Amelioration of Acetaminophen-Induced Hepatic Oxidative Stress and Inflammation by RNAi Targeting Cyp2e1 In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of LNPs
2.3. Animal Experiments
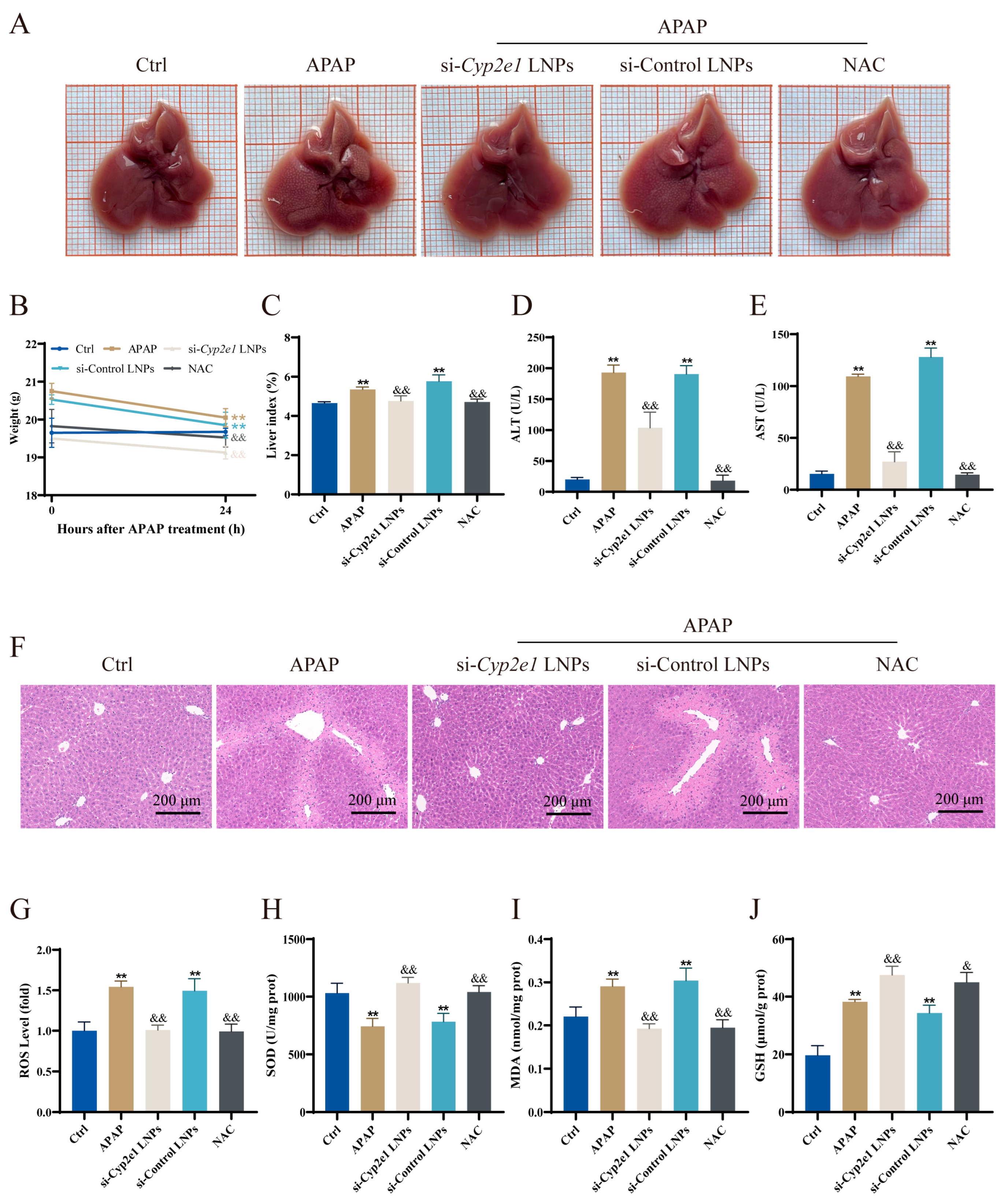
2.4. Serum and Liver Analysis
2.5. Liver Pathology
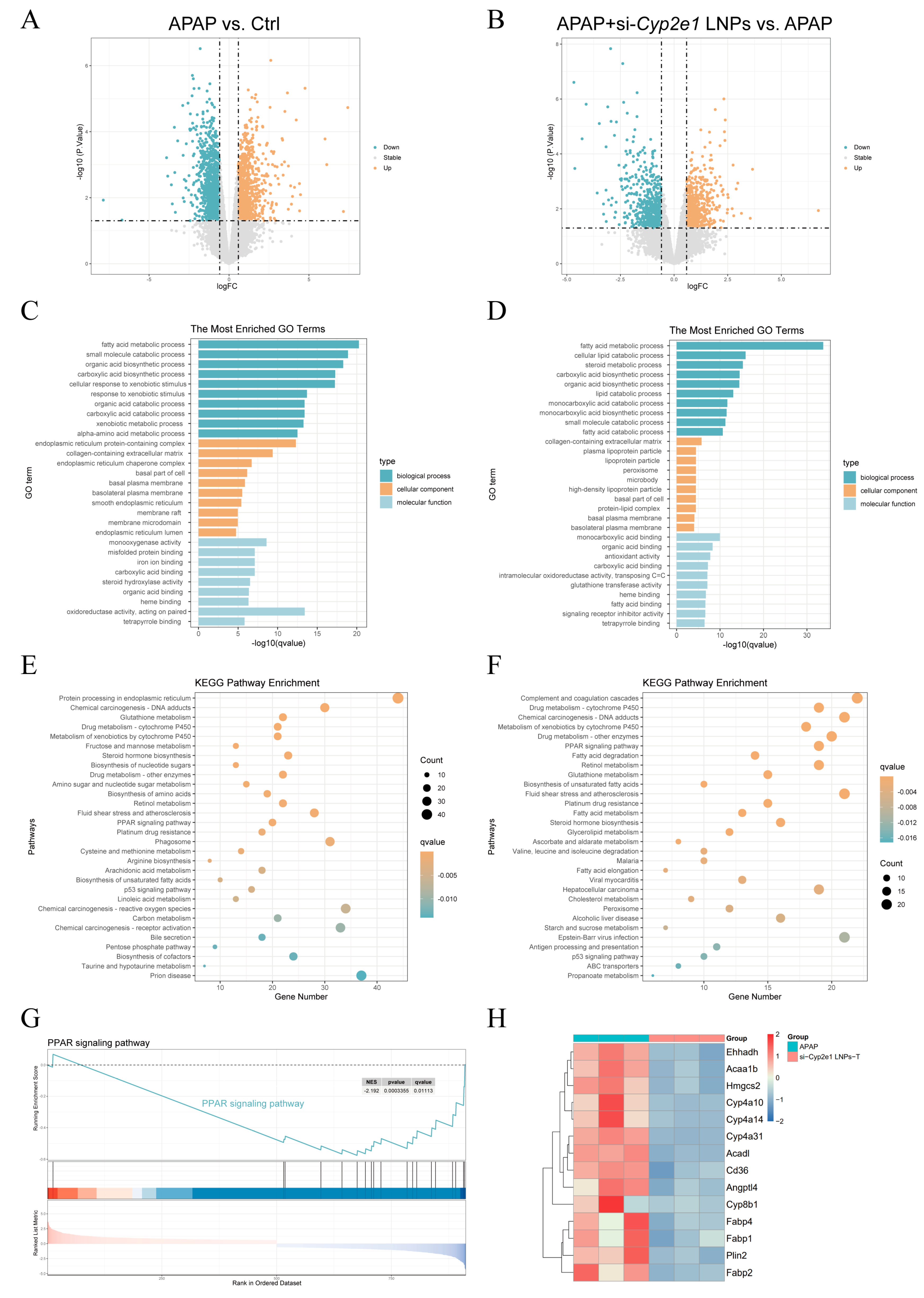
2.6. RNA Sequencing and Enrichment Analysis
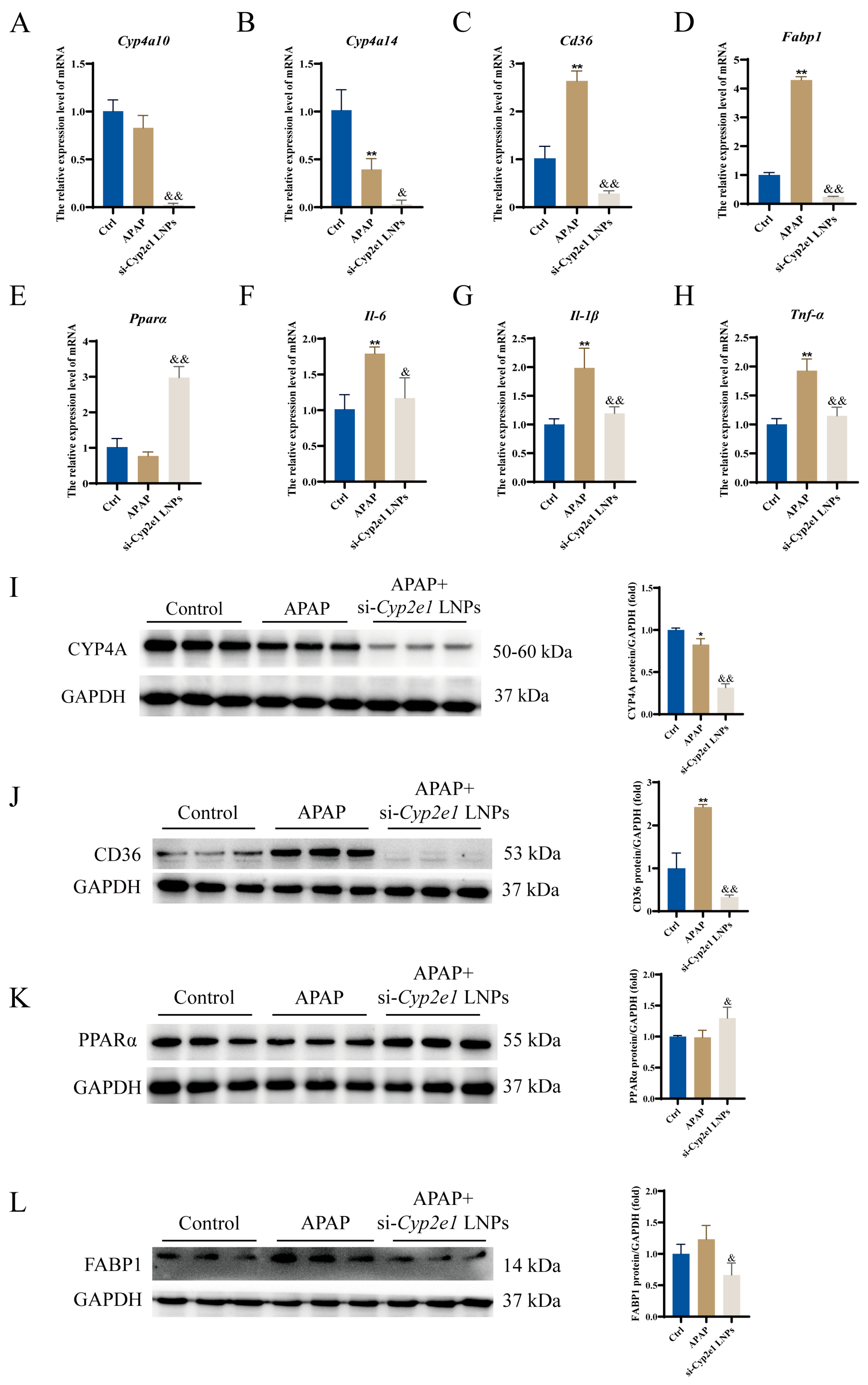
2.7. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
3.1. Characterization and Validation of si-Cyp2e1 LNPs In Vivo
3.2. Immediate Treatment with si-Cyp2e1 LNPs Could Alleviate AILI in Mice
3.3. Transcriptomics Revealed the Possible Mechanism by Which si-Cyp2e1 LNPs Attenuate AILI in Mice
3.4. si-Cyp2e1 LNPs Could Regulate the PPAR Signaling Pathway
3.5. Delayed Treatment with si-Cyp2e1 LNPs for 2 h Did Not Ameliorate AILI in Time
3.6. si-Cyp2e1 LNPs Might Accelerate Liver Recovery Compared with NAC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APAP | acetaminophen |
| AILI | APAP-induced liver injury |
| NAPQI | N-acetyl-p-benzoquinone imine |
| CYPs | cytochrome P450 enzymes |
| RNAi | RNA interference |
| siRNA | small interfering RNA |
| LNPs | lipid nanoparticles |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| GSH | glutathione |
| H&E | hematoxylin and eosin |
| DEGs | differentially expressed genes |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| GSEA | gene set enrichment analysis |
| qPCR | quantitative real-time polymerase chain reaction |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| PPAR | peroxisome proliferator-activated receptor |
| CD36 | cluster of differentiation 36 |
| FABP1 | fatty acid binding protein 1 |
| IL | interleukin |
| TNF | tumor necrosis factor |
References
- Fu, L.; Qian, Y.; Shang, Z.; Sun, X.; Kong, X.; Gao, Y. Antibiotics enhancing drug-induced liver injury assessed for causality using Roussel Uclaf Causality Assessment Method: Emerging role of gut microbiota dysbiosis. Front. Med. 2022, 9, 972518. [Google Scholar] [CrossRef]
- LaForge, J.M.; Urso, K.; Day, J.M.; Bourgeois, C.W.; Ross, M.M.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. Non-steroidal Anti-inflammatory Drugs: Clinical Implications, Renal Impairment Risks, and AKI. Adv. Ther. 2023, 40, 2082–2096. [Google Scholar] [CrossRef]
- Ostapowicz, G.; Fontana, R.J.; Schiødt, F.V.; Larson, A.; Davern, T.J.; Han, S.H.; McCashland, T.M.; Shakil, A.O.; Hay, J.E.; Hynan, L.; et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 2002, 137, 947–954. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen (APAP or N-Acetyl-p-Aminophenol) and Acute Liver Failure. Clin. Liver Dis. 2018, 22, 325–346. [Google Scholar] [CrossRef]
- Lee, S.S.; Buters, J.T.; Pineau, T.; Fernandez-Salguero, P.; Gonzalez, F.J. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 1996, 271, 12063–12067. [Google Scholar] [CrossRef]
- Yan, M.; Huo, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018, 17, 274–283. [Google Scholar] [CrossRef]
- Saito, C.; Lemasters, J.J.; Jaeschke, H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010, 246, 8–17. [Google Scholar] [CrossRef]
- Heard, K.J. Acetylcysteine for acetaminophen poisoning. New Engl. J. Med. 2008, 359, 285–292. [Google Scholar] [CrossRef]
- Akakpo, J.Y.; Ramachandran, A.; Curry, S.C.; Rumack, B.H.; Jaeschke, H. Comparing N-acetylcysteine and 4-methylpyrazole as antidotes for acetaminophen overdose. Arch. Toxicol. 2022, 96, 453–465. [Google Scholar] [CrossRef]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010, 196, 369–405. [Google Scholar] [CrossRef]
- van Rongen, A.; Välitalo, P.A.J.; Peeters, M.Y.M.; Boerma, D.; Huisman, F.W.; van Ramshorst, B.; van Dongen, E.P.A.; van den Anker, J.N.; Knibbe, C.A.J. Morbidly Obese Patients Exhibit Increased CYP2E1-Mediated Oxidation of Acetaminophen. Clin. Pharmacokinet. 2016, 55, 833–847. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Cederbaum, A.I. Induction of cytochrome P450 2E1 increases hepatotoxicity caused by Fas agonistic Jo2 antibody in mice. Hepatology 2005, 42, 400–410. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, D.; Wang, X.; Ward, S.C.; Cederbaum, A.I. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic. Biol. Med. 2010, 49, 1406–1416. [Google Scholar] [CrossRef]
- Schattenberg, J.M.; Czaja, M.J. Regulation of the effects of CYP2E1-induced oxidative stress by JNK signaling. Redox Biol. 2014, 3, 7–15. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Meng, Q.; Du, Y.; Xiao, F.; Zhang, Q.; Yu, J.; Li, K.; Chen, S.; Huang, Z.; et al. ATF4 deficiency protects hepatocytes from oxidative stress via inhibiting CYP2E1 expression. J. Cell. Mol. Med. 2014, 18, 80–90. [Google Scholar] [CrossRef]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Wu, S.; Sun, X.; Yin, R.; Ouyang, Z.; Yin, H.; Wei, Y. Amelioration of ethanol-induced oxidative stress and alcoholic liver disease by in vivo RNAi targeting Cyp2e1. Acta Pharm. Sin. B 2023, 13, 3906–3918. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Q.; Wang, Y.; Yin, H.; Wei, Y. Lipid nanoparticle delivery of siRNA targeting Cyp2e1 gene attenuates subacute alcoholic liver injury in mice. J. Zhejiang Univ. (Med. Sci.) 2023, 52, 306–317. [Google Scholar] [CrossRef]
- Bogorad, R.L.; Yin, H.; Zeigerer, A.; Nonaka, H.; Ruda, V.M.; Zerial, M.; Anderson, D.G.; Koteliansky, V. Nanoparticle-formulated siRNA targeting integrins inhibits hepatocellular carcinoma progression in mice. Nat. Commun. 2014, 5, 3869. [Google Scholar] [CrossRef]
- Zeigerer, A.; Gilleron, J.; Bogorad, R.L.; Marsico, G.; Nonaka, H.; Seifert, S.; Epstein-Barash, H.; Kuchimanchi, S.; Peng, C.G.; Ruda, V.M.; et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature 2012, 485, 465–470. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Animal models of drug-induced liver injury. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1031–1039. [Google Scholar] [CrossRef]
- Yang, R.; Miki, K.; He, X.; Killeen, M.E.; Fink, M.P. Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit. Care 2009, 13, R55. [Google Scholar] [CrossRef]
- Huan, L.; Zhang, Y.; Liu, W.; Lu, H.; Zhang, C.; Yin, R.; Ouyang, Z.; Wei, Y. In vivo inhibition of CYP2E1 fails to reduce pulegone-induced liver injury in mice. Xenobiotica 2025, 1–10. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Diboun, I.; Wernisch, L.; Orengo, C.A.; Koltzenburg, M. Microarray analysis after RNA amplification can detect pronounced differences in gene expression using limma. BMC Genom. 2006, 7, 252. [Google Scholar] [CrossRef]
- Zimmerman, H.J.; West, M. Serum Enzyme Levels in the Diagnosis of Hepatic Disease. Am. J. Gastroenterol. 1963, 40, 387–404. [Google Scholar]
- Wei, Y.; Li, M.; Feng, Z.; Zhang, D.; Sun, M.; Wang, Y.; Chen, X. The Protective Effects of Corn Oligopeptides on Acute Alcoholic Liver Disease by Inhibiting the Activation of Kupffer Cells NF-κB/AMPK Signal Pathway. Nutrients 2022, 14, 4194. [Google Scholar] [CrossRef]
- Park, J.E.; Ahn, C.H.; Lee, H.J.; Sim, D.Y.; Park, S.Y.; Kim, B.; Shim, B.S.; Lee, D.Y.; Kim, S.H. Antioxidant-Based Preventive Effect of Phytochemicals on Anticancer Drug-Induced Hepatotoxicity. Antioxid. Redox Signal. 2023, 38, 1101–1121. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, M.; Li, J.; Chen, Y.; Sun, Q.; Li, H.; Sun, W. Hepatoprotective effects of Di Wu Yang Gan: A medicinal food against CCl(4)-induced hepatotoxicity in vivo and in vitro. Food Chem. 2020, 327, 127093. [Google Scholar] [CrossRef]
- Athuraliya, T.N.; Jones, A.L. Prolonged N-acetylcysteine therapy in late acetaminophen poisoning associated with acute liver failure—A need to be more cautious? Crit. Care 2009, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Aubert, J.; Begriche, K.; Delannoy, M.; Morel, I.; Pajaud, J.; Ribault, C.; Lepage, S.; McGill, M.R.; Lucas-Clerc, C.; Turlin, B.; et al. Differences in early acetaminophen hepatotoxicity between obese ob/ob and db/db mice. J. Pharmacol. Exp. Ther. 2012, 342, 676–687. [Google Scholar] [CrossRef]
- Lam, K.; Schreiner, P.; Leung, A.; Stainton, P.; Reid, S.; Yaworski, E.; Lutwyche, P.; Heyes, J. Optimizing Lipid Nanoparticles for Delivery in Primates. Adv. Mater. 2023, 35, e2211420. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Bai, Q.; Yang, X.; Akakpo, J.Y.; Ji, L.; Yang, L.; Rülicke, T.; Zatloukal, K.; Jaeschke, H.; Ni, H.M.; et al. Dual roles of p62/SQSTM1 in the injury and recovery phases of acetaminophen-induced liver injury in mice. Acta Pharm. Sin. B 2021, 11, 3791–3805. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.M.; McGill, M.R.; Chao, X.; Du, K.; Williams, J.A.; Xie, Y.; Jaeschke, H.; Ding, W.X. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J. Hepatol. 2016, 65, 354–362. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013, 30, 2174–2187. [Google Scholar] [CrossRef]
- Adas, F.; Salaün, J.P.; Berthou, F.; Picart, D.; Simon, B.; Amet, Y. Requirement for omega and (omega;-1)-hydroxylations of fatty acids by human cytochromes P450 2E1 and 4A11. J. Lipid Res. 1999, 40, 1990–1997. [Google Scholar] [CrossRef]
- Ip, E.; Farrell, G.C.; Robertson, G.; Hall, P.; Kirsch, R.; Leclercq, I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology 2003, 38, 123–132. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Farrell, G.C.; Field, J.; Bell, D.R.; Gonzalez, F.J.; Robertson, G.R. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J. Clin. Investig. 2000, 105, 1067–1075. [Google Scholar] [CrossRef]
- Hardwick, J.P.; Osei-Hyiaman, D.; Wiland, H.; Abdelmegeed, M.A.; Song, B.J. PPAR/RXR Regulation of Fatty Acid Metabolism and Fatty Acid omega-Hydroxylase (CYP4) Isozymes: Implications for Prevention of Lipotoxicity in Fatty Liver Disease. PPAR Res. 2009, 2009, 952734. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S.; Chen, Q.; Zhang, Y.; Zhang, C.; Yin, R.; Ouyang, Z.; Wei, Y. Reducing Oxidative Stress-Mediated Alcoholic Liver Injury by Multiplexed RNAi of Cyp2e1, Cyp4a10, and Cyp4a14. Biomedicines 2024, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y. CD36 tango in cancer: Signaling pathways and functions. Theranostics 2019, 9, 4893–4908. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, P.F.; Byun, J.H.; Platko, K.; Al-Hashimi, A.A.; Lhoták, Š.; MacDonald, M.E.; Mejia-Benitez, A.; Prat, A.; Igdoura, S.A.; Trigatti, B.; et al. Pcsk9 knockout exacerbates diet-induced non-alcoholic steatohepatitis, fibrosis and liver injury in mice. JHEP Rep. 2019, 1, 418–429. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Chen, X.; Zhao, L.; Guo, Y. Involvement of CYP2E1-ROS-CD36/DGAT2 axis in the pathogenesis of VPA-induced hepatic steatosis in vivo and in vitro. Toxicology 2020, 445, 152585. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, X.; Su, Z.; Hu, C.; Mu, X.; Pan, J.; Li, M.; Teng, F.; Ling, T.; Zhao, T.; et al. CD36 deficiency ameliorates drug-induced acute liver injury in mice. Mol. Med. 2021, 27, 57. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Sun, C.; Li, R.; Li, W.; Ge, Z.; Adu-Frimpong, M.; Xu, X.; Yu, J. Amelioration action of gastrodigenin rhamno-pyranoside from Moringa seeds on non-alcoholic fatty liver disease. Food Chem. 2022, 379, 132087. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, T.; Wang, T.; Liu, X.; Hamada, K.; Sun, D.; Sun, Y.; Yang, Y.; Wang, J.; Takahashi, S.; et al. Crosstalk between CYP2E1 and PPARα substrates and agonists modulate adipose browning and obesity. Acta Pharm. Sin. B 2022, 12, 2224–2238. [Google Scholar] [CrossRef]
- Wan, Y.Y.; Cai, Y.; Li, J.; Yuan, Q.; French, B.; Gonzalez, F.J.; French, S. Regulation of peroxisome proliferator activated receptor alpha-mediated pathways in alcohol fed cytochrome P450 2E1 deficient mice. Hepatol. Res. 2001, 19, 117–130. [Google Scholar] [CrossRef]
- Manautou, J.E.; Tveit, A.; Hoivik, D.J.; Khairallah, E.A.; Cohen, S.D. Protection by clofibrate against acetaminophen hepatotoxicity in male CD-1 mice is associated with an early increase in biliary concentration of acetaminophen-glutathione adducts. Toxicol. Appl. Pharmacol. 1996, 140, 30–38. [Google Scholar] [CrossRef]
- Chen, C.; Hennig, G.E.; Whiteley, H.E.; Corton, J.C.; Manautou, J.E. Peroxisome proliferator-activated receptor alpha-null mice lack resistance to acetaminophen hepatotoxicity following clofibrate exposure. Toxicol. Sci. 2000, 57, 338–344. [Google Scholar] [CrossRef]
- Mukai, T.; Egawa, M.; Takeuchi, T.; Yamashita, H.; Kusudo, T. Silencing of FABP1 ameliorates hepatic steatosis, inflammation, and oxidative stress in mice with nonalcoholic fatty liver disease. FEBS Open Bio 2017, 7, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Aoyagi, Y.; Fukuishi, N.; Gui, M.Y.; Jin, Y.R.; Li, X.W.; Adachi, Y.; Ohno, N.; Takeya, K.; Hitotsuyanagi, Y.; et al. Suppressive effect of kamebakaurin on acetaminophen-induced hepatotoxicity by inhibiting lipid peroxidation and inflammatory response in mice. Pharmacol. Rep. 2017, 69, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]

| Name | Size (nm) | Polydispersity Index | Zeta Potential (mV) | Encapsulation Rate (%) |
|---|---|---|---|---|
| si-Cyp2e1 LNPs | 78.04 ± 0.29 | 0.143 ± 0.031 | −0.45 ± 0.1 | 89.05 ± 0.38 |
| si-Control LNPs | 72.47 ± 2.00 | 0.144 ± 0.013 | −0.37 ± 0.1 | 85.86 ± 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Huan, L.; Zhang, C.; Yin, R.; Ouyang, Z.; Wei, Y. Amelioration of Acetaminophen-Induced Hepatic Oxidative Stress and Inflammation by RNAi Targeting Cyp2e1 In Vivo. Curr. Issues Mol. Biol. 2025, 47, 372. https://doi.org/10.3390/cimb47050372
Liu W, Huan L, Zhang C, Yin R, Ouyang Z, Wei Y. Amelioration of Acetaminophen-Induced Hepatic Oxidative Stress and Inflammation by RNAi Targeting Cyp2e1 In Vivo. Current Issues in Molecular Biology. 2025; 47(5):372. https://doi.org/10.3390/cimb47050372
Chicago/Turabian StyleLiu, Wenwen, Liwen Huan, Cai Zhang, Runting Yin, Zhen Ouyang, and Yuan Wei. 2025. "Amelioration of Acetaminophen-Induced Hepatic Oxidative Stress and Inflammation by RNAi Targeting Cyp2e1 In Vivo" Current Issues in Molecular Biology 47, no. 5: 372. https://doi.org/10.3390/cimb47050372
APA StyleLiu, W., Huan, L., Zhang, C., Yin, R., Ouyang, Z., & Wei, Y. (2025). Amelioration of Acetaminophen-Induced Hepatic Oxidative Stress and Inflammation by RNAi Targeting Cyp2e1 In Vivo. Current Issues in Molecular Biology, 47(5), 372. https://doi.org/10.3390/cimb47050372







