Herbal Medicine in Breast Cancer Therapy: Mechanisms, Evidence, and Future Perspectives
Abstract
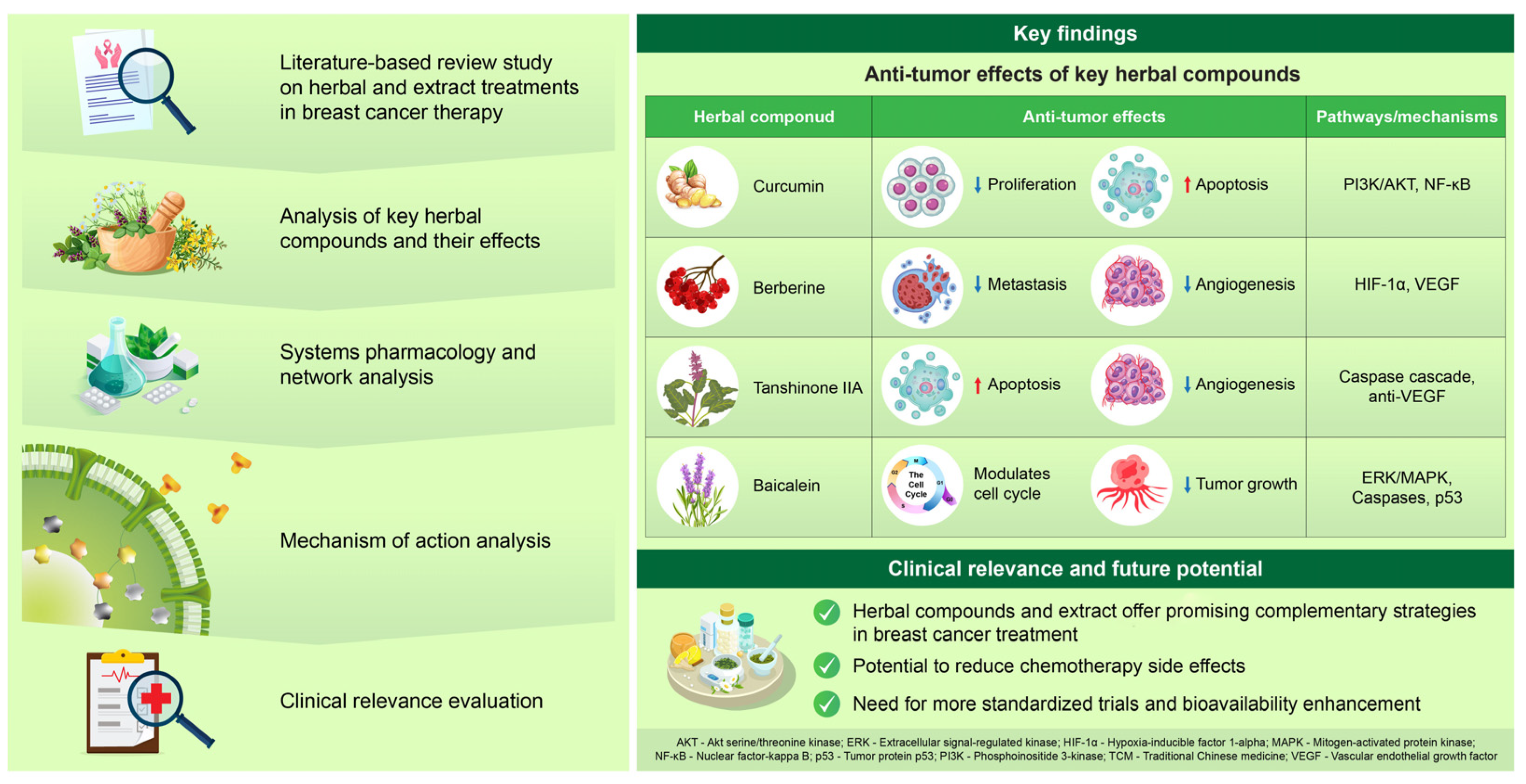
1. Introduction
2. Traditional Herbal Medicines in Breast Cancer Treatment: Mechanisms and Therapeutic Potential of Curcumin, Scutellaria baicalensis, Oldenlandia diffusa, and Salvia miltiorrhiza
3. Discussions
3.1. Variability in Experimental Design
3.2. Inconsistencies in Dosing and Bioavailability
3.3. Variability in Outcomes
4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALDH | Aldehyde dehydrogenase |
| Bcl-2 | B-cell lymphoma 2 |
| BAX | B-cell lymphoma 2-associated X protein |
| BCL | B-cell lymphoma 2 |
| CAF | Cancer-associated fibroblasts |
| CHM | Chinese herbal medicine |
| CHOP | C-EBP homologous protein |
| CHP | Chinese herbal prescription |
| CMH | Chinese medicinal herbs |
| Elk1 | ETS-like transcription factor 1 |
| DRP1 | Dynamin-related protein 1 |
| EGCG | Epigallocatechin gallate |
| EMT | Epithelial–mesenchymal transition |
| ER | Estrogen receptor |
| Erα | Estrogen receptor alpha |
| Fen1 | Flap Endonuclease 1 |
| IBC | Inflammatory breast cancer |
| IL-12 | Interleukin 12 |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinase |
| MCF-7 | Michigan Cancer Foundation-7 (human breast cancer cell line) |
| MDA-MB-231 | A human breast cancer cell line |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| ODE | Oldenlandia diffusa extract |
| OPA | Optic atrophy protein |
| ORR | Overall response rate |
| POLD1 | DNA polymerase delta catalytic subunit gene |
| p-eIF2α | Phosphorylated eukaryotic initiation factor 2 alpha |
| p-PERK | Phosphorylated PKR-like ER kinase |
| ERK | Phosphorylated extracellular signal-Regulated kinase |
| PMNs | Pre-Metastatic niches |
| Qi | Vital energy (in Traditional Chinese medicine) |
| ROS | Reactive oxygen species |
| RSK | Ribosomal S6 kinase |
| RSV | Resveratrol |
| SBGE | Scutellaria baicalensis Georgi extract |
| SbE | Scutellaria baicalensis extract |
| SG | Salvia miltiorrhiza–Ginseng |
| SGLXD | Shu-Gan-Liang-Xue Decoction |
| STS | Steroid sulfatase |
| TCA | Tricarboxylic acid |
| TCM | Traditional Chinese medicine |
| VEGF-A | Vascular endothelial growth factor A |
References
- Sha, R.; Kong, X.M.; Li, X.Y.; Wang, Y.B. Global burden of breast cancer and attributable risk factors in 204 countries and territories, from 1990 to 2021: Results from the Global Burden of Disease Study 2021. Biomark. Res. 2024, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.Q.; Li, D.H.; Liu, X.K.; Zhao, X.H.; Wen, Q.E.; Yang, Y. Traditional Chinese Medicine for Breast Cancer: A Review. Breast Cancer Targets Ther. 2023, 15, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Izah, S.C.; Ogidi, O.I.; Ogwu, M.C.; Salimon, S.S.; Yusuf, Z.M.; Akram, M.; Raimi, M.O.; Iyingiala, A.-A. Historical Perspectives and Overview of the Value of Herbal Medicine. In Herbal Medicine Phytochemistry: Applications and Trends; Izah, S.C., Ogwu, M.C., Akram, M., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 3–35. [Google Scholar]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef]
- Malcangi, G.; Inchingolo, A.M.; Casamassima, L.; Trilli, I.; Ferrante, L.; Inchingolo, F.; Palermo, A.; Inchingolo, A.D.; Dipalma, G. Effectiveness of Herbal Medicines with Anti-Inflammatory, Antimicrobial, and Antioxidant Properties in Improving Oral Health and Treating Gingivitis and Periodontitis: A Systematic Review. Nutrients 2025, 17, 762. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef]
- Wali, A.F.; Pillai, J.R.; Talath, S.; Shivappa, P.; Sridhar, S.B.; El-Tanani, M.; Rangraze, I.R.; Mohamed, O.I.; Al Ani, N.N. Phytochemicals in Breast Cancer Prevention and Treatment: A Comprehensive Review. Curr. Issues Mol. Biol. 2025, 47, 30. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Li, Y.; Li, S.; Liu, J.; Yang, X.; Xia, G.; Wang, G. Natural products and derivatives for breast cancer treatment: From drug discovery to molecular mechanism. Phytomedicine 2024, 129, 155600. [Google Scholar] [CrossRef]
- Li, S.; So, T.H.; Tang, G.; Tan, H.Y.; Wang, N.; Ng, B.F.L.; Chan, C.K.W.; Yu, E.C.; Feng, Y. Chinese Herbal Medicine for Reducing Chemotherapy-Associated Side-Effects in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 599073. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Li, J.; He, L.; Tripathy, D. Chinese medicinal herbs to treat the side-effects of chemotherapy in breast cancer patients. Cochrane Database Syst. Rev. 2007, 2007, CD004921. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Sanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, K.A.; Mendonca, C.R.; Noll, M.; Botelho, A.F.; Francischini, C.R.D.; Silva, M.A.M. Antitumor Properties of Curcumin in Breast Cancer Based on Preclinical Studies: A Systematic Review. Cancers 2022, 14, 2165. [Google Scholar] [CrossRef] [PubMed]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Y.; Wang, Y.; Rao, J.; Jiang, X.; Xu, Z. Curcumin inhibits proliferation of breast cancer cells through Nrf2-mediated down-regulation of Fen1 expression. J. Steroid Biochem. Mol. Biol. 2014, 143, 11–18. [Google Scholar] [CrossRef]
- Kang, Q.; Chen, A. Curcumin inhibits srebp-2 expression in activated hepatic stellate cells in vitro by reducing the activity of specificity protein-1. Endocrinology 2009, 150, 5384–5394. [Google Scholar] [CrossRef]
- Jiang, H.; Li, M.; Du, K.; Ma, C.; Cheng, Y.; Wang, S.; Nie, X.; Fu, C.; He, Y. Traditional Chinese Medicine for adjuvant treatment of breast cancer: Taohong Siwu Decoction. Chin. Med. 2021, 16, 129. [Google Scholar] [CrossRef]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, X.; Guan, S.; Yan, Y.; Lin, H.; Hua, Z.C. Curcumin inhibits angiogenesis and improves defective hematopoiesis induced by tumor-derived VEGF in tumor model through modulating VEGF-VEGFR2 signaling pathway. Oncotarget 2015, 6, 19469–19482. [Google Scholar] [CrossRef]
- Oh, E.T.; Kim, C.W.; Kim, S.J.; Lee, J.S.; Hong, S.S.; Park, H.J. Docetaxel induced-JNK2/PHD1 signaling pathway increases degradation of HIF-1α and causes cancer cell death under hypoxia. Sci. Rep. 2016, 6, 27382. [Google Scholar] [CrossRef]
- Mayo, B.; Penroz, S.; Torres, K.; Simon, L. Curcumin Administration Routes in Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 11492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Q.; Wu, Z.; Xu, Y.; Jiang, R. Curcumin for Treating Breast Cancer: A Review of Molecular Mechanisms, Combinations with Anticancer Drugs, and Nanosystems. Pharmaceutics 2024, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Belhaj, N.; Benachour, H.; Barberi-Heyob, M.; Kahn, C.J.; Jabbari, E.; Linder, M.; Arab-Tehrany, E. Liposome encapsulation of curcumin: Physico-chemical characterizations and effects on MCF7 cancer cell proliferation. Int. J. Pharm. 2014, 461, 519–528. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gong, J.; Deng, X.; Shen, L.; Wu, S.; Fan, H.; Liu, L. Curcumin and nanodelivery systems: New directions for targeted therapy and diagnosis of breast cancer. Biomed. Pharmacother. 2024, 180, 117404. [Google Scholar] [CrossRef]
- Bardania, H.; Baneshi, M.; Mahmoudi, R.; Khosravani, F.; Safari, F.; Khalvati, B.; Poursamad, A.; Alipour, M. Synergistic breast cancer therapy with RGD-decorated liposomes co-delivering mir-34a and cisplatin. Cancer Nanotechnol. 2024, 15, 60. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Ashraf Mirahmadi-Babaheidri, S.; Delaviz, H.; Fouani, M.H.; Alipour, M.; Jafari Barmak, M.; Christiansen, G.; Bardania, H. RGD peptide-mediated liposomal curcumin targeted delivery to breast cancer cells. J. Biomater. Appl. 2021, 35, 743–753. [Google Scholar] [CrossRef]
- Ye, X.; Chen, X.; He, R.; Meng, W.; Chen, W.; Wang, F.; Meng, X. Enhanced anti-breast cancer efficacy of co-delivery liposomes of docetaxel and curcumin. Front. Pharmacol. 2022, 13, 969611. [Google Scholar] [CrossRef]
- Yeo, S.; Kim, M.J.; Shim, Y.K.; Yoon, I.; Lee, W.K. Solid Lipid Nanoparticles of Curcumin Designed for Enhanced Bioavailability and Anticancer Efficiency. ACS Omega 2022, 7, 35875–35884. [Google Scholar] [CrossRef]
- Jiang, M.; Huang, O.; Zhang, X.; Xie, Z.; Shen, A.; Liu, H.; Geng, M.; Shen, K. Curcumin induces cell death and restores tamoxifen sensitivity in the antiestrogen-resistant breast cancer cell lines MCF-7/LCC2 and MCF-7/LCC9. Molecules 2013, 18, 701–720. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, T.; Qiu, Y.; Yang, Z.; Wang, D.; Wang, Z.; Zeng, J.; Bi, Z. Mechanisms of traditional Chinese medicine overcoming of radiotherapy resistance in breast cancer. Front. Oncol. 2024, 14, 1388750. [Google Scholar] [CrossRef]
- Liang, Z.J.; Wan, Y.; Zhu, D.D.; Wang, M.X.; Jiang, H.M.; Huang, D.L.; Luo, L.F.; Chen, M.J.; Yang, W.P.; Li, H.M.; et al. Resveratrol Mediates the Apoptosis of Triple Negative Breast Cancer Cells by Reducing POLD1 Expression. Front. Oncol. 2021, 11, 569295. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Shen, Y.C.; Wu, C.Y.; Tsai, Y.Y.; Yang, Y.H.; Lin, Y.Y.; Kuan, F.C.; Lu, C.N.; Chang, G.H.; Tsai, M.S.; et al. Danshen Improves Survival of Patients with Breast Cancer and Dihydroisotanshinone I Induces Ferroptosis and Apoptosis of Breast Cancer Cells. Front. Pharmacol. 2019, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic Galovic, A.; Jovanovic Ljeskovic, N.; Vidovic, S.; Vladic, J.; Jojic, N.; Ilic, M.; Srdic Rajic, T.; Kojic, V.; Jakimov, D. The Effects of Resveratrol-Rich Extracts of Vitis vinifera Pruning Waste on HeLa, MCF-7 and MRC-5 Cells: Apoptosis, Autophagia and Necrosis Interplay. Pharmaceutics 2022, 14, 2017. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, L.; Chalabi, N.; Delort, L.; Bignon, Y.J.; Bernard-Gallon, D.J. Resveratrol and breast cancer chemoprevention: Molecular mechanisms. Mol. Nutr. Food Res. 2005, 49, 462–471. [Google Scholar] [CrossRef]
- Kim, J.M.; Noh, E.M.; Song, H.K.; Lee, M.; Lee, S.H.; Park, S.H.; Ahn, C.K.; Lee, G.S.; Byun, E.B.; Jang, B.S.; et al. Salvia miltiorrhiza extract inhibits TPA-induced MMP-9 expression and invasion through the MAPK/AP-1 signaling pathway in human breast cancer MCF-7 cells. Oncol. Lett. 2017, 14, 3594–3600. [Google Scholar] [CrossRef]
- Vinod, B.S.; Nair, H.H.; Vijayakurup, V.; Shabna, A.; Shah, S.; Krishna, A.; Pillai, K.S.; Thankachan, S.; Anto, R.J. Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2-Akt axis. Cell Death Discov. 2015, 1, 15061. [Google Scholar] [CrossRef]
- Kim, T.H.; Shin, Y.J.; Won, A.J.; Lee, B.M.; Choi, W.S.; Jung, J.H.; Chung, H.Y.; Kim, H.S. Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim. Biophys. Acta 2014, 1840, 615–625. [Google Scholar] [CrossRef]
- Özdemi, R.F.; Sever, A.; Keçeci, Y.; Incesu, Z. Resveratrol increases the sensitivity of breast cancer MDA-MB-231 cell line to cisplatin by regulating intrinsic apoptosis. Iran. J. Basic. Med. Sci. 2021, 24, 66–72. [Google Scholar] [CrossRef]
- Behroozaghdam, M.; Dehghani, M.; Zabolian, A.; Kamali, D.; Javanshir, S.; Hasani Sadi, F.; Hashemi, M.; Tabari, T.; Rashidi, M.; Mirzaei, S.; et al. Resveratrol in breast cancer treatment: From cellular effects to molecular mechanisms of action. Cell. Mol. Life Sci. 2022, 79, 539. [Google Scholar] [CrossRef]
- Suh, J.; Kim, D.H.; Surh, Y.J. Resveratrol suppresses migration, invasion and stemness of human breast cancer cells by interfering with tumor-stromal cross-talk. Arch. Biochem. Biophys. 2018, 643, 62–71. [Google Scholar] [CrossRef]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.K.; Dim, D.C.; Bhat, H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 2014, 35, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Andreani, C.; Bartolacci, C.; Wijnant, K.; Crinelli, R.; Bianchi, M.; Magnani, M.; Hysi, A.; Iezzi, M.; Amici, A.; Marchini, C. Resveratrol fuels HER2 and ERalpha-positive breast cancer behaving as proteasome inhibitor. Aging 2017, 9, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef] [PubMed]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef]
- Stuart, E.C.; Scandlyn, M.J.; Rosengren, R.J. Role of epigallocatechin gallate (EGCG) in the treatment of breast and prostate cancer. Life Sci. 2006, 79, 2329–2336. [Google Scholar] [CrossRef]
- Mineva, N.D.; Paulson, K.E.; Naber, S.P.; Yee, A.S.; Sonenshein, G.E. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLoS ONE 2013, 8, e73464. [Google Scholar] [CrossRef]
- Somers-Edgar, T.J.; Scandlyn, M.J.; Stuart, E.C.; Le Nedelec, M.J.; Valentine, S.P.; Rosengren, R.J. The combination of epigallocatechin gallate and curcumin suppresses ER alpha-breast cancer cell growth in vitro and in vivo. Int. J. Cancer 2008, 122, 1966–1971. [Google Scholar] [CrossRef]
- Chung, S.S.; Vadgama, J.V. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NFkappaB signaling. Anticancer. Res. 2015, 35, 39–46. [Google Scholar]
- Zhao, H.; Zhu, W.; Zhao, X.; Li, X.; Zhou, Z.; Zheng, M.; Meng, X.; Kong, L.; Zhang, S.; He, D.; et al. Efficacy of Epigallocatechin-3-Gallate in Preventing Dermatitis in Patients With Breast Cancer Receiving Postoperative Radiotherapy: A Double-Blind, Placebo-Controlled, Phase 2 Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 779–786. [Google Scholar] [CrossRef]
- Banerjee, S.; Mandal, A.K.A. Role of epigallocatechin-3- gallate in the regulation of known and novel microRNAs in breast carcinoma cells. Front. Genet. 2022, 13, 995046. [Google Scholar] [CrossRef]
- Atteeq, M. Evaluating anticancer properties of Withaferin A-a potent phytochemical. Front. Pharmacol. 2022, 13, 975320. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rehman, A.U.; Siddiqui, S.; Khan, J.; Massey, S.; Singh, P.; Saluja, D.; Husain, S.A.; Iqbal, M.A. Withaferin A decreases glycolytic reprogramming in breast cancer. Sci. Rep. 2024, 14, 23147. [Google Scholar] [CrossRef] [PubMed]
- Thaiparambil, J.T.; Bender, L.; Ganesh, T.; Kline, E.; Patel, P.; Liu, Y.; Tighiouart, M.; Vertino, P.M.; Harvey, R.D.; Garcia, A.; et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer 2011, 129, 2744–2755. [Google Scholar] [CrossRef]
- Hahm, E.R.; Moura, M.B.; Kelley, E.E.; Van Houten, B.; Shiva, S.; Singh, S.V. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS ONE 2011, 6, e23354. [Google Scholar] [CrossRef]
- Zuniga, R.; Concha, G.; Cayo, A.; Cikutovic-Molina, R.; Arevalo, B.; Gonzalez, W.; Catalan, M.A.; Zuniga, L. Withaferin A suppresses breast cancer cell proliferation by inhibition of the two-pore domain potassium (K2P9) channel TASK-3. Biomed. Pharmacother. 2020, 129, 110383. [Google Scholar] [CrossRef]
- Sehrawat, A.; Samanta, S.K.; Hahm, E.R.; St Croix, C.; Watkins, S.; Singh, S.V. Withaferin A-mediated apoptosis in breast cancer cells is associated with alterations in mitochondrial dynamics. Mitochondrion 2019, 47, 282–293. [Google Scholar] [CrossRef]
- Hahm, E.R.; Lee, J.; Huang, Y.; Singh, S.V. Withaferin a suppresses estrogen receptor-alpha expression in human breast cancer cells. Mol. Carcinog. 2011, 50, 614–624. [Google Scholar] [CrossRef]
- Nagalingam, A.; Kuppusamy, P.; Singh, S.V.; Sharma, D.; Saxena, N.K. Mechanistic elucidation of the antitumor properties of withaferin a in breast cancer. Cancer Res. 2014, 74, 2617–2629. [Google Scholar] [CrossRef]
- Kim, S.H.; Hahm, E.R.; Arlotti, J.A.; Samanta, S.K.; Moura, M.B.; Thorne, S.H.; Shuai, Y.; Anderson, C.J.; White, A.G.; Lokshin, A.; et al. Withaferin A inhibits in vivo growth of breast cancer cells accelerated by Notch2 knockdown. Breast Cancer Res. Treat. 2016, 157, 41–54. [Google Scholar] [CrossRef]
- Muniraj, N.; Siddharth, S.; Nagalingam, A.; Walker, A.; Woo, J.; Gyorffy, B.; Gabrielson, E.; Saxena, N.K.; Sharma, D. Withaferin A inhibits lysosomal activity to block autophagic flux and induces apoptosis via energetic impairment in breast cancer cells. Carcinogenesis 2019, 40, 1110–1120. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Havakhah, S.; Boskabady, M.H. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. J. Ethnopharmacol. 2016, 190, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Shabani, H.; Karami, M.H.; Kolour, J.; Sayyahi, Z.; Parvin, M.A.; Soghala, S.; Baghini, S.S.; Mardasi, M.; Chopani, A.; Moulavi, P.; et al. Anticancer activity of thymoquinone against breast cancer cells: Mechanisms of action and delivery approaches. Biomed. Pharmacother. 2023, 165, 114972. [Google Scholar] [CrossRef] [PubMed]
- Alshaibi, H.F.; Aldarmahi, N.A.; Alkhattabi, N.A.; Alsufiani, H.M.; Tarbiah, N.I. Studying the Anticancer Effects of Thymoquinone on Breast Cancer Cells through Natural Killer Cell Activity. BioMed Res. Int. 2022, 2022, 9218640. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.C.; Hsu, A.; Kumar, A.P.; Sethi, G.; Tan, K.H. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: The role of p38 MAPK and ROS. PLoS ONE 2013, 8, e75356. [Google Scholar] [CrossRef]
- Woo, C.C.; Loo, S.Y.; Gee, V.; Yap, C.W.; Sethi, G.; Kumar, A.P.; Tan, K.H. Anticancer activity of thymoquinone in breast cancer cells: Possible involvement of PPAR-gamma pathway. Biochem. Pharmacol. 2011, 82, 464–475. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, C.; Sun, Y. Unraveling the Role of Scutellaria baicalensis for the Treatment of Breast Cancer Using Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. Int. J. Mol. Sci. 2023, 24, 3594. [Google Scholar] [CrossRef]
- Zieniuk, B.; Ugur, S. The Therapeutic Potential of Baicalin and Baicalein in Breast Cancer: A Systematic Review of Mechanisms and Efficacy. Curr. Issues Mol. Biol. 2025, 47, 181. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, A.; Kuang, G.; Gong, X.; Jiang, R.; Lin, D.; Li, J.; Li, H.; Zhang, X.; Wan, J.; et al. Baicalin inhibits the metastasis of highly aggressive breast cancer cells by reversing epithelial-to-mesenchymal transition by targeting beta-catenin signaling. Oncol. Rep. 2017, 38, 3599–3607. [Google Scholar] [CrossRef]
- Zhou, Q.M.; Wang, S.; Zhang, H.; Lu, Y.Y.; Wang, X.F.; Motoo, Y.; Su, S.B. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol. Sin. 2009, 30, 1648–1658. [Google Scholar] [CrossRef]
- Park, J.R.; Lee, M.C.; Moon, S.C.; Kim, J.; Ha, K.T.; Park, E.J.; Hong, C.; Seo, B.D.; Kim, B.J. Scutellaria baicalensis Georgi induces caspase-dependent apoptosis via mitogen activated protein kinase activation and the generation of reactive oxygen species signaling pathways in MCF-7 breast cancer cells. Mol. Med. Rep. 2017, 16, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Li, X.L.; Wang, Q.F.; Mehendale, S.R.; Yuan, C.S. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine 2010, 17, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shao, D.; Zhao, Y.; Zhang, F.; Zheng, X.; Tan, Y.; He, K.; Li, J.; Chen, L. Berberine Reverses Hypoxia-induced Chemoresistance in Breast Cancer through the Inhibition of AMPK- HIF-1alpha. Int. J. Biol. Sci. 2017, 13, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Oh, S.J.; Lee, J.; Han, J.; Jeon, M.; Jung, T.; Lee, S.K.; Bae, S.Y.; Kim, J.; Gil, W.H.; et al. Berberine suppresses TPA-induced fibronectin expression through the inhibition of VEGF secretion in breast cancer cells. Cell. Physiol. Biochem. 2013, 32, 1541–1550. [Google Scholar] [CrossRef]
- Zhang, R.; Qiao, H.; Chen, S.; Chen, X.; Dou, K.; Wei, L.; Zhang, J. Berberine reverses lapatinib resistance of HER2-positive breast cancer cells by increasing the level of ROS. Cancer Biol. Ther. 2016, 17, 925–934. [Google Scholar] [CrossRef]
- Chen, H.; Shang, X.; Yuan, H.; Niu, Q.; Chen, J.; Luo, S.; Li, W.; Li, X. Total flavonoids of Oldenlandia diffusa (Willd.) Roxb. suppresses the growth of hepatocellular carcinoma through endoplasmic reticulum stress-mediated autophagy and apoptosis. Front. Pharmacol. 2022, 13, 1019670. [Google Scholar] [CrossRef]
- Al-Shuhaib, M.B.S.; Al-Shuhaib, J.M.B. Phytochemistry, pharmacology, and medical uses of Oldenlandia (family Rubaceae): A review. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 2021–2053. [Google Scholar] [CrossRef]
- Gu, G.; Barone, I.; Gelsomino, L.; Giordano, C.; Bonofiglio, D.; Statti, G.; Menichini, F.; Catalano, S.; Ando, S. Oldenlandia diffusa extracts exert antiproliferative and apoptotic effects on human breast cancer cells through ERalpha/Sp1-mediated p53 activation. J. Cell. Physiol. 2012, 227, 3363–3372. [Google Scholar] [CrossRef]
- Chung, T.W.; Choi, H.; Lee, J.M.; Ha, S.H.; Kwak, C.H.; Abekura, F.; Park, J.Y.; Chang, Y.C.; Ha, K.T.; Cho, S.H.; et al. Oldenlandia diffusa suppresses metastatic potential through inhibiting matrix metalloproteinase-9 and intercellular adhesion molecule-1 expression via p38 and ERK1/2 MAPK pathways and induces apoptosis in human breast cancer MCF-7 cells. J. Ethnopharmacol. 2017, 195, 309–317. [Google Scholar] [CrossRef]
- Wang, S.; Chang, X.; Zhang, J.; Li, J.; Wang, N.; Yang, B.; Pan, B.; Zheng, Y.; Wang, X.; Ou, H.; et al. Ursolic Acid Inhibits Breast Cancer Metastasis by Suppressing Glycolytic Metabolism via Activating SP1/Caveolin-1 Signaling. Front. Oncol. 2021, 11, 745584. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; He, J.; Tong, X.; Tang, L.; Liu, M. The Hedyotis diffusa Willd. (Rubiaceae): A Review on Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics. Molecules 2016, 21, 710. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Xue, L.; Severino, R.P.; Gao, S.; Niu, J.; Qin, L.P.; Zhang, D.; Bromme, D. Salvia miltiorrhiza: An ancient Chinese herbal medicine as a source for anti-osteoporotic drugs. J. Ethnopharmacol. 2014, 155, 1401–1416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Han, B.; Li, X.; Sun, C.; Zhai, Y.; Li, M.; Jiang, M.; Zhang, W.; Liang, Y.; Kai, G. Salvia miltiorrhiza in Breast Cancer Treatment: A Review of Its Phytochemistry, Derivatives, Nanoparticles, and Potential Mechanisms. Front. Pharmacol. 2022, 13, 872085. [Google Scholar] [CrossRef]
- Han, H.; Qian, C.; Zong, G.; Liu, H.; Wang, F.; Tao, R.; Cheng, P.; Wei, Z.; Zhao, Y.; Lu, Y. Systemic pharmacological verification of Salvia miltiorrhiza-Ginseng Chinese herb pair in inhibiting spontaneous breast cancer metastasis. Biomed. Pharmacother. 2022, 156, 113897. [Google Scholar] [CrossRef]
- Onaciu, A.; Munteanu, R.; Munteanu, V.C.; Gulei, D.; Raduly, L.; Feder, R.I.; Pirlog, R.; Atanasov, A.G.; Korban, S.S.; Irimie, A.; et al. Spontaneous and Induced Animal Models for Cancer Research. Diagnostics 2020, 10, 660. [Google Scholar] [CrossRef]
- Larkin, J.O.; Collins, C.G.; Aarons, S.; Tangney, M.; Whelan, M.; O’Reily, S.; Breathnach, O.; Soden, D.M.; O’Sullivan, G.C. Electrochemotherapy: Aspects of preclinical development and early clinical experience. Ann. Surg. 2007, 245, 469–479. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin-An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef]
- Kroon, M.; Berbee, J.K.; Majait, S.; Swart, E.L.; van Tellingen, O.; van Laarhoven, H.W.M.; Kemper, E.M. Non-therapeutic plasma levels in individuals utilizing curcumin supplements in daily life. Front. Nutr. 2023, 10, 1267035. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, K.; Bhaskaran, M.; Selvam, C.; Thilagavathi, R. Nano formulation approaches for curcumin delivery—A review. J. Drug Deliv. Sci. Technol. 2023, 82, 104326. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Sahoo, S.K. Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin. Expert. Opin. Drug Deliv. 2012, 9, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Limami, Y.; Duval, R.E. Curcumin-Based Nanoparticles: Advancements and Challenges in Tumor Therapy. Pharmaceutics 2025, 17, 114. [Google Scholar] [CrossRef]
- McGrowder, D.A.; Miller, F.G.; Nwokocha, C.R.; Anderson, M.S.; Wilson-Clarke, C.; Vaz, K.; Anderson-Jackson, L.; Brown, J. Medicinal Herbs Used in Traditional Management of Breast Cancer: Mechanisms of Action. Medicines 2020, 7, 47. [Google Scholar] [CrossRef]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for radiation dermatitis: A randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
| Study Type | Findings | Mechanism/Outcome | References |
|---|---|---|---|
| Preclinical studies | Enhances effect of tamoxifen, reverses endocrine resistance | Inhibition of proliferation, promotion of apoptosis, targeting of survival pathways | [31] |
| Preclinical studies | Inhibits proliferation, induces apoptosis, suppresses metastasis | Modulation of multiple cancer-related molecular pathways | [14] |
| Preclinical study | Suppresses breast cancer cell proliferation | Downregulation of Flap endonuclease 1 via NRF2 signaling | [16] |
| Animal study | Intravenous curcumin inhibits tumor growth and metastasis in mice | Significant tumor suppression and antimetastatic activity | [17] |
| Clinical trial | Combination of curcumin with docetaxel suppresses breast cancer progression | Reduction in tumor marker levels | [19] |
| Clinical trial | Combination of curcumin with paclitaxel improves overall response rate and physical performance | Better treatment outcomes, reduced fatigue, and good tolerability | [15] |
| Pharmacokinetic study | Limitations in the clinical applicability of curcumin | Poor bioavailability, rapid metabolism, low water solubility | [22] |
| Drug delivery research | Development of advanced deliverysystems | Use of nanoparticles (polymeric nanoparticles, carbon nanotubes, liposomes) to enhance therapeutic efficacy | [23] |
| Herbal Compound | Clinical Trial Status | Key Findings | References |
|---|---|---|---|
| Curcumin (Curcuma longa) | Phase II randomized, double-blind, placebo-controlled trial (NCT03072992) | Intravenous curcumin (300 mg/week) combined with paclitaxel significantly improved objective response rate (ORR: 51% vs. 33%, p < 0.01) and physical performance in patients with advanced/metastatic breast cancer. Treatment was well-tolerated with reduced fatigue. | [15] |
| Scutellaria baicalensis | No registered clinical trials; preclinical studies available | Wogonin, a flavone from S. baicalensis, demonstrated induction of apoptosis and inhibition of proliferation in breast cancer cell lines. However, it also induced radioresistance in MCF-7 cells, indicating complex interactions. | [32] |
| Oldenlandia diffusa | Early genetic marker for progression | Aqueous extracts of O. diffusa induced apoptosis in breast cancer cell lines (MDA-MB-157 and 93B) via modulation of pro- and anti-apoptotic proteins, suggesting potential therapeutic effects. | [33] |
| Salvia miltiorrhiza | Observational study using Taiwan’s National Health Insurance Research Database (NHIRD) | Use of Danshen (S. miltiorrhiza) was associated with improved survival in breast cancer patients. Dihydroisotanshinone I, a compound from Danshen, induced ferroptosis and apoptosis in breast cancer cells in vitro and inhibited tumor growth in vivo. | [34] |
| Compound/Extract | Active Components/Source | Mechanisms of Action | Breast Cancer Cell Line(s) | Key Outcomes | References |
|---|---|---|---|---|---|
| Scutellaria baicalensis (whole plant) | Traditional East Asian herb | Modulates transcription and kinase activity; alters phosphorylation in signaling pathways | Not specified | General anti-breast cancer effect via pathway modulation | [69,70] |
| Baicalin | Flavonoid from Scutellaria baicalensis | Inhibits β-catenin signaling, reverses EMT | MDA-MB-231 | Suppresses metastasis in aggressive breast cancer | [72] |
| Baicalin + baicalein | Flavonoids from Scutellaria baicalensis | Activates caspases 9 and 3, downregulates BCL-2, upregulates BAX, p53; via ERK/p38 MAPK pathway | MCF-7 | Synergistically enhance apoptosis | [73] |
| Scutellaria baicalensis Georgi extract | Ethanol extract | Increases ROS, activates MAPK/JNK, modulates BCL-2/BAX, activates caspases | Not specified | Inhibits proliferation and induces apoptosis; MAPK-dependent | [74] |
| Scutellaria baicalensis extract | Whole-plant extract | No effect with whole-plant extract, chemoprevention with specific fraction | MCF-7 | Fractionated Scutellaria baicalensis extract shows selective antitumor effect | [75] |
| Compound/Extract | Active Components/Source | Mechanisms of Action | Breast Cancer Cell Line(s) | Key Outcomes | References |
|---|---|---|---|---|---|
| Oldenlandia diffusa extract | Whole-plant extract | Upregulates p53 via the ERα/SP1 pathway | Not specified | Inhibits proliferation, induces apoptosis | [81] |
| Ursolic acid and oleanolic acid (from Oldenlandia diffusa extract) | Bioactive triterpenoids | Induce p53, exert antiproliferative and proapoptotic effects | Not specified | Identified as active antitumor agents in Oldenlandia diffusa extract | [81] |
| Oldenlandia diffusa | Whole-plant extract | Inhibits p-ERK, p38, NF-κB; downregulates MMP-9 and ICAM-1 | MCF-7 | Reduces metastasis and invasion, promotes apoptosis | [82] |
| Ursolic acid (from O. diffusa) | Isolated by bioactivity-guided fractionation | Suppresses glycolytic metabolism via SP1/caveolin-1 signaling | Not specified | Inhibits metastasis | [83] |
| Hedyotis diffusa (synonym of Oldenlandia diffusa) | Traditional use | Used in treatment of diseases with inflammation (e.g., hepatitis, appendicitis) | Not specified | Anti-inflammatory and broad pharmacologic effects | [84] |
| Compound/Extract | Active Components | Mechanisms of Action | Breast Cancer Cell Line(s) | Key Outcomes | References |
|---|---|---|---|---|---|
| Salvia miltiorrhiza extract | Liposoluble tanshinones (e.g., dihydrotanshinone I, tanshinone I, tanshinone IIA, cryptotanshinone); phenolic acids (e.g., salvianolic acid A/B/C, rosmarinic acid) | Inhibits TPA-induced invasion | MCF-7 | Reduces cell metastasis potential | [37] |
| Salvia miltiorrhiza extract–ginseng combination | Multi-herb formulation | Increases VEGF-A and MMP-9 expression, thereby enhancing vascular basement membrane integrity; inhibits EMT | Not specified | Inhibits lung metastasis, suppresses pre-metastatic niche formation | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-C.; Tsai, C.-C.; Hsu, P.-C.; Kuo, C.-Y. Herbal Medicine in Breast Cancer Therapy: Mechanisms, Evidence, and Future Perspectives. Curr. Issues Mol. Biol. 2025, 47, 362. https://doi.org/10.3390/cimb47050362
Wu H-C, Tsai C-C, Hsu P-C, Kuo C-Y. Herbal Medicine in Breast Cancer Therapy: Mechanisms, Evidence, and Future Perspectives. Current Issues in Molecular Biology. 2025; 47(5):362. https://doi.org/10.3390/cimb47050362
Chicago/Turabian StyleWu, Hsien-Chang, Chung-Che Tsai, Po-Chih Hsu, and Chan-Yen Kuo. 2025. "Herbal Medicine in Breast Cancer Therapy: Mechanisms, Evidence, and Future Perspectives" Current Issues in Molecular Biology 47, no. 5: 362. https://doi.org/10.3390/cimb47050362
APA StyleWu, H.-C., Tsai, C.-C., Hsu, P.-C., & Kuo, C.-Y. (2025). Herbal Medicine in Breast Cancer Therapy: Mechanisms, Evidence, and Future Perspectives. Current Issues in Molecular Biology, 47(5), 362. https://doi.org/10.3390/cimb47050362








