Exploration of the Mechanisms of Acorus tatarinowii in the Treatment of Major Depressive Disorder Based on Network Pharmacology and Molecular Docking Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Active Compounds and Target Genes
2.2. Identification of Major Depressive Disorder-Related Targets and Venny Analysis
2.3. Construction of Protein–Protein Interaction (PPI) Network
2.4. Network Construction and Analysis
2.5. GO Enrichment and KEGG Pathway Analysis
2.6. Molecular Docking
3. Results
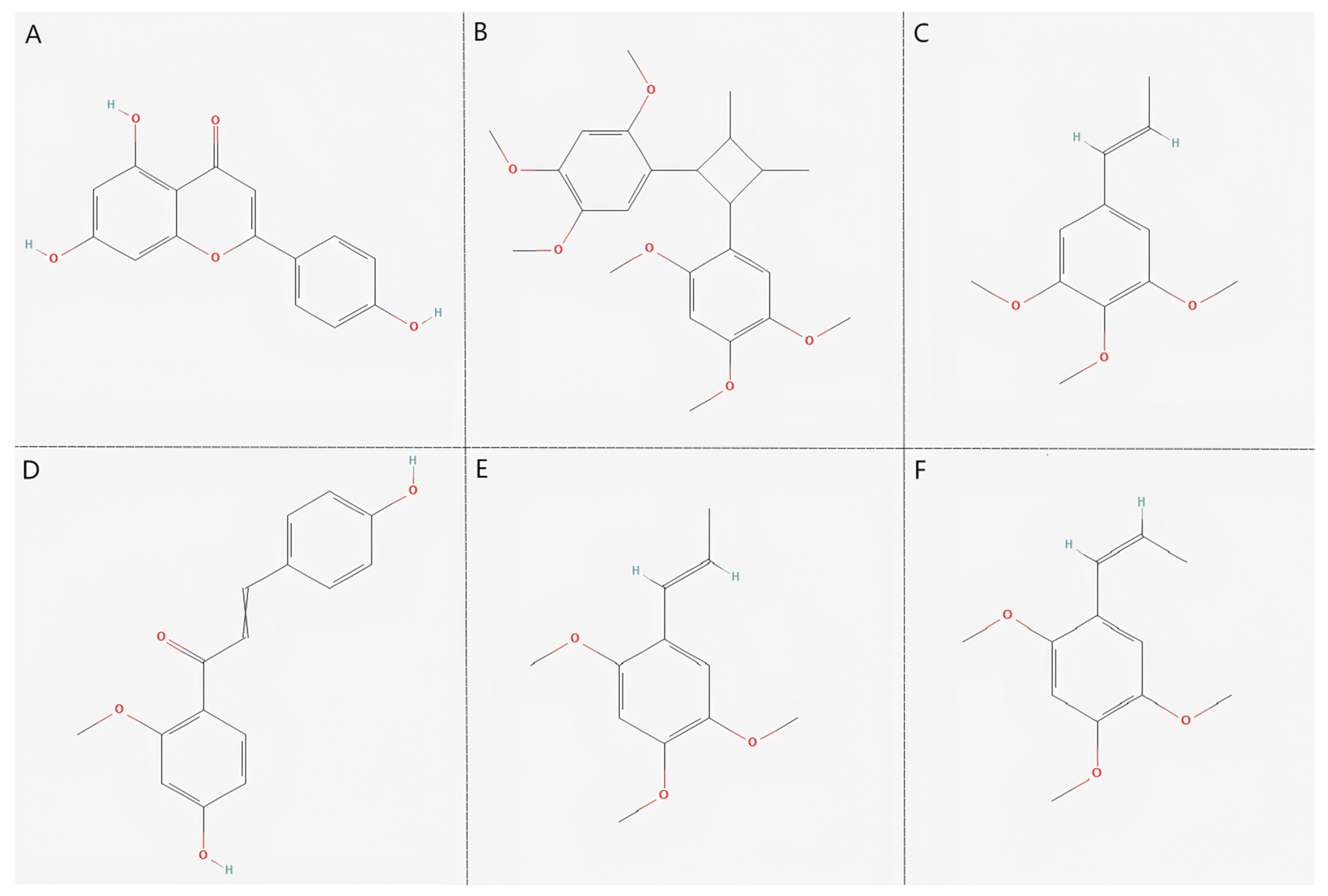
3.1. Active Components of Acorus tatarinowii and Their Potential Targets
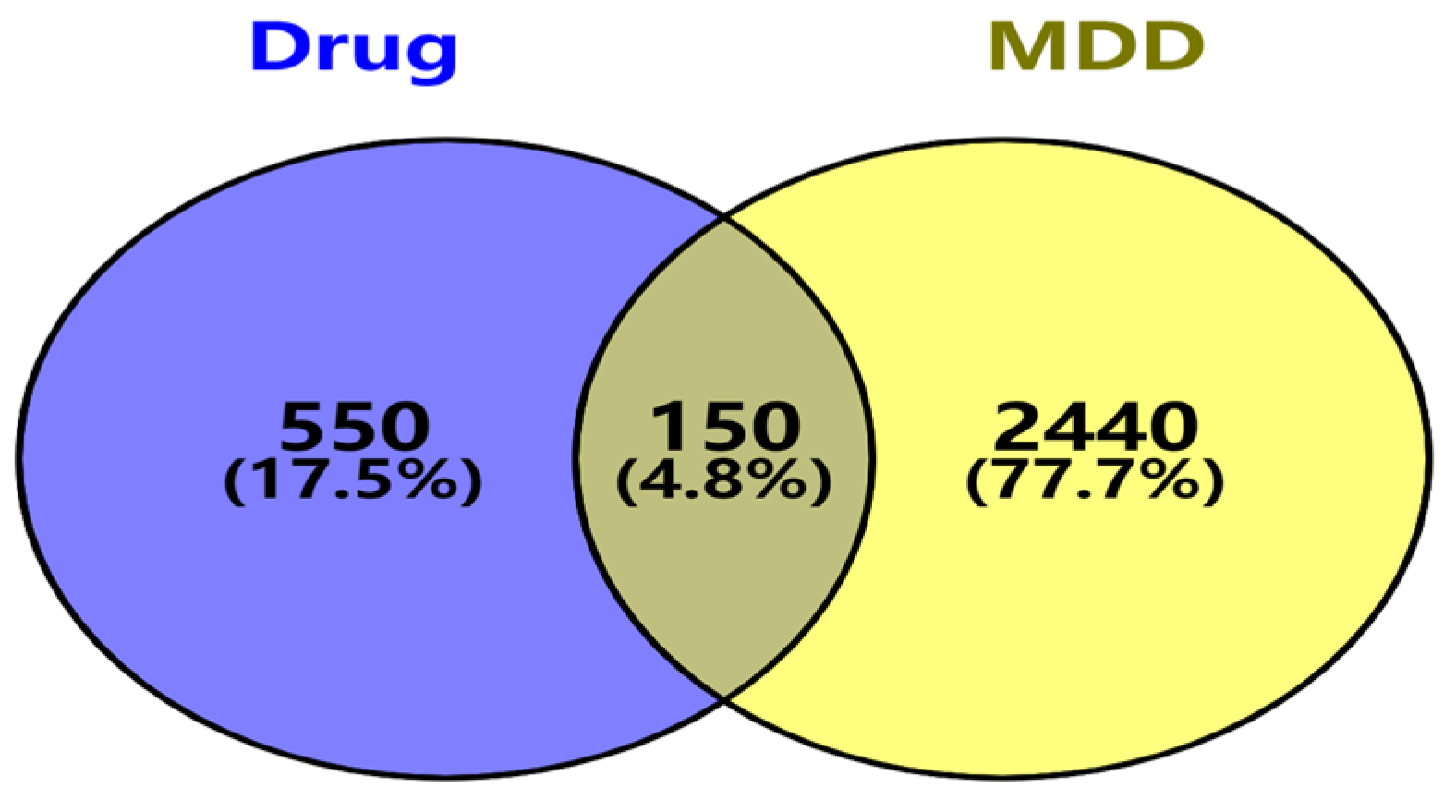
3.2. Identification of Targets Related to Major Depressive Disorder (MDD)
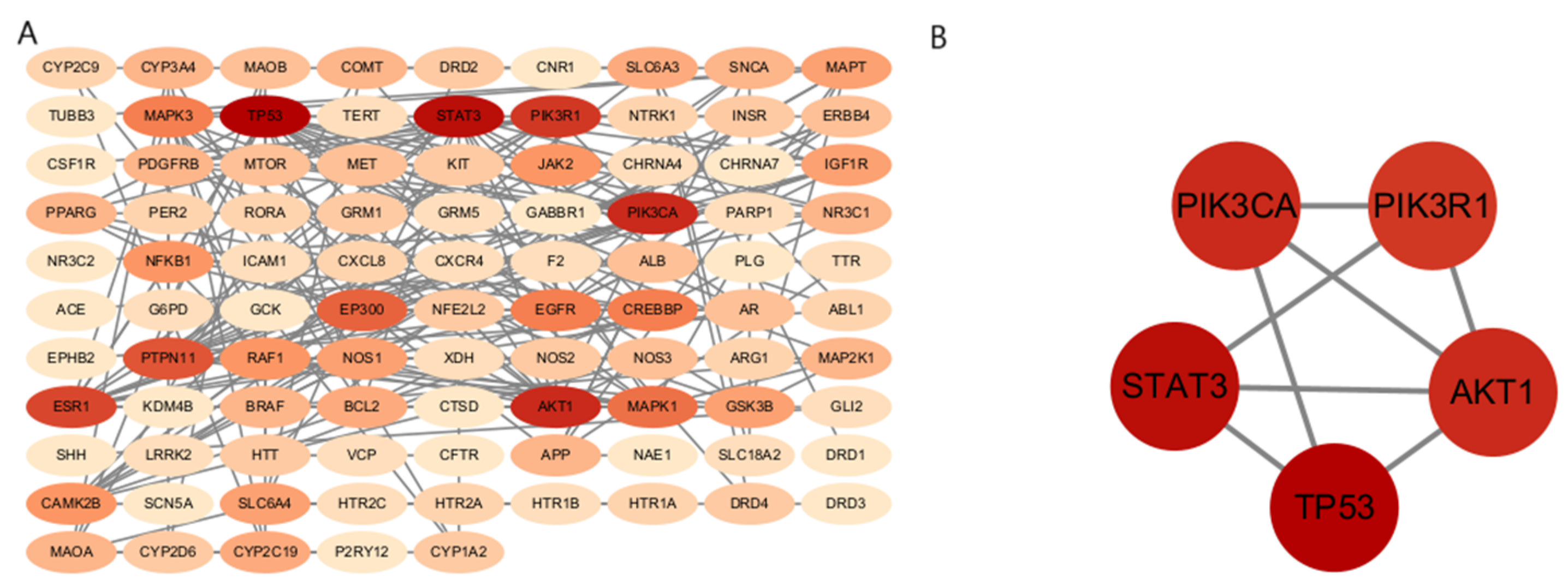
3.3. Construction of the Potential-Target Protein–Protein Interaction (PPI) Network
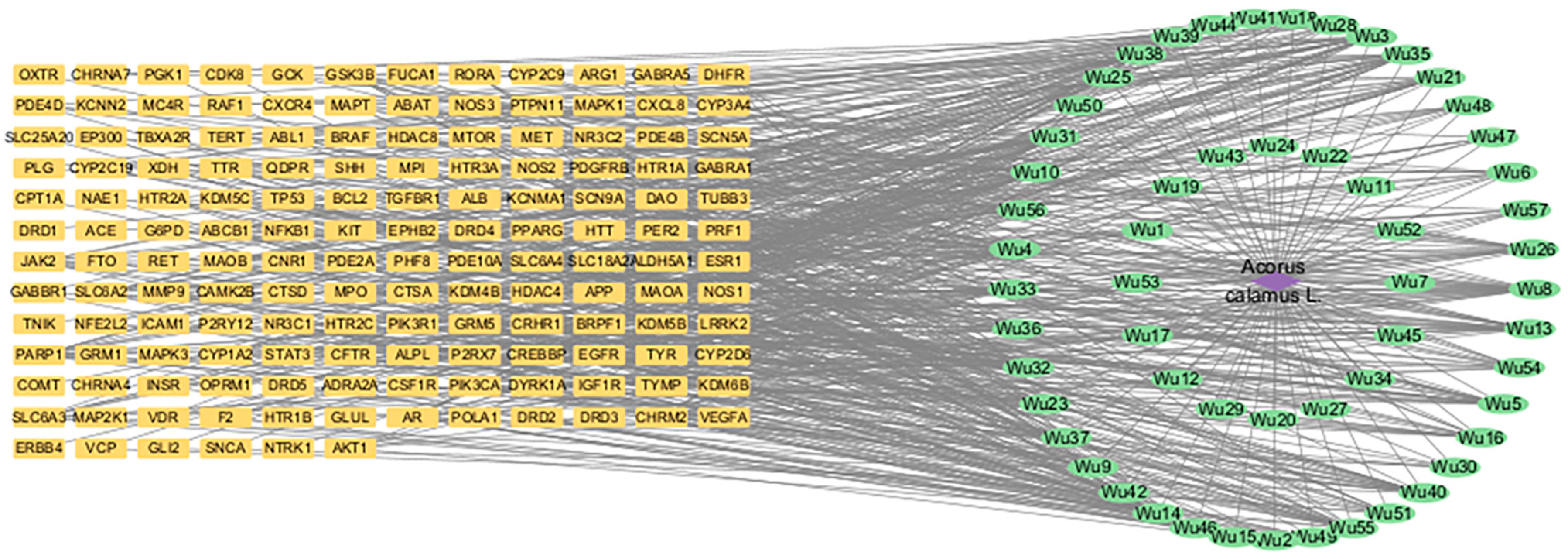
3.4. Network of Active Components and Targets of Acorus tatarinowii
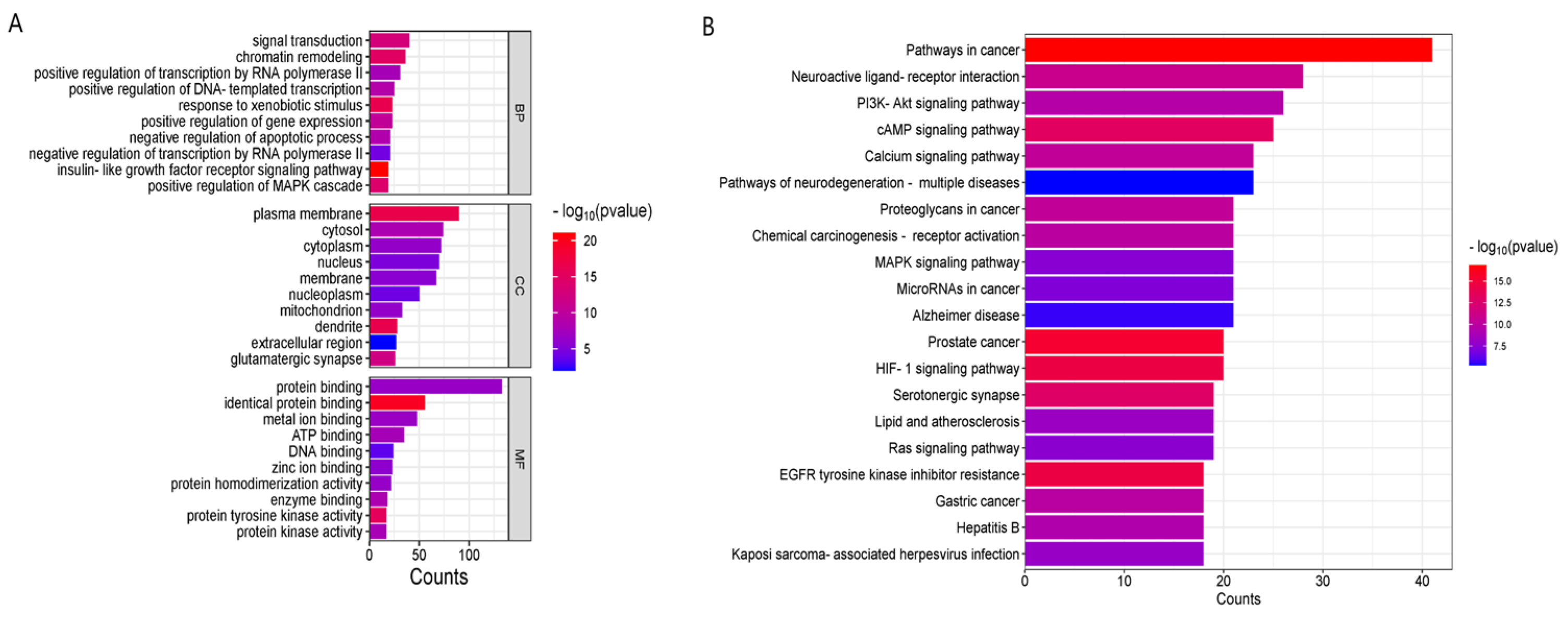
3.5. GO Enrichment Analysis and KEGG Pathway Enrichment Analysis
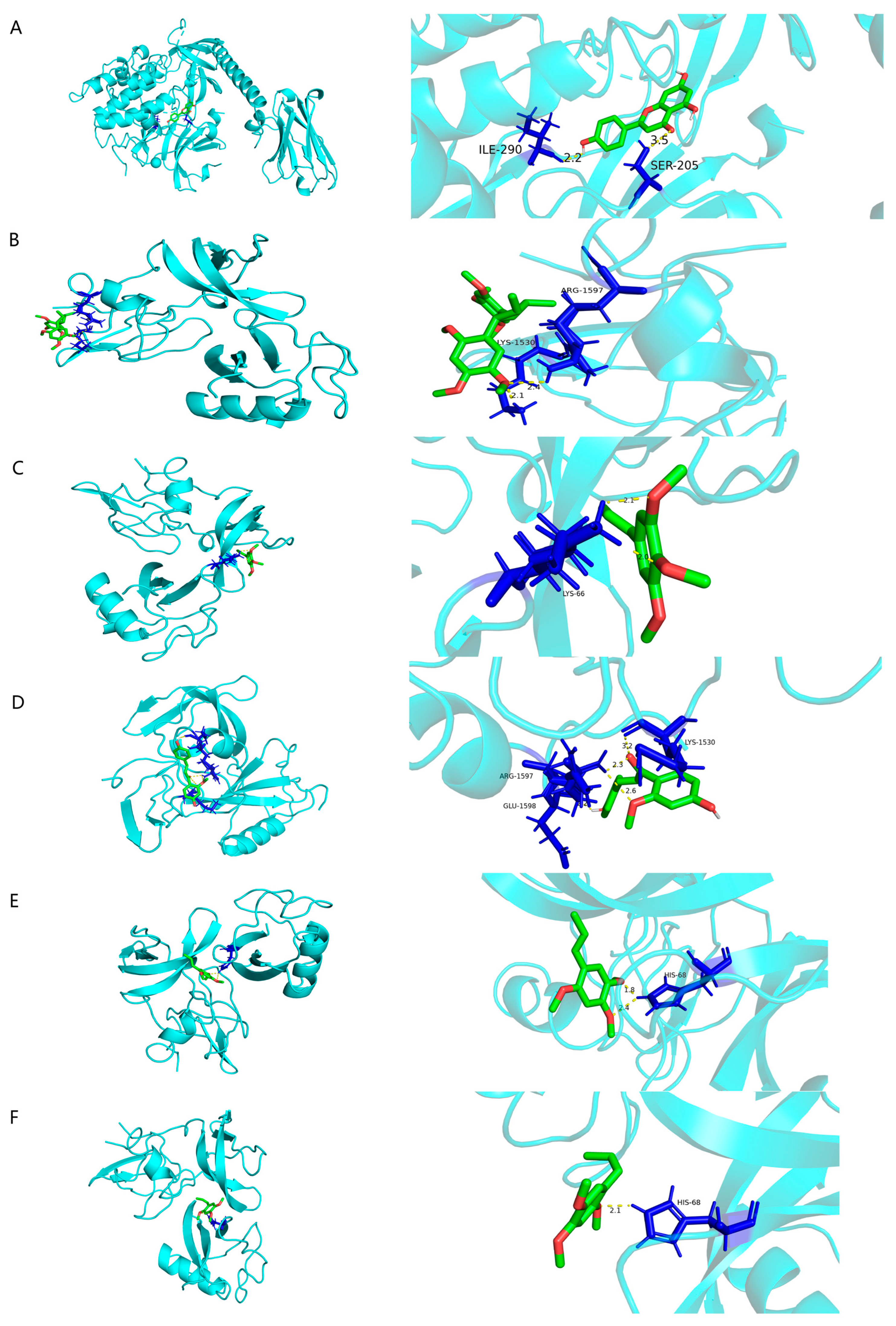
3.6. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Trivedi, M.H. Major Depressive Disorder in Primary Care: Strategies for Identification. J. Clin. Psychiatry 2020, 81, UT17042BR1C. [Google Scholar] [CrossRef]
- Shorey, S.; Ng, E.D.; Wong, C.H. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br. J. Clin. Psychol. 2022, 61, 287–305. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target Ther. 2024, 9, 30. [Google Scholar]
- Zhang, M.; Yang, Y.; Zhao, Y.; Sui, C.; Sui, Y.; Jiang, Y.; Liu, K.; Yang, S.; Wang, L.; Chen, B.; et al. The application of integrating electroencephalograph-based emotion recognition technology into brain–computer interface systems for the treatment of depression: A narrative review. Adv. Technol. Neurosci. 2024, 1, 188–200. [Google Scholar] [CrossRef]
- Yan, L.; Mahady, G.; Qian, Y.; Song, P.; Jian, T.; Ding, X.; Guan, F.; Shan, Y.; Wei, M. The Essential Oil from Acori Tatarinowii Rhizome (the Dried Rhizome of Acorus tatarinowii Schott) Prevents Hydrogen Peroxide-Induced Cell Injury in PC12 Cells: A Signaling Triggered by CREB/PGC-1α Activation. Evid. Based Complement. Altern. Med. 2020, 2020, 4845028. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ku, C.F.; Wang, H.Y.; Chan, G.K.; Yao, P.; Lin, H.Q.; Dong, T.T.; Zhang, H.J.; Tsim, K.W. Authentication of Acori Tatarinowii Rhizoma (Shi Chang Pu) and its adulterants by morphological distinction, chemical composition and ITS sequencing. Chin. Med. 2016, 11, 41. [Google Scholar] [CrossRef]
- Li, L.; Wu, M.; Wang, C.; Yu, Z.; Wang, H.; Qi, H.; Xu, X. β-Asarone Inhibits Invasion and EMT in Human Glioma U251 Cells by Suppressing Splicing Factor HnRNP A2/B1. Molecules 2018, 23, 671. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.Y.; Yun, B.S.; Hwang, B.K. Antifungal activity of beta-asarone from rhizomes of Acorus gramineus. J. Agric. Food Chem. 2004, 52, 776–780. [Google Scholar] [CrossRef]
- Lam, K.Y.; Wu, Q.Y.; Hu, W.H.; Yao, P.; Wang, H.Y.; Dong, T.T.; Tsim, K.W. Asarones from Acori Tatarinowii Rhizoma stimulate expression and secretion of neurotrophic factors in cultured astrocytes. Neurosci. Lett. 2019, 707, 134308. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Ye, K.; Jiang, Q.; Wang, M.; Wen, X.; Yang, J. Chemical profile of Xian-He-Cao-Chang-Yan formula and its effects on ulcerative colitis. J. Ethnopharmacol. 2021, 267, 113517. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.X.; Sun, Q.M.; Chen, M.; Liu, S.L.; Zhang, X.; Zhou, J.Y.; Zou, X. β-Asarone inhibits gastric cancer cell proliferation. Oncol. Rep. 2015, 34, 3043–3050. [Google Scholar] [CrossRef]
- Shi, B.; Liu, J.; Zhang, Q.; Wang, S.; Jia, P.; Bian, L.; Zheng, X. Effect of co-administration of Acori Tatarinowii Rhizoma volatile oil on pharmacokinetic fate of xanthotoxol, oxypeucedanin hydrate, and byakangelicin from Angelicae Dahuricae Radix in rat. J. Sep. Sci. 2020, 43, 2349–2362. [Google Scholar] [CrossRef]
- Zhang, W.; He, J.; Hu, Y.; Lu, J.; Zhao, J.; Li, P. Chemical Structure and Immune Activation of a Glucan from Rhizoma Acori Tatarinowii. Front. Nutr. 2022, 9, 942241. [Google Scholar] [CrossRef]
- Jin, B.; Xu, X. Price forecasting through neural networks for crude oil, heating oil, and natural gas. Meas. Energy 2024, 1, 100001. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 2012, 13, 6964–6982. [Google Scholar] [CrossRef]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Lamers, F.; Berk, M.; Penninx, B.W. Depression Heterogeneity and Its Biological Underpinnings: Toward Immunometabolic Depression. Biol. Psychiatry 2020, 88, 369–380. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.H.; Wang, Z.M.; Liu, Y.T.; Huang, J.S.; Liang, S.; Wu, H.H.; Xu, Y.T. Bioactivities of serotonin transporter mediate antidepressant effects of Acorus tatarinowii Schott. J. Ethnopharmacol. 2019, 241, 111967. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Luo, H.; Xiang, Y.; Qu, X.; Liu, H.; Liu, C.; Li, G.; Han, L.; Qin, X. Apelin-13 Suppresses Neuroinflammation Against Cognitive Deficit in a Streptozotocin-Induced Rat Model of Alzheimer’s Disease Through Activation of BDNF-TrkB Signaling Pathway. Front. Pharmacol. 2019, 10, 395. [Google Scholar] [CrossRef]
- Feng, D.; Li, P.; Xiao, W.; Pei, Z.; Chen, P.; Hu, M.; Yang, Z.; Li, T.; Xia, Z.; Cui, H.; et al. N(6)-methyladenosine profiling reveals that Xuefu Zhuyu decoction upregulates METTL14 and BDNF in a rat model of traumatic brain injury. J. Ethnopharmacol. 2023, 317, 116823. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Gu, X.; Tong, G.; Han, L. Research progress of apelin in acute ischemic brain injury. Am. J. Transl. Res. 2022, 14, 7260–7267. [Google Scholar] [PubMed]
- Zuo, J.; Zhang, T.H.; Xiong, L.; Huang, L.; Peng, C.; Zhou, Q.M.; Dai, O. Two Pairs of 7,7′-Cyclolignan Enantiomers with Anti-Inflammatory Activities from Perilla frutescens. Molecules 2022, 27, 6102. [Google Scholar] [CrossRef]
- Fu, H.; Liu, Y. Complex gut–brain interactions underlying inflammatory bowel disease-related depression have translational implications in regenerative medicine: A narrative review. Regen. Med. Rep. 2024, 1, 59–75. [Google Scholar] [CrossRef]
- Voskarides, K.; Giannopoulou, N. The Role of TP53 in Adaptation and Evolution. Cells 2023, 12, 512. [Google Scholar] [CrossRef]
- Wu, B.; Xiao, Q.; Zhu, L.; Tang, H.; Peng, W. Icariin targets p53 to protect against ceramide-induced neuronal senescence: Implication in Alzheimer’s disease. Free. Radic. Biol. Med. 2024, 224, 204–219. [Google Scholar] [CrossRef]
- Zare, A.; Ghanbari, A.; Hoseinpour, M.J.; Boroujeni, M.E.; Alimohammadi, A.; Abdollahifar, M.A.; Aliaghaei, A.; Mansouri, V.; Arani, H.Z. Methamphetamine-Triggered Neurotoxicity in Human Dorsolateral Prefrontal Cortex. Galen Med. J. 2021, 10, e2016. [Google Scholar] [CrossRef]
- Martínez, M.-A.; Rodríguez, J.-L.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Maximiliano, J.-E.; Anadón, A.; Ares, I. Use of human neuroblastoma SH-SY5Y cells to evaluate glyphosate-induced effects on oxidative stress, neuronal development and cell death signaling pathways. Environ. Int. 2020, 135, 105414. [Google Scholar] [CrossRef]
- Aloi, M.S.; Su, W.; Garden, G.A. The p53 Transcriptional Network Influences Microglia Behavior and Neuroinflammation. Crit. Rev. Immunol. 2015, 35, 401–415. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Wang, Q.; Liu, M.; Sun, A.; Li, X. Possible Involvement of the IL-6/JAK2/STAT3 Pathway in the Hypothalamus in Depressive-Like Behavior of Rats Exposed to Chronic Mild Stress. Neuropsychobiology 2021, 80, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.; Sucic, S.; Monje, F.J.; Reisinger, S.N.; Savalli, G.; Diao, W.; Khan, D.; Ronovsky, M.; Cabatic, M.; Koban, F.; et al. STAT3 controls IL6-dependent regulation of serotonin transporter function and depression-like behavior. Sci. Rep. 2015, 5, 9009. [Google Scholar] [CrossRef]
- Aldossary, K.M.; Ali, L.S.; Abdallah, M.S.; Bahaa, M.M.; Elmasry, T.A.; Elberri, E.I.; Kotkata, F.A.; El Sabaa, R.M.; Elmorsi, Y.M.; Kamel, M.M.; et al. Effect of a high dose atorvastatin as added-on therapy on symptoms and serum AMPK/NLRP3 inflammasome and IL-6/STAT3 axes in patients with major depressive disorder: Randomized controlled clinical study. Front. Pharmacol. 2024, 15, 1381523. [Google Scholar]
- Frederick, M.I.; Siddika, T.; Zhang, P.; Balasuriya, N.; Turk, M.A.; O’donoghue, P.; Heinemann, I.U. miRNA-Dependent Regulation of AKT1 Phosphorylation. Cells 2022, 11, 821. [Google Scholar] [CrossRef]
- Cui, Y.H.; Fu, A.; Wang, X.Q.; Tu, B.X.; Chen, K.Z.; Wang, Y.K.; Hu, Q.G.; Wang, L.F.; Hu, Z.L.; Pan, P.H.; et al. Hippocampal LASP1 ameliorates chronic stress-mediated behavioral responses in a mouse model of unpredictable chronic mild stress. Neuropharmacology 2021, 184, 108410. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Perroud, N.; Burkhardt, S.; Schwald, M.; Ballmann, E.; La Harpe, R.; Malafosse, A. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol. Psychiatry 2007, 61, 240–245. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Huang, J.; Chen, R.; Zhang, Y.; He, X.; Duan, W.X.; Zou, Y.L.; Sun, M.M.; Sun, H.L.; Cheng, S.M.; et al. Autophagy dysfunction contributes to NLRP1 inflammasome-linked depressive-like behaviors in mice. J. Neuroinflammation 2024, 21, 6. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, W.; Wang, M.-L.; Lin, X.-Y. PI3K-AKT/mTOR Signaling in Psychiatric Disorders: A Valuable Target to Stimulate or Suppress? Int. J. Neuropsychopharmacol. 2024, 27, pyae010. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Li, M.; Xing, Z.; Li, X.; Qing, J.; Zhang, Y.; Zhu, L.; Qi, M.; Zou, X. Mechanism of action of quercetin in regulating cellular autophagy in multiple organs of Goto-Kakizaki rats through the PI3K/Akt/mTOR pathway. Front. Med. 2024, 11, 1442071. [Google Scholar] [CrossRef]
- Guo, N.; Wang, X.; Xu, M.; Bai, J.; Yu, H.; Zhang, L. PI3K/AKT signaling pathway: Molecular mechanisms and therapeutic potential in depression. Pharmacol. Res. 2024, 206, 107300. [Google Scholar] [CrossRef]
- Hou, T.; Qin, Y.; Zhang, Y.; Wen, G.; Qi, M.; Dong, W. Teriparatide regulates osteoblast differentiation in high-glucose microenvironment through the cAMP/PKA/CREB signaling pathway. J. South. Med. Univ. 2023, 43, 39–45. [Google Scholar]
- Cheng, X.; Huang, J.; Li, H.; Zhao, D.; Liu, Z.; Zhu, L.; Zhang, Z.; Peng, W. Quercetin: A promising therapy for diabetic encephalopathy through inhibition of hippocampal ferroptosis. Phytomedicine 2024, 126, 154887. [Google Scholar] [CrossRef]
- Bolger, G.B. The cAMP-signaling cancers: Clinically-divergent disorders with a common central pathway. Front. Endocrinol. 2022, 13, 1024423. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Deng, L.; Zhang, H.; He, B.; Cao, W.; Cui, Y. Pexidartinib (PLX3397) through restoring hippocampal synaptic plasticity ameliorates social isolation-induced mood disorders. Int. Immunopharmacol. 2022, 113, 109436. [Google Scholar] [CrossRef]
- Wang, C.; Guo, J.; Guo, R. Effect of XingPiJieYu decoction on spatial learning and memory and cAMP-PKA-CREB-BDNF pathway in rat model of depression through chronic unpredictable stress. BMC Complement. Altern. Med. 2017, 17, 73. [Google Scholar] [CrossRef]
| Node | Average Shortest Path Length | Betweenness Centrality | Closeness Centrality | Clustering Coefficient | Degree | Eccentricity |
|---|---|---|---|---|---|---|
| Apigenin | 2.469 | 0.0482 | 0.405 | 0 | 27 | 3 |
| Heterotropan | 2.469 | 0.0852 | 0.405 | 0 | 27 | 3 |
| Isoelemicin | 2.469 | 0.0470 | 0.405 | 0 | 27 | 3 |
| 1-(4-Hydroxy-2-methoxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one | 2.478 | 0.0977 | 0.404 | 0 | 26 | 3 |
| Alpha-asarone | 2.488 | 0.0299 | 0.402 | 0 | 25 | 3 |
| Beta-asarone | 2.488 | 0.0308 | 0.402 | 0 | 25 | 3 |
| Potential Core Targets | Binding Affinity Values (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|
| Target | PDB ID | Apigenin | Heterotropan | Isoelemicin | (1-(4-Hydroxy-2-methoxyphenyl)-3-(4-hydroxy-phenyl)prop-2-en-1-one) | α-Asarone | β-Asarone |
| TP53 | 8SVI | −5.07 | −3.95 | −3.78 | −4.68 | −4.35 | −4.21 |
| STAT3 | 6NJS | −3.96 | −1.98 | −3.14 | −3.3 | −2.38 | −2.65 |
| AKT1 | 8UW9 | −6.34 | −2.91 | −3.3 | −3.61 | −4.17 | −3.12 |
| PIK3CA | 9ASF | −5.34 | −2.91 | −2.94 | −4.06 | −3.29 | −3.03 |
| PIK3R1 | 6G6W | −2.83 | −1.89 | −2.46 | −2.23 | −2.29 | −2.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Wei, S.; Wang, R.; Liu, Y.; Zhong, Y.; Luo, H. Exploration of the Mechanisms of Acorus tatarinowii in the Treatment of Major Depressive Disorder Based on Network Pharmacology and Molecular Docking Techniques. Curr. Issues Mol. Biol. 2025, 47, 342. https://doi.org/10.3390/cimb47050342
Han L, Wei S, Wang R, Liu Y, Zhong Y, Luo H. Exploration of the Mechanisms of Acorus tatarinowii in the Treatment of Major Depressive Disorder Based on Network Pharmacology and Molecular Docking Techniques. Current Issues in Molecular Biology. 2025; 47(5):342. https://doi.org/10.3390/cimb47050342
Chicago/Turabian StyleHan, Li, Siwen Wei, Rong Wang, Yiran Liu, Yi Zhong, and Huaiqing Luo. 2025. "Exploration of the Mechanisms of Acorus tatarinowii in the Treatment of Major Depressive Disorder Based on Network Pharmacology and Molecular Docking Techniques" Current Issues in Molecular Biology 47, no. 5: 342. https://doi.org/10.3390/cimb47050342
APA StyleHan, L., Wei, S., Wang, R., Liu, Y., Zhong, Y., & Luo, H. (2025). Exploration of the Mechanisms of Acorus tatarinowii in the Treatment of Major Depressive Disorder Based on Network Pharmacology and Molecular Docking Techniques. Current Issues in Molecular Biology, 47(5), 342. https://doi.org/10.3390/cimb47050342






