New Insights into the Interplay Between Simple Sugars and Liver Diseases
Abstract
1. Introduction
2. Definition and Classification
3. Diet
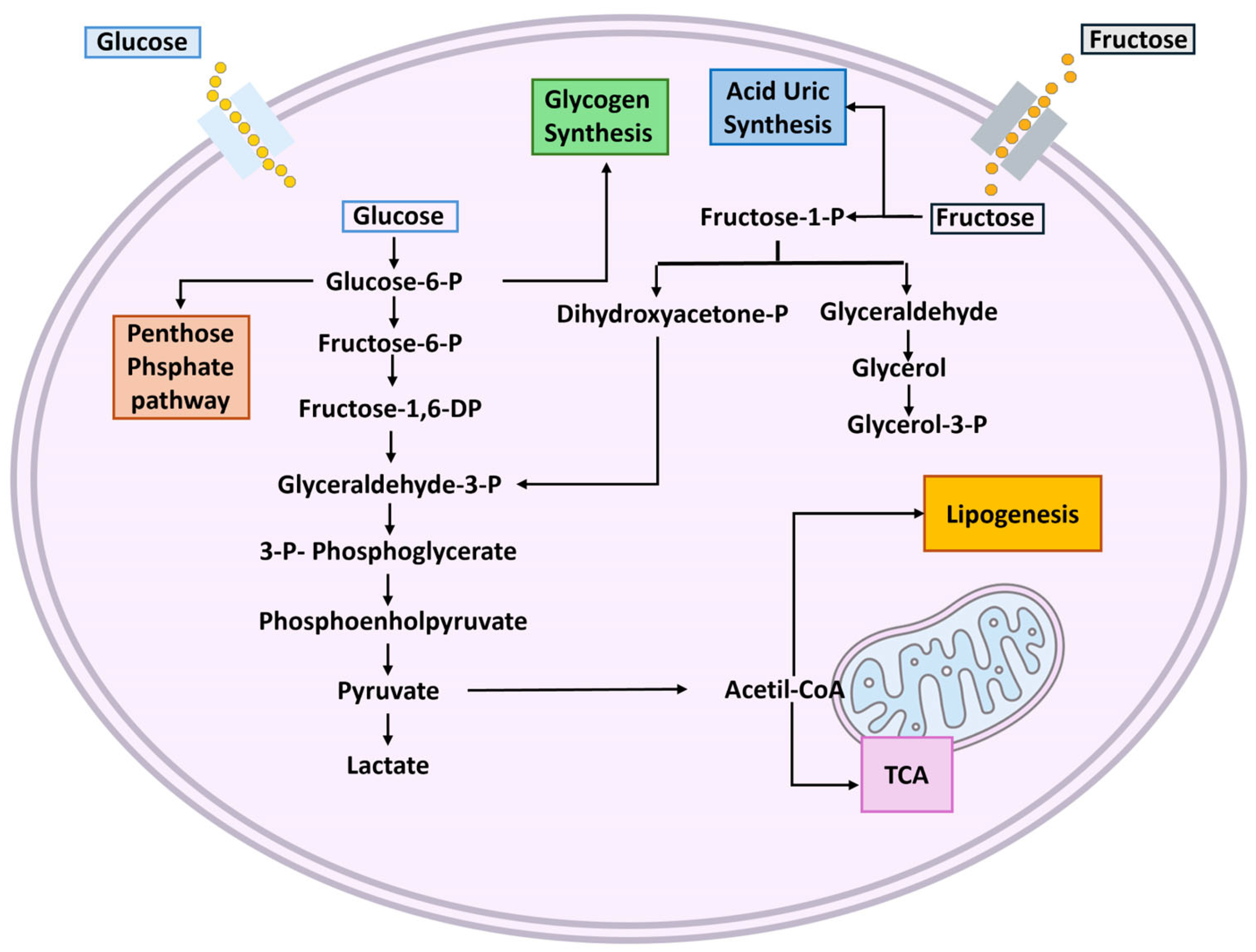
4. Metabolism
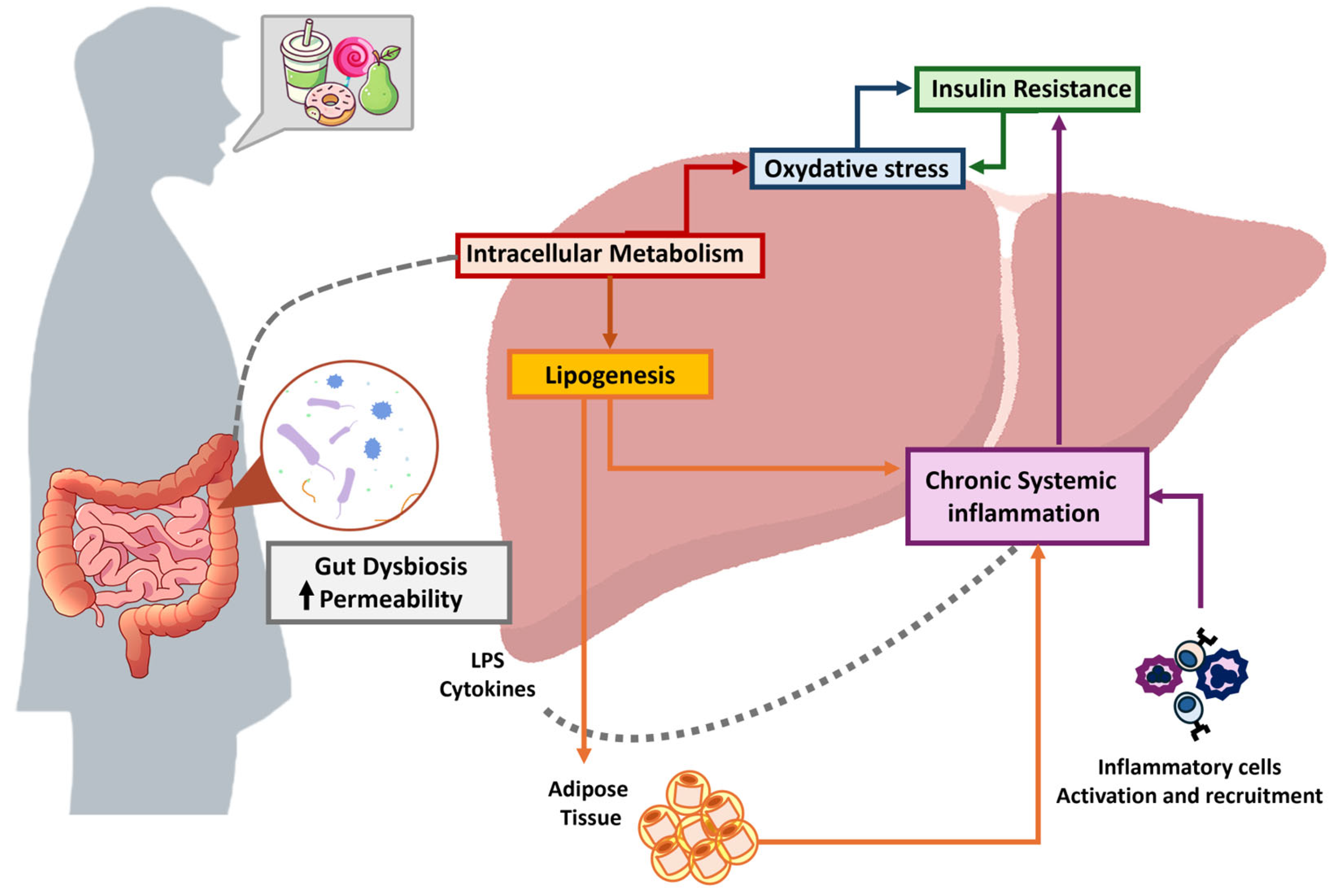
5. Mechanism of Liver Damage
5.1. Oxidative Stress
5.2. Inflammation
5.3. Intestinal Permeability and Gut Microbiota
5.4. Insulin Resistance
6. Liver Disease Development and Worsening
6.1. Metabolic Dysfunction-Associated Steatotic Liver Disease and Metabolic Dysfunction-Associated Steatohepatits: Preclinical Data
6.2. Metabolic Dysfunction-Associated Steatohepatitis: Data on Humans
6.3. Metabolic Dysfunction and Alcohol-Related Liver Disease: Preclinical Data
6.4. Metabolic Dysfunction and Alcohol-Related Liver Disease: Data on Humans
6.5. Cirrhosis: Preclinical Data
6.6. Cirrhosis: Data on Humans
6.7. Hepatocellular Carcinoma: Preclinical Data
6.8. Hepatocellular Carcinoma: Data on Humans
7. Dietary Alternatives to Simple Sugars
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic Hormone |
| ALDOB | Aldolase B |
| ASB | Artificial Sweetened Beverage |
| ATP | Adenosine Triphosphate |
| ChREBP | Carbohydrate Response Element Binding Protein |
| CRH | Corticotropin-Releasing Hormone |
| CRP | C Reactive Protein |
| DP | Degree of Polymerization |
| ECM | Extracellular Matrix |
| ER | Endoplasmatic Reticulum |
| HBV | Hepatitis B Virus |
| HCC | Hepatocellular Carcinoma |
| HCV | Hepatitis C Virus |
| HFCS | High Fructose Corn Syrup |
| IL | Interleukin |
| IR | Insulin Resistance |
| IRS1 | Insulin Receptor Substrate 1 |
| G6P | Glucose-6-Posphate |
| LPS | Lipopolysaccharide |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MASH | Metabolic Dysfunction-Associated Steatohepatitis |
| METAld | Metabolic Dysfunction and Alcohol-Related Liver Disease |
| MRI | Magnetic Resonance Imaging |
| NAFLD | Nonalcoholic fatty liver disease |
| PNPLA3 | patatin-like phospholipase domain-containing protein 3 |
| ROS | Reactive oxygen species |
| SREBP-1c | Sterol regulatory element binding transcription factor 1 |
| T2DM | Type 2 Diabetes Mellitus |
| WHO | World Health Organization |
References
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global epidemiology of cirrhosis—Aetiology, trends and predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Singal, A.G.; Kono, Y.; Tan, D.J.H.; El-Serag, H.B.; Loomba, R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022, 34, 969–977.e2. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Svegliati-Baroni, G.; Ortolani, A.; Cucco, M.; Dalla Riva, G.V.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.; Di Marco, M.; Caturelli, E.; et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002–2033: The ITA.LI.CA database. Gut 2023, 72, 141–152. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Federico, A.; Rosato, V.; Masarone, M.; Torre, P.; Dallio, M.; Romeo, M.; Persico, M. The Role of Fructose in Non-Alcoholic Steatohepatitis: Old Relationship and New Insights. Nutrients 2021, 13, 1314. [Google Scholar] [CrossRef]
- Jung, S.; Bae, H.; Song, W.S.; Jang, C. Dietary Fructose and Fructose-Induced Pathologies. Annu. Rev. Nutr. 2022, 42, 45–66. [Google Scholar] [CrossRef]
- Qi, X.; Tester, R.F. Fructose, galactose and glucose—In health and disease. Clin. Nutr. ESPEN 2019, 33, 18–28. [Google Scholar] [CrossRef]
- Yki-Järvinen, H.; Luukkonen, P.K.; Hodson, L.; Moore, J.B. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 770–786. [Google Scholar] [CrossRef]
- Drożdż, K.; Nabrdalik, K.; Hajzler, W.; Kwiendacz, H.; Gumprecht, J.; Lip, G.Y.H. Metabolic-Associated Fatty Liver Disease (MAFLD), Diabetes, and Cardiovascular Disease: Associations with Fructose Metabolism and Gut Microbiota. Nutrients 2021, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Jung, S.; Wellen, K.E.; Jang, C. The interaction between the gut microbiota and dietary carbohydrates in nonalcoholic fatty liver disease. Exp. Mol. Med. 2021, 53, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D. A periodic table of monosaccharides. Glycobiology 2024, 34, cwad088. [Google Scholar] [CrossRef]
- Chandel, N.S. Carbohydrate Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040568. [Google Scholar] [CrossRef]
- Cummings, J.H.; Stephen, A.M. Carbohydrate terminology and classification. Eur. J. Clin. Nutr. 2007, 61 (Suppl. S1), S5–S18. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Mielgo-Ayuso, J.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Redondo-Flórez, L.; Tornero-Aguilera, J.F. The Burden of Carbohydrates in Health and Disease. Nutrients 2022, 14, 3809. [Google Scholar] [CrossRef]
- Judge, A.; Dodd, M.S. Metabolism. Essays Biochem. 2020, 64, 607–647. [Google Scholar] [CrossRef]
- Basaranoglu, M.; Basaranoglu, G.; Bugianesi, E. Carbohydrate intake and nonalcoholic fatty liver disease: Fructose as a weapon of mass destruction. Hepatobiliary Surg. Nutr. 2015, 4, 109–116. [Google Scholar]
- Azaïs-Braesco, V.; Sluik, D.; Maillot, M.; Kok, F.; Moreno, L.A. A review of total & added sugar intakes and dietary sources in Europe. Nutr. J. 2017, 16, 6. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Correa-Delgado, M.; Córdova-Almeida, R.; Lara-Nava, D.; Chávez-Muñoz, M.; Velásquez-Chávez, V.F.; Hernández-Torres, C.E.; Gontarek-Castro, E.; Ahmad, M.Z. Natural sweeteners: Sources, extraction and current uses in foods and food industries. Food Chem. 2022, 370, 130991. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Karin, M. “Sweet death”: Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021, 33, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Godos, J.; Bonaccio, M.; Vitaglione, P.; Grosso, G. Ultra-Processed Foods and Nutritional Dietary Profile: A Meta-Analysis of Nationally Representative Samples. Nutrients 2021, 13, 3390. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; Baker, P.; Lawrence, M.; Rebholz, C.M.; Srour, B.; et al. Ultra-processed food exposure and adverse health outcomes: Umbrella review of epidemiological meta-analyses. BMJ 2024, 384, e077310. [Google Scholar] [CrossRef]
- Zhang, Y.; Giovannucci, E.L. Ultra-processed foods and health: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2023, 63, 10836–10848. [Google Scholar] [CrossRef]
- Grembecka, M. Natural sweeteners in a human diet. Rocz. Panstw. Zakl. Hig. 2015, 66, 195–202. [Google Scholar]
- Khorshidian, N.; Shadnoush, M.; Zabihzadeh Khajavi, M.; Sohrabvandi, S.; Yousefi, M.; Mortazavian, A.M. Fructose and high fructose corn syrup: Are they a two-edged sword? Int. J. Food Sci. Nutr. 2021, 72, 592–614. [Google Scholar] [CrossRef]
- White, J.S.; Hobbs, L.J.; Fernandez, S. Fructose content and composition of commercial HFCS-sweetened carbonated beverages. Int. J. Obes. 2015, 39, 176–182. [Google Scholar] [CrossRef]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.A. Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Dills, W.L. Sugar Alcohols as Bulk Sweeteners. Annu. Rev. Nutr. 1989, 9, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Msomi, N.Z.; Erukainure, O.L.; Islam, M.d.S. Suitability of Sugar Alcohols as Antidiabetic Supplements: A Review. J. Food Drug Anal. 2021, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, J.; Chen, Q.; Wu, H.; Mu, W. Sugar alcohols derived from lactose: Lactitol, galactitol, and sorbitol. Appl. Microbiol. Biotechnol. 2020, 104, 9487–9495. [Google Scholar] [CrossRef]
- Hong, S.J.; Ahn, M.H.; Sangshetti, J.; Arote, R.B. Sugar alcohol-based polymeric gene carriers: Synthesis, properties and gene therapy applications. Acta Biomater. 2019, 97, 105–115. [Google Scholar] [CrossRef]
- Gasmi Benahmed, A.; Gasmi, A.; Arshad, M.; Shanaida, M.; Lysiuk, R.; Peana, M.; Pshyk-Titko, I.; Adamiv, S.; Shanaida, Y.; Bjørklund, G. Health benefits of xylitol. Appl. Microbiol. Biotechnol. 2020, 104, 7225–7237. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Nagai, R. Insights from the fructose-derived product glucoselysine: Revisiting the polyol pathway in diabetic complications. J. Diabetes. Investig. 2025, 16, 569–577. [Google Scholar] [CrossRef]
- Delannoy, P.; Tolan, D.R.; Lanaspa, M.A.; San Millán, I.; Bae, S.Y.; Johnson, R.J. Aldose reductase, fructose and fat production in the liver. Biochem. J. 2025, 482, 295–307. [Google Scholar] [CrossRef]
- Fotros, D.; Hekmatdoost, A.; Pashayee-Khamene, F.; Karimi, S.; Ahmadzadeh, S.; Saberifiroozi, M.; Hatami, B.; Yari, Z. Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) and mortality among survivors of liver cirrhosis: A prospective cohort study. Nutr. J. 2025, 24, 15. [Google Scholar] [CrossRef]
- Word Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Young, J.; Scott, S.; Clark, L.; Lodge, J.K. Associations between free sugar intake and markers of health in the UK population: An analysis of the National Diet and Nutrition Survey rolling programme. Br. J. Nutr. 2022, 128, 225–236. [Google Scholar] [CrossRef]
- Wright, E.M.; Martín, M.G.; Turk, E. Intestinal absorption in health and disease—Sugars. Best Pr. Res. Clin. Gastroenterol. 2003, 17, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Choe, J.Y.; Patel, C.R. Intestinal Absorption of Fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; McGraw, T.E.; Kahn, B.B. Insulin action in adipocytes, adipose remodeling, and systemic effects. Cell Metab. 2021, 33, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L. Metabolism of sugars: A window to the regulation of glucose and lipid homeostasis by splanchnic organs. Clin. Nutr. 2021, 40, 1691–1698. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2014; pp. 177–197. [Google Scholar]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef]
- Lodge, M.; Dykes, R.; Kennedy, A. Regulation of Fructose Metabolism in Nonalcoholic Fatty Liver Disease. Biomolecules 2024, 14, 845. [Google Scholar] [CrossRef]
- Westerbeke, F.H.M.; Rios-Morales, M.; Attaye, I.; Nieuwdorp, M. Fructose catabolism and its metabolic effects: Exploring host–microbiota interactions and the impact of ethnicity. J. Physiol. 2025. [Google Scholar] [CrossRef]
- Diggle, C.P.; Shires, M.; Leitch, D.; Brooke, D.; Carr, I.M.; Markham, A.F.; Hayward, B.E.; Asipu, A.; Bonthron, D.T. Ketohexokinase: Expression and Localization of the Principal Fructose-metabolizing Enzyme. J. Histochem. Cytochem. 2009, 57, 763–774. [Google Scholar] [CrossRef]
- Geidl-Flueck, B.; Gerber, P. Insights into the Hexose Liver Metabolism—Glucose versus Fructose. Nutrients 2017, 9, 1026. [Google Scholar] [CrossRef]
- Ziolkowska, S.; Binienda, A.; Jabłkowski, M.; Szemraj, J.; Czarny, P. The Interplay between Insulin Resistance, Inflammation, Oxidative Stress, Base Excision Repair and Metabolic Syndrome in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2021, 22, 11128. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Ortega, V.E.; Moroni-González, D.; Diaz, A.; García-González, M.Á.; Brambila, E.; Treviño, S. Hepatic Insulin Resistance Model in the Male Wistar Rat Using Exogenous Insulin Glargine Administration. Metabolites 2023, 13, 572. [Google Scholar] [CrossRef] [PubMed]
- Jegatheesan, P.; De Bandt, J. Fructose and NAFLD The Multifaceted Aspects of Fructose Metabolism. Nutrients 2017, 9, 230. [Google Scholar] [CrossRef]
- Feillet-Coudray, C.; Fouret, G.; Vigor, C.; Bonafos, B.; Jover, B.; Blachnio-Zabielska, A.; Rieusset, J.; Casas, F.; Gaillet, S.; Landrier, J.F.; et al. Long-Term Measures of Dyslipidemia, Inflammation, and Oxidative Stress in Rats Fed a High-Fat/High-Fructose Diet. Lipids 2019, 54, 81–97. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, D.; Seo, W.; Gao, B.; Yoo, S.; Song, B. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis Through Ethanol-Inducible Cytochrome P450-2E1–Mediated Oxidative and Nitrative Stress. Hepatology 2021, 73, 2180–2195. [Google Scholar] [CrossRef]
- Kanazawa, J.; Kakisaka, K.; Suzuki, Y.; Yonezawa, T.; Abe, H.; Wang, T.; Takikawa, Y. Excess fructose enhances oleatic cytotoxicity via reactive oxygen species production and causes necroptosis in hepatocytes. J. Nutr. Biochem. 2022, 107, 109052. [Google Scholar] [CrossRef]
- Campos-Maldonado, F.; González-Dávalos, M.L.; Piña, E.; Anaya-Loyola, M.A.; Shimada, A.; Varela-Echavarria, A.; Mora, O. Fructose promotes more than glucose the adipocytic differentiation of pig mesenchymal stem cells. J. Food Biochem. 2022, 46, e14429. [Google Scholar] [CrossRef]
- Wang, L.; Ji, T.; Yuan, Y.; Fu, H.; Wang, Y.; Tian, S.; Hu, J.; Wang, L.; Wang, Z. High-fructose corn syrup promotes proinflammatory Macrophage activation via ROS-mediated NF-κB signaling and exacerbates colitis in mice. Int. Immunopharmacol. 2022, 109, 108814. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive intake of sugar: An accomplice of inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef] [PubMed]
- Jameel, F.; Phang, M.; Wood, L.G.; Garg, M.L. Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation. Lipids Health Dis. 2014, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Kuzma, J.N.; Cromer, G.; Hagman, D.K.; Breymeyer, K.L.; Roth, C.L.; Foster-Schubert, K.E.; Holte, S.E.; Weigle, D.S.; Kratz, M. No differential effect of beverages sweetened with fructose, high-fructose corn syrup, or glucose on systemic or adipose tissue inflammation in normal-weight to obese adults: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Tamer, F.; Ulug, E.; Akyol, A.; Nergiz-Unal, R. The potential efficacy of dietary fatty acids and fructose induced inflammation and oxidative stress on the insulin signaling and fat accumulation in mice. Food Chem. Toxicol. 2020, 135, 110914. [Google Scholar] [CrossRef]
- Lin, W.T.; Kao, Y.H.; Li, M.S.; Luo, T.; Lin, H.Y.; Lee, C.H.; Seal, D.W.; Hu, C.Y.; Chen, L.S.; Tseng, T.S. Sugar-Sweetened Beverages Intake, Abdominal Obesity, and Inflammation among US Adults without and with Prediabetes—An NHANES Study. Int. J. Environ. Res. Public. Health 2022, 20, 681. [Google Scholar] [CrossRef]
- Hou, J.; Cui, Y.; Gao, J.; Rong, M. Dietary simple sugar intake, metabolic indicators, markers of inflammation, and injury among semi-professional football players. Food Nutr. Res. 2025, 69, 11036. [Google Scholar] [CrossRef]
- Todoric, J.; Di Caro, G.; Reibe, S.; Henstridge, D.C.; Green, C.R.; Vrbanac, A.; Ceteci, F.; Conche, C.; McNulty, R.; Shalapour, S.; et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat. Metab. 2020, 2, 1034–1045. [Google Scholar] [CrossRef]
- Chavakis, T.; Alexaki, V.I.; Ferrante, A.W. Macrophage function in adipose tissue homeostasis and metabolic inflammation. Nat. Immunol. 2023, 24, 757–766. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; He, Y.; Hwang, S.; Bertola, A.; Mackowiak, B.; Ahmed, Y.A.; Seo, W.; Ma, J.; Wang, X.; Park, S.H.; et al. E-Selectin-Dependent Inflammation and Lipolysis in Adipose Tissue Exacerbate Steatosis-to-NASH Progression via S100A8/9. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 151–171. [Google Scholar] [CrossRef]
- Jaiswal, N.; Agrawal, S.; Agrawal, A. High fructose-induced metabolic changes enhance inflammation in human dendritic cells. Clin. Exp. Immunol. 2019, 197, 237–249. [Google Scholar] [CrossRef]
- Tan, J.; Ni, D.; Wali, J.A.; Cox, D.A.; Pinget, G.V.; Taitz, J.; Daïen, C.I.; Senior, A.; Read, M.N.; Simpson, S.J.; et al. Dietary carbohydrate, particularly glucose, drives B cell lymphopoiesis and function. iScience 2021, 24, 102835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, C.; Huang, P.; Chen, L.; Ma, Y.; Ding, H. Effects of chronic exposure to a high fat diet, nutritive or non-nutritive sweeteners on hypothalamic-pituitary-adrenal (HPA) and -gonadal (HPG) axes of male Sprague-Dawley rats. Eur. J. Nutr. 2024, 63, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Cawley, N.X. Sugar Making Sugar: Gluconeogenesis Triggered by Fructose via a Hypothalamic-Adrenal-Corticosterone Circuit. Endocrinology 2012, 153, 3561–3563. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Rivard, C.; Lanaspa, M.A.; Otabachian-Smith, S.; Ishimoto, T.; Cicerchi, C.; Cheeke, P.R.; Macintosh, B.; Hess, T. Fructokinase, Fructans, Intestinal Permeability, and Metabolic Syndrome: An Equine Connection? J. Equine. Vet. Sci. 2013, 33, 120–126. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Niu, D.; Zhang, S.; Wang, H.; Zhang, X.; Nan, F.; Jiang, S.; Wang, B. Gut microbiota induces hepatic steatosis by modulating the T cells balance in high fructose diet mice. Sci. Rep. 2023, 13, 6701. [Google Scholar] [CrossRef]
- Romualdo, G.R.; Valente, L.C.; Sprocatti, A.C.; Bacil, G.P.; de Souza, I.P.; Rodrigues, J.; Rodrigues, M.A.M.; Vinken, M.; Cogliati, B.; Barbisan, L.F. Western diet–induced mouse model of non-alcoholic fatty liver disease associated with metabolic outcomes: Features of gut microbiome-liver-adipose tissue axis. Nutrition 2022, 103, 111836. [Google Scholar] [CrossRef]
- Kizilaslan, N.; Zekiye Erdem, N.; Katar, M.; Gevrek, F. The Effects of Probiotics and Omega-3 Fatty Acids in Liver Steatosis Induced in Rats by High-Fructose Corn Syrup. Int. J. Clin. Pract. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef]
- Quarta, C.; Stemmer, K.; Novikoff, A.; Yang, B.; Klingelhuber, F.; Harger, A.; Bakhti, M.; Bastidas-Ponce, A.; Baugé, E.; Campbell, J.E.; et al. GLP-1-mediated delivery of tesaglitazar improves obesity and glucose metabolism in male mice. Nat. Metab. 2022, 4, 1071–1083. [Google Scholar] [CrossRef]
- Brown, M.; Dainty, S.; Strudwick, N.; Mihai, A.D.; Watson, J.N.; Dendooven, R.; Paton, A.W.; Paton, J.C.; Schröder, M. Endoplasmic reticulum stress causes insulin resistance by inhibiting delivery of newly synthesized insulin receptors to the cell surface. Mol. Biol. Cell 2020, 31, 2597–2629. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Herzig, S.; Kulkarni, R.N.; Montminy, M. TRB3: A tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 2003, 300, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef]
- Malhi, H.; Gores, G. Molecular Mechanisms of Lipotoxicity in Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2008, 28, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Ackerman, Z.; Oron-Herman, M.; Grozovski, M.; Rosenthal, T.; Pappo, O.; Link, G.; Sela, B.A. Fructose-Induced Fatty Liver Disease. Hypertension 2005, 45, 1012–1018. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Mu, W.; Roncal, C.; Sautin, Y.Y.; Abdelmalek, M.; Reungjui, S.; Le, M.; Nakagawa, T.; Lan, H.Y.; Yu, X.; et al. Comparison of free fructose and glucose to sucrose in the ability to cause fatty liver. Eur. J. Nutr. 2010, 49, 1–9. [Google Scholar] [CrossRef]
- Tseng, T.S.; Lin, W.T.; Ting, P.S.; Huang, C.K.; Chen, P.H.; Gonzalez, G.V.; Lin, H.Y. Sugar-Sweetened Beverages and Artificially Sweetened Beverages Consumption and the Risk of Nonalcoholic Fatty Liver (NAFLD) and Nonalcoholic Steatohepatitis (NASH). Nutrients 2023, 15, 3997. [Google Scholar] [CrossRef]
- Valle, M.; St-Pierre, P.; Pilon, G.; Marette, A. Differential Effects of Chronic Ingestion of Refined Sugars versus Natural Sweeteners on Insulin Resistance and Hepatic Steatosis in a Rat Model of Diet-Induced Obesity. Nutrients 2020, 12, 2292. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol.-Ren. Physiol. 2006, 290, F625–F631. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Neil, D.; Aguila, M.; Mandarim-de-Lacerda, C. Hepatic Adverse Effects of Fructose Consumption Independent of Overweight/Obesity. Int. J. Mol. Sci. 2013, 14, 21873–21886. [Google Scholar] [CrossRef] [PubMed]
- Roncal-Jimenez, C.A.; Lanaspa, M.A.; Rivard, C.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Jalal, D.; Andres-Hernando, A.; Tanabe, K.; Madero, M.; Li, N.; et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism 2011, 60, 1259–1270. [Google Scholar] [CrossRef]
- Deng, S.; Ge, Y.; Zhai, Z.; Liu, H.; Zhang, X.; Chen, Y.; Yang, Y.; Wu, Z. Fructose induces hepatic steatosis in adolescent mice linked to the disorders of lipid metabolism, bile acid metabolism, and autophagy. J. Nutr. Biochem. 2024, 129, 109635. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, A.; Ahmad, S.; Guru, B.; Gulzar, F.; Kumar, P.; Ahmad, I.; Tamrakar, A.K. Convergence of Fructose-Induced NLRP3 Activation with Oxidative Stress and ER Stress Leading to Hepatic Steatosis. Inflammation 2023, 46, 217–233. [Google Scholar] [CrossRef]
- Raza, S.; Shahi, A.; Medhe, P.; Tewari, A.; Gupta, P.; Rajak, S.; Chakravarti, B.; Sinha, R.A. Fructose-induced perturbation in cellular proteostasis via RPS6KB1 promotes hepatic steatosis. Biochim. Et Biophys. Acta BBA Mol. Cell Res. 2024, 1871, 119597. [Google Scholar] [CrossRef]
- Bhat, S.F.; Pinney, S.E.; Kennedy, K.M.; McCourt, C.R.; Mundy, M.A.; Surette, M.G.; Sloboda, D.M.; Simmons, R.A. Exposure to high fructose corn syrup during adolescence in the mouse alters hepatic metabolism and the microbiome in a sex-specific manner. J. Physiol. 2021, 599, 1487–1511. [Google Scholar] [CrossRef]
- Velázquez, A.M.; Bentanachs, R.; Sala-Vila, A.; Lázaro, I.; Rodríguez-Morató, J.; Sánchez, R.M.; Alegret, M.; Roglans, N.; Laguna, J.C. ChREBP-driven DNL and PNPLA3 Expression Induced by Liquid Fructose are Essential in the Production of Fatty Liver and Hypertriglyceridemia in a High-Fat Diet-Fed Rat Model. Mol. Nutr. Food Res. 2022, 66, 2101115. [Google Scholar] [CrossRef]
- Papadopoulos, G.; Legaki, A.I.; Georgila, K.; Vorkas, P.; Giannousi, E.; Stamatakis, G.; Moustakas, I.I.; Petrocheilou, M.; Pyrina, I.; Gercken, B.; et al. Integrated omics analysis for characterization of the contribution of high fructose corn syrup to non-alcoholic fatty liver disease in obesity. Metabolism 2023, 144, 155552. [Google Scholar] [CrossRef]
- Ferri, F.; Carotti, S.; Carpino, G.; Mischitelli, M.; Cantafora, A.; Molinaro, A.; Argenziano, M.E.; Parisse, S.; Corsi, A.; Riminucci, M.; et al. The Propensity of the Human Liver to Form Large Lipid Droplets Is Associated with PNPLA3 Polymorphism, Reduced INSIG1 and NPC1L1 Expression and Increased Fibrogenetic Capacity. Int. J. Mol. Sci. 2021, 22, 6100. [Google Scholar] [CrossRef] [PubMed]
- Geidl-Flueck, B.; Hochuli, M.; Németh, Á.; Eberl, A.; Derron, N.; Köfeler, H.C.; Tappy, L.; Berneis, K.; Spinas, G.A.; Gerber, P.A. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: A randomized controlled trial. J. Hepatol. 2021, 75, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Sigala, D.M.; Hieronimus, B.; Medici, V.; Lee, V.; Nunez, M.V.; Bremer, A.A.; Cox, C.L.; Price, C.A.; Benyam, Y.; Abdelhafez, Y.; et al. The Dose-Response Effects of Consuming High Fructose Corn Syrup-Sweetened Beverages on Hepatic Lipid Content and Insulin Sensitivity in Young Adults. Nutrients 2022, 14, 1648. [Google Scholar] [CrossRef] [PubMed]
- Simons, N.; Veeraiah, P.; Simons, P.I.H.G.; Schaper, N.C.; Kooi, M.E.; Schrauwen-Hinderling, V.B.; Feskens, E.J.M.; van der Ploeg, E.M.C.L.; Van den Eynde, M.D.G.; Schalkwijk, C.G.; et al. Effects of fructose restriction on liver steatosis (FRUITLESS); A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 391–400. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Ugalde-Nicalo, P.; Welsh, J.A.; Angeles, J.E.; Cordero, M.; Harlow, K.E.; Alazraki, A.; Durelle, J.; Knight-Scott, J.; Newton, K.P.; et al. Revised Dietary Intake Estimates for Protein and Fat. JAMA 2019, 322, 469. [Google Scholar]
- Lee, D.; Chiavaroli, L.; Ayoub-Charette, S.; Khan, T.A.; Zurbau, A.; Au-Yeung, F.; Cheung, A.; Liu, Q.; Qi, X.; Ahmed, A.; et al. Important Food Sources of Fructose-Containing Sugars and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Controlled Trials. Nutrients 2022, 14, 2846. [Google Scholar] [CrossRef]
- Buziau, A.M.; Eussen, S.J.P.M.; Kooi, M.E.; van der Kallen, C.J.H.; van Dongen, M.C.J.M.; Schaper, N.C.; Henry, R.M.A.; Schram, M.T.; Dagnelie, P.C.; van Greevenbroek, M.M.J.; et al. Fructose Intake from Fruit Juice and Sugar-Sweetened Beverages Is Associated With Higher Intrahepatic Lipid Content: The Maastricht Study. Diabetes Care 2022, 45, 1116–1123. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Gu, Y.; Zhang, T.; Wang, X.; Zhang, J.; et al. Added sugar intake and its forms and sources in relation to risk of non-alcoholic fatty liver disease: Results from the Tianjin Chronic Low-grade Systemic Inflammation and Health cohort study. Br. J. Nutr. 2023, 129, 2094–2101. [Google Scholar] [CrossRef]
- Pixner, T.; Stummer, N.; Schneider, A.M.; Lukas, A.; Gramlinger, K.; Julian, V.; Thivel, D.; Mörwald, K.; Maruszczak, K.; Mangge, H.; et al. The Role of Macronutrients in the Pathogenesis, Prevention and Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD) in the Paediatric Population—A Review. Life 2022, 12, 839. [Google Scholar] [CrossRef]
- Paik, J.M.; Mir, S.; Alqahtani, S.A.; Younossi, Y.; Ong, J.P.; Younossi, Z.M. Dietary Risks for Liver Mortality in NAFLD: Global Burden of Disease Data. Hepatol. Commun. 2022, 6, 90–100. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, C.; Liu, L.; Li, L. Sugar-sweetened beverage intake and long-term mortality in individuals with metabolic dysfunction-associated steatotic liver disease: A longitudinal analysis of the National Health and Nutrition Examination Survey database. Eur. J. Gastroenterol. Hepatol. 2024, 36, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, K.; Swaminathan, K.; Mathan Kumar, S.; Clemens, D.L.; Dey, A. In Vitro Evidence for Chronic Alcohol and High Glucose Mediated Increased Oxidative Stress and Hepatotoxicity. Alcohol. Clin. Exp. Res. 2012, 36, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Thomes, P.G.; Benbow, J.H.; Brandon-Warner, E.; Thompson, K.J.; Jacobs, C.; Donohue, T.M., Jr.; Schrum, L.W. Dietary fructose augments ethanol-induced liver pathology. J. Nutr. Biochem. 2017, 43, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Xu, M.; Schultze, F.C.; Wilting, J.; Mihm, S.; Raddatz, D.; Ramadori, G. Combination of Alcohol and Fructose Exacerbates Metabolic Imbalance in Terms of Hepatic Damage, Dyslipidemia, and Insulin Resistance in Rats. PLoS ONE 2014, 9, e104220. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Lacerda-Queiroz, N.; Sabino, J.F.L.; Marques, P.E.; Galvão, I.; Gamba, C.O.; Cassali, G.D.; de Carvalho, L.M.; da Silva, D.A.; Versiani, A.; et al. Evaluating the effects of refined carbohydrate and fat diets with acute ethanol consumption using a mouse model of alcoholic liver injury. J. Nutr. Biochem. 2017, 39, 93–100. [Google Scholar] [CrossRef]
- Babuta, M.; Morel, C.; de Carvalho Ribeiro, M.; Datta, A.A.; Calenda, C.; Copeland, C.; Nasser, I.; Szabo, G. A novel experimental model of MetALD in male mice recapitulates key features of severe alcohol-associated hepatitis. Hepatol. Commun. 2024, 8, e0450. [Google Scholar] [CrossRef]
- Li, M.; Chen, W.; Deng, Y.; Xie, W. Impacts of cardiometabolic risk factors and alcohol consumption on all-cause mortality among MASLD and its subgroups. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 2085–2094. [Google Scholar] [CrossRef]
- Aboona, M.B.; Danpanichkul, P.; Chen, V.L.; Rangan, P.; Kim, D.; Alkhouri, N.; Fallon, M.B.; Noureddin, M.; Arab, J.P.; Wijarnpreecha, K. Mortality outcomes in individuals with MASLD versus MASLD and increased alcohol intake. J. Gastroenterol. Hepatol. 2024, 39, 2456–2463. [Google Scholar] [CrossRef]
- Marti-Aguado, D.; Calleja, J.L.; Vilar-Gomez, E.; Iruzubieta, P.; Rodríguez-Duque, J.C.; Del Barrio, M.; Puchades, L.; Rivera-Esteban, J.; Perelló, C.; Puente, A.; et al. Low-to-moderate alcohol consumption is associated with increased fibrosis in individuals with metabolic dysfunction-associated steatotic liver disease. J. Hepatol. 2024, 81, 930–940. [Google Scholar] [CrossRef]
- van Eekelen, E.; Beulens, J.W.J.; Geelen, A.; Schrauwen-Hinderling, V.B.; Lamb, H.; de Roos, A.; Rosendaal, F.; de Mutsert, R. Consumption of Alcoholic and Sugar-Sweetened Beverages is Associated with Increased Liver Fat Content in Middle-Aged Men and Women. J. Nutr. 2019, 149, 649–658. [Google Scholar] [CrossRef]
- Åberg, F.; Puukka, P.; Salomaa, V.; Männistö, S.; Lundqvist, A.; Valsta, L.; Perola, M.; Färkkilä, M.; Jula, A. Risks of Light and Moderate Alcohol Use in Fatty Liver Disease: Follow-Up of Population Cohorts. Hepatology 2020, 71, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.C.; Hagaman, J.A. The interplay of Western diet and binge drinking on the onset, progression, and outlook of liver disease. Nutr. Rev. 2022, 80, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Lodge, M.; Scheidemantle, G.; Adams, V.R.; Cottam, M.A.; Richard, D.; Breuer, D.; Thompson, P.; Shrestha, K.; Liu, X.; Kennedy, A. Fructose regulates the pentose phosphate pathway and induces an inflammatory and resolution phenotype in Kupffer cells. Sci. Rep. 2024, 14, 4020. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Duan, Q.; Wu, R.; Harris, E.N.; Su, Q. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Adv. Drug Deliv. Rev. 2021, 176, 113869. [Google Scholar] [CrossRef]
- Oliveira-Cordeiro, B.; Fernandes-DA-Silva, A.; Silva-Veiga, F.M.; Miranda, C.S.; Martins, F.F.; Souza-Mello, V. Long-term hepatic damage in high-fructose-fed C57BL/6 mice: Hepatic fibrogenesis, endoplasmic reticulum stress markers, and fibrosis. An. Acad. Bras. Cienc. 2023, 95 (Suppl. S2), e20220784. [Google Scholar]
- Xin, X.; Cai, B.Y.; Chen, C.; Tian, H.J.; Wang, X.; Hu, Y.Y.; Feng, Q. High-trans fatty acid and high-sugar diets can cause mice with non-alcoholic steatohepatitis with liver fibrosis and potential pathogenesis. Nutr. Metab. 2020, 17, 40. [Google Scholar] [CrossRef]
- Georgiou, A.; Yannakoulia, M.; Papatheodoridis, G.V.; Deutsch, M.; Alexopoulou, A.; Vlachogiannakos, J.; Ioannidou, P.; Papageorgiou, M.V.; Voulgaris, T.; Papadopoulos, N.; et al. Assessment of dietary habits and the adequacy of dietary intake of patients with cirrhosis-the KIRRHOS study. Clin. Nutr. 2021, 40, 3992–3998. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef]
- Parisse, S.; Carnevale, S.; Di Bartolomeo, F.; Poli, E.; Miceli, F.; Ferri, F.; Mischitelli, M.; Rocco, B.; Lai, Q.; Lucatelli, P.; et al. A Low Daily Intake of Simple Sugars in the Diet Is Associated with Improved Liver Function in Cirrhotic Liver Transplant Candidates. Nutrients 2023, 15, 1575. [Google Scholar] [CrossRef]
- Hasan, N.; Yazdanpanah, O.; Khaleghi, B.; Benjamin, D.J.; Kalebasty, A.R. The role of dietary sugars in cancer risk: A comprehensive review of current evidence. Cancer Treat. Res. Commun. 2024, 43, 100876. [Google Scholar] [CrossRef]
- Li, X.; Qian, X.; Peng, L.X.; Jiang, Y.; Hawke, D.H.; Zheng, Y.; Xia, Y.; Lee, J.H.; Cote, G.; Wang, H.; et al. A splicing switch from ketohexokinase-C to ketohexokinase-A drives hepatocellular carcinoma formation. Nat. Cell Biol. 2016, 18, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.M.; Li, Q.; Zhang, P.F.; Shen, S.L.; Xie, W.X.; Chen, B.; Wu, J.; Hu, W.J.; Huang, X.Y.; Peng, B.G. Restoration of FBP1 suppressed Snail-induced epithelial to mesenchymal transition in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.S.; Kim, N.; Cullen, Q.; Eskandari, R.; Mamakhanyan, A.; Srouji, R.M.; Chirayil, R.; Jeong, S.; Shakiba, M.; Kastenhuber, E.R.; et al. Ketohexokinase-mediated fructose metabolism is lost in hepatocellular carcinoma and can be leveraged for metabolic imaging. Sci. Adv. 2022, 8, eabm7985. [Google Scholar] [CrossRef] [PubMed]
- Parks, S.K.; Mueller-Klieser, W.; Pouysségur, J. Lactate and Acidity in the Cancer Microenvironment. Annu. Rev. Cancer Biol. 2020, 4, 141–158. [Google Scholar] [CrossRef]
- Dewdney, B.; Roberts, A.; Qiao, L.; George, J.; Hebbard, L. A Sweet Connection? Fructose’s Role in Hepatocellular Carcinoma. Biomolecules 2020, 10, 496. [Google Scholar] [CrossRef]
- Dewdney, B.; Alanazy, M.; Gillman, R.; Walker, S.; Wankell, M.; Qiao, L.; George, J.; Roberts, A.; Hebbard, L. The effects of fructose and metabolic inhibition on hepatocellular carcinoma. Sci. Rep. 2020, 10, 16769. [Google Scholar] [CrossRef]
- Li, M.; He, X.; Guo, W.; Yu, H.; Zhang, S.; Wang, N.; Liu, G.; Sa, R.; Shen, X.; Jiang, Y.; et al. Aldolase B suppresses hepatocellular carcinogenesis by inhibiting G6PD and pentose phosphate pathways. Nat. Cancer 2020, 1, 735–747. [Google Scholar] [CrossRef]
- Liu, G.; Wang, N.; Zhang, C.; Li, M.; He, X.; Yin, C.; Tu, Q.; Shen, X.; Zhang, L.; Lv, J.; et al. Fructose-1, 6-Bisphosphate Aldolase B Depletion Promotes Hepatocellular Carcinogenesis Through Activating Insulin Receptor Signaling and Lipogenesis. Hepatology 2021, 74, 3037–3055. [Google Scholar] [CrossRef]
- Tao, Q.F.; Yuan, S.X.; Yang, F.; Yang, S.; Yang, Y.; Yuan, J.H.; Wang, Z.G.; Xu, Q.G.; Lin, K.Y.; Cai, J.; et al. Aldolase B inhibits metastasis through Ten–Eleven Translocation 1 and serves as a prognostic biomarker in hepatocellular carcinoma. Mol. Cancer 2015, 14, 170. [Google Scholar] [CrossRef]
- Syamprasad, N.P.; Jain, S.; Rajdev, B.; Panda, S.R.; Kumar, G.J.; Shaik, K.M.; Shantanu, P.A.; Challa, V.S.; Jorvekar, S.B.; Borkar, R.M.; et al. AKR1B1 drives hyperglycemia-induced metabolic reprogramming in MASLD-associated hepatocellular carcinoma. JHEP Rep. 2024, 6, 100974. [Google Scholar] [CrossRef]
- Zhou, P.; Chang, W.Y.; Gong, D.A.; Xia, J.; Chen, W.; Huang, L.Y.; Liu, R.; Liu, Y.; Chen, C.; Wang, K.; et al. High dietary fructose promotes hepatocellular carcinoma progression by enhancing O-GlcNAcylation via microbiota-derived acetate. Cell Metab. 2023, 35, 1961–1975.e6. [Google Scholar] [CrossRef] [PubMed]
- Esquea, E.M.; Young, R.G.; Reginato, M.J. Fructose promotes liver cancer via microbial acetate-induced O-GlcNAcylation. Trends Endocrinol. Metab. 2024, 35, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.J.H.; Ng, C.H.; Lin, S.Y.; Pan, X.H.; Tay, P.; Lim, W.H.; Teng, M.; Syn, N.; Lim, G.; Yong, J.N.; et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: A systematic review and meta-analysis. Lancet Oncol. 2022, 23, 521–530. [Google Scholar] [CrossRef]
- Toh, M.R.; Wong, E.Y.T.; Wong, S.H.; Ng, A.W.T.; Loo, L.H.; Chow, P.K.; Ngeow, J. Global Epidemiology and Genetics of Hepatocellular Carcinoma. Gastroenterology 2023, 164, 766–782. [Google Scholar] [CrossRef]
- Ha, S.; Wong, V.W.S.; Zhang, X.; Yu, J. Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut 2025, 74, 141–152. [Google Scholar] [CrossRef]
- Mullish, B.H. Risk Factors for Liver Cancer and Chronic Liver Disease-related Death: Are Sugar Substitutes Better Than the Real Thing? Gastroenterology 2024, 166, 213–214. [Google Scholar] [CrossRef]
- Fedirko, V.; Lukanova, A.; Bamia, C.; Trichopolou, A.; Trepo, E.; Nöthlings, U.; Schlesinger, S.; Aleksandrova, K.; Boffetta, P.; Tjønneland, A.; et al. Glycemic index, glycemic load, dietary carbohydrate, and dietary fiber intake and risk of liver and biliary tract cancers in Western Europeans. Ann. Oncol. 2013, 24, 543–553. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Coday, M.; Garcia, D.O.; Li, X.; Mossavar-Rahmani, Y.; Naughton, M.J.; Lopez-Pentecost, M.; Saquib, N.; Shadyab, A.H.; et al. Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Liver Cancer and Chronic Liver Disease Mortality. JAMA 2023, 330, 537. [Google Scholar] [CrossRef]
- Rossi, M.; Lipworth, L.; Maso, L.D.; Talamini, R.; Montella, M.; Polesel, J.; McLaughlin, J.K.; Parpinel, M.; Franceschi, S.; Lagiou, P.; et al. Dietary glycemic load and hepatocellular carcinoma with or without chronic hepatitis infection. Ann. Oncol. 2009, 20, 1736–1740. [Google Scholar] [CrossRef]
- Lagiou, P.; Rossi, M.; Tzonou, A.; Georgila, C.; Trichopoulos, D.; La Vecchia, C. Glycemic load in relation to hepatocellular carcinoma among patients with chronic hepatitis infection. Ann. Oncol. 2009, 20, 1741–1745. [Google Scholar] [CrossRef]
- Yang, L.S.; Yan, L.J.; Meng, G.X.; Ding, Z.N.; Yao, S.Y.; Li, H.C.; Dong, Z.R.; Chen, Z.Q.; Hong, J.G.; Li, T. The Association of Glycemic Index, Glycemic Load, and Daily Carbohydrates Intake with the Risk of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Nutr. Cancer 2023, 75, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Cohen, Y.; Valdés-Mas, R.; Mor, U.; Dori-Bachash, M.; Federici, S.; Zmora, N.; Leshem, A.; Heinemann, M.; Linevsky, R.; et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 2022, 185, 3307–3328.e19. [Google Scholar] [CrossRef]
- Tsai, M.J.; Li, C.H.; Wu, H.T.; Kuo, H.Y.; Wang, C.T.; Pai, H.L.; Chang, C.J.; Ou, H.Y. Long-Term Consumption of Sucralose Induces Hepatic Insulin Resistance through an Extracellular Signal-Regulated Kinase 1/2-Dependent Pathway. Nutrients 2023, 15, 2814. [Google Scholar] [CrossRef] [PubMed]
- Golzan, S.A.; Movahedian, M.; Haghighat, N.; Asbaghi, O.; Hekmatdoost, A. Association between non-nutritive sweetener consumption and liver enzyme levels in adults: A systematic review and meta-analysis of randomized clinical trials. Nutr. Rev. 2023, 81, 1105–1117. [Google Scholar] [CrossRef]
- Chi, L.; Yifei, Y.; Bian, X.; Gao, B.; Tu, P.; Ru, H.; Lu, K. Chronic sucralose consumption inhibits farnesoid X receptor signaling and perturbs lipid and cholesterol homeostasis in the mouse livers, potentially by altering gut microbiota functions. Sci. Total Environ. 2024, 919, 169603. [Google Scholar] [CrossRef]
- Wu, H.T.; Lin, C.H.; Pai, H.L.; Chen, Y.C.; Cheng, K.P.; Kuo, H.Y.; Li, C.H.; Ou, H.Y. Sucralose, a Non-nutritive Artificial Sweetener Exacerbates High Fat Diet-Induced Hepatic Steatosis Through Taste Receptor Type 1 Member 3. Front Nutr. 2022, 9, 823723. [Google Scholar] [CrossRef]
- Finamor, I.; Pérez, S.; Bressan, C.A.; Brenner, C.E.; Rius-Pérez, S.; Brittes, P.C.; Cheiran, G.; Rocha, M.I.; da Veiga, M.; Sastre, J.; et al. Chronic aspartame intake causes changes in the trans-sulphuration pathway, glutathione depletion and liver damage in mice. Redox Biol. 2017, 11, 701–707. [Google Scholar] [CrossRef]
- Finamor, I.A.; Bressan, C.A.; Torres-Cuevas, I.; Rius-Pérez, S.; da Veiga, M.; Rocha, M.I.; Pavanato, M.A.; Pérez, S. Long-Term Aspartame Administration Leads to Fibrosis, Inflammasome Activation, and Gluconeogenesis Impairment in the Liver of Mice. Biology 2021, 10, 82. [Google Scholar] [CrossRef]
- Sergi, C.M. MASLD and aspartame: Are new studies in the horizon? Front. Med. 2023, 10, 1266918. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisse, S.; Coltorti, E.; Mischitelli, M.; Ferri, F.; Ginanni Corradini, S. New Insights into the Interplay Between Simple Sugars and Liver Diseases. Curr. Issues Mol. Biol. 2025, 47, 390. https://doi.org/10.3390/cimb47060390
Parisse S, Coltorti E, Mischitelli M, Ferri F, Ginanni Corradini S. New Insights into the Interplay Between Simple Sugars and Liver Diseases. Current Issues in Molecular Biology. 2025; 47(6):390. https://doi.org/10.3390/cimb47060390
Chicago/Turabian StyleParisse, Simona, Erika Coltorti, Monica Mischitelli, Flaminia Ferri, and Stefano Ginanni Corradini. 2025. "New Insights into the Interplay Between Simple Sugars and Liver Diseases" Current Issues in Molecular Biology 47, no. 6: 390. https://doi.org/10.3390/cimb47060390
APA StyleParisse, S., Coltorti, E., Mischitelli, M., Ferri, F., & Ginanni Corradini, S. (2025). New Insights into the Interplay Between Simple Sugars and Liver Diseases. Current Issues in Molecular Biology, 47(6), 390. https://doi.org/10.3390/cimb47060390





