Pharmacological Evaluation of Angelica keiskei Extract: Molecular Interaction Analysis in Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Crude Extraction (Sample Treatment and Subcritical Water Extraction Conditions)
2.3. GC-MS
2.4. Cell Culture
2.5. Solution Preparation
2.6. MTT Assay
2.7. Human Apoptotic Array Screening
Sample Preparation
2.8. Antibody Array Assay
2.8.1. Blocking and Incubation
2.8.2. Incubation with Biotinylated Antibody Cocktail and Wash
2.8.3. Incubation with Cy3 Equivalent Dye-Streptavidin and Wash
2.8.4. Data Analysis
2.9. Apoptotic Array Data Analysis
2.9.1. Cluster Heat Map
2.9.2. Volcano Scatter Plot
2.9.3. Protein/Protein Interaction Network Investigation
2.9.4. Principle Component Analysis
2.9.5. Glimma Plot
2.9.6. Voom
2.10. Molecular Docking Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Extraction of Angelica keiskei and Compound Identification Using GCMS
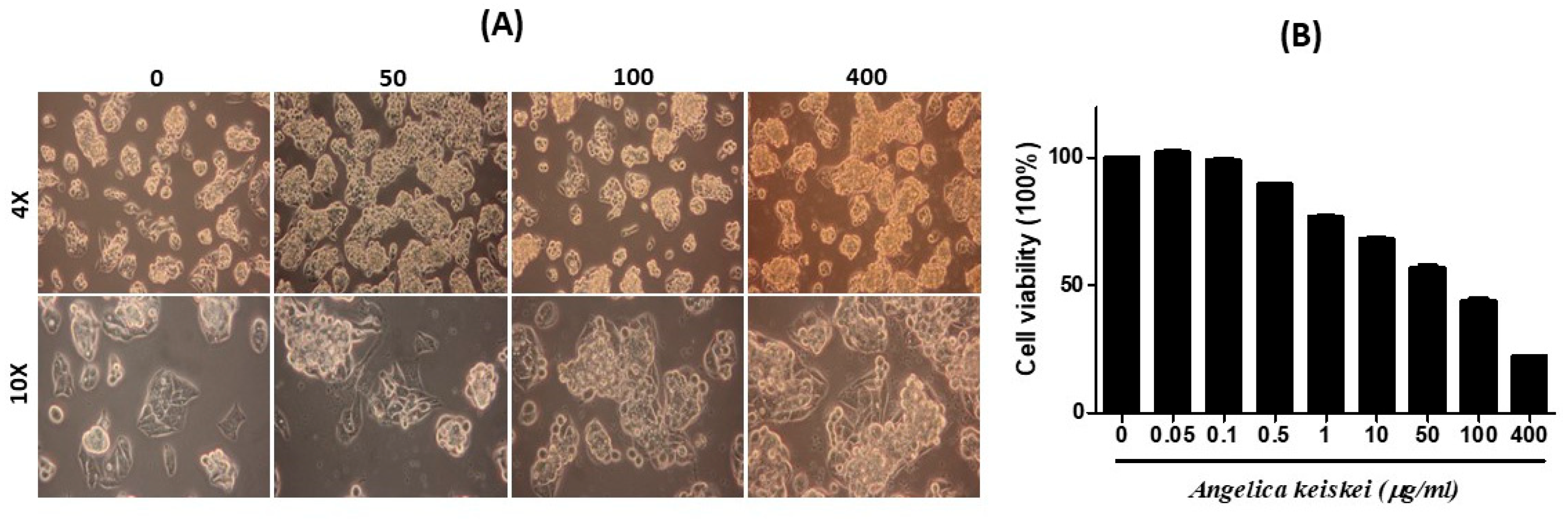
3.2. Cytotoxic Effect and Morphological Alteration of Angelica keiskei on HepG2 Cells
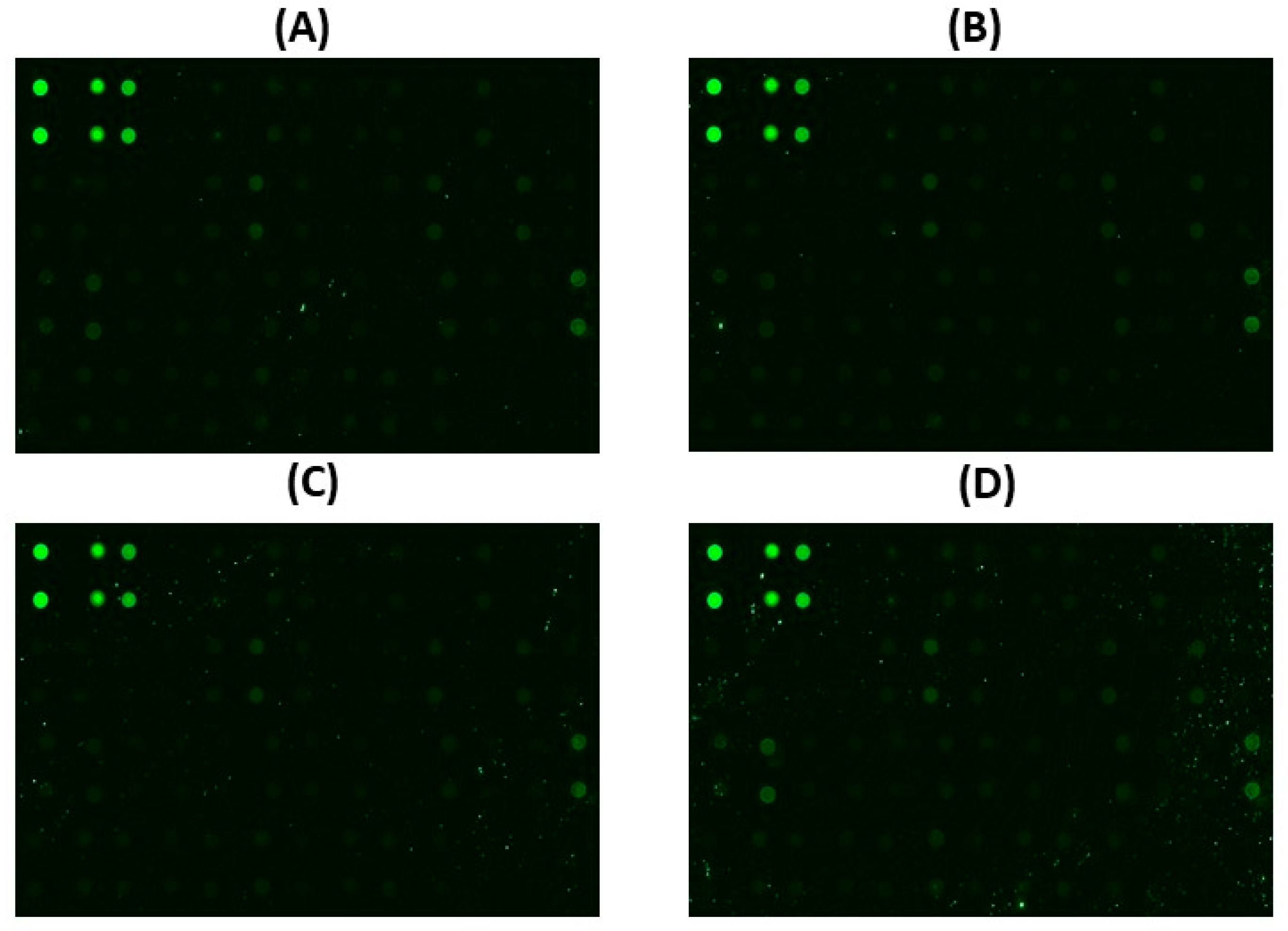
3.3. Protein Expression Alteration Using the Apoptotic Array Analysis
3.4. Identification of Differentially Expressed Apoptotic Proteins Using Cluster Heatmap
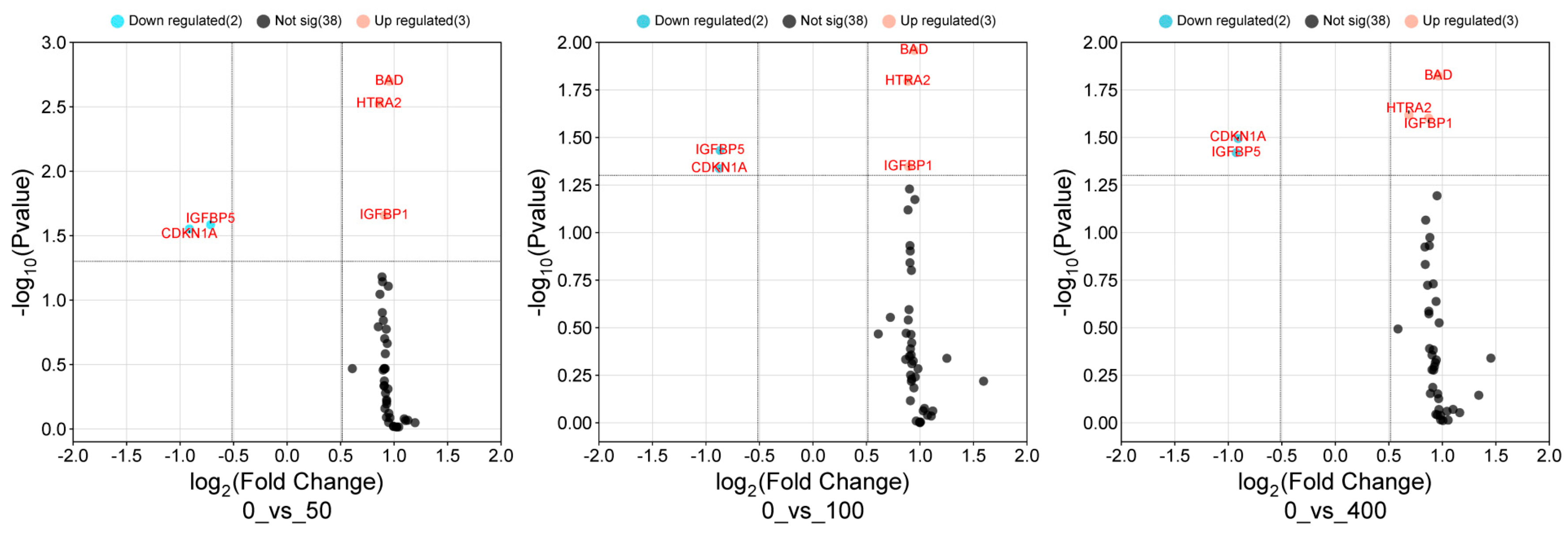
3.5. Volcano Scatter Plot for Differentially Expressed Cell Death Proteins
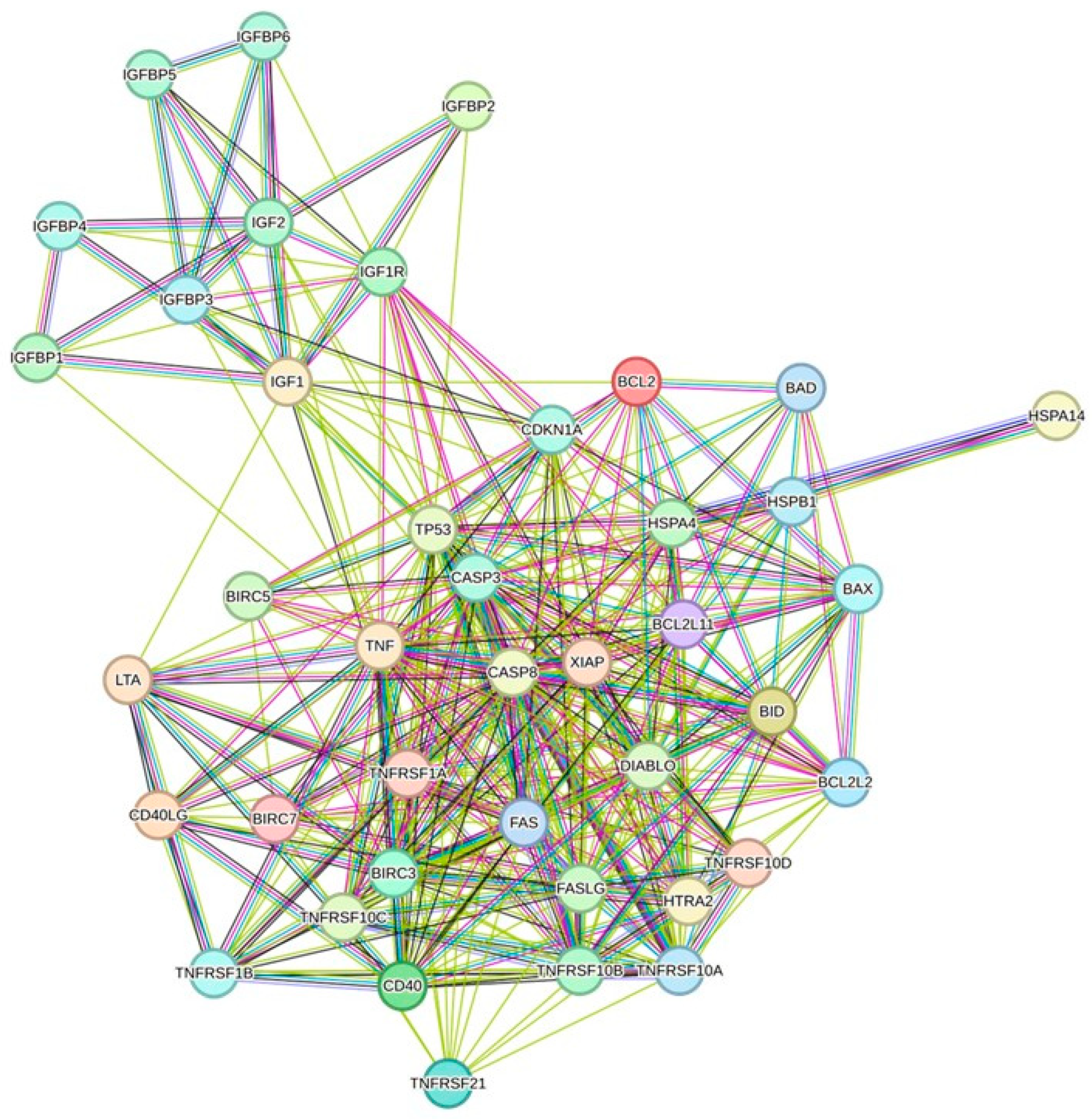
3.6. Protein/Protein Interaction Network Investigation
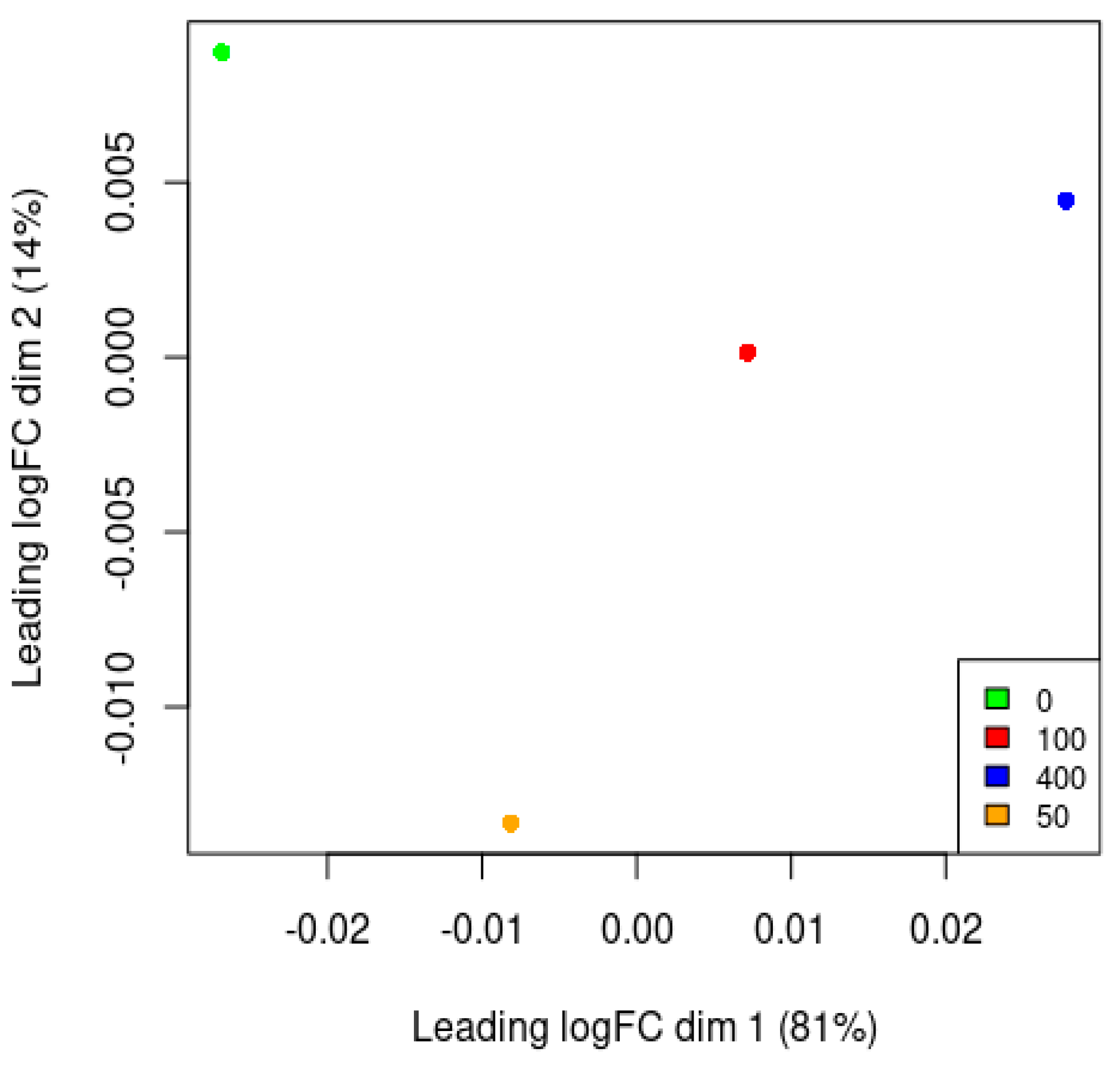
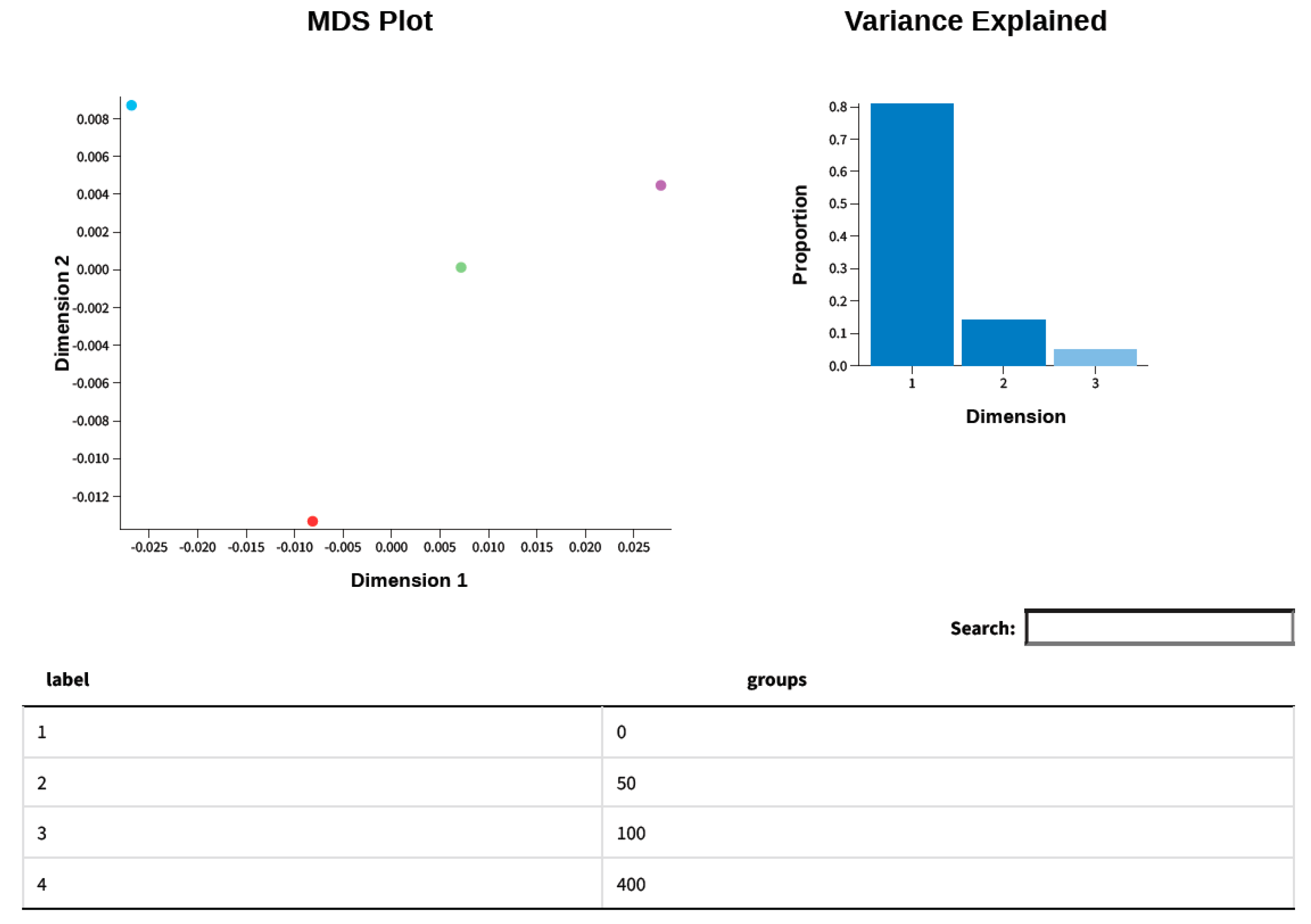
3.7. Principle Component Analysis
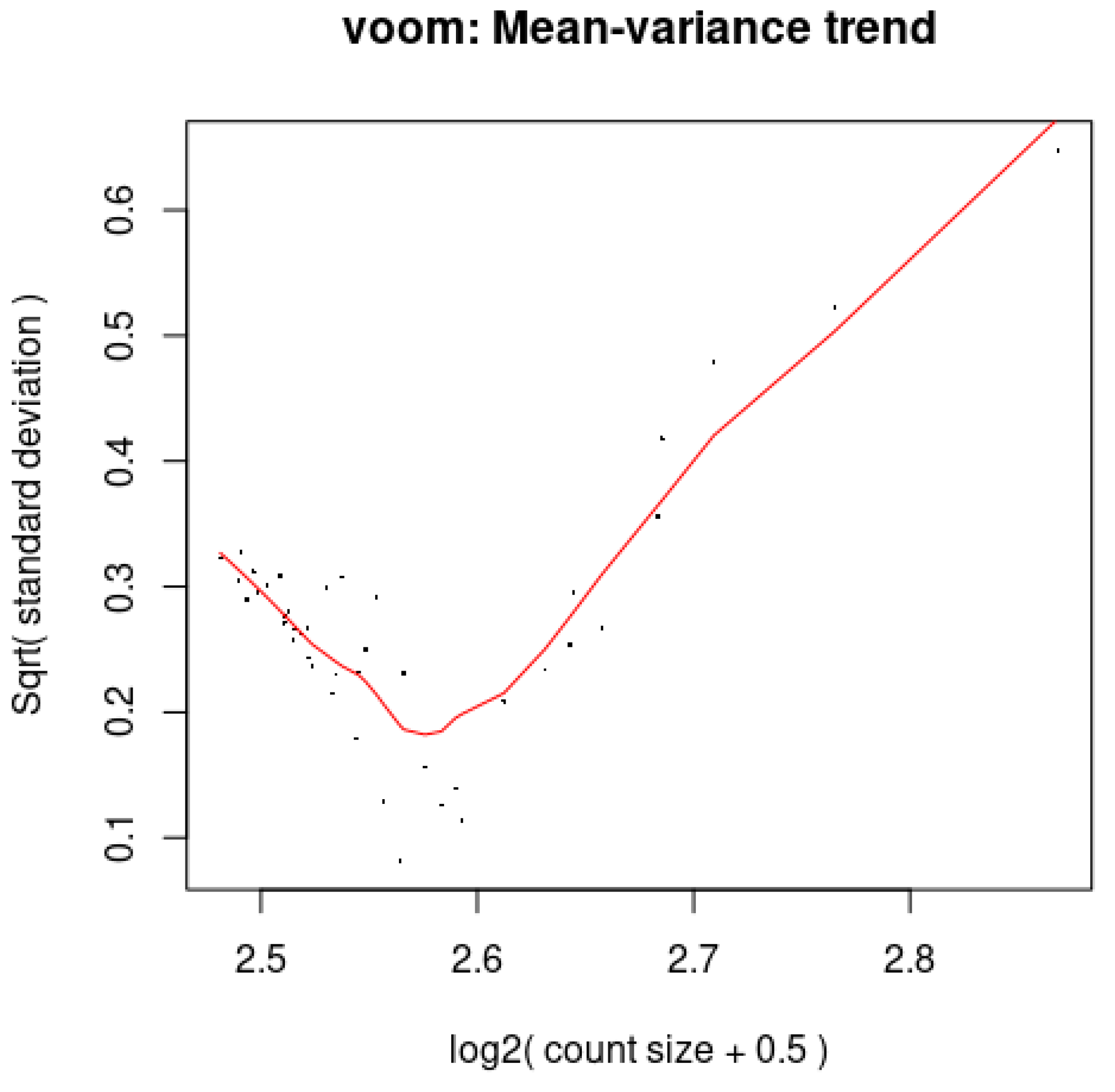
3.8. Voom: The Association Between the Mean and Variance
3.9. Glimma Plots
3.10. Computational Analysis of 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-2-one and Insulin Growth Factor Binding Protein 1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A.K. | Angelica keiskei |
| BAD | BCL2 antagonist of cell death |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A |
| DDMP | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one |
| GC-MS | Gas chromatography-mass spectrometry |
| HCC | Hepatocellular carcinoma |
| HepG2 | Hepatoblastoma cell line |
| HTRA2 | HtrA Serine Peptidases 2 |
| IGFBP1 | Insulin-like growth factor binding proteins1 |
| IGFBP5 | Insulin-like growth factor binding proteins5 |
| MEM | Minimum Essential Medium |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
References
- Moiseeva, E.P.; Almeida, G.M.; Jones, G.D.; Manson, M.M. Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol. Cancer Ther. 2007, 6, 3071–3079. [Google Scholar] [CrossRef]
- Song, T.; Lang, M.; Ren, S.; Gan, L.; Lu, W. The past, present and future of conversion therapy for liver cancer. Am. J. Cancer Res. 2021, 11, 4711. [Google Scholar]
- Global, B. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar]
- Rizvi, S.; Wang, J.; El-Khoueiry, A.B. Liver cancer immunity. Hepatology 2021, 73, 86–103. [Google Scholar]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273.e1261. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, D.; Jung, K.-W.; Won, Y.-J.; Cho, H. Cause of death and cause-specific mortality for primary liver cancer in South Korea: A nationwide population-based study in hepatitis B virus-endemic area. Clin. Mol. Hepatol. 2022, 28, 242. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. A review of the medicinal uses and pharmacology of ashitaba. Planta Medica 2016, 82, 1236–1245. [Google Scholar] [CrossRef]
- Nishimura, R.; Tabata, K.; Arakawa, M.; Ito, Y.; Kimura, Y.; Akihisa, T.; Nagai, H.; Sakuma, A.; Kohno, H.; Suzuki, T. Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol. Pharm. Bull. 2007, 30, 1878–1883. [Google Scholar] [CrossRef]
- Enoki, T.; Ohnogi, H.; Nagamine, K.; Kudo, Y.; Sugiyama, K.; Tanabe, M.; Kobayashi, E.; Sagawa, H.; Kato, I. Antidiabetic activities of chalcones isolated from a Japanese Herb, Angelica keiskei. J. Agric. Food Chem. 2007, 55, 6013–6017. [Google Scholar] [CrossRef]
- Kil, Y.-S.; Pham, S.T.; Seo, E.K.; Jafari, M. Angelica keiskei, an emerging medicinal herb with various bioactive constituents and biological activities. Arch. Pharmacal Res. 2017, 40, 655–675. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Taniguchi, M.; Nakata, K. Studies on Angelica Keiskei “Ashitaba”. Foods Food Ingred. J. Jpn. 1998, 1998, 52–60. [Google Scholar]
- Tu, L.; Wang, R.; Fang, Z.; Sun, M.; Sun, X.; Wu, J.; Dang, Y.; Liu, J. Assessment of the hypoglycemic and hypolipidemic activity of flavonoid-rich extract from Angelica keiskei. Molecules 2022, 27, 6625. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, T.; Takata, M.; Takayasu, J.; Hasegawa, T.; Tokuda, H.; Nishino, A.; Nishino, H.; Iwashima, A. Anti-tumor-promotion by principles obtained from Angelica keiskei. Planta Medica 1991, 57, 242–246. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Kang, K.-J. Effect of Angelica keiskei extract on apoptosis of MDA-MB-231 human breast cancer cells. J. Korean Soc. Food Sci. Nutr. 2011, 40, 1654–1661. [Google Scholar] [CrossRef]
- Kimura, Y.; Baba, K. Antitumor and antimetastatic activities of Angelica keiskei roots, part 1: Isolation of an active substance, xanthoangelol. Int. J. Cancer 2003, 106, 429–437. [Google Scholar] [CrossRef]
- Kim, A.; Lim, J.W.; Kim, H.; Kim, H. Supplementation with Angelica keiskei inhibits expression of inflammatory mediators in the gastric mucosa of Helicobacter pylori–infected mice. Nutr. Res. 2016, 36, 488–497. [Google Scholar] [CrossRef]
- Yun, H.-M.; Kim, B.; Kim, S.H.; Kwon, S.-H.; Park, K.-R. Xanol promotes apoptosis and autophagy and inhibits necroptosis and metastasis via the inhibition of AKT signaling in human oral squamous cell carcinoma. Cells 2023, 12, 1768. [Google Scholar] [CrossRef]
- Bae, U.-J.; Ryu, J.-H.; Park, B.-H.; Bae, E.J. Angelica keiskei root extract attenuates bile duct ligation-induced liver injury in mice. J. Med. Food 2022, 25, 435–442. [Google Scholar] [CrossRef]
- Amalia, R.; Aulifa, D.L.; Zain, D.N.; Pebiansyah, A.; Levita, J. The Cytotoxicity and Nephroprotective Activity of the Ethanol Extracts of Angelica keiskei Koidzumi Stems and Leaves against the NAPQI-Induced Human Embryonic Kidney (HEK293) Cell Line. Evid.-Based Complement. Altern. Med. 2021, 2021, 6458265. [Google Scholar] [CrossRef]
- Nkurunziza, D.; Pendleton, P.; Sivagnanam, S.P.; Park, J.-S.; Chun, B.S. Subcritical water enhances hydrolytic conversions of isoflavones and recovery of phenolic antioxidants from soybean byproducts (okara). J. Ind. Eng. Chem. 2019, 80, 696–703. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Y.; Sheng, Q.; Shyr, Y. Advanced heat map and clustering analysis using heatmap3. BioMed Res. Int. 2014, 2014, 986048. [Google Scholar] [CrossRef]
- Sharan, R.; Elkon, R.; Shamir, R. Cluster analysis and its applications to gene expression data. In Bioinformatics and Genome Analysis; Ernst Schering Research Foundation Workshop; Springer: Berlin/Heidelberg, Germany, 2002; Volume 38, pp. 83–108. [Google Scholar]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef]
- Su, S.; Law, C.W.; Ah-Cann, C.; Asselin-Labat, M.-L.; Blewitt, M.E.; Ritchie, M.E. Glimma: Interactive graphics for gene expression analysis. Bioinformatics 2017, 33, 2050–2052. [Google Scholar] [CrossRef]
- Ali, M.U.; Ahmed, S.; Ferzund, J.; Mehmood, A.; Rehman, A. Using PCA and factor analysis for dimensionality reduction of bio-informatics data. arXiv 2017, arXiv:1707.07189. [Google Scholar]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.W.; Simon, R.M. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics 2003, 19, 2448–2455. [Google Scholar] [CrossRef]
- DeWys, W.D. Studies correlating the growth rate of a tumor and its metastases and providing evidence for tumor-related systemic growth-retarding factors. Cancer Res. 1972, 32, 374–379. [Google Scholar]
- Noh, H.-M.; Ahn, E.-M.; Yun, J.-M.; Cho, B.-L.; Paek, Y.-J. Angelica keiskei Koidzumi extracts improve some markers of liver function in habitual alcohol drinkers: A randomized double-blind clinical trial. J. Med. Food 2015, 18, 166–172. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Rego, R.L.; Foster, N.R.; Thibodeau, S.N.; Alberts, S.R.; Windschitl, H.E.; Sargent, D.J. Proapoptotic Bad and Bid protein expression predict survival in stages II and III colon cancers. Clin. Cancer Res. 2008, 14, 4128–4133. [Google Scholar] [CrossRef]
- Yang, E.; Zha, J.; Jockel, J.; Boise, L.H.; Thompson, C.B.; Korsmeyer, S.J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 1995, 80, 285–291. [Google Scholar] [CrossRef]
- Fernando, R.; Foster, J.S.; Bible, A.; Ström, A.; Pestell, R.G.; Rao, M.; Saxton, A.; Baek, S.J.; Yamaguchi, K.; Donnell, R. Breast cancer cell proliferation is inhibited by BAD: Regulation of cyclin D1. J. Biol. Chem. 2007, 282, 28864–28873. [Google Scholar] [CrossRef]
- Ahn, J.; Won, M.; Choi, J.-H.; Kim, Y.S.; Jung, C.-R.; Im, D.-S.; Kyun, M.-L.; Lee, K.; Song, K.-B.; Chung, K.-S. Reactive oxygen species-mediated activation of the Akt/ASK1/p38 signaling cascade and p21 Cip1 downregulation are required for shikonin-induced apoptosis. Apoptosis 2013, 18, 870–881. [Google Scholar] [CrossRef]
- Baxter, R.C. Insulin-like growth factor binding protein-3 (IGFBP-3): Novel ligands mediate unexpected functions. J. Cell Commun. Signal. 2013, 7, 179–189. [Google Scholar] [CrossRef]
- Geis, T.; Popp, R.; Hu, J.; Fleming, I.; Henke, N.; Dehne, N.; Brüne, B. HIF-2α attenuates lymphangiogenesis by up-regulating IGFBP1 in hepatocellular carcinoma. Biol. Cell 2015, 107, 175–188. [Google Scholar] [CrossRef]
- Dai, B.; Ruan, B.; Wu, J.; Wang, J.; Shang, R.; Sun, W.; Li, X.; Dou, K.; Wang, D.; Li, Y. Insulin-like growth factor binding protein-1 inhibits cancer cell invasion and is associated with poor prognosis in hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 5645. [Google Scholar]
- Nel, I.; Baba, H.A.; Weber, F.; Sitek, B.; Eisenacher, M.; Meyer, H.E.; Schlaak, J.F.; Hoffmann, A.-C. IGFBP1 in epithelial circulating tumor cells as a potential response marker to selective internal radiation therapy in hepatocellular carcinoma. Biomark. Med. 2014, 8, 687–698. [Google Scholar] [CrossRef]
- Yang, L.; Tang, Q.; Wu, J.; Chen, Y.; Zheng, F.; Dai, Z.; Hann, S.S. Inter-regulation of IGFBP1 and FOXO3a unveils novel mechanism in ursolic acid-inhibited growth of hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 2016, 35, 1–13. [Google Scholar]
- Colak, Y.; Senates, E.; Ozturk, O.; Yilmaz, Y.; Zemheri, E.; Enc, F.Y.; Ulasoglu, C.; Aksaray, S.; Bozbeyoglu, S.G.; Kiziltas, S. Serum concentrations of human insulin-like growth factor-1 and levels of insulin-like growth factor-binding protein-5 in patients with nonalcoholic fatty liver disease: Association with liver histology. Eur. J. Gastroenterol. Hepatol. 2012, 24, 255–261. [Google Scholar] [CrossRef]
- Umemura, A.; Itoh, Y.; Itoh, K.; Yamaguchi, K.; Nakajima, T.; Higashitsuji, H.; Onoue, H.; Fukumoto, M.; Okanoue, T.; Fujita, J. Association of gankyrin protein expression with early clinical stages and insulin-like growth factor-binding protein 5 expression in human hepatocellular carcinoma. Hepatology 2008, 47, 493–502. [Google Scholar] [CrossRef]
- Li, B.; Li, A.; You, Z.; Xu, J.; Zhu, S. Epigenetic silencing of CDKN1A and CDKN2B by SNHG1 promotes the cell cycle, migration and epithelial-mesenchymal transition progression of hepatocellular carcinoma. Cell Death Dis. 2020, 11, 823. [Google Scholar] [CrossRef]
- Fornari, F.; Milazzo, M.; Chieco, P.; Negrini, M.; Marasco, E.; Capranico, G.; Mantovani, V.; Marinello, J.; Sabbioni, S.; Callegari, E.; et al. In hepatocellular carcinoma miR-519d is up-regulated by p53 and DNA hypomethylation and targets CDKN1A/p21, PTEN, AKT3 and TIMP2. J. Pathol. 2012, 227, 275–285. [Google Scholar] [CrossRef]
- Feng, L.; Li, Z.; Xiong, Y.; Yan, T.; Fu, C.; Zeng, Q.; Wang, H. HtrA2 Independently Predicts Poor Prognosis and Correlates with Immune Cell Infiltration in Hepatocellular Carcinoma. J. Oncol. 2023, 2023, 4067418. [Google Scholar] [CrossRef]
- Karunakaran, D.; Turner, A.W.; Duchez, A.C.; Soubeyrand, S.; Rasheed, A.; Smyth, D.; Cook, D.P.; Nikpay, M.; Kandiah, J.W.; Pan, C.; et al. RIPK1 gene variants associate with obesity in humans and can be therapeutically silenced to reduce obesity in mice. Nat. Metab. 2020, 2, 1113–1125. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Guan, Z.; Li, H.; Ye, M.; Chen, X.; Shen, J.; Zhou, Y.; Shi, Z.L.; Zhou, P.; et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Target. Ther. 2020, 5, 235. [Google Scholar] [CrossRef]
- Sachdeva, M.M.; Claiborn, K.C.; Khoo, C.; Yang, J.; Groff, D.N.; Mirmira, R.G.; Stoffers, D.A. Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. Proc. Natl. Acad. Sci. USA 2009, 106, 19090–19095. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Peng, C.; Liu, E.; Li, Y.; Li, C.; Niu, J. Hypoxia-inducible factor-1alpha suppressed hepatocellular carcinoma cell apoptosis through influencing on Omi/HtrA2 expression and its releasing from the mitochondrion. Oncol. Res. 2012, 20, 213–220. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, M.; Yan, Q.; Budachetri, K.; Hou, L.; Sahni, A.; Liu, H.; Han, N.C.; Lakritz, J.; Pei, D.; et al. An intracellular nanobody targeting T4SS effector inhibits Ehrlichia infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2024102118. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, L.; Ni, Q.; Tao, R.; Qin, J. Transcription factor PAX4 facilitates gastric cancer progression through interacting with miR-27b-3p/Grb2 axis. Aging (Albany NY) 2021, 13, 16786–16803. [Google Scholar] [CrossRef]
- Zakim, D.; Boyer, T.D. Hepatology: A Textbook of Liver Disease; Saunders: Philadelphia, PA, USA, 1990. [Google Scholar]
- Rutkute, K.; Nikolova-Karakashian, M.N. Regulation of insulin-like growth factor binding protein-1 expression during aging. Biochem. Biophys. Res. Commun. 2007, 361, 263–269. [Google Scholar] [CrossRef]
- Link, T.; Iwakuma, T. Roles of p53 in extrinsic factor-induced liver carcinogenesis. Hepatoma Res. 2017, 3, 95. [Google Scholar] [CrossRef]
- Hiramoto, K.; Nasuhara, A.; Michikoshi, K.; Kato, T.; Kikugawa, K. DNA strand-breaking activity and mutagenicity of 2, 3-dihydro-3, 5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP), a Maillard reaction product of glucose and glycine. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1997, 395, 47–56. [Google Scholar] [CrossRef]
- Carlsson, M.J.; Vollmer, A.S.; Demuth, P.; Heylmann, D.; Reich, D.; Quarz, C.; Rasenberger, B.; Nikolova, T.; Hofmann, T.G.; Christmann, M. p53 triggers mitochondrial apoptosis following DNA damage-dependent replication stress by the hepatotoxin methyleugenol. Cell Death Dis. 2022, 13, 1009. [Google Scholar] [CrossRef]
- Xie, Y.; Xie, W. The role of sulfotransferases in liver diseases. Drug Metab. Dispos. 2020, 48, 742–749. [Google Scholar] [CrossRef]
- Derry, W.B.; Putzke, A.P.; Rothman, J.H. Caenorhabditis elegans p53: Role in apoptosis, meiosis, and stress resistance. Science 2001, 294, 591–595. [Google Scholar] [CrossRef]
- Williams, A.B.; Schumacher, B. p53 in the DNA-damage-repair process. Cold Spring Harb. Perspect. Med. 2016, 6, a026070. [Google Scholar] [CrossRef]


| Temperature (°C) | Pressure (MPa) | Reaction Time (min) | Solid/Liquid Ratio (mg/mL) | Ethanol | Acronyms | |
|---|---|---|---|---|---|---|
| 1 | 160 | 30 | 10 | 30 | No | B1 |
| 2 | 160 | 30 | 10 | 45 | No | B2 |
| 3 | 170 | 30 | 10 | 30 | No | B3 |
| 4 | 140 | 30 | 10 | 30 | No | B4 |
| 5 | 150 | 30 | 10 | 30 | No | B5 |
| 6 | 160 | 30 | 10 | 30 | 50% | B-E-1 |
| RayBio® Human Apoptosis Antibody Array G1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detect 43 Apoptotic Markers in One Experiment | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Pos 1 | Pos 2 | Pos 3 | Neg | Neg | bad | bax | bcl-2 | bcl-w | BID | BIM | Casp 3 | Casp 8 |
| Pos 1 | Pos 2 | Pos 3 | Neg | Neg | bad | bax | bcl-2 | bcl-w | BID | BIM | Casp 3 | Casp 8 |
| CD40 | CD40L | cIAP-2 | cytoC | DR6 | Fas | FasL | NEG | HSP27 | HSP60 | HSP70 | HTRA | IGF-I |
| CD40 | CD40L | cIAP-2 | cytoC | DR6 | Fas | FasL | NEG | HSP27 | HSP60 | HSP70 | HTRA | IGF-I |
| IGF-II | IGFBP-1 | IGFBP-2 | IGFBP-3 | IGFBP4 | IGFBP-5 | IGFBP-6 | IGF-1sR | Iivin | p21 | p27 | p53 | SMAC |
| IGF-II | IGFBP-1 | IGFBP-2 | IGFBP-3 | IGFBP-4 | IGFBP-5 | IGFBP-6 | IGF-1sR | livin | p21 | p27 | p53 | SMAC |
| Survivin | sTNF-R1 | sTNF-R2 | TNF-alpha | TNF-beta | TRAIL R1 | TRAIL R-2 | TRAIL R-3 | TRAIL R-4 | XIAP | NEG | NEG | NEG |
| Survivin | sTNF-R1 | sTNF-R2 | TNF-alpha | TNF-beta | TRAIL R1 | TRAIL R-2 | TRAIL R-3 | TRAIL R-4 | XIAP | NEG | NEG | NEG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.A.; Song, M.; Kim, G.-D. Pharmacological Evaluation of Angelica keiskei Extract: Molecular Interaction Analysis in Hepatocellular Carcinoma. Curr. Issues Mol. Biol. 2025, 47, 401. https://doi.org/10.3390/cimb47060401
Singh AA, Song M, Kim G-D. Pharmacological Evaluation of Angelica keiskei Extract: Molecular Interaction Analysis in Hepatocellular Carcinoma. Current Issues in Molecular Biology. 2025; 47(6):401. https://doi.org/10.3390/cimb47060401
Chicago/Turabian StyleSingh, Alka Ashok, Minseok Song, and Gun-Do Kim. 2025. "Pharmacological Evaluation of Angelica keiskei Extract: Molecular Interaction Analysis in Hepatocellular Carcinoma" Current Issues in Molecular Biology 47, no. 6: 401. https://doi.org/10.3390/cimb47060401
APA StyleSingh, A. A., Song, M., & Kim, G.-D. (2025). Pharmacological Evaluation of Angelica keiskei Extract: Molecular Interaction Analysis in Hepatocellular Carcinoma. Current Issues in Molecular Biology, 47(6), 401. https://doi.org/10.3390/cimb47060401








