Could Fingolimod Combined with Bevacizumab Be a New Hope in Glioblastoma Treatment?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Culture of C6 Cells
2.3. Preparation and Applications of Drugs to C6 Cells
2.4. MTT Colorimetric Assay
2.5. Annexin V–FITC Staining
2.6. Caspase-3/7 Activity Analysis
2.7. Mitochondrial Membrane Potential Analysis
2.8. Colony Formation Assay
2.9. Migration Assay
2.10. Evaluation of Morphological Changes
2.11. Statistical Analysis
3. Results
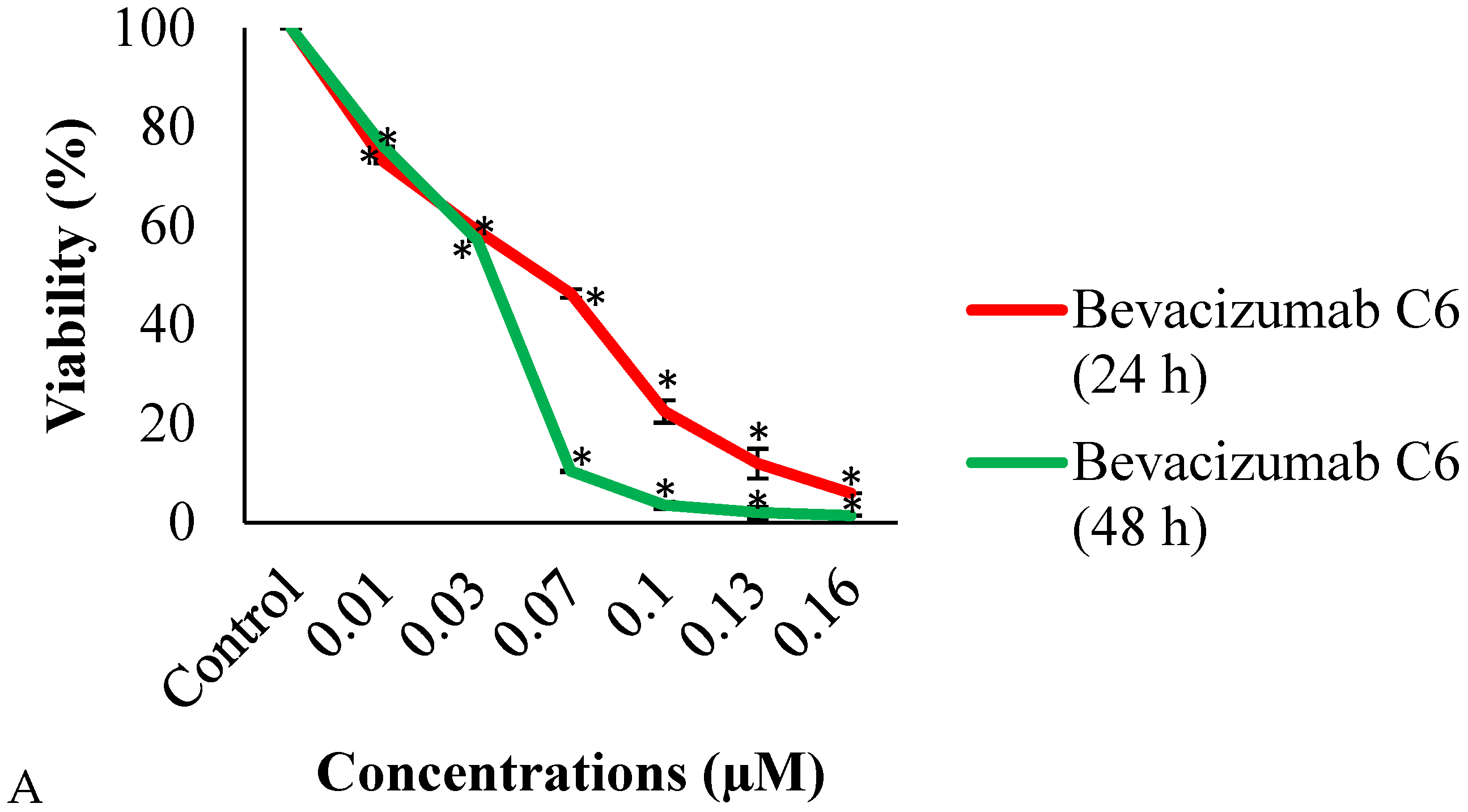
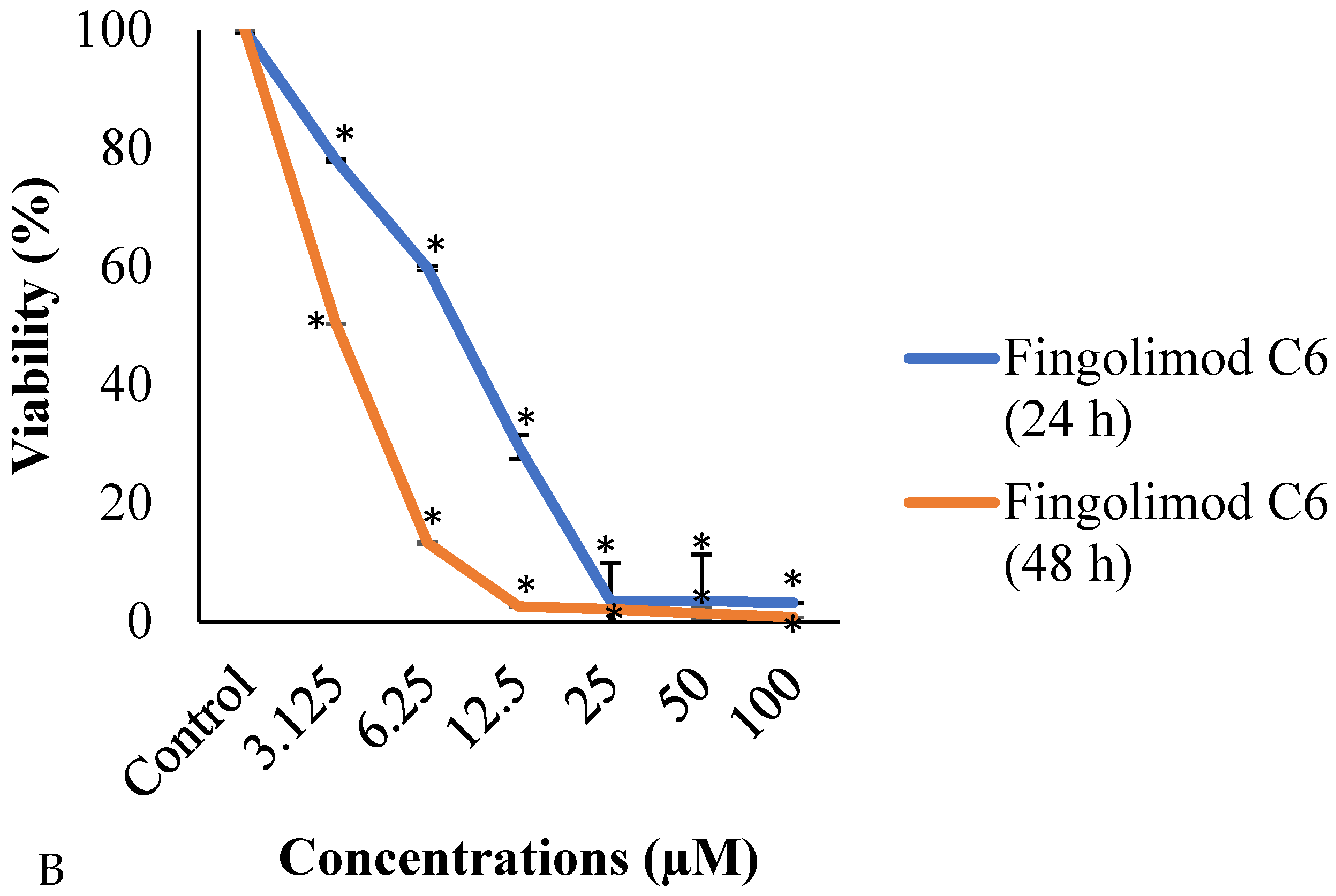
3.1. MTT Assay
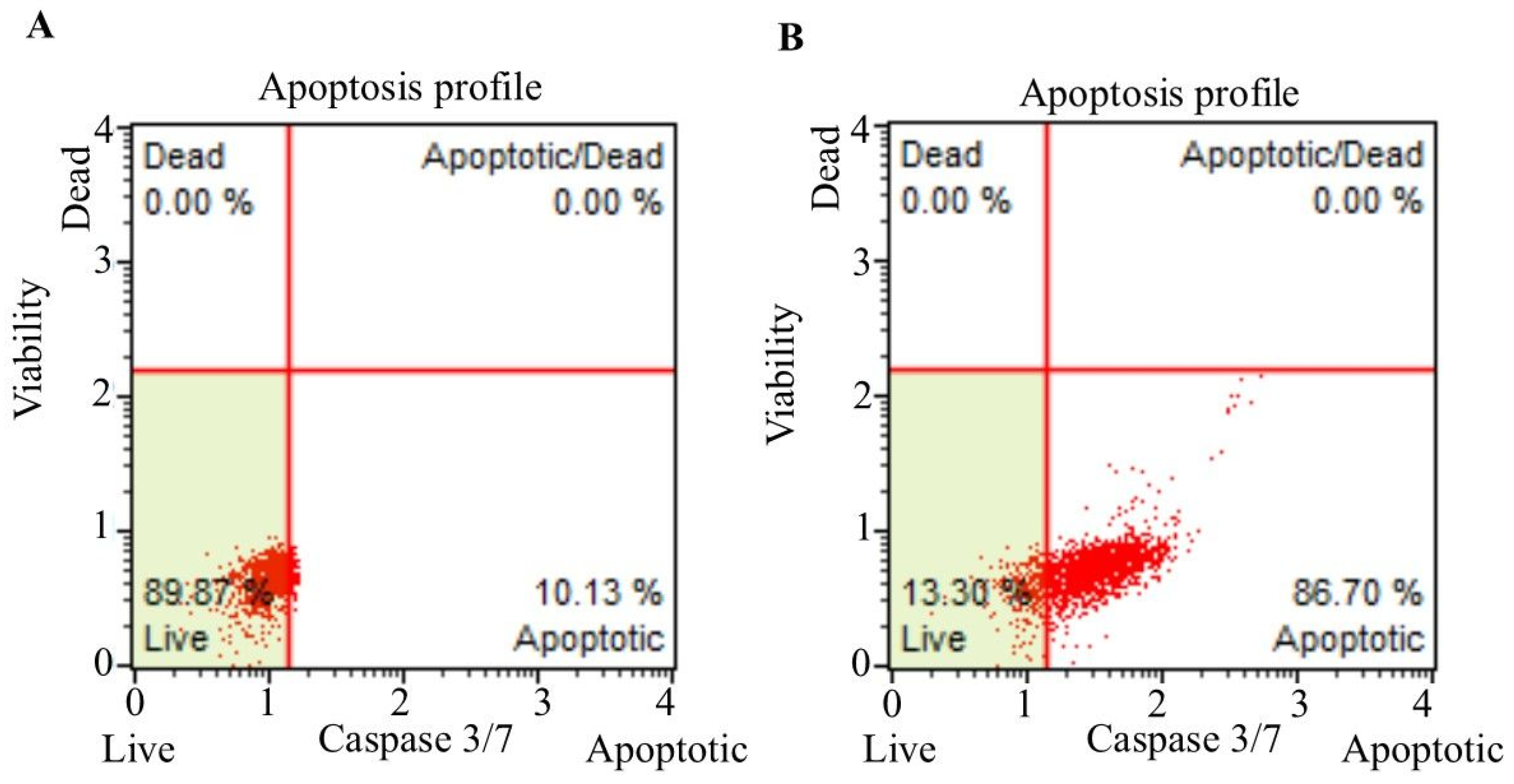
3.2. Annexin V–FITC Analysis
3.3. Caspase-3/7 Staining
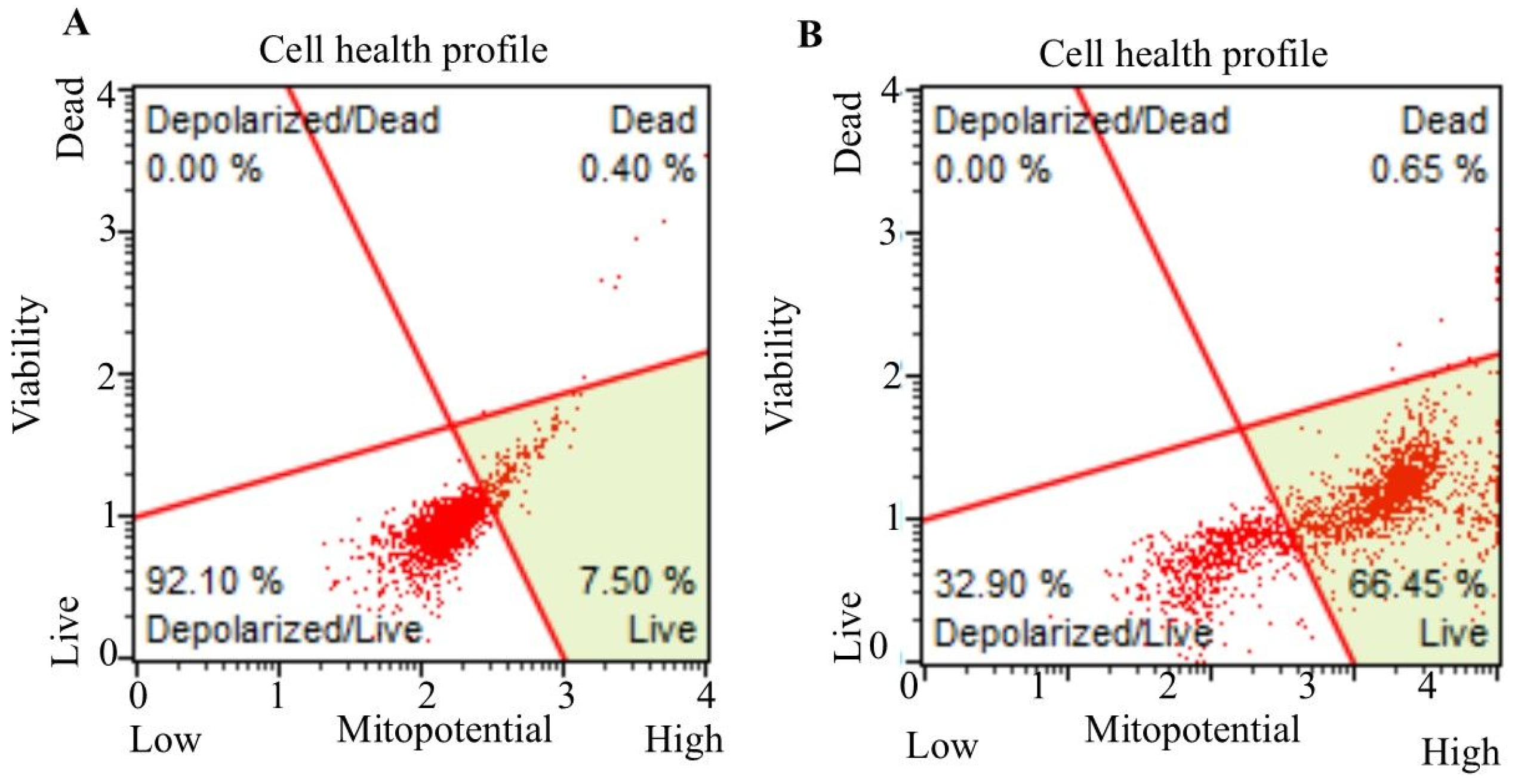
3.4. Mitochondrial Membrane Potential Measurement
3.5. Colony Formation Assay Results
3.6. Migration Assay Results
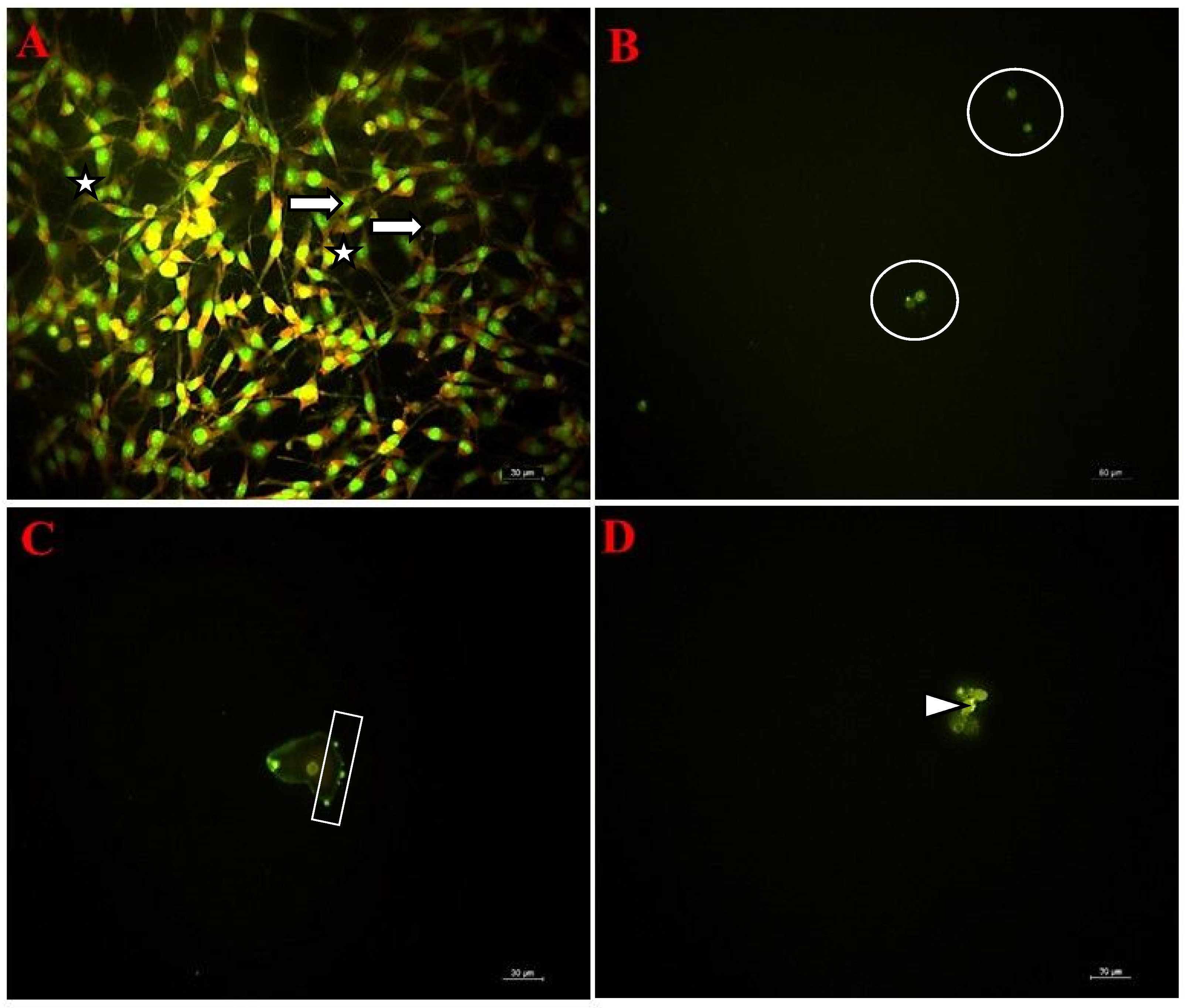
3.7. Fluorescent Microscopy Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| EGFR | Epidermal growth factor receptor |
| GBM | Glioblastoma grade IV |
| MAPK | Mitogen-activated protein kinase |
| mTOR | Mammalian target of rapamycin |
| PD-L1 | Programmed death ligand-1 |
| VEGF | Vascular endothelial growth factor |
References
- Sahoo, L.; Tripathy, N.S.; Dilnawaz, F. Naringenin nanoformulations for neurodegenerative diseases. Curr. Pharm. Biotechnol. 2024, 25, 2108–2124. [Google Scholar] [CrossRef]
- Mohammed, S.; Dinesan, M.; Ajayakumar, T. Survival and quality of life analysis in glioblastoma multiforme with adjuvant chemoradiotherapy: A retrospective study. Rep. Pract. Oncol. Radiother. 2022, 27, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Reid, T.R.; Oronsky, A.; Sandhu, N.; Knox, S.J. A review of newly diagnosed glioblastoma. Front. Oncol. 2021, 10, 574012. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.M.; Shaweis, H.; Han, C.; Sivasubramaniam, V.; Brazil, L.; Beaney, R.; Sadler, G.; Al-Sarraj, S.; Hampton, T.; Logan, J.; et al. Has the survival of patients with glioblastoma changed over the years? Br. J. Cancer 2016, 114, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, M.; Dogonchi, A.F.; Shafiei, M.; Tehrani, D.S. Survival rate of patient with glioblastoma: A population-based study. Egypt J. Neurosurg. 2024, 39, 42. [Google Scholar] [CrossRef]
- Burnet, N.G.; Jefferies, S.J.; Benson, R.J.; Hunt, D.P.; Treasure, F.P. Years of life lost (YLL) from cancer is an important measure of population burden--and should be considered when allocating research funds. Br. J. Cancer 2005, 92, 241–245. [Google Scholar] [CrossRef]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising incidence of glioblastoma multiforme in a well-defined population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef]
- Rios, S.A.; Oyervides, S.; Uribe, D.; Reyes, A.M.; Fanniel, V.; Vazquez, J.; Keniry, M. Emerging therapies for glioblastoma. Cancers 2024, 16, 1485. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Inoue, K.; Yamamoto, S.; Ikumoto, T.; Sasaki, S.; Toyama, R.; Chiba, K.; Hoshino, Y.; Okumoto, T. Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J. Antibiot. 1994, 47, 208–215. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H. FTY720 inhibits the Nrf2/ARE pathway in human glioblastoma cell lines and sensitizes glioblastoma cells to temozolomide. Pharmacol. Rep. 2017, 69, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Kataoka, H.; Seki, N.; Shimano, K.; Koyama, M.; Fukunari, A.; Sugahara, K.; Sugita, T. Fingolimod (FTY720), sphingosine 1-phosphate receptor modulator, shows superior efficacy as compared with interferon-β in mouse experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2011, 11, 366–372. [Google Scholar] [CrossRef]
- Singh, I.N.; Hall, E.D. Multifaceted roles of sphingosine-1-phosphate: How does this bioactive sphingolipid fit with acute neurological injury? J. Neurosci. Res. 2008, 86, 1419–1433. [Google Scholar] [CrossRef]
- Aslan, J.E.; You, H.; Williamson, D.M.; Endig, J.; Youker, R.T.; Thomas, L.; Shu, H.; Du, Y.; Milewski, R.L.; Brush, M.H.; et al. Akt and 14-3-3 control a PACS-2 homeostatic switch that integrates membrane traffic with TRAIL-induced apoptosis. Mol. Cell 2009, 34, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Decker, C.C.; Zechner, L.; Krstin, S.; Wink, M. In vitro wound healing of tumor cells: Inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacol. Toxicol. 2019, 20, 4. [Google Scholar] [CrossRef]
- Li, J.H.; Li, S.Y.; Shen, M.X.; Qiu, R.Z.; Fan, H.W.; Li, Y.B. Anti-tumor effects of Solanum nigrum L. extraction on C6 high-grade glioma. J. Ethnopharmacol. 2021, 274, 114034. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Budman, D.R.; Calabro, A. In vitro search for synergy and antagonism: Evaluation of docetaxel combinations in breast cancer cell lines. Breast Cancer Res. Treat 2002, 74, 41–46. [Google Scholar] [CrossRef]
- Celik, A.; Bakar-Ates, F. Alpha-lipoic acid induced apoptosis of PC3 prostate cancer cells through an alteration on mitochondrial membrane depolarization and MMP-9 mRNA expression. Med. Oncol. 2023, 40, 244. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Jia, D.; Liu, S.; Wang, F.; Li, G.; Zhang, Y.; Cao, X.; Ling, E.A.; Hao, A. Oct4 is expressed in human gliomas and promotes colony formation in glioma cells. Glia 2009, 57, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Vejselova Sezer, C. Escin induces cell death in human skin melanoma cells through apoptotic mechanisms. Toxicol. Res. 2024, 13, tfae124. [Google Scholar] [CrossRef]
- Spallotta, F.; Illi, B. The role of HDAC6 in glioblastoma multiforme: A new avenue to therapeutic interventions? Biomedicines 2024, 12, 2631. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.; Liu, Z.; Xi, K.; Zhang, Y.; Zhao, D.; Feng, F.; Geng, H.; Liu, M.; Lou, J.; et al. Implantable microneedle-mediated eradication of postoperative tumor foci mitigates glioblastoma relapse. Adv. Mater. 2024, 36, e2409857. [Google Scholar] [CrossRef] [PubMed]
- Laverty, D.J.; Gupta, S.K.; Bradshaw, G.A.; Hunter, A.S.; Carlson, B.L.; Calmo, N.M.; Chen, J.; Tian, S.; Sarkaria, J.N.; Nagel, Z.D. ATM inhibition exploits checkpoint defects and ATM-dependent double strand break repair in TP53-mutant glioblastoma. Nat. Commun. 2024, 15, 5294. [Google Scholar] [CrossRef]
- Zhang, J.F.; Okai, B.; Iovoli, A.; Goulenko, V.; Attwood, K.; Lim, J.; Hess, R.M.; Abad, A.P.; Prasad, D.; Fenstermaker, R.A. Bevacizumab and gamma knife radiosurgery for first-recurrence glioblastoma. J. Neurooncol. 2024, 166, 89–98. [Google Scholar] [CrossRef]
- Pournajaf, S.; Afsordeh, N.; Bayat, H.; Pourgholami, M.H. Fingolimod inhibits C6 rat glioma proliferation and migration, induces sub-G1 cell cycle arrest, mitochondrial and extrinsic apoptosis in vitro and reduces tumour growth in vivo. Clin. Exp. Pharmacol. Physiol. 2025, 52, e70012. [Google Scholar] [CrossRef]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef]
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and bevacizumab in progressive glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963. [Google Scholar] [CrossRef]
- Johnson, D.R.; Leeper, H.E.; Uhm, J.H. Glioblastoma survival in the United States improved after Food and Drug Administration approval of bevacizumab: A population-based analysis. Cancer 2013, 119, 3489–3495. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; Omuro, A.M.P.; Ravelo, A.; Sommer, N.; Guerin, A.; Ionescu-Ittu, R.; Shi, S.; Macalalad, A.; Uhm, J.H. Overall survival in patients with glioblastoma before and after bevacizumab approval. Curr. Med. Res. Opin. 2018, 34, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Marcus, A.; Eshhar, Z. Allogeneic chimeric antigen receptor-modified cells for adoptive cell therapy of cancer. Expert Opin. Biol. Ther. 2014, 14, 947–954. [Google Scholar] [CrossRef] [PubMed]
- LaMontagne, K.; Littlewood-Evans, A.; Schnell, C.; O’Reilly, T.; Wyder, L.; Sanchez, T.; Probst, B.; Butler, J.; Wood, A.; Liau, G.; et al. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006, 66, 221–231. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhu, J.; Ding, K.; Xu, J. FTY720 reduces migration and invasion of human glioblastoma cell lines via inhibiting the PI3K/AKT/mTOR/p70S6K signaling pathway. Tumour Biol. 2014, 35, 10707–10714. [Google Scholar] [CrossRef]
- Touat, M.; Idbaih, A.; Sanson, M.; Ligon, K.L. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann. Oncol. 2017, 28, 1457–1472. [Google Scholar] [CrossRef]
- Kim, M.M.; Umemura, Y.; Leung, D. Bevacizumab and glioblastoma: Past, present, and future directions. Cancer J. 2018, 24, 180–186. [Google Scholar] [CrossRef]
- Yilmaz, I.; Karaarslan, N. Examining the effects of HMG-CoA reductase inhibitors on anabolic and catabolic signaling pathway proteins associated with degenerative disc disease. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2990–3000. [Google Scholar]
- Yilmaz, I.; Akalan, H.; Yasar Sirin, D.; Karaarslan, N.; Kaplan, N.; Ozbek, H. Effects of an acetylcholinesterase inhibitor and an N-methyl-D-aspartate receptor antagonist on inflammation and degeneration of the nucleus pulposus. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4409–4419. [Google Scholar] [PubMed]



| Groups | Contents | Final Concentration |
|---|---|---|
| Group 1 (Control) | None | - |
| Group 2 | Treated with bevacizumab | 0.06 µM |
| Group 3 | Treated with fingolimod | 8.27 µM |
| Group 4 | Treated with bevacizumab + fingolimod | 0.06 µM + 8.27 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baloglu, M.; Vejselova Sezer, C.; Izgördü, H.; Yilmaz, I.; Kutlu, H.M. Could Fingolimod Combined with Bevacizumab Be a New Hope in Glioblastoma Treatment? Curr. Issues Mol. Biol. 2025, 47, 394. https://doi.org/10.3390/cimb47060394
Baloglu M, Vejselova Sezer C, Izgördü H, Yilmaz I, Kutlu HM. Could Fingolimod Combined with Bevacizumab Be a New Hope in Glioblastoma Treatment? Current Issues in Molecular Biology. 2025; 47(6):394. https://doi.org/10.3390/cimb47060394
Chicago/Turabian StyleBaloglu, Murat, Canan Vejselova Sezer, Hüseyin Izgördü, Ibrahim Yilmaz, and Hatice Mehtap Kutlu. 2025. "Could Fingolimod Combined with Bevacizumab Be a New Hope in Glioblastoma Treatment?" Current Issues in Molecular Biology 47, no. 6: 394. https://doi.org/10.3390/cimb47060394
APA StyleBaloglu, M., Vejselova Sezer, C., Izgördü, H., Yilmaz, I., & Kutlu, H. M. (2025). Could Fingolimod Combined with Bevacizumab Be a New Hope in Glioblastoma Treatment? Current Issues in Molecular Biology, 47(6), 394. https://doi.org/10.3390/cimb47060394






