Light-Regulated Gene Expression Patterns During Conidial Formation in Aspergillus oryzae
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Species
2.2. Culture Conditions
2.3. Light Conditions and Apparatus
2.4. RNA Extraction
2.5. Library Construction and Sequencing
2.6. Bioinformatics Analysis in RNA Sequencing
2.7. Relative Expression of Genes by qRT-PCR
2.8. Statistical Analyses of qRT-PCR
3. Results
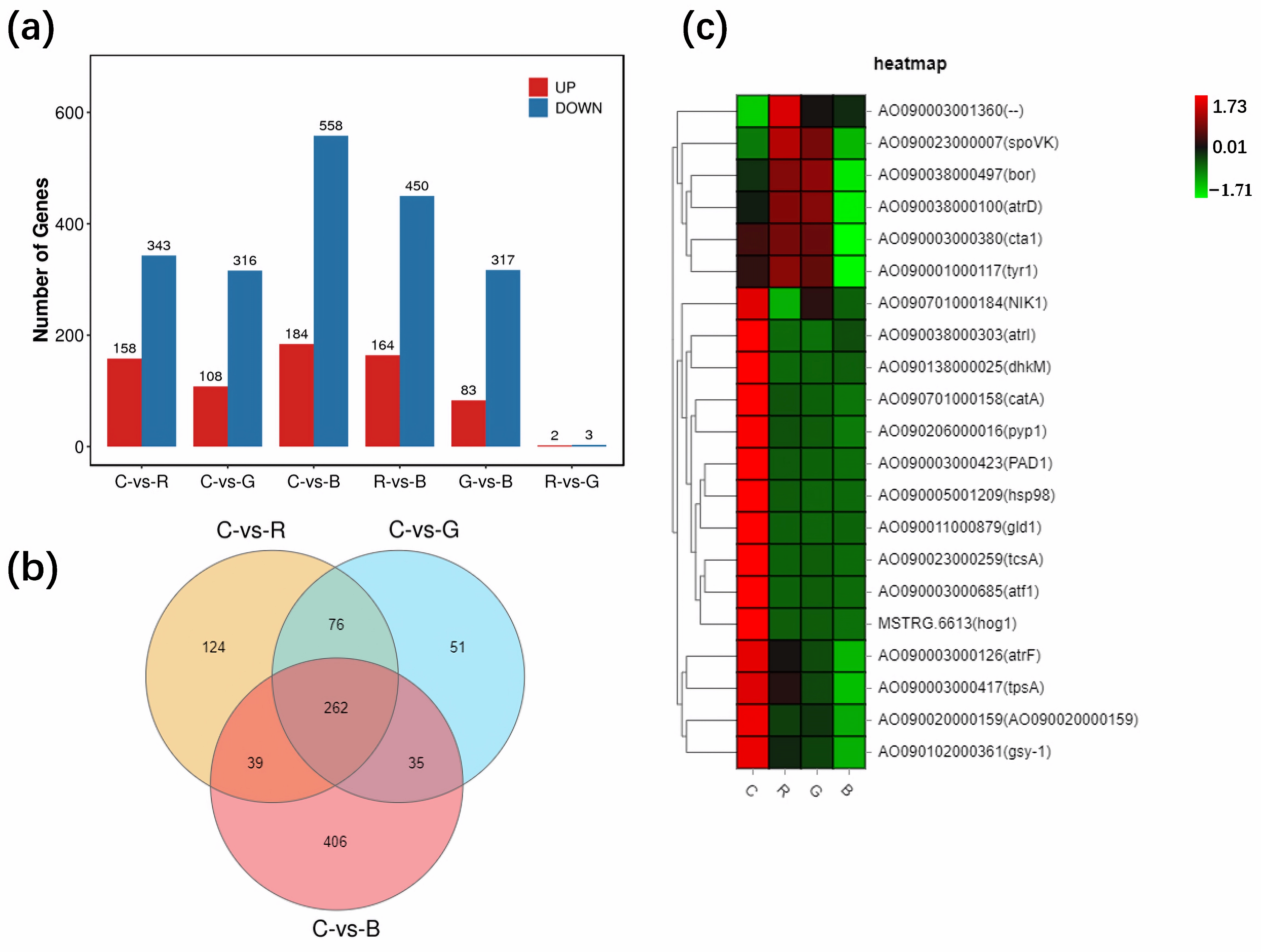
3.1. Overall Regulation of Gene Expression by Light
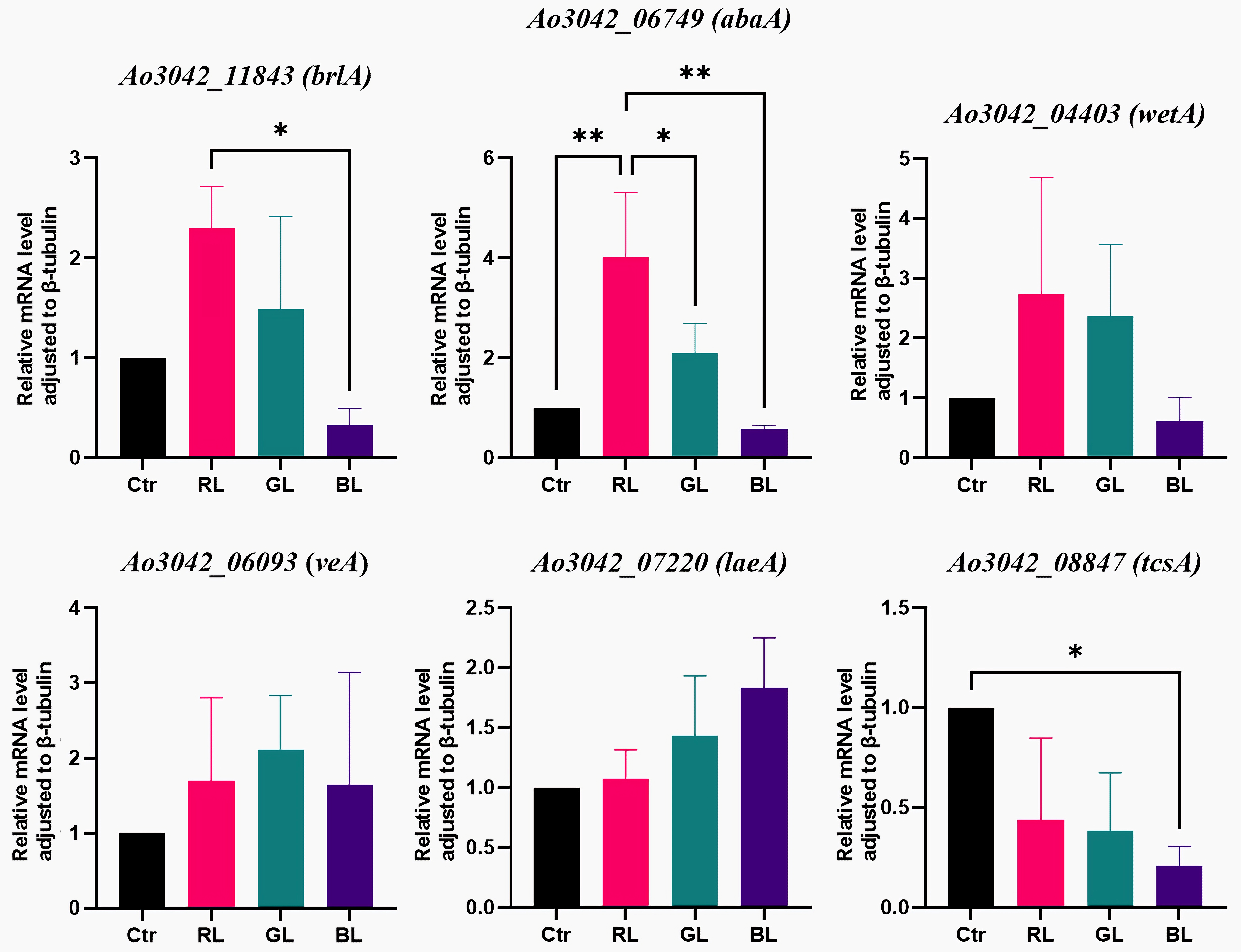
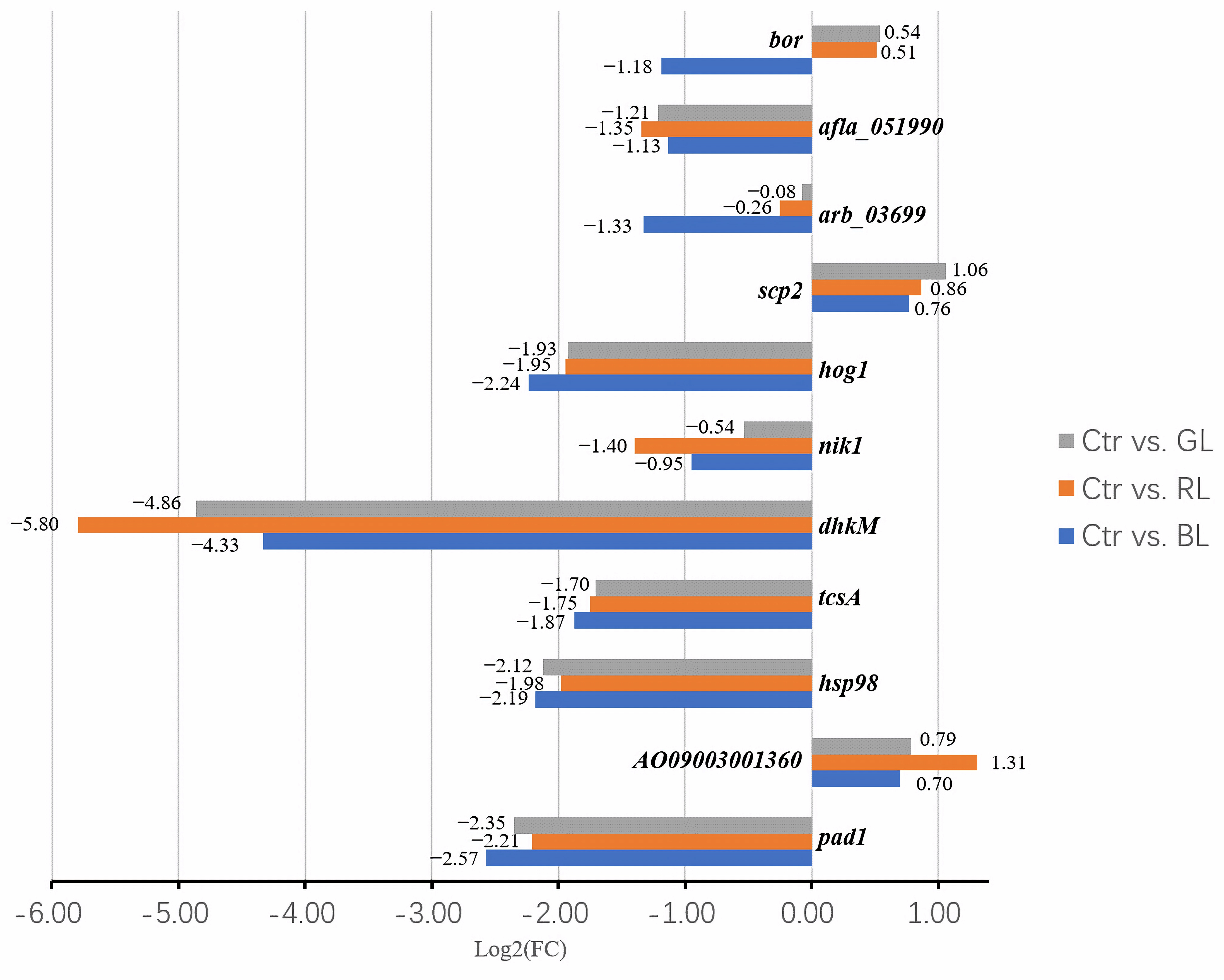
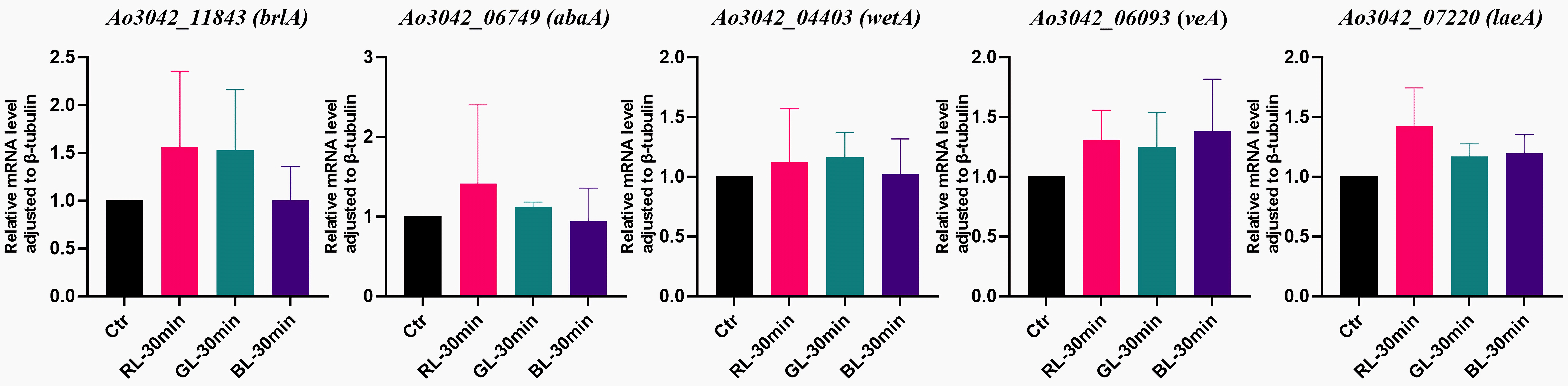
3.2. Light-Regulated Genes Related to Conidial Formation
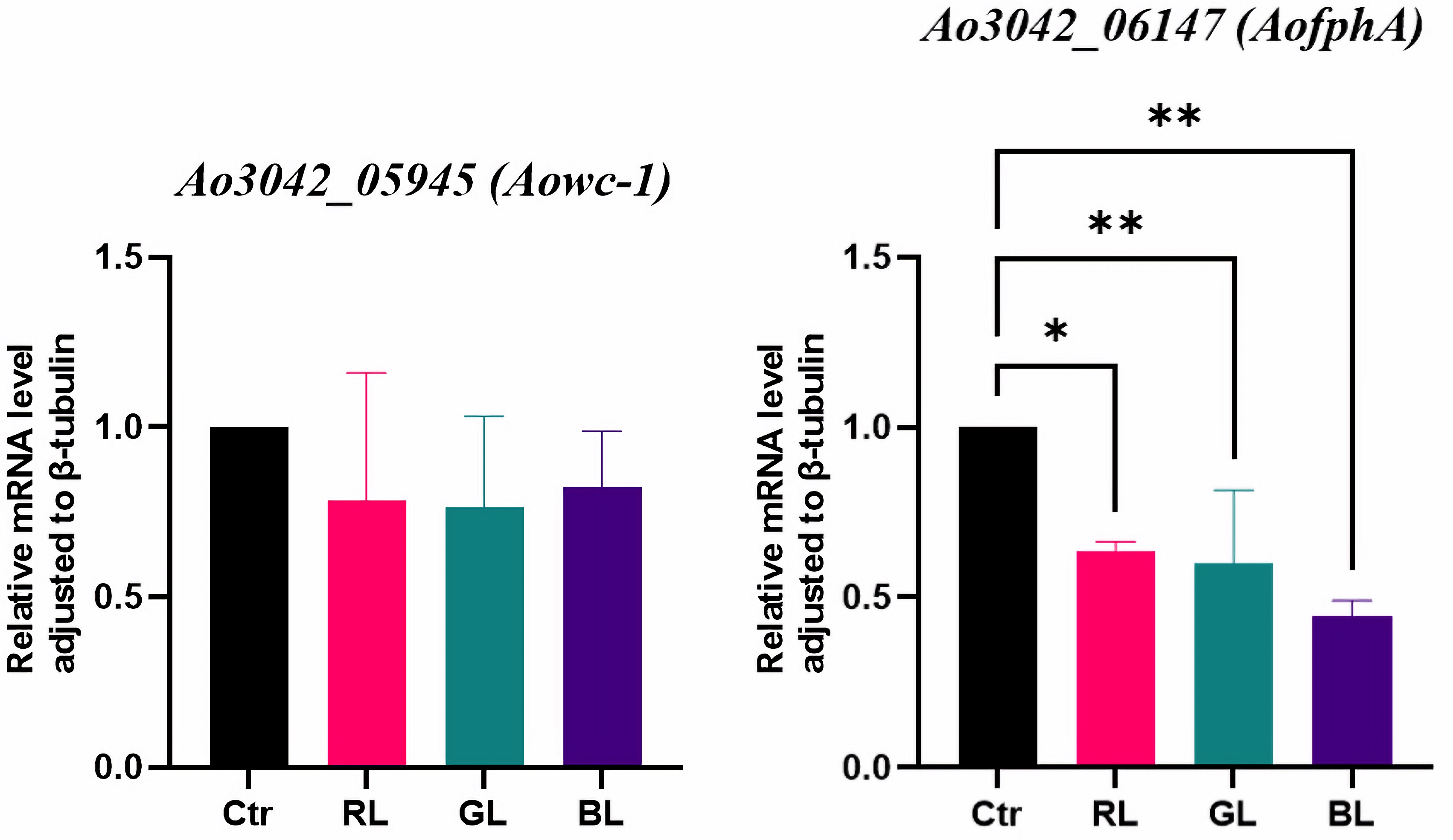
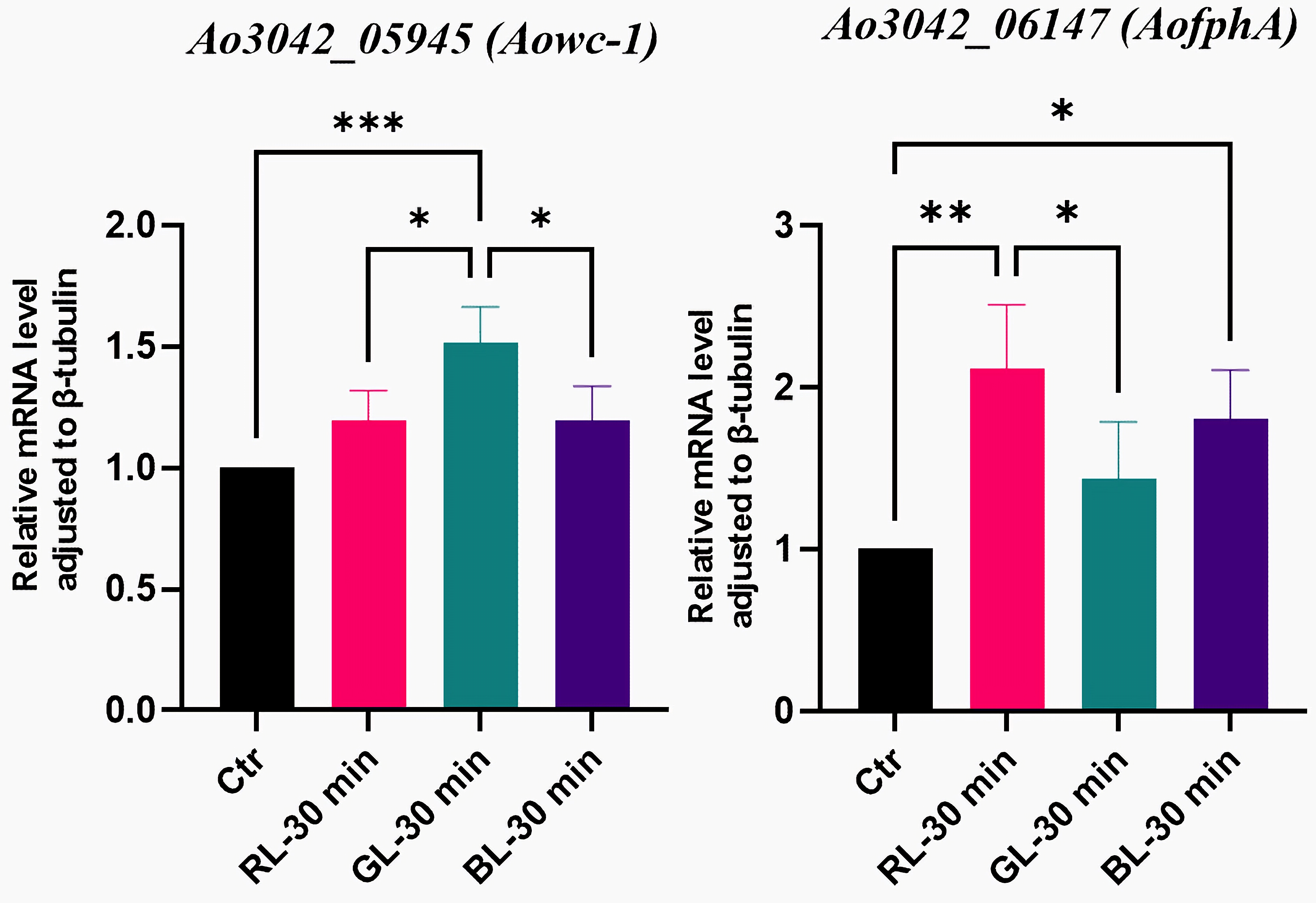
3.3. Light-Regulated Genes Among the Potential Photoreceptor-like Genes
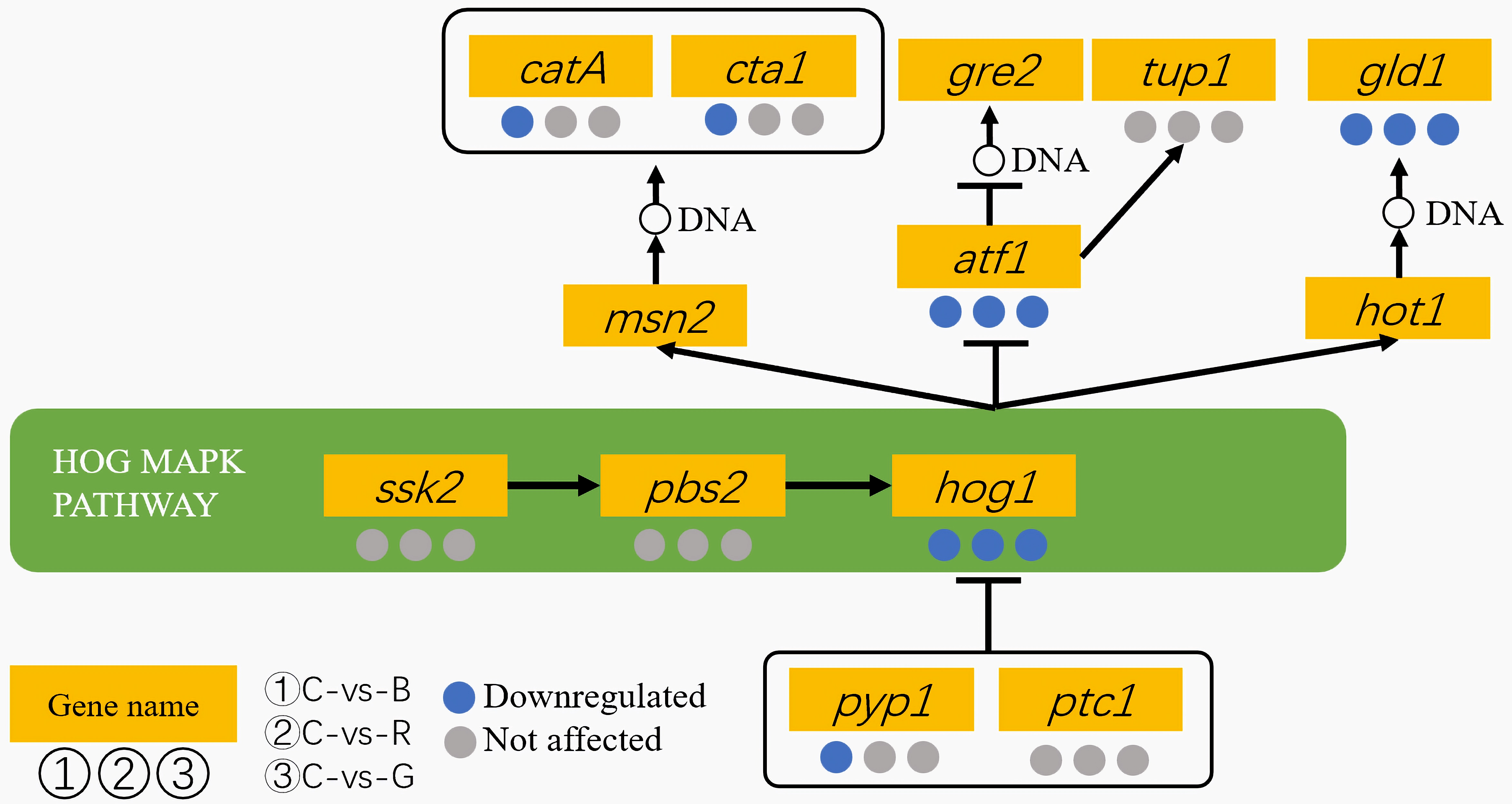
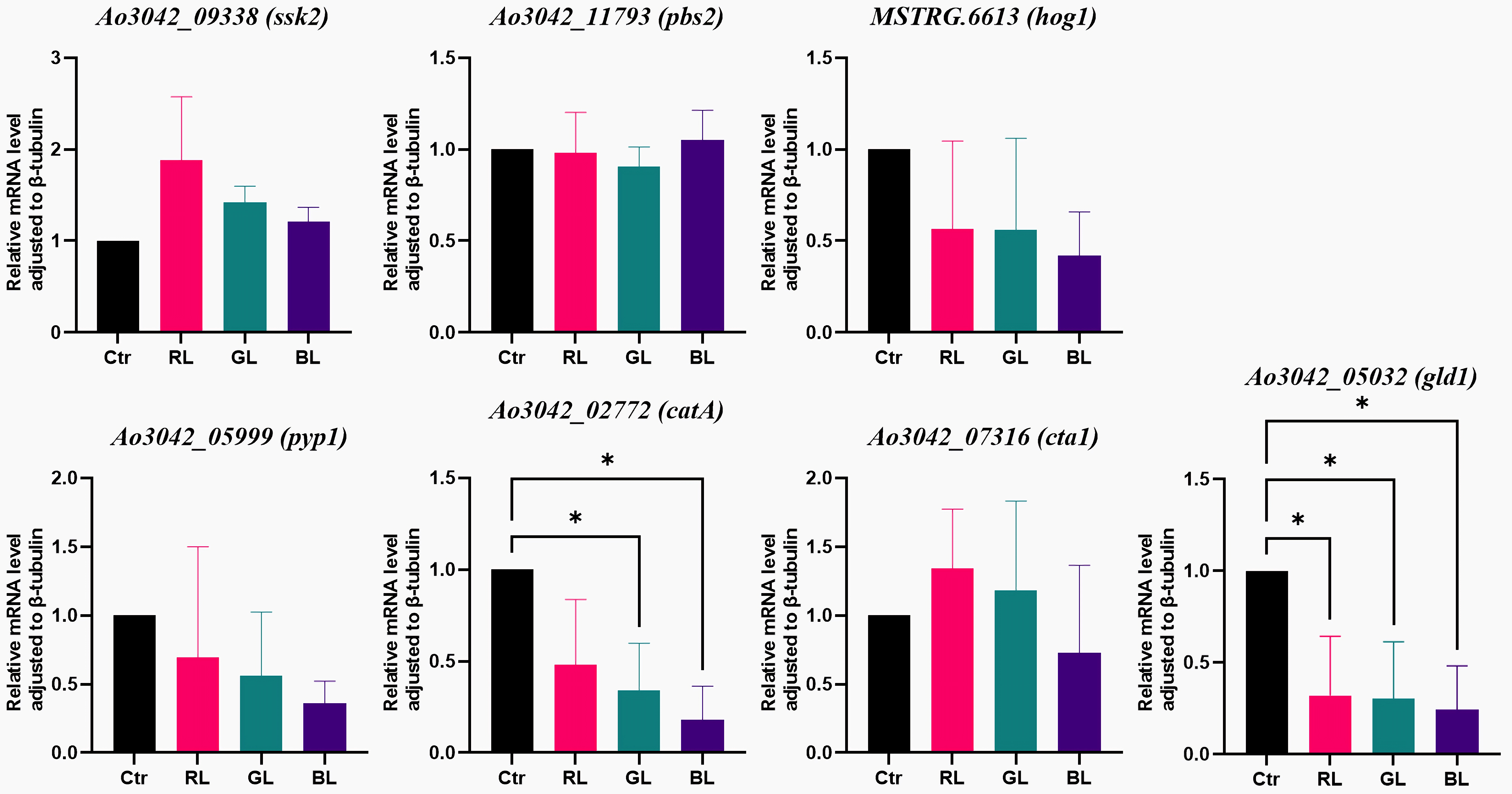
3.4. Results of Genes in the MAPK Pathway
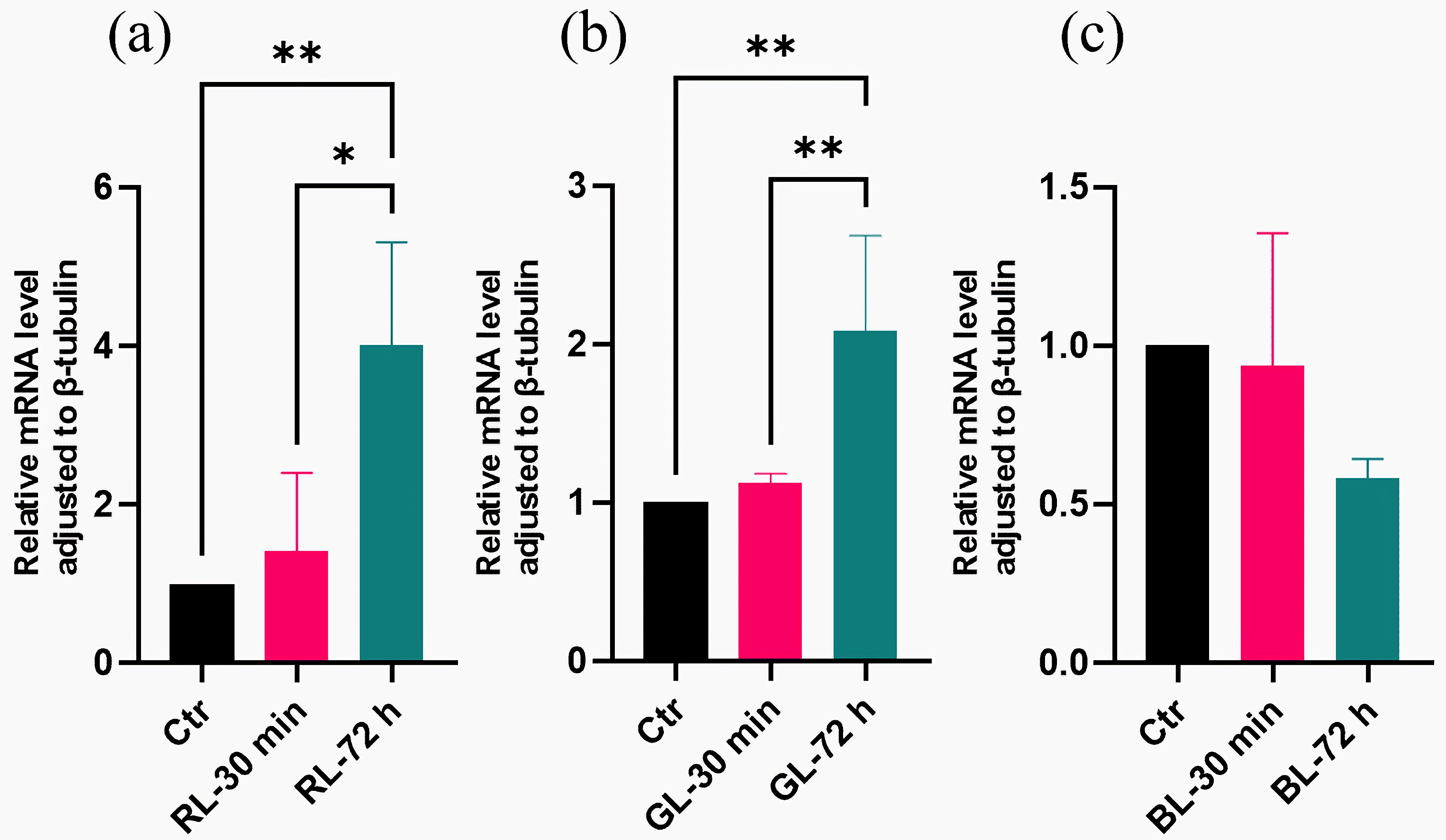
3.5. Light-Regulated Genes Were Sensitive to Light Dose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.; Hao, C.; Niu, C.; Lu, J.; Wang, L.; Shi, Y.; Li, Q. Insight into the Keystones of Chinese Broad Bean Paste Fermentation: Brewing Techniques, Chemosensory Characteristics, and Microbial Community. Trends Food Sci. Technol. 2024, 154, 104775. [Google Scholar] [CrossRef]
- Uwineza, C.; Bouzarjomehr, M.; Parchami, M.; Sar, T.; Taherzadeh, M.J.; Mahboubi, A. Evaluation of in Vitro Digestibility of Aspergillus oryzae Fungal Biomass Grown on Organic Residue Derived-VFAs as a Promising Ruminant Feed Supplement. J. Anim. Sci. Biotechnol. 2023, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, Y.; Long, S.; Feng, S.; Jia, X.; Hu, Y.; Ma, M.; Liu, J.; Zeng, B. Aspergillus oryzae as a Cell Factory: Research and Applications in Industrial Production. J. Fungi 2024, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Song, J.; Wu, Y.; Feng, S.; Sun, Z.; Hu, Y.; Yu, M.; Han, R.; Zeng, B. Strategies for the Enhancement of Secondary Metabolite Production via Biosynthesis Gene Cluster Regulation in Aspergillus oryzae. J. Fungi 2024, 10, 312. [Google Scholar] [CrossRef]
- Lin, S.; Qin, H.; Zhang, X.; Li, W.; Liu, M. Inhibition of Aspergillus oryzae Mycelium Growth and Conidium Production by Irradiation with Light at Different Wavelengths and Intensities. Microbiol. Spectr. 2021, 9, e00213-21. [Google Scholar] [CrossRef]
- Berni, E.; Brutti, A. Electromagnetic Radiations and Their Effect on Filamentous Fungi and Mycotoxins: Recent Advances and Perspectives. Curr. Opin. Food Sci. 2023, 52, 101073. [Google Scholar] [CrossRef]
- Bayram, O.S.; Bayram, O. An Anatomy of Fungal Eye: Fungal Photoreceptors and Signalling Mechanisms. J. Fungi 2023, 9, 591. [Google Scholar] [CrossRef]
- Lin, S.; Jiang, H.; Fu, Q.; Huang, S.; Tang, L.; Li, A.; Liu, M. Effects of Pulsed Light on Mycelium Growth and Conidiation in Aspergillus oryzae. Fermentation 2023, 9, 674. [Google Scholar] [CrossRef]
- Yu, Z.; Streng, C.; Seibeld, R.F.; Igbalajobi, O.A.; Leister, K.; Ingelfinger, J.; Fischer, R. Genome-Wide Analyses of Light-Regulated Genes in Aspergillus nidulans Reveal a Complex Interplay between Different Photoreceptors and Novel Photoreceptor Functions. PLoS Genet. 2021, 17, e1009845. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhou, A.; Neng, J.; Wu, D.; Shen, X.L.; Lou, X.; Yang, K. Transcriptomic Analysis Reveals the Inhibition Mechanism of Pulsed Light on Fungal Growth and Ochratoxin A Biosynthesis in Aspergillus Carbonarius. Food Res. Int. 2023, 165, 112501. [Google Scholar] [CrossRef]
- Galagan, J.E.; Calvo, S.E.; Cuomo, C.; Ma, L.J.; Wortman, J.R.; Batzoglou, S.; Lee, S.I.; Bastürkmen, M.; Spevak, C.C.; Clutterbuck, J.; et al. Sequencing of Aspergillus nidulans and Comparative Analysis with A-Fumigatus and A-Oryzae. Nature 2005, 438, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Tanaka, Y.; Okabe, T.; Nakamura, H.; Fujii, W.; Kitamoto, K.; Maruyama, J. Development of a Genome Editing Technique Using the CRISPR/Cas9 System in the Industrial Filamentous Fungus Aspergillus oryzae. Biotechnol. Lett. 2016, 38, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Chen, Z.; Fan, J.; Chen, T.; Xiao, Y.; Jie, J.; Zeng, B.; Zhang, Z. Overexpression of a Novel Gene Aokap2 Affects the Growth and Kojic Acid Production in Aspergillus oryzae. Mol. Biol. Rep. 2022, 49, 2745–2754. [Google Scholar] [CrossRef] [PubMed]
- Corrochano, L.M. Light in the Fungal World: From Photoreception to Gene Transcription and Beyond. Annu. Rev. Genet. 2019, 53, 149–170. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Z.; Fan, J.; Chen, T.; Zeng, B.; Zhang, Z. Identification and Characterization of a Novel Gene Aokap1 Involved in Growth and Kojic Acid Synthesis in Aspergillus oryzae. Arch. Microbiol. 2022, 204, 67. [Google Scholar] [CrossRef]
- Ogawa, M.; Tokuoka, M.; Jin, F.J.; Takahashi, T.; Koyama, Y. Genetic Analysis of Conidiation Regulatory Pathways in Koji-Mold Aspergillus oryzae. Fungal Genet. Biol. 2010, 47, 10–18. [Google Scholar] [CrossRef]
- Tao, L.; Yu, J.-H. AbaA and WetA Govern Distinct Stages of Aspergillus fumigatus Development. Microbiology 2011, 157, 313–326. [Google Scholar] [CrossRef]
- Appleyard, M.; McPheat, W.L.; Stark, M.J.R. A Novel “two-Component” Protein Containing Histidine Kinase and Response Regulator Domains Required for Sporulation in Aspergillus nidulans. Curr. Genet. 2000, 37, 364–372. [Google Scholar] [CrossRef]
- Calvo, A.M.; Gardner, H.W.; Keller, N.P. Genetic Connection between Fatty Acid Metabolism and Sporulation in Aspergillus nidulans. J. Biol. Chem. 2001, 276, 25766–25774. [Google Scholar] [CrossRef]
- Sugui, J.A.; Pardo, J.; Chang, Y.C.; Muellbacher, A.; Zarember, K.A.; Galvez, E.M.; Brinster, L.; Zerfas, P.; Gallin, J.I.; Simon, M.M.; et al. Role of laeA in the Regulation of Alb1, gliP, Conidial Morphology, and Virulence in Aspergillus fumigatus. Eukaryot. Cell 2007, 6, 1552–1561. [Google Scholar] [CrossRef]
- Hatakeyama, R.; Nakahama, T.; Higuchi, Y.; Kitamoto, K. Light Represses Conidiation in Koji Mold Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2007, 71, 1844–1849. [Google Scholar] [CrossRef]
- Suzuki, S.; Kusumoto, K. Transcriptome Analysis of Two Strains of Aspergillus oryzae with Different Responses to Light. Jarq-Jpn. Agric. Res. Q. 2020, 54, 13–20. [Google Scholar] [CrossRef][Green Version]
- Ruger-Herreros, C.; Rodriguez-Romero, J.; Fernandez-Barranco, R.; Olmedo, M.; Fischer, R.; Corrochano, L.M.; Canovas, D. Regulation of Conidiation by Light in Aspergillus nidulans. Genetics 2011, 188, 809–822. [Google Scholar] [CrossRef]
- Yu, W.; Pei, R.; Zhang, Y.; Tu, Y.; He, B. Light Regulation of Secondary Metabolism in Fungi. J. Biol. Eng. 2023, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Medina, H.R.; Rangel, D.E.N. Light Enhances the Production of Conidia and Influences Their Hydrophobicity in Tolypocladium inflatum. Fungal Biol. 2025, 129, 101483. [Google Scholar] [CrossRef] [PubMed]
- Blumenstein, A.; Vienken, K.; Tasler, R.; Purschwitz, J.; Veith, D.; Frankenberg-Dinkel, N.; Fischer, R. The Aspergillus nidulans Phytochrome FphA Represses Sexual Development in Red Light. Curr. Biol. 2005, 15, 1833–1838. [Google Scholar] [CrossRef]
- Brandt, S.; von Stetten, D.; Guenther, M.; Hildebrandt, P.; Frankenberg-Dinkel, N. The Fungal Phytochrome FphA from Aspergillus nidulans. J. Biol. Chem. 2008, 283, 34605–34614. [Google Scholar] [CrossRef]
- Wen, P.; Tan, F.; Lei, M.; Khan, M.S.A.; Chen, W.; Hu, X. Wavelengths and Irradiances Modulate the Circadian Rhythm of Neurospora crassa. PLoS ONE 2022, 17, e0266266. [Google Scholar] [CrossRef]
- Ma, Y.; Mackon, E.; Mackon, G.C.J.D.E.; Zhao, Y.; Li, Q.; Dai, X.; Yao, Y.; Xia, X.; Nong, B.; Liu, P. Combined Analysis of BSA-Seq Based Mapping, RNA-Seq, and Metabolomic Unraveled Candidate Genes Associated with Panicle Grain Number in Rice (Oryza sativa L.). Biomolecules 2022, 12, 918. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Dong, W.; Tang, X.; Yu, Y.; Nilsen, R.; Kim, R.; Griffith, J.; Arnold, J.; Schuttler, H. Systems Biology of the Clock in Neurospora crassa. PLoS ONE 2008, 3, e3105. [Google Scholar] [CrossRef]
- Sun, W.; Yu, Y.; Chen, J.; Yu, B.; Chen, T.; Ying, H.; Zhou, S.; Ouyang, P.; Liu, D.; Chen, Y. Light Signaling Regulates Aspergillus niger Biofilm Formation by Affecting Melanin and Extracellular Polysaccharide Biosynthesis. mBio 2021, 12, e03434-20. [Google Scholar] [CrossRef] [PubMed]
- Calcaneo-Hernandez, G.; Landeros-Jaime, F.; Cervantes-Chavez, J.A.; Mendoza-Mendoza, A.; Esquivel-Naranjo, E.U. Osmotic Stress Responses, Cell Wall Integrity, and Conidiation Are Regulated by a Histidine Kinase Sensor in Trichoderma atroviride. J. Fungi 2023, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Sano, M.; Hattori, R.; Kusumoto, K.; Suzuki, S. Aspergillus oryzae Strain with Improved Conidiation after Light Stimulation. Jarq-Jpn. Agric. Res. Q. 2018, 52, 23–28. [Google Scholar] [CrossRef]
- Sewall, T.C. Cellular Effects of Misscheduled brlA, abaA, and wetA Expression in Aspergillus nidulans. Can. J. Microbiol. 1994, 40, 1035–1042. [Google Scholar] [CrossRef]
- Fernandez, E.Q.; Moyer, D.L.; Maiyuran, S.; Labaro, A.; Brody, H. Vector-Initiated Transitive RNA Interference in the Filamentous Fungus Aspergillus oryzae. Fungal Genet. Biol. 2012, 49, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Yu, J.-H.; Chen, F.; Chen, W. Characterization of the Asexual Developmental Genes brlA and wetA in Monascus ruber M7. Fungal Genet. Biol. 2021, 151, 103564. [Google Scholar] [CrossRef]
- Cho, H.-J.; Son, S.-H.; Chen, W.; Son, Y.-E.; Lee, I.; Yu, J.-H.; Park, H.-S. Regulation of Conidiogenesis in Aspergillus flavus. Cells 2022, 11, 2796. [Google Scholar] [CrossRef]
- Suzuki, S.; Kusumoto, K.-I. Effect of Light Exposure on Enzyme Activity in Wheat Bran Solid-State Fermentation by Aspergillus oryzae. Food Sci. Technol. Res. 2023, 29, 475–479. [Google Scholar] [CrossRef]
- Oster, H.; Avivi, A.; Joel, A.; Albrecht, U.; Nevo, E. A Switch from Diurnal to Nocturnal Activity in S-Ehrenbergi Is Accompanied by an Uncoupling of Light Input and the Circadian Clock. Curr. Biol. 2002, 12, 1919–1922. [Google Scholar] [CrossRef]
- Sanz, C.; Benito, E.P.; Orejas, M.; Isabel Alvarez, M.; Eslava, A.P. Protein-DNA Interactions in the Promoter Region of the Phycomyces carB and carRA Genes Correlate with the Kinetics of Their mRNA Accumulation in Response to Light. Fungal Genet. Biol. 2010, 47, 773–781. [Google Scholar] [CrossRef]
- Vijayan, V.; O’Shea, E.K. Sequence Determinants of Circadian Gene Expression Phase in Cyanobacteria. J. Bacteriol. 2013, 195, 665–671. [Google Scholar] [CrossRef] [PubMed]
| Parameter | High Light Dose Treatment | Low Light Dose Treatment | ||||
|---|---|---|---|---|---|---|
| Light source | Blue | Green | Red | Blue | Green | Red |
| Light wavelength (nm) | 475 | 520 | 630 | 475 | 520 | 630 |
| Full width at half maximum | 25 | 37 | 21 | 25 | 37 | 21 |
| Average irradiance (W·m–2) | 19.87 | 17.87 | 15.63 | 19.87 | 17.87 | 15.63 |
| Irradiated time | 72 h | 30 min | ||||
| Light dose (kJ·m–2) | 5150 | 4632 | 4051 | 35.8 | 32.2 | 28.1 |
| Light intensity (μmol photon m−2 s−1) | 80 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Yang, J.; Wang, A.; Fu, Q.; Huang, S.; Liu, M. Light-Regulated Gene Expression Patterns During Conidial Formation in Aspergillus oryzae. Curr. Issues Mol. Biol. 2025, 47, 373. https://doi.org/10.3390/cimb47050373
Lin S, Yang J, Wang A, Fu Q, Huang S, Liu M. Light-Regulated Gene Expression Patterns During Conidial Formation in Aspergillus oryzae. Current Issues in Molecular Biology. 2025; 47(5):373. https://doi.org/10.3390/cimb47050373
Chicago/Turabian StyleLin, Shangfei, Jiali Yang, Aixia Wang, Qiqi Fu, Shijie Huang, and Muqing Liu. 2025. "Light-Regulated Gene Expression Patterns During Conidial Formation in Aspergillus oryzae" Current Issues in Molecular Biology 47, no. 5: 373. https://doi.org/10.3390/cimb47050373
APA StyleLin, S., Yang, J., Wang, A., Fu, Q., Huang, S., & Liu, M. (2025). Light-Regulated Gene Expression Patterns During Conidial Formation in Aspergillus oryzae. Current Issues in Molecular Biology, 47(5), 373. https://doi.org/10.3390/cimb47050373


_Kim.png)




