell, S.; Müller, B.; Leister, D.; Durner, J. Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell 2010, 22, 2894–2907. [Google Scholar] [CrossRef]
- Cui, B.; Pan, Q.; Clarke, D.; Villarreal, M.O.; Umbreen, S.; Yuan, B.; Shan, W.; Jiang, J.; Loake, G.J. S-nitrosylation of the zinc finger protein SRG1 regulates plant immunity. Nat. Commun. 2018, 9, 4226. [Google Scholar] [CrossRef]
- Cui, B.; Xu, S.; Li, Y.; Umbreen, S.; Frederickson, D.; Yuan, B.; Jiang, J.; Liu, F.; Pan, Q.; Loake, G.J.; et al. The Arabidopsis zinc finger proteins SRG2 and SRG3 are positive regulators of plant immunity and are differentially regulated by nitric oxide. New Phytol. 2021, 230, 259–274. [Google Scholar] [CrossRef]
- Loake, G.; Grant, M. Salicylic acid in plant defence—The players and protagonists. Curr. Opin. Plant Biol. 2007, 10, 466–472. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Feechan, A.; Yun, B.W.; Shafiei, R.; Hofmann, A.; Taylor, P.; Xue, P.; Yang, F.Q.; Xie, Z.S.; Pallas, J.A.; et al. S-nitrosylation of AtSABP3 antagonizes the expression of plant immunity. J. Biol. Chem. 2009, 284, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Skelly, M.J.; Malik, S.I.; Le Bihan, T.; Bo, Y.; Jiang, J.; Spoel, S.H.; Loake, G.J. A role for S-nitrosylation of the SUMO-conjugating enzyme SCE1 in plant immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 17090–17095. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Bellin, D.; Vandelle, E.; Imanifard, Z.; Delledonne, M. Host-mediated S-nitrosylation disarms the bacterial effec- tor HopAI1 to reestablish immunity. Plant Cell 2017, 29, 2871–2881. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.W.; Feechan, A.; Yin, M.; Saidi, N.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Malvankar, M.R.; Karle, S.B.; Kumar, K. Reactive nitrogen species: Paradigms of cellular signaling and regulation of salt stress in plants. Environ. Exp. Bot. 2018, 161, 86–97. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Wei, S.; Wang, C.; Deng, Y.; Hu, D.; Liu, H.; Gong, W.; Pan, Y.; Liao, W.; et al. Nitric oxide alleviates salt stress through protein S-nitrosylation and transcriptional regulation in tomato seedlings. Planta 2022, 256, 101. [Google Scholar] [CrossRef]
- Wawer, I.; Bucholc, M.; Astier, J.; Anielska-Mazur, A.; Dahan, J.; Kulik, A.; Wysłouch-Cieszynska, A.; Zaręba-Kozioł, M.; Krzywinska, E.; Dadlez, M.; et al. Regulation of Nicotiana tabacum osmotic stress-activated protein kinase and its cellular partner GAPDH by nitric oxide in response to salinity. Biochem. J. 2010, 429, 73–83. [Google Scholar] [CrossRef]
- Liu, M.; Wei, J.W.; Liu, W.; Gong, B. S-nitrosylation of ACO homolog 4 improves ethylene synthesis and salt tolerance in tomato. New Phytol. 2023, 239, 159–173. [Google Scholar] [CrossRef]
- Lin, W.; Wang, Y.; Li, X.; Huang, X.; Wang, Y.; Shang, J.X.; Zhao, L. S-nitrosylation of RABG3E positively regulates vesicle trafficking to promote salt tolerance. Plant Cell Environ. 2023, 46, 3858–3870. [Google Scholar] [CrossRef]
- Liu, W.; Wei, J.W.; Shan, Q.; Liu, M.; Xu, J.; Gong, B. Genetic engineering of drought- and salt-tolerant tomato via Δ1-pyrroline-5-carboxylate reductase S-nitrosylation. J. Integr. Plant Biol. 2024, 195, 1038–1052. [Google Scholar] [CrossRef]
- Li, B.; Gao, K.; Ren, H.; Tang, W. Molecular mechanisms governing plant responses to high temperatures. J. Integr. Plant Biol. 2018, 60, 757–779. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhu, T.; Yang, T.; Yang, Z.; Ren, A.; Shi, L.; Zhu, J.; Yu, H.; Zhao, M. Nitric oxide regulates ganoderic acid biosynthesis by the S-nitrosylation of aconitase under heat stress in Ganoderma lucidum. Environ. Microbiol. 2021, 23, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ma, J.; Tie, J.; Li, Y.; Hu, L.; Yu, J. BR-Mediated protein S-nitrosylation alleviated low-temperature stress in Mini Chinese cabbage (Brassica rapa ssp. pekinensis). Int. J. Mol. Sci. 2022, 23, 10964. [Google Scholar] [CrossRef] [PubMed]
- Abat, J.K.; Deswal, R. Differential modulation of S-nitrosoproteome of Brassica juncea by low temperature: Change in S-nitrosylation of Rubisco is responsible for the inactivation of its carboxylase activity. Proteomics 2009, 9, 4368–4380. [Google Scholar] [CrossRef]
- Perazzolli, M.; Dominici, P.; Romero-Puertas, M.C.; Zago, E.; Zeier, A.; Sonoda, M.; Lamb, C.; Delledonne, M. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell 2004, 16, 2785–2794. [Google Scholar] [CrossRef]
- Zhan, N.; Wang, C.; Chen, L.; Yang, H.; Feng, J.; Gong, X.; Ren, B.; Wu, B.; Mu, J.; Li, Y.; et al. S-nitrosylation targets GSNO reductase for selective autophagy during hypoxia responses in plants. Mol. Cell 2018, 71, 142–154. [Google Scholar] [CrossRef]
- Yang, H.; Mu, J.; Chen, L.; Feng, J.; Hu, J.; Li, L.; Zhou, J.M.; Zuo, J. S-nitrosylation positively regulates ascorbate peroxidase activity during plant stress responses. Plant Physiol. 2015, 167, 1604–1615. [Google Scholar] [CrossRef]
- Jain, P.; von Toerne, C.; Lindermayr, C.; Bhatla, S.C. S-nitrosylation/denitrosylation as a regulatory mechanism of salt stress sensing in sunflower seedlings. Plant Physiol. 2018, 162, 49–72. [Google Scholar] [CrossRef]
- Allagulova, C.R.; Lubyanova, A.R.; Avalbaev, A.M. Multiple ways of nitric oxide production in plants and its functional activity under abiotic stress conditions. Int. J. Mol. Sci. 2023, 24, 11637. [Google Scholar] [CrossRef]
- Zhou, X.; Joshi, S.; Khare, T.; Patil, S.; Shang, J.; Kumar, V. Nitric oxide, crosstalk with stress regulators and plant abiotic stress tolerance. Plant Cell Rep. 2021, 40, 1395–1414. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Borhannuddin Bhuyan, M.H.M.; Mahmud, J.; Baluska, F.; Fujita, M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Becana, M. Molecular responses of legumes to abiotic stress: Post-translational modifications of proteins and redox signaling. J. Exp. Bot. 2021, 72, 5876–5892. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, M.A.; Saiz, A.; Peñuelas, M.; Bustos-Sanmamed, P.; Mulet, J.M.; Barja, M.V.; Rouhier, N.; Moore, M.; James, E.K.; Dietz, K.J.; et al. Function of glutathione peroxidases in legume root nodules. J. Exp. Bot. 2015, 66, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.M.; Silva, L.S.; Ribeiro, I.; Seabra, A.R.; Carvalho, H.G. Glutamine synthetase is a molecular target of nitric oxide in root nodules of Medicago truncatula and is regulated by tyrosine nitration. Plant Physiol. 2011, 157, 1505–1517. [Google Scholar] [CrossRef]
- Benhar, M.; Forrester, M.; Stamler, J. Protein denitrosylation: Enzymatic mechanisms and cellular functions. Nat. Rev. Mol. Cell Biol. 2009, 10, 721–732. [Google Scholar] [CrossRef]
- Wu, C.; Liu, T.; Chen, W.; Oka, S.; Fu, C.; Jain, M.R.; Parrott, A.M.; Baykal, A.T.; Sadoshima, J.; Li, H. Redox regulatory mechanism of transnitrosylation by thioredoxin. Mol. Cell Proteom. 2010, 9, 2262–2275. [Google Scholar] [CrossRef]
- Kneeshaw, S.; Gelineau, S.; Tada, Y.; Loake, G.J.; Spoel, S.H. Selective protein denitrosylation activity of thioredoxin-h5 modulates plant immunity. Mol. Cell 2014, 56, 153–162. [Google Scholar] [CrossRef]
- Chen, L.; Wu, R.; Feng, J.; Feng, T.; Wang, C.; Hu, J.; Zhan, N.; Li, Y.; Ma, X.; Ren, B.; et al. Transnitrosylation mediated by the non-canonical catalase ROG1 regulates nitric oxide signaling in plants. Dev. Cell 2020, 53, 444–457. [Google Scholar] [CrossRef]
- Mengel, A.; Ageeva, A.; Georgii, E.; Bernhardt, J.; Wu, K.; Durner, J.; Lindermayr, C. Nitric oxide modulates histone acetylation at stress genes by inhibition of histone deacetylases. Plant Physiol. 2017, 173, 1434–1452. [Google Scholar] [CrossRef]
- Wang, P.; Fang, H.; Gao, R.; Liao, W. Protein persulfidation in plants: Function and mechanism. Antioxidants 2021, 10, 1631. [Google Scholar] [CrossRef]
- Munoz-Vargas, M.A.; Gonzalez-Gordo, S.; Canas, A.; Lopez-Jaramillo, J.; Palma, J.M.; Corpas, F.J. Endogenous hydrogen sulfide (H2S) is up-regulated during sweet pepper (Capsicum annuum L.) fruit ripening. In vitro analysis shows that NADP-dependent isocitrate dehydrogenase (ICDH) activity is inhibited by H2S and NO. Nitric Oxide 2018, 81, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yang, L.; Tian, M.; Chen, J.; Shi, J.; Yang, Y.; Hu, X. Nitric oxide enhances desiccation tolerance of recalcitrant Antiaris toxicaria seeds via protein S-nitrosylation and carbonylation. PLoS ONE 2011, 6, e20714. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, C. S-nitrosylation control of ROS and RNS homeostasis in plants: The switching function of catalase. Mol. Plant 2020, 13, 946–948. [Google Scholar] [CrossRef] [PubMed]
- Gietler, M.; Nykiel, M.; Orzechowski, S.; Fettke, J.; Zagdańska, B. Proteomic analysis of S-nitrosylated and S-glutathionylated proteins in wheat seedlings with different dehydration tolerances. Plant Physiol. Biochem. 2016, 108, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Padilla, M.N.; Barroso, J.B. The function of S-nitrosothiols during abiotic stress in plants. J. Exp. Bot. 2019, 70, 4429–4439. [Google Scholar] [CrossRef]
- Huang, B.; Chen, C. Detection of protein S-nitrosation using irreversible biotinylation procedures (IBP). Free Radic. Biol. Med. 2010, 49, 447–456. [Google Scholar] [CrossRef]
- Forrester, M.T.; Foster, M.W.; Stamler, J.S. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J. Biol. Chem. 2007, 282, 13977–13983. [Google Scholar] [CrossRef]
| BST Techniques | FAT-Switch Techniques | |
|---|---|---|
| Sensitivity and efficiency | Low | High |
| Specificity | High | High |
| Cost | Middle | Middle |
| Limitation of method | Time-consuming and tedious; Unsuitable for monitoring dynamic changes in S-nitrosylation | Lack of commercial antibodies; Unsuitable for monitoring dynamic changes in S-nitrosylation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Shang, J.-X.; Li, X.-Y.; Zhou, X.-F.; Zhao, L.-Q. Nitric Oxide Regulates Multiple Signal Pathways in Plants via Protein S-Nitrosylation. Curr. Issues Mol. Biol. 2025, 47, 407. https://doi.org/10.3390/cimb47060407
Lin W, Shang J-X, Li X-Y, Zhou X-F, Zhao L-Q. Nitric Oxide Regulates Multiple Signal Pathways in Plants via Protein S-Nitrosylation. Current Issues in Molecular Biology. 2025; 47(6):407. https://doi.org/10.3390/cimb47060407
Chicago/Turabian StyleLin, Wei, Jian-Xiu Shang, Xiao-Ying Li, Xue-Feng Zhou, and Li-Qun Zhao. 2025. "Nitric Oxide Regulates Multiple Signal Pathways in Plants via Protein S-Nitrosylation" Current Issues in Molecular Biology 47, no. 6: 407. https://doi.org/10.3390/cimb47060407
APA StyleLin, W., Shang, J.-X., Li, X.-Y., Zhou, X.-F., & Zhao, L.-Q. (2025). Nitric Oxide Regulates Multiple Signal Pathways in Plants via Protein S-Nitrosylation. Current Issues in Molecular Biology, 47(6), 407. https://doi.org/10.3390/cimb47060407






