The Role of Mucins in Cancer and Cancer Progression: A Comprehensive Review
Abstract
1. Introduction to Mucins and Cancer
1.1. Definition and Classification of Mucins
1.2. Overview of Cancer and Cancer Progression
2. Structure and Function of Mucins
2.1. General Features of Transmembrane Mucins
2.1.1. Extracellular Domain
2.1.2. SEA Domain
2.1.3. EGF-like Domains
2.1.4. Intracellular Domain
2.1.5. Glycosylation
2.1.6. Signaling Roles
2.1.7. Cleavage and Shedding
2.2. Role of Mucins in Normal Physiology
3. Altered Mucin Expression in Cancer
3.1. Upregulated Mucins in Cancer
3.2. Downregulated Mucins in Cancer
4. Mucins in Cancer Metastatic Cascade
4.1. Cancer Cell Invasion and Migration
4.2. Cancer Cell Adhesion and Colonization
4.3. Immune Evasion and Tumor Microenvironment
4.4. Mucins and Angiogenesis
5. Mucins in Different Types of Cancer
5.1. Breast Cancer
5.2. Colorectal Cancer
5.3. Pancreatic Cancer
| Cancer | Mucin and Mucin-Associated Deregulation | Proposed Mechanisms |
|---|---|---|
| Breast | MUC1: GalNAcT14 [187], GALNT6 [186] MEK/ERK [58], MAPK, JAK/STAT, and PI3K/AKT/mTOR pathways [144,180], neuropilin-1 [187,215] MUC16: Sialyl Lewis x and [165], and JAK2 [65,102] | Stabilization of MUC1 [186,187]. Increase tumorigenesis [58,187], inhibit apoptosis [65], angiogenesis [215]. Enhance cellular adhesion for distal metastasis [165]. Increase G2/M transition for cellular proliferation [65]. |
| Colorectal | MUC2: IL-6 overexpression [201] MUC5AC: CD44/β-catenin/p53/p21 signaling pathway [201,202] MUC16 [196]: Sialyl Lewis x epitope [195] | Inflammation and tumor growth [200] Tumorigenesis and chemoresistance [204] Invasion and metastasis [195], attenuation of NK and T cell [196] |
| Pancreatic | MUC1: β-catenin and EGFR, AKT and BCL-2, BRCA1, MAPK, JAK/STAT and the PI3K/AKT/mTOR pathways, and neuropilin-1 [210,213,214] MUC4: integrin-mediated cell adhesion [134], HER2/neu [160], MUC16: PI3K/AKT/mTOR pathways [180], Treg [216] | Enhance cell proliferation, motility, chemoresistance, glucose utilization, and angiogenesis. [210,213,214] Inhibit integrin-mediated cell adhesion [134], increased cellular proliferation and metastasis [160]. Increase tumor survival [180], promote immunosuppressive tumor microenvironment [216] |
6. Experimental Models and Techniques in Mucin Research
7. Mucins as Cancer Biomarkers
7.1. Current Mucin Biomarker
7.2. Limitation of Mucin as Biomarker
8. Therapeutic Targeting of Mucins in Cancer
8.1. Current Strategies and Challenges
8.2. Novel Approaches and Future Directions
9. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta 2015, 1850, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Webber, S.E. Receptors mediating the effects of substance P and neurokinin A on mucus secretion and smooth muscle tone of the ferret trachea: Potentiation by an enkephalinase inhibitor. Br. J. Pharmacol. 1989, 98, 1197–1206. [Google Scholar] [CrossRef]
- Bansil, R.; Stanley, E.; LaMont, J.T. Mucin biophysics. Annu. Rev. Physiol. 1995, 57, 635–657. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.W. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef]
- Pai, P.; Rachagani, S.; Dhawan, P.; Batra, S.K. Mucins and Wnt/β-catenin signaling in gastrointestinal cancers: An unholy nexus. Carcinogenesis 2016, 37, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Pothuraju, R.; Krishn, S.R.; Gautam, S.K.; Pai, P.; Ganguly, K.; Chaudhary, S.; Rachagani, S.; Kaur, S.; Batra, S.K. Mechanistic and Functional Shades of Mucins and Associated Glycans in Colon Cancer. Cancers 2020, 12, 649. [Google Scholar] [CrossRef]
- Cox, K.E.; Liu, S.; Lwin, T.M.; Hoffman, R.M.; Batra, S.K.; Bouvet, M. The Mucin Family of Proteins: Candidates as Potential Biomarkers for Colon Cancer. Cancers 2023, 15, 1491. [Google Scholar] [CrossRef]
- Strous, G.J.; Dekker, J. Mucin-type glycoproteins. Crit. Rev. Biochem. Mol. Biol. 1992, 27, 57–92. [Google Scholar] [CrossRef]
- Hattrup, C.L.; Gendler, S.J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 2008, 70, 431–457. [Google Scholar] [CrossRef]
- Demouveaux, B.; Gouyer, V.; Gottrand, F.; Narita, T.; Desseyn, J. Gel-forming mucin interactome drives mucus viscoelasticity. Adv. Colloid Interface Sci. 2018, 252, 69–82. [Google Scholar] [CrossRef]
- Desseyn, J.L.; Aubert, J.P.; Porchet, N.; Laine, A. Evolution of the large secreted gel-forming mucins. Mol. Biol. Evol. 2000, 17, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Javitt, G.; Khmelnitsky, L.; Albert, L.; Bigman, L.S.; Elad, N.; Morgenstern, D.; Ilani, T.; Levy, Y.; Diskin, R.; Fass, D. Assembly Mechanism of Mucin and von Willebrand Factor Polymers. Cell 2020, 183, 717–729.e16. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Mucins and the Microbiome. Annu. Rev. Biochem. 2020, 89, 769–793. [Google Scholar] [CrossRef] [PubMed]
- Bobek, L.A.; Liu, J.; Sait, S.N.; Shows, T.B.; Bobek, Y.A.; Levine, M.J. Structure and chromosomal localization of the human salivary mucin gene, MUC7. Genomics 1996, 31, 277–282. [Google Scholar] [CrossRef]
- Higuchi, T.; Orita, T.; Nakanishi, S.; Katsuya, K.; Watanabe, H.; Yamasaki, Y.; Waga, I.; Nanayama, T.; Yamamoto, Y.; Munger, W.; et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J. Biol. Chem. 2004, 279, 1968–1979. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Fekete, E.; Buret, A.G. The role of mucin O-glycans in microbiota dysbiosis, intestinal homeostasis, and host-pathogen interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G452–G465. [Google Scholar] [CrossRef]
- McKenzie, C.; Thim, L.; Parsons, M.E. Topical and intravenous administration of trefoil factors protect the gastric mucosa from ethanol-induced injury in the rat. Aliment. Pharmacol. Ther. 2000, 14, 1033–1040. [Google Scholar] [CrossRef]
- Bruce, J.N.; Oldfield, E.H. Method for sequential sampling of cerebrospinal fluid in humans. Neurosurgery 1988, 23, 788–790. [Google Scholar] [CrossRef]
- van Putten, J.P.M.; Strijbis, K. Transmembrane Mucins: Signaling Receptors at the Intersection of Inflammation and Cancer. J. Innate Immun. 2017, 9, 281–299. [Google Scholar] [CrossRef]
- Du, J.; Zhou, S.; Cheng, Z.; Xu, W.; Zhang, R.; Wang, L.; Guo, J. MUC1 Specific Immune Responses Enhanced by Coadministration of Liposomal DDA/MPLA and Lipoglycopeptide. Front. Chem. 2022, 10, 814880. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.W. Barrier function of epithelia. Am. J. Physiol. 1981, 241, 275. [Google Scholar] [CrossRef]
- Cavey, M.; Lecuit, T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb. Perspect. Biol. 2009, 1, a002998. [Google Scholar] [CrossRef] [PubMed]
- Tepass, U.; Tanentzapf, G.; Ward, R.; Fehon, R. Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 2001, 35, 747–784. [Google Scholar] [CrossRef]
- Vermeer, P.D.; Einwalter, L.A.; Moninger, T.O.; Rokhlina, T.; Kern, J.A.; Zabner, J.; Welsh, M.J. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 2003, 422, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef]
- Ganguly, K.; Rauth, S.; Marimuthu, S.; Kumar, S.; Batra, S.K. Unraveling mucin domains in cancer and metastasis: When protectors become predators. Cancer Metastasis Rev. 2020, 39, 647–659. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006, 22, 207–235. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 622–634. [Google Scholar] [CrossRef]
- Yamamoto, M.; Bharti, A.; Li, Y.; Kufe, D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J. Biol. Chem. 1997, 272, 12492–12494. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, W.; Ren, J.; Chen, W.; Huang, L.; Kharbanda, S.; Loda, M.; Kufe, D. Heregulin targets gamma-catenin to the nucleolus by a mechanism dependent on the DF3/MUC1 oncoprotein. Mol. Cancer Res. 2003, 1, 765–775. [Google Scholar]
- Hassan, C.; Zullo, A.; Risio, M.; Rossini, F.P.; Morini, S. Histologic risk factors and clinical outcome in colorectal malignant polyp: A pooled-data analysis. Dis. Colon Rectum 2005, 48, 1588–1596. [Google Scholar] [CrossRef]
- Deschner, E.E. Cell turnover and colon tumor development. Prev. Med. 1987, 16, 580–585. [Google Scholar] [CrossRef]
- Cappell, M.S. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol. Clin. N. Am. 2008, 37, 1–24. [Google Scholar] [CrossRef]
- Gerken, T.A.; Butenhof, K.J.; Shogren, R. Effects of glycosylation on the conformation and dynamics of O-linked glycoproteins: Carbon-13 NMR studies of ovine submaxillary mucin. Biochemistry 1989, 28, 5536–5543. [Google Scholar] [CrossRef]
- Shogren, R.; Gerken, T.A.; Jentoft, N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: Light-scattering studies of ovine submaxillary mucin. Biochemistry 1989, 28, 5525–5536. [Google Scholar] [CrossRef] [PubMed]
- Gum, J.R.J.; Hicks, J.W.; Toribara, N.W.; Rothe, E.M.; Lagace, R.E.; Kim, Y.S. The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J. Biol. Chem. 1992, 267, 21375–21383. [Google Scholar] [CrossRef] [PubMed]
- Meezaman, D.; Charles, P.; Daskal, E.; Polymeropoulos, M.H.; Martin, B.M.; Rose, M.C. Cloning and analysis of cDNA encoding a major airway glycoprotein, human tracheobronchial mucin (MUC5). J. Biol. Chem. 1994, 269, 12932–12939. [Google Scholar] [CrossRef]
- Jin, C.; Kenny, D.T.; Skoog, E.C.; Padra, M.; Adamczyk, B.; Vitizeva, V.; Thorell, A.; Venkatakrishnan, V.; Lindén, S.K.; Karlsson, N.G. Structural Diversity of Human Gastric Mucin Glycans. Mol. Cell Proteom. 2017, 16, 743–758. [Google Scholar] [CrossRef]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Teh, C.; Kishore, U.; Reid, K.B.M. Collectins and ficolins: Sugar pattern recognition molecules of the mammalian innate immune system. Biochim. Biophys. Acta 2002, 1572, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Thornton, D.J.; Rousseau, K.; McGuckin, M.A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008, 70, 459–486. [Google Scholar] [CrossRef]
- Fernández-Blanco, J.A.; Fakih, D.; Arike, L.; Rodríguez-Piñeiro, A.M.; Martínez-Abad, B.; Skansebo, E.; Jackson, S.; Root, J.; Singh, D.; McCrae, C.; et al. Attached stratified mucus separates bacteria from the epithelial cells in COPD lungs. J. Clin. Investig. 2018, 3, e120994. [Google Scholar] [CrossRef]
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, Mucus, and Goblet Cells. Chest 2018, 154, 169–176. [Google Scholar] [CrossRef]
- Joshi, S.; Kumar, S.; Bafna, S.; Rachagani, S.; Wagner, K.; Jain, M.; Batra, S.K. Genetically engineered mucin mouse models for inflammation and cancer. Cancer Metastasis Rev. 2015, 34, 593–609. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Ocansey, D.K.W.; Wang, B.; Wang, L.; Xu, Z. Mucin-Type O-Glycans: Barrier, Microbiota, and Immune Anchors in Inflammatory Bowel Disease. J. Inflamm. Res. 2021, 14, 5939–5953. [Google Scholar] [CrossRef]
- Schwerbrock, N.M.J.; Makkink, M.K.; van der Sluis, M.; Büller, H.A.; Einerhand, A.W.C.; Sartor, R.B.; Dekker, J. Interleukin 10-deficient mice exhibit defective colonic Muc2 synthesis before and after induction of colitis by commensal bacteria. Inflamm. Bowel Dis. 2004, 10, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Ambort, D.; Pelaseyed, T.; Schütte, A.; Gustafsson, J.K.; Ermund, A.; Subramani, D.B.; Holmén-Larsson, J.M.; Thomsson, K.A.; Bergström, J.H.; et al. Composition and functional role of the mucus layers in the intestine. Cell Mol. Life Sci. 2011, 68, 3635–3641. [Google Scholar] [CrossRef]
- Bergstrom, K.; Fu, J.; Johansson, M.E.V.; Liu, X.; Gao, N.; Wu, Q.; Song, J.; McDaniel, J.; McGee, S.; Chen, W.; et al. Core 1- and 3-derived O-glycans collectively maintain the colonic mucus barrier and protect against spontaneous colitis in mice. Mucosal Immunol. 2017, 10, 91–103. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yamamoto, K. Mucin glycans and their degradation by gut microbiota. Glycoconj. J. 2023, 40, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009, 19, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Palmai-Pallag, T.; Khodabukus, N.; Kinarsky, L.; Leir, S.; Sherman, S.; Hollingsworth, M.A.; Harris, A. The role of the SEA (sea urchin sperm protein, enterokinase and agrin) module in cleavage of membrane-tethered mucins. FEBS J. 2005, 272, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Bork, P.; Patthy, L. The SEA module: A new extracellular domain associated with O-glycosylation. Protein Sci. 1995, 4, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Wreschner, D.H.; McGuckin, M.A.; Williams, S.J.; Baruch, A.; Yoeli, M.; Ziv, R.; Okun, L.; Zaretsky, J.; Smorodinsky, N.; Keydar, I.; et al. Generation of ligand-receptor alliances by “SEA” module-mediated cleavage of membrane-associated mucin proteins. Protein Sci. 2002, 11, 698–706. [Google Scholar] [CrossRef]
- Macao, B.; Johansson, D.G.A.; Hansson, G.C.; Härd, T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat. Struct. Mol. Biol. 2006, 13, 71–76. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Zäch, M.; Petersson, A.C.; Svensson, F.; Johansson, D.G.A.; Hansson, G.C. Unfolding dynamics of the mucin SEA domain probed by force spectroscopy suggest that it acts as a cell-protective device. FEBS J. 2013, 280, 1491–1501. [Google Scholar] [CrossRef]
- Beatson, R.; Tajadura-Ortega, V.; Achkova, D.; Picco, G.; Tsourouktsoglou, T.-D.; Klausing, S.; Hillier, M.; Maher, D.A.J.; Noll, S.K.T.; Crocker, P.R.; et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat. Immunol. 2016, 17, 1273–1281. [Google Scholar] [CrossRef]
- Yin, B.W.T.; Dnistrian, A.; Lloyd, K.O. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int. J. Cancer 2002, 98, 737–740. [Google Scholar] [CrossRef]
- Morrison, C.B.; Markovetz, M.R.; Ehre, C. Mucus, mucins, and cystic fibrosis. Pediatr. Pulmonol. 2019, 54 (Suppl. S3), S84–S96. [Google Scholar] [CrossRef]
- Kudelka, M.R.; Stowell, S.R.; Cummings, R.D.; Neish, A.S. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 597–617. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.B.; Lavrsen, K.; Steentoft, C.; Vester-Christensen, M.B.; Clausen, H.; Wandall, H.H.; Pedersen, A.E. Glycan elongation beyond the mucin associated Tn antigen protects tumor cells from immune-mediated killing. PLoS ONE 2013, 8, e72413. [Google Scholar] [CrossRef]
- Sagar, S.; Leiphrakpam, P.D.; Thomas, D.; McAndrews, K.L.; Caffrey, T.C.; Swanson, B.J.; Clausen, H.; Wandall, H.H.; Hollingsworth, M.A.; Radhakrishnan, P. MUC4 enhances gemcitabine resistance and malignant behaviour in pancreatic cancer cells expressing cancer-associated short O-glycans. Cancer Lett. 2021, 503, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Alos, L.; Lujan, B.; Castillo, M.; Nadal, A.; Carreras, M.; Caballero, M.; de Bolos, C.; Cardesa, A. Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am. J. Surg. Pathol. 2005, 29, 806–813. [Google Scholar] [CrossRef]
- Das, S.; Rachagani, S.; Torres-Gonzalez, M.P.; Lakshmanan, I.; Majhi, P.D.; Smith, L.M.; Wagner, K.; Batra, S.K. Carboxyl-terminal domain of MUC16 imparts tumorigenic and metastatic functions through nuclear translocation of JAK2 to pancreatic cancer cells. Oncotarget 2015, 6, 5772–5787. [Google Scholar] [CrossRef] [PubMed]
- Carraway, K.L.; Ramsauer, V.P.; Haq, B.; Carothers Carraway, C.A. Cell signaling through membrane mucins. Bioessays 2003, 25, 66–71. [Google Scholar] [CrossRef]
- Savage, C.R.J.; Hash, J.H.; Cohen, S. Epidermal growth factor. Location of disulfide bonds. J. Biol. Chem. 1973, 248, 7669–7672. [Google Scholar] [CrossRef]
- Winkler, M.E.; Bringman, T.; Marks, B.J. The purification of fully active recombinant transforming growth factor alpha produced in Escherichia coli. J. Biol. Chem. 1986, 261, 13838–13843. [Google Scholar] [CrossRef]
- Nadel, J.A. Role of epidermal growth factor receptor activation in regulating mucin synthesis. Respir. Res. 2001, 2, 85–89. [Google Scholar] [CrossRef]
- Moniaux, N.; Nollet, S.; Porchet, N.; Degand, P.; Laine, A.; Aubert, J.P. Complete sequence of the human mucin MUC4: A putative cell membrane-associated mucin. Biochem. J. 1999, 338 Pt 2, 325–333. [Google Scholar] [CrossRef]
- Malmberg, E.K.; Pelaseyed, T.; Petersson, A.C.; Seidler, U.E.; De Jonge, H.; Riordan, J.R.; Hansson, G.C. The C-terminus of the transmembrane mucin MUC17 binds to the scaffold protein PDZK1 that stably localizes it to the enterocyte apical membrane in the small intestine. Biochem. J. 2008, 410, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, G.; Seidler, U. The emerging role of PDZ adapter proteins for regulation of intestinal ion transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, 766. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Hansson, G.C. CFTR anion channel modulates expression of human transmembrane mucin MUC3 through the PDZ protein GOPC. J. Cell Sci. 2011, 124, 3074–3083. [Google Scholar] [CrossRef]
- Huang, L.; Chen, D.; Liu, D.; Yin, L.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005, 65, 10413–10422. [Google Scholar] [CrossRef]
- Tailford, L.E.; Crost, E.H.; Kavanaugh, D.; Juge, N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 2015, 6, 81. [Google Scholar] [CrossRef]
- Kato, K.; Jeanneau, C.; Tarp, M.A.; Benet-Pagès, A.; Lorenz-Depiereux, B.; Bennett, E.P.; Mandel, U.; Strom, T.M.; Clausen, H. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 2006, 281, 18370–18377. [Google Scholar] [CrossRef]
- Ringot-Destrez, B.; Kalach, N.; Mihalache, A.; Gosset, P.; Michalski, J.; Léonard, R.; Robbe-Masselot, C. How do they stick together? Bacterial adhesins implicated in the binding of bacteria to the human gastrointestinal mucins. Biochem. Soc. Trans. 2017, 45, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.; Wakarchuk, W. O-glycosylation and its role in therapeutic proteins. Biosci. Rep. 2022, 42, BSR20220094. [Google Scholar] [CrossRef] [PubMed]
- Znamenskaya, Y.; Sotres, J.; Gavryushov, S.; Engblom, J.; Arnebrant, T.; Kocherbitov, V. Water sorption and glass transition of pig gastric mucin studied by QCM-D. J. Phys. Chem. B 2013, 117, 2554–2563. [Google Scholar] [CrossRef]
- Svensson, O.; Lindh, L.; Cárdenas, M.; Arnebrant, T. Layer-by-layer assembly of mucin and chitosan—Influence of surface properties, concentration and type of mucin. J. Colloid Interface Sci. 2006, 299, 608–616. [Google Scholar] [CrossRef]
- Hanson, R.L.; Hollingsworth, M.A. Functional Consequences of Differential O-glycosylation of MUC1, MUC4, and MUC16 (Downstream Effects on Signaling). Biomolecules 2016, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Wen, Y.; Swanson, B.J.; Shanmugam, K.; Kazlauskas, A.; Cerny, R.L.; Gendler, S.J.; Hollingsworth, M.A. Platelet-derived growth factor receptor beta-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res. 2007, 67, 5201–5210. [Google Scholar] [CrossRef]
- Schroeder, J.A.; Thompson, M.C.; Gardner, M.M.; Gendler, S.J. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J. Biol. Chem. 2001, 276, 13057–13064. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Behrens, M.E.; Eggers, J.P.; Cerny, R.L.; Bailey, J.M.; Shanmugam, K.; Gendler, S.J.; Bennett, E.P.; Hollingsworth, M.A. Phosphorylation of MUC1 by Met modulates interaction with p53 and MMP1 expression. J. Biol. Chem. 2008, 283, 26985–26995. [Google Scholar] [CrossRef]
- Wei, X.; Xu, H.; Kufe, D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell 2005, 7, 167–178. [Google Scholar] [CrossRef]
- Li, Y.; Kufe, D. The Human DF3/MUC1 carcinoma-associated antigen signals nuclear localization of the catenin p120(ctn). Biochem. Biophys. Res. Commun. 2001, 281, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yi, C.; Wen, Y.; Radhakrishnan, P.; Tremayne, J.R.; Dao, T.; Johnson, K.R.; Hollingsworth, M.A. Interactions between MUC1 and p120 catenin regulate dynamic features of cell adhesion, motility, and metastasis. Cancer Res. 2014, 74, 1609–1620. [Google Scholar] [CrossRef]
- Kufe, D.W. MUC1-C oncoprotein as a target in breast cancer: Activation of signaling pathways and therapeutic approaches. Oncogene 2013, 32, 1073–1081. [Google Scholar] [CrossRef]
- Khodarev, N.; Ahmad, R.; Rajabi, H.; Pitroda, S.; Kufe, T.; McClary, C.; Joshi, M.D.; MacDermed, D.; Weichselbaum, R.; Kufe, D. Cooperativity of the MUC1 oncoprotein and STAT1 pathway in poor prognosis human breast cancer. Oncogene 2010, 29, 920–929. [Google Scholar] [CrossRef]
- Ahmad, R.; Raina, D.; Joshi, M.D.; Kawano, T.; Ren, J.; Kharbanda, S.; Kufe, D. MUC1-C oncoprotein functions as a direct activator of the nuclear factor-kappaB p65 transcription factor. Cancer Res. 2009, 69, 7013–7021. [Google Scholar] [CrossRef]
- Ahmad, R.; Rajabi, H.; Kosugi, M.; Joshi, M.D.; Alam, M.; Vasir, B.; Kawano, T.; Kharbanda, S.; Kufe, D. MUC1-C oncoprotein promotes STAT3 activation in an autoinductive regulatory loop. Sci. Signal 2011, 4, ra9. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, S.; Ramasamy, S.; Kharbanda, S.; Kufe, D. Distinct evolution of the human carcinoma-associated transmembrane mucins, MUC1, MUC4 and MUC16. Gene 2006, 373, 28–34. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Singh, A.P.; Batra, S.K. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008, 22, 966–981. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, I.; Seshacharyulu, P.; Haridas, D.; Rachagani, S.; Gupta, S.; Joshi, S.; Guda, C.; Yan, Y.; Jain, M.; Ganti, A.K.; et al. Novel HER3/MUC4 oncogenic signaling aggravates the tumorigenic phenotypes of pancreatic cancer cells. Oncotarget 2015, 6, 21085–21099. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Singh, A.P.; Chakraborty, S.; Chauhan, S.C.; Bafna, S.; Meza, J.L.; Singh, P.K.; Hollingsworth, M.A.; Mehta, P.P.; Batra, S.K. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008, 68, 2065–2070. [Google Scholar] [CrossRef]
- Nagy, P.; Friedländer, E.; Tanner, M.; Kapanen, A.I.; Carraway, K.L.; Isola, J.; Jovin, T.M. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005, 65, 473–482. [Google Scholar] [CrossRef]
- Zhi, X.; Tao, J.; Xie, K.; Zhu, Y.; Li, Z.; Tang, J.; Wang, W.; Xu, H.; Zhang, J.; Xu, Z. MUC4-induced nuclear translocation of β-catenin: A novel mechanism for growth, metastasis and angiogenesis in pancreatic cancer. Cancer Lett. 2014, 346, 104–113. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, Y.; Xie, K.; Zhang, X.; Zhi, X.; Wang, W.; Li, Z.; Zhang, Q.; Wang, L.; Wang, J.; et al. The role of the AMOP domain in MUC4/Y-promoted tumour angiogenesis and metastasis in pancreatic cancer. J. Exp. Clin. Cancer Res. 2016, 35, 91. [Google Scholar] [CrossRef]
- O’Brien, T.J.; Beard, J.B.; Underwood, L.J.; Dennis, R.A.; Santin, A.D.; York, L. The CA 125 gene: An extracellular superstructure dominated by repeat sequences. Tumour Biol. 2001, 22, 348–366. [Google Scholar] [CrossRef]
- Haridas, D.; Ponnusamy, M.P.; Chugh, S.; Lakshmanan, I.; Seshacharyulu, P.; Batra, S.K. MUC16: Molecular analysis and its functional implications in benign and malignant conditions. FASEB J. 2014, 28, 4183–4199. [Google Scholar] [CrossRef]
- Das, S.; Majhi, P.D.; Al-Mugotir, M.H.; Rachagani, S.; Sorgen, P.; Batra, S.K. Membrane proximal ectodomain cleavage of MUC16 occurs in the acidifying Golgi/post-Golgi compartments. Sci. Rep. 2015, 5, 9759. [Google Scholar] [CrossRef]
- Lakshmanan, I.; Ponnusamy, M.P.; Das, S.; Chakraborty, S.; Haridas, D.; Mukhopadhyay, P.; Lele, S.M.; Batra, S.K. MUC16 induced rapid G2/M transition via interactions with JAK2 for increased proliferation and anti-apoptosis in breast cancer cells. Oncogene 2012, 31, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Akita, K.; Tanaka, M.; Tanida, S.; Mori, Y.; Toda, M.; Nakada, H. CA125/MUC16 interacts with Src family kinases, and over-expression of its C-terminal fragment in human epithelial cancer cells reduces cell-cell adhesion. Eur. J. Cell Biol. 2013, 92, 257–263. [Google Scholar] [CrossRef]
- Thériault, C.; Pinard, M.; Comamala, M.; Migneault, M.; Beaudin, J.; Matte, I.; Boivin, M.; Piché, A.; Rancourt, C. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol. Oncol. 2011, 121, 434–443. [Google Scholar] [CrossRef]
- Ligtenberg, M.J.; Kruijshaar, L.; Buijs, F.; van Meijer, M.; Litvinov, S.V.; Hilkens, J. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J. Biol. Chem. 1992, 267, 6171–6177. [Google Scholar] [CrossRef] [PubMed]
- Levitin, F.; Stern, O.; Weiss, M.; Gil-Henn, C.; Ziv, R.; Prokocimer, Z.; Smorodinsky, N.I.; Rubinstein, D.B.; Wreschner, D.H. The MUC1 SEA module is a self-cleaving domain. J. Biol. Chem. 2005, 280, 33374–33386. [Google Scholar] [CrossRef]
- Escande, F.; Lemaitre, L.; Moniaux, N.; Batra, S.K.; Aubert, J.; Buisine, M. Genomic organization of MUC4 mucin gene. Towards the characterization of splice variants. Eur. J. Biochem. 2002, 269, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Thathiah, A.; Carson, D.D. MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. Biochem J. 2004, 382, 363–373. [Google Scholar] [CrossRef]
- Zhang, L.; Muirhead, K.J.; Syed, Z.A.; Dimitriadis, E.K.; Ten Hagen, K.G. A novel cysteine-rich adaptor protein is required for mucin packaging and secretory granule stability in vivo. Proc. Natl. Acad. Sci. USA 2024, 121, e2314309121. [Google Scholar] [CrossRef]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef]
- Roberton, A.M.; McKenzie, C.G.; Sharfe, N.; Stubbs, L.B. A glycosulphatase that removes sulphate from mucus glycoprotein. Biochem. J. 1993, 293 Pt 3, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Nordman, H.; Davies, J.R.; Lindell, G.; Carlstedt, I. Human gastric mucins--a major population identified as MUC5. Biochem Soc. Trans. 1995, 23, 533S. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.B.; Roberton, A.M.; Shekels, L.L.; Lyftogt, C.T.; Niehans, G.A.; Toribara, N.W. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology 1995, 109, 735–747. [Google Scholar] [CrossRef]
- Bhaskar, K.R.; Garik, P.; Turner, B.S.; Bradley, J.D.; Bansil, R.; Stanley, H.E.; LaMont, J.T. Viscous fingering of HCl through gastric mucin. Nature 1992, 360, 458–461. [Google Scholar] [CrossRef]
- Cunningham, M.; Beachey, E.H. Immunochemical properties of streptococcal M protein purified by isoelectric focusing. J. Immunol. 1975, 115, 1002–1006. [Google Scholar] [CrossRef]
- Cebo, C.; Dambrouck, T.; Maes, E.; Laden, C.; Strecker, G.; Michalski, J.C.; Zanetta, J.P. Recombinant human interleukins IL-1alpha, IL-1beta, IL-4, IL-6, and IL-7 show different and specific calcium-independent carbohydrate-binding properties. J. Biol. Chem. 2001, 276, 5685–5691. [Google Scholar] [CrossRef]
- Dabbagh, K.; Takeyama, K.; Lee, H.M.; Ueki, I.F.; Lausier, J.A.; Nadel, J.A. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J. Immunol. 1999, 162, 6233–6237. [Google Scholar] [CrossRef] [PubMed]
- Enss, M.L.; Cornberg, M.; Wagner, S.; Gebert, A.; Henrichs, M.; Eisenblätter, R.; Beil, W.; Kownatzki, R.; Hedrich, H.J. Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm Res. 2000, 49, 162–169. [Google Scholar] [CrossRef]
- Thim, L.; Madsen, F.; Poulsen, S.S. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur. J. Clin. Investig. 2002, 32, 519–527. [Google Scholar] [CrossRef]
- Tran, C.P.; Cook, G.A.; Yeomans, N.D.; Thim, L.; Giraud, A.S. Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. Gut 1999, 44, 636–642. [Google Scholar] [CrossRef]
- Taupin, D.; Wu, D.C.; Jeon, W.K.; Devaney, K.; Wang, T.C.; Podolsky, D.K. The trefoil gene family are coordinately expressed immediate-early genes: EGF receptor- and MAP kinase-dependent interregulation. J. Clin. Investig. 1999, 103, 31. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.A.; Hoffmann, W.; Otto, W.R.; Rio, M.C.; Thim, L. Rolling in the clover: Trefoil factor family (TFF)-domain peptides, cell migration and cancer. FEBS Lett. 1997, 408, 121–123. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ogata, H.; Morikawa, M.; Iijima, S.; Harada, N.; Yoshida, T.; Brown, W.R.; Inoue, N.; Hamada, Y.; Ishii, H.; et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut 2002, 51, 169–176. [Google Scholar] [CrossRef]
- Phalipon, A.; Cardona, A.; Kraehenbuhl, J.P.; Edelman, L.; Sansonetti, P.J.; Corthésy, B. Secretory component: A new role in secretory IgA-mediated immune exclusion in vivo. Immunity 2002, 17, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Niv, Y.; Rokkas, T. Mucin Expression in Colorectal Cancer (CRC): Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2019, 53, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Chen, F.-Y.; Wu, S.-Y.; Hu, B.; Liang, X.-L.; Yang, H.-Q.; Cheng, J.-W.; Wang, P.-X.; Guo, W.; Zhou, J.; et al. Mucin 1 promotes tumor progression through activating WNT/β-catenin signaling pathway in intrahepatic cholangiocarcinoma. J. Cancer 2021, 12, 6937–6947. [Google Scholar] [CrossRef] [PubMed]
- Gundamaraju, R.; Chong, W.C. Consequence of distinctive expression of MUC2 in colorectal cancers: How much is actually bad? Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188579. [Google Scholar] [CrossRef]
- Zhang, C.; He, K.; Pan, F.; Li, Y.; Wu, J. Prognostic value of Muc5AC in gastric cancer: A meta-analysis. World J. Gastroenterol. 2015, 21, 10453–10460. [Google Scholar] [CrossRef]
- Rathee, R.; Devi, A.; Narwal, A.; Kamboj, M.; Singh, S. Immunohistochemical Coexpression of MUC1 and MUC4 in Oral Leukoplakia and Oral Squamous Cell Carcinoma. Head Neck Pathol. 2021, 15, 831–842. [Google Scholar] [CrossRef]
- Albrecht, H.; Carraway, K.L. MUC1 and MUC4: Switching the emphasis from large to small. Cancer Biother. Radiopharm. 2011, 26, 261–271. [Google Scholar] [CrossRef]
- Kaur, S.; Momi, N.; Chakraborty, S.; Wagner, D.G.; Horn, A.J.; Lele, S.M.; Theodorescu, D.; Batra, S.K. Altered expression of transmembrane mucins, MUC1 and MUC4, in bladder cancer: Pathological implications in diagnosis. PLoS ONE 2014, 9, e92742. [Google Scholar] [CrossRef]
- Yokoyama, S.; Higashi, M.; Kitamoto, S.; Oeldorf, M.; Knippschild, U.; Kornmann, M.; Maemura, K.; Kurahara, H.; Wiest, E.; Hamada, T.; et al. Aberrant methylation of MUC1 and MUC4 promoters are potential prognostic biomarkers for pancreatic ductal adenocarcinomas. Oncotarget 2016, 7, 42553–42565. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kumar, S.; Momi, N.; Sasson, A.R.; Batra, S.K. Mucins in pancreatic cancer and its microenvironment. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Singh, A.P.; Moniaux, N.; Senapati, S.; Chakraborty, S.; Meza, J.L.; Batra, S.K. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol. Cancer Res. 2007, 5, 309–320. [Google Scholar] [CrossRef]
- Ponnusamy, M.P.; Singh, A.P.; Jain, M.; Chakraborty, S.; Moniaux, N.; Batra, S.K. MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. Br. J. Cancer 2008, 99, 520–526. [Google Scholar] [CrossRef]
- Singh, P.K.; Hollingsworth, M.A. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006, 16, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Besmer, D.M.; Curry, J.M.; Roy, L.D.; Tinder, T.L.; Sahraei, M.; Schettini, J.; Hwang, S.; Lee, Y.Y.; Gendler, S.J.; Mukherjee, P. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res. 2011, 71, 4432–4442. [Google Scholar] [CrossRef]
- Nath, S.; Daneshvar, K.; Roy, L.D.; Grover, P.; Kidiyoor, A.; Mosley, L.; Sahraei, M.; Mukherjee, P. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis 2013, 2, e51. [Google Scholar] [CrossRef]
- Li, Y.; Ren, J.; Yu, W.; Li, Q.; Kuwahara, H.; Yin, L.; Carraway, K.L. 3.; Kufe, D. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. J. Biol. Chem. 2001, 276, 35239–35242. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Y.; Chen, W.; Liang, T. Epigenetic deregulation of MLF1 drives intrahepatic cholangiocarcinoma progression through EGFR/AKT and Wnt/β-catenin signaling. Hepatol. Commun. 2023, 7, e0204. [Google Scholar] [CrossRef]
- Rajabi, H.; Alam, M.; Takahashi, H.; Kharbanda, A.; Guha, M.; Ahmad, R.; Kufe, D. MUC1-C oncoprotein activates the ZEB1/miR-200c regulatory loop and epithelial-mesenchymal transition. Oncogene 2014, 33, 1680–1689. [Google Scholar] [CrossRef]
- Zhai, Y.; Lu, Q.; Lou, T.; Cao, G.; Wang, S.; Zhang, Z. MUC16 affects the biological functions of ovarian cancer cells and induces an antitumor immune response by activating dendritic cells. Ann. Transl. Med. 2020, 8, 1494–6388. [Google Scholar] [CrossRef]
- Rajabi, H.; Tagde, A.; Alam, M.; Bouillez, A.; Pitroda, S.; Suzuki, Y.; Kufe, D. DNA methylation by DNMT1 and DNMT3b methyltransferases is driven by the MUC1-C oncoprotein in human carcinoma cells. Oncogene 2016, 35, 6439–6445. [Google Scholar] [CrossRef]
- Woo, J.K.; Choi, Y.; Oh, S.; Jeong, J.; Choi, D.; Seo, H.; Kim, C. Mucin 1 enhances the tumor angiogenic response by activation of the AKT signaling pathway. Oncogene 2012, 31, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Zrihan-Licht, S.; Baruch, A.; Elroy-Stein, O.; Keydar, I.; Wreschner, D.H. Tyrosine phosphorylation of the MUC1 breast cancer membrane proteins. Cytokine receptor-like molecules. FEBS Lett. 1994, 356, 130–136. [Google Scholar] [CrossRef]
- Rachagani, S.; Torres, M.P.; Kumar, S.; Haridas, D.; Baine, M.; A Macha, M.; Kaur, S.; Ponnusamy, M.P.; Dey, P.; Seshacharyulu, P.; et al. Mucin (Muc) expression during pancreatic cancer progression in spontaneous mouse model: Potential implications for diagnosis and therapy. J. Hematol. Oncol. 2012, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Andrianifahanana, M.; Moniaux, N.; Schmied, B.M.; Ringel, J.; Friess, H.; Hollingsworth, M.A.; Büchler, M.W.; Aubert, J.P.; Batra, S.K. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: A potential role of MUC4 as a tumor marker of diagnostic significance. Clin. Cancer Res. 2001, 7, 4033–4040. [Google Scholar] [PubMed]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Betge, J.; Schneider, N.I.; Harbaum, L.; Pollheimer, M.J.; Lindtner, R.A.; Kornprat, P.; Ebert, M.P.; Langner, C. MUC1, MUC2, MUC5AC, and MUC6 in colorectal cancer: Expression profiles and clinical significance. Virchows Arch. 2016, 469, 255–265. [Google Scholar] [CrossRef]
- Wang, J.; Chang, C.; Hsieh, J.; Lee, L.; Huang, T.; Chai, C.; Lin, S. Role of MUC1 and MUC5AC expressions as prognostic indicators in gastric carcinomas. J. Surg. Oncol. 2003, 83, 253–260. [Google Scholar] [CrossRef]
- Kocer, B.; Soran, A.; Kiyak, G.; Erdogan, S.; Eroglu, A.; Bozkurt, B.; Solak, C.; Cengiz, O. Prognostic significance of mucin expression in gastric carcinoma. Dig. Dis. Sci. 2004, 49, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Yamashita, N.; Daimon, T.; Hirose, H.; Yamano, S.; Haratake, N.; Ishikawa, S.; Bhattacharya, A.; Fushimi, A.; Ahmad, R.; et al. MUC1-C is a master regulator of MICA/B NKG2D ligand and exosome secretion in human cancer cells. J. Immunother. Cancer 2023, 11, e006238. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.W. Emergence of MUC1 in Mammals for Adaptation of Barrier Epithelia. Cancers 2022, 14, 4805. [Google Scholar] [CrossRef]
- Cadoux, M.; Caruso, S.; Pham, S.; Gougelet, A.; Pophillat, C.; Riou, R.; Loesch, R.; Colnot, S.; Nguyen, C.T.; Calderaro, J.; et al. Expression of NKG2D ligands is downregulated by β-catenin signalling and associates with HCC aggressiveness. J. Hepatol. 2021, 74, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, L.; Yu, W.; Wu, Q.; Lian, J.; Li, F.; Liu, S.; Li, A.; He, Z.; Liu, J.; et al. Colorectal cancer cell-derived CCL20 recruits regulatory T cells to promote chemoresistance via FOXO1/CEBPB/NF-κB signaling. J. Immunother. Cancer 2019, 7, 215. [Google Scholar] [CrossRef]
- Ganguly, K.; Shah, A.; Atri, P.; Rauth, S.; Ponnusamy, M.P.; Kumar, S.; Batra, S.K. Chemokine-mucinome interplay in shaping the heterogeneous tumor microenvironment of pancreatic cancer. Semin. Cancer Biol. 2022, 86, 511–520. [Google Scholar] [CrossRef]
- Shimizu, A.; Hirono, S.; Tani, M.; Kawai, M.; Okada, K.; Miyazawa, M.; Kitahata, Y.; Nakamura, Y.; Noda, T.; Yokoyama, S.; et al. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci. 2012, 103, 739–746. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Guruvayoorappan, C. Pathological Implications of Mucin Signaling in Metastasis. Curr. Cancer Drug Targets 2023, 23, 585–602. [Google Scholar] [CrossRef]
- Singh, A.P.; Moniaux, N.; Chauhan, S.C.; Meza, J.L.; Batra, S.K. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004, 64, 622–630. [Google Scholar] [CrossRef]
- Ohira, G.; Kimura, K.; Yamada, N.; Amano, R.; Nakata, B.; Doi, Y.; Murata, A.; Yashiro, M.; Tanaka, S.; Ohsawa, M.; et al. MUC1 and HER2 might be associated with invasive phenotype of intraductal papillary mucinous neoplasm. Hepatogastroenterology 2013, 60, 1067–1072. [Google Scholar]
- Pang, Z.; Dong, X.; Deng, H.; Wang, C.; Liao, X.; Liao, C.; Liao, Y.; Tian, W.; Cheng, J.; Chen, G.; et al. MUC1 triggers lineage plasticity of Her2 positive mammary tumors. Oncogene 2022, 41, 3064–3078. [Google Scholar] [CrossRef] [PubMed]
- Muniyan, S.; Haridas, D.; Chugh, S.; Rachagani, S.; Lakshmanan, I.; Gupta, S.; Seshacharyulu, P.; Smith, L.M.; Ponnusamy, M.P.; Batra, S.K. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes. Cancer 2016, 7, 110–124. [Google Scholar] [CrossRef]
- Lakshmanan, I.; Marimuthu, S.; Chaudhary, S.; Seshacharyulu, P.; Rachagani, S.; Muniyan, S.; Chirravuri-Venkata, R.; Atri, P.; Rauth, S.; Nimmakayala, R.K.; et al. Muc16 depletion diminishes KRAS-induced tumorigenesis and metastasis by altering tumor microenvironment factors in pancreatic ductal adenocarcinoma. Oncogene 2022, 41, 5147–5159. [Google Scholar] [CrossRef]
- Geng, Y.; Yeh, K.; Takatani, T.; King, M.R. Three to Tango: MUC1 as a Ligand for Both E-Selectin and ICAM-1 in the Breast Cancer Metastatic Cascade. Front. Oncol. 2012, 2, 76. [Google Scholar] [CrossRef] [PubMed]
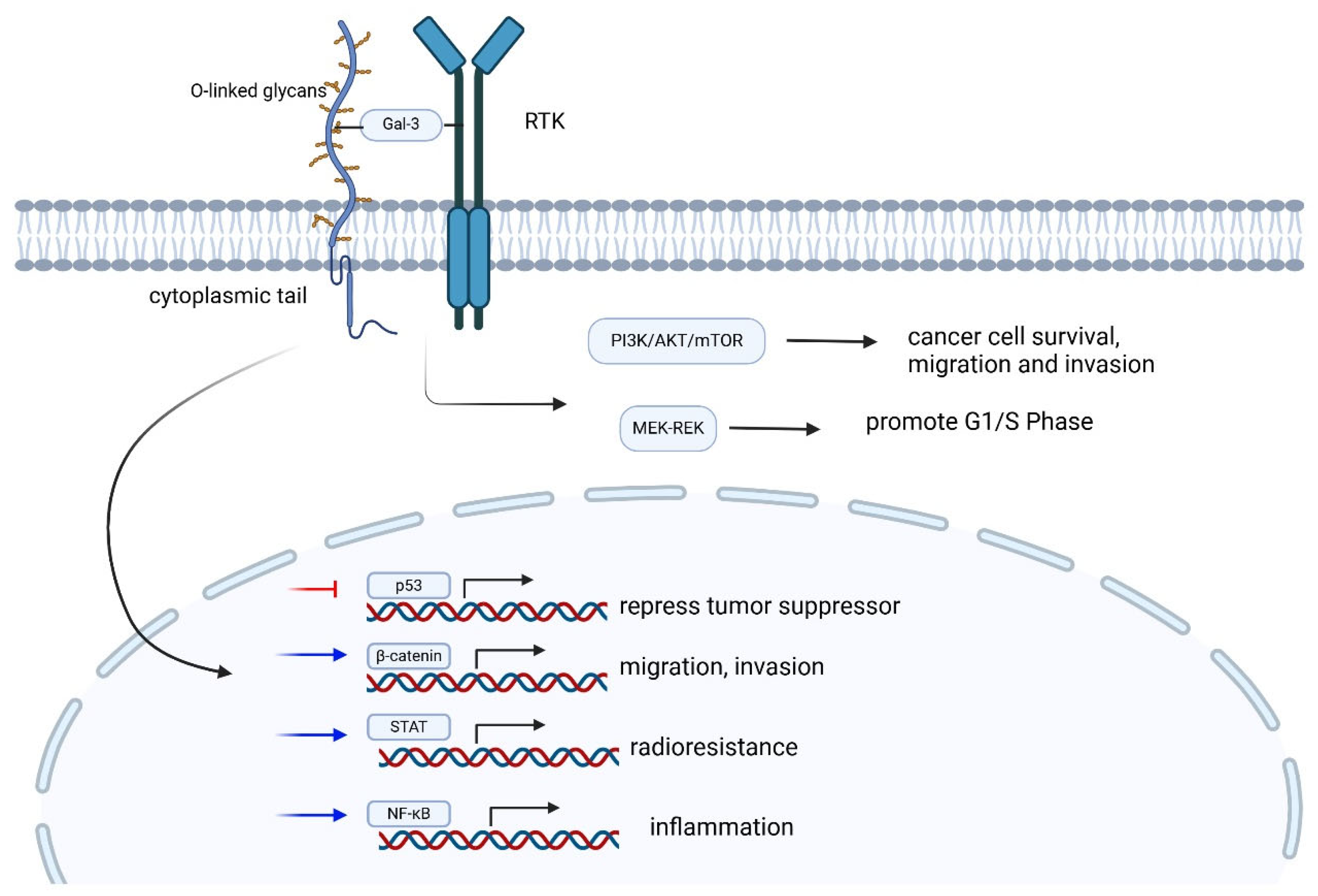
- Coppin, L.; Vincent, A.; Frénois, F.; Duchêne, B.; Lahdaoui, F.; Stechly, L.; Renaud, F.; Villenet, C.; Van Seuningen, I.; Leteurtre, E.; et al. Galectin-3 is a non-classic RNA binding protein that stabilizes the mucin MUC4 mRNA in the cytoplasm of cancer cells. Sci. Rep. 2017, 7, 43927. [Google Scholar] [CrossRef]
- Yu, L.-G.; Andrews, N.; Zhao, Q.; McKean, D.; Williams, J.F.; Connor, L.J.; Gerasimenko, O.V.; Hilkens, J.; Hirabayashi, J.; Kasai, K.; et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J. Biol. Chem. 2007, 282, 773–781. [Google Scholar] [CrossRef]
- Zhao, Q.; Barclay, M.; Hilkens, J.; Guo, X.; Barrow, H.; Rhodes, J.M.; Yu, L. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 2010, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guo, X.; Nash, G.B.; Stone, P.C.; Hilkens, J.; Rhodes, J.M.; Yu, L. Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 2009, 69, 6799–6806. [Google Scholar] [CrossRef]
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef]
- Ogata, S.; Maimonis, P.J.; Itzkowitz, S.H. Mucins bearing the cancer-associated sialosyl-Tn antigen mediate inhibition of natural killer cell cytotoxicity. Cancer Res. 1992, 52, 4741–4746. [Google Scholar]
- van de Wiel-van Kemenade, E.; Ligtenberg, M.J.; de Boer, A.J.; Buijs, F.; Vos, H.L.; Melief, C.J.; Hilkens, J.; Figdor, C.G. Episialin (MUC1) inhibits cytotoxic lymphocyte-target cell interaction. J. Immunol. 1993, 151, 767–776. [Google Scholar] [CrossRef]
- Bhatia, R.; Gautam, S.K.; Cannon, A.; Thompson, C.; Hall, B.R.; Aithal, A.; Banerjee, K.; Jain, M.; Solheim, J.C.; Kumar, S.; et al. Cancer-associated mucins: Role in immune modulation and metastasis. Cancer Metastasis Rev. 2019, 38, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Maman, S.; Witz, I.P. A history of exploring cancer in context. Nat. Rev. Cancer 2018, 18, 359–376. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Li, J.; Du, W.; Li, R.; Yang, J.; Li, J.; Li, F.; Tan, H. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Lyssiotis, C.A.; Kimmelman, A.C. Metabolic Interactions in the Tumor Microenvironment. Trends Cell Biol. 2017, 27, 863–875. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Kitamoto, S.; Yokoyama, S.; Higashi, M.; Yamada, N.; Takao, S.; Yonezawa, S. MUC1 enhances hypoxia-driven angiogenesis through the regulation of multiple proangiogenic factors. Oncogene 2013, 32, 4614–4621. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.D.; Sahraei, M.; Subramani, D.B.; Besmer, D.; Nath, S.; Tinder, T.L.; Bajaj, E.; Shanmugam, K.; Lee, Y.Y.; Hwang, S.I.L.; et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011, 30, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Zadeh, L.R.; Baradaran, B.; Molavi, O.; Ghesmati, Z.; Sabzichi, M.; Ramezani, F. Up-down regulation of HIF-1α in cancer progression. Gene 2021, 798, 145796. [Google Scholar] [CrossRef] [PubMed]
- Aubert, S.; Fauquette, V.; Hémon, B.; Lepoivre, R.; Briez, N.; Bernard, D.; Van Seuningen, I.; Leroy, X.; Perrais, M. MUC1, a new hypoxia inducible factor target gene, is an actor in clear renal cell carcinoma tumor progression. Cancer Res. 2009, 69, 5707–5715. [Google Scholar] [CrossRef]
- Jing, X.; Liang, H.; Hao, C.; Yang, X.; Cui, X. Overexpression of MUC1 predicts poor prognosis in patients with breast cancer. Oncol. Rep. 2019, 41, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Yang, J.M.; Burchell, J.; Whitehouse, C.; Taylor-Papadimitriou, J. Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. Eur. J. Biochem. 1995, 233, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Burchell, J.; Poulsom, R.; Hanby, A.; Whitehouse, C.; Cooper, L.; Clausen, H.; Miles, D.; Taylor-Papadimitriou, J. An alpha2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology 1999, 9, 1307–1311. [Google Scholar] [CrossRef]
- Park, J.; Nishidate, T.; Kijima, K.; Ohashi, T.; Takegawa, K.; Fujikane, T.; Hirata, K.; Nakamura, Y.; Katagiri, T. Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010, 70, 2759–2769. [Google Scholar] [CrossRef]
- Wu, C.; Guo, X.; Wang, W.; Wang, Y.; Shan, Y.; Zhang, B.; Song, W.; Ma, S.; Ge, J.; Deng, H.; et al. N-Acetylgalactosaminyltransferase-14 as a potential biomarker for breast cancer by immunohistochemistry. BMC Cancer 2010, 10, 123. [Google Scholar] [CrossRef]
- Picco, G.; Julien, S.; Brockhausen, I.; Beatson, R.; Antonopoulos, A.; Haslam, S.; Mandel, U.; Dell, A.; Pinder, S.; Taylor-Papadimitriou, J.; et al. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology 2010, 20, 1241–1250. [Google Scholar] [CrossRef]
- Tiainen, S.; Tumelius, R.; Rilla, K.; Hämäläinen, K.; Tammi, M.; Tammi, R.; Kosma, V.; Oikari, S.; Auvinen, P. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 2015, 66, 873–883. [Google Scholar] [CrossRef]
- Smarr, K.L.; Parker, J.C.; Wright, G.E.; Stucky-Ropp, R.C.; Buckelew, S.P.; Hoffman, R.W.; O’Sullivan, F.X.; Hewett, J.E. The importance of enhancing self-efficacy in rheumatoid arthritis. Arthritis Care Res. 1997, 10, 18–26. [Google Scholar] [CrossRef]
- Brockhausen, I. Mucin-type O-glycans in human colon and breast cancer: Glycodynamics and functions. EMBO Rep. 2006, 7, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I. Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta 1999, 1473, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Shibao, K.; Izumi, H.; Nakayama, Y.; Ohta, R.; Nagata, N.; Nomoto, M.; Matsuo, K.; Yamada, Y.; Kitazato, K.; Itoh, H.; et al. Expression of UDP-N-acetyl-alpha-D-galactosamine-polypeptide galNAc N-acetylgalactosaminyl transferase-3 in relation to differentiation and prognosis in patients with colorectal carcinoma. Cancer 2002, 94, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Itzkowitz, S.H.; Bloom, E.J.; Kokal, W.A.; Modin, G.; Hakomori, S.; Kim, Y.S. Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer 1990, 66, 1960–1966. [Google Scholar] [CrossRef]
- Nakamori, S.; Kameyama, M.; Imaoka, S.; Furukawa, H.; Ishikawa, O.; Sasaki, Y.; Kabuto, T.; Iwanaga, T.; Matsushita, Y.; Irimura, T. Increased expression of sialyl Lewis x antigen correlates with poor survival in patients with colorectal carcinoma: Clinicopathological and immunohistochemical study. Cancer Res. 1993, 53, 3632–3637. [Google Scholar]
- Belisle, J.A.; Horibata, S.; Jennifer, G.A.; Petrie, S.; Kapur, A.; André, S.; Gabius, H.-J.; Rancourt, C.; Connor, J.; Paulson, J.C.; et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol. Cancer 2010, 9, 118. [Google Scholar] [CrossRef]
- Kang, H.; Min, B.S.; Lee, K.Y.; Kim, N.K.; Kim, S.N.; Choi, J.; Kim, H. Loss of E-cadherin and MUC2 expressions correlated with poor survival in patients with stages II and III colorectal carcinoma. Ann. Surg. Oncol. 2011, 18, 711–719. [Google Scholar] [CrossRef]
- Ajioka, Y.; Allison, L.J.; Jass, J.R. Significance of MUC1 and MUC2 mucin expression in colorectal cancer. J. Clin. Pathol. 1996, 49, 560–564. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Shan, Y.; Hsu, H.; Lai, M.; Yen, M.; Fang, J.; Weng, T.; Chen, Y. Suppression of mucin 2 promotes interleukin-6 secretion and tumor growth in an orthotopic immune-competent colon cancer animal model. Oncol. Rep. 2014, 32, 2335–2342. [Google Scholar] [CrossRef]
- Wang, H.; Jin, S.; Lu, H.; Mi, S.; Shao, W.; Zuo, X.; Yin, H.; Zeng, S.; Shimamoto, F.; Qi, G. Expression of survivin, MUC2 and MUC5 in colorectal cancer and their association with clinicopathological characteristics. Oncol. Lett. 2017, 14, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Yamagishi, H.; Fukuda, K.; Ono, Y.; Inoue, T.; Ueda, Y. Differential mucin phenotypes and their significance in a variation of colorectal carcinoma. World J. Gastroenterol. 2013, 19, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.D.; Clendenning, M.; Williamson, E.; Pearson, S.-A.; Walters, R.J.; Nagler, B.; Packenas, D.; Win, A.K.; Hopper, J.L.; Jenkins, M.A.; et al. Expression of MUC2, MUC5AC, MUC5B, and MUC6 mucins in colorectal cancers and their association with the CpG island methylator phenotype. Mod. Pathol. 2013, 26, 1642–1656. [Google Scholar] [CrossRef]
- Pothuraju, R.; Rachagani, S.; Krishn, S.R.; Chaudhary, S.; Nimmakayala, R.K.; Siddiqui, J.A.; Ganguly, K.; Lakshmanan, I.; Cox, J.L.; Mallya, K.; et al. Molecular implications of MUC5AC-CD44 axis in colorectal cancer progression and chemoresistance. Mol. Cancer 2020, 19, 37. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Kannagi, R. Carbohydrate antigen sialyl Lewis a—Its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med. J. 2007, 30, 189–209. [Google Scholar]
- Ballehaninna, U.K.; Chamberlain, R.S. Serum CA 19-9 as a Biomarker for Pancreatic Cancer—A Comprehensive Review. Indian J. Surg. Oncol. 2011, 2, 88–100. [Google Scholar] [CrossRef]
- Liu, J.F.; Moore, K.N.; Birrer, M.J.; Berlin, S.; Matulonis, U.A.; Infante, J.R.; Wolpin, B.; Poon, K.A.; Firestein, R.; Xu, J.; et al. Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody-drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann. Oncol. 2016, 27, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, X.; Lu, S.; Chen, C.; Wang, J.; Zheng, Y.; Ren, B.; Xu, L. Clinicopathological and prognostic significance of MUC4 expression in cancers: Evidence from meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 10274–10283. [Google Scholar]
- Klöppel, G.; Kosmahl, M. Is the intraductal papillary mucinous neoplasia of the biliary tract a counterpart of pancreatic papillary mucinous neoplasm? J. Hepatol. 2006, 44, 249–250. [Google Scholar] [CrossRef]
- Fu, X.; Tang, N.; Xie, W.; Mao, L.; Qiu, Y. MUC1 promotes glycolysis through inhibiting BRCA1 expression in pancreatic cancer. Chin. J. Nat. Med. 2020, 18, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Levi, E.; Klimstra, D.S.; Andea, A.; Basturk, O.; Adsay, N.V. MUC1 and MUC2 in pancreatic neoplasia. J. Clin. Pathol. 2004, 57, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Roy, L.D.; Grover, P.; Rao, S.; Mukherjee, P. Mucin 1 Regulates Cox-2 Gene in Pancreatic Cancer. Pancreas 2015, 44, 909–917. [Google Scholar] [CrossRef]
- Trehoux, S.; Duchene, B.; Jonckheere, N.; Van Seuningen, I. The MUC1 oncomucin regulates pancreatic cancer cell biological properties and chemoresistance. Implication of p42-44 MAPK, Akt, Bcl-2 and MMP13 pathways. Biochem. Biophys. Res. Commun. 2015, 456, 757–762. [Google Scholar] [CrossRef]
- Zhou, R.; Curry, J.M.; Roy, L.D.; Grover, P.; Haider, J.; Moore, L.J.; Wu, S.-T.; Kamesh, A.; Yazdanifar, M.; A Ahrens, W.; et al. A novel association of neuropilin-1 and MUC1 in pancreatic ductal adenocarcinoma: Role in induction of VEGF signaling and angiogenesis. Oncogene 2016, 35, 5608–5618. [Google Scholar] [CrossRef]
- Fan, K.; Yang, C.; Fan, Z.; Huang, Q.; Zhang, Y.; Cheng, H.; Jin, K.; Lu, Y.; Wang, Z.; Luo, G.; et al. MUC16 C terminal-induced secretion of tumor-derived IL-6 contributes to tumor-associated Treg enrichment in pancreatic cancer. Cancer Lett. 2018, 418, 167–175. [Google Scholar] [CrossRef]
- Gagnon, M.; Zihler Berner, A.; Chervet, N.; Chassard, C.; Lacroix, C. Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J. Microbiol. Methods 2013, 94, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Andrianifahanana, M.; Moniaux, N.; Batra, S.K. Regulation of mucin expression: Mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Biophys. Acta 2006, 1765, 189–222. [Google Scholar] [CrossRef]
- Ehre, C.; Ridley, C.; Thornton, D.J. Cystic fibrosis: An inherited disease affecting mucin-producing organs. Int. J. Biochem. Cell Biol. 2014, 52, 136–145. [Google Scholar] [CrossRef]
- Leong, D.T.; Ng, K.W. Probing the relevance of 3D cancer models in nanomedicine research. Adv. Drug Deliv. Rev. 2014, 79-80, 95–106. [Google Scholar] [CrossRef]
- Nederman, T.; Norling, B.; Glimelius, B.; Carlsson, J.; Brunk, U. Demonstration of an extracellular matrix in multicellular tumor spheroids. Cancer Res. 1984, 44, 3090–3097. [Google Scholar] [PubMed]
- Rotin, D.; Robinson, B.; Tannock, I.F. Influence of hypoxia and an acidic environment on the metabolism and viability of cultured cells: Potential implications for cell death in tumors. Cancer Res. 1986, 46, 2821–2826. [Google Scholar] [PubMed]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef]
- Agrawal, B.; Krantz, M.J.; Parker, J.; Longenecker, B.M. Expression of MUC1 mucin on activated human T cells: Implications for a role of MUC1 in normal immune regulation. Cancer Res. 1998, 58, 4079–4081. [Google Scholar]
- Treon, S.P.; Maimonis, P.; Bua, D.; Young, G.; Raje, N.; Mollick, J.; Chauhan, D.; Tai, Y.T.; Hideshima, T.; Shima, Y.; et al. Elevated soluble MUC1 levels and decreased anti-MUC1 antibody levels in patients with multiple myeloma. Blood 2000, 96, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Fremd, C.; Stefanovic, S.; Beckhove, P.; Pritsch, M.; Lim, H.; Wallwiener, M.; Heil, J.; Golatta, M.; Rom, J.; Sohn, C.; et al. Mucin 1-specific B cell immune responses and their impact on overall survival in breast cancer patients. Oncoimmunology 2015, 5, e1057387. [Google Scholar] [CrossRef]
- Matsuda, A.; Kuno, A.; Matsuzaki, H.; Kawamoto, T.; Shikanai, T.; Nakanuma, Y.; Yamamoto, M.; Ohkohchi, N.; Ikehara, Y.; Shoda, J.; et al. Glycoproteomics-based cancer marker discovery adopting dual enrichment with Wisteria floribunda agglutinin for high specific glyco-diagnosis of cholangiocarcinoma. J. Proteom. 2013, 85, 1–11. [Google Scholar] [CrossRef]
- Kim, Y.; Pecha, R.L.; Keihanian, T.; Mercado, M.; Pena-Munoz, S.V.; Lang, K.; Van Buren, G.; Dhingra, S.; Othman, M.O. MUC1 Expressions and Its Prognostic Values in US Gastric Cancer Patients. Cancers 2023, 15, 998. [Google Scholar] [CrossRef]
- Strawbridge, R.J.; Nistér, M.; Brismar, K.; Grönberg, H.; Li, C. MUC1 as a Putative Prognostic Marker for Prostate Cancer. Biomark. Insights 2008, 3, 303–315. [Google Scholar] [CrossRef]
- Li, C.; Liu, T.; Yin, L.; Zuo, D.; Lin, Y.; Wang, L. Prognostic and clinicopathological value of MUC1 expression in colorectal cancer: A meta-analysis. Medicine 2019, 98, e14659. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, C.; Xu, J.; Luo, Y.; Xia, Q.; He, K. MUC1 promotes lung metastases of liver cancer by impairing anti-tumor immunity. Discov. Oncol. 2023, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Shering, S.; Sherry, F.; McDermott, E.; O’Higgins, N. CA 15-3: A prognostic marker in breast cancer. Int. J. Biol. Markers 2000, 15, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Li, Q.; Yang, Y.; Xu, W.; Dong, Z. A prognosis marker MUC1 correlates with metabolism and drug resistance in bladder cancer: A bioinformatics research. BMC Urol. 2022, 22, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Long, M.; Fushimi, A.; Yamamoto, M.; Hata, T.; Hagiwara, M.; Bhattacharya, A.; Hu, Q.; Wong, K.-K.; Liu, S.; et al. MUC1-C integrates activation of the IFN-γ pathway with suppression of the tumor immune microenvironment in triple-negative breast cancer. J. Immunother. Cancer 2021, 9, e002115. [Google Scholar] [CrossRef]
- Beckwith, D.M.; Cudic, M. Tumor-associated O-glycans of MUC1: Carriers of the glyco-code and targets for cancer vaccine design. Semin. Immunol. 2020, 47, 101389. [Google Scholar] [CrossRef]
- Kato, T.; Ujiie, H.; Hatanaka, K.C.; Nange, A.; Okumura, A.; Tsubame, K.; Naruchi, K.; Sato, M.; Kaga, K.; Matsuno, Y.; et al. A novel Tn antigen epitope-recognizing antibody for MUC1 predicts clinical outcome in patients with primary lung adenocarcinoma. Oncol. Lett. 2021, 21, 202. [Google Scholar] [CrossRef]
- Lüttges, J.; Zamboni, G.; Longnecker, D.; Klöppel, G. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am. J. Surg. Pathol. 2001, 25, 942–948. [Google Scholar] [CrossRef]
- Banerjee, S.; Mujumdar, N.; Dudeja, V.; Mackenzie, T.; Krosch, T.K.; Sangwan, V.; Vickers, S.M.; Saluja, A.K. MUC1c regulates cell survival in pancreatic cancer by preventing lysosomal permeabilization. PLoS ONE 2012, 7, e43020. [Google Scholar] [CrossRef]
- Scholz, C.; Heublein, S.; Lenhard, M.; Friese, K.; Mayr, D.; Jeschke, U. Glycodelin A is a prognostic marker to predict poor outcome in advanced stage ovarian cancer patients. BMC Res. Notes 2012, 5, 551. [Google Scholar] [CrossRef]
- Stojnev, S.; Ristic-Petrovic, A.; Velickovic, L.J.; Krstic, M.; Bogdanovic, D.; Khanh, D.T.; Ristic, A.; Conic, I.; Stefanovic, V. Prognostic significance of mucin expression in urothelial bladder cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 4945–4958. [Google Scholar]
- Mirili, C.; Paydas, S.; Kilic, E.B.; Seydaoglu, G.; Ogul, A.; Gokcay, S.; Buyuksimsek, M.; Yetisir, A.E.; Karaalioglu, B.; Tohumcuoglu, M.; et al. Prognostic significance of EGFR, MUC1 and PD-L1 expressions in cases with triple negative breast cancer. J. BUON 2020, 25, 159–167. [Google Scholar] [PubMed]
- Wang, Q.; Zou, H.; Wang, Y.; Shang, J.; Yang, L.; Shen, J. CCR7-CCL21 axis promotes the cervical lymph node metastasis of tongue squamous cell carcinoma by up-regulating MUC1. J Craniomaxillofac Surg. 2021, 49, 562–569. [Google Scholar] [CrossRef]
- Kahkhaie, K.R.; Moaven, O.; Abbaszadegan, M.R.; Montazer, M.; Gholamin, M. Specific MUC1 splice variants are correlated with tumor progression in esophageal cancer. World J. Surg. 2014, 38, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Ro, J.Y.; Grignon, D.J.; Troncoso, P.; Ayala, A.G. Mucin in prostatic adenocarcinoma. Semin. Diagn. Pathol. 1988, 5, 273–283. [Google Scholar] [PubMed]
- Castrillon, D.H.; Lee, K.R.; Nucci, M.R. Distinction between endometrial and endocervical adenocarcinoma: An immunohistochemical study. Int. J. Gynecol. Pathol. 2002, 21, 4–10. [Google Scholar] [CrossRef]
- Kong, C.S.; Beck, A.H.; Longacre, T.A. A panel of 3 markers including p16, ProExC, or HPV ISH is optimal for distinguishing between primary endometrial and endocervical adenocarcinomas. Am. J. Surg. Pathol. 2010, 34, 915–926. [Google Scholar] [CrossRef]
- Ronnett, B.M. Endocervical adenocarcinoma: Selected diagnostic challenges. Mod. Pathol. 2016, 29 (Suppl. S1), 12. [Google Scholar] [CrossRef]
- Fawzy, A.; Mohamed, M.R.; Ali, M.A.M.; Abd El-Magied, M.H.; Helal, A.M. Tissue CA125 and HE4 Gene Expression Levels Offer Superior Accuracy in Discriminating Benign from Malignant Pelvic Masses. Asian Pac. J. Cancer Prev. 2016, 17, 323–333. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Ramm, L.E.; Joy, G.J.; Free, K.E.; Ward, B.C. Preoperative discrimination between ovarian carcinoma, non-ovarian gynecological malignancy and benign adnexal masses using serum levels of CA125 and the polymorphic epithelial mucin antigens CASA, OSA and MSA. Int. J. Gynecol. Cancer 1992, 2, 119–128. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.; Chen, R.; He, J.; Wang, Y.; Huang, L.; Sun, L.; Duan, C.; Luo, X.; Yan, H. Development of a multimarker assay for differential diagnosis of benign and malignant pelvic masses. Clin. Chim. Acta 2015, 440, 57–63. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Hu, G. Potential application of alternatively glycosylated serum MUC1 and MUC5AC in gastric cancer diagnosis. Biologicals 2009, 37, 18–25. [Google Scholar] [CrossRef]
- Roșu, M.C.; Mihnea, P.D.; Ardelean, A.; Moldovan, S.D.; Popețiu, R.O.; Totolici, B.D. Clinical significance of tumor necrosis factor-alpha and carcinoembryonic antigen in gastric cancer. J. Med. Life 2022, 15, 4–6. [Google Scholar] [CrossRef]
- Bagaria, B.; Sood, S.; Sharma, R.; Lalwani, S. Comparative study of CEA and CA19-9 in esophageal, gastric and colon cancers individually and in combination (ROC curve analysis). Cancer Biol. Med. 2013, 10, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, M.; Noaro, G.; Saadeh, L.; Cavallin, F.; Cagol, M.; Alfieri, R.; Plebani, M.; Castoro, C. Esophageal cancer management: Preoperative CA19.9 and CEA serum levels may identify occult advanced adenocarcinoma. World J. Surg. 2015, 39, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Esnakula, A.; Abushahin, L.; Tsung, A. Understanding the Clinical Impact of MUC5AC Expression on Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 3059. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhou, L.; Zhao, D.; Li, K.; Liu, Z.; Che, N.; Liu, H. MUC5AC enhances tumor heterogeneity in lung adenocarcinoma with mucin production and is associated with poor prognosis. Jpn. J. Clin. Oncol. 2020, 50, 701–711. [Google Scholar] [CrossRef]
- Liu, D.; Chang, C.; Gold, D.V.; Goldenberg, D.M. Identification of PAM4 (clivatuzumab)-reactive epitope on MUC5AC: A promising biomarker and therapeutic target for pancreatic cancer. Oncotarget 2015, 6, 4274–4285. [Google Scholar] [CrossRef]
- Javanbakht, M.; Akhavanmoghadam, J.; Talaei, A.J.; Aghyani, M.; Mozafari, M.; Khedmat, L.; Mohebbi, M. Differential expression of two genes Oct-4 and MUC5AC associates with poor outcome in patients with gastric cancer. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1099–1105. [Google Scholar] [CrossRef]
- Pinto, R.; Carvalho, A.S.; Conze, T.; Magalhães, A.; Picco, G.; Burchell, J.M.; Taylor-Papadimitriou, J.; Reis, C.A.; Almeida, R.; Mandel, U.; et al. Identification of new cancer biomarkers based on aberrant mucin glycoforms by in situ proximity ligation. J. Cell Mol. Med. 2012, 16, 1474–1484. [Google Scholar] [CrossRef]
- Dikmen, Z.G.; Colak, A.; Dogan, P.; Tuncer, S.; Akbiyik, F. Diagnostic performances of CA125, HE4, and ROMA index in ovarian cancer. Eur. J. Gynaecol. Oncol. 2015, 36, 457–462. [Google Scholar]
- Ferraro, S.; Braga, F.; Lanzoni, M.; Boracchi, P.; Biganzoli, E.M.; Panteghini, M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: A systematic review. J. Clin. Pathol. 2013, 66, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Mungul, A.; Cooper, L.; Brockhausen, I.; Ryder, K.; Mandel, U.; Clausen, H.; Rughetti, A.; Miles, D.W.; Taylor-Papadimitriou, J.; Burchell, J.M. Sialylated core 1 based O-linked glycans enhance the growth rate of mammary carcinoma cells in MUC1 transgenic mice. Int. J. Oncol. 2004, 25, 937–943. [Google Scholar] [CrossRef]
- Paschos, K.A.; Canovas, D.; Bird, N.C. The role of cell adhesion molecules in the progression of colorectal cancer and the development of liver metastasis. Cell Signal 2009, 21, 665–674. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Li, W.; Zhang, J.; Zhang, Y. Mucin Glycans: A Target for Cancer Therapy. Molecules 2023, 28, 7033. [Google Scholar] [CrossRef]
- Pegram, M.D.; Borges, V.F.; Ibrahim, N.; Fuloria, J.; Shapiro, C.; Perez, S.; Wang, K.; Schaedli Stark, F.; Courtenay Luck, N. Phase I dose escalation pharmacokinetic assessment of intravenous humanized anti-MUC1 antibody AS1402 in patients with advanced breast cancer. Breast Cancer Res. 2009, 11, R73. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K.; Yariz, K.O.; Bondarenko, I.; Manikhas, A.; Semiglazov, V.; Alyasova, A.; Komisarenko, V.; Shparyk, Y.; Murray, J.L.; Jones, D.; et al. Randomized phase II trial of letrozole plus anti-MUC1 antibody AS1402 in hormone receptor-positive locally advanced or metastatic breast cancer. Clin. Cancer Res. 2011, 17, 6822–6830. [Google Scholar] [CrossRef]
- Bose, M.; Sanders, A.; De, C.; Zhou, R.; Lala, P.; Shwartz, S.; Mitra, B.; Brouwer, C.; Mukherjee, P. Targeting tumor-associated MUC1 overcomes anoikis-resistance in pancreatic cancer. Transl. Res. 2023, 253, 41–56. [Google Scholar] [CrossRef]
- Gao, T.; Cen, Q.; Lei, H. A review on development of MUC1-based cancer vaccine. Biomed. Pharmacother. 2020, 132, 110888. [Google Scholar] [CrossRef]
- Julien, S.; Picco, G.; Sewell, R.; Vercoutter-Edouart, A.; Tarp, M.; Miles, D.; Clausen, H.; Taylor-Papadimitriou, J.; Burchell, J.M. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br. J. Cancer 2009, 100, 1746–1754. [Google Scholar] [CrossRef]
- Fang, F.; Ma, J.; NI, W.; Wang, F.; Sun, X.; Li, Y.; Li, Q.; Xie, F.; Wang, J.; Zhai, R.; et al. MUC1 and maltose-binding protein recombinant fusion protein combined with Bacillus Calmette-Guerin induces MUC1-specific and nonspecific anti-tumor immunity in mice. Mol. Med. Rep. 2014, 10, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ni, W.; Zhao, X.; Wang, F.; Gao, Z.; Tai, G. Synergistic antitumor effects of Escherichia coli maltose binding protein and Bacillus Calmette-Guerin in a mouse lung carcinoma model. Immunol. Lett. 2011, 136, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, H.; Zhang, P.; Wu, X.; Wei, H.; Wang, L.; Wan, M.; Deng, P.; Zhang, Y.; Wang, J.; et al. Heat shock fusion protein induces both specific and nonspecific anti-tumor immunity. Eur. J. Immunol. 2006, 36, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.; Parker, J.; Modi, S.; Butts, C.; Smylie, M.; Meikle, A.; Kehoe, M.; MacLean, G.; Longenecker, M. Phase I study of the BLP25 (MUC1 peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin. Lung Cancer 2001, 3, 49–57. [Google Scholar] [CrossRef]
- North, S.A.; Graham, K.; Bodnar, D.; Venner, P. A pilot study of the liposomal MUC1 vaccine BLP25 in prostate specific antigen failures after radical prostatectomy. J. Urol. 2006, 176, 91–95. [Google Scholar] [CrossRef]
- Beatty, P.L.; van der Geest, R.; Hashash, J.G.; Kimura, T.; Gutkin, D.; Brand, R.E.; Finn, O.J. Immunobiology and immunosurveillance in patients with intraductal papillary mucinous neoplasms (IPMNs), premalignant precursors of pancreatic adenocarcinomas. Cancer Immunol. Immunother. 2016, 65, 771–778. [Google Scholar] [CrossRef]
- Messager, M.; Lefevre, J.H.; Pichot-Delahaye, V.; Souadka, A.; Piessen, G.; Mariette, C.; FREGAT working group—FRENCH. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: A multicenter comparative study. Ann. Surg. 2011, 254, 684–693; discussion 693. [Google Scholar] [CrossRef]
- Khanvilkar, K.; Donovan, M.D.; Flanagan, D.R. Drug transfer through mucus. Adv. Drug Deliv. Rev. 2001, 48, 173–193. [Google Scholar] [CrossRef]
- Rao, C.V.; Janakiram, N.B.; Mohammed, A. Molecular Pathways: Mucins and Drug Delivery in Cancer. Clin. Cancer Res. 2017, 23, 1373–1378. [Google Scholar] [CrossRef]
- Okarvi, S.M. Peptide-based radiopharmaceuticals and cytotoxic conjugates: Potential tools against cancer. Cancer Treat. Rev. 2008, 34, 13–26. [Google Scholar] [CrossRef]
- Salouti, M.; Rajabi, H.; Babaei, M.H.; Rasaee, M.J. Breast tumor targeting with (99m)Tc-HYNIC-PR81 complex as a new biologic radiopharmaceutical. Nucl. Med. Biol. 2008, 35, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Y.; Li, J.; Hang, Y.; Jaramillo, L.; Wehrkamp, C.J.; Phillippi, M.A.; Mohr, A.M.; Chen, Y.; Talmon, G.A.; et al. Cholangiocarcinoma therapy with nanoparticles that combine downregulation of MicroRNA-210 with inhibition of cancer cell invasiveness. Theranostics 2018, 8, 4305–4320. [Google Scholar] [CrossRef]
- Posey, A.D., Jr.; Schwab, R.D.; Boesteanu, A.C.; Steentoft, C.; Mandel, U.; Engels, B.; Stone, J.D.; Madsen, T.D.; Schreiber, K.; Haines, K.M.; et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 2016, 44, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Croci, D.O. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity 2012, 36, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Jia, L.; Li, Y.; Gong, Y.; Liu, C.; Zhang, X.; Wang, N.; Zhao, Y. ST6GalNAcII mediates the invasive properties of breast carcinoma through PI3K/Akt/NF-κB signaling pathway. IUBMB Life 2014, 66, 300–308. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdanifar, M.; Roy, L.D.; Whilding, L.M.; Gavrill, A.; Maher, J.; Mukherjee, P. CAR T Cells Targeting the Tumor MUC1 Glycoprotein Reduce Triple-Negative Breast Cancer Growth. Front. Immunol. 2019, 10, 1149. [Google Scholar] [CrossRef]
- Mao, L.; Su, S.; Li, J.; Yu, S.; Gong, Y.; Chen, C.; Hu, Z.; Huang, X. Development of Engineered CAR T Cells Targeting Tumor-Associated Glycoforms of MUC1 for the Treatment of Intrahepatic Cholangiocarcinoma. J. Immunother. 2023, 46, 89–95. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Ma, P.; Zha, Y.; Zhang, J.; Lei, A.; Li, N. CAR-macrophage: An extensive immune enhancer to fight cancer. EBioMedicine 2022, 76, 103873. [Google Scholar] [CrossRef]
- Niu, Z.; Chen, G.; Chang, W.; Sun, P.; Luo, Z.; Zhang, H.; Zhi, L.; Guo, C.; Chen, H.; Yin, M.; et al. Chimeric antigen receptor-modified macrophages trigger systemic anti-tumour immunity. J. Pathol. 2021, 253, 247–257. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Su, H.; Liu, Q.; Shen, J.; Dai, H.; Zheng, W.; Lu, Y.; Zhang, W.; Bei, Y.; et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br. J. Cancer 2019, 121, 837–845. [Google Scholar] [CrossRef]
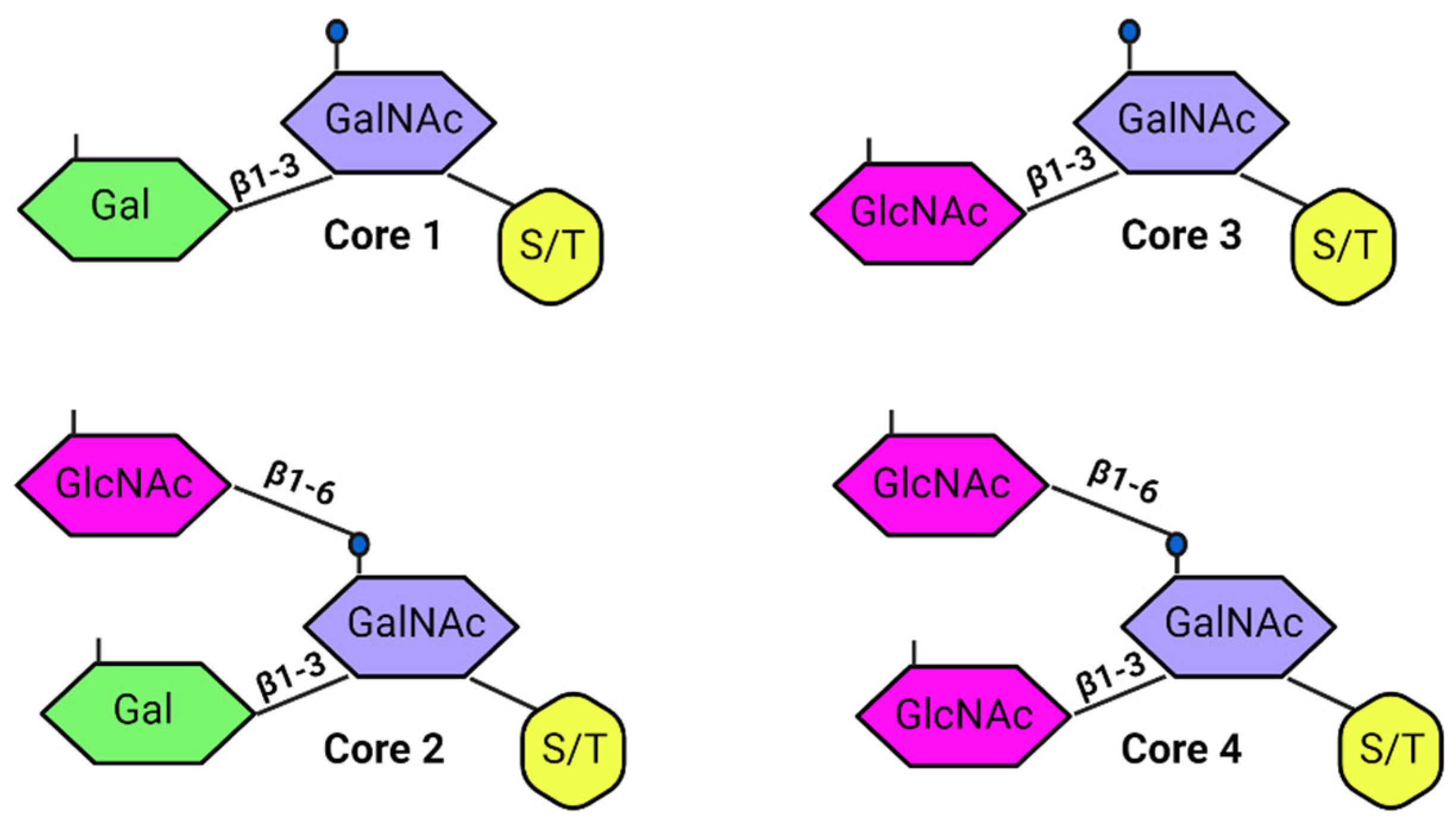

| Feature | Description | Mucin Examples |
|---|---|---|
| Structural Composition | Extracellular Domain: Rich in serine, threonine, and proline residues, heavily O-glycosylated. Forms a protective barrier against proteolytic enzymes, binds water, and forms gels. SEA Domain: Undergoes autoproteolytic cleavage, resulting in an extracellular α-chain and a transmembrane/intracellular β-chain. | Extracellular Domain (membrane-bound): MUC 1, 4, 16 [62,63,64,65] Extracellular Domain (secreted): MUC 2, 6, 19 [1,9] |
| SEA Domain: MUC 1, 12, 13, [1] | ||
| Intracellular Domain: MUC 1, 3, 12, 17, [20] | ||
| ECF-like Domain: MUC 4, 12, 13, 17, [1] | ||
| Glycosylation | Extensively glycosylated to shield the protein backbone and maintain hydration. Glycosylation patterns vary, leading to different functional properties. | MUC 1, 4, 16 [1] |
| Signaling Roles | Intracellular tails participate in signaling pathways, influencing cellular responses. For example, the MUC1 tail can be phosphorylated, affecting cell adhesion and proliferation. | MUC 1 and 4 [66] |
| Shedding and Cleavage | Extracellular domains can be shed from the cell surface, especially during inflammatory responses or in cancer. Shedding is mediated by proteases and modulates signaling and interactions. | MUC 1, 4, 16 [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Patel, A.; Demirkhanyan, L.; Gondi, C.S. The Role of Mucins in Cancer and Cancer Progression: A Comprehensive Review. Curr. Issues Mol. Biol. 2025, 47, 406. https://doi.org/10.3390/cimb47060406
Chen C, Patel A, Demirkhanyan L, Gondi CS. The Role of Mucins in Cancer and Cancer Progression: A Comprehensive Review. Current Issues in Molecular Biology. 2025; 47(6):406. https://doi.org/10.3390/cimb47060406
Chicago/Turabian StyleChen, Clare, Ameena Patel, Lusine Demirkhanyan, and Christopher S. Gondi. 2025. "The Role of Mucins in Cancer and Cancer Progression: A Comprehensive Review" Current Issues in Molecular Biology 47, no. 6: 406. https://doi.org/10.3390/cimb47060406
APA StyleChen, C., Patel, A., Demirkhanyan, L., & Gondi, C. S. (2025). The Role of Mucins in Cancer and Cancer Progression: A Comprehensive Review. Current Issues in Molecular Biology, 47(6), 406. https://doi.org/10.3390/cimb47060406






